Abstract
Objectives
This analysis evaluated the variability of isavuconazole plasma concentrations between subjects and between sampling times, and assessed their relationship to outcomes for subjects with invasive fungal disease (IFD) in the SECURE trial.
Methods
Isavuconazole-treated subjects received 372 mg of isavuconazonium sulphate (corresponding to 200 mg of isavuconazole) three times daily for 2 days, then once daily. Plasma samples were collected after day 4 and analysis sets were constructed as follows: analysis set 1 included all samples from subjects with proven/probable/possible IFD who received ≥1 dose of isavuconazole; analysis set 2 included samples from subjects in analysis set 1 who had provided >1 sample; and analysis set 3 included samples from subjects in analysis set 1 with proven/probable invasive aspergillosis. Assessments included overall distributions of plasma concentrations and variability between samples (analysis sets 1 and 2) as well as relationships to outcomes [all-cause mortality (day 42), overall response (end of treatment) and treatment-emergent adverse events; analysis sets 1 and 3].
Results
Analysis sets 1, 2 and 3 included samples from 160, 97 and 98 subjects, respectively. Trough concentrations for each were distributed similarly [mean (SD): 3406.6 (1511.5), 3495.6 (1503.3) and 3368.1 (1523.2) ng/mL, respectively]. The mean coefficient of variation between samples in analysis set 2 was 23.2%; differences between concentrations in first samples and subsequent samples were <2-fold for 85/97 subjects. In quartiles of subject data, no concentration-dependent relationships were observed for efficacy or safety.
Conclusions
Plasma concentrations of isavuconazole were reasonably consistent between subjects and sampling times, and were not associated with differences in outcomes.
Introduction
Profoundly immunocompromised patients have an elevated risk of developing invasive fungal disease (IFD) such as invasive aspergillosis (IA).1 Triazole antifungal drugs are first-line agents for the prevention and treatment of IFDs.2,3 However, the pharmacokinetics of triazole agents active against Aspergillus spp. are typically highly variable and the therapeutic window may be narrow. As a result, therapeutic drug monitoring (TDM) of triazole antifungal agents is frequently recommended to achieve safe and effective drug exposures.3–8 Guidelines issued by the Sixth European Conference on Infections in Leukaemia (ECIL-6)6 and joint guidelines from ESCMID, the European Confederation of Medical Mycology and the European Respiratory Society (ESCMID-ECMM-ERS)9 contain recommendations regarding the need for TDM and the therapeutic windows for itraconazole, posaconazole and voriconazole for which the strength is based on the clinical history with each of those agents.
The most recently developed triazole antifungal agent, isavuconazole (active moiety of the prodrug isavuconazonium sulphate), which has both intravenous and oral formulations, is now also included among first-line treatment recommendations in recent IA guidelines2,3 based on Phase 3 clinical trials in adults with IA10 or mucormycosis.11 Isavuconazole has linear pharmacokinetics and may be less variable and/or prone to food effects compared with other triazoles that are used for treatment of IA,12–14 but the potential need for TDM and the therapeutic window are not yet well defined in guidelines.6,9 In a recent analysis of data from patients in the SECURE trial, subject exposures were estimated from a population pharmacokinetic (PPK) model to assess possible associations with efficacy and safety outcomes.15 No relationship was found between the modelled exposures and efficacy outcomes [all-cause mortality (ACM) at day 42 and overall response at the end of treatment (EOT)] or elevation of liver enzyme test results.
The current post hoc analysis was conducted to examine more closely the distribution and variability of isavuconazole exposure both between and within subjects using available samples from the SECURE trial. We also aimed to determine whether the lack of a relationship between plasma concentrations and efficacy could be confirmed directly from the clinical trial samples and to determine whether there were any relationships between plasma concentrations and the incidence of treatment-emergent adverse events (TEAEs).
