Abstract
Background
Early administration of antibiotics in septic shock is associated with decreased mortality. Promptly identifying sepsis and eliciting a response are necessary to reduce time to antibiotic administration.
Methods
A best-practice advisory was introduced in the surgical intensive care unit to identify patients with septic shock and promote timely action. The best-practice advisory is triggered by blood culture orders and vasopressor administration within 24 h. The nurse or provider who triggers the alert may send an automatic notification to the intensive care unit resident, clinical pharmacist, and charge nurse, prompting bedside response and closer evaluation. Patients who met best-practice advisory criteria in the surgical intensive care unit from May 2016 through March 2017 were included. Outcomes included changes in antibiotics within 24 h, response to best-practice advisory, and time-to-antibiotics. Time-to-antibiotics was compared between a retrospective pre-intervention period and a six-month prospective post-intervention period defined by launch of the new best-practice advisory in September 2016. Data were analyzed by chi square, Mann–Whitney U, and Kruskal-Wallis.
Results
During the first six months of best-practice advisory implementation, 191 alerts were triggered by 97 unique patients. Alert notification was transmitted in 79 best-practice advisories (41%), with pharmacist bedside response in 53 (67%). New antibiotics were started within 24 h following 83 best-practice advisories (43%). There was a trend toward decreased time-to-antibiotics following implementation of the best-practice advisory (7.4 vs. 4.2 h, p = 0.057). Compared to the entire cohort, time-to-antibiotics was shorter when the team was notified and when a pharmacist responded to the bedside (4.2 vs. 1.6 vs. 1.2 hours).
Conclusions
A new best-practice advisory has been effective at eliciting a rapid response and reducing the time-to-antibiotics in surgical intensive care unit patients with septic shock. Team notification and pharmacist response are associated with decreased time-to-antibiotics.
Keywords: Sepsis, septic shock, electronic alert, antibiotics, intensive care unit
Background
Multiple studies have shown that early administration of antibiotics in patients with severe sepsis and septic shock is associated with a decreased risk of mortality.1–3 Guidelines recommended administration of effective antibiotics within the first hour of recognition of sepsis or septic shock.4,5 However, many institutions have struggled to meet this target.1,2,4,6 Promptly identifying patients with sepsis and eliciting a rapid response is necessary to reduce the time to antibiotic administration. Previous consensus conference definitions and guidelines used systemic inflammatory response syndrome (SIRS) criteria for identifying and defining sepsis.5,7 However, many patients presenting to the hospital do not meet the diagnostic SIRS criteria until hours after arrival.8 Furthermore, recent literature suggests that SIRS criteria lack sensitivity and validity.9 In surgical patients, diagnosing and initiating treatment for sepsis present additional challenges. Nearly all surgical ICU patients meet SIRS criteria, rendering this method unhelpful at predicting severe sepsis and septic shock.10
Currently at our institution, a best-practice advisory (BPA) fires when a patient in non-intensive care unit (ICU) areas meets four of four SIRS criteria (temperature >38.3 or <36.0℃; heart rate >90; respiratory rate >20; white blood cell [WBC] >12,000 or <4000, or >10% bands) within 6 h of each other. Provider teams are automatically notified when a patient meets these criteria, prompting timely action. This BPA does not apply to patients in ICU areas, due to lack of specificity for septic shock. In September 2016, a new BPA was introduced in the surgical/trauma/burn ICU (STBICU) to help identify patients with septic shock requiring provider attention and possible medical intervention. The purpose of this study was to evaluate the use of this new sepsis BPA on identification and response to suspected septic shock. We hypothesized that this BPA would prompt a reassessment of need for therapy adjustment, and improve response in patients requiring intervention.
Methods
This was a prospective study of patients in the STBICU with septic shock as identified by a new BPA during a six-month post-intervention BPA study period from 13 September 2016 to 12 March 2017. The BPA is triggered to a licensed independent practitioner (LIP) or nurse if a patient has blood cultures ordered and vasopressors ordered or administered within 24 h of each other. A lockout period prevents repeat alerts within 36 h. When a BPA fires, the LIP/nurse has the option to send an automatic page to the trauma/SICU residents, clinical pharmacist, and charge nurse. The team responds to the bedside to assess the patient, obtain blood cultures and labs as appropriate, administer fluids and medications, provide recommendations, and help improve time to antibiotic selection and administration. Alternatively, the LIP/nurse may choose not to notify the team if they feel this is not appropriate, and is then prompted to provide an explanation. In the event that a nurse chooses not to notify the team, they are encouraged to still notify the LIP, although this does not happen automatically via the computer system.
