Abstract
Objective
Osteoarthritis (OA) is one of the leading causes of disability in the adult population. Common nonoperative treatment options include nonsteroidal anti-inflammatory drugs (NSAIDs), intra-articular corticosteroids, and intra-articular injections of hyaluronic acid (HA). HA is found intrinsically within the knee joint providing viscoelastic properties to the synovial fluid. HA therapy provides anti-inflammatory relief through a number of different pathways, including the suppression of pro-inflammatory cytokines and chemokines.
Methods
We conducted a systematic review to summarize the published literature on the anti-inflammatory properties of hyaluronic acid in osteoarthritis. Included articles were categorized based on the primary anti-inflammatory responses described within them, by the immediate cell surface receptor protein assessed within the article, or based on the primary theme of the article. Key findings aimed to describe the macromolecules and inflammatory-mediated responses associated with the cell transmembrane receptors.
Results
Forty-eight articles were included in this systematic review that focused on the general anti-inflammatory effects of HA in knee OA, mediated through receptor-binding relationships with cluster determinant 44 (CD44), toll-like receptor 2 (TLR-2) and 4 (TLR-4), intercellular adhesion molecule-1 (ICAM-1), and layilin (LAYN) cell surface receptors. Higher molecular weight HA (HMWHA) promotes anti-inflammatory responses, whereas short HA oligosaccharides produce inflammatory reactions.
Conclusions
Intra-articular HA is a viable therapeutic option in treating knee OA and suppressing inflammatory responses. HMWHA is effective in suppressing the key macromolecules that elicit the inflammatory response by short HA oligosaccharides.
Keywords: osteoarthritis, hyaluronic acid, knee, anti-inflammatory
Introduction
Osteoarthritis (OA) is one of the leading causes of disability in the adult population.1 OA is most often a slow progressive joint disorder, characterized by joint pain, cartilage degeneration, and decreased joint function.2 Common nonoperative treatment options include nonsteroidal anti-inflammatory drugs (NSAIDs), intra-articular corticosteroids, and intra-articular injections of hyaluronic acid (IA-HA).3 HA is found intrinsically within the knee joint providing viscoelastic properties to the synovial fluid, and the onset of knee OA is associated with reduced HA synthesis and increased HA degradation leading to a shift in distribution toward a lower average molecular weight in the synovium, synovial cavity, and cartilage.3
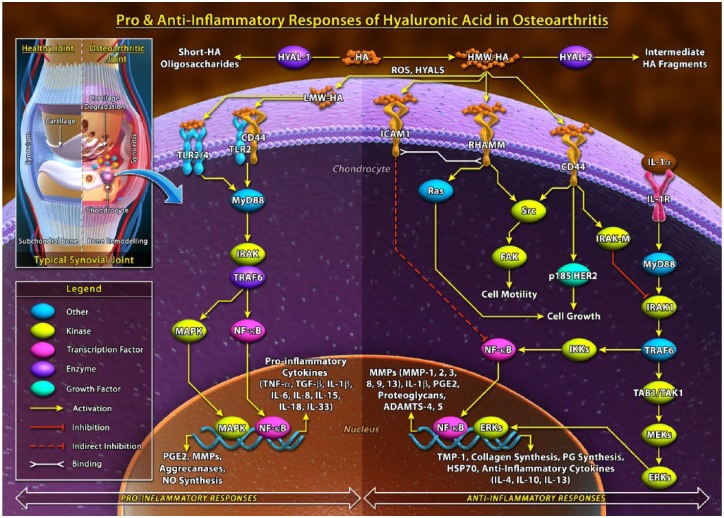
Many of the biological actions of HA are dependent on the molecular size of the ligand ( Fig. 1 ). HA in its native form is synthesized by hyaluronic acid synthases (HAS) into long polymers often ranging from lower molecular weight to higher molecular weight HA, as high as 5 × 106 kDa.4 Hyaluronidase-1 (Hyal-1) and hyaluronidase-2 (Hyal-2) enzymes are responsible for HA degradation through fragmentation of HA from both ends toward the center of the molecule, which decreases the molecular weight and creates HA oligosaccharide fragments within the synovial fluid.5 Several studies have found significant differences in the inherent properties of high molecular weight HA (HMWHA) versus short HA oligosaccharide chains, and it has been proposed that long polymer HA displays anti-inflammatory properties, whereas short HA oligosaccharides act as ligands that produce a pro-inflammatory response through mediated receptor signaling pathways.6 The molecular weight distribution of HA products is important to consider, as there is potential for short oligosaccharides to potentially be included within an HA product if the molecular weight distribution is wide and around a low average molecular weight.
Figure 1.
Summary of the pro-inflammatory and anti-inflammatory responses of hyaluronic acid.
CD44 = cluster determinant 44; ECM = extracellular matrix; ERKs = extracellular signal-regulated kinases; FAK = focal adhesion kinase; HA = hyaluronic acid; HER2 = human epidermal growth factor receptor 2; HYAL = hyaluronidase; ICAM = intercellular adhesion molecule–1; ICM = intracellular matrix; IL = interleukin; IRAK = interleukin-1 receptor-associated kinase; MAPK = mitogen activated protein (MAP) kinase; MMP = matrix metalloproteinase; MyD88 = myeloid differentiation primary response 88, NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells; NO = nitric oxide; PG = proteoglycan; PGE = prostaglandin E2; TAK1 = transforming growth factor-β (TGF-β)-activated kinase; TAL1 = T-cell acute lymphocytic leukemia protein 1; TIMP = tissue inhibitor of metalloproteinases; TLR = toll like receptor; TNF = tumor necrosis factor; TRAF6 = TNF receptor associated factors.
