Abstract
In biology, models are experimental systems meant to recreate aspects of diseases or human tissue with the goal of generating inferences and approximations that can contribute to the resolution of specific biological problems. Although there are many models for studying intracellular parasites, their data have produced critical contradictions, especially in immunological assays. Peripheral blood mononuclear cells (PBMCs) represent an attractive tissue source in pharmacogenomics and in molecular and immunologic studies, as these cells are easily collected from patients and can serve as sentinel tissue for monitoring physiological perturbations due to disease. However, these cells are a very sensitive model due to variables such as temperature, type of stimulus and time of collection as part of posterior processes. PBMCs have been used to study Toxoplasma gondii and other apicomplexan parasites. For instance, this model is frequently used in new therapies or vaccines that use peptides or recombinant proteins derived from the parasite. The immune response to T. gondii is highly variable, so it may be necessary to refine this cellular model. This mini review highlights the major approaches in which PBMCs are used as a model of study for T. gondii and other apicomplexan parasites. The variables related to this model have significant implications for data interpretation and conclusions related to host-parasite interaction.
Keywords: PBMCs (peripheral blood mononuclear cells), immunologic research, toxoplasma gondii, model of study, apicomplexa
Introduction
The phylum Apicomplexa consists of approximately 6,000 species of intracellular protozoan parasites, including various important human and animal pathogens such as Plasmodium, the causative agent of malaria; Cryptosporidium, the causative agent of cryptosporidiosis; Theileria, Babesia and Eimeria, which are important pathogens in cattle and fowl; and T. gondii, which is responsible for toxoplasmosis in birds, marsupials and mammals including humans (Tenter et al., 2000; Dubey, 2010). T. gondii has emerged as a model system for the study of intracellular parasitism; it is one of the most studied parasites due to its medical and veterinary importance, its wide range of distribution and its suitability as a model of study in pharmacogenomics, cell biology, molecular genetics and immunology. T. gondii infections are generally subclinical in healthy individuals but can be major problems for immunosuppressed adults and fetuses (Dubey, 2008). The severity of such infections can vary greatly, perhaps based on the status of the host immune system (Lahmar et al., 2009), the genotype of the infective parasite strain (Ferreira et al., 2011) and the host's genetic background (Sullivan and Jeffers, 2012). There has been significant progress, but no vaccine is currently available that will prevent T. gondii infection; indeed, very few drugs effectively reduce T. gondii's presence in infected individuals (Zhou et al., 2016a). Researchers frequently select models for studying T. gondii based on their similarity to humans in terms of genetics, anatomy, and physiology; this includes the cellular models that have been used to study T. gondii (Szabo and Finney, 2017). However, many studies make use of mouse models (Alfonzo et al., 2002; Martens et al., 2005; Tanaka et al., 2013; Unno et al., 2013; Dzitko et al., 2015; He et al., 2015). There have been highly controversial results associated with some of these models because they do not completely mimic human toxoplasmosis (Hunter and Sibley, 2012; Niedelman et al., 2012; Seok et al., 2013). In fact, some scientists have argued that new approaches must be explored; some have even proposed new models for studying T. gondii (Cornelissen et al., 2014; Tanaka et al., 2016; Nau et al., 2017).
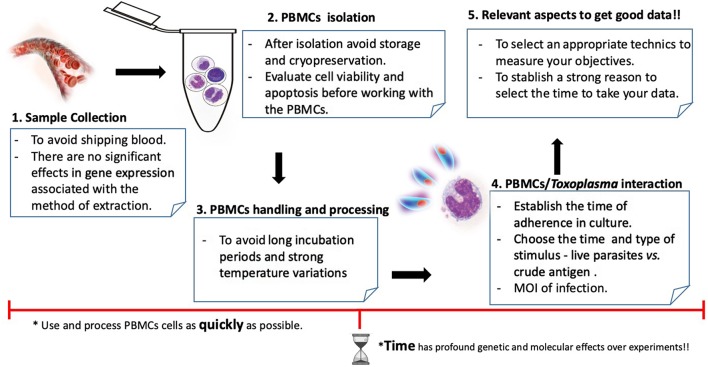
The PBMC cellular model includes T and B cells (~80%), natural killer cells (~10%) and monocytes (~10%) (Autissier et al., 2010). These blood cells play an important role in the immune response that is meant to preserve the host's homeostasis and defend it against parasite infection (Zhou et al., 2016a). Researchers have used PBMC to study T. gondii with various goals, but especially to improve diagnostic, drug-screening and immunogenetic approaches (Vendrell et al., 1992; Dzitko et al., 2015). Although PBMCs do not completely mimic an infection in vivo, they can be taken directly from affected individuals and can generate certain qualities that when added to the recommendations (Figure 1) discussed below can improve the quality of the experimental data regarding toxoplasmosis. Therefore, understanding and improving models is imperative to the appropriate interpretation and translation of this work into clinical setting (Szabo and Finney, 2017). In this review, we present a summary of how PBMCs have been used to study T. gondii and other apicomplexan parasites, discuss some controversies related to this cellular model and then describe possible improvements to the related protocols.
Figure 1.
Workflow and aspects to keep in mind to work with PBMCs and Toxoplasma gondii.
Immune Response in PBMCs Stimulated With T. gondii
PBMCs have mainly been used to model T. gondii as part of the evaluation of potential new vaccines or drugs, as well as to understand the relationships between the host's immune system and the parasite (see Table 1). The studies using these models have shown that cytokine levels can vary according to the evaluated clinical condition, the type of strain and the culture conditions which can include the type of media culture supplement; the time of data collection; and the temperature variations during storage, shipping and handling (Weinberg et al., 2010). In chronic asymptomatic individuals, the PBMCs' immune responses against total lysate antigen and against peptides derived from T. gondii are predominantly characterized by high levels of interferon gamma (IFN-γ) (Prigione et al., 2006; Bayram Delibaş et al., 2009; Cong et al., 2011; Cardona et al., 2015; Meira et al., 2015); in ocular toxoplasmosis and Toxoplasma-seronegative individuals, however, the level of this cytokine is much lower (Alfonzo et al., 2005; Meira et al., 2014; Maia et al., 2017). PBMCs have also been useful in studying the immune response of HIV-infected individuals and of pregnant women with toxoplasmosis. In one study on HIV patients who had been coinfected with T. gondii, researchers measured the IFN-γ expression of stimulated total lysate antigen using PBMCs, both before and after treatment with antiparasitic drugs (sulfadiazine, pyrimethamine, folinic acid, trimethoprim-sulfa-methoxazole, and corticosteroids); the infection's evolution was correlated with the restoration of the IFN-γ response and with decreased inflammation (Meira et al., 2015). In another study, researchers showed that, during pregnancy, tumor necrosis factor alpha (TNF-α) and interleukin (IL)-12 had decreased expression when cells were stimulated with live tachyzoites (Rezende-Oliveira et al., 2012). Interestingly, the addition of the prolactin hormone to the cells seemed to restrict the parasite's proliferation (Dzitko et al., 2012). In similar works, researchers have shown the importance of IFN-γ production in the congenital transmission of T. gondii through the upregulation of intercellular adhesion molecular 1 (ICAM-1) (Pfaff et al., 2005). Stimulating PBMCs with complete or partial antigens of T. gondii seems to reveal important aspects of the host's immune response. However, T. gondii and other apicomplexans secrete proteins in a highly regulated manner that is involved in the parasite's immune evasion mechanisms (Tosh et al., 2016). These processes are not seen when parasite antigens are used, so we recommend the use of live parasites to stimulate PBMCs (Figure 1).
