Abstract
Toxic leukoencephalopathy associated with heroin inhalation has been extensively described in the literature. This syndrome is characterized by progressive motor symptoms and dysautonomia that develop over weeks to months. We present three cases of abrupt-onset inhaled heroin-associated toxic leukoencephalopathy, a previously undescribed entity. These likely represent a subset of “found down” patients with acute neurologic changes not attributable to hypoxemic encephalopathy. All three had magnetic resonance imaging findings characteristic of toxic leukoencephalopathy, which has a relatively favorable prognosis. Prolonged unresponsiveness in patients “found down” after heroin overdose is often attributed to hypoxic brain injury. Brain magnetic resonance imaging is not generally included as part of routine workup. It should, however, be considered in patients with suspected inhaled heroin use due to the relatively favorable prognosis of toxic leukoencephalopathy. This is especially relevant in the setting of the current opioid epidemic and related increase in overdose-related intensive care unit admissions. The result may have significant impact on decisions about therapeutic options or continuation of care.
Keywords: Heroin, toxic leukoencephalopathy, heroin spongiform leukoencephalopathy, “chasing the dragon, ” magnetic resonance imaging
Introduction
The practice of smoking heroin originated in Shanghai in the 1920s and in Hong Kong was later refined into the method now known as “chasing the dragon.”1 It was not until the early 1990s, however, that heroin inhalation began to gain popularity in the United States.2,3 This phenomenon is attributed to increased availability of heroin’s water-insoluble freebase form, as well concern over safe injection practices associated with the burgeoning HIV/AIDS epidemic.1,4,5
Since then, progressive toxic leukoencephalopathy (TLE) associated with heroin inhalation has been extensively described in the literature.6–11 This syndrome is characterized by motor symptoms and dysautonomia that develop gradually over weeks to months.11,12 Acute TLE has not previously been reported in patients who have been seen normal within hours or days of onset.
We present three cases of abrupt rather than progressive-onset inhaled heroin-associated TLE with characteristic magnetic resonance imaging (MRI) findings. Acute neurologic changes in patients “found down” after drug overdose are often attributed to hypoxemic encephalopathy. These cases present an alternative diagnosis in patients who present with overdose but without identifiable apneic period. This has distinct implications for predicting neurologic outcomes in patients who present after acute heroin overdose.
