Abstract
Introduction
Temporary tracheostomy is commonly used in patients admitted to intensive care units. Cuffed tubes prevent laryngeal airflow, preventing vocalisation. Sub-glottic suction tubes such as the ‘Blue Line Ultra Suctionaid™’ are used primarily to remove sub-glottic secretions, but retrograde gas flows via the suction port can facilitate above cuff vocalisation. The aims were to assess whether patients could achieve an audible voice using above cuff vocalisation, to demonstrate the safe use of the Blue Line Ultra Suctionaid™ tracheostomy tube for above cuff vocalisation, and to assess potential benefits of above cuff vocalisation for communication, secretion management and swallowing.
Methods
Our study (Reference 15/NW/0464, IRAS 178997) recruited adult intensive care unit patients who were alert, able to participate in an above cuff vocalisation trial and dependent on an inflated Blue Line Ultra Suctionaid™ cuff for ventilatory support. Consenting participants underwent fibreoptic endoscopic assessment of swallow by experienced Speech & Language Therapy staff with and without above cuff vocalisation. Clinical and fibreoptic endoscopic assessment of swallow, assessment of voice quality, swallowing and secretion management were recorded and scored. Median differences between paired observations and scores were analysed with and without above cuff vocalisation. Adverse events were identified by follow up fibreoptic endoscopic assessment of swallow and patient accounts.
Results
Ten patients completed the study. Above cuff vocalisation was used for a median of 15 min, during a median of nine episodes, over a median of three days. Above cuff vocalisation resulted in an audible voice in eight of the 10 patients, during 66 out of 91 above cuff vocalisation attempts. There improvements in unstimulated dry cough and swallow frequency and aspiration ratings measured by fibreoptic endoscopic assessment of swallow. No complications were reported or observed in 66 attempts with only one episode terminated prematurely.
Conclusions
Above cuff vocalisation can achieve effective, safe, well-tolerated vocalisation in ventilator-dependant intensive care unit patients. Above cuff vocalisation has the potential to aid earlier, more effective communication and may improve laryngeal function and rehabilitation.
Keywords: Tracheostomy, ventilator-dependent, vocalisation, fibreoptic endoscopic assessment of swallow, swallowing
Introduction
Between 10 and 15% of all patients admitted to intensive care units (ICUs) in the Western world will require a temporary tracheostomy.1–4 These artificial airways are most commonly required to facilitate prolonged artificial ventilation, typically requiring an inflated cuff to ‘seal’ the trachea and allow positive pressure ventilation (Figure 1). Gas does not therefore flow through the upper airways (nose and mouth) and also bypasses the larynx, meaning that whilst the tracheostomy tube cuff is inflated, the patient cannot vocalise and the ability to communicate is impaired. The cuff is usually required to be inflated to facilitate artificial ventilation or to offer a degree of ‘protection’ to the airway against aspiration.
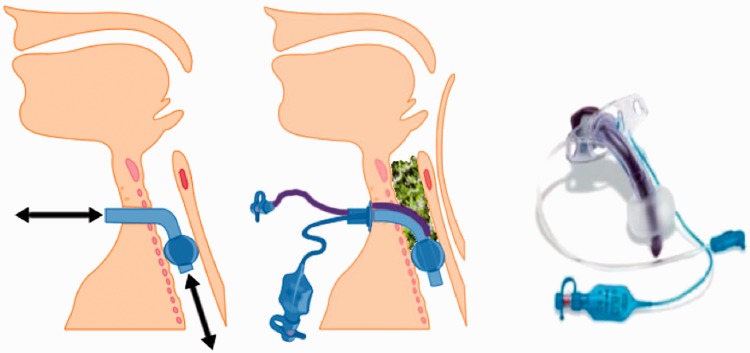
Figure 1.
(Left) Respiratory gas flow during cuff inflation. (Centre) Schematic to demonstrate aspiration of material aspirated distal to the larynx, but contained above the cuff. (Right) Smith-Medical ‘Blue Line Ultra Suctionaid™’ (BLUS) tracheostomy tube.