Table 1.
Characteristics and use of PBMC as a study model for T. gondii and other Apicomplexan parasites during the last 10 years.
| Organism | Technical observations | PBMC culture (number of cells/final volume/culture plates) | Main findings | References |
|---|---|---|---|---|
| Human PBMC and Toxoplasma gondii |
Stimulus: RH strain (TLA, 1 μg/mL) Technic: RT-qPCR Process time: 48 h after collection Cryopreserved: No Supplemented: 10% FCS |
1 × 106/500 μL/48-well | High levels of TGF-β, IL-6, IL-10 in OT individuals | Maia et al., 2017 |
|
Stimulus: RH strain (TLA, 1 μg/mL) Technic: RT-qPCR Process time: 48 h after collection Cryopreserved: No Supplemented: 10% FBS |
1 × 106/500 μL/48-well | TATA box-binding protein (TBP) and ubiquitin C (UBC) are the most stable genes for mRNA analysis in PBMCs | Meira-Strejevitch et al., 2017 | |
|
Stimulus: ND Technic: Radioactivity Process time: 168 h in incubation Cryopreserved: No Supplemented: 5% human AB serum |
ND | Despite high proliferation, lymphocytes from meth users had a lower proliferative capacity | Massanella et al., 2015 | |
|
Stimulus: Peptides from P30 and ROP 18 (10 μg/mL) Technic: ELISPOT Process time: 24 h in incubation Cryopreserved: Yes Supplemented: No |
2 × 105/100 μL/96-well | Four peptides induced IFN-γ expression | Cardona et al., 2015 | |
|
Stimulus: RH strain (TLA, 1 μg/mL) Technic: ELISA Process time: 48 h in incubation Cryopreserved: No Supplemented: 10% FBS |
1 × 106/500 μL/48-well | Restoration of IFN-γ response and a decrease of the inflammatory cytokines TNF-α and IL-10 | Meira et al., 2015 | |
|
Stimulus: RH strain (Live) Technic: Radioactivity Process time: ND Cryopreserved: ND Supplemented: ND |
2.5 × 105/100 μL/96-well | Phytoecdysteroids did not inhibit Toxoplasma and did not affect the cytokine response (IFN-γ. IL-12, IL-10) | Dzitko et al., 2015 | |
|
Stimulus: TLA (ND) Technic: ELISPOT Process time: 120 h in incubation Cryopreserved: No Supplemented: No |
3 × 105/200 μL/ND | No association was observed when PBMCs were stimulated with TLA or mitogen | Nogueira et al., 2014 | |
|
Stimulus: BK strain (Live) Technic: ELISA Process time: 48 h in incubation Cryopreserved: No Supplemented: 0.1% BSA |
2.5 × 106/ND/ND | Correlation between Prolactine and the level of IL-10, but not with IFN-γ | Dzitko et al., 2012 | |
|
Stimulus: RH and ME49 strains (Live) Technic: ELISA Process time: 48 h in incubation Cryopreserved: No Supplemented: No |
2 × 106/ND/24-well | T. gondii-seronegative non-pregnant women produced significantly higher levels of TNF-a and IL-12 | Rezende-Oliveira et al., 2012 | |
|
Stimulus:
T. gondii peptides Technic: ELISPOT Process time: ND Cryopreserved: Yes Supplemented: No |
2 × 105/100 μL/96-well | Peptides induced significant IFN-γ production by PBMCs from 4 HLA-A*0201 persons infected with T. gondii | Cong et al., 2011 | |
|
Stimulus: TRRH strain (TLA, 5 mg/mL) Technic: ELISA Process time: 72 h in incubation Cryopreserved: No Supplemented: 10% FBS |
1 × 106/200 μL/96-well | IL-5 was higher than IFN-γ in the initial phase of the infection; as the IgG started to rise, IFN-γ increased and suppressed the synthesis of IL-5 | Bayram Delibaş et al., 2009 | |
| Pigs PBMC and Toxoplasma gondii |
Stimulus: IPB-G/LR strain (TLA, 10 μg/mL) Technic: Flow cytometry Process time: 72 h in incubation Cryopreserved: No Supplemented: 10% FBS |
1 × 106/ND/ND | High levels of IFN-γ | Jennes et al., 2017 |
|
Stimulus: RH strain (TLA) Technic: RNAseq Process time: 8, 24, 48 h in incubation Cryopreserved: No Supplemented: 10% FBS |
ND | More than 2,400 differentially expressed genes | Zhou et al., 2016a | |
| Human PBMC and Plasmodium sp. |
Stimulus:
P. falciparum crude lysate (50 mg) Technic: Flow cytometry and RT-qPCR Process time: 168 h in incubation Cryopreserved: No Supplemented: 10% FCS |
1 × 106/ND/ND | Crude antigens exhibited strong heterogeneity in the cytokine production | Kijogi et al., 2018 |
|
Stimulus:
P. falciparum crude lysate (50 mg) Technic: Flow cytometry and RT-qPCR Process time: 10 h in incubation Cryopreserved: No Supplemented: 2% FCS |
ND | Decreased parasite growth and expression of PD-1 and IL-10 genes using L-citrulline supplemented media | Awasthi et al., 2017 | |
|
Stimulus: Peptide Pooling Scheme Technic: ELISPOT Process time: 18 h in incubation Cryopreserved: ND Supplemented: 10% FCS |
1 × 106/ND/96-well | Highest immunogenicity was identified at 7 days after boosting with 932 SFC compared with 57 SFC among control vaccinees | Mensah et al., 2016 | |
|
Stimulus: Recombinant RAMA protein Technic: ELISA Process time: ND Cryopreserved: ND Supplemented: ND |
ND | High levels of interferon (IFN)-γ and interleukin (IL)-10 cytokines were detected | Changrob et al., 2016 | |
|
Stimulus: Peptides from CSP and AMA1 protein (10 μg/ml) Technic: ELISPOT Process time: 36 h in incubation Cryopreserved: No Supplemented: No |
1 × 106/100 μL/96-well | CSP and AMA1 peptides recalled IFN-γ responses from naturally exposed individuals | Ganeshan et al., 2016 | |
|
Stimulus: TLR1/2 ligand PAM3CSK4 (20 ng) Technic: ND Process time: 72 h in incubation Cryopreserved: Yes Supplemented: ND |
2 × 105/100 μL/96-well | IL-1β and TNF-α were significantly higher in severe malaria cases compared with healthy controls | Manning et al., 2016 | |
