Abstract
Purpose:
The relative afferent pupillary defect (RAPD) is an important sign of asymmetrical retinal ganglion cell damage. The purpose of this study was to quantify RAPD by a pupillometer (RAPiDo, Neuroptics) and assess its correlation with asymmetric glaucoma and manual pupillary assessment.
Methods:
A total of 173 subjects were enrolled in the study and categorized into glaucoma, n = 130, and control, n = 43. Subjects were all recruited in the Glaucoma Clinic of the Aravind Eye Hospital in Madurai during their follow-up. They were 18 years and older, with best corrected visual acuity of 6/36 or better. Exclusion criteria included all retinal pathologies, optic atrophies, ocular injuries, severe uveitis, cloudy corneas, dense cataracts, or use of mydriatics or miotic drugs. RAPD was assessed in all subjects using an automated pupillometer and the results were compared with the swinging flash light test conducted on the same subjects by an experienced ophthalmologist. We looked at the correlation between RAPD and the intereye difference in cup-to-disc ratio (CDR), mean deviation (MD) of visual field testing, and retinal nerve fiber layer (RNFL) thickness. Sensitivity and specificity were assessed by area under the receiver operator characteristic (AUROC) analysis.
Results:
Glaucoma patients had significant RAPD (0.55 ± 0.05 log units) when compared with the controls (0.25 ± 0.05 log units), P < 0.001. Significant intereye differences in CDR, MD, and RNFL between glaucoma and control (P < 0.001) were seen. There was a good correlation between the magnitude and sign of RAPD and these intereye differences in CDR (r = 0.52, P < 0.001), MD (r = 0.44, P < 0.001) and RNFL thickness (r = 0.59, P < 0.001). When compared with the experienced ophthalmologist, AUROC was 0.94, with 89% sensitivity and 91.7% specificity.
Conclusion:
The good correlation between the magnitude of RAPD, as measured by the automated pupillometer, and intereye differences in MD, CDR, and RNFL thickness in glaucomatous, and the good sensitivity and specificity when compared with the experienced ophthalmologist, suggest that pupillometry may be useful as a screening tool to assess asymmetric glaucoma.
Keywords: Automated pupillometry, glaucoma, relative afferent pupillary defect, screening glaucoma, swinging flashlight test
Glaucoma refers to a broad spectrum of chronic and degenerative optic neuropathies discernable by the appearance of the optic nerve head (cupping) and visual-field damage.[1] It is the third major cause of blindness in India, responsible for 5.9% of blindness.[2,3,4] More importantly, it has been estimated that there are more than 60 million cases in a survey conducted by Saxena et al.[5] It was observed that more than 90% cases of glaucoma were undiagnosed and identified only at the time of the survey. Worldwide, the number of people with glaucoma is estimated to be more than 60 million and is projected to increase to 80 million by 2020.[6,7] Detection and mass screening of glaucoma remains a challenge; hence, advanced methods that could be applied worldwide is the need of the hour as patients are asymptomatic until late in the disease process.[8]
Asymmetric optic nerves are often the first sign of glaucoma and damage in one eye significantly increases the risk of subsequent damage in the contralateral eye.[9,10,11,12] In a study by Sarezky et al.[13] unilateral involvement was seen in 27.9% of patients. In fact, asymmetry analysis of any sort is used to identify patients with field loss or considered at elevated risk for glaucoma before subjective field loss occurs.[12,14] In this study we hypothesize that asymmetric optic nerves or visual loss to be directly translated into the presence of asymmetric pupillary responses.