It is increasingly recognised that trans-laryngeal aspiration and micro-aspiration of oropharyngeal or gastric contents into the airway can contribute to ventilator-associated pneumonia (VAP).5 Amongst the published strategies to mitigate the risks of developing VAP, subglottic suction ports have been added to a range of endotracheal and tracheostomy tubes, designed to facilitate the removal of secretions that may accumulate above the cuff.6 The inflated cuff reduces the amount of aspirated material entering the lungs and accumulated material can be removed by suctioning through the additional port (Figure 1). The Smith-Medical (Ashford, Kent, UK) ‘Blue Line Ultra Suctionaid™’ (BLUS) tracheostomy tube is one such tube, with the distal opening for aspiration of secretions situated just proximal to (above) the tracheostomy tube cuff. Because of this configuration, it is possible to attach a gas flow to this ‘suctionaid’ port, so that instead of suctioning material from above the cuff, gas flow is instead directed in a retrograde direction. This has the effect of directing an external flow of gas, separate to that used for ventilation, through the vocal cords and outwards via the upper airways. This technique has been described previously, for the BLUS tube7–9 and for similar devices,10–18 and can allow the patient to vocalise and articulate speech (Figure 2). The BLUS tube is marketed by the manufacturer describing use of the suctionaid port for ‘speech’ as possible, but the company does not currently promote the tube for this purpose as there is no supporting evidence other than anecdotal and case reports.
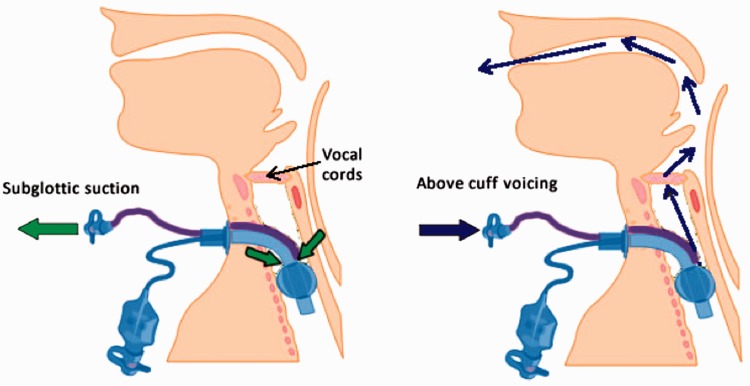
Figure 2.
Left-hand figure demonstrates the use of the BLUS subglottic suction port for the aspiration/removal of material that collects above the cuff. The right-hand figure demonstrates the flow of gas if the suction system is reversed and gas is delivered via the suctionaid port to exit via the larynx, above the cuff. The gas will escape through the upper airways and pass through the vocal cords, potentially allowing vocalization. Ventilation of the lungs can continue independently.
BLUS: Blue Line Ultra Suctionaid™.
There are clear communication and psychological benefits in facilitating vocalisation in patients who may be fully alert and yet unable to talk due to the ongoing need for cuff inflation.19,20 However, our previous experience of using above cuff vocalisation (ACV) also additionally signalled an improvement in laryngeal function, perhaps improving laryngeal sensitivity by restoration of trans-laryngeal airflow.8 We hypothesised that ACV might increase cough response to pooled laryngeal secretions, improved swallow response, improve indices of ‘airway protection’ and reduce aspiration risks.
Despite a growing interest in ACV, especially in critical care, there is currently a lack of research in this area. The aims of this feasibility study were to:
Determine whether patients achieve a functional voice with ACV (primary outcome)
Demonstrate the safe use of the BLUS tracheostomy tube for ACV in ventilator-dependant ICU patients, documenting any intolerance or adverse effects of ACV
Establish potential benefits of ACV for communication, secretion management and swallowing
Contribute to the emerging evidence base for the safe use of ACV, working towards developing guidelines for:
a. Timing for commencement and terminating ACV
b. Optimal ACV gas flows
c. Optimal duration of use
d. Indications and contraindications for use
These aims can be addressed by clinical bedside assessment (objectively for voice quality, adverse effects and swallow; subjectively for intolerance), supplemented by fibreoptic endoscopic evaluation of swallow (FEES) for secretion management and detailed swallowing assessment, and detection of laryngeal complications.
Methods
The UK Medicines and Healthcare Products Regulatory Agency (MHRA) were contacted and opined that the BLUS device could be used for ACV in line with the device’s current CE marking. The study was subsequently granted a favourable ethical opinion from the national Research Ethics Committee (Reference 15/NW/0464, IRAS Project ID 178997). The study was funded by an unrestricted grant from Smiths-Medical International Ltd (Ashford, Kent, UK).
