Abstract
Objective:
To determine if higher potency cannabis is associated with earlier progression to regular cannabis use, daily cannabis use, and cannabis use disorder symptom onset.
Methods:
Data sources were the Michigan Longitudinal Study, an ongoing prospective, high-risk family study investigating the course and predictors for substance use disorders among youth beginning prior to school entry and time-parallel national average trends in delta-9-tetrahydrocannabinol (i.e., psychoactive compound in cannabis). The national average trends in delta-9-tetrahydrocannabinol were used to estimate potency level for the individual. Only cannabis users were included in analyses (n=527).
Results:
Cox regression showed an increased risk of progression from cannabis initiation to cannabis use disorder symptom onset by 1.41 times (p<.001) for each unit increase in national average delta-9-tetrahydrocannabinol as compared to those not endorsing CUD symptom onset, adjusting for sex, regular use, and cohort effects. Accounting for regular use, individuals initiating cannabis at national average 4.9% delta-9-tetrahydrocannabinol were at 1.88 times (p=.012) higher risk for cannabis use disorder symptom onset within one year compared to those who did not endorse CUD symptom onset, while those initiating cannabis at national average 12.3% delta-9-tetrahydrocannabinol were at 4.85 times (p=.012) higher risk within one year.
Conclusions:
This study provides prospective evidence suggesting higher potency cannabis, on average in the U.S., increases risk for onset of first cannabis use disorder symptom. Development of guidelines regarding cannabis potency is critical for reducing the costs associated with negative health outcomes.
Keywords: Cannabis, Cannabis Potency, Cannabis Use Disorder, Cannabis Use Patterns, Delta-9-Tetrahydrocannabinol, High Potency Cannabis
1. Introduction
Development of guidelines and regulations regarding cannabis potency (i.e., delta-9- tetrahydrocannabinol [THC]%, psychoactive compound in cannabis) has been slow in comparison to the quickly changing legislative landscape of cannabis. Since California legalized medical cannabis in 1996, 33 states and Washington D.C. have legalized medical cannabis, and nine states have legalized recreational cannabis. However, none of the states that legalized medical or recreational cannabis have developed regulations regarding THC levels (Coombes, 2014). Uruguay, the first country to fully legalize cannabis, considered but has not instituted an upper limit for THC% (Coombes, 2014) and the Netherlands has discussed making >15% THC cannabis a “hard” drug (Rolles, 2014). The stated reasoning for this limit was decried as arbitrary because available cannabis had THC levels higher than the suggested 15% cutoff; further, little research has examined whether higher potency cannabis posed more risk for negative consequences (Niesink and Rigter, 2013). One major problem in developing these guidelines has been the inability to determine whether potency increases the risk of regular/daily cannabis use or cannabis use disorder (CUD) symptom onset.
National epidemiological surveys show an increasing prevalence of cannabis use among adults (Hasin et al., 2015; Hasin et al., 2017; Martins et al., 2016). Furthermore, there have been increasing rates of cannabis initiation among college attendees since 2013 (Miech et al., 2017). Findings are mixed regarding changes in the prevalence of CUD. The National Epidemiologic Survey of Alcohol Related Conditions (NESARC) indicated an approximate 20% increase from 2002/2003 to 2012/2013 (Hasin et al., 2015), and concomitantly, CUD diagnosis increased over 50% between 2002 and 2009 in Veteran Affairs (VA) hospitals (Bonn-Miller et al., 2012). In contrast, the National Survey on Drug Use and Health (NSDUH) suggests CUD rates remained stable over the same time period (Grucza et al., 2016). Inconsistencies in prevalence rates could be due to methodological differences associated with data collection (i.e., face-to-face interview (NESARC) versus computer-based assessment (NSDUH) versus changes in CUD assessment and diagnosis during the study period (VA). Meanwhile, THC has been linearly increasing for over two decades (Cascini et al., 2012; ElSohly et al., 2016), with mean THC% in cannabis flower at 3.50% in 1994 and 12.30% in 2012 (ElSohly et al., 2016). There has also been speculation that increases in potency are partly responsible for the increase in CUD prevalence (Copeland and Pokorski, 2016) or at the very least increased addiction potential (National Insitute on Drug Abuse [NIDA], 2017).
There have been concerns that acute harms associated with cannabis use such as increased motor vehicle accidents (Brady and Li, 2014) and increased emergency room visits (Substance Abuse and Mental Health Services Administration [SAMHSA], 2013) are related to the increases in THC% (Volkow et al., 2014). While the specific role of cannabis potency in health outcomes is understudied and difficult to substantiate (NIDA, 2017), one argument for cannabis regulation is the potential association of cannabis potency with psychosis. A handful of studies have examined cannabis potency and focused specifically on risk for psychosis or psychotic symptoms. Extant studies distinguished high- versus low-potency cannabis from self-reported types of cannabis used showing that high-potency cannabis is associated with a faster onset and greater risk of psychosis (Di Forti et al., 2009; Di Forti et al., 2014), and frequent use of high-potency cannabis is associated with altered brain structure (Rigucci et al., 2016). One cross-sectional study found that high-potency cannabis was associated with increased CUD severity among young adults (Freeman and Winstock, 2015). Although these findings have provided preliminary evidence to support further examination of potency as a predictor for negative outcomes, they are limited by using cross-sectional data, retrospective recall, possible reverse causality, categorical representations of THC levels (high versus low), and self-report of illegally obtained cannabis that may not accurately represent the THC levels of the cannabis used.
