Abstract
Changes in chromatin and epigenetic modifications have been associated with aging and aging-associated neurodegenerative diseases, although the causal relationship between these changes and disease-related pathology has been unclear. Recent studies have now made direct connections between neurodegeneration-associated proteins and derepression of repetitive element transcription due to changes in heterochromatin. We suggest that this derepression leads to an increased accumulation of intracellular double-stranded RNA (dsRNA), with an attendant induction of innate immune responses that contribute to the neuroinflammation found in essentially all age-associated neurodegenerative diseases.
Keywords: Frontotemporal dementia, amyotrophic lateral sclerosis (ALS), Alzheimer disease, tauopathy, repetitive elements, retro-transposons
Tar DNA binding protein 43 kDa (TDP-43) is an RNA-binding protein identified as a key component of ubiquitinated cytoplasmic deposits observed in affected neurons in both frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS).1 TDP-43 is mutated in ~5% of familial ALS cases (fALS),2 implying a causal role in the disease. Our previous work has shown that deletion of the Caenorhabditis elegans TDP-43 ortholog (TDP-1) results in the increased accumulation of dsRNA composed, in part, of repetitive element sequences.3 Our recent study demonstrates that TDP-1 directly interacts with HPL-2 (heterochromatin protein-like 2), a C elegans ortholog of HP1 (heterochromatin protein 1).4 Furthermore, ChIP-seq (chromatin immunoprecipitation characterized by high-throughput sequencing) experiments demonstrate that deletion of TDP-1 leads to a dramatic chromatin relocalization of HPL-2. Heterochromatin protein-like 2, like its mammalian counterpart HP1 (α, β, and γ) associates with heterochromatic regions and plays a role in suppressing repetitive element expression.5,6 Deletion of HPL-2 also leads to an increase in the dsRNA, suggesting a model in which the co-transcriptional association of TDP-1 with chromatin leads to recruitment of HPL-2, resulting in repression of repetitive element transcription and suppression of dsRNA formation. This model is summarized in Figure 1. Knockdown of TDP-43 in mammalian models also leads to a profound increase in the accumulation of repetitive element transcripts,7 suggesting that this model may also apply in human cells.
Figure 1.
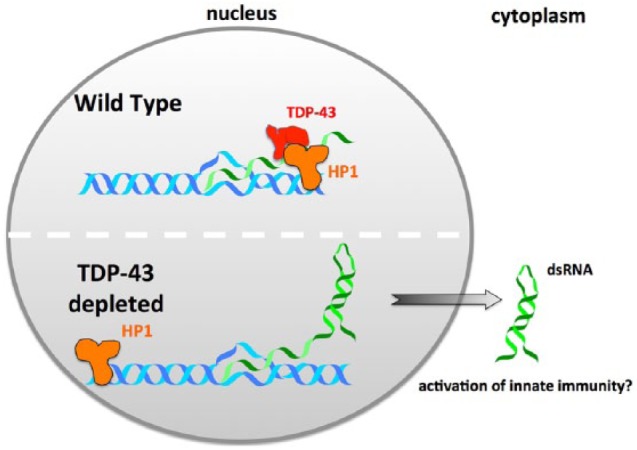
Model for how depletion of TDP-43 leads to increased accumulation of dsRNA. Speculative model based on C elegans studies illustrating how the nuclear depletion of TDP-43 observed in neurons and glia in ALS/FTD patients could lead to accumulation of dsRNA and upregulation of innate immune responses. ALS indicates amyotrophic lateral sclerosis; dsRNA, double-stranded RNA; FTD, frontotemporal dementia; TDP-43, Tar DNA binding protein 43 kd.
FTD can also be caused by inherited mutations in the tau gene (microtubule-associated protein tau [MAPT]). Although tau is a well-established microtubule-binding protein, work with Drosophila tauopathy models has demonstrated that expression of FTD-associated R406W mutant tau leads to chromatin decondensation,8 likely driven by lamin dysfunction.9 Recent studies now show that fly models expressing R406W tau also have an increase in the transposable element transcripts.10,11 Similar observations have been made in a Drosophila TDP-43 model, in which retrotransposon transcripts are increased and appear to contribute to pathology.12 Although the cause of increased retrotransposon expression in various neurodegeneration models is unclear, Sun et al10 showed that expression of R406W tau reduced the abundance of PIWI-interacting RNAs (piRNAs) targeting retrotransposons. In addition, knockdown of the piRNA-specific Argonaute protein PIWI was synthetic lethal within the context of transgenic tau expression, supporting the view that piRNAs are a causal mediator of neuronal death in tauopathy. Considering that the recruitment of HP1 can be facilitated by piRNAs, it is possible that piRNA dysfunction/repression is a major contributor to RE-mediated neuronal death in multiple forms of neurodegeneration. Whether TDP-43-opathies also disrupt piRNAs is an open question.
