Abstract
Toxoplasmosis is an important zoonosis caused by an obligate intracellular parasitic protozoan, Toxoplasma gondii. The disease is distributed worldwide and can affect all warm-blooded vertebrates, including humans. The present review aimed to collect, compile and summarize the data on the prevalence of T. gondii infection in humans and animals in the five North African countries (Morocco, Algeria, Tunisia, Libya and Egypt). Published data from national and international databases were used. Distribution patterns and risk factors for T. gondii infection are discussed, focusing on biotic and abiotic factors. This review is a comprehensive epidemiological analysis of T. gondii infection in North Africa and will therefore be a useful tool for researchers. It can also be used to propose or enhance appropriate national toxoplasmosis control programs.
Keywords: Toxoplasma gondii, North Africa, Humans, Animals
Abstract
La toxoplasmose est une zoonose importante causée par un protozoaire parasite intracellulaire obligatoire, Toxoplasma gondii. La maladie est répandue dans le monde entier, chez tous les vertébrés à sang chaud, y compris les humains. La présente étude visait à collecter, compiler et résumer les données sur la prévalence de l’infection par T. gondii chez l’homme et les animaux dans les cinq pays d’Afrique du Nord (Maroc, Algérie, Tunisie, Libye et Égypte). Les données publiées dans des bases de données nationales et internationales ont été utilisées. Les schémas de distribution et les facteurs de risque d’infection par T. gondii sont discutés, en se concentrant sur les facteurs biotiques et abiotiques. Cette synthèse est une analyse épidémiologique complète de l’infection par T. gondii en Afrique du Nord et sera donc un outil intéressant pour les chercheurs. Elle peut également être utilisée pour proposer ou renforcer des programmes nationaux appropriés de contrôle de la toxoplasmose.
Abstract
داء المقوسات هو مرض مشترك هام يسببه طفيلي وحيد الخلية اجبارى داخل خلوى، توكسوبلازما غوندي ( Toxoplasma gondii ). تنتشر هذه الاصابة في جميع أنحاء العالم وعند جميع الفقريات ذوات الدم الحار بما في ذالك الانسان. تهدف هذه الدراسة الببليوغرافية إلى جمع وتصنيف وتلخيص البيانات الحالية حول نسبة الاصابة بالطفيلي توكسوبلازما غوندي ( Toxoplasma gondii ) لدى الانسان والحيوان في بلدان شمال إفريقيا الخمسة (المغرب والجزائر وتونس وليبيا ومصر) .تم استعمال المعلومات المنشورة بقاعدات البيانات الوطنية و العالمية كما تمت مناقشة أنماط التوزيع وعوامل خطر الاصابة بالطفيلي توكسوبلازما غوندي ( Toxoplasma gondii ) مع التركيز على العوامل الحياتية و الغير حياتية.
تمثل هذه الدراسة تحليلاً وبائياً شاملاً للإصابة بالطفيلي توكسوبلازما غوندي ( Toxoplasma gondii ) في شمال إفريقيا. وبذلك فإن هذه الدراسة هي أداة مفيدة للباحثين. كما يمكن استعمالها كي يتم اقتراح او تعزيز برامج وطنية مناسبة لمكافحة داء المقوسات.
Introduction
Toxoplasma gondii was discovered in North Africa, more precisely in the Pasteur Institute of Tunis. In fact, during experiments on leishmaniosis, Nicolle and Manceaux observed an arc-shaped protozoan in tissues of a North African rodent, the gundis (Ctenodactylus gundi) [40]. It was named Toxoplasma gondii (T. gondii) based on its morphology (Toxon: arc, plasma: form) and its host.
The protozoan T. gondii is the agent of toxoplasmosis. It infects all warm-blooded animals including birds and mammals [41]. Toxoplasmosis is an important health problem worldwide [119]. The history, the epidemiological status, the life cycle, and the development of this parasite have been well studied around the world [40, 98, 126].
North Africa, a south Mediterranean region, lies between Sub-Saharan Africa and Europe. It represents a specific agro-ecological and socio-economic context, leading to specific epidemiological patterns for several human and animal diseases. The past and current status of Toxoplasma infection in North African countries is not well understood since few studies are available for the whole region.
This review aimed to collect, update and analyse the epidemiological data on Toxoplasma infection in five North African countries (Morocco, Algeria, Tunisia, Libya and Egypt), where several studies have been published in the grey literature but are not available to international readers.
