Abstract
Objectives
To investigate the impact of a six-week supervised exercise programme on cardiopulmonary fitness, balance, muscle strength and anxiety and depression in patients who have been discharged home from hospital following an intensive care unit length of stay of greater than 48 h. To investigate patients' perceptions of a six-week supervised exercise programme delivered at three months post hospital discharge.
Design
A single centre parallel, randomised controlled trial.
Setting
Outpatient department of a university teaching hospital in the UK.
Participants
Sixty adult survivors of critical illness, at three months post-hospital discharge.
Intervention
A six-week individually prescribed and supervised exercise program, with associated advice to home exercise modification. Twice weekly exercise sessions were individualised to participant's functional status and included cardiopulmonary, balance and strengthening exercises. Follow up at seven weeks, six months and 12 months.
Outcome measures
Six-Minute Walk Test, BERG balance test, grip strength and Hospital Anxiety and Depression Scale. A pre-designed survey was used to explore patient perceptions of the programme.
Results
Sixty participants (n = 30 received allocated programme in both control and treatment groups) were randomised. Loss to follow up resulted in n = 34 participants for intention to treat analysis at 12 months follow up (leaving n = 19 in control group, n = 15 in treatment group). Median participant age at enrolment was 62 years (interquartile range: 49–72), with a median intensive care unit length of stay of nine days (interquartile range: 4–17). No significant differences were found for the Six-Minute Walk Test at any time point (p > 0.05). Anxiety levels and balance were significantly improved in the treatment group at 12 months (p = 0.006 and p = 0.040, respectively).
Conclusions
Further research is needed into appropriate interventions and outcome measures, target patient populations and timing of such intervention post-hospital discharge.
Keywords: Critical illness, rehabilitation, physical fitness
The number of admissions to critical care units in the UK has increased, with approximately 163,900 adults affected per year.1 While patient survival rates are improving, it is well recognised that survivors of critical illness experience a range of profound physical and functional deficits that can persist for a number of years following hospital discharge.2–6 As described by Herridge,7 extensive research has consistently demonstrated the diversity of both physical and neuropsychological disabilities sustained by patients following critical illness.7 Risk factors for skeletal muscle wasting and weakness include immobility, severity of illness and multi-organ failure.8 These physical sequelae add to the burden of illness for not only the survivors and their families but also the healthcare system.
The Rehabilitation after Critical Illness in Adults Guidelines published by National Institute for Health and Care Excellence (NICE) in 20099 has led to a number of studies investigating the impact of exercise in the post-hospital discharge phase.10–18 The general aim of post-ICU exercise-based rehabilitation is to address the effects of the physical deficits experienced by survivors of critical illness, through the delivery of a pre-designed exercise programme, that is progressed according to participant response to exercise. These exercise programmes may be delivered on an individual or group basis and may target different components of physical fitness. It has been suggested that there may also be additional psychological and cognitive benefits gained from such exercise programmes.11,14,17,18
In a recent Cochrane review by Connolly et al.,19 it was concluded that it was not possible to determine an overall effect on functional exercise capacity using an exercise-based intervention initiated post-ICU discharge for survivors of critical illness. Reasons included differences in outcome measures used in the studies, how the results were reported, lack of detail reported regarding usual care in the control groups and overall heterogeneity in study populations.19 Further conclusions stated that no study had included an evaluation of acceptance of the treatment by patients or the experience of the patient participation in the exercise programme, providing the justification for the inclusion of a qualitative component in this trial.19 A qualitative investigation may provide some answers as to the lack of reported effect in functional status with rehabilitation in this patient cohort.
The first aim of this study was to investigate the impact of a six-week supervised exercise programme on cardiopulmonary fitness in patients who have been discharged home from hospital following an intensive care unit (ICU) length of stay of greater than 48 h. The second aim was to investigate the impact of a six-week supervised exercise programme on balance, muscle strength, anxiety and depression levels in patients who have been discharged home from hospital following an ICU length of stay of greater than 48 h. The final aim was to explore participants' perceptions of the exercise programme.
Materials and methods
Ethical approval for this trial was obtained from the Wales Research Ethics Committee 6 and informed written consent was obtained from each patient prior to trial enrolment. The trial was registered on the ISRCTN database (ISRCTN11853373, Retrospectively registered 07/02/2012 due to lack of study funding. First patient recruited 01 November 2011). https://www.isrctn.com/ISRCTN11853373?q=epic
We conducted a single-centre, assessor-blinded, parallel group, randomised controlled trial in a large teaching hospital in Wales. All participants recruited had been a patient on the medical and surgical ICU (with paediatric, burns and cardiac being managed on separate units and therefore excluded from the trial) for a length of stay of 48 h or more. Exclusion criteria included participants aged less than 18 years (no upper age limit), living outside of a commutable area (as expressed by the participant during the recruitment process), any medical contraindications (defined a priori and confirmed by the consultant running the follow up clinic during recruitment) to exercise and participation in any other concurrent rehabilitation programme.
