Abstract
Heart failure (HF) is one of the most important healthcare issues due to its prevalence, high morbidity and mortality, as well as its economic burden. A shift in the healthcare model towards reducing inpatient hospitalizations might have a significant impact on HF-related costs and quality of life. Recently, wireless monitoring has begun to be an essential part of the management in the patient with HF. The CardioMEMS HF system is one of the best examples pertaining to the success in this field. This article will discuss the CardioMEMS HF system and the rationale behind its development.
Keywords: heart failure, microelectrical mechanical system, MEMS, New York Heart Association
Introduction
Heart failure (HF) is one of the most important healthcare issues because of its high morbidity and mortality rates, prevalence, as well as its economic and psychological burden. As of 2012, approximately 2.4% of the United States (US) population were affected by HF. The prevalence is increasing with age, as almost 12% of both men and women ⩾75 years of age are living with HF.1 With the growth of the US population, both the prevalence and the total number of HF-labeled patients is expected to increase by 23% and 46%, respectively by 2030.2
The mortality rate is higher after a HF hospitalization. Although HF hospitalizations have declined in recent years, HF remains the leading cause of in-hospital death in the US. The number of HF deaths was as high in 2006 as it was in 1995. By 2035, >130 million adults in the US population (45.1%) are projected to have some form of cardiovascular disease (CVD).3
The cost of HF care is high and causes a significant burden on the US healthcare system. With the assumption of the continuation of present care practices, a marked increase in healthcare costs is imminent due to the longer survival and consequent increase in the aging population, as a result of the development and implementation of life-prolonging therapies. Subsequently, this will ultimately lead to more patients at risk for development of HF.
More specifically, the cost of HF management is projected to increase markedly; a 2.5-fold increase from US$20.9 billion in 2012 to US$53.1 billion by 2030. Of note, 80% of the costs are related to HF hospitalizations. The total cost, including indirect costs, is estimated to increase from US$31 billion in 2012 to US$70 billion by 2030. The estimated average cost for patients with HF during the final 2 years of life is more than US$156,000, and 75% of this cost is attributed to HF-related hospital admissions during the last 6 months of life.4
The best solution is prevention, which is through management of predisposing factors, such as diabetes mellitus, hypertension and ischemic heart disease. A shift in the healthcare model towards reducing inpatient hospitalizations might have a significant impact on HF-related costs, quality of life and mortality.
Nowadays, the revolution in communication technology and digital connectivity have significantly changed the way of living. The vast majority of US citizens now own a cellular telephone (95%) and 77% own a smart phone.5 Healthcare facilities and networks have been slow to follow this revolution. However, wireless monitoring has recently emerged to be an essential part in the management of the HF patient. The revolution of social media and applications is becoming a potential rich resource in patient-centered healthcare systems. Cost-effectiveness analyses for the care of HF patients have been favorable towards remote monitoring.6,7
Approximately 50% of patients with heart failure have preserved ejection fraction (EF) with prevalence varying from 40% to 71%.8 In heart failure with preserved ejection fraction (HFpEF) due to increased myocardial stiffness, small changes in volume can cause large increase in filling pressures. Chronic elevation in left atrial pressures can cause pulmonary hypertension which is associated with an increased risk of death. Also, right ventricular dysfunction is common in HFpEF and is associated with increased mortality independent of pulmonary hypertension.
Guidelines
The American College of Cardiology (ACC) and the American Heart Association (AHA) released guidelines8 on the diagnosis and management of HF. The guidelines are based on HF’s four progressive stages. Progression from one stage to the next is accompanied by a reduction in 5-year survival.
Stage A describes patients at risk for HF who are asymptomatic and do not have structural heart disease. Stage B includes patients with structural heart disease who are asymptomatic and do not have symptoms of HF; it includes New York Heart Association (NYHA) functional class I. Stage C contains patients with a history of structural heart disease with and without symptoms, including NYHA functional class I–IV: I in which there are no symptoms; II in which there is slight limitation with normal physical activity; III in which there is marked limitation with less than normal physical activity and IV, labeled for those who are symptomatic at rest or unable to engage in trivial amounts of physical activity. Stage D includes patients with refractory HF who have failed conventional guideline-directed device and medical therapies, now requiring specialized interventions. This is solely NYHA functional class IV. Interventions at each stage are aimed at modifying risk factors in stage A, treating underlying heart disease in stage B, as well as decreasing morbidity and mortality in stages C and D.