Considering no guidelines exist regarding cannabis potency, one major area of concern in rising THC levels is addiction potential, which has significant implications for treatment and intervention effectiveness. If higher levels of THC increase the likelihood of CUD symptom onset, then clinicians need to intervene early, targeting not only cannabis use patterns, but also the type of cannabis being used. Hypothetically, individuals who regularly use cannabis flower at 15% THC (or THC concentrates up to 80% THC) would experience more acute harms associated with use, faster onset of CUD symptoms, and more impairment associated with withdrawal symptoms, relative to the regular use of cannabis flower at a lower level of THC. If potency is influential in the experience of acute harms, national trends in increasing potency have the potential to increase the costs associated with more individuals seeking treatment for CUD and longer times to CUD remission. Indeed, research has shown that cannabis use was associated with an increase in emergency department utilization from 2004 to 2011 (SAMHSA, 2016; Zhu and Wu, 2016). Therefore, it is critical to identify the role that increasing cannabis potency plays in the development of CUD in order to reduce the impact on utilization of healthcare resources.
Cannabis research has been hindered by difficulties in determining the longitudinal effects of increasing cannabis potency. Although the best solution is to obtain potency levels of actual cannabis used, the feasibility of obtaining samples is limited by the federal illegality of cannabis, and historically, has been impossible to achieve. Another approach is to use average national levels of THC to estimate the effects of greater potency by integrating mean THC levels with existing longitudinal studies. This approach provides a proxy for understanding possible links between cannabis potency and CUD symptom onset. We used this integrative approach to fill a gap in the literature regarding the effect of increasing potency on patterns of cannabis use and CUD symptom onset. The Michigan Longitudinal Study, an ongoing study for 30 years, was leveraged to examine the effects of potency at cannabis-use initiation on the progression from recreational to regular/daily use in conjunction with mean national levels of THC. Higher potency levels at the initiation of cannabis use were hypothesized to be associated with progression to first regular cannabis use, first daily cannabis use, and CUD symptom onset.
2. Method
2.1. Participants and procedure
Participants were offspring in families participating in the Michigan Longitudinal Study (MLS), an ongoing, multi-wave, prospective study investigating families at elevated risk for substance use disorders (Zucker et al., 1996; Zucker et al., 2000). High-risk families were recruited via father’s drunk-driving conviction records, and a contrast sample of ecologically comparable families, albeit without a substance use disorder, were recruited through door-to-door community canvassing in the same neighborhoods as the high-risk families in a four-county wide area in central Michigan. Initial recruitment required the father to be living with a 3–5-year-old male child and the child’s biological mother. Siblings within 8-years of age from the initial male child were also recruited into the study approximately two years after the initial recruitment. In-depth assessments were conducted at 3-year intervals, and shorter annual assessments were conducted between the ages of 11 and 26. Informed consent and assent were obtained from parents and children, respectively, after receiving a complete, age-appropriate description of the study. The University Institutional Review Board approved all procedures.
This study used longitudinal data from in-depth assessments conducted at 3-year intervals from ages 12–14 to ages 24–26 and the annual assessments from ages 11–26. Only those participants who initiated cannabis between 1994 and 2012 were included in the current study. The subsample of the MLS participants with complete annual and 3-year interval assessment data and any lifetime cannabis use (n=527) were included in analyses. Because of study design consideration (Zucker et al., 2000), 71.5% of the sample was male, and 89.4% was Caucasian, which was representative of the Midwest area where the study was conducted (see Table 1 for sample characteristics). Additionally, national average cannabis potency (THC%) was obtained from a publication from ElSohly and colleagues (2016) that tracks cannabinoid concentrations from cannabis confiscated by the Drug Enforcement Agency, where data from 1994–2012 were used in the current study.
Table 1.
Sample Characteristics
| Male | Female | Overall | |
|---|---|---|---|
| M (SD) or n % | M (SD) or n % | M (SD) or n % | |
| Total Sample | 377 71.5% | 150 28.5% | 527 |
| Race | |||
| White | 339 64.3% | 134 25.4% | 473 89.4% |
| Black/African American | 20 3.8% | 5 1.0% | 25 4.7% |
| Native American | 3 0.6% | 0 0% | 3 0.6% |
| Asian/Asian American | 5 0.9% | 3 0.6% | 8 1.5% |
| Other Race/Ethnicity | 10 1.9% | 8 1.5% | 18 3.8% |
| Age first cannabis use | 16.66 (4.06) | 16.65 (3.85) | 16.66 (4.00) |
| Age first regular cannabis use | 22.42 (4.50) | 22.85 (4.51) | 22.54 (4.50) |
| Age first daily cannabis use | 22.89 (4.49) | 23.01 (4.60) | 22.92 (4.51) |
| Age first CUD symptom | 20.93 (4.42) | 21.81 (4.50) | 21.18 (4.46) |
| Regular user (Yes) | 174 32.9% | 42 7.9% | 216 40.83% |
| Regular user (No) | 204 38.6% | 109 20.6% | 313 59.17% |
| Daily user (Yes) | 138 26.1% | 33 6.2% | 171 32.32% |
| Daily user (No) | 240 45.4% | 118 22.3% | 358 67.67% |
| First cannabis use to regular use (years) | 6.21 (4.42) | 6.61 (4.57) | 6.32 (4.47) |
| First cannabis use to daily use (years) | 6.67 (4.50) | 6.76 (4.59) | 6.69 (4.52) |
| First cannabis use to CUD symptom (years) | 5.30 (4.17) | 5.98 (4.40) | 5.49 (4.45) |
Note. M=mean; SD=standard deviation; CUD=Cannabis Use Disorder.