The intervention investigated in this trial was a six-week, individualised, supervised exercise programme. Cardiopulmonary exercises included using the cycle ergometers (upper and lower limb as appropriate), treadmill, rowing and stepping machines. Specialist bariatric equipment was used for participants with a high body mass index. Strengthening exercises were progressed from global exercises using functional type activities such as sit to stand or step up exercises to more specific muscle strengthening using hand-held weights, theraband and weighted balls. The larger upper limb (deltoids, biceps and triceps) and lower limb (quadriceps, gluteals, hamstrings and calves) muscle groups were targeted with strengthening exercises. Balance exercises were also progressed from global, functional exercises in sitting/standing through to more advanced exercises using wobble boards and gym balls.
The intervention was individualised and graded to each patient based on results of baseline measurements. For example, if a patient's greatest limitation on baseline testing was evident on the Six Minute Walk Test, then the emphasis in the exercise programme would be placed on cardiopulmonary exercise. Intensity and duration of all exercises were increased incrementally according to individual patient progression over the six-week period. All exercises were progressed during the six weeks according to individual patient ability, rather than a pre-designed protocol for progression, according to number of sets or repetitions.
Over the course of the trial period, two therapists (physiotherapy technicians, both with over five years' experience of exercise prescription, able to access senior qualified physiotherapy staff for advice as required) delivered the sessions; each participant had a single therapist responsible for their program prescription and progression. Participants were seen on an individual basis (rather than as a group), due to the complexity and heterogeneity of the patient cohort. Attendance and reasons for non-compliance were recorded. Participants in the exercise group were also advised on completing an additional home exercise session, based on their personalised programme. The participants in the exercise group would attend the physiotherapy outpatient gym (starting within one week from recruitment) for two sessions of up to 1 h (according to exercise capacity), twice a week, for six consecutive weeks. Sessions were delivered on a one to one basis, which often proved difficult due to the intensive use of resources needed to achieve this.
The control group participants received usual care. Outpatient classes or community-based exercise programmes are not routinely offered to survivors of critical care, so usual care involves no formal intervention for these patients. Any patient participating in a concurrent formal exercise programme such as pulmonary or cardiac rehabilitation were excluded from the trial. Participants were not excluded if they exercised on their own volition.
The primary outcome measure used was the Six-Minute Walk Test (6MWT) in order to evaluate cardiopulmonary fitness.20 This test was selected as it is commonly used as a test of physical function in critically ill patients, from ICU stay through to an outpatient setting. The test was completed on a flat 10-m track, with a chair at one end in case the participant needed a rest, using standardised instructions for completion. This method of completing the test was used throughout the trial. Secondary outcome measures used to test balance, grip strength and anxiety and depression included the Berg Balance score,21 the Jamar Dynamometer (to measure grip strength using the American Society of Hand Therapists protocol22) and the Hospital Anxiety and Depression Scale, respectively.
Baseline measurements (at week one) were recorded following recruitment and consent in the ICU Follow-up Clinic. Measurements were then repeated at seven weeks (completed in the next appointment, immediately following the final exercise session), six months and 12 months. Organisational outcomes, such as adherence to the programme, withdrawal, loss to follow-up and adverse events (decided a priori and including onset of acute illness or injuries sustained during exercise) were also recorded and analysed.
At the end of the 12-month period, the exercise group were asked to complete a short pre-designed survey. Due to the lack of a validated survey for this patient group, we designed and piloted a new survey on two trial patient representatives and adapted according it to their feedback, prior to use in the trial. Seven closed, multiple choice style questions were included (as presented in the results) that explored the patients' perceptions of the exercise sessions.
Sample size was calculated using data collected from our earlier pilot study (completed in authors' hospital in 2009 and 2010 by the same research team) in which 24 post ICU patients completed the 6MWT pre and post a six-week supervised exercise programme.23 The mean improvement in the 6MWT was 128 m (standard deviation [SD]: 78 m). For this study, we used a conservative estimate of the smallest medically relevant difference of 60 m, to calculate the standardised difference, (giving 0.76), which resulted in a suggested total sample size of approximately 60, with a Type II error rate of 0.20 (80% power).
Consecutive eligible patients were recruited from our ICU Follow-up clinic to the trial at 12 weeks post-hospital discharge. Participants were randomly assigned to either exercise or control group using a stratification method (Minim software), which ensured the groups were evenly matched. The three stratification factors were gender, age (18–64 vs. 65 years or more) and Acute Physiology and Chronic Health Evaluation II (APACHE II) score (1–19 vs. 20 or more). The allocation sequence was held by an independent administration staff member (not involved in the study) who kept the allocation concealed from the investigators until the baseline measurements had been completed however formal blinding was not possible.
