Abstract
Design
In the process of cell division, the extremes of the eukaryotic chromosomes are progressively shortening, and this phenomenon is related to cell degeneration and senescence. The treatment of cartilage lesions with autologous chondrocytes implies that cells proliferate in an artificial environment. We have studied the viability of cultured chondrocytes after measurement of their telomere length before implantation.
Methods
Articular cartilage biopsies (B1, B2, and B3) were obtained from 3 patients (2 males and 1 female) with knee cartilage defects, who were going to be treated with chondrocyte implantation. Chondrocytes were cultured in DMEM with autologous serum. After the third passage, an aliquot of 1 million cells was removed to estimate the telomere length and the remaining cells were implanted. Telomere length was measured by quantitative fluorescent in situ hybridization (Q-FISH). Patients’ clinical outcome was determined preoperatively, and 12 and 24 months postimplantation with the International Knee Documentation Committee (IKDC) questionnaire.
Results
After chondrocyte implantation, IKDC score doubled at 12 and 24 months with regard to the basal value. After 3 passages, chondrocytes were cultured for a mean of 45.67 days, the mean duplication time being 4.53 days and the mean number of cell divisions being 10.04 during the culture period. The 20th percentile of telomere lengths were 6.84, 6.96, and 7.06 kbp and the median telomere lengths 10.30, 10.47, and 10.73 kbp, respectively. No significant correlation was found between IKDC score and telomere length.
Conclusion
Culturing autologous chondrocytes for implantation is not related to cell senescence in terms of telomere length.
Keywords: telomere length, telomerase, high-density autologous chondrocyte implantation, quantitative fluorescent in situ hybridization
Introduction
Articular cartilage is an aneural, avascular, and alymphatic tissue located at the end of the bones, whose main function is to preserve joints. Because of the aforementioned characteristics, articular cartilage has limited potential to self-repair.1,2 Articular cartilage injuries are quite common in joints such as knee, ankle, or hip, and if left untreated, may progress to osteoarthritis.3,4 Current therapies, such as transplantation of healthy cartilage, mosaicplasty, or microfracture of the subchondral bone plate have many limitations.5-7 In past decades, research focused on developing techniques to stimulate cartilage repair and tissue regeneration, in particular cell therapy techniques such as autologous chondrocyte implantation (ACI).8 The technique involves harvesting and expanding articular chondrocytes from a minor load-bearing area and reimplantation of the cultured cells in the damaged area of the cartilage.8-11
Cell culture is an artificial system in which cells are maintained outside their natural environment. In the case of chondrocyte cultures, a cell type that usually remains without proliferating or dividing at a low rate in the intact tissue, is induced to expand under in vitro culture conditions. The aim of a cell culture for cell therapy use is to have a high enough number of functional cells at the end of the process to be implanted to the patient. To achieve this, cells are divided several times and in each cell cycle, DNA is also replicated. Eukaryotic genome is linear and is organized in individual chromosomes. During chromosome replication, the enzymes that duplicate DNA cannot continue their duplication all the way to the end of a chromosome. Chromosomes are therefore shortened by 50 to 150 bp after each replication cycle.12-14 Telomeres are capping structures at the ends of eukaryotic chromosomes composed of TTAGGG repeats bound to an array of specialized proteins.15 Telomeres protect the end of the chromosome from deterioration or from fusion with neighbouring chromosomes.16,17 Telomerase is a ribonucleoprotein complex that regulates chromosome length by protecting chromosome ends from shortening.18,19 Telomerase has been shown to be specially active during embrionic development, protecting telomeres, but this enzyme loses its catalytic activity over time and becomes inactive when the organism is completely developed.20,21
Telomere shortening is related to cell senescence, viability, and ageing.22,23 Even more, short telomere length is associated with the development of several diseases such as osteoarthritis.24,25 It has been published that chondrocytes may dedifferentiate under in vitro culture conditions. Furthermore, this dedifferentiation could affect cell phenotype and cells’ “biological” age because of the fact that cells are divided several times when they are cultured.26 Chondrocytes used in the ACI technique are usually cultured for no more than 3 passages and some of their phenotypic characteristics (gene expression of type II collagen and aggrecan and lack of type I collagen) are studied after their implantation to patients.27 However, as the tissue synthetized after cell implantation is hyaline cartilage, we can presume that cells’ senescence is not affected by their in vitro manipulation.