General geographical context
North Africa includes five countries: Morocco, Algeria, Tunisia, Libya and Egypt (Table 1). All countries have a predominantly semi-arid to arid bioclimate with large desert areas covering more than 75% of the region, mainly in the centre and southern parts. The summer season is hot and dry. The rainy season is from October to April with maximum precipitation from December to February [55, 88]. High variability of inter-annual precipitation is observed. Rain is scarce in the Sahara, where temperatures reach up to 55 °C during the day and drop to below 0 °C at night.
Table 1.
Characteristics of North African countries.
| Country | Capital city | Area (km2) | Sahara surface (km2) | Population (2017) | Density (inhabitants/km2 in 2017) | Percentage of total world population (%) | Percentage urban population (2017) (%) | Median age (years) |
|---|---|---|---|---|---|---|---|---|
| Morocco | Rabat | 446,300 | – | 35,913,182 | 80 | 0.47 | 59.6 | 28.3 |
| Algeria | Algiers | 2,381,740 | 2,000,000 | 41,582,461 | 17 | 0.55 | 73 | 27.8 |
| Tunisia | Tunis | 155,360 | 90,000 | 11,580,938 | 74 | 0.15 | 66.9 | 31.4 |
| Libya | Tripoli | 1,759,540 | 700,000 | 6,411,555 | 4 | 0.08 | 80.3 | 27.6 |
| Egypt | Cairo | 995,450 | 3,000,000 | 98,250,741 | 98 | 1.29 | 38.8 | 24.8 |
Source: Worldmeters [127].
Based on the latest United Nations estimates, the current population of North Africa is 192,517,616 as of November 2017 [127] (Table 1). The most heavily populated area is the coastal strip because of its fertility and mild weather.
In North African cuisine, the most common staple foods are fish, seafood, goat meat, lamb, beef, dates, almonds, olives, various vegetables, and fruits. Because the region is predominantly Muslim, pork is not consumed, and animals are totally bled when slaughtered. Meat is predominantly consumed cooked in sauce, but undercooked grilled lamb is consumed during the Muslims’ sacrifice feast, and very often in restaurants at the side of the road [125].
Livestock (cattle, buffaloes, camels, sheep, goats, and poultry) play an important role in food security, nutrition, and the economies of North African countries by supporting rural livelihoods and employment, and ensuring access to animal source foods (ASF) [58]. In the near East and North Africa, consumption of ASF has risen by 4% over the past two decades to reach 13.4 million tonnes for meat in 2014 and 35 million tonnes (in milk equivalents) for milk and dairy products (Table 2) [58].
Table 2.
Livestock indicators in North Africa, 2014.
| Country | Livestock/TLU (2013) | Livestock as share of gross value of agricultural production (constant 2004-2006 US$) | Total meat production (1000 tonnes) |
|---|---|---|---|
| Morocco | 4,897,310 | 40% | 1,077 |
| Algeria | 4,829002 | 35% | 681 |
| Tunisia | 1,499,680 | 24% | 320 |
| Libya | 1,176,450 | NA | 176 |
| Egypt | 7,527,000 | 43% | 1,810 |
Source: These data were compiled from different sources [58].
TLU: Tropical Livestock Units.
NA: Not Available.
Life cycle of Toxoplasma gondii in the North African context
Toxoplasma gondii is an obligate apicomplexan intracellular protozoan; it has a cosmopolitan distribution [106]. The life cycle of T. gondii involves (i) felines, essentially domestic cats, as definitive hosts in which sexual reproduction occurs, and (ii) intermediate hosts, where asexual reproduction occurs; the latter consist of all warm-blooded animals, including birds and mammals, with T. gondii being most common in sheep [120]. Felines can host both sexual and asexual reproduction and are also referred to as integral hosts. The infective stages of T. gondii consist of three forms: (i) tachyzoites present during the early infection period, (ii) bradyzoites present in the intermediate hosts as tissue cysts, and (iii) sporulated oocysts containing sporozoites, shed as non-sporulated oocysts by the final hosts with feces [43]. In North Africa, there are seven species of wild felids that may be involved in the life cycle of T. gondii (Table 3). To the best of our knowledge, the population of domestic cats in North Africa has never been estimated even though it is reported to be very high, especially in urban areas.
Table 3.