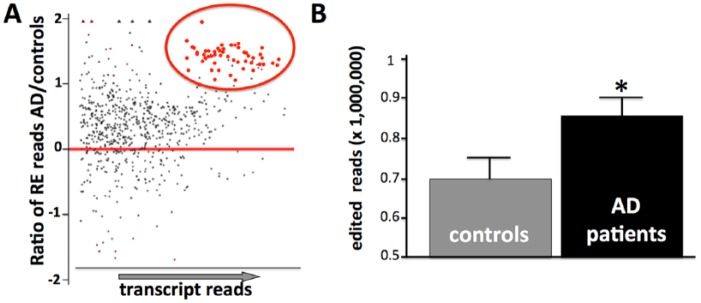
Multiple lines of evidence suggest that the chromatin changes and increased accumulation of repetitive element transcripts identified in the invertebrate models also occur in the associated neurodegenerative diseases. Repetitive element transcripts are increased in the brains of ALS patients, in particular in patients with an expansion of the C9orf72 hexanucleotide repeat.13 Increases in the endogenous retroviral class of transposable elements have been observed in the brains of Alzheimer disease (AD) patients11,12 as has increased expression of genes usually silenced by heterochromatin.8 Although it has been reported that long interspersed nuclear elements (LINE) transcripts do not increase in the AD,14 our reanalysis (Figure 2A) of published RNA-seq (global transcriptome analysis high-throughput sequencing) data15 from AD patients and controls is consistent with increased levels of transcripts from multiple classes of repetitive elements, including LINEs, short interspersed nuclear elements (SINEs), and satellite sequences. Interestingly, analysis of this data also reveals an increase in the adenosine-to-inosine RNA editing, a post-transcriptional modification of transcripts that only occurs on dsRNA (Figure 2B).
Figure 2.
Increased repetitive elements and RNA editing in AD frontal cortex. (A) MA plot of relative RE abundance based on reanalysis of published RNA-seq data (Scheckel et al16) from postmortem frontal cortex. MA plots are typically used to visualize gene expression changes by plotting the log of the expression ratios between experimental and control samples against mean average expression levels for each gene. Here we plot the log ratio of expression for individual repetitive element classes calculated from patient and control RNA-seq data. Note both the overall shift of the expression ratios to positive log values (ie, up in AD patients) and the set of RE with statistically-significant transcript abundance over-represented in AD brains (red circle). (B) Average number of adenosine-to-inosine edited reads in the same RNA-seq data. AD indicates Alzheimer disease; RNA-seq, global transcriptome analysis high-throughput sequencing. Error bar = SEM; *P < .05.
Unresolved is the degree to which heterochromatin changes and increased levels of repetitive element transcripts contribute to disease-relevant pathology. Long interspersed nuclear element 1 (LINE-1) can undergo somatic transposition in neuronal precursor cells,16 and therefore, the increased levels of retrotransposon transcripts in ALS and AD patient brains could lead to transposition events that alter gene expression and/or induce a DNA damage response. However, identification and quantification of transposition events in patient samples is technically challenging, and has not been directly demonstrated in neurodegenerative diseases. Alternatively, as repetitive element transcripts can form both intrastrand (eg, from inverted repeats of Alu elements in 3′ untranslated regions [UTRs]) and interstrand (eg, from complementary base-pairing of LINEs transcribed from opposite strands) dsRNA, the increased repetitive element transcripts in ALS and AD could lead to an increase in cytoplasmic dsRNA. As dsRNA is a classic inducer of interferon and anti-viral innate immune responses, increased dsRNA from endogenous transcripts could lead to inappropriate, and potentially pathogenic, immune responses. Indeed, accumulation of dsRNA composed of Alu sequences has been claimed to underlie geographic atrophy, an untreatable advanced form of age-related macular degeneration that results from degeneration of retinal pigmented epithelium cells.17 Very recently, a study from the Albers lab18 has demonstrated that transgenic expression of dsRNA in mice can directly induce central nervous system (CNS) neurodegeneration. In addition, these investigators provide evidence that dsRNA can be specifically detected in the brains of familial ALS patients containing the C9orf72 hexanucleotide expansion, which we believe to be the first direct demonstration of increased dsRNA in a neurodegenerative disease.