In humans, telomeres are 5 to 15 kbp of the TTAGGG sequence repeats that terminate in a single-stranded 3′ G-rich overhang of 50 to 300 bases.28 Although a critically short telomere length has not been established, the relationship between cell senescence and defined telomere lengths has been published. Thus, cultured fibroblasts appear to senescence when telomere length is around 4 to 6 kbp.29 These short telomeres are related to chromosome fusion phenomena and therefore with the cell viability.30 Telomere length measurement could thus give us a perspective about the functional status of ACI used chondrocytes.
The aim of this work is to estimate the telomere length of cultured chondrocytes before implantation in patients with articular cartilage defects in order to determine the critically short telomere proportion while trying to establish a relationship between the presence of these critically short telomeres and cell viability.
Material and Methods
Patients
Biopsies (B1, B2, and B3) from 3 patients: 2 males and 1 female were included in the study. Patient ages were 36, 40, and 42 years and all of them had cartilage lesions of the knee, Outerbridge grades III or IV. In all 3 cases, biopsies were taken from a nonbearing area of the medial condyle. All patients signed an informed consent and the study was approved by the Education and Research Committee of the hospital. The subjective perception of the symptoms and function of the operated knee was assessed at baseline, 12 months postimplantation, and 24 months post-implantation using the International Knee Documentation Committee (IKDC) score.
Cell Cultures
Cartilage biopsies were placed in sterile tubes containing Dulbecco’s Modified Eagle Medium (DMEM; Lonza Group Ltd., Basel, Switzerland) and processed to isolate the chondrocytes following the previously published procedures in a sterile GMP (Good Manufacturing Practice) certified room, approved by the Spanish Health Authorities.31 Briefly, chondrocytes were isolated after overnight digestion at 37°C with 1 mg/mL collagenase A (Roche Diagnostics GmbH, Mannheim, Germany). Cells were cultured in DMEM supplemented with 10% of autologous serum, l-glutamine, and penicillin-streptomycin and incubated at 37°C, 5% CO2, and 95% relative humidity. The culture was revised daily and every 3 days the old medium was removed and changed for fresh medium. When the culture reached 80% confluence cells were detached with 0.05 mL/cm2 of 200 mg/L trypsin–ethylenediaminetetraacetic acid. A maximum of 3 passages were performed in each culture until 40 to 50 million cells were obtained. After the third passage, cells were harvested and an aliquot of 1 million chondrocytes was used to estimate telomere length. The remaining cells were implanted to patients. In each culture, the doubling time and the number of cells in each division were estimated by the trypan-blue exclusion method, assuming that cells growth exponentially.32
Telomere Length Determination
Telomere length was estimated by quantitative in situ fluorescent hybridization method (Q-FISH) studying 10 metaphases following the method described by Canela et al.33 Each telomere length determination was repeated 5 times.
Q-FISH is based on the hybridization of telomeres with a fluorescent Peptide Nucleic Acid (PNA) probe that recognizes 3 telomere repeats. Images of nuclei and telomeres are captured by a high-content screen system. The intensity of the fluorescent signal from telomeric PNA probes that hybridizes to a given telomere is linearly proportional to the length of the telomere. Intensities of fluorescence are translated to telomere length by comparing the obtained intensities of fluorescence versus standard regression curve built with control cell lines of known telomere length (Life Length, Madrid, Spain). To perform the Q-FISH procedure, cells were seeded in a clear bottom black-walled 384-well plate at a density of 30,000 cells per well with 8 replicates of each control cell line. Two identical independent plates were prepared for each set of samples. Cells were fixed with methanol/acetic acid (3:1, vol:vol). On the next day, fixed cells were treated with pepsin to digest cytoplasm. Nuclei were processed for hybridization in situ with the PNA probe. After several washing steps following standard 4′,6-diamidino-2-phenylindole (DAPI) incubation for DNA staining, the wells were filled up with mounting medium and the plate was sored overnight at 4°C. Ultraviolet and 488 nm wavelengths were used to detect DAPI and PNA probe signals, respectively.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics v. 22 software for Windows. Telomere length distribution and median telomere length were calculated by in-house software. In all cases, 20th percentile and coefficient of variation were calculated.