Wild felid species present in North Africa.
| Species | Description | Geographic distribution | Reference |
|---|---|---|---|
| Caracal | Body length: 61–105 cm | Mauritania, Morocco, | [89] |
| Caracal caracal Schreber, 1776 | Height: 40–50 cm | Algeria, Tunisia, Libya, | |
| Weight: 8–20 kg | Egypt | ||
| In captivity, average lifespan as long as 16 years | |||
| Chaus | Relatively short tail, long legs, big | Morocco, Algeria, Egypt | [95] |
| Felis chaus Schreber, 1777 | pointed ears | ||
| Sand cat | Weight: 2–3 kg | Morocco, Algeria, | [100] |
| Felis margarita Loche, 1858 | Living in arid areas with temperatures ranging from 0 °C to 58 °C | Tunisia, Libya, Egypt | |
| Serval | Medium sized African cats | Morocco, Algeria, Tunisia | [89] |
| Felis serval Schrever, 1776 | Body length: approximately 60 cm | ||
| Weight: on average 14 kg | |||
| Average life in the wild is 10 years | |||
| African wildcat | Body length: 40–66 cm | Morocco, Algeria, | |
| Felis silvestris lybica Forster, 1780 | Tail length: 24–37 cm | Tunisia, Libya, Egypt | |
| Weight: 2.4–6.4 kg |
Transmission of the infection to humans occurs through three main routes: (i) ingestion of oocysts of T. gondii shed by felids [41, 102], (ii) ingestion of tissue bradyzoites in undercooked or raw infected meat, and (iii) vertical transmission across the placenta from the mother to the fetus [90, 120]. If the parasite is contracted for the first time during pregnancy, it may be transmitted to the fetus [120]. This vertical or congenital transmission could result in the invasion of the placenta by tachyzoites which may cross the placenta and enter fetal tissues or the bloodstream [99]. Congenital toxoplasmosis may cause abortion, neonatal death, or fetal abnormalities mainly in the neuromuscular system and eyes [70, 103, 104]. Even though infection with T. gondii is very common in humans, clinical signs are uncommon in immunocompetent people. In risk groups such as immunocompromized persons and newborns with congenital infection, clinical signs such as encephalitis, pneumonia and ophthalmologic disorders can occur [120]. Toxoplasma gondii infection can also rarely be transmitted by tissue or organ transplants [106].
In pregnant animals, primary infection can lead to abortion, hence causing high economic losses [22]. In ewes, if the infection occurs between 50 and 120 days of pregnancy, it induces abortion, expulsion of mummified fetuses, or the birth of stillborn and weak lambs. After 120 days of pregnancy, the infection generally leads to apparently normal lambs that can survive for a few days or grow normally and become protected against re-infections [23].
Toxoplasmosis in rabbits and poultry has not been well studied; nevertheless, these two species represent a potential source of T. gondii infection [122]. Transplacental transmission of T. gondii has been reported in rabbits since more than 40 years [122]. Clinical toxoplasmosis in rabbits is apparently rare and not specific [38, 117].
Biotic and abiotic factors play important roles in T. gondii transmission and thus in the epidemiology of T. gondii infection. These factors determine host geographic distribution, density, and interactions [120]. Temperate areas with sufficient rainfall located in the coastal area and the Atlas mountains of North Africa are the most favorable for the survival and spreading of oocysts shed by the definitive hosts. In fact, if the temperature and hygrometry are high, the viability of the oocysts increases, leading to higher contamination rates of intermediate hosts [128]. In such areas, the number of different herbivore species is also high, creating further favorable conditions for T. gondii transmission (Table 4).
Table 4.
Estimated domestic herbivore population in North Africa (1000 heads).
| Country | Sheep | Goats | Cattle | Camels | Equines |
|---|---|---|---|---|---|
| Morocco | 17,078 | 5118 | 2814 | 70 | NA |
| Algeria | 20,000 | 3800 | 1650 | 290 | 218 |
| Tunisia | 7616 | 1550 | 1400 | 200 | 187 |
| Libya | 4500 | 1265 | 130 | 47 | NA |
| Egypt | 2258 | 1054 | 2810 | 68 | 1072 |
| Overall | 60,302 | 18,387 | 10,494 | 2275 | NA |
Main findings of the surveys carried out in North Africa
A literature review on the seroprevalence and the molecular prevalence of T. gondii among human and animals in North African countries was conducted. Publications related to T. gondii infection and toxoplasmosis in North Africa were collected from two literature databases including PubMed and Google Scholar. Keywords used for the bibliographic search were “Morocco, Algeria, Tunisia, Libya, Egypt, human, animal, toxoplasmosis”. No time limitation was imposed and the search took place in 2015, with an update in 2018. The selected articles respected six criteria: (i) study was performed in humans and animals from five North African countries; (ii) both serologic and molecular techniques were considered; (iii) only natural infection by T. gondii was taken into consideration; (iv) studies carried out with vaccine assays were not taken into consideration; (v) in each country, information regarding prevalences of infection by T. gondii were organized by species, starting with humans then animals; and (vi) only articles written in English and French were considered.