|
Stimulus: CelTOS (10 ng) or other single (1.25 μg/ml) peptide pools Technic: ELISPOT Process time: 36 h in incubation Cryopreserved: No Supplemented: No |
4 × 105/100 μL/96-well | Natural malaria transmission induces CelTOS-specific ex vivo IFN-γ | Anum et al., 2015 | |
| Calves PBMC and Cryptosporidium parvum |
Stimulus: Recombinant C. parvum p23 vaccine antigen Technic: Flow Cytometry Process time: ND Cryopreserved: ND Supplemented: ND |
ND | Recombinant p23 vaccine antigen can stimulate a Type-1-like immune response | Wyatt et al., 2005 |
| Human PBMC and Eimeria sp. |
Stimulus: Recombinant Eimeria Antigen (rEA) Technic: Flow Cytometry Process time: 72 h in incubation Cryopreserved: ND Supplemented: 5%FCS |
25 × 106/ND/6-well | rEA stimulates human NK cell effector functions including increasing levels of IFN-γ and Granzyme B | Aylsworth et al., 2013 |
| Sheep PBMC and Babesia sp. |
Stimulus: BdE or BQ1E (10 μg/well) Technic: ELISA Process time: 120 h in incubation Cryopreserved: No Supplemented: 10% autologous plasma |
2 × 105/ND/96-well | Production of IFN-γ and IL10 have key roles in the course of infection by Babesia sp. | Guan et al., 2010 |
| Bovines PBMC and Theileria sp. |
Stimulus: Sporozoites in homogenized infected tick Technic: RT-qPCR Process time: 48 h in incubation Cryopreserved: No Supplemented: 40% FBS |
4 × 106/ND/6-well | MHC-DQ, SIRPA, PRNP, TLR10, cMAF and MAFB genes showed no change in mRNA expression after T. annulata infection | Panigrahi et al., 2016 |
|
Stimulus: Sporozoites in homogenized infected tick Technic: RT-qPCR Process time: 48 h in incubation Cryopreserved: No Supplemented: 40% FBS |
2 × 106/ND/6-well | Up-regulation in SIRPA, PRNP and MHC DQα genes and down-regulation in TLR10, cMAF and MAFB genes in crossbreds as compared to indigenous cattle was observed | Dewangan et al., 2015 | |
|
Stimulus: MPSP Peptides Technic: ELISPOT Process time: 42 h in incubation Cryopreserved: Yes Supplemented: No |
1 × 106/100 μL/96-well | IFN-γ and IL-10 were detected in infected Holsteins but weak responses were exhibited by infected Angus and Japanese Black cattle | Yamaguchi et al., 2010 |
TLA, T. gondii Lysate Antigen; ND, Not determine; OT, Ocular Toxoplasmosis; NC, Negative Control; FCS, Fetal Calf Serum; MPSP, Major piroplasm surface protein; RAMA, Rhoptry-associated membrane antigen; SFC, Spot Forming Cells (SFC)/106; CSP, Plasmodium falciparum circum- sporozoite protein; AMA-1, Apical membrane antigen-1.
On the other hand, the vaccine candidates for T. gondii are typically parasite proteins or the peptides that elicit protective immune responses in mice. However, vaccine candidates that are effective in mice are not necessarily effective in humans. PBMCs are potentially very useful tools for identifying and characterizing novel vaccine candidates for T. gondii. Cells from individuals with varied genetic and immunological backgrounds can be easily isolated and stimulated with the antigens of interest, thus allowing measurement of the desired cytokine profile or cell response. However, to our knowledge, few researchers have used this strategy (Tan et al., 2010; Cong et al., 2012; Cardona et al., 2015). The studies mentioned above have identified novel parasite derived peptides that induce strong production of IFN-γ in people who express one of the most common human leukocyte antigen (HLA) supertypes (HLA-A02, HLA-B07, and HLA-A11), making those peptides attractive vaccine candidates. PBMCs have also been used to evaluate the efficacy of two phytoecdysteroids (α-ecdysone and 20-hydroxyecdysone) in controlling T. gondii infections. These drugs are effective against Babesia gibsoni but have no effect on T. gondii's proliferation and do not elicit a Th1 protective immune response against the parasite (Dzitko et al., 2015). Thus, all these studies show that PBMCs may represent an important and undervalued model for vaccine and drug development.
Technical Aspects to Consider Before Working With PBMCs and T. gondii
To obtain scientifically valid data, the experimental conditions and the study's model must be closely controlled. PBMCs are one of the best sources for assessing the differences or changes associated with diseases or with drug responses and therapies; in addition, these cells are relatively easy to obtain from whole blood through isolation (Burczynski and Dorner, 2006). A major challenge in the monitoring of PBMCs' quality is establishing protocols that define the proper isolation, shipping and storage methods so that they can be tested without changes in cellular functionality. For researchers who work with PBMCs, it is very important to note that many variables affect these cells. This model can be used to study Toxoplasma under clinical or non-clinical conditions; however, the whole procedure—from blood withdrawal to experimentation—must be highly standardized. PBMCs are perishable living cells, and some of them begin to die immediately after their isolation from whole blood. Scientists have compared isolation techniques, but they have found no differences with respect to the generally used methods, which include Ficoll-Paque density-gradient centrifugation and BD Vacutainer cell-preparation tubes (Corkum et al., 2015).