Case 1
A 21-year-old male was found unresponsive but breathing spontaneously after being seen neurologically normal the night prior. Upon his arrival to the emergency department, he exhibited tonic-clonic seizure activity. He was intubated for airway protection and sedated with a propofol drip. Toxicology screen revealed opiates consistent with known inhaled heroin use.
Head CT without contrast was normal. Brain MRI showed pronounced abnormal white matter signal with restricted diffusion involving the splenium of the corpus callosum (Figure 1). This was thought to represent TLE and he was started on high-dose vitamin C.
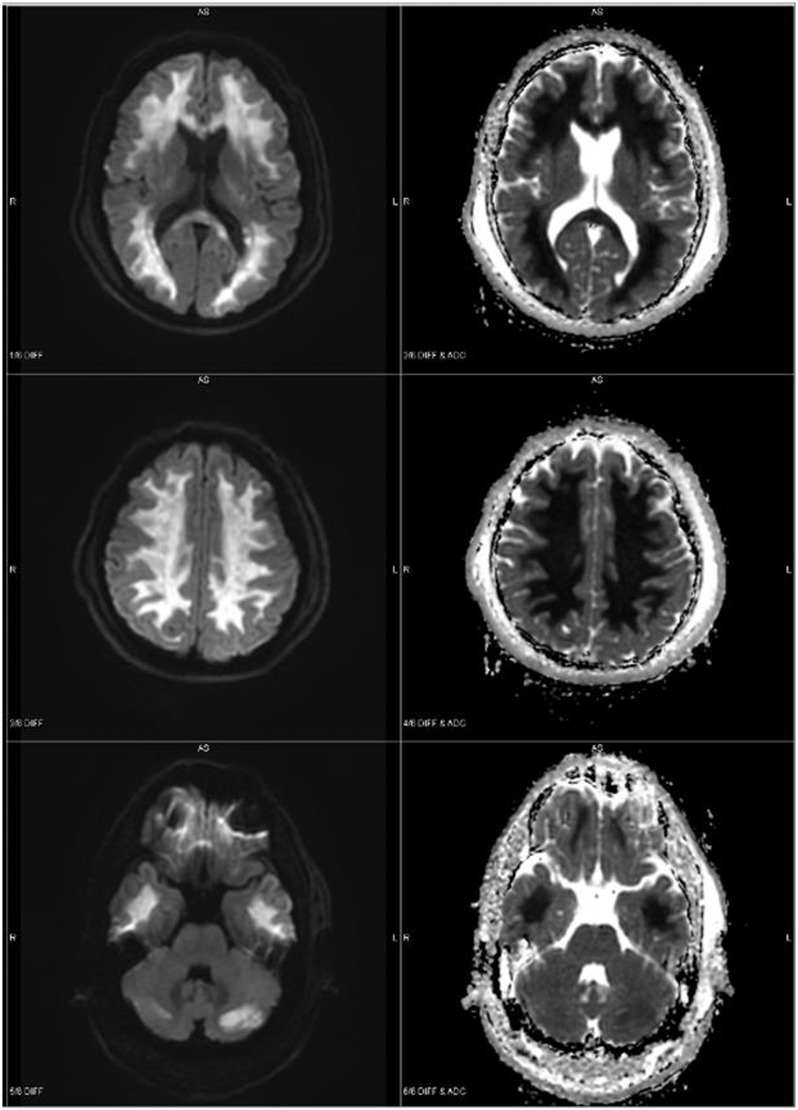
Figure 1.
MRI brain demonstrating pronounced abnormal white matter signal with restricted diffusion involving the splenium of the corpus callosum and cerebral white matter with relative sparing of the capsular white matter. Additional areas of restricted diffusion in the cerebellum (case 1).
Throughout his first week of hospitalization, the patient remained unresponsive despite repeated trials off sedation. He was tremulous with extensor posturing of the upper extremities to stimulation and exhibited frequent periods of dysautonomia with agitation, rigidity, hyperthermia, and tachycardia. Dantrolene was started with some improvement. Continuous video EEG showed no evidence of seizure activity.
At hospital day 7, the patient began to spontaneously open his eyes and exhibit roving conjugate eye movements without tracking. He underwent tracheostomy and was transferred to a long-term care facility. In the intervening five years, he has nearly returned to his cognitive and functional baseline with the exception of some residual motor deficits.
Case 2
A 34-year-old man was found unresponsive after being seen normal the day prior. Burnt foil and butane were found at the scene. Upon arrival in the emergency department, his Glasgow Coma Scale was 3 but exhibited spontaneous respirations. He was given naloxone without improvement and intubated for airway protection. Cooling measures were applied for temperature of 39.5℃. Toxicology was positive for opiates and amphetamines.
Upon medical intensive care unit arrival, he exhibited extensor posturing in response to oral care and painful stimuli. Pupillary, corneal, occulocephalic, and cough/gag reflexes were intact. CT of the brain without contrast showed no acute intracranial pathology. MRI brain showed pattern consistent with TLE rather than anoxic brain injury (Figure 2), and friends confirmed history of inhaled heroin use. He was started on vitamins C and E; coenzyme Q was considered but could not be obtained for therapeutic use.