Relative afferent pupillary defect (RAPD) or Marcus Gunn pupil is a clinical sign whereupon the patient's pupils constrict relatively less when a bright light is swung from the unaffected eye to the affected eye:[15] the “swinging flashlight test” is a noninvasive, simple, and relatively inexpensive procedure for assessing RAPD. It was first introduced by Levatin et al.[15] and then consolidated by Thompson et al.[16] The use of neutral density filters was later introduced to quantify RAPD.[17] However, the swinging flashlight test is subjected to various levels of variability: ambient light conditions, the experience of the clinician performing the test, and lack of a precise criteria for quantification, relegate the test to a small niche of physicians well trained for this specific task.[18,19]
Automatic high-resolution infrared pupillometry introduces objectivity and allows all clinical personnel to conduct the test reliably and objectively in many conditions. Examples of lab custom-made binocular pupillometers have been proposed in the literature and used in different studies and types of application.[20,21,22,23] To our knowledge, there are only two computerized pupillometers on the market capable of performing automatic RAPD analysis. One, RAPDx (Konan Medical Inc., Irvine, CA, USA) is a binocular desktop system that administrates a multichromatic swinging flashlight test for a duration varying from a minimum of 38 s to up approximately 7 min.[24,25,26]
The new, commercially available binocular system RAPiDo (Neuroptics Inc., Irvine, CA, USA [Fig. 1]) is the direct consequence of a servo-analytic modeling of the pupil, initiated by the pioneering work of Sherman and Stark[27] and recently consolidated by Privitera and Stark.[28] It is a portable, battery-operated binocular pupillometer. In this study it is applied to a population of glaucoma patients and a control group and compared with pupillary evaluation conducted by an ophthalmologist with experience in performing the swinging flashlight test. Correlation with intereye difference of the cup-to-disc ratio (CDR, fundoscopy), retinal nerve fiber layer (RNFL, optical coherence tomography (OCT)) thickness and mean deviation (MD) of visual field testing as well as comparison with the ophthalmologist clinical evaluation will be reported and discussed.
Figure 1.
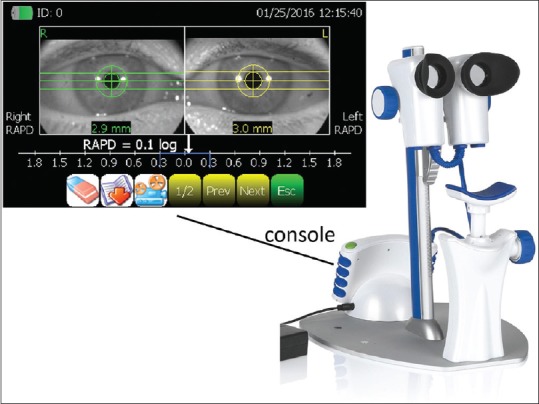
Binocular pupillometer. The binocular RAPiDo pupilometer attached to its stand with the chinrest (right). The console display unit at the base and the two cameras could be removed and used without the stand on supine subjects. The result display after a measurement (top-left) – in the example, an RAPD of 0.1 log units is reported on the left eye and displayed in the console LCD screen
Methods
A total of 173 subjects were enrolled in the study and categorized into the glaucoma group (n = 130) and control group (n = 43). They were all recruited in the Glaucoma Clinic of the Aravind Eye Hospital in Madurai during their follow-up visit between March 1 and May 31, 2017. The study protocol was approved by the Institutional Review Board of Aravind Eye Care System and the methods adhered to the Declaration of Helsinki. The procedure was explained in detail and informed consent was obtained from all the subjects.
All subjects included in the study were of age 18 years and above, with best-corrected visual acuity (BCVA) of 6/36 or better. Glaucoma is defined as a disturbance in structural or functional integrity of the optic nerve and RNFL, which was consistent with visual field abnormalities and changes in RNFL thickness (according to the AAO directives[12]; see also Jonas et al.[1]). Patients with no abnormality in ophthalmological examination with normal visual fields were defined as normal population.
We enrolled patients with glaucoma of any cause (primary open angle glaucoma, primary angle closure glaucoma post Yag laser peripheral iridotomy, and pseudo exfoliation glaucoma) in at least one eye.
Patients with retinal pathologies such as vessel occlusions, retinal detachment, severe nonproliferative diabetic retinopathy, and proliferative diabetic retinopathy with extensive panretinal photocoagulation were excluded from the study. Other causes of exclusion were: optic atrophies, blunt ocular injuries; cloudy corneas and dense cataracts affecting the measurement of RAPD and visual fields; patients on drugs affecting the pupillary response such as mydriatics, miotics, brimonidine, and systemic alpha-blockers; conditions affecting the shape of pupil as in post cataract surgery (damage to sphincter papillae); congenital pupillary abnormalities; and severe uveitis.
