Abstract
In the past few decades, cardiac ultrasound has become a widely available, easy-to-use diagnostic tool in many scenarios in acute cardiac care. The introduction of microbubbles extended its diagnostic value. Not long thereafter, several investigators explored the therapeutic potential of contrast ultrasound on thrombus dissolution. Despite large improvements in therapeutic options, acute ST elevation myocardial infarction remains one of the main causes of mortality and morbidity in the western world. The therapeutic effect of contrast ultrasound on thrombus dissolution might prove to be a new, effective treatment strategy in this group of patients. With the recent publication of human studies scrutinising the therapeutic options of ultrasound and microbubbles in ST elevation myocardial infarction, we have entered a new stage in this area of research. This therapeutic effect is based on biochemical effects both at macrovascular and microvascular levels, of which the exact working mechanisms remain to be elucidated in full. This review will give an up-to-date summary of our current knowledge of the therapeutic effects of contrast ultrasound and its potential application in the field of ST elevation myocardial infarction, along with its future developments.
Keywords: ST elevation myocardial infarction, echocardiography, microbubbles, microvascular obstruction, coronary thrombosis
Introduction
Due to its low cost, its bedside applicability and its non-invasiveness, cardiac ultrasound has developed into a widely used diagnostic tool in many heart diseases. The diagnostic possibilities increased even further with the introduction of microbubbles. In 1968, Gramiak and Shah were the first to report the use of microbubbles.1 In general, the microbubble currently consists of a high-molecular-weight, gas-filled core to prevent it from dissolving in the pulmonary vasculature. This gas is encapsulated in a lipid or albumin shell, depending on the manufacturer.2
The composition of microbubbles makes them very good scatterers of ultrasound in contrast to erythrocytes, resulting in enhancement of the ultrasound image, thereby increasing diagnostic possibilities.3
Recently, attention has shifted to potential therapeutic applications of contrast ultrasound not only in the field of cardiology, but also in cerebrovascular4 and peripheral vascular disease.5 It was known that local application of ultrasound can cause bioeffects.6,7 The addition of microbubbles lowers the threshold for these effects to take place, thereby potentially enhancing these bioeffects. It has been demonstrated in vitro and in vivo that contrast ultrasound has a potential therapeutic effect in acute ST elevation myocardial infarction (STEMI).
Coronary thrombosis
STEMI is most often caused by the acute formation of a thrombus blocking an epicardial coronary artery, and current treatment strategies are focused on the prompt revascularisation of the occluded coronary artery. According to American College of Cardiology/American Heart Association (ACC/AHA) and European Society of Cardiology (ESC) guidelines, the first choice of treatment is primary percutaneous coronary intervention (PCI).8,9 However, in rural areas, primary PCI is not available within 90 minutes of first patient contact. In those areas, thrombolysis is the next best therapy. Worldwide, thrombolysis remains the most used therapy in STEMI patients, despite its lower recanalisation rate and higher haemorrhagic complication rate compared to PCI. Hence, there is a continuing search for an easily applicable, non-invasive treatment strategy resulting in higher recanalisation rates with lower complication rates.
Pathophysiology
Low-frequency, high-intensity ultrasound
Hong et al. were one of the first to prove the potential for ultrasound-induced cavitation to dissolve thrombi in vitro.10 They used low-frequency, high-intensity ultrasound with different-sized probes to treat blood clots, and observed that all clots were disrupted in less than 3 minutes. The size of the particles of the disrupted blood clot ranged from 2.5 to 80 µm. The addition of Streptokinase®, a thrombolytic agent, did not seem to have a measurable effect on the particle size distribution. Another study performed by Rosenschein and colleagues confirmed these results in vitro.11 They also conducted an in vivo study in dogs, which led to a significant decrease in thrombus obstruction, without an increase in wall injury of the treated vessel.12
After these successful in vitro and in vivo studies with high-intensity ultrasound, two small non-randomized studies presented good results regarding safety, feasibility and thrombus dissolution based on Thrombolysis in Myocardial Infarction (TIMI) flow.13,14 However, since it was an invasive approach that did not significantly improve recanalisation rates compared to PCI, further development of high-intensity ultrasound to dissolve thrombi came to a halt.
