Abstract
Background
Early life exposure to tobacco smoke has been extensively studied but the role of second-hand smoke (SHS) for new-onset respiratory symptoms and lung function decline in adulthood has not been widely investigated in longitudinal studies. Our aim is to investigate the associations of exposure to SHS in adults with respiratory symptoms, respiratory conditions and lung function over 20 years.
Methods
We used information from 3011 adults from 26 centres in 12 countries who participated in the European Community Respiratory Health Surveys I-III and were never or former smokers at all three surveys. Associations of SHS exposure with respiratory health (asthma symptom score, asthma, chronic bronchitis, COPD) were analysed using generalised linear mixed-effects models adjusted for confounding factors (including sex, age, smoking status, socioeconomic status and allergic sensitisation). Linear mixed-effects models with additional adjustment for height were used to assess the relationships between SHS exposure and lung function levels and decline.
Results
Reported exposure to SHS decreased in all 26 study centres over time. The prevalence of SHS exposure was 38.7% at baseline (1990–1994) and 7.1% after the 20-year follow-up (2008–2011). On average 2.4% of the study participants were not exposed at the first, but were exposed at the third examination. An increase in SHS exposure over time was associated with doctor-diagnosed asthma (odds ratio (OR): 2.7; 95% confidence interval (95%-CI): 1.2–5.9), chronic bronchitis (OR: 4.8; 95%-CI: 1.6–15.0), asthma symptom score (count ratio (CR): 1.9; 95%-CI: 1.2–2.9) and dyspnoea (OR: 2.7; 95%-CI: 1.1–6.7) compared to never exposed to SHS. Associations between increase in SHS exposure and incidence of COPD (OR: 2.0; 95%-CI: 0.6–6.0) or lung function (β: − 49 ml; 95%-CI: -132, 35 for FEV1 and β: − 62 ml; 95%-CI: -165, 40 for FVC) were not apparent.
Conclusion
Exposure to second-hand smoke may lead to respiratory symptoms, but this is not accompanied by lung function changes.
Electronic supplementary material
The online version of this article (10.1186/s12931-019-0996-z) contains supplementary material, which is available to authorized users.
Keywords: Adults, Smoking, Lung function, Asthma, Respiratory symptoms, Bronchitis, ECRHS
Introduction
Exposure to second-hand smoke remains one of the most common indoor pollutants worldwide. In an overview paper from 2011 as many as 40% of children, 35% of women, and 33% of men were regularly exposed to second-hand smoke indoors worldwide [1]. Children exposed to passive smoke have deficits in lung growth [2–5]. However, the effect of environmental tobacco smoke on respiratory disorders and lung function has not been widely investigated and the associations are less clear in adults [6–8].
Emerging evidence indicates that exposure to second-hand smoke is related to the development of chronic obstructive pulmonary disease (COPD). Based on three studies [8–10], a meta-analysis [11] found an increased relative risk (RR = 1.7, 95% CI: 1.4–2.0) of COPD defined by spirometry in people exposed to passive smoking. A link between exposure to second-hand smoke and an accelerated loss of lung function [6, 8] was suggested, but the evidence is not strong. Results from cross-sectional analyses of data from middle aged adults participating in the European Community Respiratory Health Survey (ECRHS) showed adverse effects of passive smoking on respiratory symptoms including increased bronchial responsiveness, but the negative association with lung function was not statistically significant [12]. In addition, a variety of early life factors including maternal smoking during pregnancy showed an association with asthma and poor lung function in adulthood [13, 14]. A recent report on life-long exposure to tobacco smoke and lung function trajectories to middle age reported accelerated lung function decline in the exposed subjects [15]. However, some research [16, 17] indicates that current or former smokers often suffer from respiratory symptoms, although lung function is still within normal range and the criteria for COPD assessed by spirometry are not met. While there is mounting evidence that second-hand smoke exposure causes respiratory symptoms and lung function deficits at younger ages including young adulthood, the impact in older age groups is less clear.
We aimed to analyse the association of exposure to second-hand smoke with respiratory diseases such as asthma, bronchitis and COPD, asthma-related symptoms and spirometric pulmonary function in long-term follow ups of young and middle aged adults within a large European multicentre study (ECRHS).
Methods
Study population
The European Community Respiratory Health Survey (ECRHS) is a multicentre population-based cohort study that began in 1990–1994. Fifty-six centres across Europe and other parts of the world from 25 countries took part. Young adults aged between 20 and 44 years were selected at random from available population-based registers to take part in the survey. It was a two-stage study, with around 200,000 participants in the questionnaire stage 1, and 26,000 in the clinical stage 2. In the follow-up survey (ECRHS II) of the clinical stage 2 more than 10,000 adults from 29 centres in 14 countries participated (1998–2001). Detailed descriptions of the methods for ECRHS I and ECRHS II have previously been published [18, 19]. ECRHS III was the third wave of data collection on the cohort, beginning in 2008. Those who took part in the clinical stages of ECRHS I and II were again contacted, with responders invited to a local fieldwork centre, situated in an outpatient clinic or lung function laboratory. Information was gathered from standardised interviews by well-trained fieldworkers.
The current analyses were restricted to 3011 never and former smoking adults from the random sample who participated in all three surveys and had information on second-hand smoke exposure at all three examinations.
Definition of smoking and second-hand smoke
At each survey participants were asked “Have you ever smoked for as long as a year?”, and if yes, “Do you smoke now as of one month ago?” Current smokers answered both questions in the affirmative and were excluded. Those who answered the lead question in the negative were classified as never smokers, and ex-smokers were those who answered they had smoked but did not in the last month. Smokers and former smokers were asked about duration of smoking and number of cigarettes smoked per day and pack years were calculated. For analytical purposes smoking status was considered as categorical variables never smoker, ex-smoker with less than 15 pack years and ex-smoker with at least 15 pack years.
Exposure to second-hand smoke was assessed by the question “Have you been regularly exposed to tobacco smoke in the last 12 months?”. Study participants answering in the affirmative were classified as being exposed to second-hand smoke.
Definition of respiratory health parameters
Information on the following respiratory symptoms and diseases were collected: physician-diagnosed asthma, chronic bronchitis and COPD, as well as on respiratory symptoms such as wheeze, dyspnoea, cough and sputum. The asthma related symptoms were combined in an asthma score [20]. The following criteria for outcome assessment were used:
Physician-diagnosed asthma: “Have you ever had asthma?” and “Was this confirmed by a doctor?” were answered in the affirmative.
Asthma symptom score: The sum of positive answers to the following five questions, i.e. the asthma score ranges from 0 to 5, according to Sunyer et al. [20]: 1) “Have you been breathless while wheezing in the last 12 months?”; 2) “Have you been woken up with a feeling of chest tightness in the last 12 months?”; 3) “Have you had an attack of shortness of breath whilst at rest in the last 12 months?”; 4) “Have you had an attack of shortness of breath after activity in the last 12 months?” and 5) “Have you been woken by an attack of shortness of breath in the last 12 months?”
Nocturnal dyspnoea: “Have you been woken by an attack of shortness of breath at any time during the last twelve months?” was answered in the affirmative.
Cough: positive answer to at least one of “Have you been woken by an attack of coughing at any time in the last twelve months?”, “Do you usually cough first thing in the morning in the winter?” and “Do you usually cough during the day or night in the winter?”
Sputum: positive answer to at least one of the following questions: “Do you usually bring up phlegm from your chest first thing in the morning in the winter?” and “Do you usually bring up any phlegm from your chest during the day or at night in the winter?”
Chronic bronchitis: “Do you usually cough during the day or night on most days for as much as three months per year?” and “Do you usually bring up any phlegm from your chest on most days for as much as three months per year?” were answered in the affirmative.
Lung function testing
Lung function testing was performed by spirometry during the clinical examination according to the ATS/ERS recommendations [21]. Lung function measures were performed in a sitting position while the subjects were wearing nose clips. At least five, but not more than nine, forced expiratory manoeuvres were performed. The maximum forced expiratory volume in 1 s (FEV1) and maximum forced vital capacity (FVC) of the technically acceptable manoeuvres were determined. Spirometric lung function measurements pre-bronchodilation were used in the current analyses. Different spirometers were used across the study centres and follow-up time points within study centres.
Standardised z-scores were calculated based on the reference equations for spirometry from the Global Lung function Initiative (GLI - https://www.ers-education.org/guidelines/global-lung-function-initiative.aspx) [22].
The presence of COPD was based on lung function testing. Study participants with a ratio of FEV1 and FVC (measured pre-bronchodilation) below the lower limit of normal (LLN) according to the reference equations for spirometry from the Global Lung function Initiative [22] were classified as COPD patients. It was also defined as the ratio of FEV1 and FVC (measured pre-bronchodilation) below 0.7.
Definition of confounders
Potential confounding variables were assessed by questionnaire or measured at the physical examination. These included sex, age, maternal smoking during pregnancy and/or childhood, paternal smoking during childhood and occupational exposure to dust and fumes. Smoking status was defined as never smoker, ex-smoker with less than 15 pack years and ex-smoker with at least 15 pack years. Socioeconomic status was defined based on the age when fulltime education was completed (less than 17 years, 17 to 20 years and more than 20 years).
Allergen specific IgE was measured at baseline against D. pteronyssinus, cat, timothy grass and Cladosporium using the Pharmacia CAP System and allergic sensitisation was defined as being sensitised to any of these allergens using a cut-off of 0.35 kUA/L.