Treatment
In stage A, diabetes mellitus, dyslipidemia and hypertension control are the cornerstones in preventing the progression to overt HF. Long-term management of hypertension leads to a risk reduction of approximately 50% in the development of HF. Diabetes mellitus is an important risk factor for developing HF, with hemoglobin A1c levels predicting HF incidents. Diuretic-based therapy has been shown to prevent HF. Angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs) and beta-adrenergic blockers also are effective.9 The treatment of dyslipidemia with β-hydroxy β-methylglutaryl-Coenzyme A (HMG-CoA) reductase inhibitors (statins) reduces the risk of HF in high-risk patients.10
Other conditions that may contribute to HF (e.g. obesity and tobacco use) should be controlled or avoided. Obesity is linked to an increased risk of HF development and progression. Tobacco use is strongly connected to the risk of incidental HF and cessation is strongly recommended.11
Similar to stage A, stage B HF patients should continue controlling risk factors, such as diabetes mellitus, dyslipidemia and hypertension. ACE inhibitors should be used to prevent symptomatic HF and reduce morbidity and mortality. ARBs are an alternative choice for those who cannot tolerate ACE inhibitors. In addition, beta-adrenergic blockers should be used to reduce morbidity and mortality in these patients. Statins have also proven to prevent symptomatic HF and cardiovascular events. ACE inhibitors or ARBs and beta-adrenergic blockers should be used in all patients with reduced left ventricular ejection fraction (LVEF) to prevent symptomatic HF and reduce morbidity and mortality. SOLVD (Studies Of LV Dysfunction) treatment showed a reduction in mortality with enalapril treatment and a SOLVD prevention trial showed a reduction in hospitalization with enalapril with a trend towards reduction in mortality.12
In stage C, in addition to all interventions mentioned for patients with stages A and B HF, patients may also need interventions for symptomatic management. If there is history or evidence of fluid retention, diuretics should be used, and the patients should be monitored for adverse effects such as electrolyte disturbances and volume depletion. Mineralocorticoid receptor antagonists should be used in NYHA functional classes II through IV HF with a LVEF of 35% or less, and the patients should be monitored for renal insufficiency and hyperkalemia. Other medications may also be useful in certain cases including combination therapy with hydralazine and isosorbide dinitrate (ISDN), digoxin and anticoagulants.13 In the V-HeFT trial, hydralazine/ISDN showed a benefit over placebo but the benefit was modest. Subsequently the V-HeFT-2 trial showed superiority of enalapril over hydralazine/ISDN and after this trial hydralazine/ISDN was suggested as an alternative to angiotensin-converting enzyme inhibitor (ACEI) among patients who cannot tolerate it. The A-HeFT trial14 showed a benefit of mortality reduction of 43% among African Americans who were treated with hydralazine/ISDN among patients with reduced LVEF or LV dilatation.
The PARADIGM-HF trial15 showed superiority of valsartan-sacubitril over enalapril in reducing the risk of death and hospitalization among patients with NYHA class 2, 3, 4 with EF less than 40%. The ivabradine shift trial16 showed the benefit of heart rate reduction with ivabradine for the improvement of clinical outcomes in heart failure
The guidelines also mention nonpharmacological interventions, such as exercise training, sodium restriction, sleep disorder treatment, as well as device therapy including automatic implantable cardioverter defibrillators (AICDs) and cardiac resynchronization therapy defibrillator (CRT-D) implantation.17,18
Patients are usually admitted to the hospital for HF due to worsening signs and symptoms of congestion.19 Previous investigations have shown that increases in the intracardiac and pulmonary artery (PA) pressures are the cause of the congestion and begin a few days to weeks prior to the onset of overt symptoms and signs of HF, thereby leading to hospitalization.20,21 Thus, early intervention aiming towards the reduction of these pressures might lead to a reduction in the risk of hospitalization for HF. In a clinical trial,22 it was shown that the increases in intracardiac and PA pressures occur independently of weight changes, which indicates that monitoring of weight alone is inadequate to identify congestion early enough to prevent HF admission. This finding might explain why patient-dependent telemonitoring systems, which report solely daily weight change and symptoms of heart failure, have not decreased hospital admission and mortality rates.23 Thus, implantable device systems for monitoring of intracardiac and PA pressures have been developed.24–28
The CardioMEMS HF system
The CardioMEMS HF System29 (Micro-Electro-Mechanical HF System, Abbott Medical, Inc., Abbott Park, Illinois, USA) provides PA hemodynamic information used for the monitoring and management of HF. It measures the changes in PA pressure, so physicians can use them to modify HF treatment. It includes a delivery catheter with a hermetically sealed implantable wireless sensor, hospital or patient electronic system and patient database (see Figures 1–4).