Our tertiary single-site hospital has separate general and cardiothoracic ICUs. Patients from either unit who required a tracheostomy as part of the management of their critical illness were screened against the following inclusion criteria:
Adult patients > 16 years old
Cuffed BLUS (suctionaid) tracheostomy tube in situ for more than 72 h
Alert patient who:
^ Can understand the consent process
^ Can actively participate in the ACV trial (awake and trying to communicate)
^ Is suitable for FEES as per the Royal College of Speech and Language Therapists (RCSLT) FEES Position Paper20 (see Appendix 2)
Patients were excluded if they refused consent, had (or were suspected to have) a potentially obstructed upper airway, or if their clinical condition was expected to progress such that they tolerate cuff deflation and a one-way speaking valve within 72 h, negating the requirement for an ACV trial. Patients were also excluded if FEES was contraindicated (adapted from RCSLT FEES Position Paper),20 namely base of skull or facial fractures, history or risk of severe epistaxis, recent sino-nasal trauma or surgery, or significant nasal stenosis.
Suitable patients underwent a bedside trial of ACV by an experienced SLT, using incremental gas flows from 1 to 5 litres/min. If vocalisation was achieved, formal FEES was performed and anonymised images recorded. FEES was initially performed without ACV, and then with the nasendoscope remaining in situ, FEES trials and measures were immediately repeated with ACV. A standard Pentax Medical Digital FEES system (Pentax Medical, Tokyo, Japan) with digital nasendoscope was used following the Langmore FEES protocol.21 Voice was evaluated by SLT using the GRBAS Scale (auditory-perceptual assessment of severity of dysphonia)22 and also by simply recording whether the vocal sounds was subjectively absent, a whisper or an audible voice. The number of spontaneous, unstimulated coughs and swallows were observed over a two-minute period during the assessment with and without ACV.
Bedside nursing staff were encouraged post FEES to use ACV for up to 15 min every two hours, particularly when the patient wished to communicate. A record of ACV use, gas flow required, quality of vocalisation and complications were kept at the bedside. Patients were asked if they experienced ACV complications, specifically discomfort, excessive oral secretions, stomal air leak, gagging or nausea, and staff recorded if the patient asked to remove or stop the ACV trial. ACV continued at the discretion of the primary clinical team until a further FEES, performed between three and seven days later, to assess for complications. ACV was also discontinued if the clinical team attempted prolonged (>12 h) periods of cuff deflation.
The FEES image recordings were later jointly analysed by two experienced SLTs, blinded as to which FEES was conducted with ACV, who agreed consensus scores for each of the following scoring systems, described in detail in Appendix 1.
Secretion Severity Rating Scale (SRSS)23
Airway Protection Scale (APS)23
Penetration–Aspiration Scale (Pen-Asp)24
Therapy Outcome Measure for Voice Impairment (TOMS)25
ICU Functional Communication Scale (FCS, devised for this study, see Appendix 1)
Results were entered anonymously into a bespoke Microsoft™ Excel spreadsheet and summary metrics were prepared. Median differences between paired observations were analysed with SPSS 23 (IBM Corp) using Wilcoxon signed-rank test for ordinal scales. If continuous data were skewed, Wilcoxon test was also used. Results are presented as median (inter-quartile range (IQR), range) unless otherwise stated. Statistical significance was accepted at p < 0.05.
The primary outcome of this study was to assess cuff-inflated, ventilator-dependent ICU patients could achieve an audible voice using ACV. Secondary outcomes included assessing the safety of the procedure (judged by patient-reported patient discomfort and FEES examination) assessment of coughing, swallowing and secretion management with and without ACV.
Results
A total of 74 patients were managed with a tracheostomy over the five-month study period. Fifty-eight tracheostomy patients either progressed to tolerating cuff deflation or did not regain sufficient consciousness to be considered for ACV. The remaining 16 patients met the criteria to be screened for inclusion. Two patients had contra-indications for ACV (existing surgical emphysema) and one patient did not have capacity following detailed evaluation. Thirteen patients met the inclusion criteria and were recruited to the study. Three patients did not participate beyond enrolment (two patients subsequently tolerated prolonged cuff deflation and were withdrawn, and one patient withdrew consent following the first FEES, not wishing to undergo a second examination), leaving 10 patients who completed the study. Seven males and three females were recruited from the Cardiothoracic (seven patients) and General ICU (three patients). Patient demographics and indication for tracheostomy are detailed in Table 1.