We enrolled normal subjects visiting Aravind Eye Hospital for routine annual examination in the control arm or from family members of the patients. We wanted to be sure that there were no glaucoma subjects in the control arm and to avoid the problem of possible interobserver variability. Thus, those subjects whose examination findings revealed CDR of >0.5, signs of retinal or optic nerve structural abnormalities, and any abnormal visual field defect in either eye were excluded from the study.
Pupillary response was assessed by swinging flashlight test (RAPD “present” vs. “not present”) in a dark room with a torch light by an experienced doctor (SS) with 8 years of experience in the procedure and before all the other pupillometric and clinical evaluations. Assessment of RAPD was done in the same room with the automated pupillometer by an ophthalmic assistant and in complete darkness.
All enrolled patients underwent a detailed ocular examination, including BCVA measured by Snellen visual acuity charts; manifest refraction using auto refractometer (Auto-Ref-Keratometer, RK-5, Canon, Tokyo, Japan). Slit lamp examination (Carl Zeiss. Oberkochen, Germany) of anterior segment and fundus examination to evaluate the CDR, using an indirect 78 D lens (Volk Optical Inc., OH, USA) was done. Intraocular pressure (IOP) was measured using Goldman Applanation tonometry, gonioscopy using a Zeiss 4 mirror prism (for Shaffer grading), and central corneal thickness measurement using a pachymeter (Pacscan 300, Sonomed, NY, USA).
Visual field examination with Humphrey field analyzer (HFA) using SITA standard 24-2 algorithm (Carl Zeiss Meditec.) was performed and the MD of reliable fields finally considered for our analysis. All glaucoma patients were on a follow-up visit and had done visual field testing earlier. Subjects in the normal group underwent repeat visual field testing if the fields were unreliable. Measurement of RNFL thickness was done by a Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany).
The algorithm implemented in the RAPiDo is based on a train of alternating left–right light flashes for a total duration that goes from a minimum of 24 s to a maximum, in the case of multiple blinks, of 31 s. A cut-off reference normative value is also provided and reported graphically in the result page for comparison with each measurement. The pupillometer has a spatial accuracy of 0.03 mm; repeatability is discussed later in the Discussions section.
The normative value, equal to 0.3 log units, was determined by an internal and unpublished study conducted by the manufacturer with a population of healthy subjects and corresponds to results reported elsewhere in the literature; see for example Wilhelm et al.[22]
Linear regression analysis was done to look at the correlation between RAPD and asymmetry of CDR, MD (HFA), and RNFL thickness. We assessed the associative model using sensitivity, specificity, and the area under the receiver operator characteristic (ROC) curve. For size, the only variable that is normally distributed, a two-sample independent t-test was used and for all the other parameters the alternative nonparametric Mann–Whitney U-test was applied.
Results
Glaucoma patients had significant RAPD (0.55 log units, confidence interval (CI) [0.44–0.65]) when compared with the controls (0.21 log units, CI [0.17–0.26], P < 0.001) [Table 1]. Significant differences were seen in intereye differences in CDR, MD, in the visual field testing (HFA), and RNFL thickness between the glaucoma and the control group (P < 0.001). Anisocoria, the difference in size between the two eyes, does not show any relevant information when the two populations are compared. For IOP, glaucoma population is only a little higher than control. This is because all patients were already under treatment for controlling and stabilizing elevated IOP, and thus a difference with a healthy subject's baseline is, in this case, not expected.
Table 1.
Glaucoma vs. Control comparison table
| Glaucoma | Control | P | |
|---|---|---|---|
| RAPD (log units) | 0.55 [0.44-0.65] | 0.21 [0.17-0.26] | <0.001* |
| Size (mm) | 4.53 [4.40-4.67] | 5.54 [5.25-5.83] | <0.001* |
| Anisocoria (mm) | 0.26 [0.22-0.30] | 0.20 [0.15-0.24] | 0.242 |
| Age | 56.9 [55.31-58.58] | 35.21 [31.03-39.39] | <0.001* |
| IOP (mmHg) | 19.88 [16.99-22.78] | 16.88 [16.09-17.68] | 0.017* |
| CDR (cup/disk ratio) | 0.09 [0.07-0.11] | 0.03 [0.01-0.04] | <0.001* |
| HFA (MD dB) | 5.52 [4.48-6.56] | 2.24 [1.03-3.44] | <0.001* |
| OCTAve. (RNFL) | 12.72 [10.48-14.97] | 5.59 [4.05-7.12] | <0.001 |
Two sample independent t-test for size, Mann-Whitney U-test for all the other variables. Asterisks indicate statistical significance. 95% CIs is reported in brackets. IOP is intraocular pressure, CDR is evaluated by fundoscopy, HFA is mean deviation of Humphrey field analyzer, OCTAve. is average of RNFL thickness measured via optical coherent tomography. All values represent delta between eyes
There was a good linear correlation between the magnitude of RAPD and its sign (indicating which eye was affected [Fig. 2]) with intereye differences in MD (r = 0.44, P < 0.001, top-right), CDR (r = 0.52, P < 0.001, bottom-left), and RNFL thickness (r = 0.59, P < 0.001, bottom-right). Anisocoria, however, was again not correlated (top-left).
