Abstract
The title compounds were found to undergo a [2+2] photocycloaddition with olefins at λ = 419 nm in CH 2 Cl 2 as the solvent. The resulting cyclobutanes were isolated in yields of 32–87% (11 examples) and showed a defined relative configuration at C1/C4 in the major diastereoisomer (nitro and aryl trans ). The analysis of side products and triplet sensitization experiments support a mechanistic scenario in which a 1,4-diradical is formed as a key intermediate.
Key words: cycloaddition, cyclobutanes, diastereoselectivity, nitro compounds, photochemistry, stereoselective synthesis, umpolung, visible light

Although [2+2] photocycloaddition chemistry 1 originates historically 2 from experiments performed with visible light, the advent of artificial UV light sources led – starting in the middle of the 20 th century – to the almost exclusive use of short-wavelength (λ = 250–380 nm) irradiation in all areas of photochemistry. Interest in reactions that were promoted by long-wavelength (λ > 380 nm) irradiation was spurred in the 1970s and in the 1980s by the desire to find suitable energy storage systems mainly based on the [2+2] photocycloaddition of norbornadienes to quadricyclenes. 3 Aromatic carbonyl compounds 4 and transition-metal salts 5 were found to act as triplet sensitizers in this transformation allowing the reaction to occur with visible light. More recently, triplet energy sensitization has been employed for enantioselective 6 [2+2] photocycloaddition reactions that are promoted by visible light 7 in the presence of an appropriate sensitizer. 8 In the context of our work on the activation of chromophors by Lewis or Brønsted acids, 9 we became interested in the photochemistry of nitrostyrenes. 10 The compound class seemed amenable to undergo direct intermolecular [2+2] photocycloaddition reaction upon excitation with visible light and we report in this communication on our preliminary results on this topic.
Already in the 19 th century, the [2+2] photodimerization of trans -β-nitrostyrene was observed to occur upon exposure to sunlight. 11 However, reactions with olefins in the spirit of an intermolecular [2+2] photocycloaddition have remained rare and were performed exclusively with short-wavelength light. Chapman et al. mentioned in a review on the photochemistry of unsaturated nitro compounds the reaction with olefins but did not provide any experimental details. 12 13
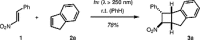
Scheme 1 [2+2] Photocycloaddition of trans -β-nitrostyrene ( 1 ) and indene ( 2a ) as reported by Majima et al. 14
Later, Majima et al. employed the reaction of trans -β-nitrostyrene ( 1 ) and indene ( 2a ) to form cyclobutane 3a (Scheme 1 ). 14 A high-pressure mercury lamp was employed as the light source in this transformation. In more recent work, pyrex-filtered irradiation was used to study the reaction of nitrostyrenes with silyl enol ethers. 15 16
Inspection of the UV-Vis spectrum 17 of trans -β-nitrostyrene in CH 2 Cl 2 (Figure 1 ) reveals a strong absorption centered at λ = 312 nm (ε = 16500 M –1 cm –1 ). This band has been previously assigned to an allowed ππ*-transition with significant charge-transfer character. 18 19 At high concentration it is evident that the absorption continues into the visible region of the electromagnetic spectrum in line with the fact that trans -β-nitrostyrene ( 1 ) is a yellow-colored solid.
Figure 1.
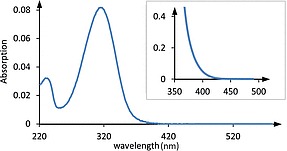
UV-Vis spectrum of trans -β-nitrostyrene in CH 2 Cl 2 solution ( c = 0.05 mM), the inset shows the long-wavelength absorption measured at c = 5 mM
In view of the apparent long-wavelength absorption of trans -β-nitrostyrene ( 1 ), its reaction with indene was revisited. The concentration, the solvent, and the stoichiometry (3.1 equiv indene) were taken from previous work, 14 and the reaction was run for 23 hours (Table 1 ). We were pleased to find that conversion was not only complete when the mixture was irradiated with fluorescent lamps 20 at λ = 300, 350, and 366 nm, but also at λ = 419 nm (Table 1 , entries 1–4). In all cases, it was observed that major diastereoisomer 3a was accompanied by a minor diastereoisomer to which structure 3a′ was assigned based on NOESY experiments. The diastereomeric ratio (d.r.) varied at around 3:1. Best yields were recorded at λ = 350 nm (Table 1 , entry 2) and λ = 419 nm (Table 1 , entry 4). Clearly, the [2+2] photocycloaddition was promoted by visible light as even long-wavelength light-emitting diodes (LEDs) led to a significant conversion at λ = 457 nm and at λ = 470 nm (Table 1 , entries 5, 6). At λ = 517 nm, there was essentially no conversion after 23 hours (Table 1 , entry 7).