End points included team notification and response to BPA, changes in antibiotics within 24 h, time of BPA and new antibiotic administration, time-to-antibiotics, ICU and hospital length of stay, and in-hospital mortality. Change in antibiotics was defined as the initiation of any new antibiotic medication, which the patient was not already on at the time of BPA. Patients requiring intervention were defined by a change in antibiotics within 24 hours of BPA. Time-to-antibiotics was measured from time of BPA trigger to administration of first new antibiotic. Prior to the implementation of this new BPA on 13 September 2016, the alert triggered silently, allowing identification and data collection for patients meeting BPA criteria, but without notification or team response. Time-to-antibiotics was compared between this pre-BPA test phase and the six-month post-intervention period.
Chi-squared test was used to analyze qualitative data. Mann–Whitney U was used to compare time-to-antibiotics between pre-and post-BPA implementation. Kruskal–Wallis was used to compare time-to-antibiotics between all post-intervention BPAs, BPAs with team notification, and BPAs with pharmacist response. All analyses were performed using Statistical Package for Social Sciences (SPSS) version 23.0 (SPSS, Chicago, IL). This study was approved by the University of Virginia Institutional Review Board for Health Sciences Research (IRB-HSR).
Results
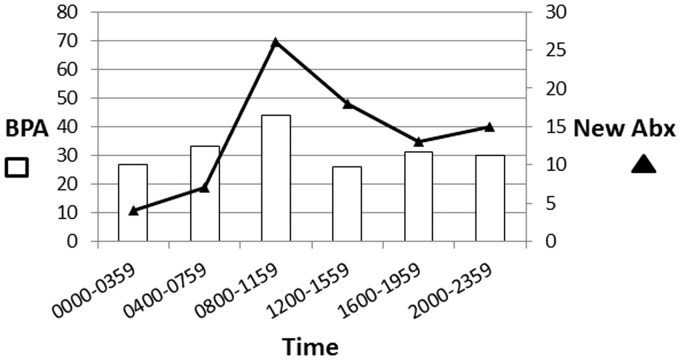
During the six-month post-intervention BPA period, 191 BPAs were triggered on 97 unique patients (Table 1). An automatic page notifying the team was sent in 79 (41%). Pharmacists responded to 53 BPAs following team notification (67%) and an additional 5 alerts when no automatic notification was sent. Patients required intervention with 83 alerts (43%). Although BPAs were triggered relatively consistently at all times of day, new antibiotics were more often started during daytime hours between 0800 and 1600 (Table 2 and Figure 1).
Table 1.
Characteristics of the best-practice advisory (BPA) cohort.
| Patients | 97 |
| Age | 62 (range 19–94) |
| Sex, m | 56 (58%) |
| ICU LOS (d) | 5 (IQR 2, 12) |
| Hospital LOS (d) | 15 (IQR 8, 32) |
| In-hospital mortality | 24 (27%) |
| BPA events | 191 |
| Repeat BPA | 94 (49%) |
| Pharmacist response when notifieda | 53 (67%) |
| Previously on antibiotics | 167 (87%) |
| Suspected sepsis | 168 (88%) |
| Pulmonary | 80 (48%) |
| Intra-abdominal | 56 (33%) |
| SSTI | 9 (5%) |
| Bone | 9 (5%) |
| Clostridium difficile/bowel | 7 (4%) |
| CNS | 4 (2%) |
| UTI | 3 (2%) |
CNS: central nervous system; ICU: intensive care unit; LOS: length of stay; IQR: interquartile range; SSTI: skin and soft tissue infection; UTI: urinary tract infection
Pharmacists documented response at five additional BPAs when team notification was not sent.
Table 2.
Best-practice advisory (BPA) and new antibiotic administration time.
| Time | BPA | New Abx |
|---|---|---|
| 0000–0359 | 27 (14%) | 4 (5%) |
| 0400–0759 | 33 (17%) | 7 (8%) |
| 0800–1159 | 44 (23%) | 26 (31%) |
| 1200–1559 | 26 (14%) | 18 (22%) |
| 1600–1959 | 31 (16%) | 13 (16%) |
| 2000–2359 | 30 (16%) | 15 (18%) |
Figure 1.