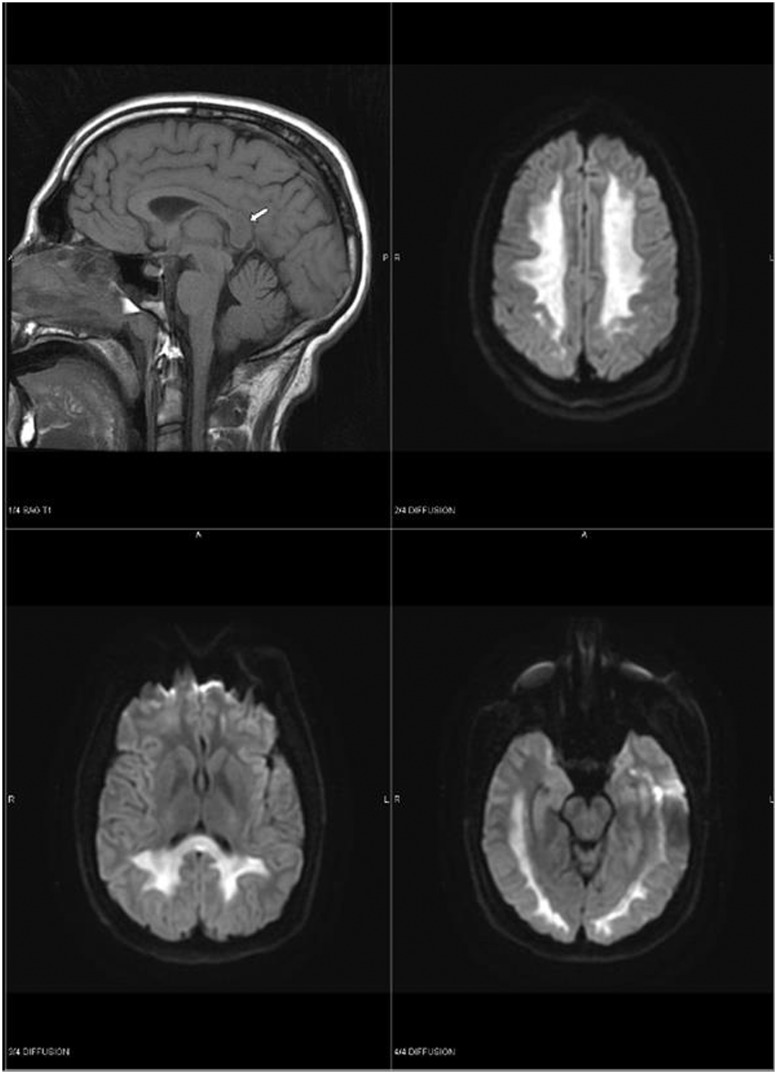
Figure 2.
MRI brain demonstrating extensive signal abnormality throughout supratentorial white matter, compatible with heroin-induced leukoencephalopathy (case 2).
The patient continued to have intermittent high-grade fever with hypertension, tachycardia, and posturing with some response to dantrolene. Video EEG showed evidence of diffuse slowing consistent with diffuse cortical dysfunction, but no epileptiform activity.
Tracheostomy and PEG tube were placed after one week. At the time of discharge to long-term care on hospital day 19, the patient displayed some slight eye opening and roving eye movements without change in responsiveness. Two months later, he had made little improvement.
Case 3
A 20-year-old male was brought in by ambulance after being found down with drug paraphernalia at his bedside. He had last been seen normal the night prior. On arrival, he was unresponsive and exhibiting extensor posturing to stimulation. He was intubated for airway protection. Toxicology was positive for opiates, benzodiazepines, cocaine, and cannabinoids. Friends confirmed the use of inhaled heroin.
Initial CT brain was concerning for hypoxic injury. MRI, however, demonstrated restricted diffusion throughout bilateral cerebral white matter including the corona radiata and corpus callosum more suggestive of TLE. Severe generalized slowing without epileptiform discharge was noted on EEG.
Autonomic symptoms were treated with cooling measures as well as meperidine, morphine, lorazepam, clonidine, and intermittent dexmedetomidine drip. On hospital day 7, the patient exhibited spontaneous eye opening, and at day 9, he appeared to track. Over the following week, he displayed small progressive improvements and at day 17 was able to intermittently follow commands and began to work with physical and occupational therapy. At the time of discharge to long-term care facility on day 34, he was speaking short sentences and tolerating a dysphagia diet.
As of eight months after discharge, the patient has recovered a great deal of motor function and is undergoing gait retraining. His cognition is grossly intact.