Toxoplasma gondii infection in Morocco
All investigations carried out in Morocco in both humans and animals were based on serological tests using enzyme-linked immunosorbent assay (ELISA). The seroprevalence of T. gondii infection in humans was studied for the first time in 1969 by Le Viguelloux and Epardeau [87]. A high infection rate of 64.9% was reported using an indirect immunofluorescence test. Shortly after, the same test was applied for the detection of T. gondii antibodies in 1,026 human sera from Rabat city [96]. Since then, congenital toxoplasmosis has been the main issue in published papers in Morocco. Using the same serological test (ELISA), the seroprevalence in pregnant women ranged between 36.7% and 62.1%, between 2007 and 2017 (Table 5). As a novel diagnostic tool, the chemiluminescent microparticle immunoassay (CMIA) was used for T. gondii antibodies detection among pregnant women in Fes city [121]. Among the risk factors, age was the most commonly reported factor in these studies and the overall conclusion is that the prevalence of Toxoplasma infection increases with age [17, 83–85]. Infection rates also varied according to the locality; reaching 50.6% in Rabat which is higher than 43.3% in Nador (North East), 42.6% in Tetouan (North) and 36.7% in Kenitra (North West) [52]. The authors attributed this difference to the temperate climate of Rabat city, which maintains the biological cycle of T. gondii (rapid and complete sporulation). Regular contact with the land (soil, gardening and agricultural activities) was retained as a major risk for T. gondii infection in Rabat city [52, 85]. In one study conducted in Rabat and concerning pregnant women, school level and knowledge of toxoplasmosis modes transmission were found to be risk factors (p < 0.01), while the consumption of raw meat, contact with cats, and level of hygiene were not significant. Toxoplasmosis was also studied in HIV-infected patients in the city of Marrakech and its surroundings [1]. The authors studied the seroprevalence of T. gondii in 95 HIV-infected adults of different ages. Seroprevalence was estimated to be 62.1%.
Table 5.
Human toxoplasmosis prevalence in North African countries.
| Country (author) | Region | Population/sample | Technique | Positive/examined (%) | Reference |
|---|---|---|---|---|---|
| Morocco (Le Viguelloux and Epardeau, 1969) | – | Patients | IFATa | 100/154 (64.9) | [87] |
| Morocco (Nejmi and Alami, 1973) | Rabat | Military personnel, schoolgirls and pregnant women | IFAT | 281/1026 (27.4) | [96] |
| Morocco (Biava et al., 1983) | Marrakech | Women | IFAT/Hemagglutination | 106/318 (33.3) | [32] |
| Morocco (Guessous-Idrissi et al., 1984) | – | Women | – | −(51.5) | [65] |
| Morocco (El Mansouri et al., 2007) | Kenitra | Pregnant women | ELISAb | −(36.7) | [52] |
| Morocco (El Mansouri et al., 2007) | Nador | Pregnant women | ELISA | −(43.3) | [52] |
| Morocco (El Mansouri et al., 2007) | Tetouan | Pregnant women | ELISA | −(42.6) | [52] |
| Morocco (El Mansouri et al., 2007) | Rabat | Pregnant women | ELISA | 1242/2456 (50.6) | [52] |
| Morocco (Laboudi et al., 2009) | Rabat | Pregnant women | ELISA | 516/1020(50.6) | [85] |