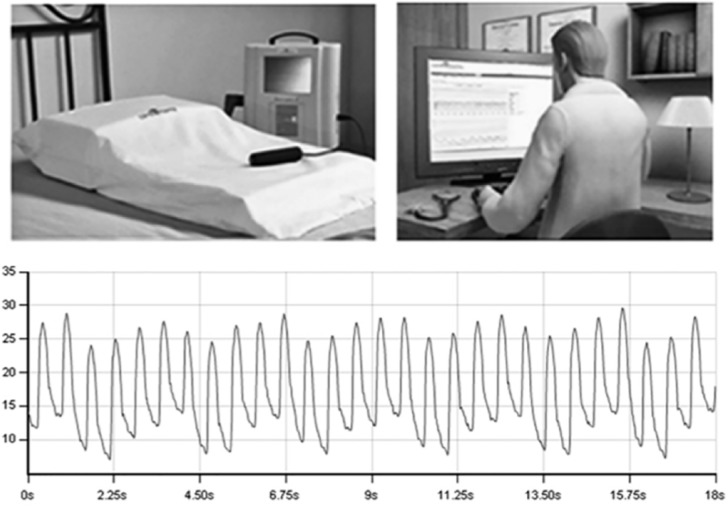
Figure 1.
CardioMEMS transducer and transmitting system.
MEMS, Micro-Electro-Mechanical System.
Figure 2.
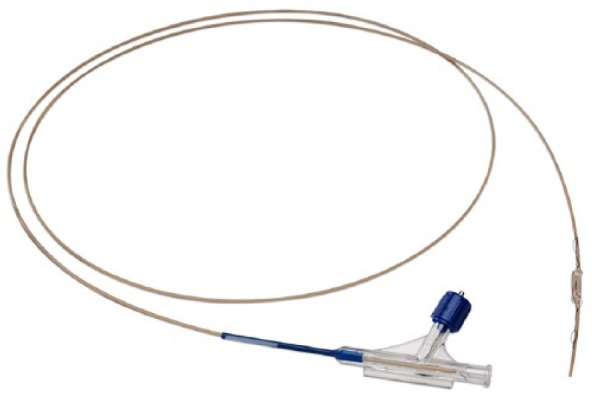
CardioMEMS delivery catheter.
MEMS, Micro-Electro-Mechanical System.
Figure 3.
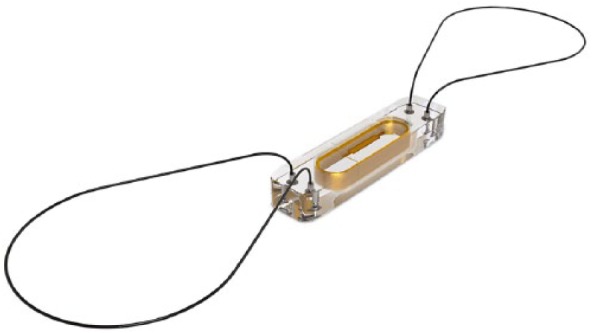
CardioMEMS PA sensor.
MEMS, Micro-Electro-Mechanical System; PA, pulmonary artery.
Figure 4.
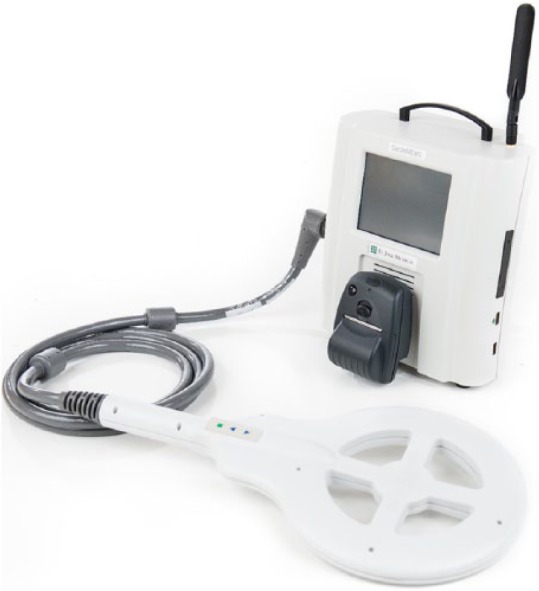
Hospital electronics system.