Primary and secondary outcomes were analysed and presented as descriptive data (medians/interquartile ranges for non-normal distributions). Data were analysed using a two-way repeated measures analysis of covariance (MANCOVA) in order to present results over time and between groups. Outcome measures were analysed using a general linear mixed model with group (control vs. treatment) and time (treated as categorical levels at baseline, week 7, six months and 12 months). Covariates (selected a priori) included in the model were ICU length of stay and number of pre-existing co-morbidities (both included as continuous variables). Linear mixed models used all outcome data available at each time point therefore imputation of missing data was not required. Intention-to-treat analysis was conducted for each of the outcomes measures, comparing exercise and control group results over each of the time points.
Organisational outcomes were presented as n(%) and comparisons between the two groups analysed using Fisher's Exact test. Responses from the closed questions in the survey results were analysed using descriptive statistics (numbers/percentages). All statistical analysis was completed using SPSS (version 23) and statistical significance was set at p < 0.05 for this trial.
Results
Participant flow and characteristics
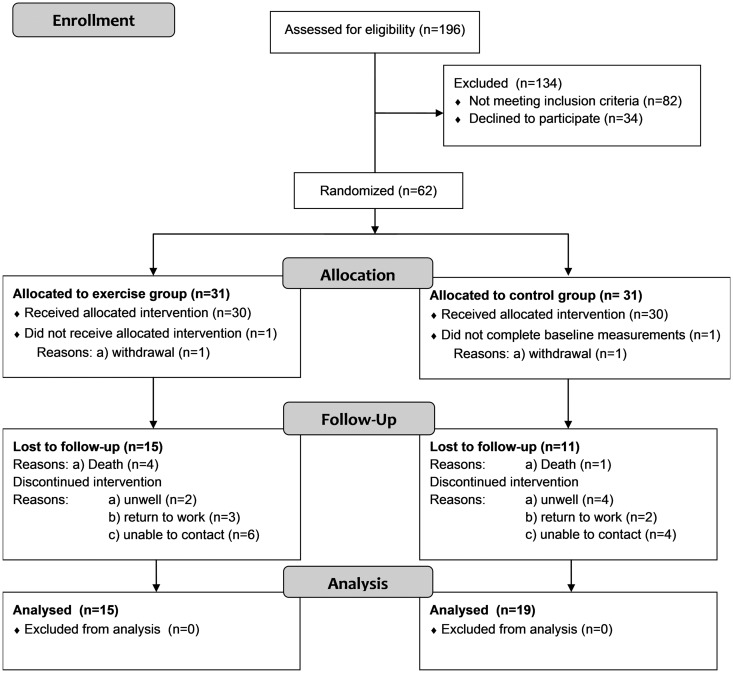
Of the 82 patients not meeting the exclusion criteria, 36 lived outside of a commutable area, 32 were not considered medically stable for participation and 14 were already participating in other rehabilitation programmes. The pre-determined sample size of 60 patients was achieved (n = 30 in each group), however, of the total trial cohort, only 34 (57%) participants completed all testing to 12 months. Participants were recruited from November 2011 until March 2015, with final 12-month testing completed in March 2016. Participant flow through the trial is outlined in Figure 1, including loss to follow up and withdrawals.
Figure 1.
Flow of patients through trial.
Median participant age at enrolment was 62 years (IQR: 49–72), with a median ICU length of stay of nine days (IQR: 4–17). Only 4 of 60 (7%) patients were not mechanically ventilated during their ICU stay and median APACHE II score was 14 (IQR: 11–19). An overall mortality rate of n = 5/60 patients (58%) was reported for the trial participants. The only differences between the groups were the number of mechanical ventilation (MV) days and overall hospital length of stay, which were significantly higher in the exercise group (p < 0.05) (Table 1).
Table 1.
Characteristics of trial participants.