| Morocco (Barkat et al., 2010) | Rabat | Pregnant women | – | 163/368 (44.3) | [17] |
| Morocco (Addebbous et al., 2012) | Marrakesh | HIV-infected adults | Indirect ELISA | 59/95 (62.1) | [1] |
| Morocco (Laboudi et al., 2014) | Rabat | Pregnant women | ELISA | 549/1169 (47) | [84] |
| Morocco (Laboudi, 2017) | Rabat | Pregnant women | ELISA | 59/128 (46.1) | [83] |
| Morocco (Tlamcani et al., 2017) | Fes | Pregnant women | CMIAc | 1367/3440 (39.7) | [121] |
| Algeria (Balozet, 1955) | Algiers | Humans | CFTd | 13/125 (10.4) | [16] |
| Algeria (Schneider et al., 1977) | Algiers | Patients | IFAT | 1297/2438 (53.2) | [110] |
| Algeria (Messserer et al., 2014) | Annaba | Pregnant women | Microparticle enzyme | 491/1028 (47.8) | [93] |
| Algeria (Berredjem et al., 2017) | Annaba | Pregnant women | ELISA | 57/143 (39.9) | [31] |
| Algeria (Berredjem et al., 2017) | Annaba | Pregnant women | PCRe (B1 gene) | 9/57 (15.8) | [31] |
| Algeria (Berredjem et al., 2017) | Annaba | Pregnant women | PCR (P30 gene) | 4/14 (28.6) | [31] |
| Tunisia (Ben Rachid and Blaha, 1970) | – | Blind children | SFDTf | 19/92 (20.6) | [30] |
| Tunisia (Ben Rachid and Blaha, 1970) | – | Adolescents | SFDT | 18/30 (60) | [30] |
| Tunisia (Messedi-Triki et al., 1982) | Tunis | – | – | 402/810 (49.6) | [92] |
| Tunisia (Jemni et al., 1985) | Sousse | Students | – | −(67) | [75] |
| Tunisia (Bchir et al., 1992) | Monastir | Pregnant women | ELISA | 195/478 (40.8) | [19] |
| Tunisia (Ben Ayed Nouira et al., 1994) | Tunis | Women | – | −(63.5) | [27] |
| Tunisia (Bouratbine et al., 2001) | Beja | Individuals | ELISA and IFAT | 830/1421 (58.4) | [35] |
| Tunisia (Sellami et al., 2010) | Sfax | Pregnant women | ELISA | 15952/40567 (39.3) | [111] |
| Tunisia (Ben Abdallah et al., 2013) | Tunis | Pregnant women | ELISA | 944/2070 (45.6) | [26] |
| Tunisia (Fakhfakh et al., 2013) | Tunis | Pregnant women | Immunocapture | 1114/2351 (47.4) | [56] |
| Tunisia (Siala et al., 2014) | Tunis | Amniotic fluid | PCR | 12/60 (20) | [115] |
| Libya (Khadre and Nageh, 1987) | Tripoli | Adult males | – | 1032/2000 (51.6) | [78] |
| Libya (Khadre and Nageh, 1987) | Tripoli | Adult females | – | 130/300 (43.3) | [78] |
| Libya (Khadre and Nageh, 1987) | Tripoli | Schoolchildren | – | 865/1980 (43.7) | [78] |
| Libya (Khadre and Nageh, 1987) | Tripoli | Female patients with abortion history | – | 1334/1921 (69.4) | [78] |
| Libya (Kassem and Morsy, 1991) | Benghazi | Pregnant women | IHATg | 176/369 (47.7) | [77] |
| Libya (Elsaid et al., 2014) | Tripoli | Control volunteers | ELISA | 3/300 (1) | [53] |
| Libya (Elsaid et al., 2014) | Tripoli | Psychiatric patients | ELISA | 151/300 (50.3) | [53] |
| Libya (Elsaid et al., 2014) | Tripoli | Control volunteers | Latex | 140/300 (46.7) | [53] |
| Libya (Elsaid et al., 2014) | Tripoli | Psychiatric patients | Latex | 185/300 (61.7) | [53] |
| Libya (Gamal and Jaroud, 2015) | Alkhoms | Pregnant women | ELISA | 142/361 (39.3) | [60] |
| Libya (Shalaka et al., 2015) | Tripoli | Patients with HIV/AIDS | – | 19/227 (8.4) | [113] |
