Abstract
It has been demonstrated that short peptides play an important role in the transmission of biological information, modulation of transcription, and restoring genetically conditioned alterations occurring with age. Peptidergic regulation of homeostasis occupies an important place in physiological processes, which lead to the aging of cells, tissues, and organs, consisting in the involution of major regulatory systems—the nervous, the endocrine, and the immune. The effect of AED (Ala-Glu-Asp), KED (Lys-Glu-Asp), KE (Lys-Glu), AEDG (Ala-Glu-Asp-Gly) peptides and their compound on neuronal differentiation of human periodontal ligament stem cells (hPDLSCs) was studied by immunofluorescence and western blot analysis. Growth-Associated Protein 43 (GAP43), which implements neurotransmission mechanisms and neuroplasticity, demonstrated an increased expression in hPDLSCs cultured with a compound of all studied peptides and with KED alone. The peptide compound and KED, increase the expression of Nestin (neurofilament protein), expressed in early neuronal precursors in hPDLSCs cultures. Thus, the compound of peptides AEDG, KE, AED, and KED could promote the neuronal differentiation of hPDLSCs and be a promising tool for the study of peptides as a modulator of neurogenesis in neurodegenerative diseases studied in animal models.
Keywords: GAP43, Nestin, neuronal differentiation, short peptides, stem cells
Introduction
To date, the treatment of neurodegenerative disorders is based on the use of mesenchymal stem cells (MSCs). The disease progression is represented by a neuronal death and the subsequently loss of brain and cognitive functions. MSCs seem to exert their actions stimulating many physiological processes, such as neurogenesis and angiogenesis, and moreover, they possess a key role in the antiapoptotic, immunomodulatory and anti-inflammatory actions. MSCs are able to differentiate into skeletal and chondrogenic tissues and also in neurons and glial cells.1 Oral tissues are considered an easy accessible source of MSCs with no ethical issues. In oral cavity, six different human adult dental stem cells have been described: dental pulp stem cells (DPSCs),2,3 exfoliated deciduous teeth stem cells (SHED),4 periodontal ligament stem cells (PDLSCs),5 apical papilla stem cells (SCAP),6 dental follicle stem cells (DFSCs),7 and gingiva stem cells.8,9
In particular, human periodontal ligament stem cells (hPDLSCs) are multipotent postnatal stem cells that have been used as a model for studying in vitro neurological diseases and to study their differentiation potential and immunomodulatory properties.10–12 hPDLSCs are obtained from healthy donors by minimally invasive procedure, and they are able to maintain the stemness features after long-term passages rather than the differentiation capacity.13 Moreover, hPDLSCs are easy to expand and manipulate in vitro and when transplanted do not trigger a host immune response.14,15
Short peptides (di, tri, and tetrapeptides) are signaling molecules capable of interacting with DNA and histone proteins, acting as regulatory factors.16 A number of studies have demonstrated the effect of short peptides on the proliferation and differentiation of stem cells. The inhibitory effect of EDP (Glu-Asp-Pro) and KED (Lys-Glu-Asp) peptides on the proliferation of embryonic and immortalized cells is previously shown, which may be evidence of their antitumor activity. EDP peptide enhances the spontaneous proliferative activity of normal lymphocytes, which may indicate the stimulating effect of peptides on non-neoplastic immune cells of adult donors.17
The effect of pineal gland and cerebral cortex synthetic peptides on the differentiation of the pluripotent ectodermal tissue of the early gastrula of the spiny frog Xenopus laevis is demonstrated. The AEDG (Ala-Glu-Asp-Gly) peptide stimulated the differentiation of the polypotent tissue into the epidermis and neural tissue, and the AEDP peptide induced the development of the mesenchyme and epidermis. The ADEL (Ala-Asp-Glu-Leu) peptide activated the expression of the proliferative markers Ki67 and Mcl-l in cultures of human bronchial epithelium when it was aging in passages. The AEDL peptide also regulated the expression of genes involved in the differentiation of bronchial epithelial cells: NKX2-1, SCGB1A1, SCGB3A2, FOXA1, and FOXA2. This peptide stimulated the expression of the genes MUC4, MUC5AC, and SFTPA1, the reduced expression of which correlates with the occurrence of pulmonary pathologies.18
EDA (Glu-Asp-Ala) and KED peptides increased proliferative activity of cortical thymocytes and activated their differentiation into regulatory T cells, preventing their apoptosis.19
KEDW(Lys-Glu-Asp-Trp) peptide has the ability to induce differentiation and functional activity of various types of endocrine pancreatic cells.20
Thus, short peptides are involved in the regulation of proliferation and differentiation processes in various types of cells and tissues. The aim of this study was to investigate the effect of short peptides AED (Ala-Glu-Asp), KED, KE (Lys-Glu), and AEDG and also their mixtures on the initial stages of neuronal differentiation and proliferation of the primary culture of human periodontal ligament hPDLSCs. In particular, KED peptide regulates stem and immune cells differentiation, showing neuroprotective, vasoprotective, and skin protective effects. AED peptide possesses protective effect to skin fibroblasts and cartilaginous tissue. KE peptide has reparative, oncostatic, and immunomodulatory activities and stimulates immune cells differentiation. AEDG peptide is a geroprotector and retinoprotector and regulates pineal gland and kidney function.