Discussion
A user “chasing the dragon,” places heroin in its non-salt freebase form (which is not soluble in water) onto a piece of aluminum foil and heats it from below. When heroin pyrolysate vapors begin to rise from the foil, these are “chased” by the user with a straw as they are inhaled.7
Despite the appearance of this method of use in the 1950s, the first major case series of heroin-induced TLE was reported in 1982 in the Netherlands (47 cases).13 Buxton et al. later described 27 cases in British Columbia with similar progression of symptoms.7
The typical time course described in these case series and others throughout the literature is a progressive one.6–10,12 Generally, heroin-induced TLE progresses through three stages over the course of weeks to months. The first stage is primarily cerebellar, typically including motor restlessness and cerebellar ataxia with pseudobulbar speech. This is followed by additional extrapyramidal symptoms such as myoclonic jerks and choreoathetoid movements, and pyramidal tract signs such as spastic paresis and hyperactive reflexes. Finally, approximately 25% of patients reach stage three with stretching spasms, akinetic mutism, extensor posturing, central pyrexia, and in some cases eventual death.8,14
MRI changes classically associated with TLE include symmetrically increased T2 and T2-FLAIR signal intensity of the cerebellar and posterior cerebral white matter.15 The posterior limb of the internal capsule is often affected, but the anterior limb and dentate nuclei are spared. These lesions do not enhance with contrast and are generally not diffusion restricted.12 Gray matter, which is extremely susceptible to hypoxic injury, is relatively spared.15 EEG reveals global slowing without epileptiform activity.8,14,16
In cases where brain biopsy is performed, histology reveals spongiform leukoencephalopathy with intracytoplasmic vacuoles.8,12 The cerebellum and limbic system, where opioid receptors have their highest concentration, tend to be preferentially affected.6
Early speculation regarding the etiology of “chasing the dragon” related TLE predominantly focused on the relative dearth of reported cases from East Asia where the practice originated.8,10 Although several large East Asian cohorts have since been identified, questions remain surrounding the relative rarity of this condition and its restriction to inhaled heroin use. This may be related to its vaporized pyrolysate form; however, the rarity of cases despite prevalence of the practice makes this unlikely.8
One case series identified in Canada included two case couples. This suggests substance-related risk factors, perhaps a “cutting agent” or adulterant. Per review of drugs seized by Canadian law enforcement smoked heroin is generally lower in purity than injected, as higher grade cuts char too quickly for effective smoking.7 However, evaluation of street heroin samples by other groups has failed to reveal neurotoxic substances.7,12 Based on the rarity of TLE and the occurrence of small epidemics, any responsible contaminant would have to be one added sporadically and late in the production process.12
TLE does not reliably occur in all persons exposed to the same heroin at the same time. Therefore, there may be a required genetic predisposition.12 The pathophysiology of TLE may be related to mitochondrial dysfunction causing oligodendrocyte apoptosis and demyelination. This thought underlies the use of antioxidants as therapeutic agents. One case–control study has identified mutations in cytochrome P450 2D6 as a potential risk factor.6 Other potential targets include caspase-9, c-jun, and mediators of the intrinsic apoptosis pathway as demonstrated in rat cerebellar granule cell preparations.17
The evidence for a dose-dependent relationship is thus far contradictory. Kriegstein et al.14 report progressive spongiform encephalopathy in three patients who used inhaled heroin together. They noted a striking difference in severity of presentation that was correlated with reported duration and frequency of use.14 Another series, however, reports a case following a single exposure.7
Relevance
Drug overdose-related deaths involving opioids have doubled since the year 2000. Heroin overdose-related deaths tripled between 2010 and 2014 alone, according to data from the CDC.18 This trend is reflected in the increase in intensive care unit admissions due to opioid overdose over the same period.19
Usage patterns have also shifted over the last two decades. Of heroin users surveyed in 2015, 30.26% smoked as compared with 17.25% who used a needle to inject. This represents a dramatic increase from 11.75% of users who responded that they had smoked heroin in 2002.20 This makes it likely that the incidence of heroin-associated TLE will also increase.
We present three cases of a previously undescribed subset of TLE cases, acute-onset toxic leukoencephalopathy associated with inhaled heroin vapor. None of the patients exhibited slowly progressive symptoms. All presented as acute overdose only hours after being seen normal, and in all cases, altered consciousness was initially attributed to residual drug. There was also initial concern for hypoxic brain injury; however, all three patients were breathing spontaneously on EMS arrival prompting further workup. Diagnosis of TLE secondary to inhaled heroin use was made after characteristic findings were noted on brain MRI. In all three cases, this diagnosis altered the course of treatment, including the use of antioxidant agents and prolongation of life-sustaining measures with expectation of slow neurologic recovery.
Patients “found down” after heroin overdose often present with decreased levels of consciousness that may persist for hours or days after admission. Due to poor prognosis after hypoxic brain injury, life-sustaining measures may be withdrawn early in the hospital course. Brain MRI is not generally included as part of routine workup for hypoxemic brain injury.21 In those with a suspected history of inhaled heroin use, especially those without a clear apneic period, TLE should be considered in the differential and MRI of the brain performed. Doing so may reveal an unsuspected heroin-associated TLE rather than hypoxemic injury, thus altering decisions about treatment and continuation of care.
