Abstract
Background
There are limited data on the pharmacogenetics and pharmacokinetics of the CNS penetration of efavirenz.
Objectives
We investigated genetic polymorphisms associated with CSF concentrations of efavirenz and its metabolites and explored the relationships with neurocognitive performance.
Methods
We included 47 HIV-infected South African black adults with and without HIV-associated neurocognitive disorder on efavirenz/tenofovir/emtricitabine and collected paired plasma–CSF samples. We considered 2049 SNPs, including SNPs known to affect plasma efavirenz exposure, from potentially relevant genes (ABCC5, ABCG2, ABCB1, SLCO2B1, SCLO1A2, ABCC4, CYP2B6 and CYP2A6) and 880 met a linkage disequilibrium (LD)-pruning threshold.
Results
We identified 9 slow, 21 intermediate and 17 extensive metabolizers. The CYP2B6 983 genotype in multivariate analyses predicted log10-transformed concentrations of plasma efavirenz (β = 0.38, P = 2.7 × 10−03), plasma 7-hydroxy-efavirenz (β = 0.59, P = 3.7 × 10−03), plasma 8-hydroxy-efavirenz:efavirenz ratio (β = −0.31, P = 1.8 × 10−04) and CSF efavirenz (β = 0.36, P = 0.01). Lower plasma 7-hydroxy-efavirenz concentrations were independently associated with CYP2A6 rs10853742 (β = −0.55, P = 3.5 × 10−05), ABCB1 rs115780656 (β = −0.65, P = 4.1 × 10−05) and CYP2A6 −48A→C (β = −0.59, P = 0.01). CYP2A6 −48A→C was independently associated with higher CSF 8-hydroxy-efavirenz:efavirenz ratio (β = 0.54, P = 0.048). CYP2B6 rs2279345 polymorphism was associated with lower plasma 7-hydroxy-efavirenz:efavirenz ratio in multivariate analyses (P < 0.05). No polymorphisms were associated with CSF:plasma ratios of efavirenz, plasma or CSF concentrations of 8-hydroxy-efavirenz or neurocognitive performance.
Conclusions
We identified novel genetic associations with plasma efavirenz, plasma 7-hydroxy-efavirenz, plasma 7-hydroxy-efavirenz:efavirenz ratio, plasma 8-hydroxy-efavirenz:efavirenz ratio, CSF efavirenz and CSF 8-hydroxy-efavirenz:efavirenz ratio.
Introduction
The fixed-dose combination of efavirenz, tenofovir and emtricitabine has been recommended as first-line ART for HIV-infected adults.1 However, prolonged ART exposure may impact cognitive function if ART neurotoxicity exceeds CNS viral suppression efficacy—a hypothesis supported by preclinical and clinical data.2 Efavirenz in particular has demonstrated neurotoxicity in in vitro studies.3 Interrupting ART after a median of 4.5 years was associated with improved cognitive function, especially among efavirenz recipients.4 In a randomized controlled trial (RCT), patients starting efavirenz, tenofovir and emtricitabine rather than PIs or all-NRTI regimens had less neurocognitive improvement after 48 weeks.5
Human genetic variants have been associated with antiretroviral pharmacokinetics and pharmacodynamics, but ART CNS-targeted strategies have not considered pharmacogenetics.6 Efavirenz is primarily metabolized by cytochrome P450 (CYP) 2B6 into 8-hydroxy-efavirenz while CYP2A6 generates the 7-hydroxy-efavirenz metabolite.7CYP2B6 516G→T (rs3745274) predicts increased plasma efavirenz exposure.8–13 The CYP2B6 516TT genotype is more common in Africans and African Americans than in Caucasians.8,14,15 Additional polymorphisms that are less frequent than 516G→T in Africans and African Americans, 983T→C (rs28399499) and CYP2B6 15582C→T (rs4803419), also predict increased plasma efavirenz exposure.7,15 The CYP2B6 983C allele is found almost exclusively with African ancestry.15,16 Polymorphisms in genes beyond CYP2B6 have been infrequently reported to be associated with efavirenz concentrations including polymorphisms in CYP2A6.12,17 Polymorphisms in CYP2B6 that predict higher efavirenz plasma concentrations predispose to efavirenz-mediated neurotoxicity.8,18 Patients with CYP2B6 slow-metabolizer genotypes also have higher CSF efavirenz exposure.19In vitro studies have implicated efavirenz and especially its metabolite 8-hydroxy-efavirenz in neuronal toxicity.3,20,21 In a CSF substudy of the ENCORE1 trial, 8-hydroxy-efavirenz exposure correlated with adverse neuropsychiatric outcomes.19 However, CYP2B6 516G→T only predicted efavirenz plasma and CSF concentrations and not 8-hydroxy-efavirenz plasma or CSF concentrations.19,22,23
Blood–brain barrier (BBB) and blood–CSF barrier (BCSFB) transporters affect influx and efflux of drugs including antiretrovirals.24,25 The superfamily of solute carrier (SLC) genes, including SLCO2B1, SLCO1A2 and SLCO1B1, influence influx transporter expression in the BBB and BCSFB.26,27 Efflux transporters in the BBB and BCSFB are influenced by ATP-binding cassette (ABC) genes including ABCB1 [which encodes P-glycoprotein (P-gp)], ABCG2, ABCC4 and ABCC5.27,28 There are conflicting data regarding whether efavirenz is a P-gp substrate and whether ABCB1 polymorphisms predict efavirenz concentrations.10,29,30ABCC4 polymorphisms (rs1751034 and rs2274407) have been associated with lower and higher maximum plasma efavirenz concentrations, respectively, and ABCG2 rs2231142 has been associated with an increased risk of abnormal dreams with efavirenz.31,32
Africans are the most genetically diverse population worldwide.33 South Africa has the world’s largest ART programme, with most patients currently receiving efavirenz/tenofovir/emtricitabine.34 Genetic polymorphisms that affect metabolizing enzymes or transporters may therefore affect efavirenz CSF penetration. We investigated associations between genetic polymorphisms and CSF exposure of efavirenz and 8-hydroxy-efavirenz in black South Africans. We also explored pharmacokinetic–pharmacodynamic relationships of CSF efavirenz and 8-hydroxy-efavirenz with neurocognitive performance.
Patients and methods
Participants
Adults (aged ≥18 and ≤70 years) who participated in an RCT (PACTR201310000635418) investigating lithium for HIV-associated neurocognitive impairment [global deficit score (GDS) of ≥0.5] were invited to participate in this study.35 We also invited participants who were screened for the RCT but were excluded based on cognitive impairment criteria. We included participants established on efavirenz-based ART for at least 6 months with suppressed plasma HIV-1 RNA. All participants provided written informed consent. The study was approved by the University of Cape Town Human Research Ethics Committee (HREC 071/2013).
Pharmacokinetic sampling
We collected paired plasma and CSF samples for assays of efavirenz and its metabolites. Participants recorded dosing time the night before and were admitted in the morning for pharmacokinetic sampling. Mid-dosing lumbar punctures were performed. Whole blood was collected within 45 min of CSF sampling, centrifuged within 1 h of collection, aliquoted and stored at −80°C until analysis. CSF was aliquoted and stored at −80°C until analysis.