2.2. Measures
2.2.1. Cannabis use.
The Drinking and Drug History Questionnaire (Zucker et al., unpublished manuscript) assessed substance use annually from ages 11–26. Age of first cannabis use was obtained through a question asking, “How old were you when you first used marijuana?” Annual cannabis use was determined by utilizing the national Monitoring the Future study measure (Johnston et al., 1979; Miech et al., 2017). Annual frequency of use was assessed by the question(s), “On how many occasions (if any) have you used marijuana (grass, pot, weed, ganga) or hashish (hash, hash oil) in the past-year and in the past 30-days?” Regular use was defined as using cannabis on 100 or more occasions in the past-year. Daily use was defined as using cannabis on 20–39 or more occasions in the past 30-days.
2.2.2. Cannabis use disorder.
Diagnostic and Statistical Manual for Mental Disorders-IV (American Psychiatric Association, 1994) symptoms of CUD and age of CUD symptom onset were assessed using the Diagnostic Interview Schedule (DIS) (Robins et al., 1996). The DIS was administered to participants by a doctoral-level clinician. At each 3-year assessment, participants were asked to report at what age any CUD symptom was first experienced.
2.2.3. Cannabis potency.
Annual national average cannabis potency (THC%) was obtained from ElSohly and colleagues (2016) who have a contract with the National Institute on Drug Abuse to track annual cannabinoid concentrations from cannabis confiscated by the Drug Enforcement Agency. For the most recent review of the protocol and cannabinoid concentrations see ElSohly and colleagues (2016). Annual national average THC percentages between 1994 and 2012 ranged from 3.50 to 12.30, respectively.
2.3. Analytic strategy
Cox regression analysis was used to investigate the number of years from first cannabis use to regular cannabis use, daily cannabis use, and first CUD symptom, using SAS 9.4. Age of first cannabis use was added to birth year to obtain the calendar year of first cannabis use. The corresponding mean THC level (Freeman and colleagues (2018) used a similar approach) from national data for year of first cannabis use was used for subsequent modeling and as the main predictor with sex included a priori in all models to reduce bias related to males using cannabis more frequently (Carliner et al., 2017) with a higher likelihood of experiencing CUD (Stinson et al,. 2006). For CUD models, regular use (Yes/No) or daily use (Yes/No) was included as a covariate to account for the effect of cannabis-use patterns on the development of CUD symptoms. Since regular users and daily users are more likely to endorse CUD symptoms, this provides a more stringent test of potency’s relation to CUD symptom endorsement. Each use-frequency covariate was included in separate models to evaluate whether the regular use or daily use influenced results; both could not be included simultaneously due to issues of multicollinearity. A similar approach was used in a previous study examining the effects of potency on progression to psychosis onset (Di Forti et al., 2009). Importantly, the birth year was included to adjust for possible cohort effects (Singer and Willett, 2009).
A robust sandwich estimator was used to account for the inclusion of siblings from the same family in the MLS data; an approach commonly used to adjust for correlated data (Lee et al., 1992). Hazard ratios above 1 indicate increased risk for experiencing the event (i.e., first occurrence of regular use, daily use, or first CUD symptom). The assumption of proportional hazards (i.e., the hazard for any individual is a fixed proportion of the hazard for any other individual, is constant over time, and the hazard functions should be parallel) is required for Cox regression; thus, formal numerical tests evaluated the tenability of this assumption by including an interaction between potency at first cannabis use and time-to-event variables (Allison, 2010).
3. Results
3.1. Cannabis use patterns and potency1
Two models examined the association between national average potency and progression to regular and daily cannabis use onset. In each model, national average potency was the focal predictor of years from first cannabis use to the onset of regular use and the onset of daily use, while sex and birth year were included as covariates to account for possible sex differences and cohort effects. The proportional hazard assumption was met in both models. Interaction terms between national average potency and number of years to regular or daily use were not significant and were removed from final models.
Results of the Cox regressions are presented in Table 2. In the first model, national average potency was not associated with the progression to first regular use (HR=1.00; 95% CI: .90–1.11; p=.948). Birth year was not significantly associated with progression to regular cannabis use onset. However, sex was associated with the progression to first regular use, where males were at a 1.95 times higher risk of regular cannabis use onset versus those not endorsing regular use onset.
Table 2.