Height and weight were measured without shoes and in light clothes at the physical examination.
Statistical analyses
We modelled the longitudinal impact of changes of second-hand smoke exposure on respiratory health outcomes. The effect of change in second-hand smoke exposure over two examinations on lung function parameters as well as respiratory symptoms and diseases at follow-up was analysed separately for ECRHS I-II, ECRHS II-III and ECRHS I-III. Therefore, study participants were categorised into four groups: those not exposed to second-hand smoke at both examinations (reference category); those not exposed to second-hand smoke at the first examination, but at the second examination (SHS increase); those exposed to second-hand smoke at the first examination, but not at the second examination (SHS decrease) and those exposed to second-hand smoke at both examinations (SHS both). Mixed effects logistic regression models and negative binomial mixed effects models with random intercept for study centre were used for respiratory symptoms/diseases and asthma symptom score, respectively. Linear mixed effects models with random intercept for study centre were used to assess the association of change in second-hand smoke exposure and lung function parameters. All models were adjusted for sex, age, weight, maternal and paternal smoking, exposure to dust/fumes, allergic sensitisation, smoking status and socioeconomic status assessed at baseline and additionally for baseline respiratory symptom/disease and lung function, respectively. The models for the association between change in second-hand smoke exposure and lung function were additionally adjusted for height, weight squared and age squared (to model the non-linear relationship of weight and age with lung function). All continuous covariates were standardised (with mean 0 and variance 1).
The associations between exposure to second-hand smoke at baseline and lung function parameters at the three surveys were analysed to evaluate the effect of second-hand smoke exposure on lung function over time. Therefore, linear mixed effects models were fitted with random intercept for study participants nested in the study centre, and an interaction term between second-hand smoke exposure and time of follow-up, i.e. the time between the particular examinations, was included to model the impact of second-hand smoke exposure on lung function decline [23].
Interaction terms with sex, maternal smoking and paternal smoking were tested. Results from stratified analyses are therefore reported. In addition, sensitivity analyses restricted to never smokers at all three surveys (n = 1974 lifetime never smokers) were performed. For the association between change in second-hand smoke exposure over time and lung function at follow-up, additional analyses using percent predicted values according to the Global Lung function Initiative [22] were conducted.
The results for the association between second-hand smoke exposure with respiratory symptoms and diseases are presented as odds ratio (OR) with corresponding 95% confidence interval (CI), whereas the results for the association of second-hand smoke exposure with lung function parameters are presented as regression coefficients (β) with corresponding 95% CI. For the asthma symptom score, the results are presented as count ratio (CR) with corresponding 95% CI.
All analyses were performed using the statistical software R, version 3.4.3 [24], and the R packages “lme4” and “lmerTest”.
Results
Description of study population and temporal changes of second-hand smoke exposure and lung function
The analyses were based on 3011 non-smoking adults from 26 study centres who participated in all three surveys and had information on second-hand smoke exposure at all three examinations (Fig. 1). The prevalence of reported exposure to second-hand smoke decreased in all participating study centres from ECRHS I to III (Table 1). Overall, at the first examination, 38.7% were exposed to second-hand smoke, 23.0% at the second examination and 7.1% at the third examination. The prevalences were highest in Spain.
Fig. 1.
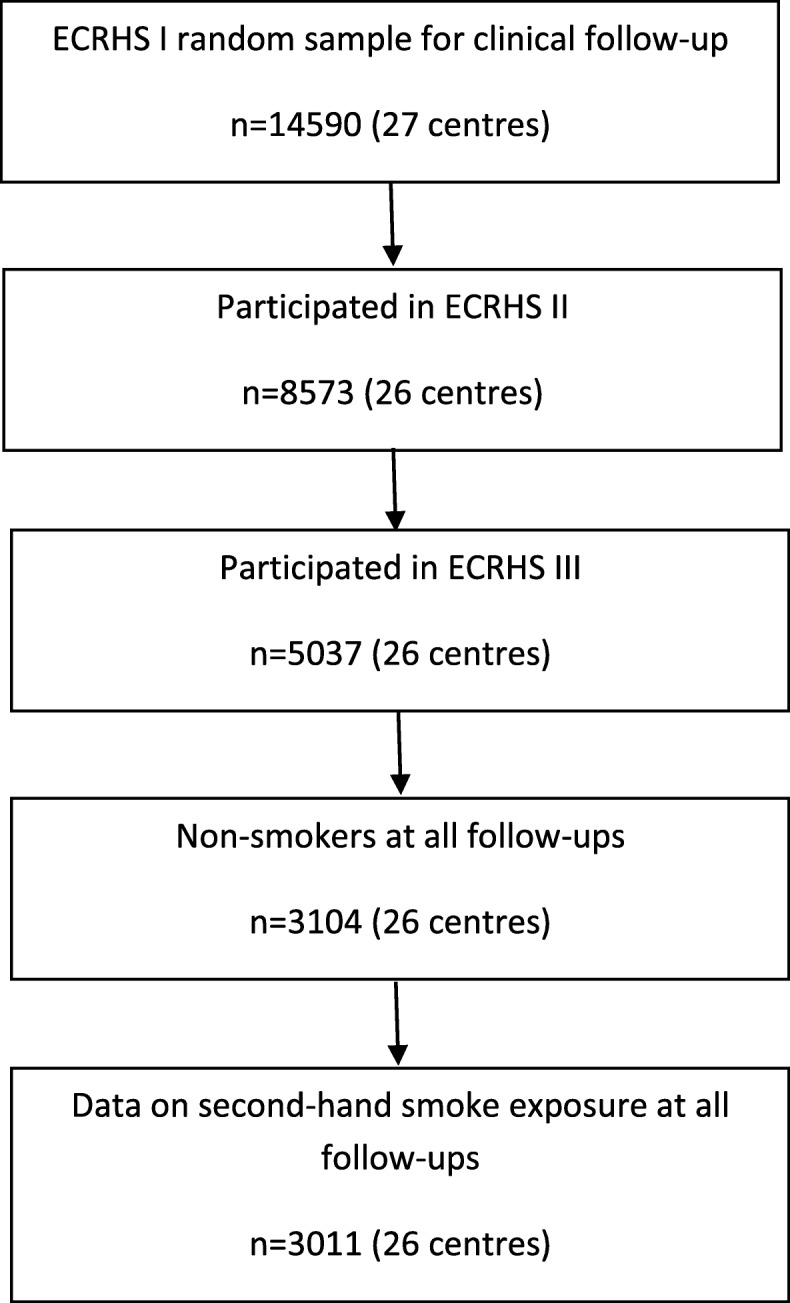
Flow chart of study population
Table 1.
Number of participants and prevalence of second-hand smoke (SHS) exposure by study centre
| n | SHS exposure in ECRHS I, % | SHS exposure in ECRHS II, % | SHS exposure in ECRHS III, % | |
|---|---|---|---|---|
| Antwerp South (Belgium) | 104 | 48.1 (50/104) | 32.7 (34/104) | 7.7 (8/104) |
| Antwerp City (Belgium) | 99 | 50.5 (50/99) | 36.4 (36/99) | 9.1 (9/99) |
| Hamburg (Germany) | 110 | 46.4 (51/110) | 26.4 (29/110) | 10.0 (11/110) |
| Erfurt (Germany) | 107 | 38.3 (41/107) | 26.2 (28/107) | 6.5 (7/107) |
| Barcelona (Spain) | 54 | 64.8 (35/54) | 40.7 (22/54) | 14.8 (8/54) |
| Galdakao (Spain) | 139 | 74.1 (103/139) | 51.1 (71/139) | 23.0 (32/139) |
| Albacete (Spain) | 69 | 68.1 (47/69) | 42.0 (29/69) | 15.9 (11/69) |
| Oviedo (Spain) | 50 | 56.0 (28/50) | 52.0 (26/50) | 28.0 (14/50) |
| Huelva (Spain) | 43 | 58.1 (25/43) | 53.5 (23/43) | 18.6 (8/43) |
| Bordeaux (France) | 63 | 50.8 (32/63) | 28.6 (18/63) | 6.3 (4/63) |
| Grenoble (France) | 212 | 34.0 (72/212) | 25.5 (54/212) | 6.6 (14/212) |
| Montpellier (France) | 88 | 34.1 (30/88) | 13.6 (12/88) | 1.1 (1/88) |
| Paris (France) | 186 | 40.3 (75/186) | 33.3 (62/186) | 4.8 (9/186) |
| Pavia (Italy) | 51 | 64.7 (33/51) | 47.1 (24/51) | 2.0 (1/51) |
| Turin (Italy) | 39 | 51.3 (20/39) | 38.5 (15/39) | 12.8 (5/39) |
| Verona (Italy) | 57 | 35.1 (20/57) | 17.5 (10/57) | 7.0 (4/57) |
| Ipswich (UK) | 93 | 31.2 (29/93) | 20.4 (19/93) | 7.5 (7/93) |
| Norwich (UK) | 88 | 36.4 (32/88) | 11.4 (10/88) | 3.4 (3/88) |
| Reykjavik (Iceland) | 197 | 47.2 (93/197) | 26.4 (52/197) | 7.1 (14/197) |
| Bergen (Norway) | 186 | 30.1 (56/186) | 11.8 (22/186) | 5.4 (10/186) |
| Gothenburg (Sweden) | 149 | 46.3 (69/149) | 6.7 (10/149) | 2.7 (4/149) |
| Umea (Sweden) | 162 | 22.2 (36/162) | 8.6 (14/162) | 4.9 (8/162) |
| Uppsala (Sweden) | 216 | 18.1 (39/216) | 3.7 (8/216) | 1.4 (3/216) |
| Basel (Switzerland) | 221 | 24.4 (54/221) | 16.3 (36/221) | 6.8 (15/221) |
| Melbourne (Australia) | 165 | 13.3 (22/165) | 8.5 (14/165) | 1.8 (3/165) |
| Tartu (Estonia) | 63 | 34.9 (22/63) | 25.4 (16/63) | 3.2 (2/63) |
| Overall | 3011 | 38.7 (1164/3011) | 23.0 (694/3011) | 7.1 (215/3011) |
The prevalence of respiratory symptoms and diseases and the distribution of lung function parameters and confounding variables in each survey are summarised in Table 2.