If our hypotheses concerning neurodegeneration, heterochromatin, and dsRNA are correct, we anticipate that accumulation of dsRNA will be discovered in additional neuro-degenerative conditions, including AD. Furthermore, we would predict that treatments that block heterochromatin derepression or dsRNA-induced innate immune activation will ameliorate the neuroinflammation associated with neurodegenerative conditions in animal models and, hopefully, in human neurodegenerative diseases.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by NINDS award R01 NS063964 to CDL.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: CDL wrote the manuscript which was revised and edited by TKS, PKG and TJL performed the bioinformatic analyses presented in Figure 2.
ORCID iD: Christopher D Link  https://orcid.org/0000-0003-2271-7697
https://orcid.org/0000-0003-2271-7697
References
- 1. Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. [DOI] [PubMed] [Google Scholar]
- 2. Ling SC, Polymenidou M, Cleveland DW. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron. 2013;79:416–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saldi TK, Ash PE, Wilson G, et al. TDP-1, the Caenorhabditis elegans ortholog of TDP-43, limits the accumulation of double-stranded RNA. EMBO J. 2014;33:2947–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saldi TK, Gonzales P, Garrido-Lecca A, et al. The Caenorhabditis elegans ortholog of TDP-43 regulates the chromatin localization of the heterochromatin protein 1 homolog HPL-2. Mol Cell Biol. 2018;38:e00668–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garrigues JM, Sidoli S, Garcia BA, Strome S. Defining heterochromatin in C. elegans through genome-wide analysis of the heterochromatin protein 1 homolog HPL-2. Genome Res. 2015;25:76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McMurchy AN, Stempor P, Gaarenstroom T, et al. A team of heterochromatin factors collaborates with small RNA pathways to combat repetitive elements and germline stress. Elife. 2017;6:e21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li W, Jin Y, Prazak L, Hammell M, Dubnau J. Transposable elements in TDP-43-mediated neurodegenerative disorders. PLoS ONE. 2012;7:e44099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frost B, Hemberg M, Lewis J, Feany MB. Tau promotes neurodegeneration through global chromatin relaxation. Nat Neurosci. 2014;17:357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frost B, Bardai FH, Feany MB. Lamin dysfunction mediates neurodegeneration in tauopathies. Curr Biol. 2016;26:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun W, Samimi H, Gamez M, Zare H, Frost B. Pathogenic tau-induced piRNA depletion promotes neuronal death through transposable element dysregulation in neurodegenerative tauopathies. Nat Neurosci. 2018;21:1038–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo C, Jeong HH, Hsieh YC, et al. Tau activates transposable elements in Alzheimer’s disease. Cell Rep. 2018;23:2874–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krug L, Chatterjee N, Borges-Monroy R, et al. Retrotransposon activation contributes to neurodegeneration in a Drosophila TDP-43 model of ALS. PLoS Genet. 2017;13:e1006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prudencio M, Gonzales PK, Cook CN, et al. Repetitive element transcripts are elevated in the brain of C9orf72 ALS/FTLD patients. Hum Mol Genet. 2017;26:3421–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Protasova MS, Gusev FE, Grigorenko AP, Kuznetsova IL, Rogaev EI, Andreeva TV. Quantitative analysis of L1-retrotransposons in Alzheimer’s disease and aging. Biochemistry (Mosc). 2017;82:962–971. [DOI] [PubMed] [Google Scholar]
- 15. Scheckel C, Drapeau E, Frias MA, et al. Regulatory consequences of neuronal ELAV-like protein binding to coding and non-coding RNAs in human brain. Elife. 2016;5:e10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muotri AR, Chu VT, Marchetto MC, Deng W, Moran JV, Gage FH. Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition. Nature. 2005;435:903–910. [DOI] [PubMed] [Google Scholar]
- 17. Tarallo V, Hirano Y, Gelfand BD, et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149:847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodriguez S, Schrank BR, Sahin A, et al. Genome-encoded cytoplasmic double-stranded RNAs, found in C9ORF72 ALS-FTD brain, provoke propagated neuronal death. BioRvix 248328 (Preprint). https://www.biorxiv.org/content/early/2018/01/19/248328. Published January 19, 2018. Accessed November 7, 2018. [DOI] [PMC free article] [PubMed]