Intra-articular HA therapy provides therapeutic relief through a number of pathways, including the suppression of pro-inflammatory cytokines and chemokines via inhibitors of the signal transduction pathways from specific cell surface receptors, as well as promotion of the synthesis of anti-inflammatory mediators.7 Evidence has shown that HA oligosaccharides and HMWHA polymer chains bind to cell surface receptors such as cluster determinant 44 (CD44), toll-like receptor 2 (TLR-2) and 4 (TLR-4), layilin (LAYN), and intercellular adhesion molecule-1 (ICAM-1). Since HA has an established impact on inflammation in osteoarthritis, the purpose of this review was to summarize the evidence within published literature regarding the anti-inflammatory properties of HA in osteoarthritis. Additionally, the review describes the macromolecules and inflammatory-mediated responses associated with the cell transmembrane receptors. Understanding the effects of HA on inflammation at the cellular level may provide further insight to into the clinical applications of HA treatment.
Methods
Literature Search and Article Screening
We conducted a comprehensive literature search using the MEDLINE (1946 to present), EMBASE (1974 to present), and PubMed databases on February 4, 2016. An updated literature search was performed on February 1, 2017. The literature search terms are listed in the appendix. The following inclusion criteria were used to determine study eligibility: (1) articles describing the anti-inflammatory mechanism of HA treatment for OA, (2) primary nonclinical basic science articles, and (3) articles with full text published in English. Studies must have focused on the inflammatory processes of osteoarthritis using HA derivatives to elucidate the anti-inflammatory mechanisms. Studies focusing on the general treatment of osteoarthritis using hyaluronic acid were excluded. Nonclinical basic science articles included experimental studies in vivo and/or in vitro, and excluded experimental studies conducted on human participants. Article screening was done in 3 stages: titles were screened for relevance, followed by abstract screening, and finally full text screening to determine eligibility.
Data Abstraction
After article screening, included articles were categorized based on the primary anti-inflammatory responses described within them based on a review of the material. Sections were derived by the immediate cell surface receptor protein discussed within the article, or based on the primary theme of the article. The inflammatory response categories used in the data abstraction process were the following: HA-CD44 receptor response, HA-TLR-4 receptor response, HA-LAYN receptor response, and HA-ICAM-1 receptor response. The key findings and conclusions from included articles were summarized in aggregate with studies of the same categorical assignment.
Results
Search Strategy
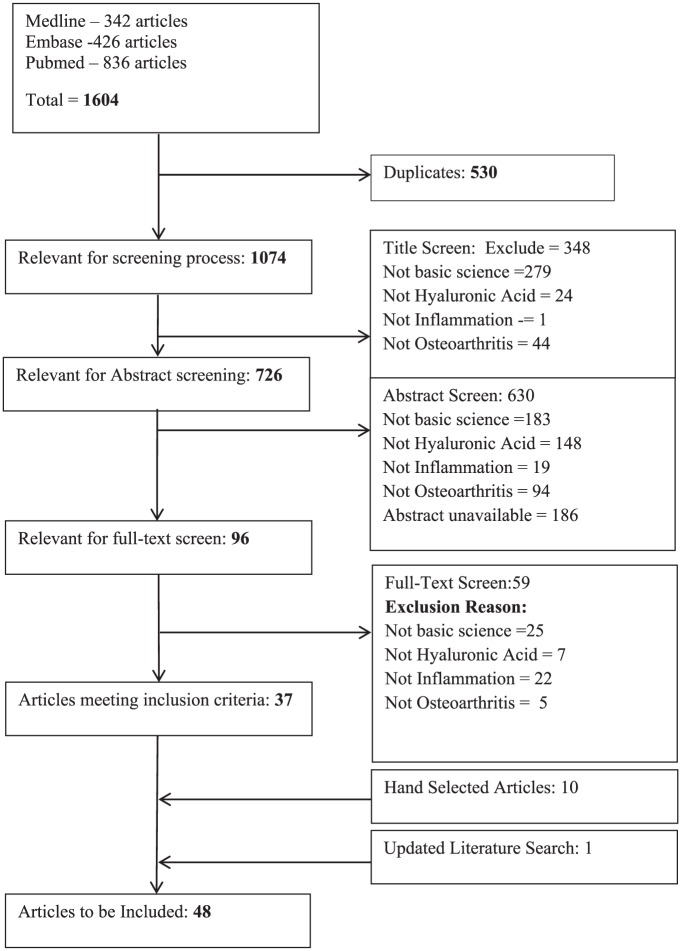
The literature search identified 1604 articles; 1074 of these articles were deemed relevant for screening ( Fig. 2 ). Of these, 37 articles met the predefined inclusion criteria and 10 additional articles were identified from our review of the reference lists of relevant articles. Therefore, 47 articles were included in this systematic review.8-54 The updated literature search identified one additional article.55
Figure 2.
Literature Search.
Study Characteristics and Area of Focus
The studies included in this review were published between 1985 and 2016 ( Table 1 ). Approximately half of the studies were published within the past decade. The majority of studies were conducted in Asia (43.8%), North America (25%), and Europe (27.1%). Multiple different factors involved in the anti-inflammatory process of HA were evaluated ( Table 2 ), with the majority of articles focusing on general anti-inflammatory effects of HA (33.3%), fragmentized HA versus HMWHA (22.9%), CD-44 receptor binding (14.6%) and pro-inflammatory macromolecules respective influences in inflammation (14.6%).
Table 1.
Study Characteristics.
| Characteristic | Total (%) (N = 48) |
|---|---|
| Year of publication | |
| 1985-1989 | 1 (2.1) |
| 1990-1994 | 4 (8.3) |
| 1995-1999 | 4 (8.3) |
| 2000-2004 | 8 (16.7) |
| 2005-2009 | 9 (18.8) |
| 2010-2014 | 19 (39.6) |
| 2015-2017 | 3 (6.3) |
| Study location | |
| Asia | 21 (43.8) |
| North America | 12 (25) |
| Europe | 13 (27.1) |
| Australia | 2 (4.2) |
| South America | 0 (0.0) |
| Africa | 0 (0.0) |
Table 2.