However, after isolation of PBMC, early apoptotic events are present in both in vitro and ex vivo experiments; as a result, it is very important to determine apoptosis before using these cells in the experiments (Wunsch et al., 2015). Various methods have been used to measure apoptosis, including the YO-PRO-1/7-AAD method, which has been proposed as a good, low-cost alternative for sensitive detection of early apoptosis in PBMCs and >80% of viability is recommended before starting to work (Glisic-Milosavljevic et al., 2005). Working with freshly isolated PBMCs is not always possible; thus, the cells are generally frozen and thawed for processing at later times (see Table 1); this allows for the batched thawing of samples and for direct comparability in assays, thus reducing inter-assay variability and allowing for future analysis of later-emerging issues (Riedhammer et al., 2016). As a consequence, both apoptosis and necrosis happen; this phenomenon has been well documented to occur during cryopreservation (Fowke et al., 2000; Baust, 2002; Cosentino et al., 2007; Mallone et al., 2011). For example, in a recent study on how storage temperature affects PBMCs and cryopreservs PBMCs' viability, recovery and gene expression patterns were all affected, as compared to those of freshly isolated PBMCs (Yang et al., 2016). In a cell infected with Toxoplasma, each hour represents a specific differential gene-expression profile (He et al., 2015; Zhou et al., 2016b), which indicates that the best possible method for handling missing data is to prevent the problem by properly planning each study and by collecting the data carefully (Wisniewski et al., 2006). The goal is to eventually have studies with comparable data. Along the same order of ideas, the time variable is important to consider when working with PBMCs, as it can lead to missing data. The problem of missing data is relatively common in most fields of research, and it can significantly affect the conclusions drawn from the data (Little et al., 2012). Accordingly, some medical researchers have focused on handling missing data and related problems using methods that prevent or minimize missing data (O'Neill and Temple, 2012). One of the main problems with using the PBMC model for T. gondii is that the majority of studies are not comparable, as researchers do not usually consider the time aspect when obtaining data. Most studies have shown that the time spent collecting data from cells after a stimulus varies; for example, no one has argued that a supernatant for cytokine measurement should be performed in an exact period of time. In the bulk of the studies, there are differences in the time accorded to the PBMC culture (varying from 48 h to 7 days) and even in the collection of supernatant for the measurement of IFN-γ after 12, 24 or 48 h (see Table 1). In this sense, the time between the making of the culture and the collection of data should be methodologically explained.
PBMCs in Other Apicomplexan Parasites
Although apicomplexans comprise a large phylum of parasitic organisms (with more than 5,000 species), only a few have been studied in detail. Most of these studies focus on parasites that produce disease in humans such as T. gondii, Plasmodium spp. and Cryptosporidium. There are fewer studies on parasites that do not affect humans directly (e.g., Eimeria, Babesia, and Theileria). One of the most studied apicomplexan parasites is Plasmodium, the pathogen that causes malaria, one of the most important public health problems worldwide. Nearly all studies regarding this parasite that have used PBMCs have focused on the development of vaccine candidates, particularly those using the peptide polling scheme (Anum et al., 2015; Ganeshan et al., 2016; Mensah et al., 2016) or recombinant proteins (Garraud et al., 2002; Garg et al., 2008; Gitau et al., 2014; Changrob et al., 2016). These studies have shown that, when PBMCs are challenged with molecules derived from Plasmodium, the immune response is characterized by Th1 cytokines such as IFN-γ, IL-1β, and TNF-α. The nuclear transcription factor kappa B (NF-kB) is what mainly regulates these proinflammatory cytokines. However, patients with complications of malaria have much lower levels of NF-kB than healthy controls do; as a consequence, these low levels limit the Th1 cell response (Punsawad et al., 2012).
Similarly, low levels of IL-1β and TNF-α have been found in patients with severe malaria. In one study, researchers analyzed 29 single-nucleotide polymorphism (SNPs) in the PBMCs of patients with various clinical conditions and found that only the “toll-like receptor-1” variant could contribute to this reduced cellular phenotype (Manning et al., 2016). Although this and other studies have shown that malaria infections inhibit the immune response, in one recent study, PBMCs from asymptomatic school children showed a strong heterogeneity of cytokine production, which suggests suppress immune responses could be related only with active infections (Kijogi et al., 2018). The donors' immunological histories could influence this strong heterogeneous response because these PBMCs could present cross-reactivity with other infections such as schistosomiasis, leishmaniosis, toxoplasmosis, and Chagas disease. For this reason, other authors have proposed alternatives to the use of PBMCs in studying the primary immune response; one such alternative are hematopoietic stem cells (naïve cells), which would reduce the discrepancies in mononuclear cell quality between studies (Chitsanoor et al., 2017).
In a study of Cryptosporidium parvum in calves recovering from cryptosporidiosis, scientists used PBMCs to evaluate the immunogenic potential of a specific protein (p23) as a vaccine antigen; in the first step, the researchers infected the calves with the parasite's oocysts, and in the next step, the PBMCs from the calves were stimulated with recombinant p23, showing that this antigen can stimulate a Type-1-like immune response among T cells (Wyatt et al., 2005). A similar response occurred in HIV-positive people infected with Cryptosporidium; their PBMCs were used to evaluate the cytokine profile after stimulation with the parasite crude extract, and this showed that INF-γ is one of the most important cytokines in the immune response against this parasite (Gomez Morales et al., 1999). With the help of IL-15, INF-γ eliminates this intracellular parasite by activating natural killer cells (Dann et al., 2005). In the case of Eimeria, PBMCs have been used to evaluate the parasite's proteins as adjuvants for vaccines or for immunostimulatory therapeutic agents in the treatment of human cancer (Aylsworth et al., 2013). For Babesia, PBMCs have been utilized to evaluate the cytokine profile response in cattle vaccination (East et al., 1997) and to identify the immune mechanisms involved in this parasite's pathogenicity (Guan et al., 2010).
With respect to Theileria, researchers have evaluated the mRNA levels of six immunological markers in PBMCs from crossbred, Tharparkar and Buffalo cattle after a parasite challenge. The markers included MHC class II DQ-α (BoLA-DQ), signal-regulatory protein alpha, prion protein, toll-like receptor 10, c-musculoaponeurotic fibrosarcoma oncogene homolog and V-maf avian musculoaponeurotic fibrosarcoma oncogene homolog B. For crossbred and Tharparkar cattle, significant differences occurred in the expression of genes in infected and uninfected cells (Dewangan et al., 2015), whereas the genes in Buffalo cattle did not show significant differences, suggesting that those genes had little effect in the progression of tropical theileriosis in the Buffalo species (Panigrahi et al., 2016). These results indicate that, although PBMCs are a good model for studying immunological phenomena, using them to make inferences or generalize results across species is not advisable, regardless of how evolutionarily close those species are.
The evidence suggests that PBMCs could be a good model for studying the immune response in apicomplexan parasites and for evaluating the efficiency of vaccine candidates, therapeutic agents and immunomodulatory molecules. However, these cells are not the most appropriate model for studying primo-infections (Chitsanoor et al., 2017). Therefore, despite all the benefits of the PBMC model, precautions should be taken related to its limitations and the type of immune response that is being evaluated.