Materials and methods
hPDLSCs culture establishment
hPDLSCs were collected from periodontal ligament biopsies after informed consent on 10 patients. In this study, we enrolled 5 male and 5 female patients (age range: 20–40 years). All volunteers were exempt from systemic and oral diseases. Biopsies were obtained from the alveolar crest and horizontal fibers of the PDL by scraping the roots of non-carious third molar teeth with Gracey’s curettes. hPDLSCs were cultured in xeno-free medium without animal-derived molecules, Mesenchymal Stem Cell Growth Medium-Chemically Defined (MSCGM-CD), according to Diomede et al.21 Briefly, plastic-adherent cells were migrated from tissue explants and isolated using 0.1% trypsin solution. Cells were plated in Petri dishes at a density of 1 × 103 cells/cm2. Cells at passage 2 were used in all experiments.
hPDLSCs characterization and differentiation
Cytofluorimetric detection was performed as previously described by Rajan et al.22 Expression of Oct 3/4, Sox-2, SSEA-4, CD14, CD29, CD34, CD44, CD45, CD73, CD90, and CD105 was evaluated on hPDLSCs. The analysis was performed by using FACStarPLUS flow cytometry system and the FlowJo™ software (TreeStar, Ashland, OR, USA).
To assess the ability to differentiate into osteogenic and adipogenic lineage, hPDLSCs were maintained under osteogenic and adipogenic conditions for 21 and 28 days, respectively, as reported by Cianci et al.23
After the differentiation time, alizarin red and adipo oil red staining were performed on undifferentiated and differentiated cells, in order to evaluate the formation of mineralized precipitates and lipid vacuoles. The observations were carried out at inverted light microscopy Leica DMIL (Leica Microsystem, Milan, Italy). Moreover, the expressions of RUNX-2, ALP, FABP4, and PPARγ genes were evaluated by reverse transcription polymerase chain reaction (RT-PCR) as reported by Cianci et al.23 T-test was used to assess the P value, considering data significant when P < 0.05.
Short peptides
In this study, we use four short peptides (AEDG, KE, AED, and KED) and their mixture. Biological activities of each peptide have been reported in Table 1.
Table 1.
AED, KED, KE, and AEDG peptides’ biological activity.
| Peptide | Biological activity |
|---|---|
| KED | 1. Decreased proliferation embrional mesenchymal stem cells, rat’s fibroblasts (line KF-1), and human eritromielosis cell line K-562.15
2. Amplified human cortical thymocytes’ differentiation toward regulatory T cells, increased proliferative activity, decreased level of its apoptosis, and stimulated proliferative and antiapoptotic activity of the mature regulatory T cells.19 3. Increased spine density up to 32% in cortical–striatal neurons in the cell culture from brain of YAC128 mice (mouse model of Huntington disease).24 4. Increased the amount of mushroom spines in hippocampal neurons in Alzheimer disease culture mouse model by 20%.25 5. Stimulated serotonin expression in neuronal cell culture.26 6. Decreased MMP9 expression and increased Ki67 and CD98hc expression in primary rat’s skin fibroblasts.27 7. Vasoprotective effect in human and rat’s vessel.28 |
| AED | 1. Normalization of cartilaginous tissue functions, for example, in human with osteoartrosis.29
2. Decreased MMP9 and Caspase-3 expression and increased Ki67 and CD98hc expression in primary rat’s skin fibroblasts.27 |
| KE | 1. Stimulated reparation processes in various tissues.30
2. Immunoprotective effects, stimulated T-cell differentiation, tumor suppressor, and geroprotector.31 3. Increased Ki67 and CD98hc expression in primary rat’s skin fibroblasts.27 |
| AEDG | 1. Prolonged life of animals, increased telomere length.31,32
2. Increased melatonin synthesis in pineal gland during aging.33,34 3. Induced retinal cells differentiation, retinaprotector.35,36 4. Normalized renal function in pathology model in rats.37 |
Experimental design
hPDLSCs were divided into six different cultures: hPDLSCs cultured without peptides (control group); hPDLSCs cultured with AEDG (the first group); hPDLSCs cultured with KE (the second group); hPDLSCs cultured with AED (the third group); hPDLSCs cultured with KED (the fourth group); and hPDLSCs cultured with a mix of all abovementioned peptides together (the fifth group). All the peptides were diluted in phosphate-buffered saline (PBS) at a concentration of 0.01 µg/mL and were added to cell medium and replaced every 3 days. The cells were placed at 37°C in a humidified 5% CO2 incubator. Cells maintained in MSCGM-CD were used as control cells. After 10 days of induction, differentiated and undifferentiated cells treated or not with peptides were collected for subsequent analysis.
MTT assay
The viability of hPDLSCs in each group was analyzed by the quantitative colorimetric MTT assay (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide test; Promega, Milan, Italy); 2 × 103 cells/well were seeded into a 96-well culture plate for 24, 48, and 72 h and 1 week of culture. At each endpoint, 20 μL of MTT solution was added at each well; after 3 h of incubation, supernatants were read at 650 nm wavelength using a ND-1000 Nano-Drop Spectrophotometer (NanoDrop Technologies, Rockland, DE, USA).38 The MTT assay was achieved in three independent experiments and three replicates for each experimental point.
Neuronal differentiation induction
hPDLSCs were seeded and maintained for 10 days in Neurobasal-A Medium (Gibco®; Life Technologies, Monza, MB, Italy) containing B27 (2%; Life Technologies), L-glutamine (2 mM; Life Technologies), penicillin (100 U/mL; Life Technologies), streptomycin (100 mg/mL; Life Technologies), amphotericin B (5 mg/mL; Life Technologies; neuroinductive medium) and supplemented with basic fibroblast growth factor (FGF; 20 ng/mL; TemaRicerca, Milan, Italy). The medium will be changed every 3 days, as previously described by Trubiani et al.39
Confocal laser scanning microscopy analysis
Control and neural differentiated cells from all groups were fixed for 30 min at room temperature with 4% of paraformaldehyde in 0.1 M sodium phosphate-buffered saline (PBS),40 pH 7.4, and permeabilized with 0.1% of Triton1-X100 in PBS for 10 min, followed by blocking with 5% skimmed milk in PBS for 30 min. Samples were incubated with rabbit primary monoclonal antibody, anti-Nestin 1:200 (Santa Cruz Biotech-nology, Inc., Dallas, TX, USA), and mouse anti-Growth-Associated Protein 43 (GAP43; Sigma Aldrich, Milan, Italy) as a primary antibody and anti-rabbit Alexa Fluor 568 probe (Molecular Probes; Life Technologies, Monza, MI, Italy) and anti-mouse Alexa Fluor 488 probe (Molecular Probes) as a secondary antibody.41 All samples were incubated with Alexa Fluor 568 phalloidin red fluorescence conjugate (1:200), as a marker of the actin cytoskeleton and with TO-PRO staining to highlight the nuclei. As markers of neuronal differentiation, the following signal molecules of GAP43 and Nestin were chosen.