Measurement of efavirenz and its metabolites
Drug assays were performed at two laboratories. The analytical laboratory in the Division of Clinical Pharmacology at the University of Cape Town quantified total efavirenz in plasma and CSF using validated LC/MS-MS assays. The lower limit of quantification (LLOQ) for plasma and CSF efavirenz was 19.5 and 0.5 ng/mL, respectively. The Bioanalytical Facility, Department of Molecular and Clinical Pharmacology at the University of Liverpool quantified total CSF 8-hydroxy-efavirenz, plasma 8-hydroxy-efavirenz and plasma 7-hydroxy-efavirenz using validated LC/MS-MS assays.22 We could not quantify CSF 7-hydroxy-efavirenz. The LLOQ for CSF 8-hydroxy-efavirenz, plasma 8-hydroxy-efavirenz and plasma 7-hydroxy-efavirenz was 3.125 ng/mL, 5.0 ng/mL and 5.0 ng/mL, respectively. Concentrations below the limit of quantification were analysed as missing data.
Characterization of genetic polymorphisms
We extracted DNA from buffy coats using QIAsymphony®. Genotyping was done using Illumina MEGAEX (Illumina, San Diego, CA, USA). SNPs that were not genotyped were imputed. SNPs were extracted for seven genes ±50 kb in each direction: ABCB1 (301 SNPs), ABCC4 (630 SNPs), ABCC5 (225 SNPs), ABCG2 (164 SNPs), CYP2A6/B6 (202 SNPs), SLCO1A2 (406 SNPs) and SLCO2B1 (118 SNPs). SNPs were excluded for genotyping efficiency less than 99%, a 5% minor allele frequency cut-off, and Hardy–Weinberg equilibrium (HWE) P values <0.00001. We further performed targeted genotyping of CYP2B6 516G→T (rs3745274) and CYP2A6 −48A→C (rs28399433) by TaqMan™ (Applied Biosystems, Foster City, CA, USA) and of CYP2B6 983T→C (rs28399499), CYP2B6 15582C→T (rs4803419), SLCO1B1 521T→C (rs4149056) and SLCO1B1 (rs4149032) by MassARRAY® iPLEX Gold (Sequenom Inc., San Diego, CA, USA). All samples were genotyped in duplicate. The final data set included 2049 SNPs from 47 participants. All genotyping was done at Vanderbilt Technologies for Advanced Genomics (VANTAGE). Laboratory personnel with no knowledge of clinical data performed the genotyping. Metabolizer genotype groups for CYP2B6 were assigned as follows: extensive metabolizer (CYP2B6 15882CC-516GG-983TT or CYP2B6 15882CT-516GG-983TT), intermediate metabolizer (CYP2B6 15882TT-516GG-983TT, CYP2B6 15882CC-516GT-983TT, CYP2B6 15882CC-516GG-983CT, CYP2B6 15882CT-516GT-983TT or CYP2B6 15882CT-516GG-983CT) or slow metabolizer (CYP2B6 15882CC-516TT-983TT, CYP2B6 15882CC-516GT-983CT or CYP2B6 15882CC-516GG-983CC). Furthermore, among participants with a slow-metabolizer genotype, additional assessment of CYP2A6 −48A→C (rs28399433) was assessed to categorize the metabolizer status into an ordinal 12-level metabolizer status as described elsewhere.7,36
Neurocognitive performance
We assessed neurocognitive impairment to provide a GDS.37 We previously reported the domains and tests included.35 We screened for symptoms of depression using the Center for Epidemiologic Studies Depression (CES-D) scale.38
Efavirenz and metabolite neurotoxicity
We compared CSF efavirenz and 8-hydroxy-efavirenz concentrations with concentrations reported to be associated with neuronal damage in vitro (31.6 ng/mL and 3.3 ng/mL, respectively).20
Viral load assessment
We determined HIV-1 RNA concentrations in plasma and CSF using the Abbott RealTime HIV-1 assay (Abbott Park, IL, USA.). We considered participants to be virologically suppressed if the viral load was <400 copies/mL. In plasma and CSF, the lower limit of detection was 40 copies/mL. In CSF, we performed a previously described nested PCR and automated DNA sequencing method to detect HIV-1 transactivator viral protein (Tat) mutations, which have been associated with HIV-1-associated neurocognitive impairment.39,40
BBB integrity
We calculated the CSF:blood albumin ratio [CSF albumin (mg/L):serum albumin (g/L)] to determine BBB integrity. The BBB was considered intact if this ratio was less than 6.8 in participants <45 years of age, and less than 10.2 in participants ≥45 years of age.41
Pharmacokinetic statistical analysis
Pharmacokinetic data were not normally distributed and were expressed as medians (IQR) and geometric means (95% CI). We corrected for plasma protein binding and estimated protein-free plasma concentrations by multiplying total plasma concentrations by the protein-free concentrations reported in the literature (efavirenz 0.22%).42 Total CSF concentrations were considered to be similar to CSF protein-free concentrations.42 Pearson’s r correlation was used to assess correlations between plasma and CSF concentrations. We performed statistical analysis using STATA version 15.0 (StataCorp, College Station, TX, USA). Graphs were created using GraphPad Prism version 7.03 for Windows (GraphPad Software, La Jolla, CA, USA).
Genetic associations
Genetic associations with pharmacokinetic parameters were analysed by multivariate linear or logistic regression. Pharmacokinetic data were log-transformed (log10) for genetic analysis. We used ratios of measured concentrations (total in CSF and plasma) without correcting for protein binding. CSF:plasma ratios were calculated using raw concentrations then log10-transformed. For efavirenz analyses we subsequently adjusted for CYP2B6 516G→T, 983T→C and 15582C→T. We performed genetic association analyses in PLINK version 1.9 (http://zzz.bwh.harvard.edu/plink/). For the primary analyses, we employed linkage disequilibrium (LD) pruning with an LD r2 threshold of 0.95 within a 50 kb window at 5 kb increments. The final analysis dataset included 880 SNPs that met the LD-pruning threshold. We used Bonferroni correction to adjust for multiple testing (P = 0.05 divided by 880 SNPs). We generated an LD plot using Haploview (https://www.broadinstitute.org/haploview/haploview).
Results
Study participant characteristics
We sampled 47 participants (Table 1), 33 (70%) of whom had mild to moderate neurocognitive impairment. All participants self-identified as black Xhosa and all were virologically suppressed in plasma. Four participants had detectable viral loads, the highest being 128 copies/mL. CSF viral loads were <40 copies/mL in all participants. Five participants had detectable HIV-1 Tat DNA, all of whom had the C30C31S substitution. We were able to determine the CSF:blood albumin ratio in 31 (66%) of 47 participants, with a median value of 2.6 (range 1.1 to 5.2), indicating an intact BBB.
Table 1.
Baseline characteristics of study participants (N = 47)
| Gender, n (%) | |
| male | 6 (13) |
| female | 41 (87) |
| Age (years), median (IQR) | 36 (32–43) |
| CD4+ T cell count (cells/mm3), median (IQR) | 470 (384–586) |
| Time on ART (months), median (IQR) | 38 (18–54) |
| BMI (kg/m2), mean ± SD | 26.3 ± 5.3 |
| ART regimen, n (%) | |
| efavirenz/tenofovir/emtricitabine | 43 (91) |
| efavirenz/tenofovir/lamivudine | 4 (9) |
| Neurocognitive impairment | |
| GDS overall, median (IQR) | 0.89 (0.22–1.5) |
| GDS ≥1, n (%) | 22 (47) |
| GDS ≥0.5 to <1, n (%) | 11 (23) |
| GDS <0.5, n (%) | 14 (30) |
| Neuromedical assessment, n (%) | |
| no disease | 26 (55) |
| mild to moderate disease | 21 (45) |
| severe disease | 0 |
| Years in education, n (%) | |
| ≥10 | 24 (51) |
| <10 | 21 (45) |
| missing information | 2 (4) |
| Employment status, n (%) | |
| employeda | 12 (26) |
| unemployed | 35 (74) |
| Depression score (CES-D scale), median (IQR) | 7 (2–11) |
Full-time or part-time work.