Figure 2.
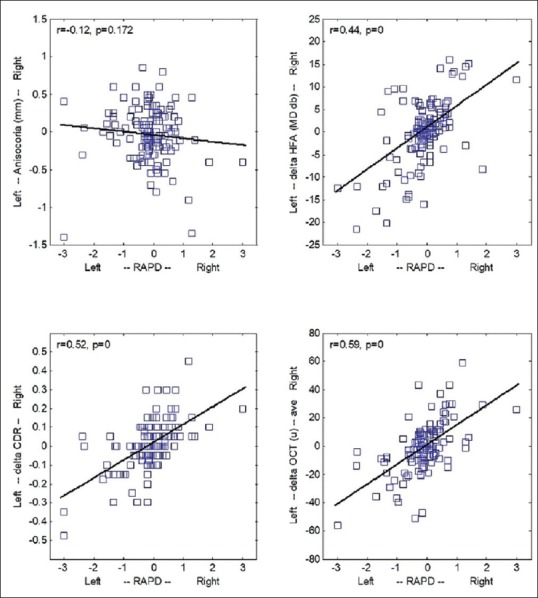
Intereye differences as a function of RAPD. Intereye difference was evaluated for: (i) pupil size (anisocoria, diameter of the right pupil minus diameter of left pupil top-left); (ii) MD of the perimetry visual field test (HFA, MD of the right eye test minus MD of the left eye test, top-right); (iii) CDR (fundoscopy, ratio in the left eye minus ratio in the right eye, bottom-left); (iv) thickness of the RNFL (OCT, thickness of the left retina minus thickness of the right retina, bottom-right). Negative values indicate a more prominent disease in the left eye. All variables, except for anisocoria, correlate with the sign and magnitude of the RAPD
Sensitivity and specificity were determined by comparing the RAPD output of the pupillometer with the binary (”present” vs. “not present”) decision of the trained clinician for the same subject using the analysis of the ROC and its area under the curve (AUC). A value of AUC = 1 denotes a perfect ideal classifier (i.e., complete agreement between the pupillometer and the clinician). Our ROC analysis resulted in an AUC [Fig. 3] of 0.94 (CI [0.86–0.99]), which indicates an excellent agreement almost identical to the ideal classifier; it corresponds to a sensitivity of 89% and a specificity of 91.7% (one-tail P value of standardized AUC < 0.001) and a most effective RAPD cut-off value equal to 0.5 log units.
Figure 3.
