Abstract
Background:
There are close links between chemotherapy-induced intestinal mucositis and microbiota dysbiosis. Previous studies indicated that D-methionine was an excellent candidate for a chemopreventive agent. Here, we investigated the effects of D-methionine on cisplatin-induced mucositis.
Materials and methods:
Male Wistar rats (176–200 g, 6 weeks old) were given cisplatin (5 mg/kg) and treated with D-methionine (300 mg/kg). Histopathological, digestive enzymes activity, oxidative/antioxidant status, proinflammatory/anti-inflammatory cytokines in intestinal tissues were measured. Next-generation sequencing technologies were also performed to investigate the gut microbial ecology.
Results:
D-methionine administration increased villus length and crypt depth and improved digestive enzyme (leucine aminopeptidase, sucrose and alkaline phosphatase) activities in the brush-border membrane of cisplatin-treated rats (p < 0.05). Furthermore, D-methionine significantly attenuated oxidative stress and inflammatory reaction and increased interleukin-10 levels in cisplatin-induced intestinal mucositis (p < 0.05). Cisplatin administration resulted in high relative abundances of Deferribacteres and Proteobacteria and a low diversity of the microbiota when compared with control groups, D-methionine only and cisplatin plus D-methionine. Cisplatin markedly increased comparative abundances of Bacteroides caccae, Escherichia coli, Mucispirillum schaedleri, Bacteroides uniformis and Desulfovibrio C21-c20, while Lactobacillus was almost completely depleted, compared with the control group. There were higher abundances of Lactobacillus, Lachnospiraceae, and Clostridium butyrium in cisplatin plus D-methionine rats than in cisplatin rats. D-methionine treatment alone significantly increased the number of Lactobacillus reuteri.
Conclusion:
D-methionine protects against cisplatin-induced intestinal damage through antioxidative and anti-inflammatory effects. By enhancing growth of beneficial bacteria (Lachnospiraceae and Lactobacillus), D-methionine attenuates gut microbiome imbalance caused by cisplatin and maintains gut homeostasis.
Keywords: cisplatin, D-methionine, gastrointestinal mucositis, Lactobacillus, next-generation sequencing
Introduction
There is mounting evidence of a close relationship between gastrointestinal (GI) mucositis and dysbiosis of the gut microbiota.1,2 Therapeutic strategies for intestinal disorders can be developed based on the etiologies of these events.
Chemotherapeutic agents effectively kill neoplastic cells, but also damage various organs, including the GI tract, due to their inability to differentiate between normal and neoplastic cells. GI mucositis, a chemotherapy-induced serious side effect, leads to functional and structural damage to the GI tract, such as shortening of intestinal villi, intestinal crypt ablation, local accumulation of inflammatory cells, impaired intestinal barrier function3,4 and decreased intestinal digestive enzyme activities.5 Clinical symptoms include nausea, vomiting, bloating, anorexia and subsequent body weight loss. Approximately 40% of chemotherapy patients6 suffer from GI mucositis which can result in further devastating effects, such as decreased quality of life, longer hospitalizations, higher health care costs, lower dosages of chemotherapeutics and even cessation of treatment.
Five important biological steps in the process of mucositis have been proposed by Sonis.7 They are initiation, primary damage response, signal amplification, ulceration and wound healing. Based on the pathobiology of mucositis, the inflammatory response plays a major role in the development of chemotherapy-induced mucositis with atrophy, thinning and ulceration of the mucosal epithelium. The loss of mucosal integrity following chemotherapy treatment results in an increase in intestinal permeability, which enables the entry of numerous microorganisms (especially pathogenic microorganisms). These microorganisms translocate into the mucosa, rapidly reproducing and releasing proinflammatory cytokines.7
The abundance and diversity of gut microbiota have been shown to be significantly altered by chemotherapeutic agents.1–3 Intestinal microbial dysbiosis is considered to be involved in the pathogenesis of GI mucositis.1 Moreover, chemotherapy efficacy and toxicity are affected by gut microbiota.8,9 It remains unknown whether intestinal microbial dysbiosis directly results in GI mucositis or merely creates a disturbance in the environment of the GI tract.3,10
Cisplatin is effective and widely used in cancer treatment, but has been demonstrated to induce various adverse effects,11–13 such as intestinal mucositis.14,15 Cisplatin-induced intestinal mucositis, characterized by epithelial sloughing and mucosal ulceration of villous tips, has been reported14,16 and the imperfection of mucus barrier is considered a ‘cause’ of cisplatin-induced mucositis.17 The intestinal mucosa acts as a barrier against invasion by pathogenic microorganisms. Loss of barrier function enables bacterial translocation and triggers the proinflammatory cascade to initiate intestinal mucositis.4 However, a causal relationship between cisplatin-induced GI mucositis and cisplatin-induced changes in the gut microbiota remains limited.18 D-methionine is a sulfur-containing essential amino acid. D-methionine protection against cisplatin-induced ototoxicity in rats was first reported by Campbell and colleagues.19 The mechanisms of D-methionine’s otoprotective action were associated with its specific antioxidant enzyme activities and its ability to counteract oxidative stress.20,21 In addition, D-methionine had a hepatoprotective effect by the prevention of the decrease of glutathione (GSH) levels in liver22 and antineurotoxicity.23 The latest double-blind placebo-controlled multicenter phase II trial revealed that oral D-methionine exhibited a protective effect against radiation and cisplatin-induced mucositis24 and did not alter tumor response to cisplatin.25,26
It remains unknown whether D-methionine exerts protective effects on cisplatin-induced GI mucositis and gut microbiota dysbiosis. To test the hypothesis that treatment with D-methionine attenuates cisplatin-induced intestinal mucositis through antioxidative and anti-inflammatory activities, as well as regulation of microbial community composition, we investigated the effects of D-methionine on oxidative stress, inflammatory markers and gut microbiota modification in cisplatin-induced intestinal mucositis. The results revealed that oral administration of D-methionine alleviates cisplatin-induced GI mucositis by suppressing oxidative stress and inflammatory mediators. Therefore, we suggest that D-methionine helps to markedly decrease lipopolysaccharide (LPS)-producing and proinflammatory bacteria (e.g. Deferribacteres and Proteobacteria) and increase anti-inflammatory bacteria (e.g. Lachnospiraceae and Lactobacillus).
Materials and methods
Reagents
Cisplatin and D-methionine were purchased from Sigma–Aldrich Co. (St. Louis, MO, USA). All other chemicals and reagents used in this study were of analytical grade.
Animals
Male Wistar rats (6 weeks old), weighing 176–200 g, were purchased from BioLASCO Taiwan Co., Ltd. and randomly divided into four groups of eight animals each. Animals were housed in cages with a maximum of four rats per cage on a 12-h light/dark cycle and allowed free access to rodent chow (LabDiet, 5001) and sterile water during the study. The experimental protocols were approved by Affidavit of Approval of Animal Use Protocol, Chung Shan Medical University Experimental Animal Center, Taichung, Taiwan (approval no: 1752).
Experimental protocol
Following 1 week of acclimatization, male Wistar rats were divided into four groups and toxicity was induced by intraperitoneal administration of cisplatin at the dose of 5 mg/kg body weight once a week for 3 weeks. In the control group, rats were given 0.9% (w/v) NaCl injection on the 1st, 8th, and 15th days and oral sterile water daily by gavage. The cisplatin group received cisplatin treatment on the 1st, 8th, and 15th days and oral sterile water daily by gavage. The cisplatin combined with D-methionine group was given cisplatin treatment on the 1st, 8th, and 15th days and D-methionine (300 mg/kg/day) daily by gavage for 3 days before the first cisplatin injection and then until the end of the experiment. D-methionine was administered at least 30 min before cisplatin injection. D-methionine group received a single intraperitoneal injection of 0.9% (w/v) NaCl solution on the 1st, 8th, and 15th days and oral D-methionine (300 mg/kg/day) by gavage 3 days before the first 0.9% NaCl injection and then until the end of the experiment. In current study, the dose of either cisplatin or D-methionine was cited from the previous published literature.19,20
Sample collection and treatment
Body weight, food and water consumption were monitored daily before cisplatin injection. Feeding efficiency was measured weekly, and calculated using the following formula: Feeding efficiency (%) = [body weight change (g)/food intake (g)] × 100%. On day 17, stool samples were collected on the grid floor, placed on a stainless-steel wire mesh, and dried in an oven to measure moisture content. Fecal pH was determined by a pH meter. At the end of the study (day 19), animals were sacrificed by CO2 asphyxiation and the cecum contents were collected immediately under laminar flow for 16S DNA (next-generation sequencing; NGS) analysis. Half of each section of duodenum, jejunum and ileum was snap-frozen in liquid nitrogen prior to storage at −80oC for biochemical analysis.