Primary Focus of the Included Studies.
| Primary Focus | Number of Studies | Reference |
|---|---|---|
| General anti-inflammatory effects of hyaluronic acid (HA) | 16 | Abatangelo et al., 1989 Yasui et al., 1992 Fraser et al., 1993 Noble et al., 1996 Takahashi et al., 2001 Tanimoto et al., 2001 Jean et al., 2006 Wang et al., 2006 Hashizume and Mihara, 2009 Brun et al., 2012 Smith et al., 2013 Chen et al., 2014 Yoshioka et al., 2014 Sundman et al., 2014 Chan et al., 2015 Aulin et al., 2016 |
| High molecular weight HA and small HA oligosaccharides | 11 | Filion and Phillips, 2001 Lajeunesse et al., 2003 Sheehan et al., 2003 Santangelo et al., 2007 Hashizume et al., 2010 Campo et al., 2012 Campo et al., 2012b Chang et al., 2012 Galois et al., 2012 Kataoka et al., 2013 Sato et al., 2014 |
| Pro-inflammatory macromolecules | 7 | Yasui et al., 1992 Shimazu et al., 1993 Goto et al., 1999 D’Souza et al., 2000 Stove et al., 2002 Greenberg et al., 2006 Oliviero et al., 2015 |
| CD44 receptor | 7 | Chow et al., 1993 Kawana et al., 2008 Yasuda, 2010 Levesque et al., 1997 Sasaki et al., 2004 Hiraiwa et al., 2011 Campo et al., 2012a |
| TLR receptors | 3 | Scheibner et al., 2006 Campo et al., 2011 Campo et al., 2012c |
| ICAM-1 receptor | 3 | Hiramitsu et al., 2006 Yasuda, 2007 Shao et al., 2013 |
| LAYN receptor | 1 | Murata et al., 2013 |
General Anti-Inflammatory Effects of HA
Several studies focused on the general effects of HA, such as its ability to inhibit inflammation, impede advances of osteoarthritis progression8,29,56 and its effect on the production of nitric oxide.47 Fraser et al.21 observed that mild acute inflammation resulted in large changes in the metabolic turnover of synovial HA. Additionally, pro-inflammatory cytokines (IL-1b, TNF-a, or IFN-y) can regulate hyaluronic acid synthase (HAS) expression17,19,48 and therefore affect the distribution of higher to lower molecular weight HA in the synovium.
Role of HA–CD44 Receptor Binding
The primary receptor of the HA ligand is the CD44 receptor. Activation of CD44 receptors initiates a signaling cascade associated with p185 human epidermal growth factor receptor 2 (HER2) and proto-oncogene c-Src kinase tyrosine kinase pathways.57 The affinity of HA to CD44 receptors is dependent on the molecular size of HA; increased avidity of HA binding to CD44 is correlated with an increase in the size of the polysaccharide chain, as larger HA oligosaccharides are capable of decreasing dissociation through divalent binding.58 Expression of the CD44 receptor is responsible for the maintenance of cartilage homeostasis, and the principal function of CD44 is to bind and internalize exogenous HA.18 Several T-cells and cytokines can assist in regulating HA-CD44 receptor binding, primarily through tumor necrosis factor–α (TNF-α) regulation.33 Kawana et al.31 conducted experiments on CD44 −/− mice and wild-type counterparts (CD44 +/+ mice) to assess the effects of HA on cytokine production. They determined that HA–CD44 binding suppressed in vivo pro-inflammatory cytokine production mediated by TLRs via nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation. An early and pivotal study conducted by Noble et al.35 examined the effects of higher molecular weight HA and lower molecular weight HA fragments on NF-κB activation in mouse macrophages. Only the smaller HA fragments were found to activate NF-κB DNA binding activity, suggesting that small HA oligosaccharide fragments elicit a pro-inflammatory response.
Several studies have further demonstrated that fragmentized HA produces a pro-inflammatory response and HMWHA produces an anti-inflammatory response in osteoarthritis.12,13,16,32,39,42,45,49,51 Treatment of normal mouse synovial fibroblasts with HA fragments significantly increased expression of TLR-4 and CD44 receptors, along with increased expression of inflammatory cytokines IL-18 and IL-33.13,14
HMWHA can occupy multiple binding sites of CD44 and promote anti-inflammatory effects within the cell, while small HA fragments bind suboptimally to a limited number of CD44 sites. HA oligosaccharides smaller than 10 units are suggested to not optimally occupy the CD44 binding site, while HA oligosaccharides of at least 20 units are required for divalent binding to multiple CD44 receptors.58 Through this action, higher molecular weight HA suppresses pro-inflammatory cyotokines,11,23,30,46 suppresses matrix metalloproteinase, proteoglycans,43 and prostaglandin E2 (PGE2) syntheses24,27,37,38,51 and production.26,52,54 HMWHA suppresses PGE2 production via CD44 through the downregulation of NF-κB.51 HMWHA also decreases levels of matrix metalloproteinase levels by increasing levels of tissue inhibitor of metalloproteinase–1 (TIMP-1).53
Campos et al.11 investigated the effects of inhibiting HA degradation on the inflammatory response in synovial fibroblasts in mice. Inhibition of HA degradation via small interference RNA (siRNAs) targeted for hyaluronidase enzymes HYAL1, HYAL2, and HYAL3 reduced TLR-4 and CD44 activation by HA fragments, suggesting that inhibition of HA degradation may contribute to reducing TLR-4 and CD44 activation and the corresponding inflammatory mediator response.11
Role of HA–TLR Receptor Binding
TLRs are highly conserved proteins that coordinate the defense against common bacteria and viruses within the immune system.4 TLRs can be activated by pathogen-associated molecular patterns (PAMPs) along with various endogenous molecules called damage-associated molecular patterns (DAMPs), which include extracellular matrix breakdown products such as low molecular weight HA fragments.4 Scheibner et al.40 demonstrated that fragmentized HA activates the innate immune response via TLR-2 through the myeloid differentiation primary response protein (MyD88)-, IL-1R-associated kinase-, TNFR-associated factor–6-, protein kinase C5-, and NF-κB-dependent pathways. Furthermore, Scheibner et al.40 found that higher molecular weight HA can inhibit TLR-2 signaling, demonstrating the anti-inflammatory ability of HMWHA therapy. A similar study was conducted by Campos et al.10 to investigate the influence of HMWHA at different concentrations on TLR-4 and TLR-2 modulation in collagen-induced arthritis (CIA) in mice. HA treatment significantly limited CIA incidence and decreased TNF-α, interleukin-1β (IL-1β), interleukin-17 (IL-17), matrix metalloprotease-13 (MMP-13), and inducible nitric oxide synthase (iNOS) levels that were initially upregulated by CIA.