Samples were observed using a Zeiss LSM510 META confocal (Zeiss, Jena, Germany) connected to an inverted Zeiss Axiovert 200 microscope equipped with a Plan Neofluar oil-immersion objective (40×/1.3 NA). Images were collected using an argon laser beam with excitation lines at 488 nm and a helium–neon source at 543 and 633 nm.
The percentages of GAP43 and Nestin-positive cells were quantified based on the 10 images randomly collected.
Western blot analysis
An amount of 30 µg of proteins obtained from undifferentiated and neurogenic-differentiated hPDLSCs of all groups were processed as previously described by Libro et al.42 Membranes were incubated with primary antibody rabbit anti-Nestin (1:750, rabbit; Sigma-Aldrich, Milan, Italy), GAP43 (1:750, rabbit; Sigma-Aldrich), and beta-actin (1:750, mouse; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After five washes in PBS containing 0.1% Tween-20, samples were incubated for 1 h at room temperature with peroxydase-conjugated secondary antibody anti-rabbit and anti-mouse diluted 1:2.000 in 1× PBS, 3% milk, and 0.1% Tween. Protein expression was detected using the enhanced chemiluminescence (ECL) detection system (Amersham Pharmacia Biotech, Pittsburgh, PA, USA) with photodocumenter Alliance 2.7 (Uvitec, Cambridge, UK). Signals were captured by ECL detection system and analyzed using an UVIband-1D gel analysis (Uvitec).
Statistical analysis
GraphPad Prism version 6.0 (GraphPad Software, La Jolla, CA, USA) was used for statistical data analysis. Data were expressed as means and standard deviation of the recorded dependent variables. The differences among the levels of the factor under investigation were evaluated performing distinct two-way analysis of variance (ANOVA) tests. Tukey tests were applied for pairwise comparisons. A value of P < 0.05 was considered statistically significant in all tests.
Results
hPDLSCs characterization and differentiation
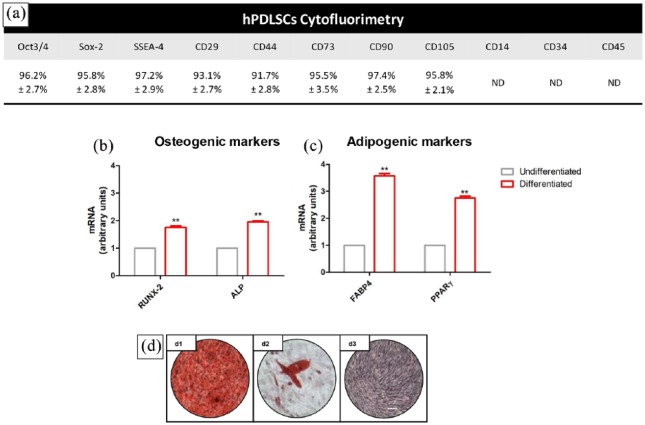
Cytofluorimetric results showed the positivity for Oct 3/4, Sox-2, SSEA-4, CD29, CD44, CD73, CD90, and CD105; meanwhile, cells showed a negativity for CD14, CD34, and CD45 (Figure 1(a)). hPDLSCs also showed the ability to differentiate toward osteogenic and adipogenic commitment as determined by RT-PCR obtained data (Figure 1(b)–(c)). In Figure 1(d), representative light microscopy pictures displayed a positive staining of alizarin red for osteogenic commitment (Figure 1(d1)) and oil red positivity for adipogenic differentiation (Figure 1(d2)).
Figure 1.
hPDLSCs characterization and differentiation. (a) Cytofluorimetric evaluation of hPDLSCs. (b) RT-PCR bar graph of osteogenic-related markers in undifferentiated and differentiated cells. (c) RT-PCR bar graph of adipogenic-related markers in undifferentiated and differentiated cells. (d) Light microscopy images of (d1) alizarin red staining, (d2) oil red staining, and (d3) toluidine blue undifferentiated cells (scale bar: 10 µm).
Proliferation rate
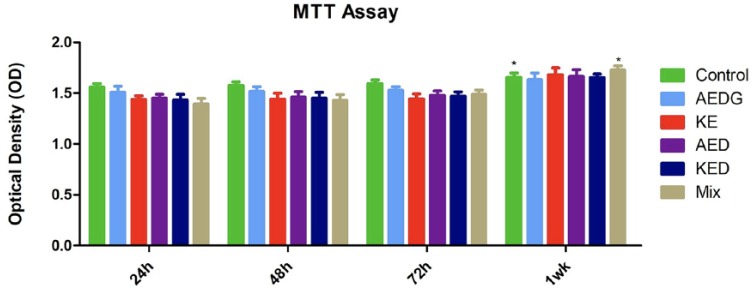
MTT assay at 24, 48, 72, and 1 week indicated that peptides did not induce an inhibition on cell proliferation, and also, the combination of these peptides showed a positive action on the proliferation rate starting at 1 week of culture (Figure 2).
Figure 2.