Low-intensity, high-frequency ultrasound
A disadvantage of transcutaneous high-intensity, low-frequency ultrasound is the fact that it is not readily available in daily clinical practice. At lower intensities and long pulse durations, a number of studies have been conducted with these ultrasound settings to evaluate the effect on thrombi. It was demonstrated that low-intensity ultrasound alone was unable to enhance clot dissolution. However, on top of a thrombolytic agent (e.g. tissue plasminogen activator [t-PA]), it was observed that thrombolysis was accelerated compared to treatment of thrombus with t-PA alone.15
Cavitation
Thus, both high- and low-intensity ultrasound seem to be able to enhance clot dissolution.16 Nevertheless, both approaches have an essentially different working mechanism. Where high-intensity ultrasound is capable of clot dissolution on its own under the influence of inertial cavitation, this effect does not occur using low-intensity ultrasound. Instead, it was thought that low-intensity ultrasound causes stable cavitation, thus creating local microstreaming. While lower-intensity ultrasound on its own may not be capable of enhancing clot dissolution, it is effective at increasing local concentrations of exogenously administered t-PA, thereby enhancing thrombolysis. This was nicely shown in an in vitro study performed by Sakharov and colleagues.17 They demonstrated when ultrasound was applied without stirring that it accelerated lysis by about two-fold. However, in the presence of mild or strong stirring around the thrombus, ultrasound only slightly enhanced thrombolysis. The fact that ultrasound still enhanced thrombolysis in the presence of strong stirring was largely explained by the local temperature rise, which is another feature of the application of low-intensity ultrasound.18
Contrast ultrasound
Tachibana and Tachibana hypothesised that the addition of microbubbles would lower the threshold for cavitation when using low-intensity ultrasound.19 They tested this by creating fresh thrombi using whole venous blood drawn from a healthy volunteer. These thrombi were divided in three groups, with each group receiving a different treatment strategy: either Urokinase® alone, Urokinase® and ultrasound or Urokinase®, ultrasound and microbubbles. Low-intensity ultrasound was applied for 3 minutes at 170 kHz and 0.5 W/cm2 in an on–off sequence (2 seconds on and 4 seconds off). After treatment, the thrombi were incubated and weighed at different time periods. After 60 minutes, a significant difference was found between the three groups. Treatment with ultrasound alone resulted in an increase in thrombolysis, as expected. However, the addition of microbubbles on top of ultrasound and Urokinase® enhanced the thrombolytic effect even further.19
In 2007, Prokop et al. carried out an experiment to test which form of cavitation results in ultrasound-accelerated, t-PA-mediated fibrinolysis.20 Although inertial cavitation was detected at the start of the experiment with contrast ultrasound on top of t-PA, this was not the dominant mechanism for enhanced fibrinolysis. When the microbubbles were pre-treated with ultrasound to prevent inertial cavitation, the reduction in clot weight was the same without pre-treatment, confirming that stable cavitation is the key mechanism for ultrasound-enhanced fibrinolysis after the addition of microbubbles.20
The different composure of microbubbles might also influence the results. However, this has never been fully explored. Therefore, clinical trials today are performed using commercially available microbubbles.
Effect of pulse duration
These results were confirmed in a study performed by Petit et al.21 Additionally, they also demonstrated that, in order for stable cavitation to enhance fibrinolysis, a longer pulse length is needed. A longer pulse duration results in a higher temporal average intensity, which can sustain any induced microbubble cavitational activity. These in vitro results were confirmed in an in vivo study performed by Xie et al.22 Using a diagnostic ultrasound system, they demonstrated in an atherosclerotic pig model with STEMI that even a slightly longer pulse duration (20 µs) resulted in higher recanalisation rates (Figure 1). When using a diagnostic ultrasound system, it was observed that thrombus age and the angle of the transducer also had influences on the thrombolytic effect of contrast ultrasound.7
Figure 1.
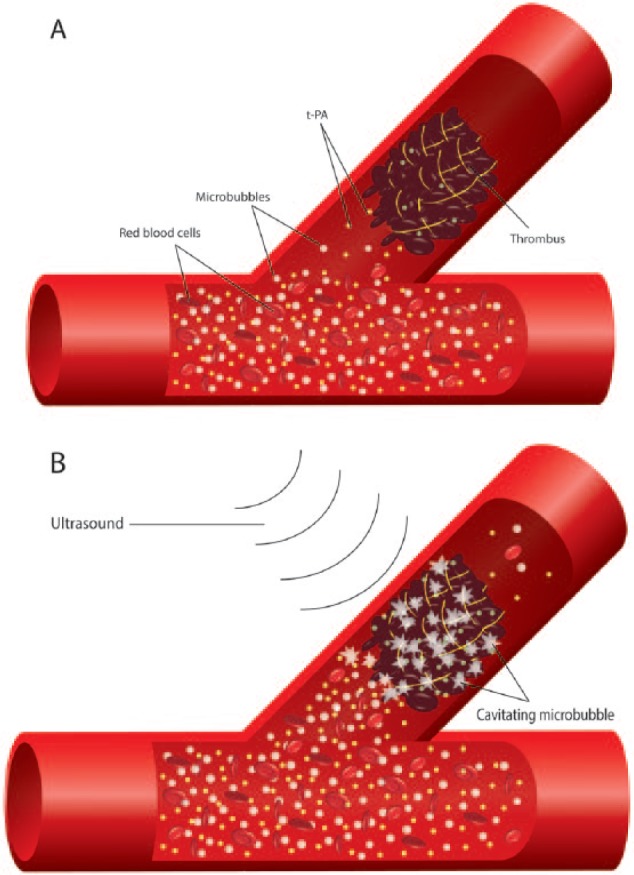
Macrovascular effect of ultrasound and microbubbles, resulting in increased concentrations of t-PA due to microstreaming and thrombus disruption due to cavitation. (A) illustrates a total occlusion of a coronary artery due to thrombus formation. (B) illustrates improvement of flow under the influence of ultrasound and cavitating microbubbles.
t-PA: tissue plasminogen activator.