Table 1 Conversion, Yield, and Diastereomeric Ratio in the Intermolecular [2+2] Photocycloaddition Reaction to Products 3a / 3a′ in Correlation to the Irradiation Wavelength .
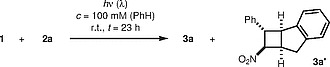 | ||||
| Entry | λ (nm) a | Conv. (%) | Yield (%) b | d.r. ( 3a / 3a′ ) c |
| 1 | 300 | 100 | 49 | 77:23 |
| 2 | 350 | 100 | 83 | 80:20 |
| 3 | 366 | 100 | 64 | 77:23 |
| 4 | 419 | 100 | 75 | 75:25 |
| 5 | 457 | 71 | 50 | 75:25 |
| 6 | 470 | 42 | 18 | 73:27 |
| 7 | 517 | <5 | – d | – |
a For the emission spectra of the light sources, see ref. 20
b Yield of isolated products 3a and 3a′ as a mixture of diastereoisomers.
c The diastereomeric ratio (d.r.) was determined by integration of appropriate 1 H NMR signals.
d No significant amounts of the respective products were isolated.
Further experiments were undertaken to identify a less problematic solvent but benzene and to optimize the reaction conditions at λ = 419 nm. While toluene was found less suited to substitute benzene, dichloromethane turned out to be an excellent solvent. A larger excess of the olefin led to higher product yields and the concentration was lowered to 20 mM in order to allow for small-scale reactions with more precious, not commercially available nitrostyrenes (vide infra). At optimized conditions 21 the [2+2] photocycloaddition products 3a / 3a′ were obtained in a yield of 87% after 24 hours of irradiation at λ = 419 nm. A variety of other olefins was employed in the reaction, and the results are summarized in Scheme 2 .
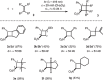
Scheme 2 Visible-light-induced [2+2] photocycloaddition of various olefins 2 to trans -β-nitrostyrene ( 1 )
It should be noted that the reactions were not always complete and that in some cases substantial amounts (up to 22%) of starting material were recovered, mostly as cis -β-nitrostyrene. Yields refer to isolated products, however, and are not corrected for conversion. With olefins 2b , d , f , g , the fact that the polarity of the excited state is opposite to the ground state polarity (photochemical umpolung ) becomes particularly apparent. C–C bond formation occurs formally between two – in the ground state – electrophilic centers (C1–C2) and between two nucleophilic centers (C3–C4). The reactions with olefins 2b – d led to a mixture of diastereoisomers the relative configuration of which could be in most cases elucidated by NOESY experiments (see Supporting Information for further details). Cyclobutanes 3e – g were obtained as single products. For the reaction of the electron rich olefin 2b , it was checked that there was no reaction in the absence of irradiation. 22
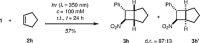
Scheme 3 Intermolecular [2+2] photocycloaddition of cyclopentene ( 2h ) to trans -β-nitrostyrene ( 1 )
Contrary to unsaturated hydrocarbons 2e and 2g , cyclopentene 2h did not react with a sufficient rate at λ = 419 nm. The [2+2] photocycloaddition could, however, be successively conducted if nitrostyrene 1 was irradiated in a solution of cyclopentene at λ = 350 nm (Scheme 3 ). The products were found to be a mixture of diastereoisomers in which product 3h with the nitro group in exo position to the cyclopentyl ring prevailed (d.r. = 87:13).
Some preliminary experiments were conducted with other aromatic nitroolefins 4 (Scheme 4 ). 2,3-Dimethyl-2-butene ( 2e ) was employed as the reaction partner since its use avoids the formation of regio- or diastereomeric cyclobutane products. It was found that electron-rich aryl groups ( para -tolyl, para -anisyl, 2-thiophenyl) in 2-position of the respective nitroethenes ( 4a , 23 4b , 23 4d 24 ) led in their [2+2] photocycloaddition to results similar to those of trans -β-nitrostyrene. Reaction times were short (2–4 h) and cyclobutanes 5a , 5b , and 5d were obtained in yields of 50–54%. The reaction with the para -cyano-substituted nitrostyrene 4c 25 was less chemoselective and gave product 5c in a yield of only 32% after a longer reaction time (6 h). A side product could be isolated (vide infra).