Histological analysis
The remaining intestinal segments were fixed in 10% buffered formalin for 24 h, embedded in paraffin, and cross-sectioned. Then 3-μm thick transverse sections were stained with hematoxylin and eosin (H&E) to observe morphology changes. For the evaluation of intestinal tissue damage quantitatively, photographs were taken using an Olympus BX60 fluorescence microscope (Olympus America, Melville, NY, USA; original magnification, 40×).
Assessment of brush-border membrane enzyme activity
Freshly excised intestines were slit and washed with ice-cold phosphate-buffered saline (PBS) solution, followed by gentle scraping of the mucosa with a glass slide. Iced saline (containing protease inhibitor) was added to the mucosa for homogenization, followed by centrifugation at 10,000 rpm at 4oC for 10 min. The supernatants were used for brush-border membrane (BBM) assays. Aliquots of mucosal homogenates were quickly frozen until further analysis. The activity of alkaline phosphatase (AP) was determined by the yellow color that appeared upon hydrolysis of p-nitrophenyl phosphate. Leucine aminopeptidase (LAP) was assayed using leucine p-nitroanilide as a substrate. Sucrase was assayed by the reduced sugars formed upon the hydrolysis of sucrose. The activities of LAP and sucrase were measured using a Randox assay kit (Randox Laboratories, Crumlin, UK) and AP activity was measured using a BioVision ALP activity colorimetric assay kit (BioVision, Inc., Milpitas, CA, USA).
Determinations of lipid peroxide, antioxidant status and inflammation in intestinal tissues
Intestinal tissue samples were frozen in liquid nitrogen and mixed in a stainless-steel mortar and pestle homogenizer. Ice-cold PBS solution was used to homogenize parts of the frozen crushed intestinal samples to determine the malondialdehyde (MDA) level, reduced GSH level, and glutathione peroxidase (GPx) activity. The MDA level was determined in intestinal homogenates after reaction with thiobarbituric acid. Total GSH was measured with a modified 5-5V-dithiobis 2-nitrobenzoic acid (DTNB)-GSSG reductase recycling assay. The GPx assay was based on the oxidation of GSH coupled to nicotinamide adenine dinucleotide phosphate by glutathione reductase (GSR). These biochemical parameters were assayed as described by Wen and colleagues.27 A solution of 2% Triton X-100 containing 0.32M sucrose was used to homogenize other parts of the frozen crushed intestinal samples for superoxide dismutase (SOD) analysis. SOD activity in intestinal tissue was measured using colorimetric assay kit (Cayman Chemical, Ann Arbor, MI, USA). Another part of the intestinal tissue samples was homogenized in 1 ml ice-cold PBS solution and then centrifuged at 12,000 g at 4°C for 30 min. Supernatants were stored at −80°C for cytokine assays. The levels of interleukin (IL)-1β, IL-6 and IL-10 in intestinal tissues were measured by specific enzyme-linked immunosorbent assay (ELISA) kits (R & D Systems, Minneapolis, MN, USA). The measurements of IL-1β, IL-6, and IL-10 were performed step by step based on the manufacturer’s standard protocol. The concentration of tumor necrosis factor (TNF)-α was measured using a commercial assay kit according to the manufacturer’s instructions (rat TNF-α, ELISA kit, BioLegend, San Diego, CA, USA).
Gut microbiota analysis
Gut bacterial DNA from the rat cecum content was extracted with commercial DNA Stool Mini extraction kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. Library sequencing was performed on Illumina HiSeq 2500 platform. On alpha diversity analysis, gut microbial composition diversities of the groups were evaluated, and various indicators were calculated using Qiime software (version 1.9.1). These indicators included Chaol (community richness), Shannon (community diversity) and Observed Species [estimated operational taxonomic unit (OTU) amounts]. Alpha diversity was also estimated using the phylogenetic diversity metric. Beta diversity analysis was used to compare gut microbiota compositions among the groups and was performed using the unweighted pair group method with arithmetic mean (UPGMA) clustering method based on weighted and unweighted UniFrac distances. Principal coordinates analysis (PCoA) was based on distance matrix. Weighted UniFrac and Unweighted UniFrac were calculated to assist PCoA. PCoA was conducted and displayed using Qiime software (version 1.7.0).
Statistical analysis
IBM SPSS Statistics 19 was used for all statistical analyses. All data are presented as mean ± standard error of the mean (SEM). Statistical comparisons were carried out by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test to measure variations between different groups; p < 0.05 was considered statistically significant.
Results
D-methionine effectively alleviates body weight loss and increases food intake and stool output
Loss of body weight and decrease in food intake are common phenomena after cisplatin treatment. They are also basic indicators of cisplatin toxicity. During the adaptation period, there were no differences in body weight or food intake among the four groups. As expected, cisplatin-treated rats and cisplatin combined with D-methionine treated rats showed decreases of 28% and 13% in body weight, respectively, compared with control animals (p < 0.05) (Table 1). In cisplatin-treated rats, there was a 95% reduction in food intake compared with the control group (p < 0.05). In contrast, co-administration of D-methionine resulted in a decline of 34% compared with the control group (p < 0.05). Body weight and food intake were unaffected in the D-methionine alone group when compared with the control group. During the experimental period, we also observed that the severity of anorexia (reduction in food intake) is associated with accumulated dose of cisplatin (data not shown).
Table 1.
Effects of D-methionine on body weight, body weight gain and food intake in cisplatin-treated rats.
| Control | D-methionine | Cisplatin | Cisplatin + D-methionine | |
|---|---|---|---|---|
| Initial body weight (g) | 304.13 ± 9.09 | 301.60 ± 10.95 | 308.63 ± 11.78 | 305.75 ± 10.79 |
| Final body weight (g) | 378.88 ± 9.85 | 370.80 ± 10.66 | 266.0 ± 11.66* | 331.63 ± 11.30* # |
| Initial food intake (g) | 28.75 ± 2.18 | 26.67 ± 0.47 | 29.75 ± 0.94 | 28.63 ± 1.19 |
| Final food intake (g) | 26.92 ± 1.40 | 25.67 ± 0.47 | 2.90 ± 2.29* | 13.78 ± 6.05* # |
Data are presented as mean ± SEM, n = 5–8. Differences were analyzed by one-way ANOVA.
represents a significant difference when compared with the control group;
represents a significant difference when compared with the cisplatin group (p < 0.05).
ANOVA, analysis of variance; SEM, standard error of the mean.
To understand whether digestion is altered by cisplatin, feeding efficiency was evaluated and calculated weekly. Supplementary Figure 1(a) shows that, in the second week, the feeding efficiency of rats treated with cisplatin was negative and decreased to −114.6% in the third week (p < 0.05). These results implied that cisplatin completely obstructs digestion. However, the decline in feeding efficiency was inhibited by D-methionine supplement, indicating that D-methionine improves digestion. Feeding efficiencies were almost the same in D-methionine alone and control groups. Cisplatin contributes to lowered food intake and feeding efficiency. Stool samples also showed reduced stool production and watery cecum content [Supplementary Figure 1(b)]. Fecal water content and pH level were not influenced by cisplatin (Supplementary Table 1). In the cisplatin + D-methionine group, stool output increased with solid cecum content compared with the cisplatin group. Nevertheless, the stool output in the cisplatin + D-methionine group was still lower than in the control and D-methionine alone groups. In the D-methionine alone group, the wet weight, dry matter, fecal water content and pH level of stool remained almost unchanged in comparison with the control group.