Best-practice advisory (BPA) and new antibiotic administration time.
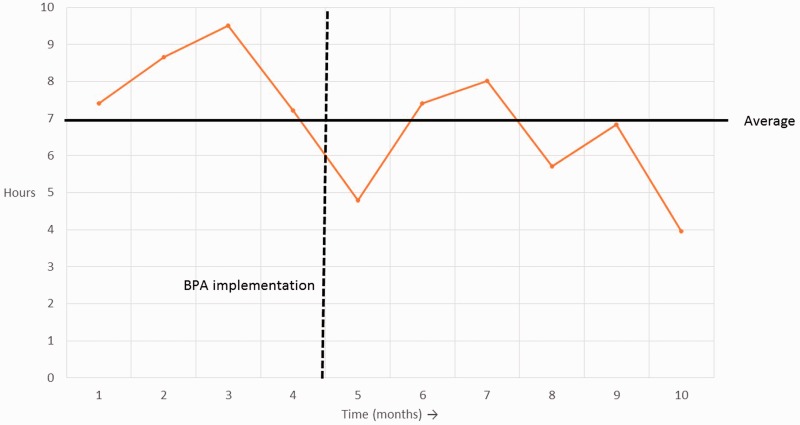
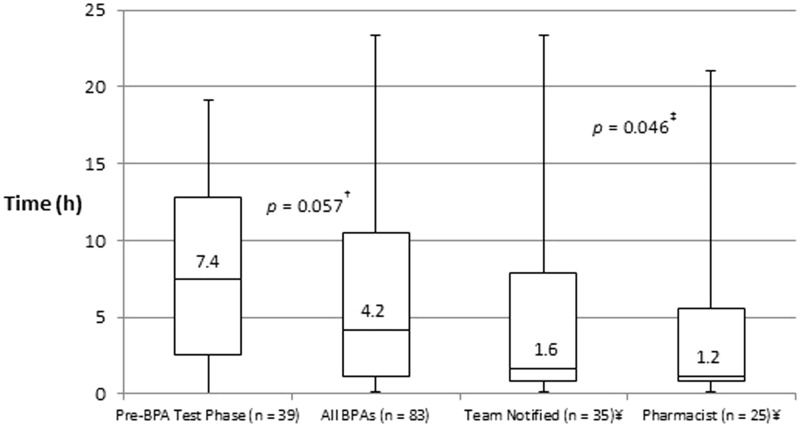
Compared to the pre-BPA test phase, time-to-antibiotics was shorter following implementation of the new septic shock BPA, although this difference did not meet statistical significance (7.4 h vs. 4.2 h, p = 0.057) (Figure 2). Compared to the entire post-intervention group, time-to-antibiotics was significantly shorter when a team notification page was disseminated, and when a pharmacist responded to the bedside (4.2 h vs. 1.6 h vs. 1.2 h, p = 0.046) (Figure 3). When a pharmacist responded, significantly more patients had antibiotics started within 1 h (36% vs. 14%, p = 0.021) and 3 h (60% vs. 34%, p = 0.031) of the BPA, compared to alerts with no pharmacist response (Table 3). There was no difference in the proportion of patients requiring intervention.
Figure 2.
Process control chart demonstrating median time to antibiotics each month, before and after implementation of BPA.
Figure 3.
Average time to new antibiotics within 24 h. ¥ Team notification and pharmacist response are subsets of the entire post-intervention BPA group. †Pairwise comparison between pre-BPA test phase and post-intervention BPA group. ‡Kruskal–Wallis comparison of all four groups.
Table 3.
Impact of pharmacist response on antibiotics.
| No response (n = 133) | RPh response (n = 58) | p | |
|---|---|---|---|
| Intervention required | 58 (44%) | 25 (43%) | 1.000 |
| Abx within 1 ha | 8 (14%) | 9 (36%) | 0.021 |
| Abx within 3 ha | 20 (34%) | 15 (60%) | 0.031 |
Including cases in which antibiotics were started within 24 h.