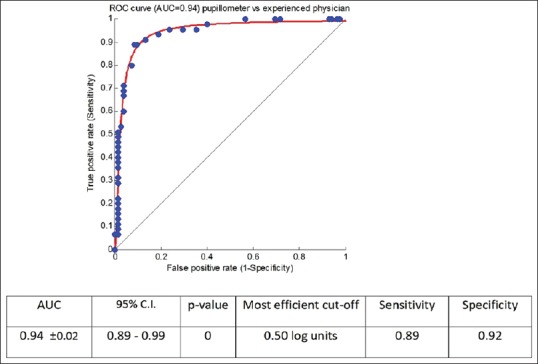
ROC curve of pupillometer vs. experienced physician. Sensitivity and specificity of the pupillometer was evaluated with ROC analysis by comparison with the manual evaluation (RAPD “present” vs. “not present”) of an experienced ophthalmologist well trained in the technique of the swinging flashlight test. An AUC of 0.94 (CI [0.89-0.99]) indicated a sensitivity of 89% and a specificity of 91.7% (one-tail P value of standardized AUC <0.001) and a most effective RAPD cut-off value equal to 0.5 log units
According to the manufacturer, a value of 0.3 log units represents the cut-off criterion for a normal RAPD (and similar conclusions are reported in the literature by other independent groups as discussed in the Discussion). If we divide the glaucomatous population based on this criterion, the two groups of patients, normal vs. abnormal, have different distributions for all glaucoma variables (CDR, MD, and OCT; Table 2). A RAPD higher than 0.3 log units corresponds to an intereye difference of (i) CDR of 0.13 (CI [0.09–0.16]), (ii) MD for HFA of 9.05 dB (CI [7.26–10.84]), (iii) thickness of the RNFL of 19.71 (CI [15.33–24.10]). Similar values are found for the clinician manual evaluation for the two groups: “present” vs. “not present” (for “present”: (i) 0.14 [0.10–0.17], (ii) 8.56 dB [6.75–10.37], (iii) 18.60 [13.87–23.33]).
Table 2.
Glaucoma variables (intereye differences) at cutoff criterion for clinician and pupillometer
| Clinician | Pupillometer | |||
|---|---|---|---|---|
| RAPD present | RAPD not present | >0.3 log | ≤0.3 log | |
| CDR (cup/disk ratio) | 0.14 [0.10-0.17] | 0.06 [0.05-0.08]* | 0.13 [0.09-0.16] | 0.07 [0.05-0.08]* |
| HFA (MD dB) | 8.56 [6.75-10.37] | 3.95 [2.80-5.10]* | 9.05 [7.26-10.84] | 3.57 [2.49-4.64]* |
| OCTAve. (RNFL m) | 18.60 [13.87-23.33] | 9.89 [7.64-12.14]* | 19.71 [15.33-24.10] | 9.10 [6.88-11.32]* |
Mann-Whitney U-test for all comparisons. *95% CIs is reported in brackets. RAPD test (present/not present) performed by the clinician (left) and by the pupillometer based on a 0.3 log units cut-off criterion (right). CDR is evaluated by fundoscopy, HFA is mean deviation of Humphrey field analyzer, OCTAve. is average of RNFL thickness measured via optical coherent tomography. All values represent delta between eyes
Discussion
RAPD is, by definition, a unilateral visual assessment directed to unilateral pathologies or cases where the severity in the two eyes is asymmetric.[29] In this paper, we referred to this unilaterality using the (intereye) “difference” between the two eyes properly ordered to make a negative value indicating a worse or more severe condition in the left eye – for example, for OCT average RNFL, it is the thickness in the left eye minus the thickness in the right eye. The same negative/positive logic was applied to the RAPD readings from the pupillometer. In our glaucomatous population, we showed correlation between the magnitude and sign of RAPD and intereye differences in perimetry MD, CDR, and average RNFL thickness. Also, when compared with manual evaluation of an expert ophthalmologist, the output of the pupillometer showed almost identical performance, a sensitivity of 89% and a specificity of 91.7%.
However, RAPD has been reported also in normal subjects; the study by Wilhelm et al. (2007) conducted with a binocular pupilometer showed a 42% of their normal population having an RAPD between 0.08 and 0.22 log units and a 6% having a RAPD between 0.23 and 0.39 log units.[22] They justified the RAPD by inaccuracy of the measurement or by synaptic asymmetries in the visual pathways and the pupillary pretectal nuclei in the midbrain. Their protocol consisted of a train of 42 pairs of stimuli for a total duration of more than 4 min. This is important to specify, as the number of repetitions of the light stimulus seems to be closely associated with the physiological variation of RAPD attributed to the normal population. In a pupillometry study, Kawasaki et al.[30] showed a confidence interval of 0.4 log units if 10 stimulus pairs were used in the protocol and only 0.1 log units if 100 stimulus pairs were used. Using a 10-s protocol, Volpe et al.[31] reported a cut-off criterion for normal population ranging between 0.3 and 0.6 log units. The protocol in the RAPiDo is based on a sequence of 10 pairs for a total duration of 24 s; with this protocol, the control population showed a mean RAPD of 0.21 log units (0.14 SD) and a CI of [0.16–0.26]. In a different repeatability study, with RAPD artificially simulated with neutral density filters on a normal population, we found the same result (paper in preparation). Interestingly, the 95% percentile equal to 0.45 log units in the control group coincides with the optimum cut-off value for sensitivity and specificity in the ROC curve analysis [0.5 log units, Fig. 3] which means that the clinician must have the same “perceptive” percentile for evaluation. If these data are confirmed, the manufacturer might need to slightly increase its normative reference.