Cox regression: Age of First Cannabis Use to Age of First Regular Use, Daily Use, and CUD Symptom
| Estimate | Standard Error | Hazard ratio | 95% Confidence Interval | |
|---|---|---|---|---|
| Years to regular Use (n=518) | ||||
| Sex | 0.67 | 0.19 | 1.95*** | 1.35–2.82 |
| Birth year | 0.02 | 0.03 | 1.02 | 0.97–1.08 |
| Potency | −0.004 | 0.05 | 1.00 | 0.90–1.11 |
| Years to Daily Use (n=517) | ||||
| Sex | 0.65 | 0.22 | 1.92** | 1.24–2.97 |
| Birth year | 0.07 | 0.03 | 1.07* | 1.01–1.13 |
| Potency | −0.09 | 0.06 | 0.91 | 0.81–1.03 |
| Years to first CUD symptom (n=480), accounting for regular use | ||||
| Sex | 0.13 | 0.17 | 1.15 | 0.82–1.59 |
| Birth year | −0.04 | 0.03 | 0.91 | 0.91–1.02 |
| Regular user | 1.41 | 0.17 | 4.11*** | 2.96–5.72 |
| Potency | 0.34 | 0.07 | 1.41*** | 1.24–1.60 |
| Potency x Years to 1st CUD symptom | −0.14 | 0.02 | 0.87*** | 0.84–0.90 |
| Years to first CUD symptom (n=480), accounting for daily use | ||||
| Sex | 0.21 | 0.16 | 1.24 | 0.90–1.71 |
| Birth year | −0.04 | 0.03 | 0.96 | 0.91–1.02 |
| Daily user | 1.14 | 0.16 | 3.14*** | 2.31–4.26 |
| Potency | 0.30 | 0.06 | 1.36*** | 1.20–1.54 |
| Potency x Years to 1st CUD symptom | −0.13 | 0.02 | 0.88*** | 0.84–0.91 |
Note. CUD=Cannabis Use Disorder. Missing values were due to reprting discrepancies regarding age of first cannabis use and age of first CUD symptom, regular use, or daily use, where negative values were treated as missing. Less than 10% of the sample was treated as missing in any given model.
p<.05,
p<.001.
In the second model, national average potency was not associated with the progression from first use to the onset of daily use (HR=.91; 95% CI: 0.81–1.03; p=.130). However, sex was associated with onset to daily use, where males had 1.92 times higher risk of daily cannabis use onset in comparison to not endorsing onset. Birth year was significantly associated to the onset of daily use with 1.07 times more risk of earlier daily use onset.
3.2. CUD symptom onset and potency
The final models tested whether national average potency was associated with the number of years from first cannabis use to first CUD symptom, accounting for regular use and daily use in separate models. The proportional hazards assumption was violated in these models, as indicated by significant interactions between national average potency and number of years from first cannabis use to first CUD symptom. When interpreting the main effect of national average potency, it is considered an average of the hazard ratios due to the inclusion of the interaction term. Interaction effects from the extended Cox regression models were examined via variance estimates at pre-specified potency levels and years to CUD to further understand the relationship of national average potency on progression to CUD symptom onset.
After adjusting for sex, being a regular user, and birth year, national average potency was associated with progression to first CUD symptom (HR=1.41; 95% CI: 1.24–1.60; p<.001). Compared to individuals not endorsing CUD symptom onset, there was 1.41 times the risk for progression to CUD symptom onset as national average potency increased by 1% point. Regular use status was associated with progression to first CUD symptom but sex and birth year were not. Those who endorsed regular use were at 4.11 times the risk of progression from initiation to CUD symptom onset, as compared to individuals not endorsing onset. In a similar model adjusting for sex, daily user status, and birth year, national average potency was associated with progression to first CUD symptom (HR=1.36, 95% CI: 1.20–1.54, p<.001). Daily use status was associated with progression to first CUD symptom, where daily users were at 3.14 times the risk of progression from first use to onset. Sex and birth year were not associated with progression to first CUD symptom.
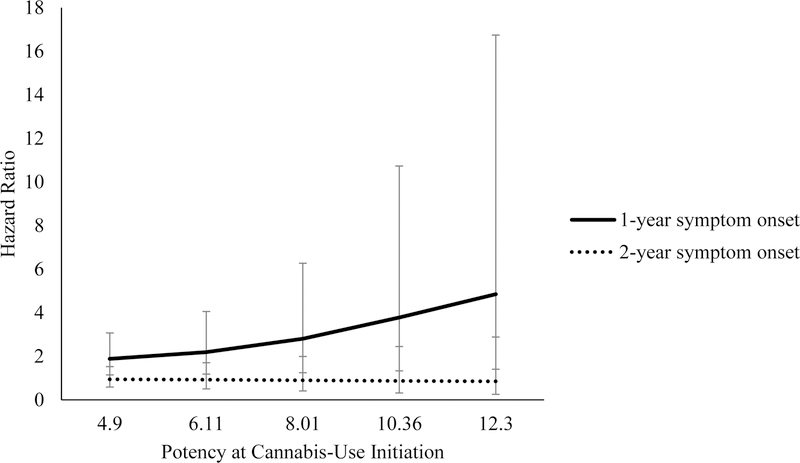
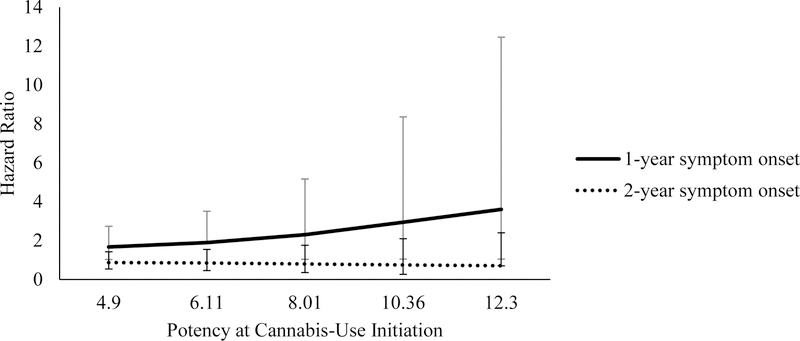