Efavirenz pharmacokinetics
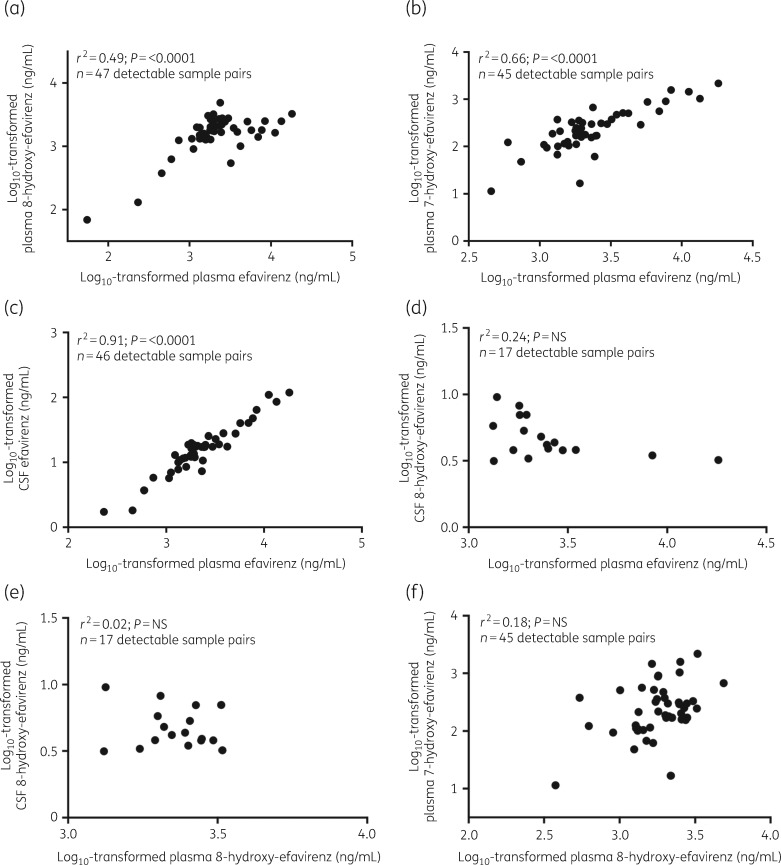
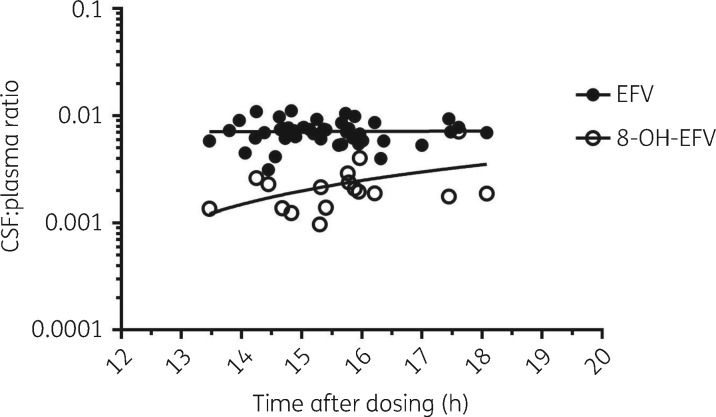
Concentrations of efavirenz (plasma and CSF), 8-hydroxy-efavirenz (plasma and CSF) and 7-hydroxy-efavirenz (plasma) are described in Table 2. Plasma 8-hydroxy-efavirenz and 7-hydroxy-efavirenz concentrations correlated with plasma efavirenz concentrations (P < 0.0001 for each) (Figure 1a and b). CSF and plasma efavirenz concentrations were correlated (P < 0.0001) (Figure 1c). There was no correlation between CSF 8-hydroxy-efavirenz and plasma efavirenz, CSF 8-hydroxy-efavirenz and plasma 8-hydroxy-efavirenz or plasma 7-hydroxy-efavirenz and plasma 8-hydroxy-efavirenz (Figure 1d–f). There was no statistically significant association of CSF:plasma ratios versus time after dosing (Figure 2). CSF efavirenz concentrations were above the IC50 (1.3 ng/mL) in all participants.42–45 CSF efavirenz concentrations and CSF 8-hydroxy-efavirenz concentrations were above the in vitro toxic concentration (CSF efavirenz, 31.6 ng/mL; CSF 8-hydroxy-efavirenz, 3.3 ng/mL) in 7 (15.2%) of 46 participants and 14 (29.8%) of 47 participants, respectively.20
Table 2.
Concentrations of efavirenz and its metabolites in plasma and CSF
| Plasma (ng/mL), total concentration | Plasma (ng/mL), protein corrected | CSF concentration (ng/mL) | CSF:plasma ratio, total concentration | CSF:plasma ratio, protein corrected | |
|---|---|---|---|---|---|
| Efavirenz pharmacokinetics (N = 47) | |||||
| undetectable samples, n/N (%) | 0 | 0 | 1/47 (2.1%) | 1/47 (2.1%) | 1/47 (2.1%) |
| median (IQR) | 1960 (1390–3200) | 4.31 (3.06–7.04) | 17.25 (10.7–19.9) | 0.71 (0.61–0.78) | 324.34 (278.29–356.44) |
| range | 55–18 100 | 0.12–39.82 | 1.73–119 | 0.31–1.12 | 142.82–508.66 |
| geometric mean concentration (95% CI) | 2081.5 (1557.8–2781.4) | 4.58 (3.43–6.12) | 15.64 (12.08–20.24) | 0.69 (0.64–0.75) | 315.54 (291.90–341.10) |
| 8-Hydroxy-efavirenz pharmacokinetics (N = 47) | |||||
| undetectable samples, n/N (%) | 0 | unknown | 30/47 (63.8%) | 30/47 (63.8%) | unknown |
| median (IQR) | 1808 (1325.5–2498.7) | 4.17 (3.80–5.79) | 0.20 (0.14–0.24) | ||
| range | 68.81–4887.5 | 3.15–9.56 | 0.10–0.72 | ||
| geometric mean concentration (95% CI) | 1570.7 (1255–1965.9) | 4.69 (3.93–5.60) | 0.21 (0.16–0.26) | ||
| 7-Hydroxy-efavirenz pharmacokinetics (N = 47) | |||||
| undetectable samples, n/N (%) | 2/47 (4.3%) | unknown | not measured | not applicable | not applicable |
| median (IQR) | 216.71 (122.91–375.43) | ||||
| range | 11.45–2181.73 | ||||
| geometric mean concentration (95% CI) | 229.17 (166.51–315.41) | ||||
Figure 1.
Pearson correlation plots for log10-transformed plasma and CSF concentrations for efavirenz and its metabolites. The relationship between (a) log10-transformed plasma efavirenz concentrations and plasma 8-hydroxy-efavirenz concentrations, (b) log10-transformed plasma efavirenz concentrations and plasma 7-hydroxy-efavirenz concentrations, (c) log10-transformed plasma efavirenz concentrations and CSF efavirenz concentrations, (d) log10-transformed plasma efavirenz concentrations and CSF 8-hydroxy-efavirenz concentrations, (e) log10-transformed plasma 8-hydroxy-efavirenz concentrations and CSF 8-hydroxy-efavirenz concentrations and (f) log10-transformed plasma 8-hydroxy-efavirenz concentrations and plasma 7-hydroxy-efavirenz concentrations. NS, not significant.