Methods
Study data
Data were used from subjects treated with isavuconazole in the SECURE trial that compared isavuconazole and voriconazole for the primary treatment of invasive mould disease caused by Aspergillus spp. and other filamentous fungi (NCT00412893).10 Briefly, isavuconazole-treated subjects were randomized to receive 372 mg of isavuconazonium sulphate (corresponding to 200 mg of isavuconazole) intravenously three times daily for 2 days (loading dose), then intravenously or orally once daily (maintenance dose). Stratification factors during randomization included geographical region, allogeneic HSCT and active malignancy at study entry.
The primary endpoint was ACM at day 42 in the ITT population (all randomized subjects who received at least one dose of study medication). Overall response at EOT in the modified ITT population [ITT subjects with proven or probable IFD, as assessed by an independent data review committee (DRC)] was a key secondary endpoint (assessed by the DRC based on a composite of clinical, mycological and radiological endpoints). Exploratory analyses included determination of trough plasma concentrations and scheduled sampling times included days 7, 14 and 42, and EOT (up to 3 days before last study dose); the study protocol stipulated that samples be drawn within a predefined window 1 h prior to the scheduled daily dose or 24 ± 1 h after dosing at EOT. Although some samples were drawn outside the strictly predefined window for trough concentrations, all were drawn during the maintenance dose (most ‘first samples’ were drawn on day 7, with the exception of seven drawn on day 6 and two drawn on day 5) and no more than 4 h prior to the next dose (or 20–25 h after dosing at EOT). This window ensured that all were taken outside the absorption and distributive phases. Plasma concentrations were determined using a validated LC with tandem MS assay as described elsewhere.16
Analysis sets
Three analysis sets were used for this analysis. The first analysis set (analysis set 1) included all subjects in the ITT population with proven, probable or possible IFD who received ≥1 dose of isavuconazole and had data for at least one plasma concentration sample. This analysis set was used in assessments of inter-subject variability, efficacy and safety. The second analysis set (analysis set 2) included subjects in analysis set 1 who had data for >1 plasma concentration sample, with the same conditions for included samples. This analysis set was used to assess intra-subject variability between sampling times by examining the distribution of the coefficient of variation (CV) and by examining the maximum changes between the first plasma concentration and subsequent concentrations. A third analysis set (analysis set 3) included all samples from subjects in analysis set 1 with proven or probable IA [subset of the mycological ITT (myITT) population] and this set was used in efficacy analyses.
Statistical analysis
The distributions of average plasma concentrations, as well as assessments of inter-subject and intra-subject variability, were assessed using descriptive statistics (for subjects who provided >1 sample, mean values were used). To assess potential relationships between plasma concentrations and efficacy, both ACM at day 42 and overall response at EOT were assessed in quartiles of subject data from analysis sets 1 and 3. Assessments were performed with quartiles that included mean values for subjects with >1 sample and were repeated with quartiles that included minimum values for those subjects to assess potential effects of including the low plasma concentrations. The incidences of TEAEs were assessed in quartiles of analysis set 1 (samples from subjects in the ITT population). Assessments were performed with quartiles that included mean values for subjects with >1 sample and were repeated with quartiles that included maximum values for those subjects to assess potential effects of including the high plasma concentrations. Analyses of TEAEs included categories of overall frequencies by system organ class (SOC), study drug-related TEAEs by SOC and study drug-related TEAEs by preferred term (PT). The Fisher–Freeman–Halton test (0.05 significance level) was used to identify associations between plasma concentration quartiles and rates of treatment success (ACM at day 42 and overall response at EOT) or TEAEs. All data analyses were performed using SAS version 9.4.
Results
Of the 258 subjects in the ITT population who received ≥1 dose of isavuconazole in the SECURE trial, samples from 160 subjects were included in analysis set 1 (subjects with proven/probable/possible IFD and ≥1 plasma concentration sample; 306 samples in total), samples from 97 subjects were included in analysis set 2 (subjects in analysis set 1 with >1 plasma concentration sample; 243 samples in total) and samples from 98 subjects were included in analysis set 3 (subjects from analysis set 1 with proven or probable IA; 191 samples in total). Demographics and characteristics of analysis sets 1–3 were similar to those of the ITT population (Table S1, available as Supplementary data at JAC Online).