D-methionine improves digestive enzyme activities
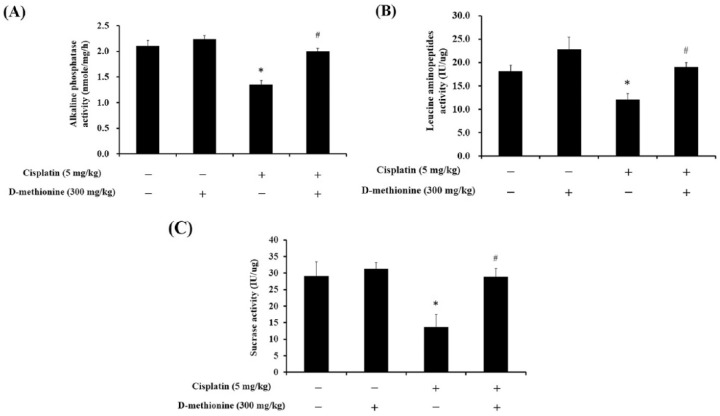
LAP, sucrase and AP were measured to evaluate digestive function and to determine whether decreases in food intake and feeding efficiency correlate with decreases in digestive enzyme activities. Cisplatin led to reductions in activities of LAP, sucrose and AP, while supplementation of D-methionine in cisplatin-treated rats significantly improved the activities of LAP, sucrase and AP (p < 0.05; Figure 1). Administration of D-methionine alone had no effect on digestive enzyme activities when compared with the control group.
Figure 1.
Effects of D-methionine treatment on BBM enzymes: AP, LAP and sucrase in cisplatin-treated rats. Data are presented as mean ± SEM, n = 5–8.
*represents a significant difference when compared with the control group;
#represents a significant difference when compared with the cisplatin group (p < 0.05).
AP, alkaline phosphatase; BBM, brush-border membrane; LAP, leucine aminopeptidase; SEM, standard error of the mean.
D-methionine attenuates cisplatin-induced oxidative stress and inflammation
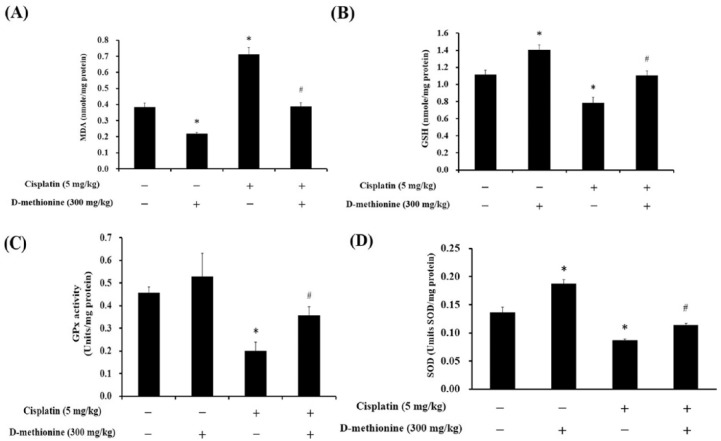
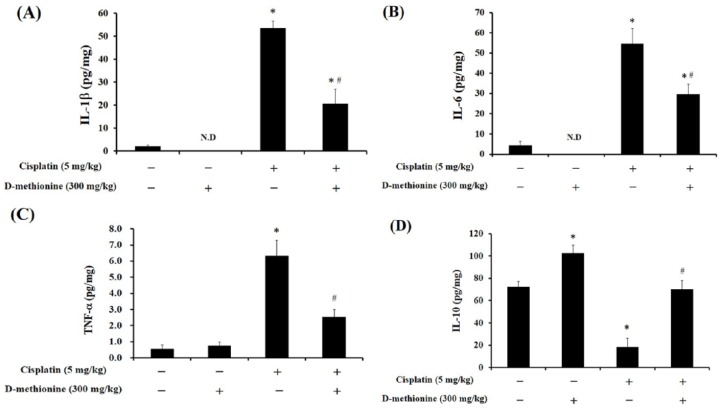
The high antioxidant efficiencies of D-methionine, also referred to as sulfur antioxidants, are well known. Several studies have revealed that the protective effect of D-methionine against cisplatin-induced ototoxicity is associated with its potent antioxidant activity.19,21,28 MDA, lipid peroxidation product, is the major biochemical consequence of oxidative attack on cell membranes and is an oxidative stress biomarker. In the present study, cisplatin elevated the levels of MDA, decreased GSH content, and reduced GPx and SOD activities in intestinal tissue (p < 0.05; Figure 2). D-methionine co-administration resulted in decreased MDA concentrations, elevated GSH levels, and increased enzyme activities of GPx and SOD after cisplatin injection. Interestingly, D-methionine alone not only diminished MDA levels, but also increased antioxidative capacity, by increasing GSH and SOD in the intestinal tissues. Due to the antioxidative property of D-methionine, we next investigated if D-methionine mitigates the production of proinflammatory cytokines in cisplatin-mediated intestinal mucositis. We also observed that D-methionine alone treatment increased IL-10 levels in the intestinal tissues. After cisplatin injection, D-methionine demonstrated beneficial effects, such as decreasing levels of TNF-α, IL-6 and IL-1β and increasing levels of IL-10, on intestinal tissues compared with cisplatin alone group (p < 0.05; Figure 3).
Figure 2.
Effects of cisplatin and D-methionine administration on MDA and GSH concentrations and GPx and SOD activities in intestinal tissue homogenates. Data are presented as mean ± SEM, n = 5–8.
*represents a significant difference when compared with the control group;
#represents a significant difference when compared with the cisplatin group (p < 0.05).
GPx, glutathione peroxidase; GSH, glutathione; MDA, malondialdehyde; SEM, standard error of the mean.
Figure 3.
Effects of D-methionine on inflammation parameters in small intestinal homogenates after treatment with cisplatin. (a) IL-1β, (b) IL-6, (c) TNF-α and (d) IL-10. Data are presented as mean ± SEM, n = 5–8.
*represents a significant difference when compared with the control group;
#represents a significant difference when compared with the cisplatin group (p < 0.05).
IL, interleukin; SEM, standard error of the mean; TNF, tumor necrosis factor.
Histological examinations of intestine
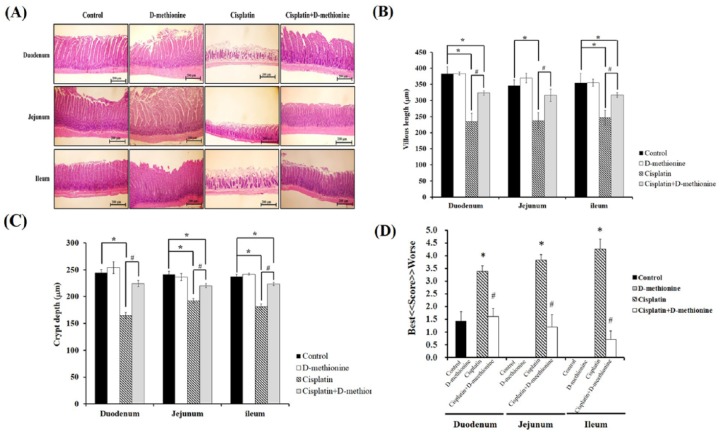
Transparency was observed in the harvested intestinal tissues of cisplatin-treated rats. Histopathological examinations of sections of duodenum, jejunum and ileum were carried out with H&E staining [Figure 4(a)]. Intact finger-like elongated structures and compactly arrayed epithelium were observed in the control and D-methionine-alone groups. However, cisplatin caused severe intestinal mucositis histologically characterized by shortened intestinal villi, shortened crypt depth and finger-like villi with broken and incomplete shapes. The most serious damage was found in the ileum. These histopathological abnormalities were restored by D-methionine supplementation. Next, villus length and crypt depth were measured, as shown in Figure 4(b). The villi of duodenum, jejunum and ileum in rats treated with cisplatin were shortened by 38.8%, 31.5% and 30.4%, respectively, compared with the control group (p < 0.05). In cisplatin combined with D-methionine treated rats, villi were shortened by 15.5%, 8.6% and 10.5%, respectively, compared with the control group (p < 0.05), which reveals significant recoveries of villus length and crypt depth. Next, quantitative histological scoring of intestinal damage was used to evaluate injury, as shown in Figure 4(c). The highest score for intestinal lesion was found in the cisplatin-treated group. This score was significantly reduced by D-methionine co-administration (p < 0.05). Together, these results demonstrated that D-methionine pretreatment protects rats from cisplatin-induced intestinal mucosal injury.
Figure 4.
Effects of D-methionine on small intestinal damage after cisplatin treatment. (a) Histological staining of representative intestine, (b) villus length, (c) crypt depth and (d) grading score of intestinal tissue damage. Data are presented as mean ± SEM, n = 5–8.
*represents a significant difference when compared with the control group;
#represents a significant difference when compared with the cisplatin group (p < 0.05).
SEM, standard error of the mean.