Figure 2.
CSF:plasma concentration ratios of detectable pairs of plasma and CSF efavirenz samples versus time after dosing. The lines are linear regression lines and were not statistically significant for efavirenz or 8-hydroxy-efavirenz (P = 0.09). EFV, efavirenz; 8-OH-EFV, 8-hydroxy-efavirenz.
Genetic associations for efavirenz
Genotyping of four polymorphisms with known effects on efavirenz (CYP2B6 516G→T, 983T→C, 15582C→T and CYP2A6 −48A→C) and two polymorphisms in SLCO1B1 (rs4149056 and rs4149032) was successful in all 47 participants. SLCO1B1 rs4149056 was monomorphic. In 43 (91%) of 47 participants, an additional 2043 polymorphisms from ABCB1, ABCC4, ABCC5, ABCG2, CYP2A6, CYP2B6, SLCO1A2 and SLCO2B1 were successfully genotyped. All 2048 polymorphisms were in HWE based on a Bonferroni-adjusted P value threshold of 0.00002; 18 had unadjusted P values <0.05 (data not shown). The 880 polymorphisms included in the final dataset based on LD pruning were in HWE based on a Bonferroni-adjusted P value threshold of 5.7 × 10−05. Ten polymorphisms had unadjusted P values <0.05.
Plasma efavirenz concentrations
Relationships between CYP2B6 slow-metabolizer genotypes and efavirenz concentrations are described in Table 3. Plasma efavirenz concentrations were significantly higher in CYP2B6 slow metabolizers compared with intermediate and extensive metabolizers (Figure S1, available as Supplementary data at JAC Online). In multivariable linear regression analysis adjusted for CYP2B6 516G→T, the polymorphism associated at P < 0.05 was CYP2B6 983T→C (β = 0.38, 95% CI = 0.14 to 0.61, P = 2.7 × 10−03). See Table S1.
Table 3.
Efavirenz metabolizer status and detectable efavirenz, 8-hydroxy-efavirenz and 7-hydroxy-efavirenz concentrations in CSF and plasma
| Metabolizer genotype | Participants, n (%) | Geometric mean concentration (95% CI) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| plasma (ng/mL) |
CSF (ng/mL) |
|||||||||
| EFV | 8-OH-EFV | 8-OH-EFV:EFV | 7-OH-EFV (n/N = 45/47 detectable) | 7-OH-EFV:EFV | EFV (n/N = 46/47 detectable) | 8-OH-EFV (n/N = 17/47 detectable) | 8-OH-EFV:EFV | |||
| Slow | 9 (19.1) | 6896.9 (3984.1–11 939.4)b | 1860.2 (1421.3–2434.6) | 0.27 (0.17–0.42)b | 810.7 (466.3–1409.6)b | 0.12 (0.10–0.14) | 45.8 (25.0–83.9)b | 1.8 (1.4–2.4) | 0.04 (0.0–3.17) | |
| Intermediate | 21 (44.7) | 1878.1 (1371.4–2572.0) | 1543.8 (1091.4–2183.8) | 0.82 (0.64–1.06) | 185.5 (108.0–318.5) | 0.11 (0.08–0.15) | 12.7 (9.3–17.4) | 2.7 (2.0–3.5) | 0.36 (0.24–0.54) | |
| Extensive | 17 (36.2) | 1253.48 (778.9–2017.2) | 1467.1 (907.9–2370.6) | 1.17 (1.03–1.33) | 89.6 (45.4–176.7) | 0.07 (0.05–0.11) | 9.0 (5.0–15.9) | 2.2 (1.6–3.0) | 0.28 (0.14–0.56) | |
| Pa | <0.01 | NS | <0.01 | <0.01 | NS | <0.01 | NS | NS | ||
EFV, efavirenz; 7-OH-EFV, 7-hydroxy-efavirenz; 8-OH-EFV, 8-hydroxy-efavirenz; NS, not significant.
P values were determined by one-way analysis of variance (ANOVA).
P < 0.01.
Plasma 8-hydroxy-efavirenz concentrations
No polymorphisms were significant after correcting for multiple testing in multivariate analyses adjusting for CYP2B6 516G→T, or CYP2B6 516G→T and 983T→C (Table S1).
Plasma 7-hydroxy-efavirenz concentrations
Plasma 7-hydroxy-efavirenz concentrations were significantly higher in CYP2B6 slow metabolizers compared with intermediate and extensive metabolizers (Table 3). After adjusting for CYP2B6 516G→T, CYP2B6 983T→C remained significant (β = 0.59, 95% CI = 0.22 to 0.96, P = 3.7 × 10−03) and ABCB1 rs11578656 became significant (β = −0.71, 95% CI = −1.01 to −0.42, P = 2.9 × 10−05). See Table S1. The association between ABCB1 rs11578656 and log10-transformed plasma 7-hydroxy-efavirenz concentrations remained significant after correcting for multiple testing in multivariate analyses adjusting for CYP2B6 516G→T and CYP2B6 983T→C (β = −0.65, 95% CI = −0.92 to −0.37, P = 4.1 × 10−05). Two additional CYP2A6 polymorphisms became significant in multivariate analyses adjusting for CYP2B6 516G→T and CYP2B6 983T→C: CYP2A6 rs10853742 (β = −0.55, 95% CI = −0.78 to −0.32, P = 3.5 × 10−05) and the known polymorphism CYP2A6 −48A→C (β = −0.59, 95% CI = −1.01 to −0.16, P = 0.01).
CSF efavirenz concentrations
CSF efavirenz concentrations were significantly higher in CYP2B6 slow metabolizers compared with intermediate and extensive metabolizers (Table 3). After adjusting for CYP2B6 516G→T, the association between CYP2B6 983T→C and log10-transformed CSF efavirenz concentrations persisted (β = 0.36, 95% CI = 0.10 to 0.62, P = 1.0 × 10−02). See Table S2.
CSF:plasma efavirenz concentration ratio, CSF:plasma 8-hydroxy-efavirenz concentration ratio and CSF 8-hydroxy-efavirenz concentrations
Linear regression analysis results for associations with CSF:plasma efavirenz concentration ratio, CSF:plasma 8-hydroxy-efavirenz concentration ratio and CSF 8-hydroxy-efavirenz concentrations are displayed in Tables 4, 5 and Table S2, respectively. No polymorphisms were significant in multivariate analyses.
Table 5.