Table 2.
Prevalence of respiratory symptoms and diseases and distribution of lung function parameters and confounding variablesa
| ECRHS I | ECRHS II | ECRHS III | ||||
|---|---|---|---|---|---|---|
| SHS exposed (n = 1164) | SHS non-exposed (n = 1847) | SHS exposed (n = 694) | SHS non-exposed (n = 2317) | SHS exposed (n = 215) | SHS non-exposed (n = 2796) | |
| Sex, female | 52.0 (605/1164) | 55.5 (1025/1847) | 51.9 (360/694) | 54.8 (1270/2317) | 58.6 (126/215) | 53.8 (1504/2796) |
| Age, years | 33.8 (7.5) | 35.1 (6.9) | 43.5 (7.3) | 43.3 (7.1) | 53.8 (6.9) | 54.7 (7.2) |
| Age completed full time education | ||||||
| < 17 years | 19.4 (212/1093) | 13.8 (232/1686) | 24.7 (160/649) | 13.3 (284/2130) | 29.6 (59/199) | 14.9 (385/2580) |
| 17–20 years | 39.1 (427/1093) | 33.6 (567/1686) | 39.8 (258/649) | 34.6 (736/2130) | 39.7 (79/199) | 35.5 (915/2580) |
| > 20 years | 41.5 (454/1093) | 52.6 (887/1686) | 35.6 (231/649) | 52.1 (1110/2130) | 30.7 (61/199) | 49.6 (1280/2580) |
| Height, cm | 170.4 (9.8) | 170.6 (9.5) | 169.6 (10.0) | 170.9 (9.5) | 167.2 (10.2) | 170.1 (9.7) |
| Weight, kg | 70.2 (14.0) | 68.7 (13.2) | 74.5 (15.0) | 73.6 (14.9) | 77.7 (14.2) | 77.6 (16.1) |
| Paternal smoking | 63.3 (714/1128) | 58.2 (1059/1819) | 67.2 (452/673) | 58.1 (1321/2274) | 72.9 (153/210) | 59.2 (1620/2737) |
| Maternal smoking | 20.5 (237/1155) | 20.3 (373/1834) | 17.8 (123/690) | 21.2 (487/2299) | 18.2 (39/214) | 20.6 (571/2775) |
| Allergic sensitisation, IgE | 26.5 (267/1007) | 33.8 (538/1593) | 26.0 (151/581) | 32.4 (654/2019) | 28.4 (50/176) | 31.1 (755/2424) |
| Dust/fumes exposure | 41.8 (484/1159) | 35.7 (653/1828) | 42.7 (296/694) | 39.0 (904/2316) | 38.5 (77/200) | 22.1 (572/2583) |
| Ex-smoker | 31.2 (363/1164) | 28.7 (531/1847) | 33.3 (231/694) | 29.5 (684/2317) | 31.6 (68/215) | 29.0 (810/2796) |
| Smoking status | ||||||
| Never smoker | 69.2 (801/1158) | 71.4 (1316/1844) | 67.9 (463/682) | 73.0 (1633/2236) | 70.0 (147/210) | 73.3 (1986/2709) |
| Ex-smoker with < 15 pack-years | 23.2 (269/1158) | 22.3 (412/1844) | 21.6 (147/682) | 21.2 (474/2236) | 16.2 (34/210) | 20.1 (544/2709) |
| Ex-smoker with > = 15 pack-years | 7.6 (88/1158) | 6.3 (116/1844) | 10.6 (72/682) | 5.8 (129/2236) | 13.8 (29/210) | 6.6 (179/2709) |
| Asthma, doctor-diagnosed | 7.1 (82/1160) | 7.4 (136/1846) | 10.4 (72/694) | 10.8 (250/2312) | 15.0 (32/213) | 13.8 (385/2792) |
| Chronic bronchitis | 1.4 (16/1162) | 1.3 (24/1846) | 2.3 (16/693) | 1.2 (27/2314) | 4.2 (9/215) | 2.1 (59/2789) |
| COPD, FEV1/FVC < 0.7 | 2.7 (29/1078) | 3.4 (59/1731) | 2.5 (16/630) | 4.4 (89/2042) | 9.3 (18/193) | 10.8 (264/2448) |
| COPD, FEV1/FVC < GLI LLN | 4.6 (50/1077) | 4.5 (78/1731) | 2.2 (14/629) | 3.8 (77/2038) | 5.7 (11/193) | 5.8 (142/2445) |
| Asthma symptom score | ||||||
| 0 | 74.8 (866/1157) | 76.0 (1392/1831) | 67.9 (468/689) | 75.0 (1723/2298) | 65.6 (137/209) | 72.7 (1974/2714) |
| 1 | 14.1 (163/1157) | 13.0 (238/1831) | 18.3 (126/689) | 13.6 (313/2298) | 16.7 (35/209) | 16.8 (455/2714) |
| 2 | 5.7 (66/1157) | 5.4 (99/1831) | 6.7 (46/689) | 5.9 (136/2298) | 7.7 (16/209) | 6.0 (163/2714) |
| 3 | 2.9 (33/1157) | 2.8 (51/1831) | 3.5 (24/689) | 3.1 (72/2298) | 4.8 (10/209) | 2.5 (67/2714) |
| 4 | 1.6 (18/1157) | 1.6 (30/1831) | 2.5 (17/689) | 1.6 (36/2298) | 3.3 (7/209) | 1.0 (26/2714) |
| 5 | 1.0 (11/1157) | 1.1 (21/1831) | 1.2 (8/689) | 0.8 (18/2298) | 1.9 (4/209) | 1.1 (29/2714) |
| Dyspnoea | 4.6 (54/1163) | 4.9 (90/1847) | 6.5 (45/694) | 5.2 (120/2310) | 9.0 (19/211) | 5.2 (142/2743) |
| Cough | 33.6 (390/1159) | 29.7 (547/1842) | 35.3 (244/692) | 31.7 (733/2312) | 40.1 (85/212) | 33.7 (936/2778) |
| Sputum | 11.2 (129/1153) | 10.1 (185/1827) | 12.0 (83/692) | 10.0 (230/2298) | 15.2 (32/211) | 10.9 (302/2769) |
| FEV1, ml | 3800 (824) | 3781 (835) | 3522 (805) | 3595 (819) | 3028 (756) | 3123 (778) |
| FVC, ml | 4573 (1032) | 4591 (1058) | 4306 (1000) | 4458(1030) | 3904 (995) | 4063 (993) |
| FEV1/FVC, % | 83.5 (6.7) | 82.8 (6.5) | 82.1 (5.9) | 80.9 (6.1) | 77.6 (5.6) | 77.0 (5.7) |
astratified by exposure to second-hand smoke (SHS) and presented as % (n/N) for categorical variables and mean (SD) for continuous variables, respectively
Table 3 shows the change in second-hand smoke exposure from ECRHS I-II, ECRHS II-III as well as ECRHS I-III. Almost 7% (ECRHS I-II) and 2.5% (ECRHS II-III) of the study participants were not exposed at the first, but were exposed at the second examination.
Table 3.
Second-hand smoke (SHS) exposure in ECRHS I-II, ECRHS II-III and ECRHS I-IIIa
| Changes between ECRHS I and II | Changes between ECRHS II and III | Changes between ECRHS I and III | |
|---|---|---|---|
| No SHS exposure at both examinations | 54.4 (1639/3011) | 74.5 (2243/3011) | 58.9 (1774/3011) |
| No SHS exposure at first examination but at second examination | 6.9 (208/3011) | 2.5 (74/3011) | 2.4 (73/3011) |
| SHS exposure at first examination but not at second examination | 22.5 (678/3011) | 18.4 (553/3011) | 33.9 (1022/3011) |
| SHS exposure at both examinations | 16.1 (486/3011) | 4.7 (141/3011) | 4.7 (142/3011) |
apresented as % (n/N)
The distribution of the lung function parameters as well as the annual decline are summarised in Table 4. All lung function parameters (FEV1, FVC and FEV1/FVC) decreased over time, with greater decline in the second 10 year follow-up period (ECRHS II-III; 42 ml/year decline in FEV1) compared to the first 10 year period (ECRHS I-II; 24 ml/year decline in FEV1) as expected with ageing of the population.
Table 4.