Results
Two patients had chondral lesions of the right knee (medial femoral condyle in one case and medial femoral condyle, trochlea, and patella in the other) and the other patient had a single lesion in the medial femoral condyle of the left knee ( Table 1 ). Patients were implanted with HD-ACI (5 million chondrocytes per cm2) and 1 million cells were used for telomere length measurement. Patients’ clinical outcome, estimated as the IKDC score at baseline, 12 months, and 24 months is depicted in Figure 1 . As shown in Figure 1 , IKDC increased in all cases from baseline to 12 and 24 months, improving each patient’s subjective perception concerning functionality of operated knee.
Table 1.
Characteristics of Patients Included in This Study.
| Biopsy ID | Gender | Age (Years) | Grade of Chondral Lesion | Location of Chondral Lesion |
|---|---|---|---|---|
| B1 | Male | 36 | IV | MFC |
| B2 | Male | 42 | III | MFC |
| B3 | Female | 40 | IV | MFC + throclea + patella |
LFC = lateral femoral condyle; MFC = medial femoral condyle.
Figure 1.
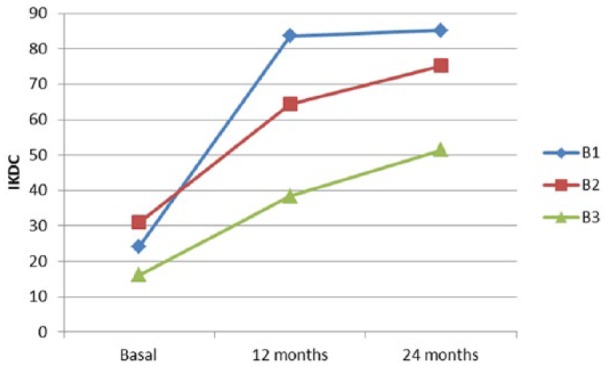
International Knee Documentation Committee (IKDC) scores at basal visit, 12-month follow-up, and 24-month follow-up for all 3 patients included in the study.
The parameters describing cell growth are shown in Table 2 . After 3 passages, cells were cultured a mean of 45.67 days, the mean time in which the number of cells was doubled was 4.53 days, and the mean number of cell divisions during this time was 10.04 ( Table 2 ).
Table 2.
Doubling Time and Number of Cell Divisions During Cultures.