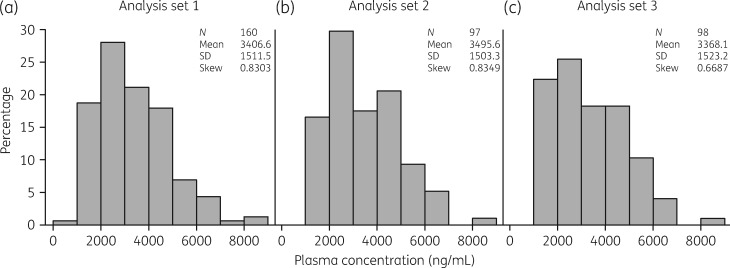
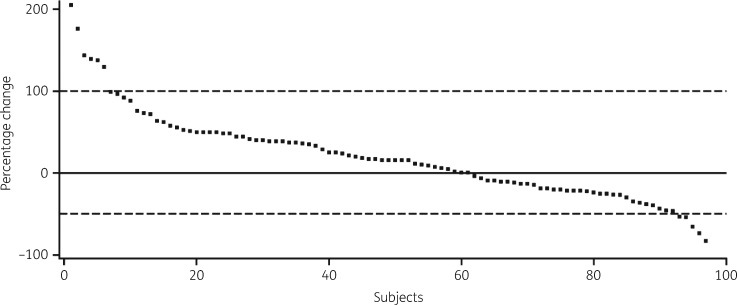
The distributions and overall consistencies of average plasma concentrations of isavuconazole were first compared by visual inspection for all patients in analysis sets 1, 2 and 3 by categorization of data into 1000 ng/mL increments. Plasma concentrations in each case demonstrated similar distributions with similar means, SD values and measures of skewness (Figure 1a–c). More than 97% of patients had concentrations >1000 ng/mL and <7000 ng/mL suggesting reasonable consistency within each analysis set and comparability of distributions between each set. In analysis set 2, the mean CV between sampling times for each subject was 23.2% (95% CI 19.9%–26.5%; Figure S1). For 85/97 subjects (87.6%), the maximum changes between the first plasma concentration and subsequent plasma concentrations were less than 2-fold (100% increase or 50% decrease; Figure 2).
Figure 1.
Distribution of average plasma concentrations. (a) Concentrations from subjects with proven/probable/possible IFD (analysis set 1). (b) Concentrations from subjects in analysis set 1 with >1 available sample (analysis set 2). (c) Concentrations from subjects with proven or probable IA in the myITT population (analysis set 3).
Figure 2.
Maximum percentage changes between the first sample and subsequent samples for analysis set 2. Broken horizontal lines represent 100% increases and 50% decreases.
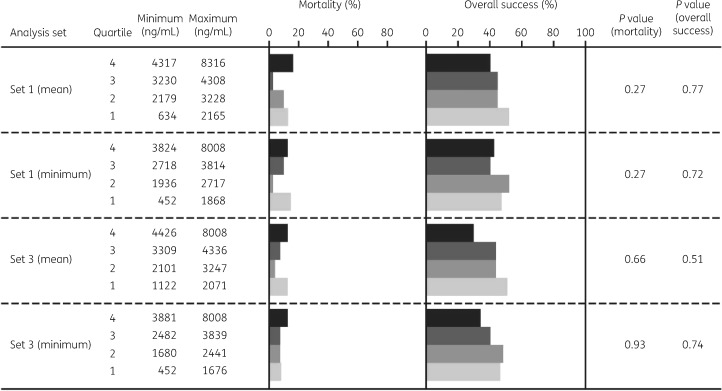
Analyses were also performed to assess the possibility of relationships between isavuconazole plasma concentrations and efficacy (day 42 ACM and overall response at EOT) in samples from subjects in analysis sets 1 and 3. As shown in Figure 3, there were no obvious trends suggesting loss of efficacy at lower plasma concentrations and no significant differences between quartiles in any of the analyses when assessed using either mean values or minimum values for subjects who provided >1 sample.