D-methionine improves gut microbiota dysbiosis in cisplatin-induced intestinal mucositis
Cisplatin caused variation in gut flora that could be prevented by D-methionine administration in cisplatin-treated rats
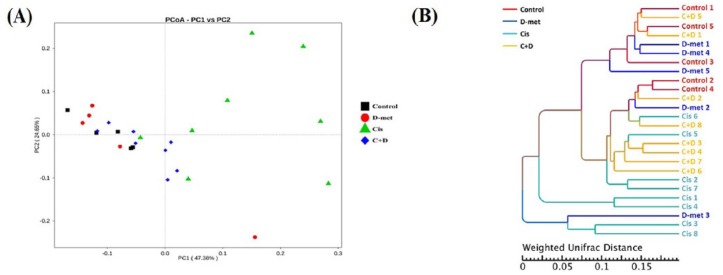
On PCoA of the caeca of 26 rats [Figure 5(a)], there were significant distances between cisplatin group and each of the other three groups, suggesting that gut microbial structures of control, D-methionine alone and cisplatin + D-methionine groups are much closer. We next used UPGMA to estimate the differences by constructing clustering trees on the basis of group phylogenetic data. From our results, the phylogenetic relationship (UPGMA clustering tree) of the cisplatin group was relatively distant from the other three groups on weighted UniFrac analysis [Figure 5(b)]. The evolutionary distances of species were quite short among control, D-methionine and cisplatin + D-methionine groups, suggesting similar microbial compositions and abundances among these three groups. These results indicated that cisplatin causes variation in gut flora.
Figure 5.
Beta diversity comparisons of gut microbiomes. PCoA analysis (a) and UPGMA (b). UPGMA clustering tree based on weighted UniFrac distance. Weighted UniFrac considers both composition and abundance of microbiomes. On PCoA, each point represents a sample, plotted by a principal component on the x-axis and another principal component on the y-axis, with each group represented by a different color. The percentage on each axis indicates the contribution value to discrepancy among samples. Different colored symbols represent rats receiving different treatments and every symbol represents individual animals. The black squares indicate control group. The red circles indicate D-methionine group. The green triangles indicate treatment with cisplatin only. The blue diamonds indicate combined treatment of cisplatin and D-methionine.
PCoA, principal coordinates analysis; UPGMA, unweighted pair group method with arithmetic mean.
D-methionine enhanced Lactobacillus and decreased Bacteroides relative abundance in cisplatin-treated rats
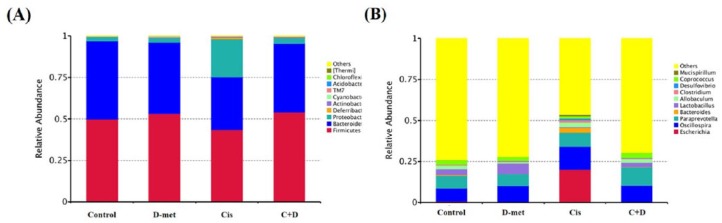
According to species annotation, the statistical numbers of sequences for each taxonomic classification level (kingdom, phylum, class, order, family, genus, species) were calculated. Accordingly, the top 10 species in the classification levels of phylum and genus were selected, and the distribution histograms of relative abundances of species were developed, as shown in Figure 6(a) for phylum and Figure 6(b) for genus. In our study, rat gut composition was dominated by three bacterial phyla: Firmicutes, Bacteroidetes and Proteobacteria. The relative abundances at the phylum level in all four groups followed the sequence Firmicutes>Bacteroidetes>Proteobacteria. In cisplatin-treated rats, the relative abundances of Bacteroidetes and Firmicutes significantly decreased, while those of Proteobacteria and Deferribacteres increased, when compared with the other three groups. However, in cisplatin + D-methionine-treated rats, the four phylum levels displayed almost the same abundances as in control rats. There was no significant change in phylum levels between control and D-methionine groups. Next, the levels at genus were compared among the four groups. Notably, the cisplatin-injected rats demonstrated greater relative abundances of Escherichia, Oscillospira, Paraprevotella, Bacteroides, Clostridium, Desulfovibrio and Mucispirillum, but lower relative abundances of Lactobacillus and Coprococcus than in the other three groups. The changes in relative abundances of Escherichia, Bacteroides and Lactobacillus were obvious, implying that cisplatin significantly alters microbiota composition. Surprisingly, in the cisplatin + D-methionine group, Escherichia and Mucispirillum were hardly rare. The relative abundance of Lactobacillus increased and the relative abundance of Bacteroides decreased. Interestingly, D-methionine alone enhanced the production of Lactobacillus compared with the control group.
Figure 6.
Phylum and genus distributions of experimental groups. The relative abundances of gut microbiota phyla (a) and genera (b) were analyzed by next-generation sequencing of bacterial 16S DNA. The y-axis and x-axis represent relative abundance and group, respectively. On the right, ‘others’ represents total relative abundances of the phyla and genera not included in the top 10. At the phylum level, abundance changes in Bacteroidetes, Proteobacteria and Firmicutes were observed.
D-methionine co-treatment can increase microbiota diversity and enhance the growth of Lachnospiraceae and Lactobacillus after cisplatin treatment.
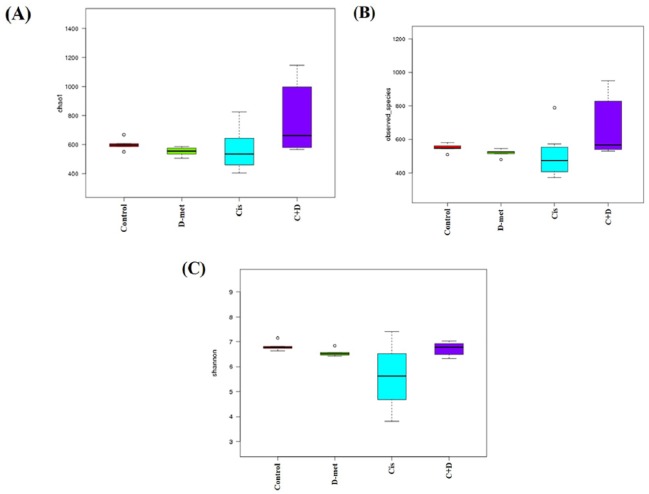
We used Chao-1, Observed species and Shannon as alpha diversity indices to represent in-group microbial community diversities. Higher index level represented higher microbial community diversity. Figure 7 shows that cisplatin led to marked decline in gut microbial diversity. Diversity increased under D-methionine co-administration. There were no significant differences in alpha diversity indices between control and D-methionine groups, suggesting that D-methionine alone does not alter gut microbiota structure.
Figure 7.
Alpha diversity indexes. Chao-1 (a), Observed species (b) and Shannon (c). The microbiota composition samples from four groups were clustered based on PCA, Pearson clustering and Shannon diversity index. Circles represent outliers. Group size is 5–8 individuals; results are shown as means ± SEM.
PCA, principal component analysis; SEM, standard error of the mean.
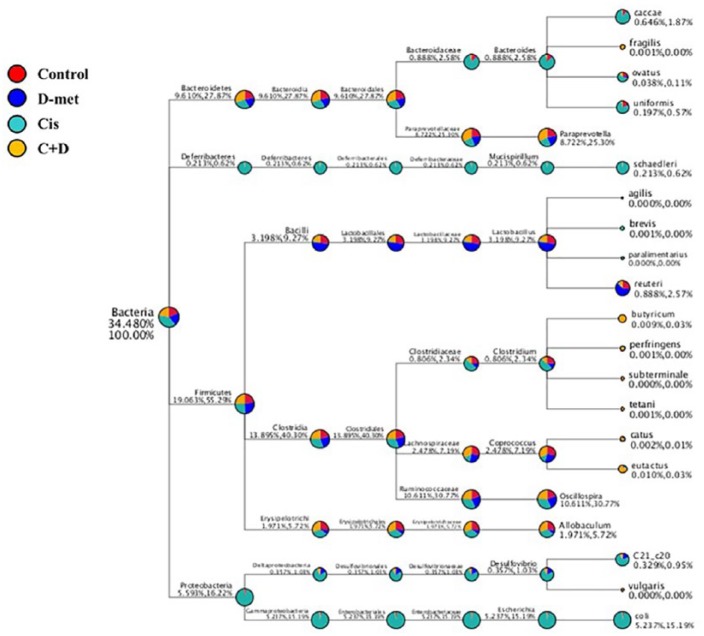
Species of interest (top 10 genera in each group by default) were selected to draw the classification tree, as shown in Figure 8. Gut bacterial communities in cisplatin-treated rats were predominantly comprised of Proteobacteria and Deferribacteres. The phylum Deferribacteres, class Deferribacteres, order Deferribacterales, family Deferribacteraceae, genus Mucispirillum and species schsedieri increased in cisplatin-treated rats. Furthermore, we observed the highest abundances of phylum Proteobacteria, class Gummaproteobacteria, order Enterobacteriales, family Enterobacteriaceae, genus Escherichia and species coli, as well as the phylum Proteobacteria, class Deltaproteobacteria, order Desulfovibrionales, family Desulfovibrionaceae, genus Desulfovibrio and species C21_c20, in cisplatin-treated rats. At the species level, Bacteroides caccae, Bacteroides ovatus and Bacteroides uniformis, Mucispirillum schaedieri, Desulfovibrio C21_c20 and Escherichia coli were more abundant in cisplatin alone group than in the other three groups. Interestingly, in terms of quantities of Lactobacillus reuteri, D-methionine > control > cisplatin + D-methionine. In the cisplatin + D-methionine group, gut microbiota composition largely shifted to Clostridium butyrium, Coprococcus catus and Coprococcus eutactus.