| Biopsy ID | Time in Culture (Days) | Doubling Time (Days) | Estimated No. of Cell Divisions |
|---|---|---|---|
| B1 | 53 | 4.83 | 10.97 |
| B2 | 49 | 5.04 | 9.72 |
| B3 | 35 | 3.72 | 9.42 |
| Mean ± SD | 45.67 ± 9.45 | 4.53 ± 0.71 | 10.04 ± 0.82 |
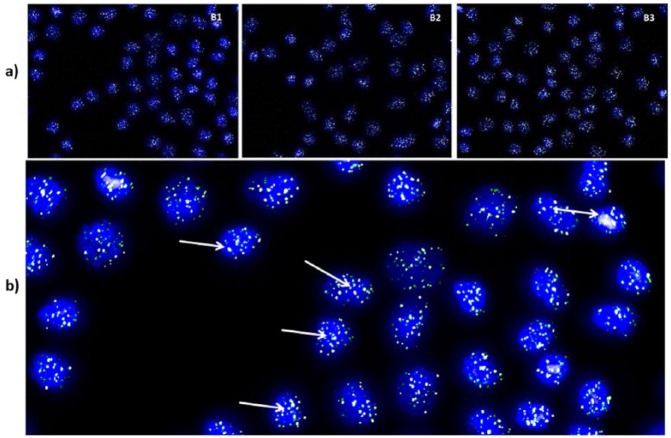
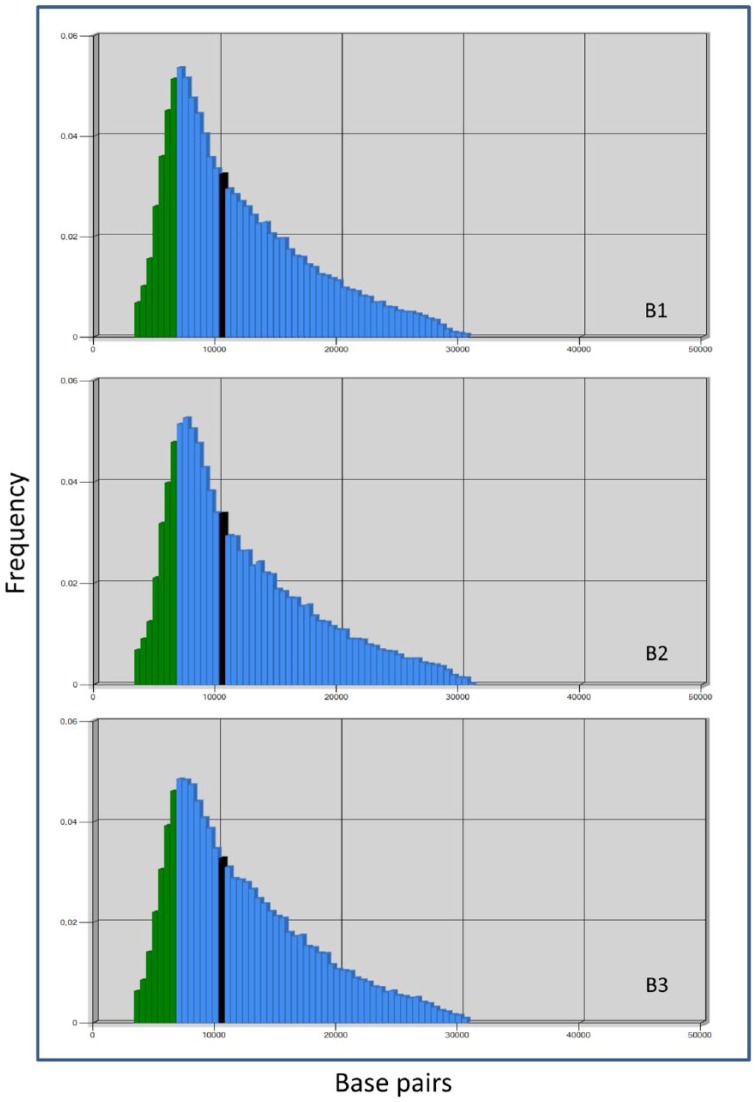
A representative metaphase of Q-FISH from each cartilage biopsy is shown in Figure 2 . Cell nuclei are stained in blue and telomeres appear in green color as a result of the hybridization of PNA probe labeled with a fluorescent decoy ( Fig. 2 ). Distribution of telomere lengths is shown in Figure 3 . As shown in Figure 3 and Table 3 , the 20th percentile of the telomere length was 6.84, 6.96, and 7.06 kbp and the median telomere span was 10.30, 10.47, and 10.73 kbp, respectively. In all 3 patients included in this study, there was no correlation between IKDC score improvement at 12 and 24 months with regard to the basal visit and the telomere length.
Figure 2.
(a) A representative metaphase from the chondrocyte cultured from each cartilage biopsy showing 4′,6-diamidino-2-phenylindole (DAPI)-stained nuclei in blue and telomeres in green/white (20× magnification). (b) One of these metaphases at higher magnification (40×). Green/white fluorescent signals corresponding to telomeres indicated by arrows.
Figure 3.
Frequency histograms of telomere length from the 3 biopsies included in the study. Blue bars represent telomere length expressed as the number of bases and green bars represent 20th percentile of length. Bold line expresses median telomere span for each case.
Table 3.