PBMCs, Omics, and Toxoplasma
Omics techniques are powerful tools in modern biology, as they enable high-throughput measurements of many genes, proteins and metabolites in samples. A limited number of studies with Toxoplasma have employed PBMCs in global transcriptional profiling, with only one using PBMCs from pigs (Zhou et al., 2016a; see Table 1). Some other studies have applied omics in research on Toxoplasma and cortical neurons, astrocytes, skeletal muscle cells, fibroblasts, and other cells; almost all of these were derived from murine models (Tanaka et al., 2013; Pittman et al., 2014; He et al., 2016; Zhou et al., 2016b). All these studies show that gene expression—and consequently, protein and metabolite levels—can undergo changes based on physiological conditions. The results of other studies suggest that the gene expression patterns in PBMCs greatly depend on temporal and interindividual variations and also show strong evidence that these cells' gene-expression profiles are very sensitive to long incubation periods (Baechler et al., 2004) in addition to being dramatically affected by cryopreservation (Yang et al., 2016). As yet, no reports in the literature exist regarding the analysis of global transcriptional profiling using human PBMCs stimulated with T. gondii. Therefore, omics techniques, including dual RNAseq (Westermann et al., 2017) in PBMCs from healthy individuals and toxoplasmosis sufferers, could provide excellent opportunities to obtain important and relevant data on this infectious disease. Given the number of methodological differences among studies, ex vivo assays can be considered a suitable model for the analysis of what occurs during infection with Toxoplasma in humans. These assays emerge as a good alternative for these main reasons: (i) an ex vivo experiment should usually be done within a 24 h period in order to minimize the effect that stress generates on the cells; (ii) PBMCs' characteristics are very interesting because they are primary cells, are not immortalized and have the genetic backgrounds of real patients; and (iii) ex vivo experiments have the advantage of being analyzable very shortly after sampling, which is particularly critical in studies of gene expression, where data can be altered dramatically with the time factor.
Conclusion
PBMCs allow for the study of the immune-system response to infections with apicomplexan parasites, the evaluation of vaccine candidates and the development of immunotherapeutic strategies. To obtain reproducible and comparable results, several variables must be optimized when working with this model. The evaluated response of human PBMCs in apicomplexans has also been restricted to a few cytokines, so it is highly advisable to include additional immunological techniques that can comprehensively reflect the host's response to the pathogen.
Author Contributions
JA and AH contributed to the conception of the publication and prepared the manuscript. Both authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the funding from the COLCIENCIAS program, project: 1113-744-55-323.
References
- Alfonzo M., Badell E., Pourcel C., Dumas G., Colle J. H., Scott-Algara D. (2005). Cell-mediated and not humoral immune response is responsible for partial protection against toxoplasmosis in SCID mice reconstituted with human PBMC. Inmunologia 24, 273–282. [Google Scholar]
- Alfonzo M., Blanc D., Troadec C., Huerre M., Eliaszewicz M., Gónzalez G., et al. (2002). Temporary restoration of immune response against Toxoplasma gondii in HIV-infected individuals after HAART, as studied in the hu-PBMC-SCID mouse model. Clin. Exp. Immunol. 129, 411–419. 10.1046/j.1365-2249.2002.01941.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anum D., Kusi K. A., Ganeshan H., Hollingdale M. R., Ofori M. F., Koram K. A., et al. (2015). Measuring naturally acquired ex vivo IFN-γ responses to Plasmodium falciparum cell-traversal protein for ookinetes and sporozoites (CelTOS) in Ghanaian adults. Malar. J. 14, 1–8. 10.1186/s12936-014-0539-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autissier P., Soulas C., Burdo T. H., Williams K. C. (2010). Evaluation of a 12-color flow cytometry panel to study lymphocyte, monocyte, and dendritic cell subsets in humans. Cytom. Part A 77, 410–419. 10.1002/cyto.a.20859 [DOI] [PubMed] [Google Scholar]
- Awasthi V., Chauhan R., Chattopadhyay D., Das J. (2017). Effect of l-arginine on the growth of Plasmodium falciparum and immune modulation of host cells. J. Vector Borne Dis. 54, 139–145. [PubMed] [Google Scholar]
- Aylsworth C. F., Aldhamen Y. A., Seregin S. S., Godbehere S., Amalfitano A. (2013). Activation of human natural killer cells by the novel innate immune modulator recombinant Eimeria antigen. Hum. Immunol. 74, 916–926. 10.1016/j.humimm.2013.04.035 [DOI] [PubMed] [Google Scholar]
- Baechler E. C., Batliwalla F. M., Karypis G., Gaffney P. M., Moser K., Ortmann W. A., et al. (2004). Expression levels for many genes in human peripheral blood cells are highly sensitive to ex vivo incubation. Genes Immun. 5, 347–353. 10.1038/sj.gene.6364098 [DOI] [PubMed] [Google Scholar]
- Baust J. M. (2002). Molecular mechanisms of cellular demise associated with cryopreservation failure. Cell Preserv. Technol. 1, 17–31. 10.1089/15383440260073266 [DOI] [Google Scholar]
- Bayram Delibaş S., Turgay N., Gürüz A. Y. (2009). The role of cytokines in the immunopathogenesis of toxoplasmosis. Turkiye Klin. J. Med. Sci. 29, 1217–1221. [Google Scholar]
- Burczynski M. E., Dorner A. J. (2006). Transcriptional profiling of peripheral blood cells in clinical pharmacogenomic studies. Pharmacogenomics 7, 187–202. 10.2217/14622416.7.2.187 [DOI] [PubMed] [Google Scholar]
- Cardona N. I., Moncada D. M., Gómez-Marin J. E. (2015). A rational approach to select immunogenic peptides that induce IFN-γ response against Toxoplasma gondii in human leukocytes. Immunobiology 220, 1337–1342. 10.1016/j.imbio.2015.07.009 [DOI] [PubMed] [Google Scholar]