| All patients N = 60 | Treatment N = 30 | Control N = 30 | P value | |
|---|---|---|---|---|
| Male | 31 (52%) | 15 (50%) | 16 (53%) | 0.797 |
| Age | 62 (49–72) | 61 (49–70) | 62.5 (46–70) | 0.503 |
| APACHE II | 14 (11–19) | 15 (12–19) | 13 (9–19) | 0.174 |
| Functional Comorbidity Index | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.741 |
| ICU LOS | 9 (4–17) | 12 (5–21) | 7 (4–15) | 0.082 |
| MV days | 4 (1–12) | 5 (2–14) | 2 (1–11) | 0.019 |
| Total hospital LOS | 20 (10–30) | 23 (15–45) | 15 (9–25) | 0.046 |
| Primary diagnosis: | ||||
| Surgical | 32 (53%) | 15 (50%) | 17 (57%) | 0.796 |
| Respiratory | 15 (25%) | 9 (30%) | 6 (20%) | 0.552 |
| Medical | 9 (15%) | 5 (17%) | 4 (13%) | >0.999 |
| Trauma | 4 (7%) | 1 (3%) | 3 (10%) | >0.999 |
| Neurology | 2 (3%) | 1 (3%) | 1 (3%) | |
| 12-Month mortality | 5 (8%) | 4 (13%) | 1 (3%) | 0.353 |
| Readmissions | 25 (42%) | 13 (43%) | 12 (40%) | >0.999 |
Values presented as number (%) and median (IQR). APACHE II: Acute Physiology and Chronic Health Evaluation II; LOS: length of stay; MV: mechanical ventilation; readmissions: readmissions to hospital during the one year follow up period. Fisher's Exact test used for comparisons.
Both control and intervention groups improved in all outcome measures between baseline and 12 months however the substantial level of variability in participants' results is reflected by the large SDs (Table 2).
Table 2.
Six-Minute Walk Test, Berg Balance, HAD scale, Grip dynamometer test raw data by study group.
| Baseline control n = 30 Treatment n = 30 |
Week 7 Control n = 26 Treatment n = 26 |
6 months Control n = 21 Treatment n = 20 |
12 months Control n = 19 Treatment n = 15 |
P value | ||
|---|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | |||
| 6MWT | Control | 269.7 (196.5) | 283.6 (229.3) | 304.8 (213.7) | 294.7 (250.5) | 0.491 |
| Treatment | 283.5 (203.2) | 379.3 (207.3) | 381.6 (207.9) | 363.6 (239.9) | ||
| HAD (A) | Control | 10.3 (4.9) | 9.3 (3.9) | 8.6 (4.5) | 8.6 (4.1) | 0.495 |
| Treatment | 5.4 (4.9) | 3.9 (4.0) | 4.9 (4.0) | 3.7 (3.0) | ||
| HAD (D) | Control | 8.3 (4.4) | 7.8 (4.3) | 7.3 (3.3) | 7.6 (4.7) | 0.761 |
| Treatment | 4.9 (2.8) | 3.7 (3.0) | 4.4 (3.7) | 4.0 (3.8) | ||
| Berg | Control | 50.7 (7.0) | 50.5 (7.6) | 49.7 (7.9) | 47.2 (11.4) | 0.990 |
| Treatment | 52.4 (4.8) | 53.4 (6.2) | 55.1 (1.9) | 54.1 (4.4) | ||
| GRIP (L) | Control | 19.1 (11.9) | 19.5 (12.5) | 20.5 (14.2) | 24.9 (16.2) | 0.283 |
| Treatment | 16.6 (9.7) | 20.7 (11.4) | 22.5 (15.6) | 20.1 (12.4) | ||
| GRIP (R) | Control | 20.1 (13.7) | 22.1 (14.1) | 22.7 (14.2) | 24.9 (16.2) | 0.807 |
| Treatment | 20.4 (11.6) | 22.4 (10.3) | 27.7 (15.9) | 20.1 (12.4) |
6MWT: Six-minute Walk Test; HAD(A): Hospital Anxiety and Depression Score (Anxiety); HAD(D): Hospital Anxiety and Depression Score (Depression); Grip L: Grip strength left; Grip R: Grip strength right; n: number; SD: Standard deviation.
Comparison between treatment and control
There were no significant differences in the 6MWT between control and treatment groups at all time points, and the confidence intervals are very wide as a result of the small sample size. There were also no significant differences within either group (Table 3).
Table 3.
Group comparisons for Six-Minute Walk Test, using model estimates.
| Time point | Control Mean 6MWT (SE) | Treatment Mean 6MWT (SE) | Mean difference from control 95% CI, p value |
|---|---|---|---|
| Baseline | 249.47 (36.28) | 232.7 (31.70) | 16.77 (−79.67 to 113.21) 0.854 |
| 7 weeks | 276.27 (42.74) | 346.81 (33.07) | −70.54 (−179.08 to 38.00) 0.112 |
| 6 months | 313.29 (45.76) | 339.63 (46.32) | −26.34 (−158.42 to 105.73) 0.596 |
| 12 months | 294.74 (57.46) | 344.68 (62.61) | −49.94 (−223.71 to 123.63) 0.373 |
6MWT: Six-Minute Walk Test; SE: standard error; CI: confidence interval; all values in metres. Means/comparisons calculated from linear mixed model, using covariates of number of co-morbidities and ICU length of stay. Repeated measure p value (results of multivariate analysis of covariance (MANCOVA) analysis showing results over time between groups): p = 0.491. Within group changes over time: control group: p = 0.452; treatment group: p = 0.546. Significance: p < 0.05.