Figure 3.
Quartile analyses of day 42 ACM and overall success at EOT in analysis sets 1 and 3 assessed using mean or minimum values for subjects who provided >1 sample.
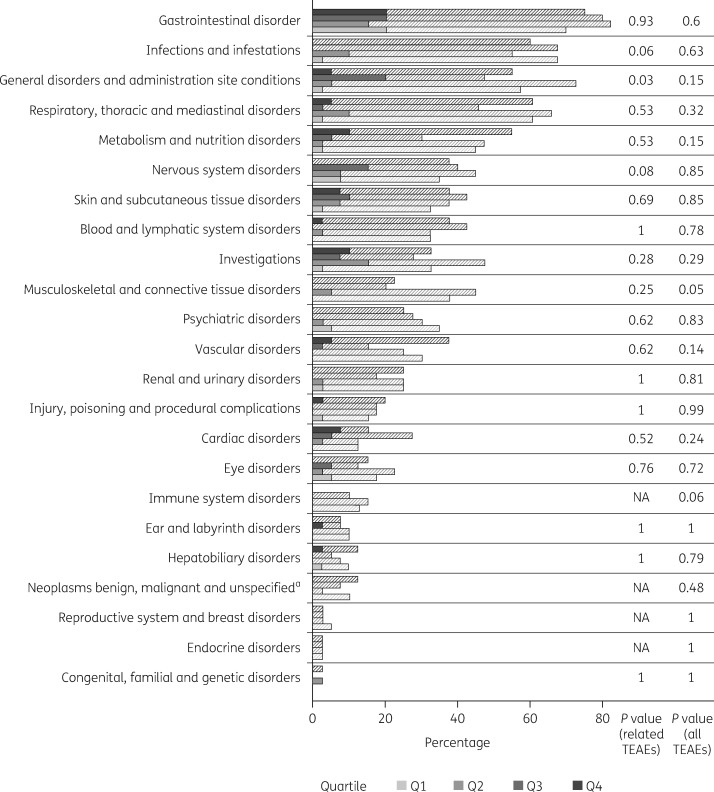
Quartile analyses were also performed to look for potential relationships between plasma concentrations and the incidence of TEAEs in samples from analysis set 1. In analyses of all TEAEs by SOC using mean values for subjects who provided >1 sample, one significant difference between quartiles was observed. Specifically, musculoskeletal and connective tissue disorders had lower incidences in quartiles 3 and 4 (highest plasma concentrations), which is not consistent with a relationship to exposure. The analyses of study drug-related TEAEs by SOC or by PT revealed significant differences between quartiles for general disorders and administration-site conditions. The highest incidence occurred in quartile 3 (second-highest plasma concentrations), which is also inconsistent with a relationship to exposure (Table S2 and Figure 4). In analyses of all TEAEs by SOC using maximum values for subjects who provided >1 sample, there were no significant differences between quartiles for any TEAE (Table S3). For study drug-related TEAEs by SOC, significant differences between quartiles were observed for general disorders and administration-site conditions, and for nervous system disorders. Also, in these two instances, the highest incidence of TEAEs occurred in quartile 3, which is not consistent with any relationship to exposure. There were no significant differences between quartiles for any of the analyses of study drug-related TEAEs by PT.
Figure 4.
Quartile analyses of all TEAEs (hatched bars) and related TEAEs (solid bars) by SOC for analysis set 1, assessed using mean values for subjects who provided >1 sample. aIncludes cysts and polyps. NA, not applicable (no TEAEs).