MTT assay. Proliferation rate of hPDLSCs after the treatment with AEDG, KE, AED, and KED and the mixture of all peptides at 24, 48, and 72 h and 1 week of culture (*P < 0.05).
Study of the effect of peptides on neuronal differentiation of hPDLSCs by immunofluorescence analyses
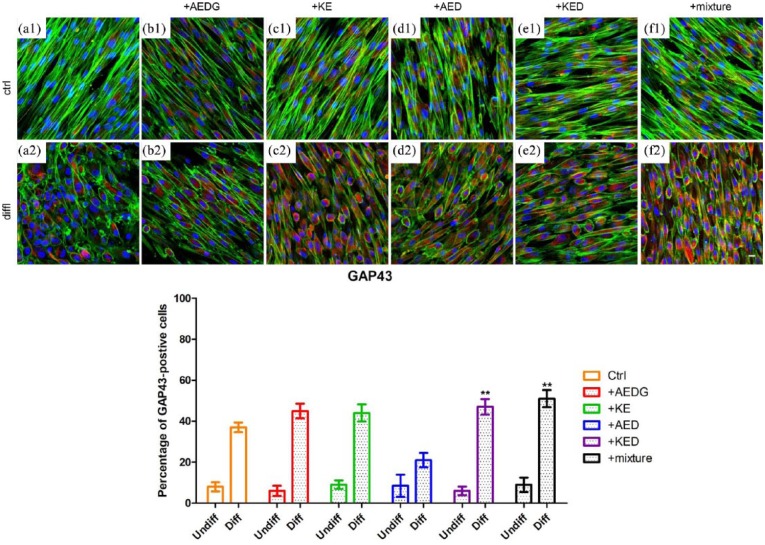
Immunofluorescence confocal microscopy images showed that the peptides AEDG, KE, AED, and KED and their mixture increased the synthesis of the GAP43 protein in hPDLSCs cultures compared to the control cells (Figure 3(a1)–(e1)). In differentiated hPDLSCs, there was an increase in GAP43, especially in cells treated with the mixture and KED (Figure 3(a2)–(e2)).
Figure 3.
Effect of peptides on the expression of GAP43 in hPDLSCs. (a1) Untreated hPDLSCs cultured in basal conditions. (a2) Untreated hPDLSCs cultured in neuroinductive medium. (b1) hPDLSCs cultured in basal conditions treated with AEDG. (b2) hPDLSCs cultured in neuroinductive medium treated with AEDG. (c1) hPDLSCs cultured in basal conditions treated with KE. (c2) hPDLSCs cultured in neuroinductive medium treated with KE. (d1) hPDLSCs cultured in basal conditions treated with AED. (d2) hPDLSCs cultured in neuroinductive medium treated with AED. (e1) hPDLSCs cultured in basal conditions treated with KED. (e2) hPDLSCs cultured in neuroinductive medium treated with KED. (f1) hPDLSCs cultured in basal conditions treated with mixture of peptides. (f2) hPDLSCs cultured in neuroinductive medium treated with mixture of peptides. The bar graphs represent the percentage of positive cells for Nestin (**P < 0.05). Cell nuclei are dye-colored DAPI—blue fluorescence, GAP43—Alexa Fluor 568—red fluorescence, and actin expression—Alexa Fluor 488—green fluorescence (bar: 5 µm).
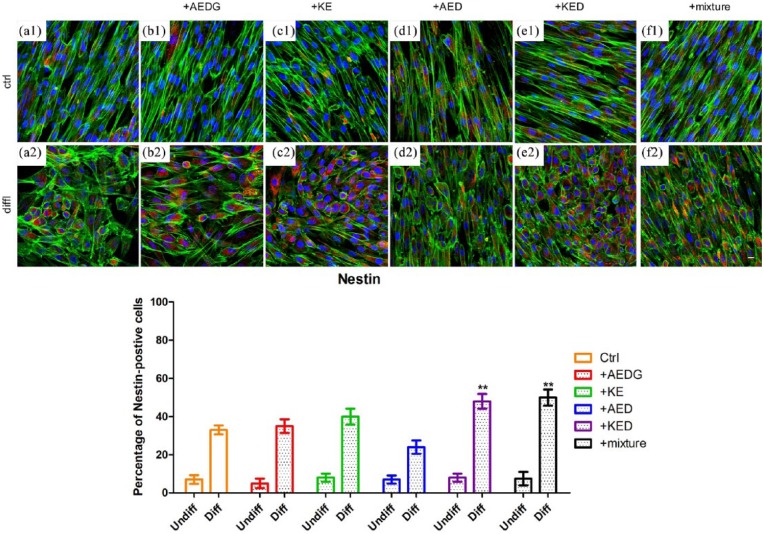
Nestin protein expression was evaluated in control and neuronal differentiated hPDLSCs treated and untreated with AEDG, KE, AED, and KED and their mixture. A higher expression of Nestin in control and differentiated cells treated with KED and the mixture was evident (Figure 4(e1), (f1), (e2), and (f2)). Bar graphs reported in Figures 3 and 4 showed that more than 50% of differentiated cells treated with peptides mixture were positive for GAP43 and Nestin, respectively, when compared to the differentiated cells with no peptides treatment.
Figure 4.