There was no difference in team notification or pharmacist response for initial BPA compared to repeat alerts in patients who had previously triggered the BPA (Table 4). Significantly more patients required intervention at the time of the initial alert (53% vs. 34%, p = 0.010), but there was no difference in number of patients receiving new antibiotics within 1 h (22% vs. 19%, p = 0.757) or 3 h (45% vs. 38%, p = 0.495).
Table 4.
Initial best-practice advisory (BPA) vs. repeat event.
| Initial BPA (n = 97) | Repeat BPA (n = 94) | p | |
|---|---|---|---|
| Team notified | 41 (42%) | 38 (40%) | 0.796 |
| RPh response | 28 (68%) | 25 (66%) | 0.813 |
| Intervention required | 51 (53%) | 32 (34%) | 0.010 |
| Abx within 1 ha | 11 (22%) | 6 (19%) | 0.757 |
| Abx within 3 ha | 23 (45%) | 12 (38%) | 0.495 |
Including cases in which antibiotics were started within 24 h.
Discussion
The results of our study demonstrate that implementation of a new BPA has led to earlier antibiotic response in surgical ICU patients with septic shock, although this difference in response time did not reach statistical significance among the entire population when including those cases in which the alert was bypassed. Team notification and pharmacist response were associated with even further reductions in time-to-antibiotics. The BPA was developed as a measure to ensure mobilization of resources for assessment, team communication and timely administration of new or modified antibiotic regimens. The alert is not necessarily intended to diagnose septic shock, but rather serve as a decision point tool reminding the team to reevaluate the patient’s current therapy including antibiotic regimen. Our results confirmed our hypothesis that this BPA would prompt a reassessment of need for therapy adjustment, and improve response in patients requiring intervention.
Computer-based alerts have been previously described for improving sepsis care. Screening tools with automatic alerts and triage model systems in emergency departments have led to improved time to antibiotics and intravenous fluids, and reduced length of stay.11–15 One study in non-ICU patients found increased therapeutic response after implementing a computerized sepsis alert.16 Within our institution, a SIRS BPA has demonstrated reduction in the sepsis mortality index in non-ICU areas. Data in ICU patients are more limited. One study evaluating an electronic sepsis evaluation and management tool found no difference in outcomes compared to usual care, but these results may have been impacted by low utilization.17 Alert fatigue and technical failure may be barriers to timely alert acknowledgment.18,19 Surveys and studies evaluating most effective or preferred methods of alert delivery (pagers, electronic health record (EHR) based, cell phones) have found mixed results, although active alerts such as computer pop-ups and pharmacy interventions may be more effective than alert systems which do not automatically fit into the clinician workflow.18–20
The development of this new BPA involved careful discussion of a number of potential triggers to identify critically ill patients in the ICU requiring attention and intervention. Our institution’s SIRS alert is not used in ICUs, because these patients may often meet these criteria for a number of reasons other than sepsis. Overidentification and alert fatigue would detract from the value of such an alert. Even among non-ICU patients, SIRS criteria are often present as a result of noninfectious causes. For this reason, the institution’s SIRS alert requires four-out-of-four SIRS criteria, as opposed to the two-out-of-four criteria needed to meet sepsis definitions.7 In designing this new BPA in an SICU, we chose factors that may be more specific to septic shock in an ICU population, and which are readily available in the EHR. In light of the most recent updates to the Surviving Sepsis Campaign Guidelines, use of Sepsis-3 triggers, based on Sequential Organ Failure Assessment (SOFA), may have been a meaningful alternative.4,21 Unfortunately, Glasgow Coma Scale (GCS) is not routinely assessed and documented in all patients at our institution. And even when used, an acute change in mental status may not be identified until the next assessment, which could be many hours later. The criteria we chose for our BPA are immediately detected by the EHR, allowing timely response.
Early recognition and response to septic shock are vital, since a delay in starting antibiotics is associated with increased mortality and other poor clinical outcomes. Kumar demonstrated a 7.6% decreased survival for each hourly delay in effective antibiotics among patients with septic shock.1 Similarly, a retrospective analysis of the Surviving Sepsis Campaign database found a linear increase in hospital mortality odds ratio as time-to-antibiotics increased from 0 to more than 6 h among patients in all areas of the hospital and across levels of illness severity.2 In addition to increased mortality, delays in starting antibiotics in sepsis have been associated with increased risk of acute kidney injury,22 increased ICU and hospital length of stay,23 and increased change in SOFA score, indicating new or progressing organ dysfunction.24 Our study was not powered to detect differences in these clinical outcomes between the pre-BPA and post-intervention groups, and unmeasured confounding factors associated with a retrospective historical cohort would have made it difficult to draw any firm conclusions regarding these endpoints. However, we did show a significant improvement in antibiotic response following implementation of this new sepsis BPA.