Table 1.
Patient characteristics at study entry.
| Age (years) | Height (cm) | Weight (kg) | APACHE II | ICU LoS (days) | Advanced respiratory support days | Days from tracheostomy to first ACV |
|---|---|---|---|---|---|---|
| 60 (26) 28–83 | 170 (25) 150–185 | 70 (22) 60–96 | 18 (5) 9–20 | 28 (36) 22–132) | 25 (26) 20–102 | 8 (9) 3–46 |
Note: Reasons for admission, all of which complicated by prolonged respiratory wean: (1) pneumonia, complicated by cardiomyopathy requiring left ventricular assist device; (2) elective right lower lobectomy; (3) emergency laparotomy for ischaemic gut; (4) elective lobectomy complicated; (5) elective lobectomy complicated; (6) respiratory syncytial viral pneumonitis requiring Extra-Corporeal Membrane Oxygenation (ECMO); (7) double lung transplant for cystic fibrosis; (8) biventricular heart failure due to hypertrophic cardiomyopathy, treated with urgent heart transplant; (9) out of hospital cardiac arrest; (10) severe interstitial lung disease treated with single lung transplant.
Values are median (IQR) and range.
ACV: above cuff vocalisation; APACHE II: Acute Physiology And Chronic Health Evaluation II; ICU: intensive care unit; IQR: inter-quartile range; LoS: length of stay.
All patients were ‘Nil by mouth’ at the time of recruitment, and all had naso-gastric tubes in situ. Nine patients had undergone percutaneous tracheostomy and one had undergone an open surgical procedure. All patients had a BLUS tracheostomy tube initially inserted, which remained in place during the study. Sizes 7 mm internal diameter tubes (one patient), 8 mm (four patients) and 9 mm (five patients) were used.
All patients who completed the study underwent all planned assessments. Following baseline FEES, patients used ACV for a median of three days (3, 1–7). A median of nine (7, 4–19) ACV episodes typically lasted for 15 (10, 1–20) min, using additional gas flows of 1–5 litres/min via the suctionaid port. ACV resulted in an audible voice (speech or whisper) in eight of the 10 patients, during 66 out of 91 attempts (72.5%) (Table 2). Where an audible voice using ACV was achieved at the time of FEES, voice quality assessment by SLT staff was possible on 13 occasions (in eight patients) using the GRBAS scale (each domain scored from 0-normal to 3-high degree of impairment). Median (IQR) scores for ACV voice were as follows: GRBAS Grade 3(1), Roughness 2(1), Breathiness 2(3), Asthenia 2(2) and Strain 2(3).
Table 2.
Effectiveness and complications of ACV.
| Count | % | |
|---|---|---|
| Effectiveness of ACV | ||
| No voice | 25 | 27.5 |
| Whisper | 29 | 31.9 |
| Speech | 37 | 40.7 |
| Speech or whisper | 66 | 72.5 |
| Total ACV episodes | 91 | 100.0 |
| Primary patient-reported complications of ACV | ||
| None | 66 | 72.5 |
| Discomfort | 10 | 11.0 |
| Excessive oral secretions | 9 | 9.9 |
| Stomal air leak | 2 | 2.2 |
| Gagging | 2 | 2.2 |
| Nausea | 1 | 1.1 |
| Patient asked to remove | 1 | 1.1 |
| Total | 91 | 100.0 |
ACV: above cuff vocalisation.
These results from the five scoring domains from the paired FEES assessments without and with ACV are presented in Table 3, with observed cough and swallow counts. Three comparisons were not possible due to secretions and laryngeal oedema obscuring the FEES images. Secretion management significantly improved with ACV when assessed by SSRS, as did TOMS and ICU FCS. There was also a significant improvement in unstimulated dry cough and swallow frequency. Additionally, there was a non-significant improvement in APS and Pen-Asp scores with ACV.
Table 3.