Genetic associations with detectable log10-transformed CSF:plasma 8-hydroxy-efavirenz concentrations in 16 South African adults
| Chromosome | Gene | Polymorphism (minor allele) | MAF | 516G→T adjusted |
516G→T and 983T→C adjusted |
516G→T and 983T→C and CYP2B6 15582C→T adjusted |
|||
|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | ||||
| 3 | ABCC5 | rs6762938 (T) | 0.31 | 0.23 (0.14–0.33) | 3.9 × 10−04 | 0.22 (0.12–0.32) | 1.1 × 10−03 | 0.22 (0.11–0.33) | 0.02 |
| 13 | ABCC4 | rs11343244 (T) | 0.10 | 0.41 (0.23–0.59) | 6.2 × 10−04 | 0.39 (0.20–0.49) | 1.1 × 10−03 | 0.41 (0.23–0.59) | 8.5 × 10−04 |
| 13 | ABCC4 | rs7997839 (G) | 0.14 | 0.41 (0.23–0.59) | 6.2 × 10−04 | 0.39 (0.20–0.49) | 1.1 × 10−03 | 0.41 (0.23–0.59) | 8.5 × 10−04 |
| 3 | ABCC5 | rs10937161 (T) | 0.20 | 0.36 (0.21–0.51) | 4.1 × 10−04 | 0.36 (0.22–0.49) | 2.3 × 10−04 | 0.35 (0.21–0.50) | 5.0 × 10−04 |
| 3 | ABCC5 | rs36092077 (A) | 0.16 | 0.39 (0.21–0.56) | 8.9 × 10−04 | 0.39 (0.23–0.54) | 3.7 × 10−04 | 0.39 (0.21–0.56) | 7.1 × 10−04 |
| 3 | ABCC5 | rs6807271 (A) | 0.31 | 0.21 (0.10–0.32) | 2.6 × 10−03 | 0.21 (0.10–0.32) | 2.6 × 10−03 | 0.20 (0.08–0.31) | 5.8 × 10−03 |
| 3 | ABCC5 | rs59309690 (A) | 0.09 | 0.60 (0.27–0.93) | 3.7 × 10−03 | 0.58 (0.26–0.90) | 4.1 × 10−03 | 0.57 (0.24–0.90) | 6.5 × 10−03 |
| 4 | ABCG2 | rs2728108 (A) | 0.08 | 0.60 (0.27–0.93) | 3.7 × 10−03 | 0.58 (0.26–0.90) | 4.1 × 10−03 | 0.57 (0.24–0.90) | 6.5 × 10−03 |
| 13 | ABCC4 | rs1678392 (A) | 0.19 | 0.32 (0.14–0.51) | 4.9 × 10−03 | 0.30 (0.11–0.50) | 0.01 | 0.31 (0.11–0.51) | 0.01 |
| 13 | ABCC4 | rs116336902 (A) | 0.07 | 0.32 (0.13–0.51) | 5.1 × 10−03 | 0.30 (0.10–0.50) | 0.01 | 0.31 (0.11–0.51) | 0.01 |
| 13 | ABCC4 | rs147385814 (C) | 0.07 | 0.32 (0.13–0.51) | 5.1 × 10−03 | 0.30 (0.10–0.50) | 0.01 | 0.31 (0.11–0.51) | 0.01 |
| 13 | ABCC4 | rs4771904 (T) | 0.27 | −0.27 (−0.44 to −0.10) | 7.8 × 10−03 | −0.26 (−0.45 to −0.07) | 0.02 | −0.27 (−0.44 to −0.10) | 0.03 |
| 3 | ABCC5 | rs6794223 (G) | 0.14 | 0.27 (0.10–0.43) | 6.7 × 10−03 | 0.25 (0.09–0.41) | 9.0 × 10−03 | 0.25 (0.08–0.42) | 0.01 |
| 11 | SLCO2B1 | rs57141326 (A) | 0.08 | 0.34 (0.12–0.56) | 9.6 × 10−03 | 0.31 (0.09–0.54) | 0.02 | 0.33 (0.11–0.56) | 0.02 |
| 7 | ABCB1 | rs28401781 (T) | 0.24 | 0.19 (0.07–0.32) | 9.0 × 10−03 | 0.19 (0.06–0.31) | 5.8 × 10−03 | 0.22 (0.11–0.33) | 2.4 × 10−03 |
| 3 | ABCC5 | rs56889675 (T) | 0.26 | 0.24 (0.09–0.38) | 7.6 × 10−03 | 0.27 (0.14–0.39) | 1.1 × 10−03 | 0.26 (0.13–0.39) | 2.1 × 10−03 |
| 3 | ABCC5 | rs10470524 (T) | 0.22 | 0.24 (0.09–0.38) | 7.6 × 10−03 | 0.27 (0.14–0.39) | 1.1 × 10−03 | 0.26 (0.13–0.39) | 2.1 × 10−03 |
| 13 | ABBC4 | rs4148551 (T) | 0.36 | −0.25 (−0.41 to −0.10) | 7.4 × 10−03 | −0.23 (−0.39 to −0.08) | 0.01 | −0.23 (−0.40 to −0.07) | 1.8 × 10−03 |
| 19 | CYP2B6 | composite CYP2B6 516/983 | 0.41 | NA | NA | NA | NA | NA | NA |
| 19 | CYP2B6 | CYP2B6 516G→Ta | 0.29 | NA | NA | NA | NA | NA | NA |
| 19 | CYP2B6 | CYP2B6 983T→Cb | 0.13 | −0.18 (−0.45–0.08) | 0.19 | NA | NA | NA | NA |
| 19 | CYP2B6 | CYP2B6 15582C→Tb | 0.10 | 0.02 (−0.24–0.28) | 0.88 | −0.05 (−0.33 to 0.22) | 0.70 | NA | NA |
| 19 | CYP2A6 | CYP2A6 −48A→Cb | 0.09 | −0.04 (−0.32–0.25) | 0.81 | 0.10 (−0.23 to 0.43) | 0.55 | 0.09 (−0.26–0.52) | 0.61 |
MAF, minor allele frequency; NA, not applicable.
For the CSF 8-hydroxy-efavirez analysis, the targeted SNPs (CYP2B6 516G→T, CYP2A6 -48A→C, CYP2B6 983T→C and CYP2B6 15582C→T) included 17 patients and the rest 16 patients.
P < 0.05 accepted as significant for SNPs with a previously described association.
SNP of interest but did not meet criteria of P < 0.01; Bonferroni-corrected P value 5.68 × 10−05.
Table 4.