Median, 20th Percentile, and Variation Coefficient of Telomere Length for All 3 Cartilage Biopsies Included in the Study.
| Biopsy ID | Median Length (bp) | 20th Percentile of Length (bp) | Variation Coefficient |
|---|---|---|---|
| B1 | 10,270 | 6840 | 3.94 |
| B2 | 10,733 | 7095 | 3.72 |
| B3 | 10,467 | 7059 | 3.54 |
Discussion
Nowadays, autologous chondrocyte implantation is the only treatment that can regenerate articular cartilage after a lesion.8-11,37 In cell therapy, the cell is the protagonist. The source tissue is manipulated to obtain cells that are going to be converted into a medicine after several in vitro divisions. It is well known that telomere shortening after cell division is related to cell senescence and telomeres shorter than 4 kbp may be considered as critically short telomeres.29 So, the question is: Have the implanted cells been substantially affected in terms of telomere length after their in vitro expansion? The present work represents a first attempt to answer this question, and therefore telomere length of implanted autologous chondrocyte and, especially, the critically short telomere (shorter than 4 kbp) proportion were studied.
To perform the present work, we have studied the telomere length of those same cells cultured from 3 patients who were going to be implanted with autologous chondrocytes. The 3 patients had chondral lesions in the patellofemoral joint. In a recent study published by Schuette et al.,34 it has been shown that patients with chondral lesions in the patellofemoral joint have good mid- and long-term clinical outcomes. In fact, IKDC improvement after 12 and 24 months was seen in the 3 patients included in our study, indicating the effectiveness of the chondrocyte implantation measured by patients’ subjective perception of their knee functionality. As mentioned above, autologous chondrocyte implantation is currently considered as an effective technique and huge scientific evidence has been published concerning the short-, mid-, and long-term effectiveness of the technique,9,10,31,35,36 so our results are in agreement with those published, despite the low number of patients included in our study.
The chondrocytes implanted in the patients included in the present study were cultured for a mean time of 45.67 days, in which 3 passages were done, with 10.04 being the mean number of cell divisions occurring during the culture period. Cultured cells have a limited capacity to divide and when this limit, called Hayflick’s limit, is reached, cells become senescent.37 This phenomenon, in which the limit has been overpassed, is in many cases the key step for the malignant transformation of the cells which is a telomere dysfunction allowing cells to divide indefinite and uncontrollably.38 In several human cells such as fibroblasts, this form of senescence linked to DNA replication and called replicative senescence occurs after 60 replicative cycles.39 In the case of chondrocytes, it is documented that these events which lead cells to senescence occur even earlier and may very well be reached just after 30 or 35 population doublings.40 The results found in chondrocytes included in the present study indicate that they have not overpassed Hayflick’s limit and are thus not senescent, since they have had a mean 10.04 cell divisions.
Replicative senescence is accompanied by telomere shortening and is related to degenerative diseases such as osteoarthritis.39 In the samples included in our study, 20% of telomere lengths were between 6.8 and 7.0 kbp long (20th percentile) for all 3 cases and 50% of them were between 10.3 and 10.7 kbp (median). This result indicates that telomere length for implanted cells is long enough to think they are not senescent from the point of view of telomere length. In fact, senescence has been shown to start in cultured fibroblasts when the telomere length is between 4 and 6 kbp.19 Although chondrocytes and fibroblasts are 2 different cell types, with different division rates under natural circumstances, we may speculate that chromosome erosion and fusion phenomena are mainly related to these extremely short telomeres and, in the case of the implanted chondrocytes, the telomere length thus obtained may guarantee that no such chromosome aberrations occur. In fact, in all 3 cases patients had excellent clinical outcomes, unrelated to telomere length. In addition, these 3 patients are around 40 years old (maximum age for chondrocyte implantation in our unit is 55 years), so (not senescent) telomere length values are not due to their age. We could hypothesize that measurement of telomere length after 3 passages during cell culture in young patients would improve our results.
Taking all these results as a whole, we can conclude that cultured chondrocytes for implantation are not senescent, at least in terms of telomere length and number of cell divisions involved in the 3 passages carried out to obtain the necessary number of cells for high-density autologous chondrocyte implantation.