Figure 8.
Classification tree. The first circle represents the kingdom level (bacteria). The second circle (in same column) represents the phylum level. The subsequent order is class, order, family, genus and species. The first number (after the taxonomic ranks) represents the relative abundance of the whole corresponding taxon, while the second number represents the relative abundance of the corresponding taxon. For example, for Firmicutes (19.05%, 55.29%), 19.05% represents the proportion of this phylum among all phylum levels; 55.29% represents the proportion of this phylum among the four phylum levels.
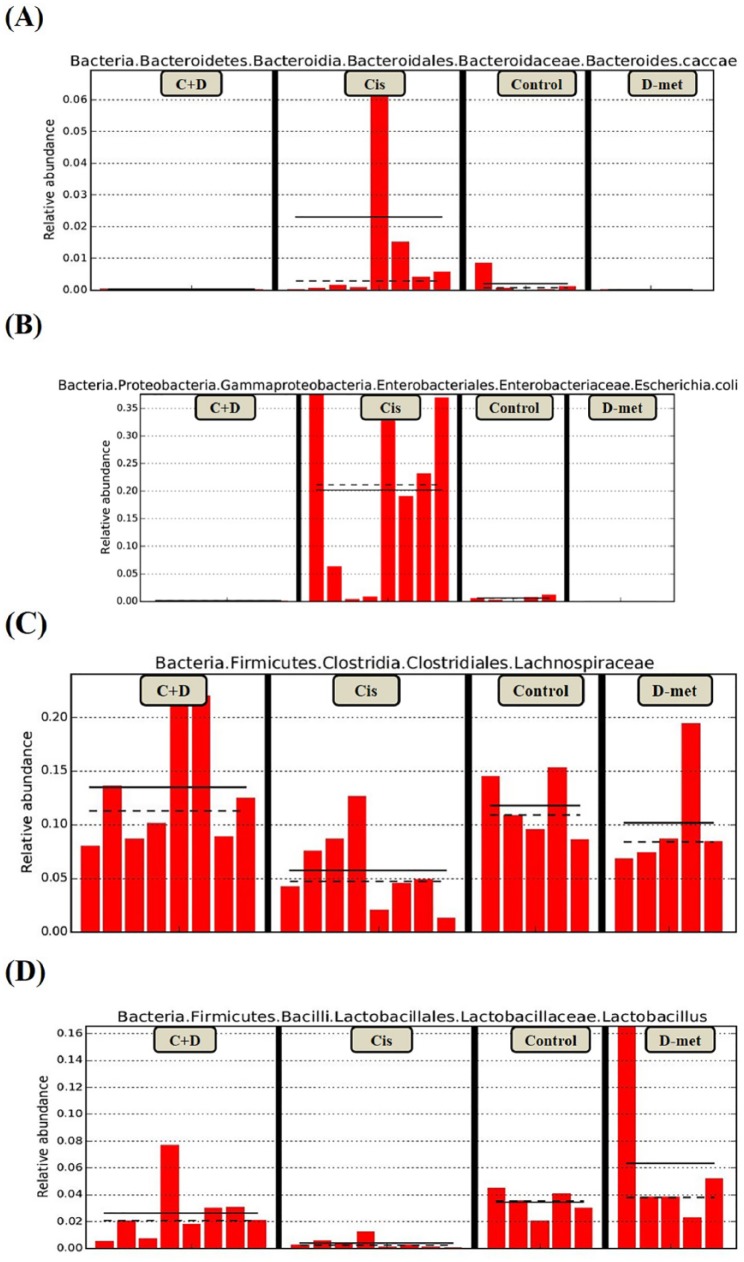
Taxonomic biomarkers were investigated based on linear discriminative analysis effect size (LEfSe) and cladogram (circular hierarchical tree; Supplementary Figure 2). Biomarkers from the four groups are shown in Figure 9. Potentially enteropathogenic bacteria, such as Bacteroides caccae of the phylum Bacteroidetes and Escherichia coli of the phylum Proteobacteria, were predominant biomarkers in cisplatin group. Family Lachnospiraceae was the predominant biomarker in cisplatin + D-methionine group. Lactobacillus was the predominant biomarker in D-methionine alone group. The abundance distributions of the 100 most dominant OTUs of the four groups are displayed on species abundance heat map and represented by color gradient. In the heat map (Supplementary Figure 3), we found obvious discrepancies in species abundance between the cisplatin alone and cisplatin + D-methionine groups.
Figure 9.
Biomarkers are represented by bar chart. The relative abundance with statistical differences biomarker (genus and species level) were compared among groups. Bacteroides caccae (a), Escherichia coli (b), Lactobacillus (c), Lachnospiraceae (d). The present study shows the proportion of Bacteroides caccae, Escherichia coli in cisplatin-treated rats were elevated than that other three groups, reflect chronic exposure to cisplatin results in a more proinflammatory microbiota growth.
Discussion
The latest clinical trial revealed that oral D-methionine exhibit a protective effect against radiation and cisplatin-induced mucositis.24 D-methionine has been demonstrated to reduce cisplatin-induced ototoxicity and neurotoxicity due to its antioxidative effect.21,28,29 However, the protective effect of D-methionine against cisplatin-induced GI mucositis has not been explored. In addition, the evidence that cisplatin affect gut microbiota composition remains limited.30 In our study, both the antioxidant and anti-inflammatory effects of D-methionine were important mediators of cisplatin-induced GI mucositis. D-methionine exhibited gastroprotective effect partly through increased richness of Lachnospiraceae and Lactobacillus to modulate gut microbiota dysbiosis.
The common problems of cancer patients receiving chemotherapy were GI disorder and weight loss. Their occurrence was about 50% and 77% respectively.31 The incidence of anorexia was up to 46% after chemotherapy, which suggested that patients with a GI disorder and weight loss may closely associate with anorexia.31 Cancer patients with weight loss have also increased risks for infection, life threat and malnutrition. Our results are consistent with those of another study32 in which cisplatin, the most potent emesis agent, caused significant weight loss and anorexia. Another study revealed that longer D-methionine pretreatment time is associated with better protective effects against cisplatin toxicity.29 Our study confirmed that pretreatment with D-methionine effectively attenuates cisplatin-induced weight loss and anorexia and increases stool output. Campbell and colleagues19 and Ekborn and colleagues28 also found that D-methionine supplementation results in less weight loss after cisplatin treatment. Thus, D-methionine potentially improves promotes GI function.
We attributed cisplatin-induced intestinal mucositis to the interaction between oxidative stress and inflammation, which is consistent with the findings of a previous study.33–35 Oral supplementation with D-methionine suppressed the oxidative stress in cisplatin-induced GI mucositis, characterized by decreased MDA levels, increased GSH concentration and enhanced SOD, CAT and GPx activities in the intestinal tissues (Figure 2).