Campos et al.13 investigated the influence of short HA oligosaccharides and HMWHA on the inflammatory response in normal mouse chondrocytes. HA fragment treatment produced a significant upregulation of TLR-4, TNF-a, IL-1b, IL-6, and IL-18. HMWHA did not exert any activity in untreated cells, although it was able to reduce the effects of HA fragments significantly. TLR-4 was confirmed as the target of HA action through TLR-4 small interference RNA experiments. Therefore, HMWHA can attenuate the inflammatory process induced by small HA fragments through TLR-4 receptor binding.
Role of HA–ICAM Receptor Binding
ICAM-1 is another cell surface receptor for HA. Upregulation in the expression of ICAM receptors has been observed in inflamed and malignant tissues.59 The NF-κB/I-κB transcriptional regulatory system is a critical component of the host inflammatory response, induced by activation of the aforementioned cell surface receptors. This system has an essential role in transducing signals leading to the expression of numerous genes involved in the inflammatory response, including the induction of the expression of pro-inflammatory cytokines such as IL-1β, IL-6, and IL-8, as well as TNF-α.4 Shao et al.41 evaluated the impact of ICAM-1 in HA therapy in an experimental rat model of severe non-bacterial cystitis. Elevated ICAM-1, TNF-α, and IL-6 levels were observed in the inflammation model. After treatment with HA, a significant decrease in ICAM-1 was observed, suggesting that a reduction of ICAM-1 may play a role in the anti-inflammatory effect of HA. Yasuda et al.50 examined the effects of HMWHA within the physiological levels on pro-inflammatory cytokine production by lipopolysaccharide (LPS)-stimulated U937 macrophages. HA was added to U937 macrophage cultures in the presence of LPS, with or without pretreatment with anti-ICAM-1 antibody. LPS stimulated production of TNFα, IL-1β, and IL-6. HMWHA inhibited LPS-induced cytokine production, whereas fragmentized HA provided no effect. Anti-ICAM-1 antibody blocked the effects of HA on the LPS actions on U937 cells, suggesting an intrinsic role of ICAM-1 receptors in the anti-inflammatory action of HA. LPS activated NF-κB and mitogen-activated protein kinases (MAPK) pathways, whereas HA downregulated p65 NF-κB and I-κBα phosphorylation by LPS without affecting MAPKs. Inhibition studies revealed the requirement of NF-κB for LPS-stimulated cytokine production. HMWHA suppresses LPS-stimulated production of pro-inflammatory cytokines via ICAM-1 through downregulation of NF-κB and I-κB.
Role of HA–Layilin Receptor Binding
LAYN’s ability to bind HA illustrates a parallel between the LAYN and CD44, as they both bind to cytoskeleton-membrane linker proteins through their cytoplasmic domains and to HA through their extracellular domains.60 Murata et al.34 examined the expression and potential function of LAYN in human articular chondrocytes and synoviocytes. LAYN was constitutively expressed in human articular chondrocytes and synoviocytes and it was shown that IL-1β significantly suppressed the expression of LAYN in these cells. HMWHA repressed IL-1β-induced MMP-1 and MMP-13 production in chondrocytes, but this was significantly abrogated in chondrocytes transfected with siRNA against LAYN, suggesting that LAYN may contribute to the regulation of HA functions in the arthritic condition.
Discussion
HA has multiple effects on inflammatory mediators involved in the osteoarthritic disease state. In vitro and animal studies have shown that HA interacts with different cell surface receptors in a molecular weight– and dose-dependent manner. The size of HA strongly influences receptor affinity, receptor activation, and downstream signaling.61 However, the distinct HA ligand-receptor relationships with CD44, TLR-2 and TLR-4 remain to be fully elucidated. Recent evidence suggests signal transduction by HA is dependent on the ability of HA to cluster the receptors on the cell membrane. For example, the amount of HA binding to CD44 receptors increases as a function of HA size. HMWHA possess multivalent sites for CD44 binding while short oligosaccharides of HA have only 1 or 2 binding sites.62 Reversible binding of HA to CD44 receptors occur with short HA oligosaccharides, and this interaction is essentially irreversible with larger HA polymers, suggesting an antagonistic relationship between short chain oligosaccharides of HA and CD44 receptors. Similar molecular weight– and size-dependent relationships to TLR receptors also exist, influencing downstream signaling cascades.56,63,64
HMWHA can bind to the sites of CD44, TLR-2, and TLR-4, to promote anti-inflammatory effects within the cell. Through CD44 receptor binding, HMWHA downregulates the expression of IL-8, IL-33, MMPs, proteoglycans and PGE2 and suppresses NF-κB activation. HMWHA also suppresses pro-inflammatory cytokine levels through interactions with ICAM-1 by downregulation of NF-κB and I-κB. Further research into the relationship between HA and LAYN is warranted to fully understand its involvement with inflammation.