- Changrob S., Wang B., Han J. H., Lee S. K., Nyunt M. H., Lim C. S., et al. (2016). Naturally-acquired immune response against Plasmodium vivax rhoptry-associated membrane antigen. PLoS ONE 11:e148723 10.1371/journal.pone.0148723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitsanoor S., Somsri S., Panburana P., Mungthin M., Ubalee R., Emyeam M., et al. (2017). A novel in vitro model reveals distinctive modulatory roles of Plasmodium falciparum and Plasmodium vivax on naïve cell-mediated immunity. Malar. J. 16, 1–10. 10.1186/s12936-017-1781-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong H., Mui E. J., Witola W. H., Sidney J., Alexander J., Sette A., et al. (2011). Towards an immunosense vaccine to prevent toxoplasmosis: protective Toxoplasma gondii epitopes restricted by HLA-A*0201. Vaccine 29, 754–762. 10.1016/j.vaccine.2010.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong H., Mui E. J., Witola W. H., Sidney J., Alexander J., Sette A., et al. (2012). Toxoplasma gondii HLA-B*0702-restricted GRA720-28 peptide with adjuvants and a universal helper T cell epitope elicits CD8+ T cells producing interferon-γ and reduces parasite burden in HLA-B*0702 mice. Hum. Immunol. 73, 1–10. 10.1016/j.humimm.2011.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkum C. P., Ings D. P., Burgess C., Karwowska S., Kroll W., Michalak T. I. (2015). Immune cell subsets and their gene expression profiles from human PBMC isolated by Vacutainer Cell Preparation Tube (CPTTM) and standard density gradient. BMC Immunol. 16, 1–18. 10.1186/s12865-015-0113-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen J. B. W. J., van der Giessen J. W. B., Takumi K., Teunis P. F. M., Wisselink H. J. (2014). An experimental Toxoplasma gondii dose response challenge model to study therapeutic or vaccine efficacy in cats. PLoS ONE 9:e104740 10.1371/journal.pone.0104740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino L. M., Corwin W., Baust J. M., Diaz-Mayoral N., Cooley H., Shao W., et al. (2007). Preliminary report: evaluation of storage conditions and cryococktails during peripheral blood mononuclear cell cryopreservation. Cell Preserv. Technol. 5, 189–204. 10.1089/cpt.2007.9987 [DOI] [Google Scholar]
- Dann S. M., Wang H., Gambarin K. J., Actor J. K., Robinson P., Lewis D. E., et al. (2005). Interleukin-15 activates human natural killer cells to clear the intestinal protozoan Cryptosporidium. J. Infect. Dis. 192, 1294–1302. 10.1086/444393 [DOI] [PubMed] [Google Scholar]
- Dewangan P., Panigrahi M., Kumar A., Saravanan B. C., Ghosh S., Asaf V. N. M., et al. (2015). The mRNA expression of immune-related genes in crossbred and Tharparkar cattle in response to in vitro infection with Theileria annulata. Mol. Biol. Rep. 42, 1247–1255. 10.1007/s11033-015-3865-y [DOI] [PubMed] [Google Scholar]
- Dubey J. P. (2008). The history of Toxoplasma gondii- The first 100 years. J. Eukaryot. Microbiol. 55, 467–475. 10.1111/j.1550-7408.2008.00345.x [DOI] [PubMed] [Google Scholar]
- Dubey J. P. (2010). Toxoplasmosis of Animals and Humans. Boca Raton, FL: CRC Press. [Google Scholar]
- Dzitko K., Grzybowski M. M., Pawełczyk J., Dziadek B., Gatkowska J., Staczek P., et al. (2015). Phytoecdysteroids as modulators of the Toxoplasma gondii growth rate in human and mouse cells. Parasit. Vect. 8:422. 10.1186/s13071-015-1019-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzitko K., Ławnicka H., Gatkowska J., Dziadek B., Komorowski J., Długonska H. (2012). Inhibitory effect of prolactin on Toxoplasma proliferation in peripheral blood mononuclear cells from patients with hyperprolactinemia. Parasite Immunol. 34, 302–311. 10.1111/j.1365-3024.2012.01359.x [DOI] [PubMed] [Google Scholar]
- East I. J., Zakrzewski H., Gale K. R., Leatch G., Dimmock C. M., Thomas M. B., et al. (1997). Vaccination against Babesia bovis: T cells from protected and unprotected animals show different cytokine profiles. Int. J. Parasitol. 27, 1537–1545. 10.1016/S0020-7519(97)00141-0 [DOI] [PubMed] [Google Scholar]
- Ferreira I. M. R., Vidal J. E., de Mattos C., de C. B., de Mattos L. C., Qu D., et al. (2011). Toxoplasma gondii isolates: multilocus RFLP-PCR genotyping from human patients in Sao Paulo State, Brazil identified distinct genotypes. Exp. Parasitol. 29, 190–195. 10.1016/j.exppara.2011.06.002 [DOI] [PubMed] [Google Scholar]
- Fowke K. R., Behnke J., Hanson C., Shea K., Cosentino L. M. (2000). Apoptosis: A method for evaluating the cryopreservation of whole blood and peripheral blood mononuclear cells. J. Immunol. Methods 244, 139–144. 10.1016/S0022-1759(00)00263-5 [DOI] [PubMed] [Google Scholar]
- Ganeshan H., Kusi K. A., Anum D., Hollingdale M. R., Peters B., Kim Y., et al. (2016). Measurement of ex vivo ELISpot interferon-gamma recall responses to Plasmodium falciparum AMA1 and CSP in Ghanaian adults with natural exposure to malaria. Malar. J. 15, 1–15. 10.1186/s12936-016-1098-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S., Chauhan S. S., Singh N., Sharma Y. D. (2008). Immunological responses to a 39.8 kDa Plasmodium vivax tryptophan-rich antigen (PvTRAg39.8) among humans. Microbes Infect. 10, 1097–1105. 10.1016/j.micinf.2008.05.008 [DOI] [PubMed] [Google Scholar]
- Garraud O., Perraut R., Diouf A., Nambei W. S., Tall A., Spiegel A., et al. (2002). Regulation of antigen-specific immunoglobulin G subclasses in response to conserved and polymorphic Plasmodium falciparum antigens in an in vitro model. Infect. Immun. 70, 2820–2827. 10.1128/IAI.70.6.2820-2827.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitau E. N., Tuju J., Karanja H., Stevenson L., Requena P., Kimani E., et al. (2014). CD4+ T Cell Responses to the Plasmodium falciparum erythrocyte membrane protein 1 in children with mild malaria. J. Immunol. 192, 1753–1761. 10.4049/jimmunol.1200547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisic-Milosavljevic S., Waukau J., Jana S., Jailwala P., Rovensky J., Ghosh S. (2005). Comparison of apoptosis and mortality measurements in peripheral blood mononuclear cells (PBMCs) using multiple methods. Cell Prolif. 38, 301–311. 10.1111/j.1365-2184.2005.00351.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Morales M. A., La Rosa G., Ludovisi A., Onori A. M., Pozio E. (1999). Cytokine profile induced by Cryptosporidium antigen in peripheral blood mononuclear cells from immunocompetent and immunosuppressed persons with cryptosporidiosis. J. Infect. Dis. 179, 967–973. [DOI] [PubMed] [Google Scholar]
- Guan G., Chauvin A., Yin H., Luo J., Moreau E. (2010). Course of infection by Babesia sp. BQ1 (Lintan) and B. divergens in sheep depends on the production of IFN? and IL10. Parasite Immunol. 32, 143–152. 10.1111/j.1365-3024.2009.01169.x [DOI] [PubMed] [Google Scholar]