The wireless sensor is designed for permanent implantation into the distal PA. The PA sensor consists of a three-dimensional coil and pressure sensitive capacitor encased between two wafers of fused silica. The fused silica assembly is encased in silicone. The coil electromagnetically couples the pressure sensitive capacitor to the electronics system, allowing the remote measurement of the resonant frequency of the circuit without the need for an on-board battery. This resonant frequency is then converted to a pressure measurement. The PA sensor provides noninvasive hemodynamic data, which are collected in the physician’s clinic, hospital or patient’s home. The data include PA pressure waveforms, heart rate, as well as systolic, diastolic and mean PA pressures (see Figure 1).
The data are transmitted to a secure website, where PA monitoring information is available at all times. Changes in PA pressure can be used in conjunction with symptoms and signs of HF to guide modifications of medical therapy.
The CardioMEMS HF system is indicated for monitoring PA pressure and heart rate in NYHA class III HF patients, who have been hospitalized for HF in the previous year. The hemodynamic data are used by physicians for management of HF and to reduce hospitalizations caused by HF exacerbation, mainly by using or increasing the dose of diuretics to reduce the congestion before causing symptoms.30
The CardioMEMS HF system may not be appropriate for implantation in the following conditions: patients with an active infection, history of recurrent deep vein thrombosis or pulmonary embolism, unable to tolerate a right heart catheterization, patients with an estimated glomerular filtration rate <25 ml/min who are unresponsive to diuretic therapy or on chronic renal dialysis, congenital heart disease or mechanical right heart valve, known coagulation disorders, hypersensitivity to aspirin or clopidogrel, patients who have undergone implantation of CRT-D within the past 3 months, body mass index (BMI) > 35 kg/m2 and chest circumference >165 cm.30
The CardioMEMS HF system was tested in a trial called ‘wireless pulmonary artery hemodynamic monitoring in chronic heart failure’ (the CHAMPION trial).30
On 8bDecember 2011, the US Food and Drug Administration (FDA) Circulatory System Devices Panel reviewed the CardioMEMS CHAMPION HF monitoring system premarket approval (PMA) application.31
Multicenter clinical trials
The CHAMPION trial30 was a prospective, randomized, multicenter, single-blind clinical trial. A total of 550 patients with NYHA functional class III heart failure, irrespective of LVEF, and a previous hospital admission for HF within the preceding 12 months were enrolled in 64 centers in the US. They were randomly assigned to management with a wireless implantable hemodynamic monitoring (W-IHM) system (treatment group) or to a control group for at least 6 months. In the treatment group, physicians used daily measurement of PA pressures in addition to standard of care, whereas standard of care therapy alone was used in the control group. The primary efficacy endpoint was HF-related hospitalization at 6 months. The safety endpoints were freedom from system-related complications at 6 months and freedom from pressure sensor failures. All analyses were by intention to treat.
In 6 months, there were 84 HF-related hospitalization in the treatment group, as compared with 120 in the control group. During the entire follow up (mean of 15 months), the treatment group had a 37% reduction in HF-related hospitalizations, compared with the control group. Overall freedom from system-related complications was 98.6%, compared with a prespecified performance criterion of 80%, while overall freedom from pressure sensor failures was 100%.
The interpretation of the results is consistent with a large and significant reduction in hospitalizations for patients with NYHA functional class III HF who were managed with a W-IHM system. The information about PA pressure added to the clinical signs and symptoms improved the overall management of HF.
The CardioMEMS device has been approved for patients with HFpEF and heart failure with reduced ejection fraction (HFrEF) by the US FDA in 2014. In the CHAMPION trial, 119 patients had HFpEF. In this subgroup, the hospitalization rate was 0.33 in the control group versus 0.18 in the treated group. This was lower when compared with the hospitalization rate in patients with HFrEF and this was statistically significant.30,32
On the other hand, there is evidence that hemodynamically guided management of patients with HFpEF decreases the incidence of decompensation leading to hospitalization, as compared with standard HF management strategies.33
The CHAMPION trial chose to examine the effect of hemodynamic monitoring on HF hospitalization rates only for NYHA functional class III patients, who are likely to have a measurable decrease in hospitalization rates. NYHA functional classes I and II are rarely hospitalized for decompensated HF and thus they are not expected to have considerable changes in the rate of hospitalizations. The patients with NYHA functional class IV usually require frequent hospitalizations despite continuous monitoring of cardiac filling pressures and therefore rates of hospitalization may be inappropriate to test. However, those patients may still benefit from hemodynamic monitoring in their management.
The CHAMPION trial overcame the limitations that other studies of implantable hemodynamic monitoring systems were affected by, such as lack of control group, small numbers of patients and statistically underpowered. This trial was not powered to detect a mortality benefit. However, the significant reduction in HF hospitalization rates would most probably lead to an indirect reduction in mortality, as with each HF hospitalization the physiological reserve of the heart decreases and the mortality increases.