Anxiety levels were significantly lower in the treatment group than the control group at seven weeks, which was not maintained at six months, but evident again at 12 months (Table 4). Balance was also significantly improved at 12 months in the treatment group compared to the control group, but not at any preceding time points (Table 4). Results of the MANCOVA analysis highlighted no significant differences in the results over time between the two groups (Table 4).
Table 4.
Group comparisons for all secondary outcome measures from the model estimates.
| Time point | Outcome measure control M (SE) | Outcome measure treatment M (SE) | Mean difference from control 95%CI p value | Repeated measures p value |
|---|---|---|---|---|
| HAD A | HAD A | |||
| Baseline | 9.3 (1.0) | 7.1 (1.0) | −2.2 (0.93–1.92) 0.142 | |
| 7 weeks | 9.0 (1.0) | 6.0 (1.0) | −3.0 (1.02–2.36) 0.043 | |
| 6 months | 8.3 (1.1) | 6.4 (1.1) | −1.9 (0.84–2.11) 0.250 | |
| 12 months | 8.5 (0.9) | 4.4 (1.0) | −4.1 (1.23–5.24) 0.006 | 0.491 |
| HAD D | HAD D | |||
| Baseline | 8.6 (0.8) | 6.7 (0.8) | −1.9 (0.95–1.77) 0.128 | |
| 7 weeks | 7.8 (0.9) | 5.3 (0.9) | −2.5 (0.99–2.34) 0.084 | |
| 6 months | 6.8 (0.8) | 5.3 (0.9) | −1.5 (0.86–2.05) 0.239 | |
| 12 months | 7.4 (1.1) | 4.7 (1.2) | −2.7 (0.90–3.41) 0.110 | 0.761 |
| BERG | BERG | |||
| Baseline | 47.3 (1.9) | 50.4 (1.9) | 3.1 (0.84–1.05) 0.279 | |
| 7 weeks | 50.0 (1.6) | 52.7 (1.6) | 2.7 (0.87–1.04) 0.264 | |
| 6 months | 50.1 (1.8) | 52.3 (1.9) | 2.2 (0.86–1.06) 0.442 | |
| 12 months | 47.2 (2.1) | 54.2 (2.4) | 7.0 (0.76–0.99) 0.040 | 0.990 |
| GRIP L | GRIP L | |||
| Baseline | 18.1 (2.0) | 16.2 (2.0) | −1.9 (0.80–1.58) 0.536 | |
| 7 weeks | 19.7 (2.5) | 20.7 (2.5) | 1.0 (0.66–1.36) 0.795 | |
| 6 months | 22.1 (3.3) | 20.4 (3.5) | −1.7 (0.68–1.78) 0.731 | |
| 12 months | 25.1 (3.5) | 19.3 (3.9) | −5.8 (0.80–2.33) 0.286 | 0.283 |
| GRIP R | GRIP R | |||
| Baseline | 20.2 (2.3) | 18.2 (2.3) | −2.0 (0.79–1.58) 0.562 | |
| 7 weeks | 22.7 (2.5) | 21.6 (2.5) | −1.1 (0.76–1.46) 0.767 | |
| 6 months | 24.3 (3.5) | 24.9 (3.7) | −0.6 (0.63–1.51) 0.912 | |
| 12 months | 26.9 (4.0) | 24.1 (4.5) | −2.8 (0.69–1.92) 0.651 | 0.807 |
HAD A: anxiety HAD D: depression; Grip L: left; Grip R: right; BERG: balance; SE: standard error; CI: confidence interval; all values in metres. Means/comparisons calculated from linear mixed model, using covariates of number of co-morbidities and ICU length of stay. Repeated measure p value (results of multivariate analysis of covariance (MANCOVA) analysis showing results over time between groups. Significance: p < 0.05.
A significant difference was reported between the two groups in mean change from baseline in the 6MWT at week 7 and six months, balance at 6 and 12 months and grip strength at week 7 (Table 5).
Table 5.
Group comparisons for mean change from baseline and effect size.