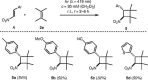
Scheme 4 Visible-light-induced [2+2] photocycloaddition of some 2-aryl-substituted nitroethenes 4 and 2,3-dimethyl-2-butene ( 2e )
If 2,3-dimethyl-2-butene was subjected to [2+2] photocycloaddition with cis -β-nitrostyrene instead of trans -β-nitrostyrene the reaction was slower. The reaction product was exclusively the trans -substituted cyclobutane 3e that was isolated in 43% yield. Irradiation of trans -β-nitrostyrene at λ = 419 nm in the absence of an olefin established an equilibrium 26 between the cis and the trans diastereoisomer in a ratio of 86:14. 27 This finding is in accord with the higher extinction coefficient of the trans diastereoisomer within the wavelength range of the light source. 20c The absorption maximum of cis -β-nitrostyrene is centered at λ = 309 nm (ε = 5200 M –1 cm –1 ) in CH 2 Cl 2 solution. 26b
Mechanistically, there is no indication for a reaction course which would deviate from the pathway of typical [2+2] photocycloaddition reactions. 1 In this regard, it seems likely that olefin 2 adds to the excited substrate, for example, trans -β-nitrostyrene ( 1 ), most likely on the triplet hypersurface (Scheme 5 ). A 1,4-diradical 6 is formed as intermediate which collapses after intersystem crossing to product 3 . Evidence for the postulated structure of diradical 6 is based on the constitution of the products and side products. Indeed, olefins such as 8 were isolated in a few instances and their formation is readily explained by a hydrogen abstraction in the intermediate 1,4-diradical. In the reaction of olefin 2e with styrene 4c , byproduct 8 was obtained in 5% yield and is putatively formed via intermediate 7 .
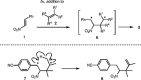
Scheme 5 Mechanistic suggestion for the reaction of trans -β-nitrostyrene ( 1 ) with olefins 2 via triplet 1,4-diradical 6 and formation of side product 8 in the reaction between 4c and 2e via 1,4-diradical 7
Further support for the hypothesis that the reaction proceeds via a triplet intermediate was obtained from the reaction of styrene 1 and olefin 2e . In the absence of an additive the reaction was complete after 12 hours (Scheme 2 ), while a significant rate increase was noted upon addition of the triplet sensitizer 9 H -thioxanthen-9-one (thioxanthone). 28 No β-nitrostyrene was detected after three hours and product 3e was obtained in 47% yield (Scheme 6 ).

Scheme 6 Rate increase of the reaction between trans -β-nitrostyrene ( 1 ) and 2e in the presence of a triplet sensitizer (TXT = thioxanthone)
In summary, we have shown that nitro-substituted cyclobutanes can be accessed by a visible-light-induced [2+2] photocycloaddition of various 2-arylnitroethenes and olefins. The yields are moderate to good (32–87%) and can possibly be further improved by adjusting the wavelength and the reaction temperature. Given the straightforward reduction of nitro compounds to amines, 11c 14 29 the method offers also access to various aminocyclobutanes. Mechanistically, it remains open to what degree a charge transfer 30 occurs upon encounter of the photoexcited nitro compound and the olefin. In addition, it might be worth to study whether other nitroethenes but nitrostyrenes are equally suited for [2+2] photocycloaddition reactions. Work along these lines is in progress in our laboratories and will be reported in due course.
Funding Statement
Financial support by the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 665951 – ELICOS) is gratefully acknowledged.