Genetic associations with detectable log10-transformed CSF:plasma efavirenz concentrations in South African adults
| Chromosome | Gene | Polymorphism (minor allele) | MAF | 516G→T adjusted |
516G→T and 983T→C adjusted |
516G→T and 983T→C and CYP2B6 15582C→T adjusted |
|||
|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | ||||
| 13 | ABCC4 | rs9584273 (T) | 0.07 | 0.14 (0.06–0.22) | 2.2 × 10−03 | 0.14 (0.05–0.22) | 2.8 × 10−03 | 0.14 (0.06–0.22) | 2.5 × 10−03 |
| 13 | ABCC4 | rs9590160 (A) | 0.09 | −0.12 (−0.19 to −0.04) | 3.2 × 10−03 | −0.12 (−0.19 to −0.04) | 3.5 × 10−03 | −0.12 (−0.19 to −0.04) | 4.3 × 10−03 |
| 13 | ABCC4 | rs74107818 (G) | 0.10 | −0.11 (−0.18 to −0.04) | 3.2 × 10−03 | −0.11 (−0.19 to −0.04) | 3.3 × 10−03 | −0.11 (−0.18 to −0.04) | 4.0 × 10−03 |
| 13 | ABCC4 | rs74107809 (A) | 0.07 | −0.13 (−0.21 to −0.04) | 5.8 × 10−03 | −0.13 (−0.21 to −0.04) | 4.8 × 10−03 | −0.13 (−0.21 to −0.04) | 5.7 × 10−03 |
| 19 | CYP2B6 | rs8100458 (C) | 0.19 | 0.07 (0.02–0.12) | 6.8 × 10−03 | 0.08 (0.03–0.14) | 6.4 × 10−03 | 0.10 (0.03–0.16) | 5.4 × 10−03 |
| 3 | ABCC5 | rs7610724 (G) | 0.07 | 0.10 (0.03–0.18) | 7.7 × 10−03 | 0.10 (0.03–0.17) | 7.6 × 10−03 | 0.11 (0.03–0.18) | 9.8 × 10−03 |
| 7 | ABCB1 | rs2235023 (T) | 0.42 | −0.06 (−0.11 to −0.02) | 7.8 × 10−03 | −0.06 (−0.11 to −0.02) | 7.6 × 10−03 | −0.06 (−0.11 to −0.02) | 9.2 × 10−03 |
| 19 | CYP2G1P | rs142357867 (T) | 0.03 | −0.16 (−0.28 to −0.05) | 8.9 × 10−03 | −0.17 (−0.29 to −0.06) | 5.5 × 10−03 | −0.18 (−0.30 to −0.06) | 5.1 × 10−03 |
| 13 | ABCC4 | rs200689258 (AC) | 0.09 | 0.11 (0.03–0.19) | 8.0 × 10−03 | 0.11 (0.03–0.18) | 0.01 | 0.11 (0.03–0.19) | 0.01 |
| 3 | ABCC5 | rs7427051(A) | 0.24 | −0.07 (−0.11 to −0.02) | 8.6 × 10−03 | −0.07 (−0.12 to −0.02) | 0.01 | −0.07 (−0.12 to −0.02) | 0.01 |
| 13 | ABCC4 | rs73548889 (C) | 0.06 | 0.13 (0.04–0.22) | 9.9 × 10−03 | 0.13 (0.03–0.22) | 0.01 | 0.14 (0.05–0.24) | 6.9 × 10−03 |
| 13 | ABCC4 | rs9524925 (G) | 0.17 | −0.07 (−0.12 to −0.02) | 7.6 × 10−03 | −0.07 (−0.13 to −0.02) | 0.01 | −0.07 (−0.12 to −0.02) | 0.01 |
| 19 | CYP2B6 | composite CYP2B6 516/983 (C) | 0.41 | NA | NA | NA | NA | NA | NA |
| 19 | CYP2B6 | CYP2B6 516G→Ta | 0.29 | NA | NA | NA | NA | NA | NA |
| 19 | CYP2B6 | CYP2B6 983T→Cb | 0.13 | −0.03 (−0.10–0.06) | 0.60 | NA | NA | NA | NA |
| 19 | CYP2B6 | CYP2B6 15582C→Tb | 0.10 | −0.03 (−0.10–0.07) | 0.68 | −0.03 (−0.12–0.07) | 0.57 | NA | NA |
| 19 | CYP2A6 | CYP2A6 −48A→Cb | 0.09 | −0.00 (−0.09–0.09) | 0.97 | 0.01 (−0.09–0.12) | 0.78 | 0.01 (−0.09–0.12) | 0.80 |
MAF, minor allele frequency; NA, not applicable.
The targeted SNPs (CYP2B6 516G→T, CYP2A6 −48A→C, CYP2B6 983T→C, CYP2B6 15582C→T, SLCO1B1 521T→ C and SLCO1B1) included 47 patients and the rest 43 patients.
P < 0.05 accepted as significant for SNPs with a previously described association.
SNP of interest but did not meet criteria of P < 0.01; Bonferroni-corrected P value 5.68 × 10−05.
Plasma 8-hydroxy-efavirenz:efavirenz ratios
After adjusting for CYP2B6 516G→T, the association between CYP2B6 983T→C and log10-transformed 8-hydroxy-efavirenz:efavirenz ratios remained significant (β = −0.31, 95% CI = −0.45 to −0.16, P = 1.8 × 10−04). See Table S3.
Plasma 7-hydroxy-efavirenz:efavirenz ratios
In multivariate linear regression models, a previously described CYP2B6 polymorphism (rs2279345) was significantly associated with lower 7-hydroxy-efavirenz:efavirenz ratios (β = −0.28, 95% CI = −0.43 to −0.12, P = 1.2 × 10−03) at P < 0.05 (Table S3). The association remained significant at P < 0.05 after adjusting for CYP2B6 516G→T, CYP2B6 516G→T and CYP2B6 983T→C.
CSF 8-hydroxy-efavirenz:efavirenz ratios
Linear regression analysis results for genetic associations with log10-transformed CSF 8-hydroxy-efavirenz:efavirenz ratios were available in 17 participants (Table S3). After adjusting for CYP2B6 516G→T and CYP2B6 983T→C, the association between CYP2A6 −48A→C and higher log10-transformed CSF 8-hydroxy-efavirenz:efavirenz ratios became significant (β = 0.54, 95% CI = 0.05 to 1.03, P = 4.8 × 10−02).
LD
To demonstrate independent associations, the LD plots are displayed in Figures S2 to S8.
Pharmacokinetic–pharmacodynamic associations with neurocognitive performance
We found no significant correlation between GDS and CSF concentrations of efavirenz or 8-hydroxy-efavirenz. Detectable CSF 8-hydroxy-efavirenz tended to be associated with impaired executive function on the Colour Trails Test (P = 0.043). Participants with detectable CSF 8-hydroxy-efavirenz had a higher GDS compared with participants without detectable CSF 8-hydroxy-efavirenz (1.0 compared with 0.82), but this was not statistically significant. The GDS in the five participants in whom HIV-1 Tat DNA was detected were similar to those in whom Tat DNA was not detected.
Discussion
We investigated whether genetic polymorphisms are associated with CSF disposition of efavirenz in black South African adults. In multivariate analysis CYP2B6 983T→C predicted plasma efavirenz, plasma 7-hydroxy-efavirenz, plasma 8-hydroxy-efavirenz:efavirenz ratio and CSF efavirenz. Lower plasma 7-hydroxy-efavirenz concentrations were independently associated with CYP2A6 rs10853742, ABCB1 rs115780656 and CYP2A6 −48A→C. The CYP2A6 −48A→C polymorphism was also independently associated with higher CSF 8-hydroxy-efavirenz:efavirenz ratio. The CYP2B6 rs2279345 polymorphism was associated with lower plasma 7-hydroxy-efavirenz:efavirenz ratio in multivariate analyses adjusting for CYP2B6 516G→T and 983T→C (P < 0.05). No polymorphisms were associated with CSF:plasma ratios of efavirenz, plasma or CSF concentrations of 8-hydroxy-efavirenz or neurocognitive performance.
We expected CSF 8-hydroxy-efavirenz concentrations to be higher in extensive metabolizers as it is formed via the CYP2B6 enzymatic pathway. However, similar to the findings of others, 8-hydroxy-efavirenz concentrations in plasma and CSF remained constant irrespective of metabolizer status.19,22,23 It is possible that the small number of participants (17 of 47) with detectable CSF 8-hydroxy-efavirenz concentrations contributed to the lack of association between the metabolizer status and CSF 8-hydroxy-efavirenz concentrations. Winston et al.19 proposed that CSF 8-hydroxy-efavirenz concentrations are independent of metabolizer status as it is 8-hydroxy-efavirenz spillover from the plasma, or that efavirenz is metabolized to 8-hydroxy-efavirenz in the CNS, trapping 8-hydroxy-efavirenz within the CNS compartment. We found that CYP2A6 −48A→C was independently associated with a higher CSF 8-hydroxy-efavirenz:efavirenz ratio, which may suggest that in CYP2B6 slow metabolizers, efavirenz may be metabolized to 8-hydroxy-efavirenz in the CNS by the accessory pathway CYP2A6. However, we did not find a statistically significant association with CYP2A6 −48A→C and CSF 8-hydroxy-efavirenz or plasma 8-hydroxy-efavirenz.