Results for the interaction effects are reported in Table 3. To better understand the interaction effects, we obtained hazard ratios for specific national average potency levels and the number of years to first CUD symptom. When first CUD symptom occurred in the first year after cannabis use initiation, the increase in national average potency was associated with larger hazard ratios (See Figures 1 and 2). For example, after adjusting for regular use, when national average potency was at 4.9%, there was a 1.88 times greater risk in CUD symptom onset in the first year after initiation for every unit increase in national average potency, compared to those who did not endorse CUD symptom onset. When national average potency was at 12.3%, the risk for experiencing the first CUD symptom within one year of initiating cannabis use was 4.85 times as likely. In the model adjusting for daily user status, at 4.9% national average potency, progression to CUD symptom onset within the first year of cannabis use initiation was 1.67 times as likely, while at 12.3% national average potency, progression to CUD symptom onset was 3.60 times as likely, as compared to those not endorsing CUD symptom onset. Within two years of initiation, after adjusting for regular use or daily use status, national average potency was not associated with progression to CUD symptom onset for either model.
Table 3.
Interactive Effects of Potency and Years to CUD Symptom Onset with Regular User Status and Daily User Status as Covariates
| Potency | Estimate | Standard Error | Hazard ratio | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Regular user: One year to CUD symptom onset | |||||
| 4.90 | 0.63 | 0.25 | 1.88* | 1.14–3.07 | |
| 6.11 | 0.78 | 0.31 | 2.19* | 1.18–4.05 | |
| 8.01 | 1.03 | 0.41 | 2.80* | 1.24–6.27 | |
| 10.36 | 1.33 | 0.53 | 3.78* | 1.33–10.73 | |
| 12.30 | 1.58 | 0.63 | 4.85* | 1.40–16.74 | |
| Regular user: Two years to CUD symptom onset | |||||
| 4.90 | −0.06 | 0.25 | 0.94 | 0.58–1.52 | |
| 6.11 | −0.08 | 0.31 | 0.92 | 0.50–1.70 | |
| 8.01 | −0.11 | 0.41 | 0.90 | 0.41–1.99 | |
| 10.36 | −0.14 | 0.52 | 0.87 | 0.31–2.44 | |
| 12.30 | −0.16 | 0.62 | 0.85 | 0.25–2.88 | |
| Daily user: One year to CUD symptom onset | |||||
| 4.90 | 0.51 | 0.25 | 1.67* | 1.02–2.73 | |
| 6.11 | 0.64 | 0.31 | 1.89* | 1.89–3.50 | |
| 8.01 | 0.84 | 0.41 | 2.30* | 1.03–5.16 | |
| 10.36 | 1.08 | 0.53 | 2.94* | 1.04–8.36 | |
| 12.30 | 1.28 | 0.63 | 3.60* | 1.04–12.45 | |
| Daily user: Two years to CUD symptom onset | |||||
| 4.90 | −0.14 | 0.25 | 0.87 | 0.53–1.42 | |
| 6.11 | −0.18 | 0.31 | 0.84 | 0.45–1.54 | |
| 8.01 | −0.23 | 0.41 | 0.79 | 0.35–1.76 | |
| 10.36 | −0.30 | 0.53 | 0.74 | 0.26–2.09 | |
| 12.30 | −0.36 | 0.63 | 0.70 | 0.70–2.40 | |
Note. CUD=Cannabis Use Disorder.
p<.05
Figure 1.
Regular user status hazard ratios representing risk of reporting a CUD symptom in the first or second year after cannabis-use initiation by potency at year of initiation. Error bars reflect 95% confidence intervals for hazard ratios.
Figure 2.
Daily user status hazard ratios representing risk of reporting a CUD symptom in the first or second year after cannabis-use initiation by potency at year of initiation. Error bars reflect 95% confidence intervals for hazard ratios.
4. Discussion
The present study is an essential first step toward understanding the influence of potency on CUD symptom onset. As cannabis policies continue to change – moving toward full recreational legalization – potency has linearly increased in parallel, with few guidelines or regulations developed in determining “safe” levels. The current study found prospective evidence that higher cannabis potency (on average in the U.S.) increased the risk for progression to CUD symptom onset. Specifically, higher potency cannabis, on average in the U.S., used at cannabis initiation was associated with over four times the risk of CUD symptom onset within the first year of initiation, as compared to those not endorsing symptom onset.