| Control |
Intervention |
Mean difference |
|||
|---|---|---|---|---|---|
| Outcome measure | Time point | Mean change (ESa) | Mean change (ESa) | Difference (95% CI) | ESb |
| 6MWT | Week 7 | 7.3 (0.04) | 104.46 (0.60) | 97.15 (−134.97 to −59.33)‡ | 1.17 |
| 6 months | 36.48 (0.18) | 97.05 (0.56) | 60.57 (−112.80 to −8.34)‡ | 0.70 | |
| 12 months | 25.0 (0.13) | 76.07 (0.44) | 51.07 (−125.53 to 23.39) | 0.48 | |
| HAD (A) | Week 7 | −0.77 (0.17) | −1.27 (0.22) | −0.50 (−1.09 to 2.09) | 0.18 |
| 6 months | −1.86 (0.41) | −0.26 (0.05) | 1.6 (−3.86 to 0.63) | 0.45 | |
| 12 months | 1.63 (0.35) | 1.40 (0.25) | −0.23 (−3.14 to 2.68) | 0.06 | |
| HAD (D) | Week 7 | −0.54 (0.12) | −1.38 (0.31) | −0.84 (−0.55 to 2.23) | 0.33 |
| 6 months | −1.24 (0.29) | −0.42 (0.09) | 0.82 (−2.62 to 0.98) | 0.30 | |
| 12 months | −0.79 (0.18) | −0.67 (0.15) | 0.12 (−1.98 to 1.74) | 0.03 | |
| Berg | Week 7 | 1.15 (0.09) | 2.92 (0.38) | 1.77 (−5.68 to 2.14) | 0.05 |
| 6 months | −1.10 (0.09) | 2.37 (0.30) | 3.47 (−6.89 to −0.05)‡ | 0.63 | |
| 12 months | −3.47 (0.28) | 2.07 (0.27) | 5.54 (−11.08 to −0.002)‡ | 0.67 | |
| GRIP (R) | Week 7 | 2.35 (0.18) | 3.85 (0.33) | 1.5 (−4.60 to 61.58) | 0.27 |
| 6 months | 3.33 (0.25) | 7.11 (0.62) | 3.78 (−9.78 to 2.23) | 0.40 | |
| 12 months | 6.58 (0.50) | 4.93 (0.43) | −1.65 (−4.65 to 7.95) | 0.19 | |
| GRIP (L) | Week 7 | 1.15 (0.10) | 4.54 (0.46) | 3.39 (−6.23 to −0.55)‡ | 0.64 |
| 6 months | 2.05 (0.15) | 6.05 (0.52) | 4.00 (−9.84 to 1.84) | 0.45 | |
| 12 months | 5.84 (0.43) | 3.33 (0.29) | −2.51 (−3.08 to 8.10) | 0.27 |
6MWT: Six Minute Walk Test; CI: confidence interval. Values for 6MWT given in metres.
ES, effect size: mean change from first assessment/SD at first assessment (based on adjusted scores).
ES, effect size: (intervention mean change − usual care mean change)/pooled SD for change (based on unadjusted scores).
‡Significant p < 0.05; significant differences between groups occur when CI does not cross 0.
Subject numbers the same as reported in Table 2.
To interpret effect size: Small: < 0.20, medium: 0.50; large: 0.80; very large: > 1.2 (based on work of Sawilowsky).24
Organisational outcomes
Organisational outcomes were analysed and there were no significant differences between the two groups in terms of loss to follow up, withdrawal or adverse events. There were no adverse events reported during the trial period. Adherence (defined as number of patients completing all exercise sessions within the intervention as described in trial protocol (but still completing the outcome measures at seven weeks) was 67% in the treatment group. In terms of withdrawal (defined as participant withdrawal following randomisation before, or during receipt of the intervention), two participants (6%) withdrew from the exercise programme prematurely due to medical reasons and three (10%) withdrew as they were returning to work. Total percentage attrition rate at 12 months in the exercise group was 50% (15/30) and 63% (19/30) in the control group. Of the withdrawals, one participant withdrew prior to starting the intervention, two participants completed 50% of sessions and two participants completed 83% of sessions.
Exercise programme perceptions
Results from the seven survey items were that participants felt that six weeks was not enough sessions (47%), but that sessions were delivered at an appropriate time post-illness (80%), at the correct intensity (80%), weekly frequency (87%), session duration (87%) and number of fitness test sessions (87%); accordingly participants would recommend to the programme to other ICU survivors (100%).
Discussion
This study of a six-week supervised, personalised exercise programme in survivors of critical illness, demonstrated no significant difference between the control and treatment group in the primary outcome measure of the 6MWT. Further information about the patients' trajectory of illness pre-ICU admission is needed to draw any definitive conclusions.
These results support previous research, which although demonstrating improvements in physical function, reported no significant improvement in 6MWT when compared to control at 12 months follow up.11,15 The results may be due to the loss to follow-up in the study, resulting in the target sample size not being achieved at later time points. Similar lack of significant results have been demonstrated using other outcome measure of physical function, including anaerobic threshold14 and the Rivermead Mobility Index.12,13
In a later secondary analysis of the 6MWT data obtained in the study by Denehy et al.,15 the authors reported that in future trials of interventions to improve outcomes in critical illness survivors, presence of pre-existing disease should be used as a stratification variable and that data should be analysed using percentage differences.25 These suggestions for rehabilitation trials may explain why the results of this study were non-significant. Another possible explanation for the lack of significant findings in physical function in this study could be that the patients in the treatment group were receiving MV for a significantly longer time and had a longer overall hospital length of stay, than the control group. This may account for the lower physical function (as evident in 6MWT and group strength) recorded at baseline in the treatment group, although this was not significant.