Distribution of lung function parameters and annual changea
| ECRHS I | ECRHS II | ECRHS III | Difference between ECRHS I and II (ml or % per year) | Difference between ECRHS II and III (ml or % per year) | Difference between ECRHS I and III (ml or % per year) | |
|---|---|---|---|---|---|---|
| FEV1 (ml) | 3789 (830) | 3578 (816) | 3116 (777) | −24 (36) | −42 (26) | −34 (17) |
| FVC (ml) | 4584 (1048) | 4422 (1025) | 4052 (994) | −19 (45) | −34 (35) | −27 (21) |
| FEV1/FVC (%) | 83 (7) | 81 (6) | 77 (6) | −0.2 (0.5) | −0.4 (0.4) | − 0.3 (0.3) |
apresented as mean (SD)
Adjusted associations between change in second-hand smoke exposure over time and respiratory symptoms and diseases at follow-up [ECRHS I-II, ECRHS II-III and ECRHS I-III]
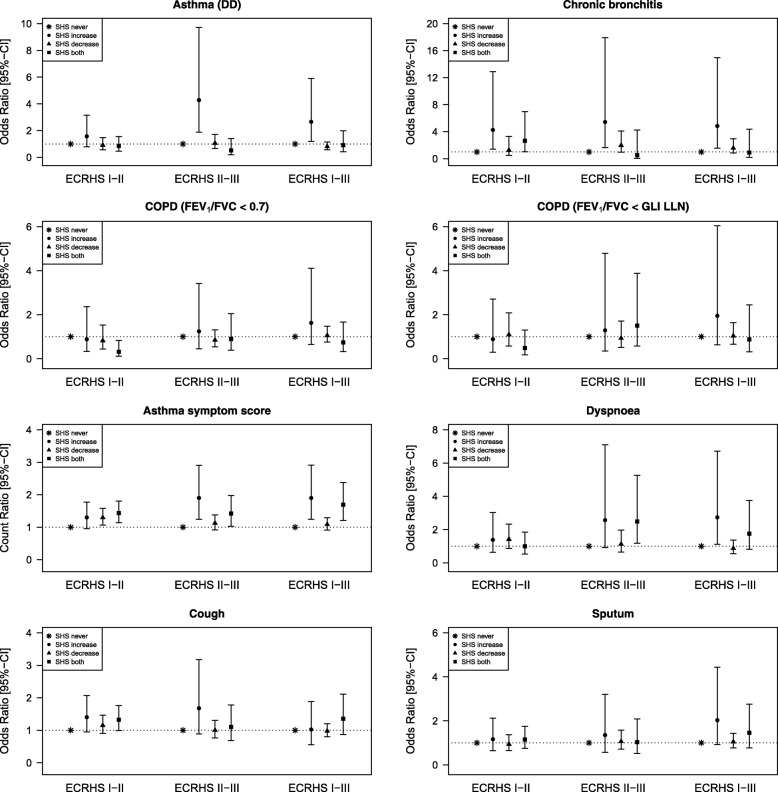
Adjusted time variant analysis of second-hand smoke exposure showed that those reporting increased second-hand smoke exposure had increased risks for the development of doctor-diagnosed asthma, chronic bronchitis and increased asthma symptom score, reaching conventional levels of significance from ECRHS II-III as well as from ECRHS I-III overall (Fig. 2). However, compared to those not exposed on both occasions there was no evidence that those reporting exposure on both occasions had an increased risk of asthma or chronic bronchitis. However, asthma score did increase in this group compared to the non-exposed. An increased risk of nocturnal dyspnoea was observed only for those reporting increased second-hand smoke exposure between the first and the third survey but not for those exposed at both surveys.
Fig. 2.
Associations between change in second-hand smoke (SHS) exposure over time and respiratory symptoms/diseases at follow-up. SHS never: no SHS exposure at both examinations (reference category); SHS increase: no SHS exposure at first examination but at second examination; SHS decrease: SHS exposure at first examination but not at second examination; SHS both: SHS exposure at both examinations. All models are adjusted for sex, age, weight, maternal smoking, paternal smoking, combination of smoking status and pack years, education, exposure to dust/fumes, allergic sensitisation (at baseline) and baseline respiratory symptom/disease
Spirometrically defined COPD was not statistically significantly associated with changes in second-hand smoke exposure at any of the examined periods over the 20 years after adjustment for several selected confounders (Fig. 2).
Adjusted associations between change in second-hand smoke exposure over time and lung function parameters at follow-up
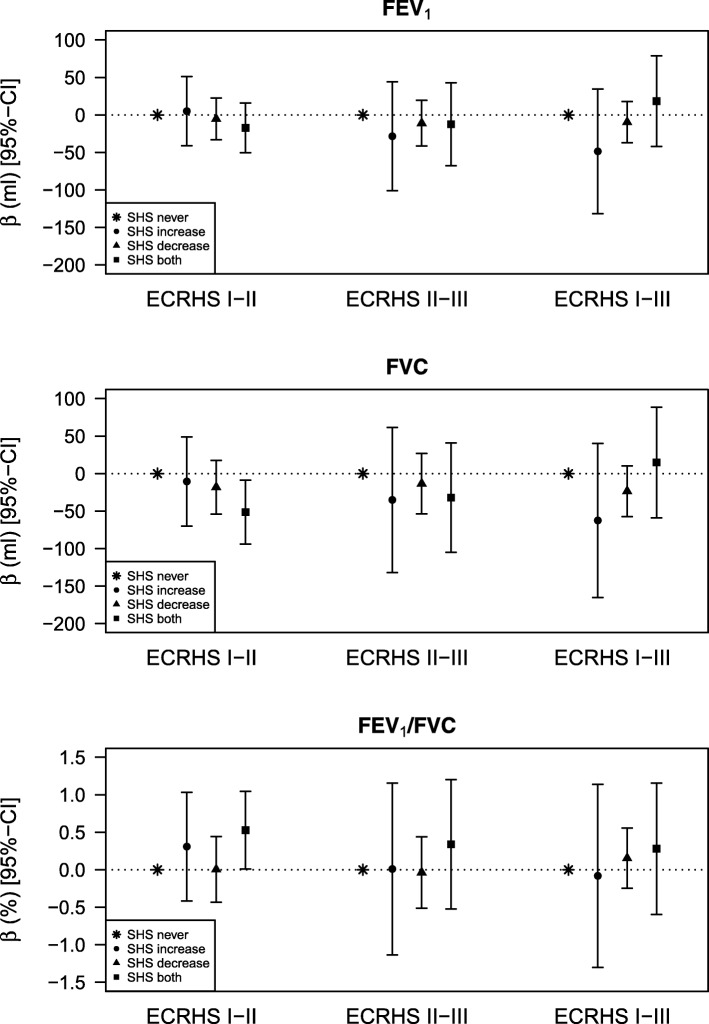
There was no association of second-hand smoke exposure with FEV1.
Study participants exposed to second-hand smoke at the first as well as at the second survey (ECRHS I-II) had a reduced forced vital capacity at the second survey (approximately 50 ml) compared to those not exposed to second-hand smoke at these two surveys (Fig. 3) – but there was no clear or consistent pattern of association over the entire study period.
Fig. 3.
Associations between change in second-hand smoke (SHS) exposure over time and lung function at follow-up. SHS never: no SHS exposure at both examinations (reference category); SHS increase: no SHS exposure at first examination but at second examination; SHS decrease: SHS exposure at first examination but not at second examination; SHS both: SHS exposure at both examinations. All models are adjusted for sex, age, age squared, weight, weight squared, height, maternal smoking, paternal smoking, combination of smoking status and pack years, education, exposure to dust/fumes, allergic sensitisation (at baseline) and baseline lung function
Similarly the ratio of FEV1/FVC showed associations with increased second-hand smoke exposure from ECRHS I-II but no consistent pattern when the data were examined over the entire period.
Sensitivity analyses using percent predicted values according to the Global Lung function Initiative showed comparable results (Additional file 1: Table S1).
Adjusted associations between second-hand smoke exposure at the first examination and lung function as well as lung function decline
The associations of exposure to second-hand smoke at the first examination (ECRHS I) with lung function as well as lung function decline from ECRHS I-III are summarised in Table 5. Exposure to second-hand smoke at the first examination resulted in reduced FEV1 and FVC over time, with stronger effects for males compared to females. It also shows that those exposed to second-hand smoke at the first examination had a slightly slower decline in lung function compared to those not exposed.
Table 5.
Associations between second-hand smoke (SHS) exposure at the first examination and lung function and decline
| Total | Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95%-CI | p-value | β | 95%-CI | p-value | β | 95%-CI | p-value | |
| FEV1a | |||||||||
| SHS exposureb | −44 | (−82, −5) | 0.03 | −75 | (− 140, − 11) | 0.02 | −13 | (− 57, 31) | 0.56 |
| SHS exposure*follow-up timec | 1 | (0, 3) | 0.03 | 2 | (0, 4) | 0.03 | 1 | (−1, 3) | 0.19 |
| FVCa | |||||||||
| SHS exposureb | − 56 | (−102, − 10) | 0.02 | − 102 | (− 179, − 24) | 0.01 | −19 | (−71, 33) | 0.47 |
| SHS exposure*follow-up timec | 2 | (0, 4) | 0.02 | 3 | (0, 6) | 0.03 | 2 | (0, 4) | 0.09 |
| FEV1/FVCa | |||||||||
| SHS exposureb | 0.0 | (− 0.5, 0.5) | 0.96 | 0.1 | (− 0.6, 0.9) | 0.71 | 0.1 | (−0.6, 0.7) | 0.88 |
| SHS exposure*follow-up timec | 0.0 | (0.0, 0.0) | 0.94 | 0.0 | (0.0, 0.0) | 0.96 | 0.0 | (0.0, 0.0) | 0.62 |
Models are adjusted for age, age squared, weight, weight squared, height, combination of smoking status and pack years, maternal smoking, paternal smoking, allergic sensitisation, education and exposure to dust/fumes as well as for sex in the total study population
aan interaction term between time between follow-ups and SHS exposure is included to determine the effect of SHS exposure on lung function decline
ba negative estimate suggests that those exposed to SHS at the first examination had lower average lung function at all three examinations than those not exposed
ca negative estimate suggests that those exposed to SHS at the first examination had a higher decline in lung function between the examinations than those not exposed
* indicating the interaction term between SHS exposure and time of follow-up
Sensitivity analyses restricted to lifetime never smokers, i.e. participants who were never smokers at all three surveys showed comparable results (data not shown).