Effect of peptides on Nestin expression in hPDLSCs. (a1) Untreated hPDLSCs cultured in basal conditions. (a2) Untreated hPDLSCs cultured in neuroinductive medium. (b1) hPDLSCs cultured in basal conditions treated with AEDG. (b2) hPDLSCs cultured in neuroinductive medium treated with AEDG. (c1) hPDLSCs cultured in basal conditions treated with KE. (c2) hPDLSCs cultured in neuroinductive medium treated with KE. (d1) hPDLSCs cultured in basal conditions treated with AED. (d2) hPDLSCs cultured in neuroinductive medium treated with AED. (e1) hPDLSCs cultured in basal conditions treated with KED. (e2) hPDLSCs cultured in neuroinductive medium treated with KED. (f1) hPDLSCs cultured in basal conditions treated with mixture of peptides. (f2) hPDLSCs cultured in neuroinductive medium treated with mixture of peptides. The bar graphs represent the percentage of positive cells for Nestin (**P < 0.05). Cell nuclei are dye-colored DAPI—blue fluorescence, Nestin—Alexa Fluor 568—red fluorescence, and actin—Alexa Fluor 488—green fluorescence (bar: 5 µm).
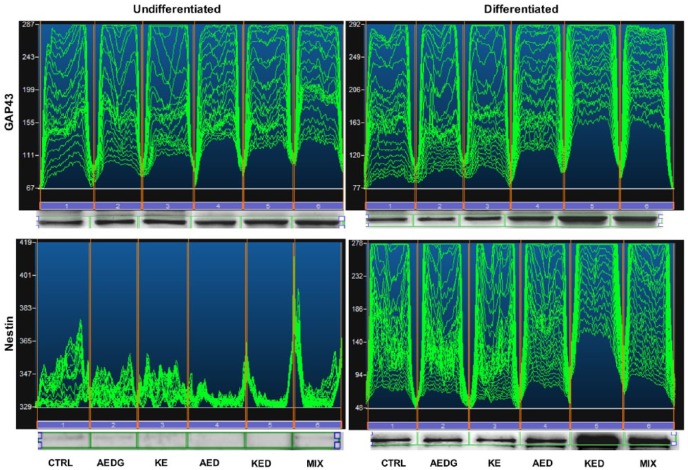
Western blot analysis
Western blot analysis confirm confocal microscopy data, and the mixture of AEDG, KE, AED, and KED peptides induced an increase of GAP43 expression in undifferentiated hPDLSCs, and this effect was more evident in differentiated hPDLSCs compared to the undifferentiated cells and to untreated differentiated hPDLSCs. Also, the results of western blot analysis indicate that the mixture of all peptides and the KED peptide affected GAP43 expression, increasing the expression of this growth factor compared with the control samples (Figure 5). The results about Nestin expression in hPDLSCs cultures confirm the immunofluorescence data. Thus, in hPDLSCs maintained under differentiation culture conditions and with KED peptide or peptide mixture treatments, the expression of Nestin significantly increased compared with the control cells (Figure 5).
Figure 5.
Western blot of GAP43 and Nestin protein expression in undifferentiated and neurogenic-differentiated hPDLSCs. Specific bands of GAP43 protein with related densitometric analyses. Specific bands of Nestin protein with related densitometric analyses.
Discussion
In the treatment of neurodegenerative diseases, tissue repair and regeneration are the best expected outcomes, as in the stem cells–based therapy. hPDLSCs possess the peculiarity features of the MSCs, as self-renewal, immunomodulatory, clonogenicity, and multi-tissue differentiation potential.23 Improved and extended survival time of stem cells in transplantation is one of the main objectives of research in regenerative medicine.43 MSCs are able to differentiate toward different lineages, as osteogenic, adipogenic, chondrogenic, and neurogenic commitment. Homeostasis is a complex process regulated by peptides that lead to the aging cells, tissues, and organs. Morphological and functional aging consists in the involution of organs and tissues, referred to nervous, endocrine, and immune systems.44 Small isolated peptides revealed a pronounced tissue-specific activity in cell cultures and in animal model.45 Peptides showed a stimulation and increase in protein synthesis in tissue-specific derived cells.46
In our study, hPDLSCs cultures have been used to evaluate the in vitro effects of AEDG, KE, AED, and KED and their mixture. MSCs derived from oral cavity become increasingly important for their neural crest origin, for their easy accessibility, and for their manipulation.47 The PDLSCs are able to differentiate into mesengenic and neurogenic lineages.48
During neurogenic differentiation, hPDLSCs change their mesenchymal phenotype and fibroblast-like shape and progressively assume neuronal-like features with cytoskeleton actin rearrangement. Neurogenic-differentiated cells showed the cytoskeleton actin, thick actin bundles at cell periphery with a rounded cell body with thin neurite-like processes.
Biological activity of natural peptides is widely studied, and it appeared to be similar in standard testing tissue cultures and in animal model,49,50 as also demonstrated in our in vitro cellular model; AEDG, KE, AED, and KED and their mixture do not negatively influence the proliferation rate, but hPDLSCs maintain the logarithmic proliferation rate at different end-point.
To validate the peptide effects on the neurogenic process, GAP43 and Nestin expression was evaluated. GAP43 (Growth-Associated Protein 43) is a protein of neuronal plasticity, since high levels of its expression are observed in the cone of axon growth during its development, in axonal regeneration, and after long-term potentiation (LTP).51 This protein is a key component of the axon and presynaptic terminus. Mutation in the gene Gap43 leads to axon atrophy a few days after its formation.52 Due to the cysteine site, GAP43 is able to bind to lipid rafts, the main components of cell membranes that coordinate neurotransmission and neuroplasticity.53,54 Participation of GAP43 protein in the learning process was demonstrated.55 GAP43 protein is a substrate for protein kinase C. Phosphorylation of protein kinase C serine at position 41 in GAP43 regulates neuron formation, regeneration, and synaptic plasticity.39,56
Nestin refers to the type VI of intermediate filament proteins (FP), and it is more expressed in neuron cells, where it is responsible for the growth of the axon in the radial direction. In most cases, type VI FP proteins in tissues are assembled into heteropolymers. It has been shown that nestin forms heterodimers and heterotetramers, but does not independently form FP in vitro.57 Nestin is expressed by various types of cells during their differentiation. Nestin is expressed in dividing cells at the early stages of their development in the central nervous system (CNS) and peripheral nervous system.58 After neuronal differentiation, nestin expression is suppressed, and it is replaced by tissue-specific proteins of neurofilaments.59 Nestin expression is re-induced in the adult body in pathological conditions, for example, in glial scar resulting from a CNS trauma.60 Thus, nestin is used as a marker of progenitor cells in the CNS.
hPDLSCs showed a high capacity to differentiate into neurogenic lineage given their embryological origin from neural crest.61 Immunofluorescence data on neurogenic committed hPDLSCs have shown that GAP43 and nestin increased their expression and the protein levels in peptide-treated cells. In particular, KED and peptide mixture showed the major effects on hPDLSCs in terms of the enhancement of GAP43 and nestin expression.