All of these studies were performed with intermittent applications of ultrasound in order for microvascular replenishment to occur in between the high mechanical index (MI) impulses. The pulse durations were not prolonged further than 20 µs in order to prevent unwanted side effects, such as vascular wall damage and haemolysis, seen with longer pulse durations or continuous-wave ultrasound.12 There is another advantage of using image-guided, interrupted ultrasound, as it permits the visual assessment of the effect of treatment (Figure 2).
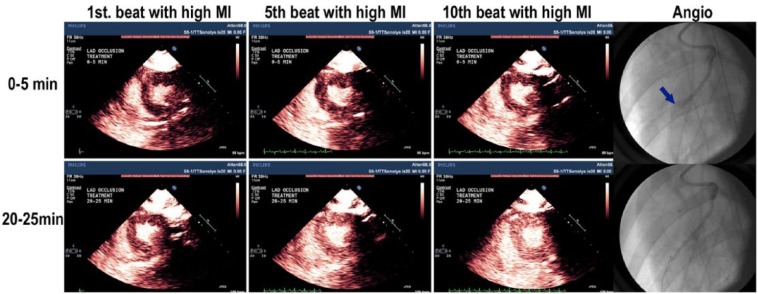
Figure 2.
Change in replenishment kinetics during treatment of an acute ST elevation myocardial infarction, confirmed on coronary angiography (total occlusion of the left anterior descending artery (LAD), pointed out by the arrow).21 (Courtesy of Dr TR Porter, University of Nebraska Medical Center, Omaha, Nebraska, USA). MI: mechanical index.
Despite these successful preliminary studies, the optimal ultrasound settings remain a matter of debate. In addition, when applying it clinically in acute STEMI, attenuation plays an important role, making it difficult to establish the optimal settings when translating these results to human studies.23
Targeted contrast ultrasound
Another potential advantage of contrast ultrasound is the possibility of targeting microbubbles to a site of interest, by conjugating the microbubble surface with agents that would adhere to upregulated receptors. At first, it was thought that microbubbles passed freely through the microcirculation. However, it was discovered that this was not the case in inflamed or injured tissue. Activated neutrophils and monocytes cause microbubbles with an albumin or phosphatidyl serine-containing lipid shell to bind and become phagocytosed. In this circumstance, the microbubble remains acoustically active and thus creates an increased ultrasound signal locally.24
More specific targeting strategies can be utilised to enhance thrombus dissolution with ultrasound and microbubbles (sonothrombolysis). By incorporating a single-chain antibody specific for activated glycoprotein IIb/IIIa into the lipid shell of the microbubble, the interaction with activated platelets can be enhanced, thereby locally increasing the concentration of microbubbles and thus increasing signal intensity.25 Positive results with targeted contrast ultrasound have already been demonstrated in an in vivo pig study by Xie et al.26 A recent study with both targeted ultrasound and targeted fibrinolytic agents in an in vivo mouse model demonstrated promising results not only with respect to increased fibrinolysis, but also with respect to lower bleeding risks.27,28
Clinical trials
The first sonothrombolysis trials in humans were performed with non-diagnostic ultrasound systems at low frequencies. Cohen and colleagues were the first to investigate the effect of ultrasound-enhanced thrombolysis in 25 patients with an acute STEMI.29 After patients were pre-treated with thrombolytics, low-frequency ultrasound was applied for 60 minutes, followed by a coronary angiogram. With 64% of the patients having TIMI III flow after treatment, results compared favourably with historical data.
However, the PLUS trial, a large, multicentre, randomised, clinical trial examining the effect of low-frequency ultrasound added to fibrinolytic agents, was halted after an interim analysis in 360 patients demonstrated a lack of treatment efficacy, both in TIMI flow grade as in ST segment resolution.30 No microbubbles or image-guided therapy were used in both trials.
Our group was the first to conduct a feasibility study using diagnostic ultrasound and commercially available microbubbles to the treatment protocol. Although it involved only 10 patients, this first trial demonstrated that sonothrombolysis was safe and feasible.31 Subsequent to this, Mathias Jr et al. published the results of a large clinical trial using contrast ultrasound with different ultrasound settings in acute STEMI patients.32 Prior to PCI, there was a significantly higher epicardial coronary recanalisation rate in the patient group receiving image-guided, high-mechanical index impulses. Interestingly, in contrast to in vitro and in vivo studies, even patients treated with a short pulse duration (3-4 µs) had a higher epicardial recanalisation rate. Microvascular flow also appeared improved, indicating that other factors, in addition to the sonothrombolytic effect, may be improving early and longer-term outcomes.32
Microvascular obstruction
The introduction of primary PCI improved revascularisation rates significantly for patients with STEMI. However, in up to 50% of patients, myocardial perfusion has remained poor in the area at risk.33 The causes of this persistent microvascular obstruction (MVO) can be categorised into four different pathogenetic components, which are distal embolisation, injury caused by ischemia, injury caused by reperfusion and individual predisposition. Contrast ultrasound may improve outcomes by affecting one or all of these components, and may potentially reduce the frequency of MVO in STEMI.