We explored pharmacokinetic–pharmacodynamic relationships of efavirenz and neurocognition. Although CSF efavirenz and 8-hydroxy-efavirenz concentrations were above the in vitro CSF toxicity threshold, in 15.2% and 29.8% of participants, respectively, we did not find a relationship between GDS performance and plasma or CSF efavirenz concentrations or CSF 8-hydroxy-efavirenz concentrations. Participants with detectable CSF 8-hydroxy-efavirenz scored worse on the Colour Trails Test (P = 0.04) and had a higher GDS (median 1.39 compared with median 1.0), which was not statistically significant. It is possible that CYP2A6 −48A→C predisposes CYP2B6 slow metabolizers to higher CSF 8-hydroxy-efavirenz concentrations and worse neurocognitive performance. Various cellular mechanisms for efavirenz toxicity have been proposed.46 Higher efavirenz concentrations, which are associated with CYP2B6 slow-metabolizer status, are associated with neurological symptoms, which may include serious presentations such as encephalopathy.18,47 In a CSF substudy of the ENCORE1 trial, 8-hydroxy-efavirenz exposure correlated with adverse neuropsychology outcomes.19
Our study has limitations. We had limited power, with 47 participants, to detect genetic associations between infrequent genotypes with small effect sizes (increase in plasma or CSF concentrations). The CYP2A6 −48A→C polymorphism has been associated with increased plasma efavirenz concentrations in CYP2B6 slow metabolizers, but we found no association as only three participants with CYP2B6 slow-metabolizer genotype carried a single CYP2A6 −48A→C allele.36 This may have also limited our ability to detect associations between CYP2B6 15582C→T and plasma efavirenz concentrations, as 15582CT heterozygosity has been associated with increased plasma efavirenz exposure, and there were no 15582TT homozygotes in our study.7 We were not able to detect pharmacokinetic–pharmacodynamic associations due to limited power to detect smaller differences in cognitive impairment. However, to our knowledge this is the largest sample size examining pharmacogenetic, pharmacokinetic and pharmacodynamic associations with CSF efavirenz. Our study was cross-sectional. Neurocognitive changes would have been better assessed longitudinally. We did not measure unbound concentrations of efavirenz, and protein-free CSF:plasma concentrations of efavirenz in particular may have more accurately reflected the pharmacodynamically active concentrations.
In summary, we identified novel genetic associations with plasma efavirenz, plasma 7-hydroxy-efavirenz, plasma 7-hydroxy-efavirenz:efavirenz ratio, plasma 8-hydroxy-efavirenz:efavirenz ratio, CSF efavirenz and CSF 8-hydroxy-efavirenz:efavirenz ratio. No polymorphisms were associated with: CSF:plasma ratios of efavirenz; plasma or CSF 8-hydroxy-efavirenz concentrations; or neurocognitive performance.
Supplementary Material
Acknowledgements
We are grateful to the study participants, the study team [in alphabetical order: Laura Comrie (clinical support), Carla Freeman (clinical support), Shahieda Isaacs (viral sequencing), Pam Jordan (data capturer), Teboho Linda (neuropsychology technician), Queen Maswana (recruiter), Nozipho Mawisa (study nurse), Rasmita Ori (clinical support), Kareema Poggenpoel (administration support) and Shireen Surtie (study coordinator)] and Cara Sutcliffe and the VANTAGE team at Vanderbilt.
Funding
This work was supported by the South African Medical Research Council and the European and Developing Countries Clinical Trials Partnership (SP.2011.41304.065). The drug assays analysed at the University of Cape Town (UCT) were supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (UM1 AI068634, UM1 AI068636, UM1 AI106701, U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (AI068632). D. W. H. is supported by National Institutes of Health grants (AI077505, AI069439, TR002243).
Transparency declarations
None to declare.
Author contributions
E. H. D.: study concept and design, data acquisition, data analysis and interpretation of data, drafting and revising the manuscript for content, study supervision and obtaining funding. P. Z. S.: data analysis, interpretation of data and revising the manuscript for content. G. U. v. Z.: sample analysis, interpretation of data and revising the manuscript for content. L. W.: sample analysis and revising the manuscript for content. S. K.: sample analysis and revising the manuscript for content. J. A. J.: study concept and design, revising the manuscript for content and study supervision. D. W. H.: sample analysis, data analysis, interpretation of data and revising the manuscript for content. G. M.: study concept and design and revising the manuscript for content.
References
- 1.WHO. First-Line ART For Adults http://www.who.int/hiv/pub/guidelines/arv2013/art/artadults/en/.
- 2. Underwood J, Robertson KR, Winston A.. Could antiretroviral neurotoxicity play a role in the pathogenesis of cognitive impairment in treated HIV disease? AIDS 2015; 29: 253–61. [DOI] [PubMed] [Google Scholar]
- 3. Robertson K, Liner J, Meeker RB.. Antiretroviral neurotoxicity. J Neurovirol 2012; 18: 388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robertson KR, Su Z, Margolis DM. et al. Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology 2010; 74: 1260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Winston A, Duncombe C, Li PCK. et al. Does choice of combination antiretroviral therapy (cART) alter changes in cerebral function testing after 48 weeks in treatment-naive, HIV-1-infected individuals commencing cART? A randomized, controlled study. Clin Infect Dis 2010; 50: 920–9. [DOI] [PubMed] [Google Scholar]
- 6. Haas DW, Tarr PE.. Perspectives on pharmacogenomics of antiretroviral medications and HIV-associated comorbidities. Curr Opin HIV AIDS 2015; 10: 116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holzinger ER, Grady B, Ritchie MD. et al. Genome-wide association study of plasma efavirenz pharmacokinetics in AIDS Clinical Trials Group protocols implicates several CYP2B6 variants. Pharmacogenet Genomics 2012; 22: 858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haas DW, Ribaudo HJ, Kim RB. et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS 2004; 18: 2391–400. [PubMed] [Google Scholar]
- 9. Viljoen M, Karlsson MO, Meyers TM. et al. Influence of CYP2B6 516G>T polymorphism and interoccasion variability (IOV) on the population pharmacokinetics of efavirenz in HIV-infected South African children. Eur J Clin Pharmacol 2012; 68: 339–47. [DOI] [PubMed] [Google Scholar]
- 10. Ngaimisi E, Habtewold A, Minzi O. et al. Importance of ethnicity, CYP2B6 and ABCB1 genotype for efavirenz pharmacokinetics and treatment outcomes: a parallel-group prospective cohort study in two sub-Saharan Africa populations. PLoS One 2013; 8: e67946.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jamshidi Y, Moreton M, McKeown DA. et al. Tribal ethnicity and CYP2B6 genetics in Ugandan and Zimbabwean populations in the UK: implications for efavirenz dosing in HIV infection. J Antimicrob Chemother 2010; 65: 2614–9. [DOI] [PubMed] [Google Scholar]
- 12. Kwara A, Lartey M, Sagoe KWC. et al. CYP2B6, CYP2A6 and UGT2B7 genetic polymorphisms are predictors of efavirenz mid-dose concentration in HIV-infected patients. AIDS 2009; 23: 2101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Swart M, Skelton M, Ren Y. et al. High predictive value of CYP2B6 SNPs for steady-state plasma efavirenz levels in South African HIV/AIDS patients. Pharmacogenet Genomics 2013; 23: 415–27. [DOI] [PubMed] [Google Scholar]