Overall, HA has a safe and tolerable profile in clinical trials and practice.1,2,7 Basic science evidence has progressed the understanding of how HA is able to inhibit the inflammatory response in knee OA, and the clinical efficacy of HA has been studied extensively and has been used in treating patients with symptomatic knee OA.65 Patients with knee OA suffer significant pain and disability. HA has been used to treat these symptoms of OA, to restore proper knee function and manage pain.66 HA is not traditionally seen as an anti-inflammatory agent; however, HA has demonstrated, in vitro and in vivo, anti-inflammatory effects within the synovial fluid at the molecular level. Further insight into the clinical efficacy of IA-HA in treating inflammation in knee OA is required. Kaneko et al. observed a decrease in synovial fluid IL-6 and IL-8 levels in patients with knee OA after being treated with sodium hyaluronate.67 Segzin et al.68 observed a significant decrease in IL-6 levels in patients receiving both hyaluronan and placebo in a randomized controlled trial; however, IL-8 and TNF-α levels did not change. A recent pilot study investigated the use of viscosupplementation on synovial fluid inflammation by examining changes in synovial fluid levels of cytokines and oxidative stress, and reductions in TNF-α and IL-1β levels were observed over a 6-month period.69 Higher quality studies are necessary to evaluate the anti-inflammatory properties of viscosupplementation treatment used in clinical practice, as well as delineate the differences between products that differ in structure from endogenous HA.
The systematic review is strengthened by the methodological approach in identifying available nonclinical basic science evidence in online databases. Multiple mechanisms of action of HA in inflammation have been identified and comprehensively reported, providing insight into the pro- and anti-inflammatory key macromolecules identified. Moreover, an in vitro comparison of the different preparations of HA further explicates the anti-inflammatory potential of intra-articular HA treatment. This review is limited in that it primarily focused on the anti-inflammatory influence of HA in the treatment of OA, and did not address the larger profile of the mechanism of action of HA. Other characteristics of viable HA treatment that should be considered include its rheological properties, subchondral protective properties, chondroprotective effects, and lubrication and shock absorption features, which may also affect inflammatory properties.70
Conclusion
HA produces molecular weight–dependent anti-inflammatory effects through a multifactorial mechanism of action via CD44, TLR, and I-CAM receptor signaling. HMWHA combats the pro-inflammatory influences of fragmentized HA, such as suppressing the expression of pro-inflammatory cytokines, matrix metalloproteinases, prostaglandins, and nitric oxide. Future studies investigating the effects of HA on cartilage damage and inflammation in well controlled clinical studies may help determine whether preparations of intra-articular HA aid in other degenerative characteristics of OA, such as chondroprotection and slowing the progression of disease.
Appendix
Literature Search Strategy.
| Medline: 342 Articles | Embase: 426 Articles | PubMed: 836 Articles |
|---|---|---|
| 1. Hyaluronic acid[title] 2. Hylan*[title] 3. Hyaluronan*[title] 4. Viscosupplementation[title] 5. 1 or 2 or 3 or 4 6. Osteoarthrit*.mp 7. Arthrit*.mp 8. Joint pain.mp or arthralgia/ 9. 6 or 7 or 8 10. Anti-inflammatory .mp or Anti-Inflammatory Agents/ 11. Inflammat*.mp 12. Inflam*.mp 13. Swell* 14. 10 or 11 or 12 or 13 15. 5 and 9 and 14 16. Limit 15 to English language |
1. Hyaluronic acid[title] 2. Hylan*[title] 3. Hyaluronan*[title] 4. Viscosupplementation[title] 5. 1 or 2 or 3 or 4 6. Osteoarthrit*.mp 7. Arthrit*.mp 8. Joint pain.mp or arthralgia/ 9. 6 or 7 or 8 10. Anti-inflammatory .mp or Anti-Inflammatory Agents/ 11. Inflammat*.mp 12. Inflam*.mp 13. Swell* 14. 10 or 11 or 12 or 13 15. 5 and 9 and 14 16. Limit 15 to English language |
1. Hyaluronic acid.mp 2. Hylan*.mp 3. Hyaluronan*.mp 4. Viscosupplementation.mp 5. 1 or 2 or 3 or 4 6. Osteoarthrit*.mp 7. Arthrit*.mp 8. Joint pain.mp 9. 6 or 7 or 8 10. Anti-Inflammatory.mp 11. Inflammat*.mp 12. Inflam*.mp 13. Swell*.mp 14. 10 or 11 or 12 or 13 15. 5 and 9 and 14 16. Limit 15 to English language |
Footnotes
Acknowledgments and Funding: The authors would like to thank Mark Gichuru and Mark Phillips of Global Research Solutions Inc. for their medical writing assistance. This study was funded by Ferring Pharmaceuticals, Inc.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Roy Altman is a consultant for Ferring Pharmaceuticals, Inc. Asheesh Bedi and Ajay Manjoo have no conflicts of interest to disclose. Peter Shaw and Faizan Niazi are paid employees of Ferring Pharmaceuticals Inc. Philip Mease is a consultant for Genentech, has a research grant with Genentech, Merck and Zynerba, and is a speaker for Lilly, Merck, Sun, and Zynerba.