One limitation of the present investigation is the comparison with human evaluation that was based on only one reference physician. Assessing inter-examiner variability of the swinging flashlight test in a population of experienced ophthalmologists and compared their performance with standard clinical practitioners (optometry technicians, nurses, etc.) and automatic pupillometry would help to better understand clinical limitations, variability, and practicality of the human or pupillometry RAPD test – so we plan to conduct this study in the future.[32]
Another point to make is about the age discrepancy in the data – our control group was mainly composed of family members accompanying patients the day of their visit and they were often younger (average, 35.2 years old) than patients (average, 56.9 years old). Aging, however, should not produce any RAPD. It is known that pupil size baseline is smaller in elderly and, consequently, some of the dynamic variables of the pupil light reflex might be different; however, when properly normalized, older and younger pupils maintain the same dynamic properties – this is largely reported in the literature and discussed not only in the seminal book of Irene Loewenfeld but also in Bremner.[33,34] An age-related component of the RAPD is thus very unlikely and a more recent study conducted by Satou et al. confirmed this critical point by showing that the relative differences of the pupil light reflex amplitude between the two eyes, something the authors called “RAPD score,” did not indeed correlate with age.[35]
Pupil testing is a crucial part of the ophthalmic examination and the swinging flashlight test is a well-established medical routine aimed to identify the presence of a RAPD – however, RAPD does not necessarily indicate glaucoma or the location of the visual field sensitivity loss. The study here reported correlation with a group of typical complications related to glaucoma, but its impact and significance in ophthalmology goes behind this specific application and it could be, in fact, used as a model for many other (neuro) ophthalmologic conditions.
This study was conceived with a serious challenge in mind: rural populations located in remote and isolated areas are not easily accessible by specialized clinical personnel and, although our well-trained clinician was well capable of detecting RAPD, her deployment outside the eye hospital is basically impossible. The same limitation applies worldwide in many similar geographic regions in developing countries. The pupillometer used in this study agrees very well with the performance of our reference clinician and it could then be used as its proxy – it is portable, automatic, battery-operated, and its use and interface are simple and straightforward for untrained/nontechnical individuals. Results, in electronic format, could be downloaded and communicated electronically to the physician for evaluation and decision-making, and thus, its deployment is well suitable for telemedicine and mass screening of all those ophthalmologic conditions associated with RAPD.
Conclusion
Good correlation was found between the magnitudes of RAPD as reported by the pupillometer and intereye differences in HFA MD, CDR, average RNFL thickness, and clinician performance; thus, it could potentially be used as a screening support to assess asymmetric glaucoma.
Commercial disclosures
CMP is the Chief Scientist of Neuroptics, the manufacturer of the device used in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors wish to thank Dr SR Krishnadas for his support in designing the study and guiding through it. They would also like to thank Mr Alagu Chidambaram Sivarama Subramanian, Ms Gracy Evangalin Vincent, Ms Iniya Paramasivam, Mrs Pandialakshmi Department, of Clinical Research, Mrs R Iswarya (Statistician) for their help with data collection and analysis, and Ms T Kumaragurpari (Chief Librarian, Aravind Eye Hospital, Madurai) for her assistance in review in literature and formatting the text and references.