Discussion
In this analysis of subjects treated with isavuconazole in the SECURE trial, the distribution of plasma concentrations was reasonably narrow. Less than 3% of patients had an average concentration outside a range of 1000–7000 ng/mL, indicating that the recommended clinical dose resulted in plasma concentrations that were largely consistent. The intra-subject variability between sampling times was ∼23% and the maximum difference between the first trough concentration and subsequent concentrations was less than 2-fold for >85% of subjects. These data provide direct support for the consistency and predictability of plasma concentrations in the majority of subjects during maintenance dosing, from day 3 onward. Furthermore, no clear correlation was observed between plasma concentrations and efficacy outcomes (ACM on day 42 and overall response at EOT). In two different analytical approaches to assess the incidence of TEAEs (using either mean or maximum concentrations), no significant instances were found in which the quartile with the highest plasma concentration also had the highest incidence. Thus, the modest variability in concentrations observed in the SECURE trial was not associated with any obvious differences in efficacy or safety outcomes.
The present analysis provides the most complete assessment to date of the variability in isavuconazole plasma concentrations and associations with outcomes in patients from the SECURE trial. The lack of a relationship of plasma concentrations with efficacy provides direct support for a similar finding in the PPK model,15 although the present analysis provides a more comprehensive analysis of associations with safety outcomes. For example, the lack of an association with hepatotoxicity inferred from liver enzyme test results in the PPK analysis are now more directly demonstrated by a lack of an association with hepatobiliary TEAEs. Furthermore, it is well established that voriconazole trough concentrations above a threshold between 4 and 6 mg/L are associated with neurotoxicity,17–19 whereas no evidence of a relationship between isavuconazole plasma concentration and neurotoxicity was evident in the present analysis. The SECURE trial also reported significantly lower incidences of eye disorders and skin and subcutaneous tissue disorders with isavuconazole versus voriconazole,10 and neither of those TEAEs demonstrated any relationship with isavuconazole plasma concentrations in the present analysis.
The overall consistency of isavuconazole plasma concentrations at the clinical dose observed in the current analysis contrasts with the variability of itraconazole, posaconazole and voriconazole concentrations observed previously. For example, a study of oral itraconazole in healthy volunteers found wide inter-subject variability and accumulation over 15 days of dosing.20 A PPK analysis of posaconazole data (oral suspension formulation) from healthy volunteers and patients found that its bioavailability was 55% lower in patients and the bioavailability was also reduced by mucositis or diarrhoea.21 In an analysis of the distribution of weekly mean plasma concentration of voriconazole from subjects in 10 Phase 2/3 studies, the distribution was highly skewed, with no clear mean, and the most frequent concentration was in the lowest interval (0–1 mg/L).22 The distributions of trough concentrations in Monte Carlo simulations for both oral and intravenous doses of voriconazole demonstrated similar distributions.23 The consistency of isavuconazole concentrations in serial samples in the current analysis also contrasts with the variability of voriconazole plasma concentrations in serial samples. For example, a study of paired voriconazole plasma concentration samples found that the concentration in the second sample differed by more than 2-fold for almost half of assessed patients (n/N = 30/64; 47%).24 The reasons for the variability of the dose–exposure relationship of voriconazole are not completely understood. They may involve saturable metabolism,25 allelic variations in cytochrome P450 2C19 (CYP2C19; the primary isoenzyme responsible for metabolism of voriconazole26) and perhaps auto-inhibition of CYP3A4.27
This analysis has some limitations. For example, the relative consistency of the relationship between the clinical dose and plasma concentrations also meant that limited data were available to properly assess efficacy or safety outcomes associated with very low or very high exposures. Therefore, it was not possible to identify any thresholds that might support recommendations for minimum or maximum concentrations that could be used as a clinical guide. The possibility that exposure–response relationships might differ by pathogen species was not excluded, although the relative proportions of most species in SECURE were not sufficiently large to have allowed any definitive conclusions. In addition, although plasma concentrations are an indicator of exposure, it is well established that the ratio of drug exposure (measured as the AUC) to the MIC of the pathogen is the most relevant pharmacokinetic-pharmacodynamic index of efficacy for triazole antifungal agents, including isavuconazole.28 Nevertheless, a PPK analysis that included data from the SECURE trial indicated that exposures achieved by the clinical dose of isavuconazole were likely to provide adequate coverage for >90% of patients with Aspergillus spp. pathogens having MICs up to 1 mg/L (EUCAST methodology) or up to 0.5 mg/L (CLSI methodology).29 Recent data has indicated that the relative consistency in isavuconazole concentrations in patients from the SECURE trial is also observed in real-world data,30 suggesting that the extent of coverage is also likely to be applicable in clinical practice.