Pathophysiology
During a study aiming for epicardial recanalisation using contrast ultrasound in combination with thrombolysis, Xie et al. encountered an improvement in microvascular flow despite an absence of epicardial coronary recanalisation.34 It was therefore hypothesised that contrast ultrasound has a potential therapeutic effect on microvascular perfusion, reducing the effect of MVO. This was confirmed in a microvascular rat model by Leeman et al.35 In this study, the authors demonstrated that both intermittently applied higher mechanical index and longer pulse duration improved microvascular blood flow in a pure model of microvascular thrombotic obstruction. In a later study, using the same model, t-PA was added, resulting in an improvement of microvascular reperfusion.36 Recently, Belcik et al. confirmed these results in a hind limb ischemia mouse model using contrast ultrasound, and the authors observed that this improvement in microvascular flow lasted for more than 24 hours.37
Even though the exact mechanism underlying this improvement in microvascular flow might not be known, a fair amount of studies with contrast ultrasound have been conducted in order to explore the effect on cellular level and thereby its potential beneficial effect on MVO.
Nitric oxide
It is known that when the balance between nitric oxide (NO) synthesis and superoxide production falls in favour of superoxide production after reperfusion, this will result in vasoconstriction and exacerbation of the inflammatory state.38 Increasing the amount of NO release might counteract this imbalance after reperfusion. Therefore, it was hypothesised that vasodilation due to the release of NO was one of the main contributors to this improvement in local perfusion. This was subsequently demonstrated by pre-treating animals with L-Nω-nitroarginine methyl ester (L-NAME), a strong inhibitor of NO synthase. This time, tissue perfusion units (TPU) and pH did not improve under the influence of ultrasound.39 These results were confirmed in another in vivo study where the coronary arteries of nine dogs and five pigs were occluded, and local myocardial perfusion improved in both groups after treatment with low-frequency ultrasound.40
In a rat study using the same pure microvascular thrombosis protocol used by Leeman et al.,35 intermittent high-mechanical index impulses and 20-µs pulse duration ultrasound were applied through a tissue-mimicking phantom in order to simulate transthoracic attenuation, and this proved effective at improving microvascular flow during a commercially available microbubble infusion.41 In this study, when the NO synthase inhibitor L-NAME was administered, there was a higher perivascular haemorrhage rate. Thus, NO production induced by diagnostic ultrasound high MI impulses may be preventing unwanted bioeffects.41
In an animal study by Xie and colleagues, microbubbles were added on top of ultrasound and t-PA.26,34 They also detected an increase in microvascular flow in the peri-infarct zone, despite the absence of epicardial coronary recanalisation. Since ultrasound-induced microbubble cavitation causes local shear stress on both thrombus and endothelial borders, it was postulated that local mechanical forces stimulate endothelial cells to produce NO.42 This mechanism of stimulating the release of NO might be due to the production of intracellular hydrogen peroxide (H2O2). In a study performed by Juffermans et al., the production of intracellular H2O2 was detected in vitro after treatment of the endothelial cell with contrast ultrasound.43 This intracellular H2O2 has been shown to stimulate the production of NO by the activation of endothelial NO synthase, counteracting the imbalance between NO synthesis and superoxide production that occurs after reperfusion (Figure 3).44 The duration and intensity of cavitation induced by ultrasound, as well as the cellular environment in which cavitation occurs, may influence what bioeffect is observed. For example, if microbubbles are phagocytosed by neutrophil granulocytes, there may be amplification of superoxide generation, even prior to ultrasound-induced cavitation.45,46 However, once high-intensity ultrasound with a long pulse duration was applied, a decrease in superoxide generation was observed. Nevertheless, the application of high-intensity ultrasound caused an increase in apoptosis, membrane injury and complete cell destruction. This effect was stronger using albumin microbubbles compared to lipid microbubbles.45 Therefore, further investigation of the optimal ultrasound intensity setting in combination with microbubbles is needed, in addition to exploring what effect that surrounding cellular environment has on outcome.
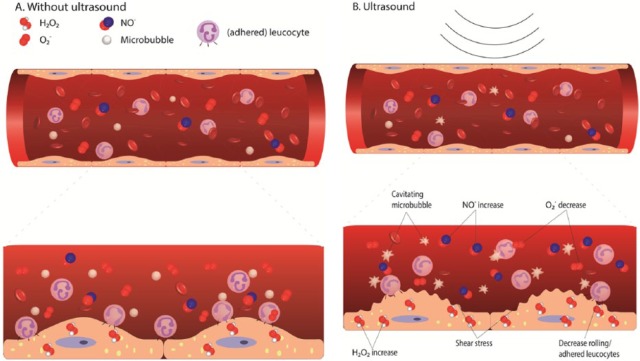
Figure 3.
Microvascular effect of ultrasound and microbubbles on an endothelial cell level, resulting in a decreased inflammatory response, leading to vasodilation through: (1) activation of shear stress resulting in an increase in intracellular H2O2, which results in the production of extracellular NO; (2) a decrease of superoxide; and (3) a decrease in rolling/adhered leukocytes. (A) The untreated situation without ultrasound; (B) treatment with ultrasound.
NO: nitric oxide; H2O2: hydrogen peroxide; O2−: superoxide.