Supporting Information
Supporting Information
Supporting Information
Primary Data
Primary Data
Primary Data
Created: 2017-03-07, Format: Zahlen, Version: 1, Discipline: Chemistry
References and Notes
- 1.Poplata S, Tröster A, Zou Y.-Q, Bach T. Chem. Rev. 2016;116:9748. doi: 10.1021/acs.chemrev.5b00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth H D. Angew. Chem., Int. Ed. Engl. 1989;28:1193. [Google Scholar]
- Lennartson A, Roffey A, Moth-Poulsen K. Tetrahedron Lett. 2015;56:1457. [Google Scholar]; Dubonosov A D, Bren V A, Chernoivanov V A. Russ. Chem. Rev. 2002;71:917. [Google Scholar]
- Barwise A J. G, Gorman A A, Leyland R L, Smith P G, Rodgers M A. J. J. Am. Chem. Soc. 1978;100:1814. [Google Scholar]; Jones G, II., Chiang S.-H, Xuan P T. J. Photochem. 1979;10:1. [Google Scholar]
- Ikezawa H, Kutal C, Yasufuku K, Yamazaki H. J. Am. Chem. Soc. 1986;108:1589. [Google Scholar]; Grutsch P A, Kutal C. J. Am. Chem. Soc. 1986;108:3108. [Google Scholar]; Sluggett G W, Turro N J, Roth H D. J. Phys. Chem. A. 1997;101:8834. [Google Scholar]
- Brimioulle R, Lenhart D, Maturi M M, Bach T. Angew. Chem. Int. Ed. 2015;54:3872. doi: 10.1002/anie.201411409. [DOI] [PubMed] [Google Scholar]; Meggers E. Chem. Commun. 2015;51:3290. doi: 10.1039/c4cc09268f. [DOI] [PubMed] [Google Scholar]
- Zou Y.-Q, Duan S.-W, Meng X.-G, Hu X.-Q, Gao S, Chen J.-R, Xiao W.-J. Tetrahedron. 2012;68:6914. [Google Scholar]; Lu Z, Yoon T P. Angew. Chem. Int. Ed. 2012;51:10329. doi: 10.1002/anie.201204835. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kumarasamy E, Raghunathan R, Jockusch S, Ugrinov A, Sivaguru J. J. Am. Chem. Soc. 2014;136:8729. doi: 10.1021/ja5034638. [DOI] [PubMed] [Google Scholar]; Hurtley A E, Lu Z, Yoon T P. Angew. Chem. Int. Ed. 2014;53:8991. doi: 10.1002/anie.201405359. [DOI] [PMC free article] [PubMed] [Google Scholar]; Liu Q, Zhu F.-P, Jin X.-L, Wang X.-J, Chen H, Wu L.-Z. Chem. Eur. J. 2015;21:10326. doi: 10.1002/chem.201501176. [DOI] [PubMed] [Google Scholar]; Mojr V, Svobodová E, Straková K, Neveselý T, Chudoba J, Dvořáková H, Cibulka R. Chem. Commun. 2015;51:12036. doi: 10.1039/c5cc01344e. [DOI] [PubMed] [Google Scholar]
- Alonso R, Bach T. Angew. Chem. Int. Ed. 2014;53:4368. doi: 10.1002/anie.201310997. [DOI] [PubMed] [Google Scholar]; Tröster A, Alonso R, Bauer A, Bach T. J. Am. Chem. Soc. 2016;138:7808. doi: 10.1021/jacs.6b03221. [DOI] [PMC free article] [PubMed] [Google Scholar]; Blum T R, Miller Z D, Bates D M, Guzei I A, Yoon T P. Science. 2016;354:1391. doi: 10.1126/science.aai8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Herdtweck E, Bach T. Angew. Chem. Int. Ed. 2010;49:7782. doi: 10.1002/anie.201003619. [DOI] [PubMed] [Google Scholar]; Brimioulle R, Bach T. Science. 2013;342:840. doi: 10.1126/science.1244809. [DOI] [PubMed] [Google Scholar]; Brimioulle R, Bauer A, Bach T. J. Am. Chem. Soc. 2015;137:5170. doi: 10.1021/jacs.5b01740. [DOI] [PubMed] [Google Scholar]; Brenninger C, Pöthig A, Bach T. Angew. Chem. Int. Ed. 2017;56:4337. doi: 10.1002/anie.201700837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reviews on the photochemistry of nitro compounds:; Ho T.-I, Chow Y L.InThe Chemistry of Functional Groups, Supplement F2: The Chemistry of Amino, Nitroso, Nitro and Related Groups Patai S.Wiley; Chichester: 1996747 [Google Scholar]; Döpp D.InCRC Handbook of Organic Photochemistry and Photobiology Horspool W M, Song P.-S.CRC Press; Boca Raton: 19951019 [Google Scholar]
- Priebs B. Justus Liebigs Ann. Chem. 1884;225:319. [Google Scholar]; Meisenheimer J, Heim F. Justus Liebigs Ann. Chem. 1907;355:260. [Google Scholar]; Miller D B, Flanagan P W, Shechter H. J. Am. Chem. Soc. 1972;94:3912. [Google Scholar]