The adverse event rates were similar to those with right heart catheterization,34 and better than those with other permanent implants used in HF management, such as cardiac defibrillators and biventricular pacemakers,35–36 because of lack of the complications associated with the placement of subcutaneous impulse generators and transvenous leads.
In 2014, the US FDA approved the CardioMEMS device for NYHA class III heart failure patients who were hospitalized for heart failure in the previous year.
In the 2016 European Society of Cardiology guidelines, CardioMEMS received a class IIb recommendation for a directed therapy management and monitoring tool in HF patients.37
The CardioMEMS HF system post-approval study38 was a prospective study aimed to enroll 1200 patients with CardioMEMS. It was started in 2015. The first 300 patients enrolled in the post-approval study were older than the CHAMPION cohort and the mean age was 69 years of age compared with about 62 years in CHAMPION trial, were more often women (38% versus 28% in CHAMPION) and were more likely to have HFpEF (41% versus about 22%). Initial data on 300 patients showed that during the first 6 months the hospitalization rate for worsening heart failure was 0.20. This was in contrast with 0.32 in the treatment group in the CHAMPION trial and 0.44 among the control group.
The following are other important studies which have addressed the telemonitoring techniques for HF.
PAPIRUS (Pulmonary Artery Pressure by Implantable device Responding to Ultrasonic Signal) II trial39 evaluated the feasibility of home monitoring of patients with chronic HF by acoustic wireless communication with an implant directly measuring PA pressure. It was a prospective, multicenter phase I study. It consisted of 31 patients with NYHA functional class III and IV HF. This study showed that home monitoring of PA pressures was feasible, well tolerated and accepted by patients in everyday ambulatory conditions. Initial data indicated that home monitoring may help in directing the management in patients with severe cardiopulmonary disease.
The COMPASS-HF (Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure) trial 40 was a multicenter, prospective, single-blind, randomized, parallel-controlled trial of 274 NYHA class III or IV HF patients who received an implantable continuous hemodynamic monitor. Patients were randomized to a chronicle or control group. Both groups received optimal medical therapy but the hemodynamic information from the monitor was used to guide patient management only in the chronicle group. There were no pressure sensor failures and system-related complications occurred in only 8% of patients. The primary efficacy endpoint was not met because, although the chronicle group had a 21% lower rate of all HF-related events compared with the control group, this difference did not reach statistical significance. A 36% reduction in the relative risk of a HF-related hospitalization in the chronicle group was shown in a retrospective analysis of the time to first HF hospitalization.
The REDUCE (reducing events in patients with chronic heart failure) II trial41 was a multicenter, prospective, single-blind, randomized, parallel-controlled trial in approximately 850 patients, which was designed to assess the safety of the chronicle ICD system, a single chamber ICD with a hemodynamic monitoring system, and the effectiveness of a management strategy guided by intracardiac pressure information among ICD-indicated NYHA functional class II or III HF patients. The patients were randomized to the chronicle group or control group. Both received optimal medical therapy but the hemodynamic information from the device was used to guide patient management only in the chronicle group. The primary safety endpoint was met, freedom from system-related complications. However, the rate of HF hospitalizations, as well as emergency department and urgent care visits, did not differ between groups. REDUCE II was unable to test clinical efficacy endpoints adequately. In contrast with the CardioMEMS device, the chronicle device measured right ventricular pressures and it was designed for HFrEF.
HF disease management trials
There have been at least six randomized control trials (RCTs) involving more than 1000 patients looking into HF disease management. Some of the RCTs showed a benefit in decreasing hospitalization while others did not. The Coordinating study evaluating Outcomes of Advising and Counseling in Heart failure (COACH) trial42 did not find any differences in mortality and hospitalization among HF patients assigned to routine care compared with 9 visits by a specialized nurse and 18 visits by a specialized nurse as well as home visits and counseling by pharmacist. The Israel heart failure disease management study (IHF-DMS) was a study which did not find any difference in mortality or hospitalization among HF patients.43 The DIAL trial44 (randomized trial of phone intervention in chronic heart failure) randomly assigned 1518 Argentinean patients with HF to either routine care or an intervention consisting of an explanatory booklet plus periodic telephone contact by a specialized nurse over the course of 1 year. The intervention resulted in a lower rate of death or hospitalization for HF compared with the usual care at a mean of 16 months. However, the benefit was small. These are complex interventions applied in very different health ecosystems and this makes it challenging to draw generalizable conclusions.