The main limitation in our study is the low number of samples included. However, given the low variation coefficient estimated from the telomere length measurement, it may be argued that these results are consistent enough to think that it can occur in all cultured and then implanted chondrocytes under the same culturing conditions, involving cell isolation, culture media, and number of passages.
Footnotes
Acknowledgments and Funding: We would like to thank Mario Wensell for carefully revising the linguistics for this article. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been financed by the Dr. Pedro Guillen Trust.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Ethical approval for this study was obtained from the Education and Research Committee of the hospital (Approval ID 15/2016).
Informed Consent: Written informed consent was obtained from all subjects before the study.
Trial Registration: Not applicable.
References
- 1. Correa D, Lietman SA. Articular cartilage repair: current needs, methods and research directions. Semin Cell Dev Biol. 2017;62:67-77. doi: 10.1016/j.semcdb.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 2. Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14:177-82. [DOI] [PubMed] [Google Scholar]
- 3. Perera JR, Gikas PD, Bentley G. The present state of treatments for articular cartilage defects in the knee. Ann R Coll Surg Engl. 2012;94:381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deng Z, Jin J, Zhao J, Xu H. Cartilage defect treatments: with or without cells? Mesenchymal Stem cells or chondrocytes? Traditional or matrix-assisted? A systematic review and meta-analyses. Stem Cells Int. 2016;2016:9201492. doi: 10.1155/2016/9201492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robert H. Chondral repair of the knee joint using mosaicplasty. Orthop Traumatol Surg Res. 2011;97:418-29. [DOI] [PubMed] [Google Scholar]
- 6. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;(391 suppl):S362-9. [DOI] [PubMed] [Google Scholar]
- 7. Eldracher M, Orth P, Cucchiarini M, Pape D, Madry H. Small subchondral drill holes improve marrow stimulation of articular cartilage defects. Am J Sports Med. 2014;42:2741-50. [DOI] [PubMed] [Google Scholar]
- 8. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95. [DOI] [PubMed] [Google Scholar]
- 9. Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212-34. [DOI] [PubMed] [Google Scholar]
- 10. Dewan AK, Gibson MA, Elisseeff JH, Trice ME. Evolution of autologous chondrocyte repair and comparison to other cartilage repair techniques. Biomed Res Int. 2014;2014:272481. doi: 10.1155/2014/272481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guillén-García P, Rodríguez-Iñigo E, Guillén-Vicente I, Caballero-Santos R, Guillén-Vicente M, Abelow S, et al. Increasing the dose of autologous chondrocytes improves articular cartilage repair: histological and molecular study in the sheep animal model. Cartilage. 2014;5:114-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458-60. [DOI] [PubMed] [Google Scholar]
- 13. Allsopp RC, Chang E, Kashefi-Aazam M, Rogaev EI, Piatyszek MA, Shay JW, et al. Telomere shortening is associated with cell division in vitro and in vivo. Exp Cell Res. 1995;220:194-200. [DOI] [PubMed] [Google Scholar]
- 14. Martin JA, Buckwalter JA. Telomere erosion and senescence in human articular cartilage chondrocytes. J Gerontol A Biol Sci Med Sci. 2001;56:B172-9. [DOI] [PubMed] [Google Scholar]
- 15. Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, et al. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988;85:6622-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569-73. [DOI] [PubMed] [Google Scholar]
- 17. Tamayo M, Mosquera A, Rego I, Blanco FJ, Gosálvez J, Fernández JL. Decreased length of telomeric DNA sequences and increased numerical chromosome aberrations in human osteoarthritic chondrocytes. Mutat Res. 2011;708:50-8. [DOI] [PubMed] [Google Scholar]
- 18. Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, et al. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen JL, Blasco MA, Greider CW. Secondary structure of vertebrate telomerase RNA. Cell. 2000;100:503-14. [DOI] [PubMed] [Google Scholar]