Inflammatory response
Another important mechanism resulting in reperfusion injury is the activation of the inflammatory response. Under the influence of changes in the local mechanical forces and the increased local release of reactive oxygen species after reperfusion, there is an increase in leukocyte adhesion. These activated leukocytes in turn enhance the inflammatory response, can form aggregates with platelets and release vasoconstrictors like superoxide.47 Decreasing the inflammatory response might have a positive effect on the microcirculation after ischemia/reperfusion, resulting in a lower prevalence of MVO (Figure 3). In several in vivo studies, it has been demonstrated that application of ultrasound alone at the area where ischemia/reperfusion was created resulted in local vasodilation and a decrease in the number of rolling and adhered leukocytes. When microbubbles were added in this setting, ultrasound application further enhanced vasodilation and decreased the number of leukocytes.48–50
Interestingly, in an in vivo mouse model, no clear disruption of leukocytes could be observed after application of ultrasound with a mechanical index of 0.9 to phagocytosed microbubbles.51
Clinical trials
The first-in-human, Phase II trial has been performed by Roos et al.52 In this study aiming at improving microvascular reperfusion with a longer pulse duration (20 µs), ultrasound in combination with microbubbles was used to treat patients at up to a maximum of 15 minutes prior to PCI and 30 minutes post-PCI. Unfortunately, the study was discontinued after six patients, as the investigators encountered coronary spasm of the culprit vessel in 50% of the patients. These results were confirmed in a pig model when a blockage of the LAD was created by balloon injury and thrombus injection and treated with long-pulse duration ultrasound.52
Although contrast ultrasound has been shown to improve microvascular perfusion despite absent epicardial coronary recanalisation, the full mechanism of this effect still remains unclear. Furthermore, pulse duration seems to play an important role in enhancing microvascular perfusion. Nevertheless, it also results in local vasoconstriction. Further research is warranted in order to investigate the working mechanism, as well as the optimal settings.
Safety and feasibility
Concerns have also risen regarding possible adverse effects of the treatment of reperfusion injury using contrast ultrasound. It has been demonstrated that high-intensity, focused ultrasound results in platelet activation, especially in the presence of inertial cavitation.53,54 When microbubbles were added, it was demonstrated that only at very high microbubble concentrations could platelets be activated, and when ultrasound was applied, there was a significant further increase in platelet activation. It must be emphasised that these results were obtained in platelet-rich plasma and with high concentrations of microbubbles. With normal concentrations of microbubbles added to samples of whole blood, no significant increase in platelet activation was observed, not even after ultrasound application.55
Furthermore, it was also shown that high-intensity ultrasound can perforate normal vessel walls.12 Nevertheless, this effect was only observed at very high intensities in an in vitro study using tissue from a necropsy. In vivo studies did not encounter this adverse effect.
Another safety issue encountered was coronary spasm during contrast ultrasound with longer pulse durations during a Phase II trial, which was therefore halted at an early stage.52 The intensity and pulse duration seem to play important roles in both mechanical effects as well as adverse effects. Nevertheless, several in-human studies have been performed thus far, demonstrating safety and feasibility (Table 1).
Table 1.
Studies assessing the effect of ultrasound on epicardial recanalisation in ST elevation myocardial infarction patients.
| First author/reference | No. of patients | Contrast used | Drugs | US settings | Outcome |
|---|---|---|---|---|---|
| Cohen et al.29 | 25 | No | Reteplase or tenecteplase | 27 kHz, continuous wave, 0.9 W/cm2 | Safe and feasible |
| Hudson et al.30 | 360 | No | ASA, heparin or enoxaparin, tenecteplase | 28 kHz, pulsed wave, 0.38 W/cm2 | No improvement |
| Slikkerveer et al.31 | 10 | Yes | ASA, heparin, alteplase | 1.6 MHz, MI 1.18, pulse duration 5 µs | Safe and feasible |
| Roos et al.52 | 6 | Yes | ASA, heparin, ticagrelor | 1.6 MHz, MI 1.3, pulse duration 20 µs | Coronary spasm |
| Mathias Jr et al.32 | 30 | Yes | ASA, heparin, clopidogrel | 1.3/1.8 MHz, MI 1.1–1.3, pulse duration 3/5/20 µs | Safe and feasible |
ASA: acetylsalicylic acid; US: ultrasound; MI: mechanical index.
Future directions
With the recent publication of the positive results by Mathias Jr et al.,32 the next step is to initiate a larger, multicentre, randomised, clinical trial aiming to improve both epicardial coronary recanalisation as well as MVO using contrast ultrasound in STEMI patients. Since the group treated with high-mechanical index impulses and shorter pulse durations demonstrated a higher epicardial recanalisation rate without signs of coronary spasm, it seems to be appropriate to use these settings in future studies. In addition to ST resolution on an electrocardiogram and epicardial recanalisation on angiography, magnetic resonance imaging and myocardial contrast echocardiography might also be performed in order to examine the effects of these impulse on the subsequent frequency of MVO at specific time points following treatment.
Technical issues
When taking all of the different settings into consideration for reaching an optimal therapeutic effect and preventing serious side effects, it raises the question as to whether this new therapy is limited to centres with extensive experience. Since the largest improvement in epicardial thrombus dissolution can be obtained in the prehospital setting, care should be taken with future research that a protocol will be developed that can be performed in this prehospital setting by non-experienced users. The same goes for treatment of MVO using contrast ultrasound after primary PCI has been performed. This also calls for the development of a device, such as an ultrasound vest, which could be transported in the prehospital setting and is easy to use.