Although these analyses do not identify a therapeutic window for isavuconazole, they do suggest that TDM may be less critical during treatment with this agent compared with other triazole antifungal agents active against Aspergillus spp. Given the predictability of exposure in the current analyses, if performance of TDM is deemed advisable, a sparse sampling schedule might be sufficient. Finally, the consistency of dose–exposure relationships is likely to maximize the potential for efficacious and safe use of isavuconazole for treatment of IFD in a real-world setting.
Supplementary Material
Acknowledgements
This study was presented in part at the Twenty-sixth European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, The Netherlands, 2016 (Abstract O424).
We thank Amit Desai, Laura Kovanda and Robert Townsend of Astellas Pharma Global Development, Inc. for critical reviews of early drafts.
Funding
This analysis was funded by Basilea Pharmaceutica International Ltd. Medical writing support (see below) was funded by Basilea Pharmaceutica International Ltd.
Transparency declarations
T. K., M. E., M. S. and P. L. are employees of Basilea Pharmaceutica International Ltd. At the time of the study, M. S. was an employee of ICON plc who were contracted to perform analyses related to the study. A. H. G. has received grants from Gilead, Merck, Sharp & Dohme, Pfizer and Schering-Plough, is a consultant to Amplyx, Astellas, Basilea, Gilead, Merck, Sharp & Dohme and Schering-Plough, and has served on the speaker’s bureau of Astellas, Basilea, Gilead, Merck, Sharp & Dohme, Pfizer, Schering-Plough and Zeneus/Cephalon. D. A.: none to declare.
Medical writing support was provided by Ed Parr, PhD, CMPP, of Envision Scientific Solutions.
References
- 1. Ruhnke M, Schwartz S.. Recent developments in the management of invasive fungal infections in patients with oncohematological diseases. Ther Adv Hematol 2016; 7: 345–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patterson TF, Thompson GR. 3rd, Denning DWet al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63: e1–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tissot F, Agrawal S, Pagano L. et al. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 2016; 102: 433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ashbee HR, Barnes RA, Johnson EM. et al. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother 2014; 69: 1162–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chau MM, Kong DC, van Hal SJ. et al. Consensus guidelines for optimising antifungal drug delivery and monitoring to avoid toxicity and improve outcomes in patients with haematological malignancy, 2014. Intern Med J 2014; 44: 1364–88. [DOI] [PubMed] [Google Scholar]
- 6. Lewis R, Brüggemann R, Padoin C. et al. Triazole antifungal therapeutic drug monitoring. Presented at the: Sixth European Conference on Infections in Leukaemia Meeting, Sophia Antipolis, France, 2015 http://www.ecil-leukaemia.com/telechargements2015/ECIL6-Triazole-TDM-07-12-2015-Lewis-R-et-al.pdf.