Discussion
This study investigated the association of exposure to second-hand smoke with respiratory symptoms and diagnoses, as well as lung function and lung function decline in never and former smoking participants in ECRHS I-III. We show that the proportion of the studied population exposed to second-hand smoke fell markedly over the follow-up period of 20 years. Individuals who became exposed to second-hand smoke over time were at increased risk of doctor-diagnosed asthma, chronic bronchitis as well as a higher asthma symptom score. Associations between increase in second-hand smoke exposure and incidence of COPD or lung function were not apparent.
Comparison with results from other epidemiology studies
Only a few studies have examined the association of exposure to second-hand smoke with onset of asthma in adulthood. In the prospective U.S. Black Women’s Health Study, Coogan et al. observed a positive association of passive smoke exposure with the incidence of adult-onset asthma over 15 years of follow-up in 46,182 women aged 21 to 69 years at baseline [25]. Non-smoking study participants who were exposed to second-hand smoke had a 21% increase (adjusted HR: 1.2; 95%-CI: 1.0–1.5) in asthma incidence compared to those not exposed. Similar findings were observed in two Finnish population-based case-control studies [26, 27]. Exposure to second-hand smoke at the workplace or at home increased the risk for the development of asthma during a period of 2.5 years [27]. In our study, an increase in second-hand smoke exposure over time was associated with an increased risk of doctor-diagnosed asthma. An increased asthma symptom score was observed for those reporting increased second-hand smoke exposure as well as for those exposed on both occasions. A decrease in second-hand smoke exposure was also associated with an increased asthma symptom score. However, this association was not consistent, being only recorded from ECRHS I to ECRHS II, and the strength of the association was rather low. Of note, the ECRHS questionnaire had not been specifically devised to assess changes in respiratory symptoms after smoking cessation or decrease in second-hand smoke exposure. Overall our study findings are consistent with results of the few other studies on second-hand smoke and asthma development in adults.
Previous studies have investigated the association of exposure to second-hand smoke with respiratory symptoms and diseases, especially COPD. For instance, Eisner et al. [6] analysed the effect of lifetime exposure to second-hand smoke on the risk for the development of COPD in 2112 adults (including current, former and never smokers) aged 55 to 75 years in the U.S. It showed a positive significant association between cumulative exposure to second-hand smoke at home (adjusted OR: 1.6; 95%-CI: 1.1–2.2) as well as at work (adjusted OR: 1.4; 95%-CI: 1.0–1.8) with self-reported doctor-diagnosed COPD. A Chinese study [8] also investigated the relationship of self-reported density and duration of exposure to passive smoking with respiratory symptoms (cough, phlegm and shortness of breath) and COPD (FEV1/FVC < 0.7 measured pre-bronchodilation) based on data from 15,379 never smoking adults in the Guangzhou Biobank Cohort Study. Exposure to second-hand smoke at home and at work was significantly associated with an increased risk of COPD (adjusted OR: 1.5; 95%-CI: 1.2–1.9) and any respiratory symptoms (adjusted OR: 1.2; 95%-CI: 1.1–1.3).
An increased risk of COPD, defined using the fixed ratio of FEV1/FVC < 0.7 measured post-bronchodilation, was seen in those with second-hand smoke exposure in a study [28] of 2182 lifelong never smokers taking part in the Obstructive Lung Disease in Northern Sweden (OLIN) studies. Exposure to second-hand smoke was categorised into several groups based on previous and current exposure to second-hand smoke at home and at work. The strongest associations were seen in those ever exposed at home and at both previous and current work (adjusted OR: 3.8; 95%-CI: 1.3–11.2) as well as for those currently exposed at home and at both previous and current work (adjusted OR: 5.7; 95%-CI: 1.5–22.5). A significant dose dependent relationship of exposure to second-hand smoke with mortality from different diseases, including COPD amongst other causes of death, could be shown in another study [10]. In contrast, a study conducted by Chan-Yeung et al. [9] found no association between exposure to second-hand smoke and an increased risk for COPD in a small sex- and age-matched case-control study comprising 289 patients and controls, respectively, in Hong Kong, China.
The different associations between exposure to second-hand smoke and COPD in the above studies might be due to the different definition of COPD as some studies used questionnaire-based information whereas others used spirometric measurements. Furthermore, some studies were restricted to lifetime never smokers compared to studies also including active smokers. We have shown no significant association between increase in second-hand smoke exposure and incidence of COPD based on lung function testing in our study, which was restricted to never and former smokers. The different observed associations might also be due to residual confounding in some studies or potential misclassification of self-reported exposure to second-hand smoke in our study.
Another study, based on Taiwan’s National Health Insurance Bureau claims data, investigated the association of exposure to second-hand smoke and chronic bronchitis in women [29] and showed that women who were exposed to second-hand smoke had a 3.7 (95%-CI: 1.2–11.3) higher risk of chronic bronchitis compared to those not exposed to second-hand smoke. Furthermore, exposure to second-hand smoke was also associated with mild (adjusted OR: 1.8; 95%-CI: 1.1–2.9) and moderate (adjusted OR: 3.8; 95%-CI: 1.7–8.6) COPD as defined by GOLD.
We have shown a significant positive association of new exposure to second-hand smoke between two surveys with chronic bronchitis at follow-up, defined as having cough and sputum. However there was no evidence of a decrease in chronic bronchitis if exposure to second-hand smoke stopped over the same time frame. In addition, exposure to second-hand smoke was not associated with COPD defined by a ratio of FEV1/FVC below 0.7 [30, 31]. According to the original classification of COPD from the Global Initiative for Chronic Obstructive Lung Disease (GOLD) in 2001 [32], stage 0 “at risk” is characterised by chronic symptoms (sputum production and cough) with still normal spirometry, i.e. the ratio between FEV1 and FVC of at least 0.7. This GOLD stage 0 would be similar to the definition of chronic bronchitis used in this analysis which requires a positive answer to both the question on cough and the question on sputum, independent of lung function. Moreover, the overlap between chronic bronchitis and COPD defined by spirometry was quite small in this study. As an effect of exposure to second-hand smoke was found in this study only for chronic bronchitis, but not for COPD, one might speculate that these results indicate a transient effect, but not structural changes in the airways as would be common in COPD patients.
Although COPD is generally considered a disease characterised by a progressive, gradually accelerating decline in FEV1 Macklem has pointed out that increase in residual volume (RV) is the first functional abnormality in chronic bronchitis [33]. Thus, gas trapping with reduction of FVC is an early abnormality because RV increases more than the total lung capacity (TLC). The observed decrease in FEV1 occurs because of a reduction in FVC. The FEV1/FVC ratio will decrease because of loss of lung elastic recoil, a sine qua non of COPD [33, 34] but early in the disease the decrease in FVC may exceed that in FEV1 with a paradoxical effect on the FEV1/FVC ratio. This may explain why we did not see an association with COPD defined by spirometric lung function parameters.
A reduced FEV1 and FVC over time was observed for those reporting exposure to second-hand smoke at the first examination, with stronger effects for males compared to females. Several studies have investigated the association between second-hand smoke exposure and lung function, suggesting sex differences in vulnerability [35–38]. However, the findings are inconsistent. Some studies found adverse effects of passive smoking on spirometric lung function parameters for both sexes [35, 38], whereas another study found stronger effects for women compared to men [36]. Our results are consistent with findings of the study conducted by Masjedi et al. [37] showing a negative association between second-hand smoke exposure and lung function among men, but not among women.
Janson et al. [39] investigated changes and determinants for changes in active as well as passive smoking in the first and second survey of the European Community Respiratory Health Survey showing that exposure to second-hand smoke was higher among subjects with lower socio-economic status and educational level. Furthermore, subjects exposed to second-hand smoke were less likely to quit smoking suggesting that a decrease in second-hand smoke exposure might be effective in decreasing active smoking. In our study, exposure to second-hand smoke decreased during the 20 years of follow-up where for many of the study centres the decrease between the second 10 year follow-up period was stronger compared to the first 10 year period. However, second-hand smoke exposure was still present in all participating centres.
Strengths and limitations
The European Community Respiratory Health Survey has a longitudinal study design with two follow-ups approximately 10 and 20 years, respectively, after the first survey and therefore we can model the association between changes in second-hand smoke exposure over time with respiratory health outcomes. We are also able to investigate the effect of exposure to second-hand smoke at baseline on lung function decline using spirometric measurements that were performed and quality controlled according to well established guidelines. Also the large study population of around 2000 never-smoking study participants and the high number of participating centres and countries are further strengths.