To extend prior research examining potential factors in the development of CUD, this study used national aggregates of potency levels combined with prospective data to support the development of future research using individual potency levels to better understand the within- person associations of cannabis potency and CUD symptom onset risk. Prior studies have suggested not all cannabis users will meet criteria for CUD (Forman-Hoffman et al., 2017). Past inconsistencies in findings regarding CUD diagnosis based on general population studies may capture transitions in cannabis use patterns and current negative life events among frequent users are potentially more important in the development of CUD (van der Pol et al., 2013). Though regular and daily cannabis use had strong associations with progression from cannabis initiation to CUD symptom onset versus those not endorsing onset, average U.S. potency accounted for substantial risk in CUD progression after controlling for these use patterns. Although national average potency increases were not associated with CUD symptom onset within two years after initiation, this could be indicative of a higher-risk individual having already experienced symptom onset, with potency being a potential driving factor for those at-risk for earlier CUD symptom onset. Relatedly, potency may be most influential in predicting the risk of earlier or faster onset to first CUD symptom among at-risk or sensitive individuals. Individuals who have not developed a CUD symptom in the first year after initiating use may reflect a lower-risk or protected group who will not ultimately develop CUD symptomatology.
Though more empirical research is needed to support the findings from this study, that higher potency cannabis, on average in the U.S., at initiation was associated with progression to CUD symptom onset (versus those not endorsing CUD symptom onset) highlights the need for early intervention and targeted prevention efforts. Potential approaches for prevention could begin with policy development, such as the establishment of guidelines and regulations regarding THC level in available cannabis, especially in states with legal medical and recreational cannabis. Future research should identify the specific CUD symptoms that are affected by increases in national average or individual potency that can be targeted in prevention programs in order to limit CUD symptom onset. By understanding the risks for CUD symptom onset based on THC%, clinicians and physicians can also develop individualized treatment plans for those using high potency THC concentrates or initiating on higher potency cannabis flower.
In comparison to individuals who did not endorse regular/daily cannabis use onset, national average potency was not associated with progression to regular cannabis use or daily cannabis use onset. This finding is not necessarily surprising. Using years and national average potency levels as metrics for examining potency and patterns of cannabis use may not capture the faster progression of this phenomenon. While potency could hasten the progression from initiation to regular/daily cannabis use onset, these use patterns may occur within weeks or months depending on the level of potency. The modeling of potency itself could have affected these results, where yearly changes in potency (or higher potency level changes between flower or concentrates) and use of national averages of potency instead of individual-level potency could potentially impact our understanding of problematic cannabis use development. Another explanation could be that problematic cannabis use patterns are related more to the number of uses per day as opposed to the number of days used to sustain the subjective and physiological effects of cannabis. Before espousing definitive statements about the possible effect of potency on the development of more problematic patterns of use, further research is needed to understand the nuances of cannabis use patterns generally.
There were limitations to this study. Although the MLS has been ongoing for more than 30 years, the sample consisted of a mostly Caucasian, high-risk population and the results may not generalize to more diverse community samples. At the same time, the utilization of a higher risk group of subjects ensures that there will be sufficient cannabis use variation to yield reliable estimates of the nature of the hypothesized relationship. Another limitation is the use of national level potency data to estimate potency variation over time. However, the fact that average regional potency levels from 1994 to 2014 were 7.35% (ElSohly et al., 2016) and within +/−1 standard deviation of the national mean potency levels for individual years is suggestive that this limitation would not introduce major confounds into the analysis. While there are obvious drawbacks to using national averages of THC levels for regional data and as a proxy for THC levels in participant’s cannabis, the regional data is unavailable and measuring THC levels in participant’s actual cannabis has been limited due to legal issues.
Average levels of cannabidiol (i.e., compound in cannabis with analgesic effects and may provide a protective effect from the psychoactive effects of THC) decreased linearly from .28% in 1995 to .15% in 2014 (ElSohly et al., 2016). This decrease in cannabidiol suggests cannabis may, in some cases, have more intoxicating effects. Additionally, the study relied on self-report and retrospective recall of CUD symptom age of onset (e.g., forward telescoping, Perra et al., 2003), which has the potential for recall and social desirability biases. A common limitation to cannabis research has been delineating quantity and type of cannabis (e.g., flower, concentrates). This is especially true for high potency cannabis, as concentrates have been reported as high as 80% THC (NIDA, 2017) and individuals have been shown to titrate when using higher potency cannabis (Freeman et al., 2014); nevertheless, the current study provides an important understanding of high potency flower (~12% THC), on average in the U.S., that is well below 80% THC and still increased risk of progression to CUD symptom onset.
4.1. Conclusions
There has been an ongoing assumption that higher THC levels may increase the addiction potential of cannabis. This study provides preliminary evidence suggesting higher potency cannabis, on average in the U.S., in fact, increases the risk of progression to CUD symptom onset, albeit within the limitations of utilizing a high-risk sample. As legal recreational and medical cannabis becomes more available to the general public, it is critical that policymakers, researchers, clinicians, and physicians understand the health outcomes associated with these increases in potency to enhance the development of future guidelines and regulations for cannabis in any form.
Highlights.
Risk to faster cannabis use disorder symptom onset increased at higher potency levels
Initiating at 4.9% showed 88% risk for symptom onset within a year
Initiating at 12.3% showed 4.85 times higher risk for symptom onset within a year
Potency did not predict progression to regular or daily cannabis use
Acknowledgments
Role of Funding Source
This study used data from the Michigan Longitudinal study, which is funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (R01 AA007065) and is conducted by the University of Michigan. Dr. Zucker has also received research support from the NIAAA (R01 AA007065). Dr. Treloar Padovano is supported by a career award from the NIAAA (K23 AA024808). Dr. Arterberry is supported by a fellowship from the NIAAA (T32 AA007477). This funding agency had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Dr. Treloar Padovano has previously been a paid statistical consultant for Zynerba® Pharmaceuticals All other authors report no financial relationships with commercial interests.