References
- 1. Cheng OT, Souzdalnitski D, Vrooman B, Cheng J. Evidence-based knee injections for the management of arthritis. Pain Med. 2012;13:740-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bellamy N, Campbell J, Welch V, Gee TL, Bourne R, Wells GA. Viscosupplementation for the treatment of osteoarthritis of the knee (Review). Cochrane Database Syst Rev. 2006;(2):CD005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther. 2003;5:54-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Litwiniuk M, Krejner A, Speyrer M, Gauto A, Grzela T. Hyaluronic acid in inflammation and tissue regeneration. Wounds. 2016;28:78-88. [PubMed] [Google Scholar]
- 5. Reed RK, Laurent UB, Fraser JR, Laurent TC. Removal rate of [3H]hyaluronan injected subcutaneously in rabbits. Am J Physiol. 1990;259:H532-5. [DOI] [PubMed] [Google Scholar]
- 6. Turley E, Noble P, Bourguignon L. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589-92. [DOI] [PubMed] [Google Scholar]
- 7. Stitik TP, Levy JA. Viscosupplementation (biosupplementation) for osteoarthritis. Am J Phys Med Rehabil. 2006;85(suppl 11):S32-50. [DOI] [PubMed] [Google Scholar]
- 8. Abatangelo G, Botti P, Del Bue M, Gei G, Samson JC, Cortivo R, et al. Intraarticular sodium hyaluronate injections in the Pond-Nuki experimental model of osteoarthritis in dogs. I. Biochemical results. Clin Orthop Relat Res. 1989;(241):278-85. [PubMed] [Google Scholar]
- 9. Brun P, Zavan B, Vindigni V, Schiavinato A, Pozzuoli A, Iacobellis C, et al. In vitro response of osteoarthritic chondrocytes and fibroblast-like synoviocytes to a 500-730 kDa hyaluronan amide derivative. J Biomed Mater Res B Appl Biomater. 2012;100:2073-81. [DOI] [PubMed] [Google Scholar]
- 10. Campo GM, Avenoso A, Nastasi G, Micali A, Prestipino V, Vaccaro M, et al. Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression. Biochim Biophys Acta. 2011;1812:1170-81. [DOI] [PubMed] [Google Scholar]
- 11. Campo GM, Avenoso A, D’Ascola A, Prestipino V, Scuruchi M, Natashi G, et al. Inhibition of hyaluronan synthesis reduced inflammatory response in mouse synovial fibroblasts subjected to collagen-induced arthritis. Arch Biochem Biophys. 2012;518:42-52. [DOI] [PubMed] [Google Scholar]
- 12. Campo GM, Avenoso A, D’Ascola A, Prestipino V, Scuruchi M, Natashi G, et al. Hyaluronan differently modulates TLR-4 and the inflammatory response in mouse chondrocytes. BioFactors. 2012;38:69-76. [DOI] [PubMed] [Google Scholar]
- 13. Campo GM, Avenoso A, D’Ascola A, Scuruchi M, Prestipino V, Calatroni A, et al. 6-Mer hyaluronan oligosaccharides increase IL-18 and IL-33 production in mouse synovial fibroblasts subjected to collagen-induced arthritis. Innate Immun. 2012;18:675-84. [DOI] [PubMed] [Google Scholar]
- 14. Campo GM, Avenoso A, D’Ascola A, Scuruchi M, Prestipino V, Nastasi G, et al. The inhibition of hyaluronan degradation reduced pro-inflammatory cytokines in mouse synovial fibroblasts subjected to collagen-induced arthritis. J Cell Biochem. 2012;113:1852-67. [DOI] [PubMed] [Google Scholar]
- 15. Chan DD, Xiao WF, Li J, de la Motte CA, Sandy JD, Plaas A. Deficiency of hyaluronan synthase 1 (Has1) results in chronic joint inflammation and widespread intra-articular fibrosis in a murine model of knee joint cartilage damage. Osteoarthritis Cartilage. 2015;23:1879-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang CC, Hsieh MS, Liao ST, Chen YH, Cheng CW, Huang PT, et al. Hyaluronan regulates PPARγ and inflammatory responses in IL-1β-stimulated human chondrosarcoma cells, a model for osteoarthritis. Carbohydr Polym. 2012;90:1168-75. [DOI] [PubMed] [Google Scholar]
- 17. Chen WH, Lo WC, Hsu WC, Wei HJ, Liu HY, Lee CH, et al. Synergistic anabolic actions of hyaluronic acid and platelet-rich plasma on cartilage regeneration in osteoarthritis therapy. Biomaterials. 2014;35:9599-607. [DOI] [PubMed] [Google Scholar]
- 18. Chow G, Nietfeld JJ, Knudson CB, Knudson W. Antisense inhibition of chondrocyte CD44 expression leading to cartilage chondrolysis. Arthritis Rheum. 1998;41:1411-9. [DOI] [PubMed] [Google Scholar]
- 19. D’Souza AL, Masuda K, Otten LM, Nishida Y, Knudson W, Thonar EJ. Differential effects of interleukin-1 on hyaluronan and proteoglycan metabolism in two compartments of the matrix formed by articular chondrocytes maintained in alginate. Arch Biochem Biophys. 2000;374:59-65. [DOI] [PubMed] [Google Scholar]
- 20. Filion MC, Phillips NC. Pro-inflammatory activity of contaminating DNA in hyaluronic acid preparations. J Pharm Pharmacol. 2001;53:555-61. [DOI] [PubMed] [Google Scholar]
- 21. Fraser JR, Kimpton WG, Pierscionek BK, Cahill RN. The kinetics of hyaluronan in normal and acutely inflamed synovial joints: observations with experimental arthritis in sheep. Semin Arthritis Rheum. 1993;6(suppl 1):9-17. [DOI] [PubMed] [Google Scholar]
- 22. Galois L, Etienne S, Henrionnet C, Scala-Bertola J. Ambivalent properties of hyaluronate and hylan during post-traumatic OA in the rat knee. Biomed Mater Eng. 2012;22:235-42. [DOI] [PubMed] [Google Scholar]
- 23. Goto H, Onodera T, Hirano H, Shimamura T. Hyaluronic acid suppresses the reduction of α2(VI) collagen gene expression caused by interleukin-1β in cultured rabbit articular chondrocytes. Tohoku J Exp Med. 1999;187:1-13. [DOI] [PubMed] [Google Scholar]