MTT test showed that AED, KED, KE, and AEDG peptides and their combination stimulate hPDLSCs proliferation. It has been reported that KE peptide and AED peptide, which stimulate proliferation of immune cells and fibroblasts,27,31 can potentiate proliferation activity and have a proliferation effect in hPDLSCs. KED peptide in previous investigation decreased stem cell proliferation, but stimulates thymocytes and skin fibroblast proliferation.19 May be that effect of this peptide is dependent of cell type and stage of its differentiation. Also, KED peptide can decrease or potentiate proliferation activity of peptides.
We can suppose that KED peptide can activate neuronal differentiation, and it is the reason of neuroplasticity processes activation. GAP43 and nestin expression have been upregulated by KED and the peptides mixture indicating their role in the enhancement of neurogenic commitment.
In conclusion, short peptides could be employed as a supplementary substance in culture medium to enhance neurogenic differentiation capacity in vitro for future regenerative cell therapy.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Oriana Trubiani  https://orcid.org/0000-0002-7459-4898
https://orcid.org/0000-0002-7459-4898
References
- 1. Diomede F, Rajan TS, D’Aurora M, et al. (2017) Stemness characteristics of periodontal ligament stem cells from donors and multiple sclerosis patients: A comparative study. Stem Cells International 2017: 1606125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diomede F, Caputi S, Merciaro I, et al. (2014) Pro-inflammatory cytokine release and cell growth inhibition in primary human oral cells after exposure to endodontic sealer. International Endodontic Journal 47(9): 864–872. [DOI] [PubMed] [Google Scholar]
- 3. Gronthos S, Mankani M, Brahim J, et al. (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America 97(25): 13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miura M, Gronthos S, Zhao MR, et al. (2003) SHED: Stem cells from human exfoliated deciduous teeth. Proceedings of the National Academy of Sciences of the United States of America 100(10): 5807–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diomede F, Zini N, Gatta V, et al. (2016) Human periodontal ligament stem cells cultured onto cortico-cancellous scaffold drive bone regenerative process. European Cells & Materials 32: 181–201. [DOI] [PubMed] [Google Scholar]
- 6. Zhang J, Wang ZH, Jiang Y, et al. (2015) Nuclear factor I-C promotes proliferation and differentiation of apical papilla-derived human stem cells in vitro. Experimental Cell Research 332(2): 259–266. [DOI] [PubMed] [Google Scholar]
- 7. Morsczeck C, Vollner F, Saugspier M, et al. (2010) Comparison of human dental follicle cells (DFCs) and stem cells from human exfoliated deciduous teeth (SHED) after neural differentiation in vitro. Clinical Oral Investigations 14(4): 433–440. [DOI] [PubMed] [Google Scholar]
- 8. Trubiani O, Toniato E, DiIorio D, et al. (2012) Morphological analysis and interleukin release in human gingival fibroblasts seeded on different denture base acrylic resins. International Journal of Immunopathology and Pharmacology 25(3): 637–643. [DOI] [PubMed] [Google Scholar]
- 9. Diomede F, Gugliandolo A, Cardelli P, et al. (2018) Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: A new tool for bone defect repair. Stem Cell Research & Therapy 9(1): 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ballerini P, Diomede F, Petragnani N, et al. (2017) Conditioned medium from relapsing-remitting multiple sclerosis patients reduces the expression and release of inflammatory cytokines induced by LPS-gingivalis in THP-1 and MO3.13 cell lines. Cytokine 96: 261–272. [DOI] [PubMed] [Google Scholar]
- 11. Rajan TS, Giacoppo S, Trubiani O, et al. (2016) Conditioned medium of periodontal ligament mesenchymal stem cells exert anti-inflammatory effects in lipopolysaccharide-activated mouse motoneurons. Experimental Cell Research 349(1): 152–161. [DOI] [PubMed] [Google Scholar]
- 12. Giacoppo S, Thangavelu SR, Diomede F, et al. (2017) Anti-inflammatory effects of hypoxia-preconditioned human periodontal ligament cell secretome in an experimental model of multiple sclerosis: A key role of IL-37. FASEB Journal 31(12): 5592–5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diomede F, Rajan TS, Gatta V, et al. (2017) Stemness maintenance properties in human oral stem cells after long-term passage. Stem Cells International 2017: 5651287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gugliandolo A, Diomede F, Cardelli P, et al. (2018) Transcriptomic analysis of gingival mesenchymal stem cells cultured on 3D bioprinted scaffold: A promising strategy for neuroregeneration. Journal of Biomedical Materials Research: Part A 106(1): 126–137. [DOI] [PubMed] [Google Scholar]
- 15. Diomede F, Gugliandolo A, Scionti D, et al. (2018) Biotherapeutic effect of gingival stem cells conditioned medium in bone tissue restoration. International Journal of Molecular Sciences 19(2): 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fedoreyeva LI, Kireev II, Khavinson V, et al. (2011) Penetration of short fluorescence-labeled peptides into the nucleus in HeLa cells and in vitro specific interaction of the peptides with deoxyribooligonucleotides and DNA. Biochemistry 76(11): 1210–1219. [DOI] [PubMed] [Google Scholar]