Consent
Written consent was obtained from the patients included in this case series or from a representative if the patient was unable to provide consent.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Strang J, Griffiths P, Gossop M. Heroin smoking by “chasing the dragon”: origins and history. Addiction 1997; 92: 673–683; discussion 685–695. [DOI] [PubMed] [Google Scholar]
- 2.Gossop M, Griffiths P, Strang J. Chasing the dragon. J Subst Abuse Treat 1991; 8: 90–91. [PubMed] [Google Scholar]
- 3.Kramer TH, Fine J, Bahari B, et al. Chasing the dragon: the smoking of heroin and cocaine. J Subst Abuse Treat 1990; 7: 65. [DOI] [PubMed] [Google Scholar]
- 4.Des Jarlais DC, Friedman SR. AIDS and the use of injected drugs. Sci Am 1994; 270: 82–88. [DOI] [PubMed] [Google Scholar]
- 5.Treaster JB. Fearing AIDS, users of heroin shift to inhaling drug. The New York Times 1991. Available at https://www.nytimes.com/1991/11/17/nyregion/fearing-aids-users-of-heroin-shift-to-inhaling-drug.html (accessed 2 May 2018).
- 6.Bach AG, Jordan B, Wegener NA, et al. Heroin spongiform leukoencephalopathy (HSLE). Clin Neuroradiol 2012; 22: 345–349. [DOI] [PubMed] [Google Scholar]
- 7.Buxton JA, Sebastian R, Clearsky L, et al. Chasing the dragon – characterizing cases of leukoencephalopathy associated with heroin inhalation in British Columbia. Harm Reduct J 2011; 8: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordova JP, Balan S, Romero J, et al. “Chasing the dragon”: new knowledge for an old practice. Am J Ther 2014; 21: 52–55. [DOI] [PubMed] [Google Scholar]
- 9.Lefaucheur R, Lebas A, Gérardin E, et al. Leucoencephalopathy following abuse of sniffed heroin. J Clin Neurosci 2017; 35: 70–72. [DOI] [PubMed] [Google Scholar]
- 10.Singh R, Saini M. Toxic leucoencephalopathy after “chasing the dragon.”. Singapore Med J 2015; 56: e102–e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan TP, Algra PR, Valk J, et al. Toxic leukoencephalopathy after inhalation of poisoned heroin: MR findings. AJNR Am J Neuroradiol 1994; 15: 175–178. [PMC free article] [PubMed] [Google Scholar]
- 12.Tormoehlen LM. Toxic leukoencephalopathies. Psychiatr Clin North Am 2013; 36: 277–292. [DOI] [PubMed] [Google Scholar]
- 13.Wolters EC, van Wijngaarden GK, Stam FC, et al. Leucoencephalopathy after inhaling “heroin” pyrolysate. Lancet 1982; 2: 1233–1237. [DOI] [PubMed] [Google Scholar]
- 14.Kriegstein AR, Shungu DC, Millar WS, et al. Leukoencephalopathy and raised brain lactate from heroin vapor inhalation (“chasing the dragon”). Neurology 1999; 53: 1765–1773. [DOI] [PubMed] [Google Scholar]
- 15.Kumar Y, Drumsta D, Mangla M, et al. Toxins in brain! Magnetic resonance (MR) imaging of toxic leukoencephalopathy – a pictorial essay. Pol J Radiol 2017; 82: 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pirompanich P, Chankrachang S. Intravenous heroin-associated delayed spongiform leukoencephalopathy: case report and reviews of the literature. J Med Assoc Thai 2015; 98: 703–708. [PubMed] [Google Scholar]
- 17.Pu H, Su L, Miao N, et al. Involvement of Bax and caspase-9 in heroin-induced apoptosis in cerebellar granule neurons via C-Jun pathway activation. Int J Clin Exp Pathol 2016; 9: 773–788. [Google Scholar]
- 18.Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths – United States, 2000–2014. MMWR Morb Mortal Wkly Rep 2016; 64: 1378–1382. [DOI] [PubMed] [Google Scholar]
- 19.Stevens JP, Wall MJ, Novack L, et al. The critical care crisis of opioid overdoses in the United States. Ann Am Thorac Soc 2017; 14: 1803–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Survey of Drug Use and Health, 2017. Available at: https://www.samhsa.gov/data/sites/default/files/NSDUH-PillImages-2017.pdf (accessed 2 May 2018).
- 21.Edlow JA, Rabinstein A, Traub SJ, et al. Diagnosis of reversible causes of coma. Lancet 2014; 384: 2064–2076. [DOI] [PubMed] [Google Scholar]