Summary
We focused on the therapeutic application of contrast ultrasound in STEMI. A considerable amount of research has been performed to demonstrate that contrast ultrasound enhances thrombolysis. Although the exact working mechanism remains to be elucidated, it is known that destruction of microbubbles and local application of ultrasound induces several bioeffects, resulting in enhanced thrombolysis. Multiple animal infarct studies confirmed the therapeutic application of contrast ultrasound in STEMI, and the first human studies show promising results.
The aforementioned animal studies also demonstrated that despite an absence of epicardial coronary recanalisation, the peripheral perfusion of the area at risk did improve, indicating microvascular effects that are independent of upstream vascular flow and obstruction. This may be mediated by cavitation-induced activation of purinergic pathways, leading to prolonged increases in NO production that have a positive influence on the imbalance between NO and superoxide production and subsequent inflammatory responses. There does appear to be an ultrasound dose–response curve in the setting of a microbubble infusion, in that inertial cavitation at a longer pulse duration may cause unwanted vasospasm in the presence of microbubbles. Pulse duration seems to be playing a major role in these side effects. Nevertheless, the local destruction of microbubbles under the influence of ultrasound might have a positive effect on MVO. Therefore, further research is warranted in order to explore the potential application of contrast ultrasound in patients suffering MVO after successful primary PCI.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
References
- 1. Gramiak R, Shah PM. Echocardiography of the aortic root. Invest Radiol 1968; 3: 356–366. [DOI] [PubMed] [Google Scholar]
- 2. Lindner JR. Microbubbles in medical imaging: Current applications and future directions. Nat Rev Drug Discov 2004; 3: 527–532. [DOI] [PubMed] [Google Scholar]
- 3. Dijkmans PA, Senior R, Becher H, et al. Myocardial contrast echocardiography evolving as a clinically feasible technique for accurate, rapid, and safe assessment of myocardial perfusion: the evidence so far. J Am Coll Cardiol 2006; 48: 2168–2177. [DOI] [PubMed] [Google Scholar]
- 4. Nacu A, Kvistad CE, Naess H, et al. NOR-SASS (Norwegian Sonothrombolysis in Acute Stroke Study): Randomized controlled contrast-enhanced sonothrombolysis in an unselected acute ischemic stroke population. Stroke 2017; 48: 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ebben HP, Nederhoed JH, Slikkerveer J, et al. Therapeutic application of contrast-enhanced ultrasound and low-dose urokinase for thrombolysis in a porcine model of acute peripheral arterial occlusion. J Vasc Surg 2015; 62: 477–485. [DOI] [PubMed] [Google Scholar]
- 6. WFUMB Symposium on Safety and Standardisation in Medical Ultrasound. Issues and Recommendations Regarding Thermal Mechanisms for Biological Effects of Ultrasound. Hornbaek, Denmark, 30 August–1 September 1991. Ultrasound Med Biol 1992; 18: 731–810. [PubMed] [Google Scholar]
- 7. Xie F, Lof J, Everbach C, et al. Treatment of acute intravascular thrombi with diagnostic ultrasound and intravenous microbubbles. JACC Cardiovasc Imaging 2009; 2: 511–518. [DOI] [PubMed] [Google Scholar]
- 8. American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions; O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61: e78–e140. [DOI] [PubMed] [Google Scholar]
- 9. Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC); Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33: 2569–2619. [DOI] [PubMed] [Google Scholar]
- 10. Hong AS, Chae JS, Dubin SB, et al. Ultrasonic clot disruption: An in vitro study. Am Heart J 1990; 120: 418–422. [DOI] [PubMed] [Google Scholar]
- 11. Rosenschein U, Bernstein JJ, DiSegni E, et al. Experimental ultrasonic angioplasty: Disruption of atherosclerotic plaques and thrombi in vitro and arterial recanalization in vivo. J Am Coll Cardiol 1990; 15: 711–717. [DOI] [PubMed] [Google Scholar]
- 12. Ernst A, Schenk EA, Gracewski SM, et al. Ability of high-intensity ultrasound to ablate human atherosclerotic plaques and minimize debris size. Am J Cardiol 1991; 68: 242–246. [DOI] [PubMed] [Google Scholar]
- 13. Hamm CW, Steffen W, Terres W, et al. Intravascular therapeutic ultrasound thrombolysis in acute myocardial infarctions. Am J Cardiol 1997; 80: 200–204. [DOI] [PubMed] [Google Scholar]
- 14. Rosenschein U, Roth A, Rassin T, et al. Analysis of coronary ultrasound thrombolysis endpoints in acute myocardial infarction (ACUTE trial). Results of the feasibility phase. Circulation 1997; 95: 1411–1416. [DOI] [PubMed] [Google Scholar]