The secondary outcome measures in this study that were found to be significantly improved at 12 months from baseline, compared to the control group, were anxiety and balance. The 95% confidence intervals were narrower for the secondary outcome measures than the 6MWT, suggesting a lesser degree of variation in results and less impact of the small sample size. Balance is a variable that has not been previously investigated in this patient population and may well be worthy of further consideration in future studies. Poor balance in this patient cohort may be due to loss of neuromuscular control, commonly reported in critically ill patients. It could be suggested that this supports more recent research, which suggests that further work is needed investigating the most appropriate outcome measures for this very heterogeneous patient population.
Improved levels of anxiety in this study may have been influenced by the use of a one to one supervised exercise programme. Participants reported that attending the sessions gave them much improved confidence in leaving their homes and even for some, returning to work, which may have influenced the withdrawal rate of the treatment group. Although feedback about the intervention was very positive, as outlined in the survey results, capturing this described benefit as an outcome measure is complex. The survey results of this study demonstrated that overall, patients were very satisfied with the intervention. It could be suggested that in future studies, there is more focus on qualitative outcome measures however alternative methods for exploring patients' perceptions should be considered. For example, the use of interviews or focus groups could provide richer data.
There were a number of limitations that may have influenced the results of this study. These limitations have been recognised through recent research, which were unknown at the time of trial design. We are unable to report the training intensity achieved in each session, by each participant, which may influence the repeatability of the trial. Timing of implementation of the treatment may have influenced the trial results. The sample size calculation did not sufficiently allow for loss to follow up. As a result, as with many previous studies, the results lack significance, despite a trend towards greater improvements in the primary outcome in the treatment group, compared to control. In future studies, it could be suggested that the clinical significance should be considered in the analysis, rather than purely statistical significance. The patients only needed to be on ICU for greater than 48 h for inclusion in the study, which is now also known to be insufficient time for this sort of trial. Lack of information regarding number of patients with ICU-acquired weakness in the sample is another limitation, as this may have provided greater understanding as to the lack of difference in baseline outcome measures, despite the longer ICU stay and MV days in the treatment group.
Those patients who returned to work (although only a small number in this trial) may influence the trial results and further research investigating the optimal timing for a post-ICU rehabilitation programme is needed. The patients were enrolled into the study from an ICU follow-up clinic, which may have led to the low adherence level to the exercise and a biased sample, as not all patients would have attended the clinic. ICU survivors who did not attend follow up in the first place may be at extremes of good function so felt no need to attend, or conversely they may have very poor function, so were unable to travel to the clinic. Furthermore, if the clinic could not be held, recruitment was inevitably halted. It could be suggested that patients who felt they had no issues since leaving hospital, may have been less inclined to attend, than those feeling they had longer-term problems, requiring the attention of the clinic.
Another limitation of the study is that participants were not asked to keep a record of the one exercise session that they were given to complete at home. It is not possible to comment therefore of the possible influence of that session or whether participants adhered to it. It was also not possible to formally blind participants or clinicians to group randomisation, however, we attempted to minimise bias by blinding the assessor of outcomes. The choice of outcome measures used in this study may have missed other clinically important effects that may have been captured with alternative measures.
The time point post-discharge at which patients were asked to attend the ICU Follow-up Clinic invariably influenced the time at which the patient commenced participation in the study. Although the aim was to enrol participants at 12 weeks post-discharge from the Follow-Up Clinic, patients did not always attend appointments at exactly 12 weeks. Variation in length of time from hospital discharge may have influenced the study's findings. Choice of stratification variables for the randomisation may also have influenced the results of the trial but recent (and future) research may inform future trials of more sensitive variables.
Despite the limitations of this study, further evidence has been added to the ever-increasing body of research that has highlighted that effectiveness of post-ICU rehabilitation programmes is a complex area. More questions are raised than answered currently, such as when the programmes should be commenced, who should be targeted, what interventions are most effective, what outcome measures should be used and many more. As with previous research, the six-week supervised exercise programme in this study did not significantly improve physical function, but this may be due to the deficit in sample size or a lack of sensitivity in the 6MWT. This study did report a significant improvement in anxiety levels and balance at 12 months, when compared with the control group.
Acknowledgments
Mrs Sandra Jones, Mrs Abigail Baglow, Mr Thomas Bromfield and Mrs Beverley Jones for running the exercise programme and assessing the patients.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Key statistics from the Case Mix Programme: 1 April 2014 to 31 March 2015. ICNARC, 2016, https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports/Summary-Statistics (2016, accessed 20th January 2017).