Scores assigned following FEES at first assessment, without and then with ACV.a
| Median values |
Number of patients |
|||||||
|---|---|---|---|---|---|---|---|---|
| Scale | Paired comparisons made | Without ACV | With ACV | Median difference | Improved with ACV | Worse with ACV | No change | Wilcoxon signed rank p |
| SSRS (0 normal – 3 worse) | 10 | 3 | 1 | 0.5 | 5 | 0 | 5 | 0.04b |
| APS (1 worse – 5 better) | 8 | 3 | 3 | 0 | 2 | 0 | 6 | 0.18 |
| Pen-Asp (1 better – 8 worse) | 9 | 8 | 7 | 0 | 4 | 1 | 4 | 0.28 |
| TOMS (0 worse – 5 better) | 10 | 0 | 1 | 1 | 8 | 0 | 2 | 0.01b |
| ICU FCS (1 worse – 4 better) | 10 | 2 | 3 | 1 | 6 | 0 | 4 | 0.02b |
| Unstimulated dry swallow frequency (per minute) | 10 | 0 | 2 | 2 | 8 | 1 | 1 | 0.02b |
| Unstimulated cough frequency (per minute) | 10 | 0 | 0.5 | 0.5 | 5 | 0 | 5 | 0.04b |
ACV: above cuff vocalisation; APS: Airway Protection Scale; FCS: Functional Communication Scale; ICU: intensive care unit; Pen-Asp: Penetration-Aspiration Scale; SSRS: Secretion Severity Rating Scale; TOMS: Therapy Outcome Measure for Voice Impairment.
Observed cough and swallow frequency (per minute) are also presented. bSignificant results are indicated by.
Discussion
Our feasibility study has demonstrated that ACV using retrograde gas flow via the subglottic suctionaid port of a BLUS tracheostomy tube can achieve effective vocalisation in around three-quarters of ventilator-dependant, cuff-inflated ICU patients who met our broad inclusion criteria and completed the study. Vocalisation was judged possible using both bedside clinical assessments (audible voice or whisper) and more detailed SLT assessments (TOMS and ICU FCS). Furthermore, the vast majority of patients tolerated up to 15 min of ACV without significant complications. Patient discomfort and excessive oral secretions were the commonest complications reported.
The patients recruited to this study were unable to tolerate tracheostomy tube cuff deflation at the time of enrolment and so ACV facilitated an effective means of verbal communication that would have otherwise been unavailable to them. There are clear benefits in facilitating effective communication for critically ill patients, for their family or carers and for their attending medical, nursing and allied healthcare staff.26,27 Delirium, agitation, pain and compliance with therapies are all likely improved with better communication.20,28
ACV also appeared to have a significant positive effect on secretion management (SSRS scale) and by increasing both the unstimulated cough and swallow frequency. Secretion management also improved when assessed by other invasive measures (Pen-Asp and APS) although not significantly. We postulate that these observations may be due to an increase in laryngeal sensitivity or afferent neural activity associated with restoration of trans-laryngeal airflow. There may also be a physical or physiological effect from the exhaled gas flows that impact upon glottic secretions facilitating upward expulsion.29 This is an area that requires further research, as there may be benefits in minimising the downregulation of laryngeal function associated with prolonged intubation or (cuff up) tracheostomy use, or may have potential in stimulating earlier rehabilitation of laryngeal function and swallowing following critical illness.30–32
ACV has been described by others, but the optimal timing to commence ACV, duration of each episode, frequency of use and overall strategy for ACV as part of a comprehensive weaning plan has not been established. Our protocol was based on the clinical experience of colleagues at the Tracheostomy Review and Management Service (TRAMS), Austin Healthcare, Melbourne Australia (personal communication, Mrs T Cameron, TRAMS). TRAMS use ACV as a step towards demonstrating that the patient can manage their tracheal and oral secretion load and therefore tolerate cuff deflation trials, pragmatically waiting 72 h post tracheostomy insertion. Stomal air leak was only observed on two occasions (in the same patient who had undergone percutaneous tracheostomy) and was easily detected clinically. Based on our observations, we suggest ACV could be attempted earlier following tracheostomy assuming the stoma site is healing well. Stomal leak may be less of a problem with purely percutaneous tracheostomy insertion.7 Earlier ACV may realize earlier vocalisation and laryngeal function benefits without significantly increasing the risk of subcutaneous air leak. ACV has been limited to 10–15 min periods by others to mitigate concerns around the gas drying the laryngeal mucosa.7 We did not observe any short-term evidence of laryngeal drying and the follow up FEES did not demonstrate any laryngeal complications. Future work could use more sensitive measures of laryngeal function with enhanced imaging technology such as narrow-band imaging (NBI) or Pentax i-SCAN and assess the effectiveness of warmed, humidified ACV gas flows on the mucosa.