- 15. Everbach EC, Francis CW. Cavitational mechanisms in ultrasound-accelerated thrombolysis at 1 MHz. Ultrasound Med Biol 2000; 26: 1153–1160. [DOI] [PubMed] [Google Scholar]
- 16. Datta S, Coussios CC, McAdory LE, et al. Correlation of cavitation with ultrasound enhancement of thrombolysis. Ultrasound Med Biol 2006; 32: 1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sakharov DV, Hekkenberg RT, Rijken DC. Acceleration of fibrinolysis by high-frequency ultrasound: The contribution of acoustic streaming and temperature rise. Thromb Res 2000; 100: 333–340. [DOI] [PubMed] [Google Scholar]
- 18. Wu J. Temperature rise generated by ultrasound in the presence of contrast agent. Ultrasound Med Biol 1998; 24: 267–274. [DOI] [PubMed] [Google Scholar]
- 19. Tachibana K, Tachibana S. Albumin microbubble echo-contrast material as an enhancer for ultrasound accelerated thrombolysis. Circulation 1995; 92: 1148–1150. [DOI] [PubMed] [Google Scholar]
- 20. Prokop AF, Soltani A, Roy RA. Cavitational mechanisms in ultrasound-accelerated fibrinolysis. Ultrasound Med Biol 2007; 33: 924–933. [DOI] [PubMed] [Google Scholar]
- 21. Petit B, Bohren Y, Gaud E, et al. Sonothrombolysis: The contribution of stable and inertial cavitation to clot lysis. Ultrasound Med Biol 2015; 41: 1402–1410. [DOI] [PubMed] [Google Scholar]
- 22. Xie F, Gao S, Wu J, et al. Diagnostic ultrasound induced inertial cavitation to non-invasively restore coronary and microvascular flow in acute myocardial infarction. PLoS One 2013; 8: e69780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xie F, Tsutsui JM, Lof J, et al. Effectiveness of lipid microbubbles and ultrasound in declotting thrombosis. Ultrasound Med Biol 2005; 31: 979–985. [DOI] [PubMed] [Google Scholar]
- 24. Lindner JR, Coggins MP, Kaul S, et al. Microbubble persistence in the microcirculation during ischemia/reperfusion and inflammation is caused by integrin- and complement-mediated adherence to activated leukocytes. Circulation 2000; 101: 668–675. [DOI] [PubMed] [Google Scholar]
- 25. Wang X, Hagemeyer CE, Hohmann JD, et al. Novel single-chain antibody-targeted microbubbles for molecular ultrasound imaging of thrombosis: Validation of a unique noninvasive method for rapid and sensitive detection of thrombi and monitoring of success or failure of thrombolysis in mice. Circulation 2012; 125: 3117–3126. [DOI] [PubMed] [Google Scholar]
- 26. Xie F, Lof J, Matsunaga T, et al. Diagnostic ultrasound combined with glycoprotein IIb/IIIa-targeted microbubbles improves microvascular recovery after acute coronary thrombotic occlusions. Circulation 2009; 119: 1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang X, Palasubramaniam J, Gkanatsas Y, et al. Towards effective and safe thrombolysis and thromboprophylaxis: Preclinical testing of a novel antibody-targeted recombinant plasminogen activator directed against activated platelets. Circ Res 2014; 114: 1083–1093. [DOI] [PubMed] [Google Scholar]
- 28. Wang X, Gkanatsas Y, Palasubramaniam J, et al. Thrombus-targeted theranostic microbubbles: A new technology towards concurrent rapid ultrasound diagnosis and bleeding-free fibrinolytic treatment of thrombosis. Theranostics 2016; 6: 726–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cohen MG, Tuero E, Bluguermann J, et al. Transcutaneous ultrasound-facilitated coronary thrombolysis during acute myocardial infarction. Am J Cardiol 2003; 92: 454–457. [DOI] [PubMed] [Google Scholar]
- 30. Hudson M, Greenbaum A, Brenton L, et al. Adjunctive transcutaneous ultrasound with thrombolysis: results of the PLUS (Perfusion by ThromboLytic and UltraSound) trial. JACC Cardiovasc Interv 2010; 3: 352–359. [DOI] [PubMed] [Google Scholar]
- 31. Slikkerveer J, Kleijn SA, Appelman Y, et al. Ultrasound enhanced prehospital thrombolysis using microbubbles infusion in patients with acute ST elevation myocardial infarction: Pilot of the Sonolysis study. Ultrasound Med Biol 2012; 38: 247–252. [DOI] [PubMed] [Google Scholar]
- 32. Mathias Jr W, Tsutsui JM, Tavares BG, et al. Diagnostic ultrasound impulses improve microvascular flow in patients with STEMI receiving intravenous microbubbles. J Am Coll Cardiol 2016; 67: 2506–2515. [DOI] [PubMed] [Google Scholar]
- 33. Niccoli G, Burzotta F, Galiuto L, et al. Myocardial no-reflow in humans. J Am Coll Cardiol 2009; 54: 281–292. [DOI] [PubMed] [Google Scholar]