- He J.-J., Ma J., Elsheikha H. M., Song H.-Q., Huang S.-Y., Zhu X.-Q., et al. (2016). Transcriptomic analysis of mouse liver reveals a potential hepato-enteric pathogenic mechanism in acute Toxoplasma gondii infection. Parasit. Vectors 9:427. 10.1186/s13071-016-1716-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J. J. A., Ma J. A. B., Song H. Q. A., Zhou D. H. A., Wang J. L. A., Huang S. Y. A., et al. (2015). Transcriptomic analysis of global changes in cytokine expression in mouse spleens following acute Toxoplasma gondii infection. Parasitol. Res. 115, 703–712. 10.1007/s00436-015-4792-5 [DOI] [PubMed] [Google Scholar]
- Hunter C. A., Sibley L. D. (2012). Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat Rev Microbiol. 10, 766–778. 10.1038/nrmicro2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennes M., De Craeye S., Devriendt B., Dierick K., Dorny P., Cox E. (2017). Strain- and dose-dependent reduction of Toxoplasma gondii burden in pigs is associated with interferon-gamma production by CD8+ lymphocytes in a heterologous challenge model. Front. Cell. Infect. Microbiol. 7:232. 10.3389/fcimb.2017.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijogi C., Kimura D., Bao L. Q., Nakamura R., Chadeka E. A., Cheruiyot N. B., et al. (2018). Modulation of immune responses by Plasmodium falciparum infection in asymptomatic children living in the endemic region of Mbita, western Kenya. Parasitol. Int. 67, 284–293. 10.1016/j.parint.2018.01.001 [DOI] [PubMed] [Google Scholar]
- Lahmar I., Abou-Bacar A., Abdelrahman T., Guinard M., Babba H., Ben Yahia S., et al. (2009). Cytokine profiles in toxoplasmic and viral uveitis. J. Infect. Dis. 199, 1239–1249. 10.1086/597478 [DOI] [PubMed] [Google Scholar]
- Little R. J., D'Agostino R., Cohen M. L., Dickersin K., Emerson S. S., Farrar J. T., et al. (2012). The prevention and treatment of missing data in clinical trials. N. Engl. J. Med. 367, 1355–1360. 10.1056/NEJMsr1203730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia M. M., Meira-Strejevitch C. S., Pereira-Chioccola V. L., de Hippólito D. D. C., Silva V. O., Brandão de Mattos C. C., et al. (2017). Evaluation of gene expression levels for cytokines in ocular toxoplasmosis. Parasite Immunol. 39:e12462. 10.1111/pim.12462 [DOI] [PubMed] [Google Scholar]
- Mallone R., Mannering S. I., Brooks-Worrell B. M., Durinovic-Bell,ó I., Cilio C. M., Wong F. S., et al. (2011). Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T cell responses: position statement of the T-cell workshop committee of the immunology of diabetes society. Clin. Exp. Immunol. 163, 33–49. 10.1111/j.1365-2249.2010.04272.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning L., Cutts J., Stanisic D. I., Laman M., Carmagnac A., Allen S., et al. (2016). A Toll-like receptor-1 variant and its characteristic cellular phenotype is associated with severe malaria in Papua New Guinean children. Genes Immun. 17, 52–59. 10.1038/gene.2015.50 [DOI] [PubMed] [Google Scholar]
- Martens S., Parvanova I., Zerrahn J., Griffiths G., Schell G., Reichmann G., et al. (2005). Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS Pathog. 1:e24. 10.1371/journal.ppat.0010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massanella M., Gianella S., Schrier R., Dan J. M., Pérez-Santiago J., Oliveira M. F., et al. (2015). Methamphetamine use in HIV-infected individuals affects T-cell function and viral outcome during suppressive antiretroviral therapy. Sci. Rep. 5:13179. 10.1038/srep13179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meira C. S., Pereira-Chioccola V. L., Vidal J. E., de Mattos C. C. B., Motoie G., Costa-Silva T. A., et al. (2014). Cerebral and ocular toxoplasmosis related with IFN-γ, TNF-α, and IL-10 levels. Front. Microbiol. 5:492. 10.3389/fmicb.2014.00492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meira C. S., Pereira-Chioccola V. L., Vidal J. E., Motoie G., da Costa-Silva T. A., Gava R., et al. (2015). Evolution of cytokine profile during the treatment of cerebral toxoplasmosis in HIV-infected patients. J. Immunol. Methods 426, 14–18. 10.1016/j.jim.2015.07.005 [DOI] [PubMed] [Google Scholar]
- Meira-Strejevitch C. S., Pereira-Chioccola V. L., Maia M. M., de Hippolito D. D. C., Wang H.-T. L., Motoie G., et al. (2017). Reference genes for studies in infectious parasitic diseases in five types of human tissues. Gene Rep. 7, 98–105. 10.1016/j.genrep.2017.03.002 [DOI] [PubMed] [Google Scholar]
- Mensah V. A., Gueye A., Ndiaye M., Edwards N. J., Wright D., Anagnostou N. A., et al. (2016). Safety, immunogenicity and efficacy of prime-Boost vaccination with chad63 and mva encoding me-trap against plasmodium falciparum infection in adults in senegal. PLoS One 11:e167951. 10.1371/journal.pone.0167951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau J., Eller S. K., Wenning J., Spekker-Bosker K. H., Schroten H., Schwerk C., et al. (2017). Experimental porcine Toxoplasma gondii infection as a representative model for human toxoplasmosis. Mediators Inflamm. 2017:3260289. 10.1155/2017/3260289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedelman W., Gold D. A., Rosowski E. E., Sprokholt J. K., Lim D., Arenas A. F., et al. (2012). The rhoptry proteins ROP18 and ROP5 mediate Toxoplasma gondii evasion of the Murine, But Not the human, interferon-gamma response. 8:e1002784 10.1371/journal.ppat.1002784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira R. S., Gomes-Silva A., Bittar R. C., Silva Mendonça D., Amato V. S., da Silva Mattos M., et al. (2014). Antigen-triggered interferon- γ and interleukin-10 pattern in cured mucosal leishmaniasis patients is shaped during the active phase of disease. Clin. Exp. Immunol. 177, 679–686. 10.1111/cei.12364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill R. T., Temple R. (2012). The prevention and treatment of missing data in clinical trials: an FDA perspective on the importance of dealing with it. Clin. Pharmacol. Ther. 91, 550–554. 10.1038/clpt.2011.34 [DOI] [PubMed] [Google Scholar]
- Panigrahi M., Kumar A., Bhushan B., Ghosh S., Saravanan B. C., Sulabh S., et al. (2016). No change in mRNA expression of immune-related genes in peripheral blood mononuclear cells challenged with Theileria annulata in Murrah buffalo (Bubalus bubalis). Ticks Tick. Borne. Dis. 7, 754–758. 10.1016/j.ttbdis.2016.03.006 [DOI] [PubMed] [Google Scholar]
- Pfaff A. W., Georges S., Abou-Bacar A., Letscher-Bru V., Klein J.-P., Mousli M., et al. (2005). Toxoplasma gondii regulates ICAM-1 mediated monocyte adhesion to trophoblasts. Immunol. Cell Biol. 83, 483–489. 10.1111/j.1440-1711.2005.01356.x [DOI] [PubMed] [Google Scholar]