- 14. Gounden V, van Niekerk C, Snyman T. et al. Presence of the CYP2B6 516G>T polymorphism, increased plasma efavirenz concentrations and early neuropsychiatric side effects in South African HIV-infected patients. AIDS Res Ther 2010; 7: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sinxadi PZ, Leger PD, McIlleron HM. et al. Pharmacogenetics of plasma efavirenz exposure in HIV-infected adults and children in South Africa. Br J Clin Pharmacol 2015; 80: 146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thorn CF, Lamba JK, Lamba V. et al. PharmGKB summary: very important pharmacogene information for CYP2B6. Pharmacogenet Genomics 2010; 20: 520–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. di Iulio J, Fayet A, Arab-Alameddine M. et al. In vivo analysis of efavirenz metabolism in individuals with impaired CYP2A6 function. Pharmacogenet Genomics 2009; 19: 300–9. [DOI] [PubMed] [Google Scholar]
- 18. Variava E, Sigauke FR, Norman J. et al. Late efavirenz-induced ataxia and encephalopathy: a case series. J Acquir Immune Defic Syndr 2017; 75: 577–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Winston A, Amin J, Clarke A. et al. Cerebrospinal fluid exposure of efavirenz and its major metabolites when dosed at 400 mg and 600 mg once daily: a randomized controlled trial. Clin Infect Dis 2015; 60: 1026–32. [DOI] [PubMed] [Google Scholar]
- 20. Tovar-y-Romo LB, Bumpus NN, Pomerantz D. et al. Dendritic spine injury induced by the 8-hydroxy metabolite of efavirenz. J Pharmacol Exp Ther 2012; 343: 696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brandmann M, Nehls U, Dringen R.. 8-Hydroxy-efavirenz, the primary metabolite of the antiretroviral drug efavirenz, stimulates the glycolytic flux in cultured rat astrocytes. Neurochem Res 2013; 38: 2524–34. [DOI] [PubMed] [Google Scholar]
- 22. Nightingale S, Chau TTH, Fisher M. et al. Efavirenz and metabolites in cerebrospinal fluid: relationship with CYP2B6 c.516G→T genotype and perturbed blood-brain barrier due to tuberculous meningitis. Antimicrob Agents Chemother 2016; 60: 4511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aouri M, Barcelo C, Ternon B. et al. In vivo profiling and distribution of known and novel phase I and phase II metabolites of efavirenz in plasma, urine, and cerebrospinal fluid. Drug Metab Dispos 2016; 44: 151–61. [DOI] [PubMed] [Google Scholar]
- 24. Suhy AM, Webb A, Papp AC. et al. Expression and splicing of ABC and SLC transporters in the human blood-brain barrier measured with RNAseq. Eur J Pharm Sci 2017; 103: 47–51. [DOI] [PubMed] [Google Scholar]
- 25. Varatharajan L, Thomas SA.. The transport of anti-HIV drugs across blood-CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res 2009; 82: A99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alfirevic A, Durocher J, Elati A. et al. Misoprostol-induced fever and genetic polymorphisms in drug transporters SLCO1B1 and ABCC4 in women of Latin American and European ancestry. Pharmacogenomics 2015; 16: 919–28. [DOI] [PubMed] [Google Scholar]
- 27. Stieger B, Gao B.. Drug transporters in the central nervous system. Clin Pharmacokinet 2015; 54: 225–42. [DOI] [PubMed] [Google Scholar]
- 28. Eyal S, Hsiao P, Unadkat JD.. Drug interactions at the blood-brain barrier: fact or fantasy? Pharmacol Ther 2009; 123: 80–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Störmer E, von Moltke LL, Perloff MD. et al. Differential modulation of P-glycoprotein expression and activity by non-nucleoside HIV-1 reverse transcriptase inhibitors in cell culture. Pharm Res 2002; 19: 1038–45. [DOI] [PubMed] [Google Scholar]
- 30. Swart M, Ren Y, Smith P. et al. ABCB1 4036A>G and 1236C>T polymorphisms affect plasma efavirenz levels in South African HIV/AIDS patients. Front Genet 2012; 3: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sánchez Martín A, Cabrera Figueroa S, Cruz Guerrero R. et al. Impact of pharmacogenetics on CNS side effects related to efavirenz. Pharmacogenomics 2013; 14: 1167–78. [DOI] [PubMed] [Google Scholar]
- 32. Sánchez-Martín A, Cabrera Figueroa S, Cruz R. et al. Gene–gene interactions between DRD3, MRP4 and CYP2B6 polymorphisms and its influence on the pharmacokinetic parameters of efavirenz in HIV infected patients. Drug Metab Pharmacokinet 2016; 31: 349–55. [DOI] [PubMed] [Google Scholar]
- 33. Campbell MC, Tishkoff SA.. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu Rev Genomics Hum Genet 2008; 9: 403–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO. Antiretroviral Therapy Coverage Data and Estimates by Country. Global Health Observatory Data Repository; 2017. http://apps.who.int/gho/data/node.main.626?lang=en. [Google Scholar]
- 35. Decloedt EH, Freeman C, Howells F. et al. Moderate to severe HIV-associated neurocognitive impairment: a randomized placebo-controlled trial of lithium. Medicine (Baltimore) 2016; 95: e5401.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haas DW, Kwara A, Richardson DM. et al. Secondary metabolism pathway polymorphisms and plasma efavirenz concentrations in HIV-infected adults with CYP2B6 slow metabolizer genotypes. J Antimicrob Chemother 2014; 69: 2175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carey CL, Woods SP, Gonzalez R. et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 2004; 26: 307–19. [DOI] [PubMed] [Google Scholar]
- 38. Myer L, Smit J, Le Roux L. et al. Common mental disorders among HIV-infected individuals in South Africa: prevalence, predictors, and validation of brief psychiatric rating scales. AIDS Patient Care STDS 2008; 22: 147–58. [DOI] [PubMed] [Google Scholar]
- 39. Mishra M, Vetrivel S, Siddappa NB. et al. Clade-specific differences in neurotoxicity of human immunodeficiency virus-1 B and C Tat of human neurons: significance of dicysteine C30C31 motif. Ann Neurol 2008; 63: 366–76. [DOI] [PubMed] [Google Scholar]
- 40. Paul RH, Joska JA, Woods C. et al. Impact of the HIV Tat C30C31S dicysteine substitution on neuropsychological function in patients with clade C disease. J Neurovirol 2014; 20: 627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blennow K, Fredman P, Wallin A. et al. Protein analysis in cerebrospinal fluid. II. Reference values derived from healthy individuals 18-88 years of age. Eur Neurol 1993; 33: 129–33. [DOI] [PubMed] [Google Scholar]
- 42. Avery LB, Sacktor N, McArthur JC. et al. Protein-free efavirenz concentrations in cerebrospinal fluid and blood plasma are equivalent: applying the law of mass action to predict protein-free drug concentration. Antimicrob Agents Chemother 2013; 57: 1409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Best BM, Koopmans PP, Letendre SL. et al. Efavirenz concentrations in CSF exceed IC50 for wild-type HIV. J Antimicrob Chemother 2011; 66: 354–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Avery LB, VanAusdall JL, Hendrix CW. et al. Compartmentalization and antiviral effect of efavirenz metabolites in blood plasma, seminal plasma, and cerebrospinal fluid. Drug Metab Dispos 2013; 41: 422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yilmaz A, Watson V, Dickinson L. et al. Efavirenz pharmacokinetics in cerebrospinal fluid and plasma over a 24-hour dosing interval. Antimicrob Agents Chemother 2012; 56: 4583–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Apostolova N, Blas-Garcia A, Galindo MJ. et al. Efavirenz: what is known about the cellular mechanisms responsible for its adverse effects. Eur J Pharmacol 2017; 812: 163–73. [DOI] [PubMed] [Google Scholar]
- 47. Kenyon C, Mfolozi S, Croxford R. et al. Severe efavirenz-induced vacuolar axonopathy complicated by fatal aspiration pneumonia. Br J Clin Pharmacol 2012; 74: 1070–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.