- 34. Xie F, Slikkerveer J, Gao S, et al. Coronary and microvascular thrombolysis with guided diagnostic ultrasound and microbubbles in acute ST segment elevation myocardial infarction. J Am Soc Echocardiogr 2011; 24: 1400–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leeman JE, Kim JS, Yu FT, et al. Effect of acoustic conditions on microbubble-mediated microvascular sonothrombolysis. Ultrasound Med Biol 2012; 38: 1589–1598. [DOI] [PubMed] [Google Scholar]
- 36. Roos ST, Yu FT, Kamp O, et al. Sonoreperfusion therapy kinetics in whole blood using ultrasound, microbubbles and tissue plasminogen activator. Ultrasound Med Biol 2016; 42: 3001–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Belcik JT, Davidson BP, Xie A, et al. Augmentation of muscle blood flow by ultrasound cavitation is mediated by ATP and purinergic signaling. Circulation 2017; 135: 1240–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carden DL, Granger DN. Pathophysiology of ischaemia–reperfusion injury. J Pathol 2000; 190: 255–266. [DOI] [PubMed] [Google Scholar]
- 39. Suchkova VN, Baggs RB, Sahni SK, et al. Ultrasound improves tissue perfusion in ischemic tissue through a nitric oxide dependent mechanism. Thromb Haemost 2002; 88: 865–870. [PubMed] [Google Scholar]
- 40. Siegel RJ, Suchkova VN, Miyamoto T, et al. Ultrasound energy improves myocardial perfusion in the presence of coronary occlusion. J Am Coll Cardiol 2004; 44: 1454–1458. [DOI] [PubMed] [Google Scholar]
- 41. Porter TR, Radio S, Lof J, et al. Diagnostic ultrasound high mechanical index impulses restore microvascular flow in peripheral arterial thromboembolism. Ultrasound Med Biol 2016; 42: 1531–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Altland OD, Dalecki D, Suchkova VN, et al. Low-intensity ultrasound increases endothelial cell nitric oxide synthase activity and nitric oxide synthesis. J Thromb Haemost 2004; 2: 637–643. [DOI] [PubMed] [Google Scholar]
- 43. Juffermans LJ, Dijkmans PA, Musters RJ, et al. Transient permeabilization of cell membranes by ultrasound-exposed microbubbles is related to formation of hydrogen peroxide. Am J Physiol Heart Circ Physiol 2006; 291: H1595–H1601. [DOI] [PubMed] [Google Scholar]
- 44. Cai H, Li Z, Dikalov S, et al. NAD(P)H oxidase-derived hydrogen peroxide mediates endothelial nitric oxide production in response to angiotensin II. J Biol Chem 2002; 277: 48311–48317. [DOI] [PubMed] [Google Scholar]
- 45. Korosoglou G, Hardt SE, Bekeredjian R, et al. Ultrasound exposure can increase the membrane permeability of human neutrophil granulocytes containing microbubbles without causing complete cell destruction. Ultrasound Med Biol 2006; 32: 297–303. [DOI] [PubMed] [Google Scholar]
- 46. Korosoglou G, da Silva KG, Hansen A, et al. Ultrasound contrast agents can influence the respiratory burst activity of human neutrophil granulocytes. Ultrasound Med Biol 2004; 30: 75–81. [DOI] [PubMed] [Google Scholar]
- 47. Schwartz BG, Kloner RA. Coronary no reflow. J Mol Cell Cardiol 2012; 52: 873–882. [DOI] [PubMed] [Google Scholar]
- 48. Camarozano AC, Garcia de Almeida Cyrino FZ, Bottino DA, et al. Effects of microbubbles and ultrasound on the microcirculation: Observation on the hamster cheek pouch. J Am Soc Echocardiogr 2010; 23: 1323–1330. [DOI] [PubMed] [Google Scholar]
- 49. Bertuglia S, Giusti A, Picano E. Effects of diagnostic cardiac ultrasound on oxygen free radical production and microvascular perfusion during ischemia reperfusion. Ultrasound Med Biol 2004; 30: 549–557. [DOI] [PubMed] [Google Scholar]
- 50. Bertuglia S. Mechanisms by which low-intensity ultrasound improve tolerance to ischemia–reperfusion injury. Ultrasound Med Biol 2007; 33: 663–671. [DOI] [PubMed] [Google Scholar]
- 51. Lindner JR, Dayton PA, Coggins MP, et al. Noninvasive imaging of inflammation by ultrasound detection of phagocytosed microbubbles. Circulation 2000; 102: 531–538. [DOI] [PubMed] [Google Scholar]
- 52. Roos ST, Juffermans LJ, van Royen N, et al. Unexpected high incidence of coronary vasoconstriction in the Reduction of Microvascular Injury Using Sonolysis (ROMIUS) trial. Ultrasound Med Biol 2016; 42: 1919–1928. [DOI] [PubMed] [Google Scholar]
- 53. Poliachik SL, Chandler WL, Mourad PD, et al. Activation, aggregation and adhesion of platelets exposed to high-intensity focused ultrasound. Ultrasound Med Biol 2001; 27: 1567–1576. [DOI] [PubMed] [Google Scholar]
- 54. Poliachik SL, Chandler WL, Ollos RJ, et al. The relation between cavitation and platelet aggregation during exposure to high-intensity focused ultrasound. Ultrasound Med Biol 2004; 30: 261–269. [DOI] [PubMed] [Google Scholar]
- 55. Shigeta K, Taniguchi N, Omoto K, et al. In vitro platelet activation by an echo contrast agent. J Ultrasound Med 2003; 22: 365–373. [DOI] [PubMed] [Google Scholar]