The brush-border enzyme is located in the epithelial cells lining of the intestine and faces the lumen. It is part of digestive system which involves in digestion and absorption of nutrients. The brush-border enzyme was directly interaction with the luminal contents. These enzyme’s activity were modified when intestine luminal exposure to ingested harmful materials. It has been shown that administration of cisplatin inhibits these brush-border enzyme activities by the cause of the production of lipid peroxidation products and reactive oxygen species (ROS), which affects membrane structure and function and leads to inactivation of these enzymes.36 In addition, cisplatin also decreases the activity of the antioxidant enzymes in the rat intestine, indicating that cisplatin can exert direct toxic effects on the intestinal mucosa by increasing the formation of ROS to disrupt the intestinal function.36 Antioxidant administration is able to restore the activities of these enzymes after exposure to hazardous substances including cisplatin.36,37 We speculated that the restoration of brush-border enzyme activity by D-methionine is in part due to its ability to increase SOD activity and GSH availability (Figure 2). The protective effect of D-methionine has also been reported to be due directly to its SOD promoting activity and through the metabolic product, GSH, which acts as a thiol-containing reducing agent to scavenge ROS.21,25 It is well known that through methionine cycle and trans-sulfuration pathway D-methionine induces cysteine and GSH production,38 and acts as an antioxidant to reduce oxidative stress. It is worth noting that administration of D-methionine alone significantly increased GSH levels [Figure 2(b)] and activity of SOD [Figure 2(d)]. Hence, the antioxidant effect of D-methionine helps to improve digestive disorders induced by cisplatin.
There was evidence that repeated cisplatin treatment induced a significant increase in the size of inflammatory nodules and Ki-67-positive cell proliferation, which implies that cisplatin increased the synthesis of proinflammatory cytokines, contributing to inflammation.39 The results of this study also revealed that cisplatin-induced inflammation is attenuated by administration of D-methionine, which inhibits cisplatin-induced generation of IL1β, IL-6 and TNF-α (Figure 3). Although the pathogenic mechanisms of chemotherapy-induced GI mucositis are still unknown, Hamouda and colleagues proposed a possible mechanism of 5-fluorouracil (5-FU)-induced intestinal mucositis in which TNF-α induces the destruction of intestinal epithelial barrier and triggers dysbiosis with increases in abundances of mostly Gram-negative bacteria to elicit secondary inflammation, ultimately resulting in intestinal mucositis.40
A recent review has indicated that chemotherapeutic agents result in gut microbiota community imbalances by depressing the growth of beneficial bacteria and promoting the growth of harmful bacteria associated with GI mucositis.1 For instance, methotrexate induces substantial decreases in lactobacilli and enterococci, but relative increases in potentially enteropathogenic bacteria Bacteroides, Enterococci and Enterobacteriaceae.41 Irinotecan regimen leads to decreases in the abundances of Clostridium cluster XIVa, Lactobacillus group and Bifidobacterium spp., and increases in the abundances of Clostridium cluster XI and Enterobacteriaceae.42 Therefore, changes in abundance and diversity of gut microbiota are greatly influenced by chemotherapy.
Our results clearly demonstrated an imbalance in gut microbiota in cisplatin-treated rats due to drastic increases in abundances of the phyla Proteobacteria (mucosa-associated inflammation-promoting bacteria) and Deferribacteres, supporting the positive correlation between intestinal inflammation and abundance of gut Proteobacteria (particularly Enterobacteriaceae). Gut Proteobacteria abundance is a potential diagnostic criterion for dysbiosis and disease.43 B. caccae leads to mucin depletion and thinner mucus layer. This contributes to the entry of pathogenic bacteria into lumen and enhances susceptibility to colitis.44 M. schaedleri control oxidative stress during inflammation through specialized systems, inhabiting mucus layer (rich in mucin) and exhibiting proinflammatory properties through activation of nuclear factor kappa B (NF-κB) and peroxisome proliferator-activated receptor (PPAR)-delta or the inhibition of epidermal growth factor.45 Inflammation creates a favorable opportunity for E. coli to exhibit an adhere-invasive effect on the intestinal mucosa.46 The levels of Proteobacteria, particularly Enterobacteriaceae (including E. coli), increase in the gut microbiota of patients with inflammatory bowel disease (IBD).46 Both Desulfovibrios and E. coli, Gram-negative bacteria, are able to produce LPS, an endotoxic component of cell walls of certain bacteria (such as Gram-negative bacteria) that is a key mediator of gut dysbiosis and inflammation. When gut bacteria generate huge amounts of LPS, LPS forms a complex which contains LPS binding proteins and pattern recognition receptor. This is recognized by Toll-like receptors to trigger the activation of NF-κB and the production of IL-6, IL-1β and TNF-α.47 Desulfovibrio subspecies are sulfate-reducing anaerobic Gram-negative bacteria associated with increased gut permeability.48 Additionally, the ompW gene is detected only in the B. caccae TonB-linked outer membrane protein, which may contribute to the pathogenesis of intestinal inflammatory-associated diseases.49 Mucin, a major component of mucus, is considered to play an important defense role in inflamed intestine. Cisplatin decreases GI mucin content, suggesting that mucus barrier dysfunction is a cause of cisplatin-induced mucositis.17 Thus, we speculated that decreased mucin content correlates with M. schaedleri and B. caccae overgrowth. Chemotherapy agent-induced upregulation of TNF-α and IL-1β may be due to gut dysbiosis.40 To sum up, we speculated that inflammatory response in intestinal tissue caused by cisplatin is correlated with overgrowth of M. schaedleri, B. caccae, and E. coli.
Cisplatin caused significant increases in bacteria of the Bacteroidaceae and Erysipelotrichaceae families, as well as in Bacteroides uniformis. Cisplatin-induced dysbiosis is associated with disruption of the intestinal mucosa. Meanwhile, cisplatin treatment also causes the depletion of health-promoting species Ruminococcus gnavus.18 Tumor with/without cisplatin treatment mediated the structural and functional composition of gut microbiota which shifted toward more Bacteroidetes and less Firmicutes and Proteobacteria.30 Cisplatin had the strong effect in reshaping the gut bacteria community structure in a different way found by the liver tumor-bearing mouse model.30 The results showed cisplatin treatment was effective in reducing liver tumor growth but led to an imbalanced gut microbiota and decreased the ecological diversity in the gut.
Intestinal bacteria can interfere with damage to intestinal tissues via the following mechanisms: (1) modulation of inflammation and oxidative stress through a variety of mechanisms by beneficial members such as Clostridiumcluster XIVa in the Lachnospiraceae family; (2) attenuation of intestinal permeability by members such as Bifidobacterium spp. and Lactobacilli which increase tight junction expression; and (3) maintenance of the mucus layer, for example, by various Lactobacillus species, which upregulate mucin production.50 Interestingly, our results demonstrated that oral daily supplementation of D-methionine inhibits the overgrowth of inflammation causing-bacteria (M. schaedleri, B. caccae, E. coli) and increases the growth of Lachnospiraceae (Clostridium cluster XIVa), C. butyricum and L. reuteri in cisplatin-induced mucositis.
Lachnospiraceae administration attenuates colitis in mice.51 Using lactate and acetate, Lachnospiraceae can produce butyrate,52 which decreases gut pH levels and prevents growth of pathogenic bacteria.53 Coprococcus eutactus is the predominant butyrate-producing bacteria.54 Acetic acid, propionic acid, and butyric acid are known as short-chain fatty acids (SCFAs). SCFAs maintain intestinal homeostasis by suppressing the growth of Gram-negative pathogens. They are also energy sources and anti-inflammatory agents and promote apoptosis of cancer cells.55 Thus, prominent SCFA-producing bacteria can act as indicators of a stable and healthy gut. IL-10 protects intestinal health and is produced via T helper 2 (Th2) cells, regulatory T-cells, dendritic cells and macrophages. Recent research has indicated that maintenance of high levels of IL-10 contributes to a reduction in damage to the intestinal epithelium caused by cisplatin.33 C. butyricum, known as a probiotic and classified in Clostridium cluster I, promotes IL-10 production by intestinal macrophages in inflamed mucosa, and has been shown to prevent experimental colitis in mice.56 Our study showed that D-methionine supplementation prior to cisplatin treatment attenuates the decreased IL-10 level [Figure 3(d)], implying anti-inflammatory effects of IL-10 in the pathogenesis of GI mucositis.33,57
Lactobacillus spp. can prevent overgrowth of potentially pathogenic bacteria58 and possess antioxidative effects.59 L. reuteri exerts anti-inflammatory effects on intestine through reduction of TLR4 signal, leading to transcriptional inhibition of NF-κB genes in necrotizing enterocolitis model.60 L. reuteri belongs to vitamin B12 activity-producer strain,60 of which cobalamin is involved in methionine metabolic pathways,61 helpful to GSH production and antioxidant activity.
A recent review has shown that imbalances in intestinal microbiota can be modulated by natural antioxidants through their antioxidant and anti-inflammatory activities.47 Our results also confirmed that the antioxidant properties of D-methionine play important roles in gut microbiota composition modulation during cisplatin treatment. It is worth further investigating whether oral supplementation with Lachnospiraceae and L. reuteri can improve cisplatin-induced mucositis.