- 24. Greenberg DD, Stoker A, Kane S, Cockrell M, Cook JL. Biochemical effects of two different hyaluronic acid products in a co-culture model of osteoarthritis. Osteoarthritis Cartilage. 2006;14:814-22. [DOI] [PubMed] [Google Scholar]
- 25. Hashizume M, Mihara M. Desirable effect of combination therapy with high molecular weight hyaluronate and NSAIDs on MMP production. Osteoarthritis Cartilage. 2009;17:1513-8. [DOI] [PubMed] [Google Scholar]
- 26. Hashizume M, Koike N, Yoshida H, Suzuki M, Mihara M. High molecular weight hyaluronic acid relieved joint pain and prevented the progression of cartilage degeneration in a rabbit osteoarthritis model after onset of arthritis. Mod Rheumatol. 2010;20:432-8. [DOI] [PubMed] [Google Scholar]
- 27. Hiraiwa H, Sakai T, Mitsuyama H, Hamada T, Yamamoto R, Omachi T, et al. Inflammatory effect of advanced glycation end products on human meniscal cells from osteoarthritic knees. Inflamm Res. 2011;60:1039-48. [DOI] [PubMed] [Google Scholar]
- 28. Hiramitsu T, Yasuda T, Ito H, Shimizu M, Julovi SM, Kakinuma T, et al. Intercellular adhesion molecule-1 mediates the inhibitory effects of hyaluronan on interleukin-1β-induced matrix metalloproteinase production in rheumatoid synovial fibroblasts via down-regulation of NF-κB and p38. Rheumatology (Oxford). 2006;45:824-32. [DOI] [PubMed] [Google Scholar]
- 29. Jean Y, Wen Z, Chang Y, Lee HS, Hsieh SP, Wu CT, et al. Hyaluronic acid attenuates osteoarthritis development in the anterior cruciate ligament-transected knee: association with excitatory amino acid release in the joint dialysate. J Orthop Res. 2006;24:1052-61. [DOI] [PubMed] [Google Scholar]
- 30. Kataoka Y, Ariyoshi W, Okinaga T, Kaneuji T, Mitsugi S, Takahashi T, et al. Mechanisms involved in suppression of ADAMTS4 expression in synoviocytes by high molecular weight hyaluronic acid. Biochem Biophys Res Commun. 2013;432:580-5. [DOI] [PubMed] [Google Scholar]
- 31. Kawana H, Karaki H, Higashi M, Miyazaki M, Hilberg F, Kitagawa M, et al. CD44 suppresses TLR-mediated inflammation. J Immunol. 2008;180:4235-45. [DOI] [PubMed] [Google Scholar]
- 32. Lajeunesse D, Delalandre A, Martel-Pelletier J, Pelletier JP. Hyaluronic acid reverses the abnormal synthetic activity of human osteoarthritic subchondral bone osteoblasts. Bone. 2003;33:703-10. [DOI] [PubMed] [Google Scholar]
- 33. Levesque MC, Haynes BF. Cytokine induction of the ability of human monocyte CD44 to bind hyaluronan is mediated primarily by TNF-α and is inhibited by IL-4 and IL-13. J Immunol. 1997;159:6184-94. [PubMed] [Google Scholar]
- 34. Murata M, Yudoh K, Shimizu H, Beppu M, Nakamura H, Kato T, et al. Layilin, a talin-binding hyaluronan receptor, is expressed in human articular chondrocytes and synoviocytes and is down-regulated by interleukin-1β. Mod Rheumatol. 2013;23:478-88. [DOI] [PubMed] [Google Scholar]
- 35. Noble BP, Mckee CM, Cowman M, Shin HS. Hyaluronan fragments activate an NF-κB/I-κB autoregulatory loop in murine macrophages. J Exp Med. 1996;183:2373-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oliviero F, Scanu A, Ramonda R, Frallonardo P, Sfriso P, Dayer JM, et al. IL-1β and IL-8 are scavenged by the hexadecylamide derivative of hyaluronic acid: a new mechanism. J Biomed Mater Res A. 2015;103:2823-9. [DOI] [PubMed] [Google Scholar]
- 37. Santangelo KS, Johnson AL, Ruppert AS, Bertone AL. Effects of hyaluronan treatment on lipopolysaccharide-challenged fibroblast-like synovial cells. Arthritis Res Ther. 2007;9:R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sasaki A, Sasaki K, Konttinen YT. Hyaluronate inhibits the interleukin-1β-induced expression of matrix metalloproteinase (MMP)-1 and MMP-3 in human synovial cells. Tohoku J Exp Med. 2004;204:99-107. [DOI] [PubMed] [Google Scholar]
- 39. Sato E, Ando T, Ichikawa J, Okita G, Sato N, Wako M, et al. High molecular weight hyaluronic acid increases the differentiation potential of the murine chondrocytic ATDC5 cell line. J Orthop Res. 2014;32:1619-27. [DOI] [PubMed] [Google Scholar]
- 40. Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272-81. [DOI] [PubMed] [Google Scholar]
- 41. Shao Y, Lu G, Shen Z-J, He H. Reduction of intercellular adhesion molecule 1 may play a role in anti-inflammatory effect of hyaluronic acid in a rat model of severe non-bacterial cystitis. World J Urol. 2013;31:535-40. [DOI] [PubMed] [Google Scholar]
- 42. Sheehan KM, DeLott LB, Day SM, DeHeer DH. Hyalgan has a dose-dependent differential effect on macrophage proliferation and cell death. J Orthop Res. 2003;21:744-51. [DOI] [PubMed] [Google Scholar]
- 43. Shimazu A, Jikko A, Iwamoto M, Koike T, Yan W, Okada Y, et al. Effects of hyaluronic acid on the release of proteoglycan from the cell matrix in rabbit chondrocyte cultures in the presence and absence of cytokines. Arthritis Rheum. 1993;36:247-53. [DOI] [PubMed] [Google Scholar]
- 44. Smith MM, Russell AK, Schiavinato A, Little CB. A hexadecylamide-derivative of hyaluronan (HYMOVIS®) has superior beneficial effects on human osteoarthritic chondrocytes and synoviocytes than unmodified hyaluronan. J Inflamm (Lond). 2013;10:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stöve J, Schöniger R, Huch K, Brenner R, Günther K, Puhl W, et al. Effects of dexamethasone on proteoglycan content and gene expression of IL-1β-stimulated osteoarthrotic chondrocytes in vitro. Acta Orthop Scand. 2002;73:562-7. [DOI] [PubMed] [Google Scholar]