However this study has some limitations. The information on second-hand smoke exposure as well as respiratory symptoms and diseases was obtained using self-administered questionnaires completed at follow-up and no biomarkers for exposure to second-hand smoke were available. Moreover, the information on second-hand some exposure was only requested for the last 12 months at each survey and not for the total study period. Furthermore, information on the number of cigarettes smoked by other people was not available. No dose-related association between second-hand smoke exposure and respiratory health has been investigated which has to be taken into account when drawing conclusions.
In addition, against a backdrop of falling smoking rates and smoke free legislation across Europe only a small proportion of the study group became newly exposed to second-hand smoke over the period of the study. Questionnaire-based information on second-hand smoke exposure might be prone to reporting bias as subjects having respiratory symptoms or diseases might tend to be more affected. Siroux et al. [40] has found no indication that asthma status influences reporting of exposure to second-hand smoke in childhood or adulthood but we cannot exclude that our results are related to reporting biases. The use of different spirometers across study centres and surveys could have resulted in temporal differences in lung function measurements. Sensitivity analyses using lung function values corrected for this change showed comparable results. Furthermore, it was difficult to disentangle the survey and age effects due to three time points comprising two follow-ups each after approximately 10 years as different findings were observed for the association of new exposure to second-hand smoke between two surveys with respiratory symptoms and diseases at follow-up.
The questions on cough and sputum were only requested for winter and not for summer, as these symptoms are often more worse during the winter months. Furthermore, no information on the change of the ventilation equipment used for air cleaning during the follow-up periods was available and thus could not be considered as potential confounding variable.
Conclusion
In a longitudinal analysis of adults, following a multi-centre cohort over twenty years, exposure to second-hand smoke decreased substantially during the study period. Second-hand smoke exposure in adults was associated with an increased risk for asthma and chronic bronchitis. Our results support further restrictions on smoking in public places.
Additional file
Table S1. Associations between change in second-hand smoke (SHS) exposure over time and lung function at follow-up [percent predicted values according to the Global Lung function Initiative – GLI]. (DOCX 18 kb)
Acknowledgements
ECRHS I coordinating centre and project management group
Coordinating Centre (London): P Burney, S Chinn, C Luczynska, D Jarvis, E Lai.
Project Management Group: P Burney (Project leader UK), S Chinn (UK), C Luczynska (UK), D Jarvis (UK), P Vermeire (Antwerp), H Kesteloot (Leuven), J Bousquet (Montpellier), D Nowak (Hamburg), J Prichard (Dublin), R de Marco (Verona), B Rijcken (Groningen), JM Anto (Barcelona), J Alves (Oporto), G Boman (Uppsala), N Nielsen (Copenhagen), P Paoletti (Pisa).
ECRHS II principal investigators and senior scientific teams
Australia (M. Abramson, E.H Walters, J. Raven); Belgium: South Antwerp and Antwerp City (P. Vermeire, J. Weyler, M. van Sprundel, V. Nelen); Estonia: Tartu (R. Jõgi, A. Soon); France: Paris (F. Neukirch, B. Leynaert, R. Liard, M. Zureik), Grenoble (I. Pin, J. Ferran-Quentin); Germany: Erfurt (J. Heinrich, M. Wjst, C. Frye, I. Meyer); Iceland: Reykjavik (T. Gislason, E. Bjornsson, D. Gislason, K.B Jörundsdóttir); Italy: Turin (R. Bono, M. Bugiani, P.Piccioni, E. Caria, A. Carosso, E. Migliore, G. Castiglioni), Verona (R. de Marco, G. Verlato, E. Zanolin, S. Accordini, A. Poli, V. Lo Cascio, M. Ferrari, I. Cazzoletti), Pavia (A. Marinoni, S. Villani, M. Ponzio, F. Frigerio, M. Comelli, M. Grassi, I. Cerveri, A. Corsico); Norway: Bergen (A. Gulsvik, E. Omenaas, C. Svanes, B. Laerum); Spain: Albacete (J. Martinez-Moratalla Rovira, E. Almar, M. Arévalo, C. Boix, G González, J.M. Ignacio García, J. Solera, J Damián), Galdakao (N. Muñozguren, J. Ramos, I. Urrutia, U. Aguirre), Barcelona (J. M. Antó, J. Sunyer, M. Kogevinas, J. P. Zock, X. Basagaña, A. Jaen, F. Burgos, C. Acosta), Huelva (J. Maldonado, A. Pereira, J.L. Sanchez), Oviedo (F. Payo, I. Huerta, A. de la Vega, L Palenciano, J Azofra, A Cañada); Sweden: Göteborg (K. Toren,L. Lillienberg, A. C. Olin, B. Balder, A. Pfeifer-Nilsson, R. Sundberg), Umea (E. Norrman, M. Soderberg, K. Franklin, B. Lundback, B. Forsberg, L. Nystrom), Uppsala (C. Janson, G. Boman, D. Norback, G. Wieslander, M. Gunnbjornsdottir); Switzerland: Basel (N. Kuenzli, B. Dibbert, M. Hazenkamp, M. Brutsche, U. Ackermann-Liebrich); United Kingdom: Ipswich (D. Jarvis, R. Hall, D. Seaton), Norwich (D. Jarvis, B. Harrison).
ECRHS III principal investigators and senior scientific teams
Australia: Melbourne (M. Abramson, G. Benke, S. Dharmage, B. Thompson, S. Kaushik); Belgium: South Antwerp & Antwerp City (J. Weyler, M. van Sprundel, V. Nelen, E. Van de Mieroop); France: Bordeaux (C. Raherison, P.O Girodet), Grenoble (I. Pin, V. Siroux, J.Ferran, J.L Cracowski), Montpellier (P. Demoly, A.Bourdin, I. Vachier), Paris (B. Leynaert, D. Soussan, D. Courbon, C. Neukirch, L. Alavoine, X. Duval, I. Poirier); Germany: Erfurt (J. Heinrich, E. Becker, G. Woelke, O. Manuwald), Hamburg (H. Magnussen, D. Nowak, A-M Kirsten); Iceland: Reykjavik (T. Gislason, B. Benediktsdottir, D. Gislason, E.S Arnardottir, M. Clausen, G. Gudmundsson, L. Gudmundsdottir, H. Palsdottir, K. Olafsdottir, S. Sigmundsdottir, K. Bara-Jörundsdottir); Italy: Pavia (I. Cerveri, A.Corsico, A. Grosso, F. Albicini, E. Gini, E.M Di Vincenzo, V. Ronzoni, S. Villani, F. Campanella, F. Manzoni, L. Rossi, O. Ferraro), Turin (M. Bugiani, R. Bono, P. Piccioni, R. Tassinari, V. Bellisario), Verona (R de Marco, S. Accordini, L. Calciano, L. Cazzoletti, M. Ferrari, A.M Fratta Pasini, F. Locatelli, P. Marchetti, A. Marcon, E. Montoli, G. Nguyen, M. Olivieri, C. Papadopoulou, C.Posenato, G. Pesce, P. Vallerio, G. Verlato, E. Zanolin); Norway: (C. Svanes, E. Omenaas, A. Johannessen, T. Skorge, F. Gomez Real); Spain: Albacete (J. Martinez-Moratalla Rovira, E. Almar, A. Mateos, S. García, A. Núñez, P.López, R. Sánchez, E Mancebo), Barcelona (J M. Antó, J.P Zock, J. Garcia-Aymerich, X. Basagaña, F. Burgos, C. Sanjuas, S Guerra), Galdakao (N. Muñozguren, I. Urrutia, U. Aguirre, S. Pascual), Huelva (J Antonio Maldonado, A. Pereira, J. Luis Sánchez, L. Palacios), Oviedo (F. Payo, I. Huerta, N. Sánchez, M. Fernández, B. Robles); Sweden: Göteborg (K. Torén, M. Holm, J-L Kim, A-C Olin, A. Dahlman-Höglund), Umea (B. Forsberg, L. Braback, E. Norrman, L. Modig, B. Järvholm, H. Bertilsson, K. Franklin, C. Wahlgreen, M. Soderberg) Uppsala (B. Andersson, D. Norback, U. Spetz Nystrom, G. Wieslander, G.M Bodinaa Lund, K. Nisser); Switzerland: Basel (N.M. Probst-Hensch, N. Künzli, D. Stolz, C. Schindler, T. Rochat, J.M. Gaspoz, E. Zemp Stutz, M. Adam, C. Autenrieth, I. Curjuric, J. Dratva, A. Di Pasquale, R. Ducret-Stich, E. Fischer, L. Grize, A. Hensel, D. Keidel, A. Kumar, M. Imboden, N. Maire, A. Mehta, H. Phuleria, M. Ragettli, M. Ritter, E. Schaffner, G.A Thun, A. Ineichen, T. Schikowski, M. Tarantino, M. Tsai); UK: Ipswich (N. Innes), Norwich (A. Wilson).
Funding
ECRHS I financial support
The following grants helped to fund the local studies.