- 2.Herridge MS, Tansey CM, Matté A, et al. Canadian Critical Care Trials Group Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364: 1293–1304. [DOI] [PubMed] [Google Scholar]
- 3.Herridge MS, Moss M, Hough CL, et al. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med 2016; 42: 725–738. [DOI] [PubMed] [Google Scholar]
- 4.Schelling G, Stoll C, Haller M, et al. Health-related quality of life and posttraumatic stress disorder in survivors of the acute respiratory distress syndrome. Crit Care Med 1998; 26: 651–659. [DOI] [PubMed] [Google Scholar]
- 5.Granja C1, Amaro A, Dias C, et al. Outcome of ICU survivors: a comprehensive review. The role of patient-reported outcome studies. Acta Anaesthesiol Scand 2012; 56: 1092–1103. [DOI] [PubMed] [Google Scholar]
- 6.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med 2011; 39: 371–379. [DOI] [PubMed] [Google Scholar]
- 7.Herridge MS. The challenge of designing a post-critical care illness rehabilitation intervention. Crit Care 2011; 15: 1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens RD, Dowdy DW, Michaels RK, et al. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med 2007; 33: 1876–1889. [DOI] [PubMed] [Google Scholar]
- 9.NICE. Rehabilitation after critical illness in adults. NICE Clinical Guidance 83. London, UK: National Institute for Health and Care Excellence, http://www.nice.org.uk/guidance/cg83 (2009, accessed 20th January 2017).
- 10.Morris PE, Berry MJ, Files DC, et al. Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure: a randomized clinical trial. JAMA 2016; 28: 2694–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott D, McKinley S, Alison J, et al. Health-related quality of life and physical recovery after a critical illness: a multi-centre randomised controlled trial of a home-based physical rehabilitation program. Crit Care 2011; 15: R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh TS, Salisbury LG, Merriweather JL, et al. RECOVER Investigators Increased hospital-based physical rehabilitation and information provision after intensive care unit discharge: The RECOVER Randomized Clinical Trial. JAMA Intern Med 2015; 175: 901–910. [DOI] [PubMed] [Google Scholar]
- 13.McDowell K, O'Neill B, Blackwood B, et al. Effectiveness of an exercise programme on physical function in patients discharged from hospital following critical illness: a randomised controlled trial (the REVIVE study). Thorax 2017; 72: 594–595. . [DOI] [PubMed] [Google Scholar]
- 14.Batterham AM, Bonner S, Wright J, et al. Effect of supervised aerobic exercise rehabilitation on physical fitness and quality-of-life in survivors of critical illness: an exploratory minimized controlled trial (PIX study). Br J Anaes 2014; 113: 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denehy L, Skinner EH, Edbrooke L, et al. Exercise rehabilitation for patients with critical illness: a randomized controlled trial with 12 months of follow-up. Crit Care 2013; 17: R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones C, Skirrow P, Griffiths R, et al. Rehabilitation after critical illness: a randomized, controlled trial. Crit Care Med 2003; 31: 2456–2461. [DOI] [PubMed] [Google Scholar]
- 17.Jackson J, Ely EW, Morey M, et al. Cognitive and physical rehabilitation of intensive care unit survivors: results of the RETURN randomized controlled pilot investigation. Crit Care Med 2012; 40: 1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen JF, Egerod I, Bestle MH, et al. A recovery program to improve quality of life, sense of coherence and psychological health in ICU survivors: a multicenter randomized controlled trial, the RAPIT study. Intensive Care Med 2016; 42: 1733–1743. [DOI] [PubMed] [Google Scholar]
- 19.Connolly B, Salisbury L, O'Neill B, et al. ERACIP Group Exercise rehabilitation following intensive care unit discharge for recovery from critical illness (Review). Cochrane Database for Systematic Reviews 2015; 6: CD008632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ATS. Statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 21.Berg K, Wood-Dauphinee S, Williams JI, et al. Measuring balance in the elderly: preliminary development of an instrument. Physiotherapy Canada 1989; 41: 304–311. [Google Scholar]
- 22.Fess EE. Grip strength. In: Casanova JS (ed). Clinical assessment recommendations 1992; vol 2, Chicago: American Society of Hand Therapists, pp. 41–45. [Google Scholar]
- 23.Battle C, James K, Temblett P, et al. Early results of a 6-week exercise programme in post-ICU patients. Critical Care 2013; 17: P541. [Google Scholar]
- 24.Sawilowsky S. New effect size rules of thumb. J Modern Appl Statist Methods 2009; 8: 467–474. [Google Scholar]
- 25.Puthucheary ZA, Denehy L. Exercise interventions in critical illness survivors: understanding inclusion and stratification criteria. Am J Respir Crit Care Med 2015; 191: 1464–1467. [DOI] [PubMed] [Google Scholar]