- 46. Sundman E, Cole B, Karas V, Della Valle C, Tetreault MW, Mohammed HO, et al. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am J Sports Med. 2014;42:35-41. [DOI] [PubMed] [Google Scholar]
- 47. Takahashi K, Hashimoto S, Kubo T, Hirasawa Y, Lotz M, Amiel D. Hyaluronan suppressed nitric oxide production in the meniscus and synovium of rabbit osteoarthritis model. J Orthop Res. 2001;19:500-3. [DOI] [PubMed] [Google Scholar]
- 48. Tanimoto K, Ohno S, Fujimoto K, Honda K, Ijuin C, Tanaka N, et al. Proinflammatory cytokines regulate the gene expression of hyaluronic acid synthetase in cultured rabbit synovial membrane cells. Connect Tissue Res. 2001;42:187-95. [DOI] [PubMed] [Google Scholar]
- 49. Wang CT, Lin YT, Chiang BL, Lin YH, Hou SM. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthritis Cartilage. 2006;14:1237-47. [DOI] [PubMed] [Google Scholar]
- 50. Yasuda T. Hyaluronan inhibits cytokine production by lipopolysaccharide-stimulated U937 macrophages through down-regulation of NF-κB via ICAM-1. Inflamm Res. 2007;56:246-53. [DOI] [PubMed] [Google Scholar]
- 51. Yasuda T. Hyaluronan inhibits prostaglandin E2 production via CD44 in U937 human macrophages. Tohoku J Exp Med. 2010;220:229-35. [DOI] [PubMed] [Google Scholar]
- 52. Yasui T, Akatsuka M, Tobetto K, Hayaishi M, Ando T. The effect of hyaluronan on interleukin-1α-induced prostaglandin E2 production in human osteoarthritic synovial cells. Agents Actions. 1992;37:155-6. [DOI] [PubMed] [Google Scholar]
- 53. Yasui T, Akatsuka M, Tobetto K, Hayakawa T. Effects of hyaluronan on the production of stromelysin and tissue inhibitor of metalloproteinase-1 (TIMP-1) in bovine articular chondrocytes. Biomed Res. 1992;13:343-8. [Google Scholar]
- 54. Yoshioka K, Yasuda Y, Kisukeda T, Nodera R, Tanaka Y, Miyamoto K. Pharmacological effects of novel cross-linked hyaluronate, Gel-200, in experimental animal models of osteoarthritis and human cell lines. Osteoarthritis Cartilage. 2014;22:879-87. [DOI] [PubMed] [Google Scholar]
- 55. Aulin C, Lundbäck P, Palmblad K, Klareskog L, Erlandsson Harris H. An in vivo cross-linkable hyaluronan gel with inherent anti-inflammatory properties reduces OA cartilage destruction in female mice subjected to cruciate ligament transection. Osteoarthritis Cartilage. 2017;25:157-65. [DOI] [PubMed] [Google Scholar]
- 56. Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, et al. Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vigetti D, Viola M, Karousou E, Rizzi M, Moretto P. Hyaluronan-CD44-ERK1/2 regulate human aortic smooth muscle cell motility during aging. J Biol Chem. 2008;283:4448-58. [DOI] [PubMed] [Google Scholar]
- 58. Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000;275:26967-75. [DOI] [PubMed] [Google Scholar]
- 59. McCourt PA, Ek B, Forsberg N, Gustafson S. Intercellular adhesion molecule-1 is a cell surface receptor for hyaluronan. J Biol Chem. 1994;269:30081-4. [PubMed] [Google Scholar]
- 60. Bono P, Rubin K, Higgins JM, Hynes RO. Layilin, a novel integral membrane protein, is a hyaluronan receptor. Mol Biol Cell. 2001;12:891-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang C, Cao M, Liu H, He Y, Xu J, Du Y, et al. The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J Biol Chem. 2012;287:43094-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wolny P, Banerji S, Gounou C, Brisson AR, Day AJ, Jackson DG, et al. Analysis of CD44-hyaluronan interactions in an artificial membrane system: insights into the distinct binding properties of high and low molecular weight hyaluronan. J Biol Chem. 2010;285:30170-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Campo G, Avenoso A, Campo S, D’Ascola A, Nastasi G, Calatroni A. Small hyaluronan oligosaccharides induce inflammation by engaging both toll-like-4 and CD44 receptors in human chondrocytes. Biochem Pharmacol. 2010;80:480-90. [DOI] [PubMed] [Google Scholar]
- 64. Higman VA, Briggs DC, Mahoney DJ, Blundell CD, Sattelle BM, Dyer DP, et al. A refined model for the TSG-6 link module in complex with hyaluronan. J Biol Chem. 2014;289:5619-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Roque VA, Agre M, Barroso J, Brito I. Managing knee ostheoarthritis: efficacy of hyaluronic acid injections. Acta Reumatol Port. 2013;38:154-61. [PubMed] [Google Scholar]
- 66. Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma J, Dieppe P, et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62:1145-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kaneko S, Satoh T, Chiba J, Ju C, Inoue K, Kagawa J. Interleukin-6 and interleukin-8 levels in serum and synovial fluid of patients with osteoarthritis. Cytokines Cell Mol Ther. 2000;6:71-9. [DOI] [PubMed] [Google Scholar]
- 68. Vincent H. Hyaluronic acid (HA) viscosupplementation on synovial fluid inflammation in knee osteoarthritis: a pilot study. Open Orthop J. 2013;7:378-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sezgin M, Demirel A, Karaca C, Ortancil O, Ulkar GB, Kanik A, et al. Does hyaluronan affect inflammatory cytokines in knee osteoarthritis?. Rheumatol Int. 2004;25:264-9. [DOI] [PubMed] [Google Scholar]
- 70. Altman R, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. doi: 10.1186/s12891-015-0775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]