In an observational study use of a PA pressure monitor (CardioMEMS, Abbott Medical, Inc.) was associated with a 30% reduction in mortality.45 In 1 year after implantation of the sensor, patients with the device had a mortality rate of 0.22 per patient-year versus 0.30 in controls, for a hazard ratio of 0.70 [95% confidence interval (CI), 0.59–0.83; p < 0.0001]. This was an observational study with its inherent limitations.
The GUIDE-HF (hemodynamic-GUIDEd management of Heart Failure) trial is a randomized control trial in NYHA functional classes II–IV, which is ongoing and likely to show directions in the future if any improvement in mortality occurs with the CardioMEMS device. The GUIDE-HF investigational device exemption (IDE) trial will include approximately 3600 patients at approximately 140 sites and is expected to be completed by 2023.
The study is intended to measure all-cause mortality and heart failure hospitalizations.
A summary of the above-mentioned trials is shown in Table 1.
Table 1.
Trials on implantable hemodynamic monitoring in heart failure patients.
| Name of trial | Type of trial | NYHA functional class | Clinical endpoints | Specific inclusion criteria | Number of patients enrolled | Duration of clinical trial | |
|---|---|---|---|---|---|---|---|
| 1. | CHAMPION trial30 |
Double-blind, randomized control study | III | Reduction in hospitalization | Diagnosis of HF for 3 months with preserved or reduced EF and BMI < 35 kg/m2 | 550 | Until last enrolled patient reached 6 months |
| 2 | PAPIRUS trial39 |
Prospective, multicenter phase I study | III, IV | Home monitoring of PAP was feasible, safe and accepted by patients in every day ambulatory conditions | Age > 18 years and followed regularly in HF clinic for 6 months | 31 | 6 months |
| 3 | REDUCE-HF trial41 | Prospective, multicenter, single-blind, randomized, parallel-controlled study | II, III | Use of a single chamber AICD with a hemodynamic monitoring system Primary endpoint was met but the rates of hospitalization and urgent care visits did not differ between groups |
Age > 18 years and eGFR > 30 ml/min/m2 | 850 | 12 months |
| 4 | COMPASS-HF trial40 | Prospective, multicenter, single-blind randomized, parallel-controlled study | III, IV | Chronicle group had an insignificant 21% lower rate of all HF-related events compared with the control group | Age > 18 years and received optimal medical HF therapy for at least 3 months prior to enrollment | 274 | 6 months |
| 5. | GUIDE-HF trial (ongoing)46 |
Prospective, multicenter, single-blind randomized, parallel-controlled study | II, III, IV | Recurrent heart failure hospitalization; death from any cause | Age > 18 years; Diagnosis and treatment for HF (regardless of LVEF) for >90 days | 3600 | 5 years |
AICD, implantable cardioverter defibrillator; BMI, body mass index; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HF, heart failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PAP, pulmonary artery pressure.
Conclusions and future directions
The best solution for HF morbidity and mortality reduction is prevention through managing the conditions predisposing to HF such as diabetes mellitus, hypertension and ischemic heart disease. Further research on HF management and prevention strategies should be expanded and applied to sex-based factors and across different ethnicities. The transition in the healthcare model is now towards a reduction in HF hospitalizations to minimize HF treatment-related costs in order to mitigate the limited healthcare resources. There are some obstacles facing these goals, such as cost-effectiveness, medico-legal issues, security of patient’s data and validation, which should be resolved.
Self-management by patients had largely been unsuccessful because of the advanced age of HF patients and the complexity of treatment. The addition of self-management counseling to an educational intervention in patients with mild to moderate HF did not reduce HF hospitalizations or mortality. All strategies should still aim to minimize patient responsibility in monitoring. A multidisciplinary approach can reduce HF hospital admissions and improve quality of life and mortality.
The generalizability of the CardioMEMS HF system results to most patients with NYHA class III HF is adequate, because of a significant reduction in HF hospitalization and very few contraindications. To date, CardioMEMS is the only device or intervention other than guideline-directed medical therapy that has been shown to impact congestive heart failure (CHF) readmission rates, and is expected to show a mortality benefit in the ongoing GUIDE-HF trial.
The major restriction of device monitoring is in patients with stage IV or V chronic kidney disease due to the limited response to diuresis, even with high PA pressure. Children under 18 years were excluded in all these clinical trials. Another limitation will be the high cost of the CardioMEMS device which likely will come down in the near future.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Puvanalingam Ayyadurai  https://orcid.org/0000-0001-7927-655X
https://orcid.org/0000-0001-7927-655X
Contributor Information
Puvanalingam Ayyadurai, Bronx-Lebanon Hospital Center, Division of Cardiology, Bronx, NY, USA.