Australia: Asthma Foundation of Victoria, Allen and Hanbury’s; Belgium: Belgian Science Policy Office, National Fund for Scientific Research; Estonia: Estonian Science Foundation, grant no 1088; France: Ministère de la Santé, Glaxo France, Insitut Pneumologique d’Aquitaine, Contrat de Plan Etat-Région Languedoc-Rousillon, CNMATS, CNMRT (90MR/10, 91AF/6), Ministre delegué de la santé, RNSP, France; GSF; Germany: Bundesminister für Forschung und Technologie; Italy: Ministero dell’Università e della Ricerca Scientifica e Tecnologica, CNR, Regione Veneto grant RSF n. 381/05.93; Norway: Norwegian Research Council project no. 101422/310; Spain: Fondo de Investigación Sanitaria (#91/0016–060-05/E, 92/0319 and #93/0393), Hospital General de Albacete, Hospital General Juan Ramón Jiménez, Dirección Regional de Salud Pública (Consejería de Sanidad del Principado de Asturias), CIRIT (1997 SGR 00079) and Servicio Andaluz de Salud; Sweden: The Swedish Medical Research Council, the Swedish Heart Lung Foundation, the Swedish Association against Asthma and Allergy; Switzerland: Swiss national Science Foundation grant 4026–28099; UK: National Asthma Campaign, British Lung Foundation, Department of Health, South Thames Regional Health Authority.
The co-ordination of this work was supported by the European Commission.
ECRHS II financial support
The following grants helped to fund the local studies
Australia: National Health and Medical Research Council; Belgium: Antwerp: Fund for Scientific Research (grant code, G.0402.00), University of Antwerp, Flemish Health Ministry; Estonia: Tartu Estonian Science Foundation grant no 4350; France: Bordeaux: Institut Pneumologique d’Aquitaine; Grenoble: Programme Hospitalier de Recherche Clinique – Direction de la Recherche Clinique (DRC) de Grenoble 2000 number 2610, Ministry of Health, Ministere de l’Emploi et de la Solidarite, Direction Generale de la Sante, Centre Hospitalier Universitaire (CHU) de Grenoble, Comite des Maladies Respiratoires de l’Isere; Montpellier: Programme Hospitalier de Recherche Clinique – DRC de Grenoble 2000 number 2610, Ministry of Health, Direction de la Recherche Clinique, CHU de Grenoble, Ministere de l’Emploiet de la Solidarite, Direction Generale de la Sante, Aventis (France), Direction Regionale des Affaires Sanitaires et Sociales Languedoc-Roussillon; Paris: Ministere de l’Emploi et de la Solidarite, Direction Generale de la Sante,Union Chimique Belge- Pharma (France), Aventis (France), Glaxo France, Programme Hospitalier de Recherche Clinique – DRC de Grenoble 2000 number 2610, Ministry of Health, Direction de la Recherche Clinique, CHU de Grenoble; Germany: Erfurt: GSF – National Research Centre for Environment and Health, Deutsche Forschungsgemeinschaft (grant code, FR1526/1–1); Hamburg: GSF – National Research Centre for Environment and Health, Deutsche Forschungsgemeinschaft (grant code, MA 711/4–1); Iceland: Reykjavik: Icelandic Research Council, Icelandic University Hospital Fund; Italy: Pavia: GlaxoSmithKline Italy, Italian Ministry of University and Scientific and Technological Research (MURST), Local University Funding for Research 1998 and 1999; Turin: Azienda Sanitaria Locale 4 Regione Piemonte (Italy), Azienda Ospedaliera Centro Traumatologico Ospedaliero/Centro Traumatologico Ortopedico—Istituto Clinico Ortopedico Regina Maria Adelaide Regione Piemonte; Verona: Ministero dell’Universita´ e della Ricerca Scientifica (MURST), Glaxo Wellcome spa: Norway: Bergen: Norwegian Research Council, Norwegian Asthma and Allergy Association, Glaxo Wellcome AS, Norway Research Fund; Spain: Fondo de Investigacion Santarias (grant codes, 97/0035–01,99/0034–01 and 99/0034 02), HospitalUniversitario de Albacete, Consejeria deSanidad; Barcelona: Sociedad Espanola de Neumologı’a y Cirugı’a Toracica, Public Health Service (grant code, R01 HL62633–01), Fondo de Investigaciones Santarias (grant codes, 97/0035–01, 99/0034–01, and 99/0034–02), Consell Interdepartamentalde Recerca i Innovacio´ Tecnolo’gica (grant code, 1999SGR 00241) Instituto de Salud Carlos III; Red deCentros de Epidemiologı’a y Salud Pu′blica, C03/09,Redde Basesmoleculares y fisiolo’gicas de lasEnfermedadesRespiratorias,C03/011and Red de Grupos Infancia y Medio Ambiente G03/176; Huelva: Fondo de Investigaciones Santarias (grant codes, 97/0035–01, 99/0034–01, and 99/0034–02); Galdakao: Basque Health Department; Oviedo: Fondo de Investigaciones Sanitaria (97/0035–02, 97/0035, 99/0034–01, 99/0034–02, 99/0034–04, 99/0034–06, 99/350, 99/0034--07), European Commission (EU-PEAL PL01237), Generalitat de Catalunya (CIRIT 1999 SGR 00214), Hospital Universitario de Albacete, Sociedad Española de Neumología y Cirugía Torácica (SEPAR R01 HL62633–01) Red de Centros de Epidemiología y Salud Pública (C03/09), Red de Bases moleculares y fisiológicas de las Enfermedades Respiratorias (C03/011) and Red de Grupos Infancia y Medio Ambiente (G03/176; 97/0035–01, 99/0034–01, and 99/0034–02); Sweden: Göteborg, Umea, Uppsala: Swedish Heart Lung Foundation, Swedish Foundation for Health Care Sciences and Allergy Research, Swedish Asthma and Allergy Foundation, Swedish Cancer and Allergy Foundation, Swedish Council for Working Life and Social Research (FAS); Switzerland: Basel: Swiss National Science Foundation, Swiss Federal Office for Education and Science, Swiss National Accident Insurance Fund; UK: Ipswich and Norwich: Asthma UK (formerly known as National Asthma Campaign).
ECRHS III financial support
The following grants helped to fund the local studies.
Australia: National Health & Medical Research Council; Belgium: Antwerp South, Antwerp City: Research Foundation Flanders (FWO), grant code G.0.410.08.N.10 (both sites); Estonia: Tartu: SF0180060s09 from the Estonian Ministry of Education; France (All): Ministère de la Santé. Programme Hospitalier de Recherche Clinique (PHRC) national 2010; Bordeaux: INSERM U897 Université Bordeaux segalen; Grenoble: Comitee Scientifique AGIRadom 2011; Paris: Agence Nationale de la Santé, Région Ile de France, domaine d’intérêt majeur (DIM); Germany: Erfurt: German Research Foundation HE 3294/10–1; Hamburg: German Research Foundation MA 711/6–1, NO 262/7–1; Iceland: Reykjavik: The Landspitali University Hospital Research Fund, University of Iceland Research Fund, ResMed Foundation, California, USA, Orkuveita Reykjavikur (Geothermal plant), Vegagerðin (The Icelandic Road Administration (ICERA). Italy: All Italian centres were funded by the Italian Ministry of Health, Chiesi Farmaceutici SpA, in addition Verona was funded by Cariverona foundation, Education Ministry (MIUR); Norway: Norwegian Research council grant no 214123, Western Norway Regional Health Authorities grant no 911631, Bergen Medical Research Foundation; Spain: Fondo de Investigación Sanitaria (PS09/02457, PS09/00716 09/01511, PS09/02185 PS09/03190), Servicio Andaluz de Salud, Sociedad Española de Neumología y Cirurgía Torácica (SEPAR 1001/2010); Barcelona: Fondo de Investigación Sanitaria (FIS PS09/00716); Galdakao: Fondo de Investigación Sanitaria (FIS 09/01511); Huelva: Fondo de Investigación Sanitaria (FIS PS09/02185) and Servicio Andaluz de Salud; Oviedo: Fondo de Investigación Sanitaria (FIS PS09/03190); Sweden: All centres were funded by The Swedish Heart and Lung Foundation, The Swedish Asthma and Allergy Association, The Swedish Association against Lung and Heart Disease. Swedish Research Council for health, working life and welfare (FORTE); Göteborg: also received further funding from the Swedish Council for Working life and Social Research; Umea: also received funding from Vasterbotten Country Council ALF grant; Switzerland: The Swiss National Science Foundation (grants no 33CSCO-134276/1, 33CSCO-108796, 3247BO-104283, 3247BO-104288, 3247BO-104284, 3247–065896, 3100–059302, 3200–052720, 3200–042532, 4026–028099) The Federal office for forest, environment and landscape, The Federal Office of Public Health, The Federal Office of Roads and Transport, the canton’s government of Aargan, Basel-Stadt, Basel-Land, Geneva, Luzern, Ticino, Valais and Zürich, the Swiss Lung League, the canton’s Lung League of Basel Stadt/Basel, Landschaft, Geneva, Ticino, Valais and Zurich, SUVA, Freiwillige Akademische Gesellschaft, UBS Wealth Foundation, Talecris Biotherapeutics GmbH, Abbott Diagnostics, European Commission 018996 (GABRIEL), Wellcome Trust WT 084703MA; UK: Medical Research Council (MRC), support of the National Institute for Health Research through the Primary Care Research Network.
Availability of data and materials
The datasets used and analysed during the current study are available from the authors upon reasonable request.