- 20. Wilson B, Novakofski KD, Donocoff RS, Liang YX, Fortier LA. Telomerase activity in articular chondrocytes is lost after puberty. Cartilage. 2014;5:215-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parsch D, Fellenberg J, Brummendorf TH, Eschlbeck AM, Richter W. Telomere length and telomerase activity during expansion and differentiation of human mesenchymal stem cells and chondrocytes. J Mol Med (Berl). 2004;82:49-55. [DOI] [PubMed] [Google Scholar]
- 22. Opresko PL, Shay JW. Telomere-associated aging disorders. Ageing Res Rev. 2017;33:52-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shay JW, Wright WE. Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis. 2005;26:867-74. [DOI] [PubMed] [Google Scholar]
- 24. Price JS, Waters JG, Darrah C, Pennington C, Edwards DR, Donell ST, et al. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002;1:57-65. [DOI] [PubMed] [Google Scholar]
- 25. Kuszel L, Trzeciak T, Richter M, Czarny-Ratajczak M. Osteoarthritis and telomere shortening. J Appl Genet. 2015;56:169-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lefebvre V, Peeters-Joris C, Vaes G. Production of collagens, collagenase and collagenase inhibitor during the dedifferentiation of articular chondrocytes by serial subcultures. Biochim Biophys Acta. 1990;1051:266-75. [DOI] [PubMed] [Google Scholar]
- 27. Guillén-García P, Rodríguez-Iñigo E, Aráuz S, Guillén-Vicente M, Guillén-Vicente I, Caballero-Santos R, et al. Experience with the matrix-induced autologous chondrocyte implantation technique for the treatment of chondral lesions: results from 50 patients at 2 years follow-up. Rev Esp Artrosc Cir Art. 2015:22:120-5. [Google Scholar]
- 28. MacNeil DE, Bensoussan HJ, Autexier C. Telomerase regulation from beginning to the end. Genes (Basel). 2016;7(9):E64. doi: 10.3390/genes7090064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349-52. [DOI] [PubMed] [Google Scholar]
- 30. Baird DM, Rowson J, Wynford-Thomas D, Kipling D. Extensive allelic variation and ultrashort telomeres in senescent human cells. Nat Genet. 2003;33:203-7. [DOI] [PubMed] [Google Scholar]
- 31. López-Alcorocho JM, Aboli L, Guillen-Vicente I, Rodriguez-Iñigo E, Guillen-Vicente M, Fernández-Jaén TF, et al. Cartilage defect treatment using high-density autologous chondrocyte implantation: two-year follow-up. Cartilage. Epub 2017. January 1. doi: 10.1177/194760351769304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sinclair WK, Ross DW. Models of growth in mammalian cells. Biophys J. 1969;9:1056-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Canela A, Vera E, Klatt P, Blasco MA. High-throughput telomere length quantification by FISH and its application to human population studies. Proc Natl Acad Sci U S A. 2007;104:5300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schuette HB, Kraeutler MJ, McCarty EC. Matrix-assisted autologous chondrocyte transplantation in the knee: a systematic review of mid- to long-term clinical outcomes. Orthop J Sports Med. 2017;5(6):2325967117709250. doi: 10.1177/2325967117709250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gille J, Behrens P, Schulz AP, Oheim R, Kienast B. Matrix-associated autologous chondrocyte implantation: a clinical follow-up at 15 years. Cartilage. 2016;7:309-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pareek A, Carey JL, Reardon PJ, Peterson L, Stuart MJ, Krych AJ. Long-term outcomes after autologous chondrocyte implantation: a systematic review at mean follow-up of 11.4 years. Cartilage. 2016;7:298-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1:72-6. [DOI] [PubMed] [Google Scholar]
- 38. Rousseau P, Autexier C. Telomere biology: rationale for diagnostics and therapeutics in cancer. RNA Biol. 2015;12:1078-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mollano AV, Martin JA, Buckwalter JA. Chondrocyte senescence and telomere regulation: implications in cartilage aging and cancer (a brief review). Iowa Orthop J. 2002;22:1-7. [PMC free article] [PubMed] [Google Scholar]
- 40. Martin JA, Mitchell CJ, Klingelhutz AJ, Buckwalter JA. Effects of telomerase and viral oncogene expression on the in vitro growth of human chondrocytes. J Gerontol A Biol Sci Med Sci. 2002;57:B48-53. [DOI] [PubMed] [Google Scholar]