Our study has demonstrated that ACV is an effective tool for communication and vocalisation in ICU patients dependent on cuff-inflated tracheostomy tubes who would otherwise not be able to vocalise. The procedure was well tolerated and accepted by both patients and staff. There may be additional benefits of ACV for secretion management and swallowing that could positively impact on laryngeal function, and merits further research.
Supplemental Material
Supplemental material, Appendices for Safety and feasibility of above cuff vocalisation for ventilator-dependant patients with tracheostomies by Brendan A McGrath, Sarah Wallace, Mark Wilson, Leanne Nicholson, Tim Felton, Christine Bowyer and Andrew M Bentley in Journal of the Intensive Care Society
Acknowledgements
We are grateful to Mrs T Cameron, Senior Clinician & Manager, Tracheostomy Review and Management Service (TRAMS), Austin Healthcare, Melbourne Australia for her review and constructive comments regarding this manuscript.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: BAM has received expenses from Smiths-Medical and Ambu for attending company educational and product evaluation events, for which he has declined personal payments.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The study was funded by an unrestricted grant from Smiths-Medical International Ltd (Ashford, Kent, UK). The funder had no influence on the study design, conduct, data analysis or the writing of this manuscript.
References
- 1.Veenith T, Ganeshamoorthy S, Standley T, et al. Intensive care unit tracheostomy: a snapshot of UK practice. Int Arch Med 2008; 1: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blot F, Melot C. Commission d'Epidémiologie et de Recherche Clinique Indications, timing, and techniques of tracheostomy in 152 French ICUs. Chest 2005; 127: 1347–1352. [DOI] [PubMed] [Google Scholar]
- 3.Halum SL, Ting JY, Plowman EK, et al. A multi-institutional analysis of tracheotomy complications. Laryngoscope 2012; 122: 38–45. [DOI] [PubMed] [Google Scholar]
- 4.Shah RK, Lander L, Berry JG, et al. Tracheotomy outcomes and complications: a national perspective. Laryngoscope 2012; 122: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klompas M, Branson R, Eichenwald EC, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014; 35: 915–936. [DOI] [PubMed] [Google Scholar]
- 6.Frost SA, Azeem A, Alexandrou E, et al. Subglottic secretion drainage for preventing ventilator associated pneumonia: a meta-analysis. Aust Crit Care 2013; 26: 180–188. [DOI] [PubMed] [Google Scholar]
- 7.Husain T, Gatward JJ, Harris RD. Use of subglottic suction port to enable verbal communication in ventilator-dependent patients. Am J Respir Crit Care Med 2011; 184: 384–384. [DOI] [PubMed] [Google Scholar]
- 8.McGrath BA, Lynch J, Wilson M, et al. Above cuff vocalisation: a novel technique for communication in the ventilator-dependent tracheostomy patient. J Intensive Care Soc 2015; 17: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandian V, Smith CP, Cole TK, et al. Optimizing communication in mechanically ventilated patients. J Med Speech Lang Pathol 2014; 21: 309–318. [PMC free article] [PubMed] [Google Scholar]
- 10.Hess DR. Facilitating speech in the patient with a tracheostomy. Respir Care 2005; 50: 519–525. [PubMed] [Google Scholar]
- 11.Egbers PH, Bultsma R, Middelkamp H, et al. Enabling speech in ICU patients during mechanical ventilation. Intensive Care Med 2014; 40: 1057–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nomori H. Tracheostomy tube enabling speech during mechanical ventilation. Chest 2004; 125: 1046–1051. [DOI] [PubMed] [Google Scholar]