References
- 1.Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390:2183–93. doi: 10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- 2.Murthy GVS, Gupta SK, Bachani D, Jose R, John N. Current estimates of blindness in India. Br J Ophthalmol. 2005;89:257–60. doi: 10.1136/bjo.2004.056937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramakrishnan R, Nirmalan PK, Krishnadas R, Thulasiraj RD, Tielsch JM, Katz J. Glaucoma in a rural population of southern India: The Aravind comprehensive eye survey. Ophthalmology. 2003;110:1484–90. doi: 10.1016/S0161-6420(03)00564-5. [DOI] [PubMed] [Google Scholar]
- 4.Vijaya L, George R, Baskaran M, Arvind H, Raju P, Ramesh SV. Prevalence of primary open-angle glaucoma in an urban south Indian population and comparison with a rural population. The Chennai Glaucoma Study. Ophthalmology. 2008;115:648–54.e1. doi: 10.1016/j.ophtha.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 5.Saxena R, Singh D, Vashist P. Glaucoma: An emerging peril. Indian J Community Med. 2013;38:135–7. doi: 10.4103/0970-0218.116348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster PJ, Johnson GJ. Glaucoma in China: How big is the problem? Br J Ophthalmol. 2001;85:1277–82. doi: 10.1136/bjo.85.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hennessy AL, Katz J, Ramakrishnan R, Krishnadas R, Thulasiraj RD, Tielsch JM, et al. The utility of relative afferent pupillary defect as a screening tool for glaucoma: Prospective examination of a large population-based study in a south Indian population. Br J Ophthalmol. 2011;95:1203–6. doi: 10.1136/bjo.2010.194217. [DOI] [PubMed] [Google Scholar]
- 9.Jonas JB, Budde WM, Panda-Jonas S. Ophthalmoscopic evaluation of the optic nerve head. Surv Ophthalmol. 1999;43:293–320. doi: 10.1016/s0039-6257(98)00049-6. [DOI] [PubMed] [Google Scholar]
- 10.Distelhorst JS, Hughes GM. Open-angle glaucoma. Am Fam Physician. 2003;67:1937–44. [PubMed] [Google Scholar]
- 11.Pro MJ, Moster MR. Asymmetric optic nerves: Advice on evaluating patients. Glaucoma Today. 2007. [Last assessed on 2018 Sep 20]. pp. 27–29. Available from: http:// glaucomatoday.com/2007/10/GT0907_04.php/
- 12.American Academy of Ophthalmology. Primary Open-Angle Glaucoma. Preferred Practice Pattern. San Francisco: American Academy of Ophthalmology; 2000. pp. 1–36. [Google Scholar]
- 13.Sarezky D, Krupin T, Cohen A, Stewart CW, Volpe NJ, Tanna AP. Correlation between intereye difference in visual field mean deviation values and relative afferent pupillary response as measured by an automated pupillometer in subjects with glaucoma. J Glaucoma. 2014;23:419–23. doi: 10.1097/IJG.0b013e31827b1522. [DOI] [PubMed] [Google Scholar]
- 14.Graham SL, Klistorner AI, Grigg JR, Billson FA. Objective VEP perimetry in glaucoma: Asymmetry analysis to identify early deficits. J Glaucoma. 2000;9:10–9. doi: 10.1097/00061198-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Levatin P. Pupillary escape in disease of the retina or optic nerve. Arch Ophthalmol. 1959;62:768–79. doi: 10.1001/archopht.1959.04220050030005. [DOI] [PubMed] [Google Scholar]
- 16.Thompson HS. Pupillary signs in the diagnosis of optic nerve disease. Trans Ophthalmol Soc U K. 1976;96:377–81. [PubMed] [Google Scholar]
- 17.Thompson HS, Corbett JJ, Cox TA. How to measure the relative afferent pupillary defect. Surv Ophthalmol. 1981;26:39–42. doi: 10.1016/0039-6257(81)90124-7. [DOI] [PubMed] [Google Scholar]
- 18.Wilhelm H, Wilhelm B. Clinical applications of pupillography. J Neuroophthalmol. 2003;23:42–9. doi: 10.1097/00041327-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Lankaranian D, Altangerel U, Spaeth GL, Leavitt JA, Steinmann WC. The usefulness of a new method of testing for a relative afferent pupillary defect in patients with ocular hypertension and glaucoma. Trans Am Ophthalmol Soc. 2005;103:200. [PMC free article] [PubMed] [Google Scholar]