- 7. Mousset S, Buchheidt D, Heinz W. et al. Treatment of invasive fungal infections in cancer patients—updated recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Hematol 2014; 93: 13–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamada Y, Tokimatsu I, Mikamo H. et al. Practice guidelines for therapeutic drug monitoring of voriconazole: a consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J Infect Chemother 2013; 19: 381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ullmann AJ, Aguado JM, Arikan-Akdagli S. et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 2018; 24 Suppl 1: e1–38. [DOI] [PubMed] [Google Scholar]
- 10. Maertens JA, Raad II, Marr KA. et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet 2016; 387: 760–9. [DOI] [PubMed] [Google Scholar]
- 11. Marty FM, Ostrosky-Zeichner L, Cornely OA. et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis 2016; 16: 828–37. [DOI] [PubMed] [Google Scholar]
- 12. Schmitt-Hoffmann A, Roos B, Heep M. et al. Single-ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother 2006; 50: 279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmitt-Hoffmann A, Roos B, Maares J. et al. Multiple-dose pharmacokinetics and safety of the new antifungal triazole BAL4815 after intravenous infusion and oral administration of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother 2006; 50: 286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmitt-Hoffmann A, Desai A, Kowalski D. et al. Isavuconazole absorption following oral administration in healthy subjects is comparable to intravenous dosing, and is not affected by food, or drugs that alter stomach pH. Int J Clin Pharmacol Ther 2016; 54: 572–80. [DOI] [PubMed] [Google Scholar]
- 15. Desai AV, Kovanda LL, Hope WW. et al. Exposure-response relationships for isavuconazole in patients with invasive aspergillosis and other filamentous fungi. Antimicrob Agents Chemother 2017; 61: e01034–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kovanda LL, Desai AV, Lu Q. et al. Isavuconazole population pharmacokinetic analysis using nonparametric estimation in patients with invasive fungal disease (results from the VITAL study). Antimicrob Agents Chemother 2016; 60: 4568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dolton MJ, McLachlan AJ.. Voriconazole pharmacokinetics and exposure–response relationships: assessing the links between exposure, efficacy and toxicity. Int J Antimicrob Agents 2014; 44: 183–93. [DOI] [PubMed] [Google Scholar]
- 18. Luong ML, Al-Dabbagh M, Groll AH. et al. Utility of voriconazole therapeutic drug monitoring: a meta-analysis. J Antimicrob Chemother 2016; 71: 1786–99. [DOI] [PubMed] [Google Scholar]
- 19. Jin H, Wang T, Falcione BA. et al. Trough concentration of voriconazole and its relationship with efficacy and safety: a systematic review and meta-analysis. J Antimicrob Chemother 2016; 71: 1772–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hardin TC, Graybill JR, Fetchick R. et al. Pharmacokinetics of itraconazole following oral administration to normal volunteers. Antimicrob Agents Chemother 1988; 32: 1310–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dolton MJ, Brüggemann RJ, Burger DM. et al. Understanding variability in posaconazole exposure using an integrated population pharmacokinetic analysis. Antimicrob Agents Chemother 2014; 58: 6879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US FDA. Drug Approval Package, Vfend (Voriconazole) Tablets and Injection; Clinical Pharmacology and Biopharmaceutics Review(s) https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-266_VFEND_biopharmr.pdf.
- 23. Hope WW. Population pharmacokinetics of voriconazole in adults. Antimicrob Agents Chemother 2012; 56: 526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trifilio SM, Yarnold PR, Scheetz MH. et al. Serial plasma voriconazole concentrations after allogeneic hematopoietic stem cell transplantation. Antimicrob Agents Chemother 2009; 53: 1793–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Purkins L, Wood N, Ghahramani P. et al. Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens. Antimicrob Agents Chemother 2002; 46: 2546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Owusu Obeng A, Egelund EF, Alsultan A. et al. CYP2C19 polymorphisms and therapeutic drug monitoring of voriconazole: are we ready for clinical implementation of pharmacogenomics? Pharmacotherapy 2014; 34: 703–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hohmann N, Kocheise F, Carls A. et al. Dose-dependent bioavailability and CYP3A inhibition contribute to non-linear pharmacokinetics of voriconazole. Clin Pharmacokinet 2016; 55: 1535–45. [DOI] [PubMed] [Google Scholar]
- 28. Lepak AJ, Andes DR.. Antifungal pharmacokinetics and pharmacodynamics. Cold Spring Harb Perspect Med 2014; 5: a019653.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Desai A, Kovanda L, Kowalski D. et al. Population pharmacokinetics of isavuconazole from phase 1 and phase 3 (SECURE) trials in adults and target attainment in patients with invasive infections due to Aspergillus and other filamentous fungi. Antimicrob Agents Chemother 2016; 60: 5483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andes D, Kovanda L, Desai A. et al. Isavuconazole concentration in real-world practice: consistency with results from clinical trials. Antimicrob Agents Chemother 2018; 62: e00585–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.