- 17. Khavinson V, Nikolsky IS, Nikolskaya VV, et al. (2011) Effect of tripeptides on lymphoid and stem cells. Bulletin of Experimental Biology and Medicine 151(6): 722–725. [DOI] [PubMed] [Google Scholar]
- 18. Khavinson V, Tendler SM, Vanyushin BF, et al. (2014) Peptide regulation of gene expression and protein synthesis in bronchial epithelium. Lung 192(5): 781–791. [DOI] [PubMed] [Google Scholar]
- 19. Khavinson V, Polyakova VO, Linkova NS, et al. (2011) Peptides regulate cortical thymocytes differentiation, proliferation, and apoptosis. Journal of Amino Acids 2011: 517137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khavinson V, Tendler SM, Kasyanenko NA, et al. (2015) Tetrapeptide KEDW interacts with DNA and regulates gene expression. American Journal of Biomedical Sciences 7: 14. [Google Scholar]
- 21. Diomede F, Merciaro I, Martinotti S, et al. (2016) miR-2861 is involved in osteogenic commitment of human periodontal ligament stem cells grown onto 3D scaffold. Journal of Biological Regulators and Homeostatic Agents 30(4): 1009–1018. [PubMed] [Google Scholar]
- 22. Rajan TS, Giacoppo S, Diomede F, et al. (2016) The secretome of periodontal ligament stem cells from MS patients protects against EAE. Scientific Reports 6: 38743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cianci E, Recchiuti A, Trubiani O, et al. (2016) Human periodontal stem cells release specialized proresolving mediators and carry immunomodulatory and prohealing properties regulated by lipoxins. Stem Cells Translational Medicine 5(1): 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khavinson V, Linkova N, Kukanova E, et al. (2017) Neuroprotective effect of EDR peptide in mouse model of Huntington’s disease. Journal of Neurology and Neuroscience 1(8): 1–11. [Google Scholar]
- 25. Kraskovskaya NA, Kukanova EO, Linkova NS, et al. (2017) Tripeptides restore the number of neuronal spines under conditions of in vitro modeled Alzheimer’s disease. Bulletin of Experimental Biology and Medicine 163(4): 550–553. [DOI] [PubMed] [Google Scholar]
- 26. Khavinson V, Linkova NS, Tarnovskaya SI, et al. (2014) Short peptides stimulate serotonin expression in cells of brain cortex. Bulletin of Experimental Biology and Medicine 157(1): 77–80. [DOI] [PubMed] [Google Scholar]
- 27. Linkova NS, Drobintseva AO, Orlova OA, et al. (2016) Peptide regulation of skin fibroblast functions during their aging in vitro. Bulletin of Experimental Biology and Medicine 161(1): 175–178. [DOI] [PubMed] [Google Scholar]
- 28. Khavinson V, Grigoriev EI, Malinin VV, et al. (2008) Peptide enhancing capillary resistancy, pharmacological substance based thereon and method of its application. Eurasia Patent N ЕА; 010158. [Google Scholar]
- 29. Khavinson V, Grigoriev EI, Malinin VV, et al. (2008) Peptide normalizing osseous and cartilaginous tissue metabolism, pharmacological substance based thereon and method of its application. Eurasia Patent N ЕА; 010574. [Google Scholar]
- 30. Khavinson V, Morozov VG, Malinin VV, et al. (2005) Use of a dipeptide for stimulating repair processes. Australia Patent N AU 776693. [Google Scholar]
- 31. Anisimov VN, Khavinson V. (2010) Peptide bioregulation of aging: Results and prospects. Biogerontology 11(2): 139–149. [DOI] [PubMed] [Google Scholar]
- 32. Khavinson V, Bondarev IE, Butyugov AA, et al. (2004) Peptide promotes overcoming of the division limit in human somatic cell. Bulletin of Experimental Biology and Medicine 137(5): 613–616. [DOI] [PubMed] [Google Scholar]
- 33. Khavinson V, Linkova NS, Kvetnoy IM, et al. (2012) Molecular cellular mechanisms of peptide regulation of melatonin synthesis in pinealocyte culture. Bulletin of Experimental Biology and Medicine 153(2): 255–258. [DOI] [PubMed] [Google Scholar]
- 34. Djeridane Y, Khavinson V, Anisimov VN, et al. (2003) Effect of synthetic pineal tetrapeptide (Ala-Glu-Asp-Gly) on melatonin secretion by the pineal gland of young and old rats. Journal of Endocrinological Investigation 26(3): 211–215. [DOI] [PubMed] [Google Scholar]
- 35. Khavinson V, Pronyaeva VE, Linkova NS, et al. (2014) Molecular-physiological aspects of peptide regulation of the function of the retina in retinitis pigmentosa. Human Physiology 40(1): 153–158. [PubMed] [Google Scholar]
- 36. Khavinson V, Pronyaeva VE, Linkova NS, et al. (2013) Peptidergic regulation of differentiation of embryonic retinal cells. Bulletin of Experimental Biology and Medicine 155(1): 172–174. [DOI] [PubMed] [Google Scholar]
- 37. Zamorskii II, Shchudrova TS, Linkova NS, et al. (2015) Peptides restore functional state of the kidneys during cisplatin-induced acute renal failure. Bulletin of Experimental Biology and Medicine 159(6): 736–739. [DOI] [PubMed] [Google Scholar]
- 38. Cavalcanti MF, Maria DA, deIsla N, et al. (2015) Evaluation of the proliferative effects induced by low-level laser therapy in bone marrow stem cell culture. Photomedicine and Laser Surgery 33(12): 610–616. [DOI] [PubMed] [Google Scholar]
- 39. Trubiani O, Guarnieri S, Diomede F, et al. (2016) Nuclear translocation of PKCalpha isoenzyme is involved in neurogenic commitment of human neural crest-derived periodontal ligament stem cells. Cellular Signalling 28(11): 1631–1641. [DOI] [PubMed] [Google Scholar]