- 20.Kardon RH, Kawasaki A, Miller NR. Origin of the relative afferent pupillary defect in optic tract lesions. Ophthalmology. 2006;113:1345–53. doi: 10.1016/j.ophtha.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 21.Kawasaki A, Miller NR, Kardon R. Pupillographic investigation of the relative afferent pupillary defect associated with a midbrain lesion. Ophthalmology. 2010;117:175–9. doi: 10.1016/j.ophtha.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 22.Wilhelm H, Peters T, Lüdtke H, Wilhelm B. The prevalence of relative afferent pupillary defects in normal subjects. J Neuroophthalmol. 2007;27:263–7. doi: 10.1097/WNO.0b013e31815bf865. [DOI] [PubMed] [Google Scholar]
- 23.Kalaboukhova L, Fridhammar V, Lindblom B. An objective method for measuring relative afferent pupillary defect in glaucomatous optic neuropathy—stimulus optimization. Neuroophthalmology. 2006;30:7–15. [Google Scholar]
- 24.Tatham AJ, Meira-Freitas D, Weinreb RN, Marvasti AH, Zangwill LM, Medeiros FA. Estimation of retinal ganglion cell loss in glaucomatous eyes with a relative afferent pupillary defect. Invest Ophthalmol Vis Sci. 2014;55:513–22. doi: 10.1167/iovs.13-12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gracitelli CP, Tatham AJ, Zangwill LM, Weinreb RN, Abe RY, Diniz-Filho A, et al. Asymmetric macular structural damage is associated with relative afferent pupillary defects in patients with glaucoma. Invest Ophthalmol Vis Sci. 2016;57:1738–46. doi: 10.1167/iovs.15-18079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao HL, Kadambi SV, Dasari S. Predicting the intereye asymmetry in functional and structural damage in glaucoma using automated pupillography. Acta Ophthalmol. 2017;95:e532. doi: 10.1111/aos.13317. [DOI] [PubMed] [Google Scholar]
- 27.Sherman PM, Stark LW. A servoanalytic study of consensual pupil reflex to light. J Neurophysiol. 1957;20:17–26. doi: 10.1152/jn.1957.20.1.17. [DOI] [PubMed] [Google Scholar]
- 28.Privitera CM, Stark LW. A binocular pupil model for simulation of relative afferent pupil defects and the swinging flashlight test. Biol Cybern. 2006;94:215–24. doi: 10.1007/s00422-005-0042-8. [DOI] [PubMed] [Google Scholar]
- 29.Kardon RH, Haupert CL, Thompson HS. The relationship between static perimetry and the relative afferent pupillary defect. Am J Ophthalmol. 1993;115:351–6. doi: 10.1016/s0002-9394(14)73587-1. [DOI] [PubMed] [Google Scholar]
- 30.Kawasaki A, Moore P, Kardon RH. Variability of the relative afferent pupillary defect. Am J Ophthalmol. 1995;120:622–33. doi: 10.1016/s0002-9394(14)72209-3. [DOI] [PubMed] [Google Scholar]
- 31.Volpe NJ, Plotkin ES, Maguire MG, Hariprasad R, Galetta SL. Portable pupillography of the swinging flashlight test to detect afferent pupillary defects. Ophthalmology. 2000;107:1913–21. doi: 10.1016/s0161-6420(00)00354-7. [DOI] [PubMed] [Google Scholar]
- 32.Meeker M, Du R, Bacchetti P, Privitera CM, Larson MD, Holland MC, et al. Pupil examination: Validity and clinical utility of an automated pupillometer. J Neurosci Nurs. 2005;37:34–40. [PubMed] [Google Scholar]
- 33.Loewenfeld IE. Oxford: Butterworth-Heinemann; 1999. The Pupil: Anatomy, Physiology, and Clinical Applications; pp. 498–517. [Google Scholar]
- 34.Bremner FD. Pupillometric evaluation of the dynamics of the pupillary response to a brief light stimulus in healthy subjects. Invest Ophthalmol Vis Sci. 2012;53:7343–7. doi: 10.1167/iovs.12-10881. [DOI] [PubMed] [Google Scholar]
- 35.Satou T, Goseki T, Asakawa K, Ishikawa H, Shimizu K. Effects of age and sex on values obtained by RAPDx pupillometer and determined the standard values for detecting relative afferent pupillary defect. Transl Vis Sci Technol. 2016;5:18. doi: 10.1167/tvst.5.2.18. [DOI] [PMC free article] [PubMed] [Google Scholar]