Hassan Alkhawam, St. Louis University School of Medicine, Division of Cardiology, St. Louis, MO, USA.
Muhammad Saad, Bronx-Lebanon Hospital Center, Division of Cardiology, Bronx, NY, USA.
Mohammed Adel Al-Sadawi, SUNY Downstate Medical Center at Brooklyn, Department of Medicine, Brooklyn, NY, USA.
Niel N. Shah, Smt NHL Municipal Medical College, Department of Medicine, Ahmedabad, Gujarat, India
Constantine E. Kosmas, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Timothy J. Vittorio, BronxCare Health System, Division of Cardiology, 1650 Grand Concourse, 12th Floor, Bronx, NY 10457, USA.
References
- 1. Roger VL, Go AS, Lloyd-Jones DM, et al. ; On behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2012 update: a report from the American Heart Association [published correction appears in Circulation. 2012; 125: e1002]. Circulation 2012; 125: e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013; 6: 606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics -2018 update: a report from the American Heart Association. Circulation 2018; 137: e67–e492. [DOI] [PubMed] [Google Scholar]
- 4. Jencks SF, Williams MV. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009; 360: 1418–1428. [DOI] [PubMed] [Google Scholar]
- 5. Pew Research Internet Project. Mobile technology, http://www.pewinternet.org/factsheet/mobile/ (January 2017, accessed 20 May 2018).
- 6. Klersy C, De Silvestri A, Gabutti G, et al. Economic impact of remote patient monitoring: an integrated economic model derived from a meta-analysis of randomized controlled trials in heart failure. Eur J Heart Fail 2011; 13: 450–459. [DOI] [PubMed] [Google Scholar]
- 7. Pekmezaris R, Mitzner I, Pecinka KR, et al. The impact of remote patient monitoring (telehealth) upon Medicare beneficiaries with heart failure. Telemed J E Health 2012; 18: 101–108. [DOI] [PubMed] [Google Scholar]
- 8. Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis 2005; 47: 320–332. [DOI] [PubMed] [Google Scholar]
- 9. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary. Circulation 2013; 128: e240–e327. [DOI] [PubMed] [Google Scholar]
- 10. Staessen JA, Li Y, Thijs L, et al. Blood pressure reduction and cardiovascular prevention; an update including the 2003–2004 secondary prevention trials. Hypertens Res 2005; 28: 385–407. [DOI] [PubMed] [Google Scholar]
- 11. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004; 364: 937. [DOI] [PubMed] [Google Scholar]
- 12. The SOLVD Investigators* 1992. Effect of Enalapril on Mortality and the Development of Heart Failure in Asymptomatic Patients with Reduced Left Ventricular Ejection Fractions; New England Journal of Medicine; Vol 327; issue 10; p685–691. 10.1056/nejm199209033271003. [DOI] [PubMed] [Google Scholar]
- 13. Yancy CW, Jessup M, Bozkurt B, et al. 2017. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 36: e137–e161. [DOI] [PubMed] [Google Scholar]
- 14. Franciosa JA, Taylor AL, Cohn JN, et al. African American Heart Failure Trial (A-HeFT): rationale, design, and methodology. J Card Fail 2002; 8: 128–135. [DOI] [PubMed] [Google Scholar]
- 15. McMurray JJ, Packer M, Desai AS, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 16. Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. 2010; 376: 875–885. [DOI] [PubMed] [Google Scholar]
- 17. Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 1996; 335: 1933–1940. [DOI] [PubMed] [Google Scholar]
- 18. Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346: 877–883. [DOI] [PubMed] [Google Scholar]
- 19. Adams KF, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005; 149: 209–216. [DOI] [PubMed] [Google Scholar]
- 20. Zile MR, Bennett TD, St John Sutton M, et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 2008; 118: 1433–1441. [DOI] [PubMed] [Google Scholar]
- 21. Ritzema J, Troughton R, Melton I, et al. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Circulation 2010; 121: 1086–1095. [DOI] [PubMed] [Google Scholar]
- 22. Bourge RC, Abraham WT, Adamson PB, et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF Study. J Am Coll Cardiol 2008; 51: 1073–1079. [DOI] [PubMed] [Google Scholar]
- 23. Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med 2010; 363: 2301–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Magalski A, Adamson PB, Gadler F, et al. Continuous ambulatory right heart pressure measurements with an implantable hemodynamic monitor: a multicenter, 12-month follow-up study of patients with chronic heart failure. J Card Fail 2002; 8: 63–70. [DOI] [PubMed] [Google Scholar]