- 12.Chapman O L, Griswold A A, Hoganson E, Lenz G, Reasoner J. Pure Appl. Chem. 1964;9:585. [Google Scholar]
- 13.Magner J T, Selke M, Russell A A, Chapman O L. In a later paper, the reaction of trans -β-nitrostyrene ( 1 ) and 2,3-dimethylbutadiene ( 2d ) was reported to be performed at λ = 300 nm: J. Chem. Ed. 199673854 [Google Scholar]
- 14.Majima T, Pac C, Sakurai H. J. Am. Chem. Soc. 1980;102:5265. [Google Scholar]
- 15.Ramkumar D, Sankararaman S. J. Chem. Soc., Perkin Trans. 2. 1996:939. [Google Scholar]
- 16.Stevenson S M, Higgins R F, Shores M P, Ferreira E M. For a Cr-photocatalytic [4+2] cycloaddition of trans -β-nitro- para -methoxystyrene and 1,3-dienes, see: Chem. Sci. 20178654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L T, Tam W, Marder S R, Stiegman A E, Rikken G, Spangler C W. J. Phys. Chem. 1991;95:10643. [Google Scholar]; Aboskalova N I, Smirnova N N, Kataeva O N, Baichurin R I, Fel'gendler A V, Berkova G A, Berestovitskaya V A. Russ. J. Gen. Chem. 2008;78:1711. [Google Scholar]
- Cowley D J. J. Chem. Soc., Perkin Trans. 2. 1975:1576. [Google Scholar]; Zhang S.-Q, Wang H.-G, Pei K.-M, Zheng X. J. Chem. Phys. 2007;126:194505. doi: 10.1063/1.2736685. [DOI] [PubMed] [Google Scholar]
- For further references to the photochemistry of nitroolefins, see:; Ried W, Wilk M. Justus Liebigs Ann. Chem. 1954;590:111. [Google Scholar]; Zimmerman H E, Roberts L C, Arnold R. J. Org. Chem. 1977;42:621. [Google Scholar]; Humphry-Baker R A, Salisbury K, Wood G P. J. Chem. Soc., Perkin Trans. 2. 1978:659. [Google Scholar]; Grant R D, Pinhey J T, Rizzardo E, Smith G C. Aust. J. Chem. 1985;38:1505. [Google Scholar]; Kassaee M Z, Nassari M A. J. Photochem. Photobiol. A. 2000;136:41. [Google Scholar]
- For the emission spectra of the lamps, see for λ = 300 nm, 366 nm:; Maturi M M, Wenninger M, Alonso R, Bauer A, Pöthig A, Riedle E, Bach T. Chem. Eur. J. 2013;19:7461. doi: 10.1002/chem.201300203. [DOI] [PubMed] [Google Scholar]; λ = 350 nm:; Rimböck K.-H, Pöthig A, Bach T. Synthesis. 2015;47:2869. [Google Scholar]; λ = 419 nm:; Alonso R, Bach T. Angew. Chem. Int. Ed. 2014;53:4368. doi: 10.1002/anie.201310997. [DOI] [PubMed] [Google Scholar]; λ = 457 nm, 470 nm:; Lenhart D, Bauer A, Pöthig A, Bach T. Chem. Eur. J. 2016;22:6519. doi: 10.1002/chem.201600600. [DOI] [PubMed] [Google Scholar]
- 21.Representative Procedure 29.8 mg of nitrostyrene 1 (199 μmol, 1.00 equiv) and 10.0 equiv of olefin 2e (168 mg, 2.00 mmol) were dissolved in degassed, dry CH 2 Cl 2 ( c = 20 mM). The reaction solution was irradiated at λ = 419 nm in a Duran tube at r.t., and the reaction progress was monitored by TLC. When no further conversion was observed by TLC ( t = 12 h), the reaction was stopped and all volatiles were removed. Purification by column chromatography (pentane/Et 2 O = 20:1) gave product 3e as a yellow oil (27.5 mg, 118 μmol, 59%). When performed on a mmol scale (150 mg 1 ), product 3e was obtained in 56% yield (132 mg). 1 H NMR (500 MHz, CDCl 3 , 300 K): δ = 0.71 (s, 3 H, CH 3 -2), 1.15 (s, 3 H, CH 3 -3), 1.19 (s, 3 H, CH 3 -2), 1.24 (s, 3 H, CH 3 -3), 3.97 (d, 3J = 10.1 Hz, 1 H, H-1), 4.91 (d, 3J = 10.1 Hz, 1 H, H-4), 7.08–7.13 (m, 2 H, ortho -H Ar ), 7.23–7.28 (m, 1 H, para -H Ar ), 7.30–7.37 (m, 2 H, meta -H Ar ) ppm. 13 C NMR (101 MHz, CDCl 3 , 300 K): δ = 19.5 (q, CH 3 -3), 21.5 (q, CH 3 -2), 22.8 (q, CH 3 -3), 24.3 (q, CH 3 -2), 39.3 (s, C-2), 44.9 (s, C-3), 49.4 (d, C-1), 84.9 (d, C-4), 127.0 ( ortho -C Ar H). 127.1 (d, para -C Ar H), 128.6 (d, meta -C Ar H), 136.4 (s, C Ar ) ppm.