Following implementation of the new BPA, time-to-antibiotics has been decreased. This response time is shorter when the team is notified, and even more so when a pharmacist responds. Even among only the cases in which the team was notified, time-to-antibiotics was shorter if a pharmacist responded to the bedside. This highlights the importance of recognition and team communication, and indicates additional value in pharmacist presence. It is important to point out, however, that we did not evaluate the appropriateness of starting antibiotics. Given an increase in antibiotics being started in response to the alerts, it is possible that the BPA may introduce overtreatment. Since overutilization of antibiotics can lead to development of resistance, clinicians must continue to use proper judgement when taking action in response to the BPA.
Currently, when the BPA is triggered, the nurse or LIP has the option whether or not to send a pre-built notification text page to the clinical pharmacist, charge nurse, and ICU resident. Time-to-antibiotics was shorter when the team was contacted, but this notification was sent in fewer than half of BPAs. It is unclear why the alert was bypassed in the majority of cases. Anecdotally, we believe that in many cases, the alert was bypassed because the patient was already receiving antibiotics. However, data were not collected to verify this assumption or identify other possible reasons why the BPA was not used. Ongoing education may be conducted to improve utilization. Our positive results have prompted discussions among unit-based leadership regarding modification of the BPA to automatically send the team notification message with all BPA events. This could reduce the time-to-antibiotics among those cases in which the notification page is not being sent. However this would further increase the burden, especially on evenings, nights, and weekends when staffing is limited.
Although pharmacist bedside response was associated with improved antibiotic administration times, a pharmacist only responded to 67% of alerts when the team was notified. Staffing limitations may be a barrier to full compliance. Our data collection for response was based on pharmacist documentation using informal handoff notes in the electronic medical record. It is also possible that we under-identified pharmacist response due to lack of documentation. Departmental analysis of workload and shift times is being conducted in efforts to optimize resources and availability.
BPA events occurred evenly throughout the day and night, but new antibiotics were most frequently started from 8 a.m. until 12 p.m. The surgical and trauma interdisciplinary medical teams generally round on patients each day during these morning hours, so it is perhaps not surprising that the most changes are occurring at this time. Consult services, including an infectious disease service, often provide recommendations to the primary team throughout the late morning and afternoon once their patients have been examined and discussed. It makes sense that a large portion of new antibiotics was also started between 12 p.m. and 4 p.m. Changes to antibiotics were much less commonly made outside of these hours, especially overnight. This new BPA was designed to prompt attention and response to patients with septic shock. Decreased staffing resources outside of normal business hours may be impacting the ability to effectively respond to these alerts.
This study had a number of limitations. The retrospective design makes it difficult to draw conclusions due to unmeasured differences between cohorts. We did not prespecify a primary outcome, so our results must be interpreted with caution. We did not compare baseline characteristics or clinical outcomes (mortality, length of stay) to the pre-BPA group. Spectrum of antibiotics was not assessed, so early administration of antibiotics may not have always correlated with early administration of effective antibiotics. Anecdotal evidence suggests that education and resource needs may be barriers to full compliance with alert response.
Conclusion
This prospective study demonstrated the effectiveness of a new sepsis alert in surgical ICU patients. Time-to-antibiotics was decreased following implementation of this BPA. Antibiotics were administered even more quickly if the team was notified and if a pharmacist responded to the bedside. Further analysis of resources and modifications to the BPA process may be conducted to increase utilization.