- 13.Kunduk M, Appel K, Tunc M, et al. Preliminary report of laryngeal phonation during mechanical ventilation via a new cuffed tracheostomy tube. Respir Care 2010; 55: 1661–1670. [PubMed] [Google Scholar]
- 14.Safar P, Grenvik A. Speaking cuffed tracheostomy tube. Crit Care Med 1975; 3: 23–26. [DOI] [PubMed] [Google Scholar]
- 15.Saul A, Bergström B. A new permanent tracheostomy tube – speech valve system. Laryngoscope 1979; 89: 980–983. [PubMed] [Google Scholar]
- 16.Kluin KJ, Maynard F, Bogdasarian RS. The patient requiring mechanical ventilatory support: use of the cuffed tracheostomy ‘talk’ tube to establish phonation. Otolaryngol Head Neck Surg 1984; 92: 625–627. [DOI] [PubMed] [Google Scholar]
- 17.Sparker AW, Robbins KT, Nevlud GN, et al. A prospective evaluation of speaking tracheostomy tubes for ventilator dependent patients. Laryngoscope 1987; 97: 89–92. [DOI] [PubMed] [Google Scholar]
- 18.Leder SB. Verbal communication for the ventilator-dependent patient: voice intensity with the Portex ‘Talk’ tracheostomy tube. Laryngoscope 1990; 100: 1116–1121. [DOI] [PubMed] [Google Scholar]
- 19.Flinterud SI, Andershed B. Transitions in the communication experiences of tracheostomised patients in intensive care: a qualitative descriptive study. J Clin Nurs 2015; 24: 2295–2304. [DOI] [PubMed] [Google Scholar]
- 20.Khalaila R, Zbidat W, Anwar K, et al. Communication difficulties and psychoemotional distress in patients receiving mechanical ventilation. Am J Crit Care 2011; 20: 470–479. [DOI] [PubMed] [Google Scholar]
- 21.Kelly AM, McLaughlin C, Wallace S, et al. Fibreoptic Endoscopic Evaluation of Swallowing (FEES): the role of speech and language therapy. Royal College of Speech and Language Therapists Position Paper RCSLT, www.rcslt.org/members/publications/publications2/fees_position_paper_300315. (2015, accessed 19 September 2017).
- 22.Hirano M. Clinical examination of voice. 81–84, New York: Springer-Verlag, 1981. [Google Scholar]
- 23.Murray JA. Manual of dysphagia assessment in adults, San Diego: Singular Pub. Group, 1999. [Google Scholar]
- 24.Rosenbek JC, Robbins JA, Roecker EB, et al. A penetration-aspiration scale. Dysphagia 1996; 11: 93–98. [DOI] [PubMed] [Google Scholar]
- 25.Enderby P, Alexander J. Therapy outcome measures: speech-therapy pathology, San Diego: Singular Pub. Group, 1997. [Google Scholar]
- 26.Breckenridge SJ, Chlan L, Savik K. Impact of tracheostomy placement on anxiety in mechanically ventilated adult ICU patients. Heart Lung 2014; 43: 392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoit JD. The gift of speech … priceless. Respir Care 2010; 55: 1760–1761. [PubMed] [Google Scholar]
- 28.Patak L, Gawlinski A, Fung NI, et al. Communication boards in critical care: patients' views. Appl Nurs Res 2006; 19: 182–190. [DOI] [PubMed] [Google Scholar]
- 29.Kothari M, Bjerrum K, Nielsen LH, et al. Influence of external subglottic air flow on dysphagic tracheotomized patients with severe brain injury. Ann Otol Rhinol Laryngol 2017; 126: 199–204. [DOI] [PubMed] [Google Scholar]
- 30.Sulica L, Hembree A, Blitzer A. Swallowing and sensation: evaluation of deglutition in the anesthetized larynx. Ann Otol Rhinol Laryngol 2002; 111: 291–294. [DOI] [PubMed] [Google Scholar]
- 31.Sörös P, Lalone E, Smith R, et al. Functional MRI of oropharyngeal air-pulse stimulation. Neuroscience 2008; 153: 1300–1308. [DOI] [PubMed] [Google Scholar]
- 32.Siebens AA, Tippett DC, Kirby N, et al. Dysphagia and expiratory air flow. Dysphagia 1993; 8: 266–269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendices for Safety and feasibility of above cuff vocalisation for ventilator-dependant patients with tracheostomies by Brendan A McGrath, Sarah Wallace, Mark Wilson, Leanne Nicholson, Tim Felton, Christine Bowyer and Andrew M Bentley in Journal of the Intensive Care Society