- For thermal [2+2] cycloaddition reactions of trans -β-nitrostyrene ( 1 ) and olefins, see: ; Brannock K C, Bell A, Burpitt R D, Kelly C A. J. Org. Chem. 1964;29:801. [Google Scholar]; Scheeren H W, Frissen A E. Synthesis. 1983:794. [Google Scholar]; Albrecht Ł, Dickmeiss G, Acosta F C, Rodríguez-Escrich C, Davis R L, Jørgensen K A. J. Am. Chem. Soc. 2012;134:2543. doi: 10.1021/ja211878x. [DOI] [PubMed] [Google Scholar]; Seebach D, Sun X, Ebert M.-O, Schweizer W B, Purkayastha N, Beck A K, Duschmalé J, Wennemers H, Mukaiyama T, Benohoud M, Hayashi Y, Reiher M. Helv. Chim. Acta. 2013;96:799. [Google Scholar]; Dobi Z, Holczbauer T, Soós T. Eur. J. Org. Chem. 2017:1391. [Google Scholar]
- 23.Yan Q, Liu M, Kong D, Zi G, Hou G. Chem. Commun. 2014;50:12870. doi: 10.1039/c4cc05815a. [DOI] [PubMed] [Google Scholar]
- 24.Quan X.-J, Ren Z.-H, Wang Y.-Y, Guan Z.-H. Org. Lett. 2014;16:5728. doi: 10.1021/ol5027975. [DOI] [PubMed] [Google Scholar]
- 25.Keene C, Kürti L. Synthesis. 2013;45:1719. [Google Scholar]
- Bluhm A L, Weinstein J. J. Am. Chem. Soc. 1965;87:5511. [Google Scholar]; Miller D B, Flanagan P W, Shechter H. J. Org. Chem. 1976;41:2112. [Google Scholar]; Desiraju G R, Pedireddi V R. J. Chem. Soc., Chem. Commun. 1989:1112. [Google Scholar]
- 27. The reaction time was 6 h. If the isomerization was performed starting from cis -β-nitrostyrene under otherwise identical conditions, the cis / trans ratio was 82:18.
- 28.Murov S L, Carmichael I, Hug G L. The tabulated triplet energy (E T ) of thioxanthone is E T = 265 kJ mol –1 : Handbook of Photochemistry2nd ed.Marcel Dekker; New York: 199380 [Google Scholar]
- 29.Xiong H, Foulk M, Aschenbrenner L, Fan J, Tiong-Yip C.-L, Johnson K D, Moustakas D, Fleming P R, Brown D G, Zhang M, Ferguson D, Wu D, Yu Q. Bioorg. Med. Chem. Lett. 2013;23:6789. doi: 10.1016/j.bmcl.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Müller F, Mattay J. Chem. Rev. 1993;93:99. [Google Scholar]; Mori T, Inoue Y. Chem. Soc. Rev. 2013;42:8122. doi: 10.1039/c3cs60117j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Primary Data
Primary Data
Created: 2017-03-07, Format: Zahlen, Version: 1, Discipline: Chemistry