Acknowledgments
The authors’ acknowledge Kristi Kimpel Wilkins, MSN, RN, CCRN, CCNS, Matt Robertson, MSN, ACNP-BC, and Jason Lyman, MD, MS.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Kumar A, Roberts D, Wood KE, et al. Duration of hypotention before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34: 1589–1596. [DOI] [PubMed] [Google Scholar]
- 2.Ferrer R, Martin-Loeches I, Philips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med 2014; 42: 1749–1755. [DOI] [PubMed] [Google Scholar]
- 3.Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013; 41: 1167–1174. [DOI] [PubMed] [Google Scholar]
- 4.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2016. Crit Care Med 2017; 45: 486–552. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41: 580–637. [DOI] [PubMed] [Google Scholar]
- 6.Puskarich MA, Trzeciak S, Shapiro NI, et al. Emergency Medicine Shock Research Network (EMSHOCKNET). Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med 2011; 39: 2066–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy MM, Fink MP, Marshall JC, et al. SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003; 31: 1250–1256. [DOI] [PubMed] [Google Scholar]
- 8.Villar J, Clement JP, Stotts J, et al. Many emergency department patients with severe sepsis and septic shock do not meet diagnostic criteria within 3 hours of arrival. Ann Emerg Med 2014; 64: 48–54. [DOI] [PubMed] [Google Scholar]
- 9.Kaukonen KM, Bailey M, Pilcher D, et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med 2015; 372: 1629–1638. [DOI] [PubMed] [Google Scholar]
- 10.Pittet D, Rangel-Frausto S, Li N, et al. Systemic inflammatory response syndrome, sepsis, severe sepsis and septic shock: incidence, morbidities and outcomes in surgical ICU patients. Intensive Care Med 1995; 21: 302–309. [DOI] [PubMed] [Google Scholar]
- 11.Rosengvist M, Fagerstrand E, Lanbeck P, et al. Sepsis alert—a triage model that reduces time to antibiotics and length of hospital stay. Infect Dis 2017; 49: 507–513. [DOI] [PubMed] [Google Scholar]
- 12.Gatewood MO, Wemple M, Greco S, et al. A quality improvement project to improve early sepsis care in the emergency department. BMJ Qual Saf 2015; 24: 787–795. [DOI] [PubMed] [Google Scholar]
- 13.Idrees M, Macdonald SP, Kodali K. Sepsis early alert tool: early recognition and timely management in the emergency department. Emerg Med Australas 2016; 28: 399–403. [DOI] [PubMed] [Google Scholar]
- 14.Hayden GE, Tuuri RE, Scott R, et al. Triage sepsis alert and sepsis protocol lower times to fluids and antibiotics in the ED. Am J Emerg Med 2016; 34: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narayanan N, Gross AK, Pintens M, et al. Effect of an electronic medical record alert for severe sepsis among ED patients. Am J Emerg Med 2016; 34: 185–188. [DOI] [PubMed] [Google Scholar]
- 16.Sawyer AM, Deal EN, Labelle AJ, et al. Implementation of a real-time computerized sepsis alert in nonintensive care unit patients. Crit Care Med 2011; 39: 469–473. [DOI] [PubMed] [Google Scholar]
- 17.Semler MW, Weavind L, Hooper MH, et al. An electronic tool for the evaluation and treatment of sepsis in the ICU: A randomized controlled trial. Crit Care Med 2015; 43: 1595–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison AM, Thongprayoon C, Aakre CA, et al. Comparison of methods of alert acknowledgement by critical care clinicians in the ICU setting. Peer J 2017; 5: e3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dziadzko MA, Harrison AM, Tiong IC, et al. Testing modes of computerized sepsis alert notification delivery systems. BMC Med Inform Decis Mak 2016; 16: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheepers-Hoeks AM, Grouls RJ, Neef C, et al. Physicians’ responses to clinical decision support on an intensive care unit—comparison of four different alerting methods. Artif Intell Med 2013; 59: 33–38. [DOI] [PubMed] [Google Scholar]
- 21.Singer M, Deutschman CS, Seymour CW, et al. The third international definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagshaw SM, Lapinsky S, Dial S, et al. Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med 2009; 35: 871–881. [DOI] [PubMed] [Google Scholar]
- 23.Zhang D, Micek ST, Kollef MH. Time to appropriate antibiotic therapy is an independent determinant of postinfection ICU and hospital lengths of stay in patients with sepsis. Crit Care Med 2015; 43: 2133–2140. [DOI] [PubMed] [Google Scholar]
- 24.Garnacho-Montero J, Aldabo-Pallas T, Garnacho-Montero C, et al. Timing of adequate antibiotic therapy is a greater determinant of outcome than are TNF and IL-10 polymorphisms in patients with sepsis. Crit Care 2016; 10: R111. [DOI] [PMC free article] [PubMed] [Google Scholar]