Demographic variables such as race/ethnicity, income, and highest grade completed were used in separate analyses. Due to missing values associated with ongoing data collection and data management, multiple imputation was conducted using Blimp version 1.1 to account for the nested data and proc mianalyze in SAS 9.4 was used to conduct analyses. Though income had a significant relationship with progression from cannabis initiation to first regular use. No other demographic variables had a relation with outcomes, and the pattern of results did not differ for the focal predictor, national average potency. Results are available on request from the author.
References
- Allison PD, 2010. Survival Analysis Using SAS: A Practical Guide, Survival SAS Institute; Available at https://www.sas.com. [Google Scholar]
- American Psychiatry Association. 1994. Diagnostic and statistical manual of mental disorders (DSM-IV) American Psychiatry Association, Washington, DC. [Google Scholar]
- Bonn-Miller MO, Harris AHS, Trafton JA 2012. Prevalence of cannabis use disorder diagnoses among veterans in 2002, 2008, and 2009. Psychol. Serv 9, 404–416. 10.1037/a0027622 [DOI] [PubMed] [Google Scholar]
- Brady JE, Li G, 2014. Trends in alcohol and other drugs detected in fatally injured drivers in the United States, 1999–2010. Am. J. Epidemiol 179, 692–699. 10.1093/aje/kwt327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carliner H, Mauro PM, Brown QL, Shmulewitz D, Rahim-Juwel R, Sarvet AL, Wall MM, Martins SS, Carliner G, Hasin DS, 2017. The widening gender gap in marijuana use prevalence in the U.S. during a period of economic change, 2002–2014. Drug Alcohol Depend 170, 51–58. 10.1016/j.drugalcdep.2016.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascini F, Aiello C, Di TG, 2012. Increasing Δ−9-tetrahydrocannabinol (Δ−9-THC) content in herbal cannabis over time: systematic review and meta-analysis. Curr. Drug Abuse Rev 5, 32–40. 10.1186/1477-7517-9-15 [DOI] [PubMed] [Google Scholar]
- Coombes R, 2014. Cannabis regulation: high time for change? BMJ 348, g3382–g3382. 10.1136/bmj.g3382 [DOI] [PubMed] [Google Scholar]
- Copeland J, Pokorski I, 2016. Progress toward pharmacotherapies for cannabis-use disorder: an evidence-based review. Subst. Abuse Rehabil 7, 41–53. 10.2147/SAR.S89857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Forti M, Morgan C, Dazzan P, Pariante C, Mondelli V, Marques TR, Handley R, Luzi S, Russo M, Paparelli A, Butt A, Stilo SA, Wiffen B, Powell J, Murray RM, 2009. High-potency cannabis and the risk of psychosis. Br. J. Psychiatry 195, 488–491. 10.1192/bjp.bp.109.064220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Forti M, Sallis H, Allegri F, Trotta A, Ferraro L, Stilo SA, Marconi A, La Cascia C, Marques TR, Pariante C, Dazzan P, Mondelli V, Paparelli A, Kolliakou A, Prata D, Gaughran F, David AS, Morgan C, Stahl D, Khondoker M, MacCabe JH, Murray RM, 2014. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr. Bull 40, 1509–1517. 10.1093/schbul/sbt181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC, 2016. Changes in cannabis potency over the last 2 decades (1995–2014): analysis of current data in the United States. Biol. Psychiatry 79, 613–619. 10.1016/j.biopsych.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman-Hoffman VL, Glasheen C, Batts KR, 2017. Marijuana use, recent marijuana initiation, and progression to marijuana use disorder among young male and female adolescents aged 12–14 living in US households. Subst. Abuse 11, 1–14. doi: 1;11:1178221817711159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman TP, Winstock AR, 2015. Examining the profile of high-potency cannabis and its association with severity of cannabis dependence. Psychol. Med 45, 3181–3189. 10.1017/S0033291715001178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman TP, van der Pol P, Kuijpers W, Wisselink J, Das RK, Rigter S, van Laar M, Griffiths P, Swift W, Niesink R, Lynskey MT, 2018. Changes in cannabis potency and first-time admissions to drug treatment: a 16-year study in the Netherlands. Psychol. Med 1–7. [DOI] [PubMed]
- Grucza RA, Agrawal A, Krauss MJ, Cavazos-Rehg PA, Bierut LJ, 2016. Recent trends in the prevalence of marijuana use and associated disorders in the United States. JAMA Psychiatry 73, 300 10.1001/jamapsychiatry.2015.3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang HT, Jung J, Pickering RP, Ruan WJ, Smith SM, Huang BJ, Grant BF, 2015. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry 72, 1235–1242. 10.1001/jamapsychiatry.2015.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Sarvet AL, Cerdá M, 2017. US adult illicit cannabis use, cannabis use disorder, and medical marijuana laws 1991–1992 to 2012–2013. JAMA Psychiatry 12, CD008940 10.1001/JAMAPSYCHIATRY.2017.0724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, Bachman JG, O’Malley PM, 1979. Drugs and the class of 1978: behaviors, attitudes, and recent national trends. Michigan Univ., Ann Arbor. Inst. for Social Research; https://eric.ed.gov/?id=ED185649 [Google Scholar]