Authors’ contributions
JH and CF designed the study. CF conducted the statistical analyses and wrote the initial draft. All authors provided substantial contributions to the conception or design of the work, the acquisition, analysis or interpretation of data, revised the manuscript and approved the final version.
Ethics approval and consent to participate
Local ethics committees at each centre approved the study protocols, and all the participants gave their written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oberg M, Jaakkola MS, Woodward A, Peruga A, Prüss-Ustün A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 2011;377(9760):139–146. doi: 10.1016/S0140-6736(10)61388-8. [DOI] [PubMed] [Google Scholar]
- 2.Strachan DP, Cook DG. Health effects of passive smoking. 5. Parental smoking and allergic sensitisation in children. Thorax. 1998;53:117–123. doi: 10.1136/thx.53.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strachan DP, Cook DG. Health effects of passive smoking. 6. Parental smoking and childhood asthma: longitudinal and case-control studies. Thorax. 1998;53:204–212. doi: 10.1136/thx.53.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook DG, Strachan DP, Carey IM. Health effects of passive smoking. 9. Parental smoking and spirometric indices in children. Thorax. 1998;53:884–893. doi: 10.1136/thx.53.10.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook DG, Strachan DP. Health effects of passive smoking. 10. Summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax. 1999;54:357–366. doi: 10.1136/thx.54.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisner MD, Balmes J, Katz PP, Trupin L, Yelin EH, Blanc PD. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ Health. 2005;4(1):7. doi: 10.1186/1476-069X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sobonya R, Burrows B. The epidemiology of emphysema. Clin Chest Med. 1983;4(3):351–358. [PubMed] [Google Scholar]
- 8.Yin P, Jiang CQ, Cheng KK, Lam TH, Lam KH, Miller MR, et al. Passive smoking exposure and risk of COPD among adults in China: the Guangzhou biobank cohort study. Lancet. 2007;370(9589):751–757. doi: 10.1016/S0140-6736(07)61378-6. [DOI] [PubMed] [Google Scholar]
- 9.Chan-Yeung M, Ho ASS, Cheung AHK, Liu RWT, Yee WKS, Sin KM, et al. Determinants of chronic obstructive pulmonary disease in Chinese patients in Hong Kong. Int J Tuberc Lung Dis. 2007;11(5):502–507. [PubMed] [Google Scholar]
- 10.McGhee SM, Ho SY, Schooling M, Ho LM, Thomas GN, Hedley AJ, et al. Mortality associated with passive smoking in Hong Kong. BMJ. 2005;330(7486):287–288. doi: 10.1136/bmj.38342.706748.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer F, Kraemer A. Meta-analysis of the association between second-hand smoke exposure and ischaemic heart diseases. COPD and stroke BMC Public Health. 2015;15:1202. doi: 10.1186/s12889-015-2489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janson C, Chinn S, Jarvis D, Zock JP, Torén K, Burney P, et al. Effect of passive smoking on respiratory symptoms, bronchial responsiveness, lung function, and total serum IgE in the European Community respiratory health survey: a cross-sectional study. Lancet. 2001;358(9299):2103–2109. doi: 10.1016/S0140-6736(01)07214-2. [DOI] [PubMed] [Google Scholar]
- 13.Accordini S, Calciano L, Johannessen A, Portas L, Benediktsdottir B, Bertelsen RJ, et al. A three-generation study on the association of tobacco smoking with asthma. Int J Epidemiol. 2018;47:1106–1117. doi: 10.1093/ije/dyy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svanes C, Sunyer J, Plana E, Dharmage S, Heinrich J, Jarvis D, et al. Early life origins of chronic obstructive pulmonary disease. Thorax. 2010;65:14–20. doi: 10.1136/thx.2008.112136. [DOI] [PubMed] [Google Scholar]
- 15.Allinson JP, Hardy R, Donaldson GC, Shaheen SO, Kuh D, Wedzicha JA. Combined impact of smoking and early-life exposures on adult lung function trajectories. Am J Respir Crit Care Med. 2017;196:1021–1030. doi: 10.1164/rccm.201703-0506OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regan EA, Lynch DA, Curran-Everett D, Curtis JL, Austin JHM, Grenier PA, et al. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med. 2015;175(9):1539–1549. doi: 10.1001/jamainternmed.2015.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374(19):1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Community Respiratory Health Survey II Steering Committee The European Community respiratory health survey II. Eur Respir J. 2002;20(5):1071–1079. doi: 10.1183/09031936.02.00046802. [DOI] [PubMed] [Google Scholar]
- 19.Burney PG, Luczynska C, Chinn S, Jarvis D. The European Community respiratory health survey. Eur Respir J. 1994;7(5):954–960. doi: 10.1183/09031936.94.07050954. [DOI] [PubMed] [Google Scholar]
- 20.Sunyer J, Pekkanen J, Garcia-Esteban R, Svanes C, Künzli N, Janson C, et al. Asthma score: predictive ability and risk factors. Allergy. 2007;62:142–148. doi: 10.1111/j.1398-9995.2006.01184.x. [DOI] [PubMed] [Google Scholar]
- 21.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 22.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuertes E, Carsin AE, Antó JM, Bono R, Corsico AG, Demoly P, et al. Leisure-time vigorous physical activity is associated with better lung function: the prospective ECRHS study. Thorax. 2018;73:376–384. doi: 10.1136/thoraxjnl-2017-210947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria; 2017. Available from: https://www.R-project.org/.
- 25.Coogan PF, Castro-Webb N, Yu J, O’Connor GT, Palmer JR, Rosenberg L. Active and passive smoking and the incidence of asthma in the black Women’s health study. Am J Respir Crit Care Med. 2015;191:168–176. doi: 10.1164/rccm.201406-1108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lajunen TK, Jaakkola JJK, Jaakkola MS. The synergistic effect of heredity and exposure to second-hand smoke on adult-onset asthma. Am J Respir Crit Care Med. 2013;188:776–782. doi: 10.1164/rccm.201304-0773OC. [DOI] [PubMed] [Google Scholar]
- 27.Jaakkola MS, Piipari R, Jaakkola N, Jaakkola JJK. Environmental tobacco smoke and adult-onset asthma: a population-based incident case-control study. Am J Public Health. 2003;93:2055–2060. doi: 10.2105/AJPH.93.12.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagstad S, Bjerg A, Ekerljung L, Backman H, Lindberg A, Rönmark E, et al. Passive smoking exposure is associated with increased risk of COPD in never smokers. Chest. 2014;145(6):1298–1304. doi: 10.1378/chest.13-1349. [DOI] [PubMed] [Google Scholar]
- 29.Wu CF. Feng NH, Chong IW, Wu KY, Lee CH, Hwang JJ, et al. Second-hand smoke and chronic bronchitis in Taiwanese women: a health-care based study BMC Public Health. 2010;10:44. doi: 10.1186/1471-2458-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Global Initiative for Chronic Obstructive Lung Disease. Pocket guide to COPD diagnosis, management, and prevention. A guide for health care professionals; 2017. Available from: https://goldcopd.org/.
- 31.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2006. Available from: https://goldcopd.org/.
- 32.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS, Committee GS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO global initiative for chronic obstructive lung disease (GOLD) workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 33.Macklem PT. Therapeutic implications of the pathophysiology of COPD. Eur Respir J. 2010;35:676–680. doi: 10.1183/09031936.00120609. [DOI] [PubMed] [Google Scholar]
- 34.Hogg JC, Paré PD, Hackett TL. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiol Rev. 2017;97:529–552. doi: 10.1152/physrev.00025.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langhammer A, Johnsen R, Gulsvik A, Holmen TL, Bjermer L. Sex differences in lung vulnerability to tobacco smoking. Eur Respir J. 2003;21:1017–1023. doi: 10.1183/09031936.03.00053202. [DOI] [PubMed] [Google Scholar]
- 36.Eisner MD. Environmental tobacco smoke exposure and pulmonary function among adults in NHANES III: impact on the general population and adults with current asthma. Environ Health Perspect. 2002;110:765–770. doi: 10.1289/ehp.02110765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masjedi MR, Kazemi H, Johnson DC. Effects of passive smoking on the pulmonary function of adults. Thorax. 1990;45:27–31. doi: 10.1136/thx.45.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janzen B, Karunanayake C, Rennie D, Pickett W, Lawson J, Kirychuk S, et al. Gender differences in the association of individual and contextual exposures with lung function in a rural Canadian population. Lung. 2017;195:43–52. doi: 10.1007/s00408-016-9950-8. [DOI] [PubMed] [Google Scholar]
- 39.Janson C, Künzli N, de Marco R, Chinn S, Jarvis D, Svanes C, et al. Changes in active and passive smoking in the European Community respiratory health survey. Eur Respir J. 2006;27(3):517–524. doi: 10.1183/09031936.06.00106605. [DOI] [PubMed] [Google Scholar]
- 40.Siroux V, Guilbert P, Le Moual N, Oryszczyn MP, Kauffmann F. Influence of asthma on the validity of reported lifelong environmental tobacco smoke in the EGEA study. Eur J Epidemiol. 2004;19:841–849. doi: 10.1023/B:EJEP.0000040528.89863.59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Associations between change in second-hand smoke (SHS) exposure over time and lung function at follow-up [percent predicted values according to the Global Lung function Initiative – GLI]. (DOCX 18 kb)
Data Availability Statement
The datasets used and analysed during the current study are available from the authors upon reasonable request.