The first limitation of our study was lacking the quantitative assessment to calculate the number of bacteria/g of feces for microbiota compositional variation. The human/animal microbial communities were analyzed using NGS to represent the percentage of various bacteria, which was mostly described by relative microbiome profiling (RMP). However, the RMP analysis might probably underestimate the microbiota richness associated with disease. Furthermore, due to the limitation in the read length of sequences, primer biases, the quality of databases, leading to the level of genus and species classifications were also difficult to measure. In our study, the species level was too little to be determined, and this restricted a better understanding of the microbial ecology of cisplatin and D-methionine treatment. Another limitation of our study was that we did not perform a tumor-bearing model. The gut microbiota composition under tumor progression may differ from tumor-free animals. The present study was only performed in a nontumor-bearing model. Above all, the current results demonstrated that D-methionine effectively alleviated cisplatin-induced mucositis and gut dysbiosis, which are provided as a reference for further research.
In conclusion, our data suggest that D-methionine dually mediates cisplatin-induced GI mucositis and microbiota community dysbiosis. D-methionine protects against cisplatin-induced gastrointestinal damage through its antioxidant and anti-inflammatory activities and enhances growth of beneficial bacteria (Lachnospiraceae and Lactobacillus), thereby regulating gut microbiome imbalance induced by cisplatin.
Supplemental Material
Supplemental material, Supporting_Information_-2018.12.03 for D-methionine alleviates cisplatin-induced mucositis by restoring the gut microbiota structure and improving intestinal inflammation by Cheng-Hsi Wu, Jiunn-Liang Ko, Jiuan-Miaw Liao, Shiang-Suo Huang, Meei-Yn Lin, Ling-Hui Lee, Li-Yu Chang and Chu-Chyn Ou in Therapeutic Advances in Medical Oncology
Acknowledgments
We thank Liao-Jiunn Wang (Graduate Institute of Veterinary Pathobiology, National Chung Hsing University of Taiwan) for the interpretation of the intestinal pathological composition.
Footnotes
Funding: This study was supported by grant no. CSMU-JAH-106-03 and NCHU-CSMU-10504 from Chung Shan Medical University.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Cheng-Hsi Wu, Department of Family Medicine, Jen-Ai Hospital, Dali, Taichung, Taiwan.
Jiunn-Liang Ko, Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan.
Jiuan-Miaw Liao, Department of Physiology, School of Medicine, Chung Shan Medical University, Taichung, Taiwan.
Shiang-Suo Huang, Department of Pharmacology and Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan.
Meei-Yn Lin, Department of Food Science and Biotechnology, National Chung Hsing University, Taichung, Taiwan.
Ling-Hui Lee, Department of Food Science and Biotechnology, National Chung Hsing University, Taichung, Taiwan.
Li-Yu Chang, Department of Nursing, Jen-Ai Hospital, Dali, Taichung, Taiwan.
Chu-Chyn Ou, School of Nutrition, Chung Shan Medical University, 110, Sec. 1, Chien-Kuo N. Road, Taichung 40203, Taiwan.
References
- 1. Montassier E, Gastinne T, Vangay P, et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment Pharmacol Ther 2015; 42: 515–528. [DOI] [PubMed] [Google Scholar]
- 2. Wang C, Yang S, Gao L, et al. Carboxymethyl pachyman (CMP) reduces intestinal mucositis and regulates the intestinal microflora in 5-fluorouracil-treated CT26 tumour-bearing mice. Food Funct 2018; 9: 2695–2704. [DOI] [PubMed] [Google Scholar]
- 3. Touchefeu Y, Montassier E, Nieman K, et al. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Aliment Pharmacol Ther 2014; 40: 409–421. [DOI] [PubMed] [Google Scholar]
- 4. Guabiraba R, Besnard AG, Menezes GB, et al. IL-33 targeting attenuates intestinal mucositis and enhances effective tumor chemotherapy in mice. Mucosal Immunol 2014; 7: 1079–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen MC, Chen YL, Lee CF, et al. Supplementation of magnolol attenuates skeletal muscle atrophy in bladder cancer-bearing mice undergoing chemotherapy via suppression of FoxO3 activation and induction of IGF-1. PLoS One 2015; 10: e0143594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elting LS, Cooksley C, Chambers M, et al. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer 2003; 98: 1531–1539. [DOI] [PubMed] [Google Scholar]
- 7. Sonis ST. The pathobiology of mucositis. Nat Rev Cancer 2004; 4: 277–284. [DOI] [PubMed] [Google Scholar]
- 8. Pouncey AL, Scott AJ, Alexander JL, et al. Gut microbiota, chemotherapy and the host: the influence of the gut microbiota on cancer treatment. Ecancermedicalscience 2018; 12: 868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alexander JL, Wilson ID, Teare J, et al. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol 2017; 14: 356–365. [DOI] [PubMed] [Google Scholar]
- 10. Zwielehner J, Lassl C, Hippe B, et al. Changes in human fecal microbiota due to chemotherapy analyzed by TaqMan-PCR, 454 sequencing and PCR-DGGE fingerprinting. PLoS One 2011; 6: e28654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Freyer DR, Chen L, Krailo MD, et al. Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017; 18: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rezaee R, Momtazi AA, Monemi A, et al. Curcumin: a potentially powerful tool to reverse cisplatin-induced toxicity. Pharmacol Res 2017; 117: 218–227. [DOI] [PubMed] [Google Scholar]
- 13. Dugbartey GJ, Peppone LJ, de Graaf IA. An integrative view of cisplatin-induced renal and cardiac toxicities: molecular mechanisms, current treatment challenges and potential protective measures. Toxicology 2016; 371: 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rebillard A, Rioux-Leclercq N, Muller C, et al. Acid sphingomyelinase deficiency protects from cisplatin-induced gastrointestinal damage. Oncogene 2008; 27: 6590–6595. [DOI] [PubMed] [Google Scholar]
- 15. Shahid F, Farooqui Z, Khan F. Cisplatin-induced gastrointestinal toxicity: an update on possible mechanisms and on available gastroprotective strategies. Eur J Pharmacol 2018; 827: 49–57. [DOI] [PubMed] [Google Scholar]
- 16. Tazuke Y, Maeda K, Wasa M, et al. Protective mechanism of glutamine on the expression of proliferating cell nuclear antigen after cisplatin-induced intestinal mucosal injury. Pediatr Surg Int 2011; 27: 151–158. [DOI] [PubMed] [Google Scholar]
- 17. Yamamoto H, Ishihara K, Takeda Y, et al. Changes in the mucus barrier during cisplatin-induced intestinal mucositis in rats. Biomed Res Int 2013; 2013: 276186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perales-Puchalt A, Perez-Sanz J, Payne KK, et al. Frontline Science: Microbiota reconstitution restores intestinal integrity after cisplatin therapy. J Leukoc Biol 2018; 103: 799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campbell KC, Rybak LP, Meech RP, et al. D-methionine provides excellent protection from cisplatin ototoxicity in the rat. Hear Res 1996; 102: 90–98. [DOI] [PubMed] [Google Scholar]
- 20. Lo WC, Chang CM, Liao LJ, et al. Assessment of D-methionine protecting cisplatin-induced otolith toxicity by vestibular-evoked myogenic potential tests, ATPase activities and oxidative state in guinea pigs. Neurotoxicol Teratol 2015; 51: 12–20. [DOI] [PubMed] [Google Scholar]
- 21. Campbell KC, Meech RP, Rybak LP, et al. The effect of D-methionine on cochlear oxidative state with and without cisplatin administration: mechanisms of otoprotection. J Am Acad Audiol 2003; 14: 144–156. [PubMed] [Google Scholar]
- 22. Liao Y, Lu X, Lu C, et al. Selection of agents for prevention of cisplatin-induced hepatotoxicity. Pharmacol Res 2008; 57: 125–131. [DOI] [PubMed] [Google Scholar]
- 23. Hinduja S, Kraus KS, Manohar S, et al. D-methionine protects against cisplatin-induced neurotoxicity in the hippocampus of the adult rat. Neurotox Res 2015; 27: 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamstra DA, Lee KC, Eisbruch A, et al. Double-blind placebo-controlled multicenter phase II trial to evaluate D-methionine in preventing/reducing oral mucositis induced by radiation and chemotherapy for head and neck cancer. Head Neck 2018; 40: 1375–1388. [DOI] [PubMed] [Google Scholar]