- 25. Verdejo HE, Castro PF, Concepcion R, et al. Comparison of a radiofrequency-based wireless pressure sensor to Swan-Ganz catheter and echocardiography for ambulatory assessment of pulmonary artery pressure in heart failure. J Am Coll Cardiol 2007; 50: 2375–2382. [DOI] [PubMed] [Google Scholar]
- 26. Castro PF, Concepcion R, Bourge RC, et al. A wireless pressure sensor for monitoring pulmonary artery pressure in advanced heart failure: initial experience. J Heart Lung Transplant 2007; 26: 85–88. [DOI] [PubMed] [Google Scholar]
- 27. Ritzema J, Melton IC, Richards AM, et al. Direct left atrial pressure monitoring in ambulatory heart failure patients: initial experience with a new permanent implantable device. Circulation 2007; 116: 2952–2959. [DOI] [PubMed] [Google Scholar]
- 28. Adamson PB, Magalski A, Braunschweig F, et al. Ongoing right ventricular hemodynamics in heart failure: clinical value of measurements derived from an implantable monitoring system. J Am Coll Cardiol 2003; 41: 565–571. [DOI] [PubMed] [Google Scholar]
- 29. CardioMEMS Inc. CardioMEMS HF system, PA sensor and delivery system. User’s manual, https://www.accessdata.fda.gov/cdrh_docs/pdf10/p100045d.pdf (May 2014, accessed 20 May 2018).
- 30. Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377: 658–666. [DOI] [PubMed] [Google Scholar]
- 31. US Food and Drug Administration. 2011. Meeting Materials of the Circulatory System Devices Panel, https://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/CirculatorySystemDevicesPanel/ucm124182.htm (October 2011, accessed 20 May 2018).
- 32. United States Food and Drug Administration. PMA P100045: FDA summary of safety and effectiveness data, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P100045 (accessed 20 May 2018).
- 33. Adamson PB, Abraham WT, Bourge RC, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 2014; 7: 935–944. [DOI] [PubMed] [Google Scholar]
- 34. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352: 225–237. [DOI] [PubMed] [Google Scholar]
- 35. Young JB, Abraham WT, Smith AL, et al. Safety and efficacy of combined cardiac resynchronization therapy and implantable cardioversion defibrillation in patients with advanced chronic heart failure. The Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) trial. JAMA 2003; 289: 2685–2694. [DOI] [PubMed] [Google Scholar]
- 36. Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of A defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346: 877–883. [DOI] [PubMed] [Google Scholar]
- 37. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 38. Zoler ML. CardioMEMS shows real-world success as use expands, https://www.mdedge.com/ecardiologynews/article/149637/heart-failure/cardiomems-shows-real-world-success-use-expands (accessed 20 May 2018).
- 39. Hoppe U C, Vanderheyden M, Sievert H, et al. Chronic monitoring of pulmonary artery pressure in patients with severe heart failure: multicenter experience of the monitoring Pulmonary Artery Pressure by Implantable device Responding to in patients Ultrasonic Signal (PAPIRUS) II study. Heart 2009; 95: 1091–1097. [DOI] [PubMed] [Google Scholar]
- 40. Bourge RC, Abraham WT, Adamson PB, et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol 2008; 51: 1073–1079. [DOI] [PubMed] [Google Scholar]
- 41. Adamson PB, Conti JB, Smith AL, et al. Reducing events in patients with chronic heart failure (REDUCEhf) study design: continuous hemodynamic monitoring with an implantable defibrillator. Clin Cardiol 2007; 30: 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jaarsma T, van der Wal MH, Lesman-Leegte I, et al. Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH). Arch Intern Med 2008; 168: 316. [DOI] [PubMed] [Google Scholar]
- 43. Kalter-Leibovici O, Freimark D, Freedman LS, et al. Disease management in the treatment of patients with chronic heart failure who have universal access to health care: a randomized controlled trial. BMC Med 2017; 15: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. GESICA Investigators. Randomised trial of telephone intervention in chronic heart failure: DIAL trial. BMJ 2005; 331: 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hughes S. CardioMEMS sensor linked to lower mortality in heart failure. Medscape, 27 March 2018. [Google Scholar]
- 46. St. Jude Medical. Hemodynamic-GUIDEd management of heart failure (GUIDE-HF), https://clinicaltrials.gov/ct2/show/NCT03387813 (January 2018, accessed 20 May 2018).