- Lee EW, Wei LJ, Amato DA, Leurgans S, 1992. Cox-type regression analysis for large numbers of small groups of correlated failure time observations, in: Survival Analysis: State of the Art pp. 237–247. 10.1007/978-94-015-7983-4_14 [DOI]
- Martins SS, Mauro CM, Santaella-Tenorio J, Kim JH, Cerda M, Keyes KM, Hasin DS, Galea S, Wall M, 2016. State-level medical marijuana laws, marijuana use and perceived availability of marijuana among the general U.S. population. Drug Alcohol Depend 169, 26–32. 10.1016/j.drugalcdep.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech RA, Patrick ME, O’Malley PM, Johnston LD, 2017. The influence of college attendance on risk for marijuana initiation in the United States: 1977 to 2015. Am. J. Public Health 107, 996–1002. 10.2105/AJPH.2017.303745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse, 2017. Drug Facts: marijuana Available at https://www.drugabuse.gov/publications/term/160/DrugFacts
- Niesink R, Rigter S, 2013. THC-concentraties in wiet, nederwiet en hasj in Nederlandse coffeeshops (2011–2012) Retrieved from https://www.trimbos.nl/?act=winkeldl.download&prod=578
- Miech RA, Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME, 2017. Monitoring the Future National Survey Results on Drug Use, 1975–2016: Volume I, Secondary school students. Institute for Social Research, The University of Michigan; Ann Arbor, MI: Retrieved from http://www.monitoringthefuture.org/pubs/monographs/mtf-vol1_2016.pdf [Google Scholar]
- Parra GR, O’Neill SE, Sher KJ 2003. Reliability of self-reported age of substance involvement onset. Psychol. Addict. Beha 17, 211–218. [DOI] [PubMed] [Google Scholar]
- Rigucci S, Marques TR, Di Forti M, Taylor H, Dell’Acqua F, Mondelli V, Bonaccorso S, Simmons A, David AS, Girardi P, Pariante CM, Murray RM, Dazzan P, 2016. Effect of high-potency cannabis on corpus callosum microstructure. Psychol. Med 46, 841–854. 10.1017/S0033291715002342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins L, Helzer J, Croughman J, Williams J, Spitzer R, 1980. The National Institute of Mental Health Diagnostic Interview Schedule, Version 3
- Robins LN, Marcus L, Reich W, Cunningham R, Gallagher T, 1996. Diagnostic Interview Schedule, Version IV
- Singer JD, Willett JB, 2009. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence, Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence 10.1093/acprof:oso/9780195152968.001.0001 [DOI]
- Stinson FS, Ruan WJ, Pickering R, Grant BF, 2006. Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychol. Med 36, 1447. 10.1017/S0033291706008361 [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2013. Drug Abuse Warning Network, 2011: National estimates of drug-related emergency department visits. HHS Publ. No. 13–4760, DAWN Series D-39 Rockville, MD: Retrieved from https://www.samhsa.gov/data/sites/default/files/DAWN2k11ED/DAWN2k11ED/DAWN2k11ED.pdf [Google Scholar]
- Rolles S, 2014. Cannabis policy in the Netherlands: moving forwards not backwards Retrieved from https://www.tdpf.org.uk/blog/cannabis-policy-netherlands-moving-forwards-not-backwards.
- van der Pol P, Liebregts N, de Graaf R, Korf DJ, van den Brink W and van Laar M, 2013. Predicting the transition from frequent cannabis use to cannabis dependence: a three-year prospective study. Drug Alcohol Depend 133,.352–359. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Compton WM, Weiss SRB, 2014. Adverse health effects of marijuana use. N. Engl. J. Med 370, 2219–27. 10.1056/NEJMra1402309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Hockenberry JM, Cummings JR, 2015. The effect of medical marijuana laws on adolescent and adult use of marijuana, alcohol, and other substances. J. Health Econ 42, 64–80. 10.1016/j.jhealeco.2015.03.007 [DOI] [PubMed] [Google Scholar]
- Zhu H, Wu LT, 2016. Trends and correlates of cannabis-involved emergency department visits: 2004 to 2011. J. Addict. Med 10, 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RA, Fitzgerald HE, Noll RB, Unpubl. manuscript. Drinking and drug history Michigan State Univ. [Google Scholar]
- Zucker RA, Ellis DA, Fitzgerald HE, Bingham CR, Sanford K, 1996. Other evidence for at least two alcoholisms II: Life course variation in antisociality and heterogeneity of alcoholic outcome. Dev. Psychopathol 8, 831 10.1017/S0954579400007458 [DOI] [Google Scholar]
- Zucker RA, Fitzgerald HE, Refior SK, Puttler LI, Pallas DM, Ellis DA, 2000. The clinical and social ecology of childhood for children of alcoholics: description of a study and implications for a differentiated social policy, in: Fitzgerald HE, Lester BM,ZB (Ed.), Children of Addiction Garland Press, pp. 109–142. [Google Scholar]