- Pittman K. J., Aliota M. T., Knoll L. J. (2014). Dual transcriptional profiling of mice and Toxoplasma gondii during acute and chronic infection. BMC Genomics 15:806. 10.1186/1471-2164-15-806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigione I., Chiesa S., Taverna P., Ceccarelli R., Frulio R., Morandi F., et al. (2006). T cell mediated immune responses to Toxoplasma gondii in pregnant women with primary toxoplasmosis. Microbes Infect. 8, 552–560. 10.1016/j.micinf.2005.08.008 [DOI] [PubMed] [Google Scholar]
- Punsawad C., Krudsood S., Maneerat Y., Chaisri U., Tangpukdee N., Pongponratn E., et al. (2012). Activation of nuclear factor kappa B in peripheral blood mononuclear cells from malaria patients. Malar. J. 11:191. 10.1186/1475-2875-11-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende-Oliveira K., Silva N. M., Mineo J. R., Rodrigues Junior V. (2012). Cytokines and chemokines production by mononuclear cells from parturient women after stimulation with live Toxoplasma gondii. Placenta 33, 682–687. 10.1016/j.placenta.2012.05.013 [DOI] [PubMed] [Google Scholar]
- Riedhammer C., Halbritter D., Weissert R. (2016). Peripheral blood mononuclear cells: isolation, freezing, thawing, and culture christine. Methods Mol. Biol. 1304, 53–61. 10.1007/7651_2014_99 [DOI] [PubMed] [Google Scholar]
- Seok J., Warren H. S., Cuenca A. G., Mindrinos M. N., Baker H. V., Xu W., et al. (2013). Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. U.S.A. 110, 3507–3512. 10.1073/pnas.1222878110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan W. J., Jeffers V. (2012). Mechanisms of Toxoplasma gondii persistence and latency. FEMS Microbiol. Rev. 36, 717–733. 10.1111/j.1574-6976.2011.00305.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo E. K., Finney C. A. M. (2017). Toxoplasma gondii: one organism, multiple models. Trends Parasitol. 33, 113–127. 10.1016/j.pt.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Tan T. G., Mui E., Cong H., Witola W. H., Montpetit A., Muench S. P., et al. (2010). Identification of T. gondii epitopes, adjuvants, and host genetic factors that influence protection of mice and humans. Vaccine 28, 3977–3989. 10.1016/j.vaccine.2010.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N., Ashour D., Dratz E., Halonen S. (2016). Use of human induced pluripotent stem cell-derived neurons as a model for Cerebral Toxoplasmosis. Microbes Infect. 18, 496–504. 10.1016/j.micinf.2016.03.012 [DOI] [PubMed] [Google Scholar]
- Tanaka S., Nishimura M., Ihara F., Yamagishi J., Suzuki Y., Nishikawa Y. (2013). Transcriptome analysis of mouse brain infected with toxoplasma gondii. Infect. Immun. 81, 3609–3619. 10.1128/IAI.00439-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenter A. M., Heckeroth A. R., Weiss L. M. (2000). Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30, 1217–1258. 10.1016/S0020-7519(00)00124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosh K. W., Mittereder L., Bonne-Annee S., Hieny S., Nutman T. B., Singer S. M., et al. (2016). The IL-12 response of primary human dendritic cells and monocytes to Toxoplasma gondii is stimulated by phagocytosis of live parasites rather than host cell invasion. J. Immunol. 196, 345–356. 10.4049/jimmunol.1501558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unno A., Kachi S., Batanova T. A., Ohno T., Elhawary N., Kitoh K., et al. (2013). Toxoplasma gondii tachyzoite-infected peripheral blood mononuclear cells are enriched in mouse lungs and liver. Exp. Parasitol. 134, 160–164. 10.1016/j.exppara.2013.03.006 [DOI] [PubMed] [Google Scholar]
- Vendrell J. P., Pratlong F., Decostert A., Boulot P., Conge A. M., Darcyt F. (1992). Secretion of Toxoplasma gondii-specific antibody in vitro by peripheral blood mononuclear cells as a new marker of acute toxoplasmosis tidreniete winuteo difficult. Clin. Exp. Immunol. 89, 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A., Song L. Y., Wilkening C. L., Fenton T., Hural J., Louzaod R., et al. (2010). Optimization of storage and shipment of cryopreserved peripheral blood mononuclear cells from HIV-infected and uninfected individuals for ELISPOT assays. J. Immunol. Methods 363, 42–50. 10.1016/j.jim.2010.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann A. J., Barquist L., Vogel J. (2017). Resolving host–pathogen interactions by dual RNA-seq. PLoS Pathog. 13:e1006033. 10.1371/journal.ppat.1006033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski S. R., Leon A. C., Otto M. W., Trivedi M. H. (2006). Prevention of missing data in clinical research studies. Biol. Psychiatry. 59, 997–1000. 10.1016/j.biopsych.2006.01.017 [DOI] [PubMed] [Google Scholar]
- Wunsch M., Caspell R., Kuerten S., Lehmann P. V., Sundararaman S. (2015). Serial measurements of apoptotic cell numbers Provide Better Acceptance Criterion for PBMC quality than a single measurement prior to the T cell assay. Cells 4, 40–55. 10.3390/cells4010040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt C. R., Lindahl S., Austin K., Kapil S., Branch J. (2005). Response of T lymphocytes from previously infected calves to recombinant Cryptosporidium parvum P23 vaccine antigen. J. Parasitol. 91, 1239–1242. 10.1645/GE-3446RN.1 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Yamanaka M., Ikehara S., Kida K., Kuboki N., Mizuno D., et al. (2010). Generation of IFN-γ-producing cells that recognize the major piroplasm surface protein in Theileria orientalis-infected bovines. Vet. Parasitol. 171, 207–215. 10.1016/j.vetpar.2010.03.038 [DOI] [PubMed] [Google Scholar]
- Yang J., Diaz N., Adelsberger J., Zhou X., Stevens R., Rupert A., et al. (2016). The effects of storage temperature on PBMC gene expression. BMC Immunol. 17, 1–15. 10.1186/s12865-016-0144-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C.-X., Zhou D.-H., Liu G.-X., Suo X., Zhu X.-Q. (2016a). Transcriptomic analysis of porcine PBMCs infected with Toxoplasma gondii RH strain. Acta Trop. 154, 82–88. 10.1016/j.actatropica.2015.11.009 [DOI] [PubMed] [Google Scholar]
- Zhou C. X., Elsheikha H. M., Zhou D. H., Liu Q., Zhu X. Q., Suo X. (2016b). Dual identification and analysis of differentially expressed transcripts of porcine PK-15 cells and Toxoplasma gondii during in vitro infection. Front. Microbiol. 7:721. 10.3389/fmicb.2016.00721 [DOI] [PMC free article] [PubMed] [Google Scholar]