- 25. Vuyyuri SB, Hamstra DA, Khanna D, et al. Evaluation of D-methionine as a novel oral radiation protector for prevention of mucositis. Clin Cancer Res 2008; 14: 2161–2170. [DOI] [PubMed] [Google Scholar]
- 26. Hamstra DA, Eisbruch A, Naidu MU, et al. Pharmacokinetic analysis and phase 1 study of MRX-1024 in patients treated with radiation therapy with or without cisplatinum for head and neck cancer. Clin Cancer Res 2010; 16: 2666–2676. [DOI] [PubMed] [Google Scholar]
- 27. Wen JJ, Vyatkina G, Garg N. Oxidative damage during chagasic cardiomyopathy development: role of mitochondrial oxidant release and inefficient antioxidant defense. Free Radic Biol Med 2004; 37: 1821–1833. [DOI] [PubMed] [Google Scholar]
- 28. Ekborn A, Laurell G, Johnstrom P, et al. D-Methionine and cisplatin ototoxicity in the guinea pig: D-methionine influences cisplatin pharmacokinetics. Hear Res 2002; 165: 53–61. [DOI] [PubMed] [Google Scholar]
- 29. Gopal KV, Wu C, Shrestha B, et al. D-Methionine protects against cisplatin-induced neurotoxicity in cortical networks. Neurotoxicol Teratol 2012; 34: 495–504. [DOI] [PubMed] [Google Scholar]
- 30. Li J, Sung CY, Lee N, et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci USA 2016; 113: E1306–E1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sanchez-Lara K, Ugalde-Morales E, Motola-Kuba D, et al. Gastrointestinal symptoms and weight loss in cancer patients receiving chemotherapy. Br J Nutr 2013; 109: 894–897. [DOI] [PubMed] [Google Scholar]
- 32. Alhadeff AL, Holland RA, Zheng H, et al. Excitatory hindbrain-forebrain communication is required for cisplatin-induced anorexia and weight loss. J Neurosci 2017; 37: 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Araujo RS, Silveira ALM, de Sales ESEL, et al. Intestinal toxicity evaluation of long-circulating and pH-sensitive liposomes loaded with cisplatin. Eur J Pharm Sci 2017; 106: 142–151. [DOI] [PubMed] [Google Scholar]
- 34. Pandit A, Kim HJ, Oh GS, et al. Dunnione ameliorates cisplatin-induced small intestinal damage by modulating NAD(+) metabolism. Biochem Biophys Res Commun 2015; 467: 697–703. [DOI] [PubMed] [Google Scholar]
- 35. Chang B, Nishikawa M, Sato E, et al. L-Carnitine inhibits cisplatin-induced injury of the kidney and small intestine. Arch Biochem Biophys 2002; 405: 55–64. [DOI] [PubMed] [Google Scholar]
- 36. Arivarasu NA, Priyamvada S, Mahmood R. Oral administration of caffeic acid ameliorates the effect of cisplatin on brush-border membrane enzymes and antioxidant system in rat intestine. Exp Toxicol Pathol 2013; 65: 21–25. [DOI] [PubMed] [Google Scholar]
- 37. Naqshbandi A, Rizwan S, Khan MW, et al. Dietary flaxseed oil supplementation ameliorates the effect of cisplatin on brush-border membrane enzymes and antioxidant system in rat intestine. Hum Exp Toxicol 2013; 32: 385–394. [DOI] [PubMed] [Google Scholar]
- 38. Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nat Rev Cancer 2016; 16: 650–662. [DOI] [PubMed] [Google Scholar]
- 39. Uranga JA, Garcia-Martinez JM, Garcia-Jimenez C, et al. Alterations in the small intestinal wall and motor function after repeated cisplatin in rat. Neurogastroenterol Motil 2017; 29: e13047–e13059. [DOI] [PubMed] [Google Scholar]
- 40. Hamouda N, Sano T, Oikawa Y, et al. Apoptosis, dysbiosis and expression of inflammatory cytokines are sequential events in the development of 5-fluorouracil-induced intestinal mucositis in mice. Basic Clin Pharmacol Toxicol 2017; 121: 159–168. [DOI] [PubMed] [Google Scholar]
- 41. Fijlstra M, Ferdous M, Koning AM, et al. Substantial decreases in the number and diversity of microbiota during chemotherapy-induced gastrointestinal mucositis in a rat model. Support Care Cancer 2015; 23: 1513–1522. [DOI] [PubMed] [Google Scholar]
- 42. Lin XB, Dieleman LA, Ketabi A, et al. Irinotecan (CPT-11) chemotherapy alters intestinal microbiota in tumour bearing rats. PLoS One 2012; 7: e39764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 2015; 33: 496–503. [DOI] [PubMed] [Google Scholar]
- 44. Desai MS, Seekatz AM, Koropatkin NM, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016; 167: 1339–1353 e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Loy A, Pfann C, Steinberger M, et al. Lifestyle and horizontal gene transfer-mediated evolution of Mucispirillum schaedleri, a core member of the murine gut microbiota. mSystems 2017; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mukhopadhya I, Hansen R, El-Omar EM, et al. IBD-what role do Proteobacteria play? Nat Rev Gastroenterol Hepatol 2012; 9: 219–230. [DOI] [PubMed] [Google Scholar]
- 47. Morais CA, de Rosso VV, Estadella D, et al. Anthocyanins as inflammatory modulators and the role of the gut microbiota. J Nutr Biochem 2016; 33: 1–7. [DOI] [PubMed] [Google Scholar]
- 48. Lam YY, Ha CW, Hoffmann JM, et al. Effects of dietary fat profile on gut permeability and microbiota and their relationships with metabolic changes in mice. Obesity (Silver Spring) 2015; 23: 1429–1439. [DOI] [PubMed] [Google Scholar]
- 49. Ashorn S, Raukola H, Valineva T, et al. Elevated serum anti-Saccharomyces cerevisiae, anti-I2 and anti-OmpW antibody levels in patients with suspicion of celiac disease. J Clin Immunol 2008; 28: 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Taur Y, Pamer EG. Microbiome mediation of infections in the cancer setting. Genome Med 2016; 8: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen L, Wilson JE, Koenigsknecht MJ, et al. NLRP12 attenuates colon inflammation by maintaining colonic microbial diversity and promoting protective commensal bacterial growth. Nat Immunol 2017; 18: 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reichardt N, Duncan SH, Young P, et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J 2014; 8: 1323–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Haenen D, Zhang J, Souza da Silva C, et al. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J Nutr 2013; 143: 274–283. [DOI] [PubMed] [Google Scholar]
- 54. Nylund L, Nermes M, Isolauri E, et al. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy 2015; 70: 241–244. [DOI] [PubMed] [Google Scholar]
- 55. Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol 2014; 12: 661–672. [DOI] [PubMed] [Google Scholar]
- 56. Hayashi A, Sato T, Kamada N, et al. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe 2013; 13: 711–722. [DOI] [PubMed] [Google Scholar]
- 57. Fang ZZ, Zhang D, Cao YF, et al. Irinotecan (CPT-11)-induced elevation of bile acids potentiates suppression of IL-10 expression. Toxicol Appl Pharmacol 2016; 291: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen SJ, Liu XW, Liu JP, et al. Ulcerative colitis as a polymicrobial infection characterized by sustained broken mucus barrier. World J Gastroenterol 2014; 20: 9468–9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hsieh FC, Lee CL, Chai CY, et al. Oral administration of Lactobacillus reuteri GMNL-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose-fed rats. Nutr Metab (Lond) 2013; 10: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu Y, Fatheree NY, Mangalat N, et al. Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NF-kappa B signaling in the intestine. Am J Physiol Gastrointest Liver Physiol 2012; 302: G608–G617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rush EC, Katre P, Yajnik CS. Vitamin B12: one carbon metabolism, fetal growth and programming for chronic disease. Eur J Clin Nutr 2014; 68: 2–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supporting_Information_-2018.12.03 for D-methionine alleviates cisplatin-induced mucositis by restoring the gut microbiota structure and improving intestinal inflammation by Cheng-Hsi Wu, Jiunn-Liang Ko, Jiuan-Miaw Liao, Shiang-Suo Huang, Meei-Yn Lin, Ling-Hui Lee, Li-Yu Chang and Chu-Chyn Ou in Therapeutic Advances in Medical Oncology