- 40. Pizzicannella J, Rabozzi R, Trubiani O, et al. (2011) Histidine-tryptophan-ketoglutarate solution helps to preserve endothelial integrity of saphenous vein: An immunohistochemical and ultrastructural analysis. Journal of Biological Regulators and Homeostatic Agents 25: 93–99. [PubMed] [Google Scholar]
- 41. Pizzicannella J, Diomede F, Merciaro I, et al. (2018) Endothelial committed oral stem cells as modelling in the relationship between periodontal and cardiovascular disease. Journal of Cellular Physiology 233: 6734–6747. [DOI] [PubMed] [Google Scholar]
- 42. Libro R, Scionti D, Diomede F, et al. (2016) Cannabidiol modulates the immunophenotype and inhibits the activation of the inflammasome in human gingival mesenchymal stem cells. Frontiers in Physiology 7: 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Romeo L, Diomede F, Gugliandolo A, et al. (2018) Moringin induces neural differentiation in the stem cell of the human periodontal ligament. Scientific Reports 8(1): 9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dilman VM, Anisimov VN, Ostroumova MN, et al. (1979) Increase in lifespan of rats following polypeptide pineal extract treatment. Experimentelle Pathologie 17(9): 539–545. [DOI] [PubMed] [Google Scholar]
- 45. Anisimov VN, Loktionov AS, Khavinson V, et al. (1989) Effect of low-molecular-weight factors of thymus and pineal-gland on life-span and spontaneous tumor-development in female mice of different age. Mechanisms of Ageing and Development 49: 245–257. [DOI] [PubMed] [Google Scholar]
- 46. Anisimov SV, Khavinson V, Anisimov VN. (2004) Elucidation of the effect of brain cortex tetrapeptide Cortagen on gene expression in mouse heart by microarray. Neuro Endocrinology Letters 25(1–2): 87–93. [PubMed] [Google Scholar]
- 47. Diomede F, Zini N, Pizzicannella J, et al. (2018) 5-Aza exposure improves reprogramming process through embryoid body formation in human gingival stem cells. Frontiers in Genetics 9: 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Diomede F, Zingariello M, Cavalcanti M, et al. (2017) MyD88/ERK/NFkB pathways and pro-inflammatory cytokines release in periodontal ligament stem cells stimulated by Porphyromonas gingivalis. European Journal of Histochemistry 61(2): 2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morozov VG, Khavinson V. (1997) Natural and synthetic thymic peptides as therapeutics for immune dysfunction. International Journal of Immunopharmacology 19(9–10): 501–505. [DOI] [PubMed] [Google Scholar]
- 50. Khavinson V, Goncharova N, Lapin B. (2001) Synthetic tetrapeptide epitalon restores disturbed neuroendocrine regulation in senescent monkeys. Neuro Endocrinology Letters 22(4): 251–254. [PubMed] [Google Scholar]
- 51. Zhao JC, Zhang LX, Zhang Y, et al. (2012) The differential regulation of Gap43 gene in the neuronal differentiation of P19 cells. Journal of Cellular Physiology 227(6): 2645–2653. [DOI] [PubMed] [Google Scholar]
- 52. Gagliardini V, Dusart I, Fankhauser C. (2000) Absence of GAP-43 can protect neurons from death. Molecular and Cellular Neurosciences 16(1): 27–33. [DOI] [PubMed] [Google Scholar]
- 53. Tong J, Nguyen L, Vidal A, et al. (2008) Role of GAP-43 in sequestering phosphatidylinositol 4,5-bisphosphate to raft bilayers. Biophysical Journal 94(1): 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mishra R, Gupta SK, Meiri KF, et al. (2008) GAP-43 is key to mitotic spindle control and centrosome-based polarization in neurons. Cell Cycle 7(3): 348–357. [DOI] [PubMed] [Google Scholar]
- 55. Rosskothen-Kuhl N, Illing RB. (2014) Gap43 transcription modulation in the adult brain depends on sensory activity and synaptic cooperation. PLoS ONE 9(3): e92624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Holahan M, Routtenberg A. (2009) The protein kinase C phosphorylation site on GAP-43 differentially regulates information storage. Hippocampus 18: 1099–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Michalczyk K, Ziman M. (2005) Nestin structure and predicted function in cellular cytoskeletal organisation. Histology and Histopathology 20(2): 665–671. [DOI] [PubMed] [Google Scholar]
- 58. Yan SX, Li PL, Wang Y, et al. (2016) Nestin regulates neural stem cell migration via controlling the cell contractility. The International Journal of Biochemistry & Cell Biology 78: 349–360. [DOI] [PubMed] [Google Scholar]
- 59. Quick Q, Paul M, Skalli O. (2015) Roles and potential clinical applications of intermediate filament proteins in brain tumors. Seminars in Pediatric Neurology 22(1): 40–48. [DOI] [PubMed] [Google Scholar]
- 60. Krishnasamy S, Weng YC, Thammisetty SS, et al. (2017) Molecular imaging of nestin in neuroinflammatory conditions reveals marked signal induction in activated microglia. Journal of Neuroinflammation 14(1): 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pizzicannella J, Cavalcanti M, Trubiani O, et al. (2018) MicroRNA 210 mediates VEGF upregulation in human periodontal ligament stem cells cultured on 3Dhydroxyapatite ceramic scaffold. International Journal of Molecular Sciences 19(12): E3916. [DOI] [PMC free article] [PubMed] [Google Scholar]