| Libya (Gashout et al., 2016) | Tripoli | Women who have had spontaneous abortions | ELISA | 54/140 (38.6) | [61] |
| Libya (Gashout et al., 2016) | Tripoli | HIV patients | ELISA | 23/26 (88.5) | [61] |
| Libya (Gashout et al., 2016) | Sabrata | Patients with leukemia or lymphoma | ELISA | 6/9 (66.7) | [61] |
| Libya (Gashout et al., 2016) | Zawia | Children with ocular infection | ELISA | 1/2 (50) | [61] |
| Libya (Haq et al., 2016) | Misurata | Pregnant women | PCR | 27/276 (9.8) | [67] |
| Egypt (Azab et al., 1992) | – | Serum of lactating women | IFAT | 22/70 (31.4) | [13] |
| Egypt (Azab et al., 1992) | – | Milk of lactating women | IFAT | 12/70 (17.1) | [13] |
| Egypt (Youssef, 1993) | Dakahlia | Inhabitants | Dot-ELISA | −(23.8) | [130] |
| Egypt (Ibrahim et al., 1997) | Gharbia | Workers | IHAT | 11/21 (52.4) | [72] |
| Egypt (Ibrahim et al., 1997) | Dakahlia | Pregnant women | ELISA | 52/101 (51.5) | [72] |
| Egypt (Amrei et al., 1999) | Zagazig | Children with intellectual disability | – | 14/32 (43.7) | [11] |
| Egypt (Amrei et al., 1999) | Zagazig | Adult females | – | −(37.5) | [11] |
| Egypt (Elsheikha et al., 2009) | Mansoura | Blood donors | ELISA | 155/260 (59.6) | [54] |
| Egypt (El-Gozamy et al., 2009) | Qualyobia | Pregnant women | ELISA | 46.5 to 57.6 | [50] |
| Egypt (Ghoneim et al., 2010) | El Fayoum | Pregnant women | ELISA IgG | 27/59 (45.8) | [64] |
| Egypt (Ghoneim et al., 2010) | El Fayoum | Pregnant women | ELISA IgM | 18/59 (30.5) | [64] |
| Egypt (Ghoneim et al., 2010) | El Fayoum | Pregnant women | SFDT | 14/ 59 (23.7) | [64] |
| Egypt (Ghoneim et al., 2010) | El Fayoum | Pregnant women | PCR | 19/59 (33.2) | [64] |
| Egypt (El Deeb et al., 2012) | Menoufia | Pregnant women | ELFAh | 218/323 (67.5) | [47] |
| Egypt (Ahmed et al., 2014) | Sharkia | Pregnant women | MATi | 82/100 (82) | [4] |
| Egypt (Kamal et al., 2015) | Minia | Women with high risk pregnancy | ELISA | 61/120 (50.8) | [76] |
| Egypt (Ibrahim et al., 2017) | Menoufia | Pregnant women | ELISA | 63/171 (36.8) | [74] |
| Egypt (Ibrahim et al., 2017) | Menoufia | Pregnant women | RT-PCRj | 24/171 (14) | [74] |
| Egypt (Ibrahim et al., 2017) | Gharbia | Pregnant women | ELISA | 60/193 (31.1) | [74] |
| Egypt (Ibrahim et al., 2017) | Gharbia | Pregnant women | RT-PCR | 19/193 (9.8) | [74] |
IFAT: ImmunoFluorescent Antibody Test.
ELISA: Enzyme Linked Immunosorbent Assay.
CMIA: Chemiluminescent Microparticle Immunoassay
CFT: Complement-Fixation Test.
PCR: Polymerase Chain Reaction.
SFDT: Sabin-Feldman Dye Test.
IHAT: Indirect Hemagglutination Antibody test.
ELFA: Enzyme-Linked Fluorescence Assay.
MAT: Modified Agglutination Test.
RT-PCR: Real-Time Polymerase Chain Reaction.
– : Not Available.
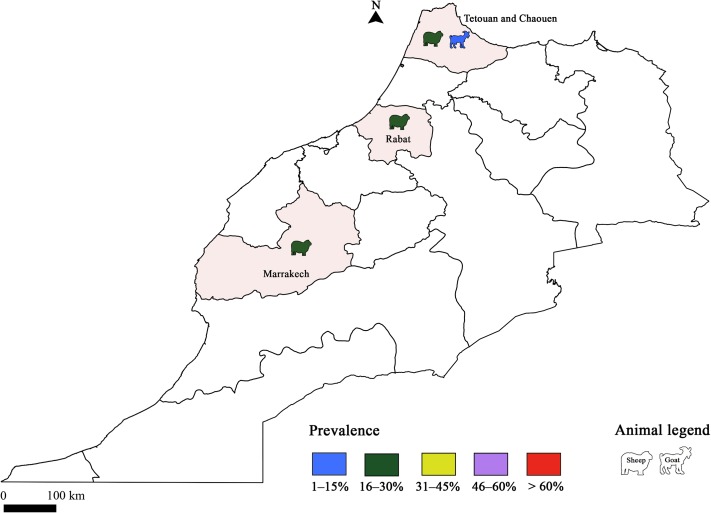
Few studies have targeted T. gondii infection in livestock species; only sheep and goats were concerned (Fig. 1). The seroprevalence of T. gondii infection in sheep was lower than 30% using ELISA (Table 6). Using the same diagnostic tool, there was no significant difference according to the locality (30% in Rabat, 27.6% and 30% in the Marrakech region, and 20.8% in Northern Morocco and Middle Atlas) [24, 28, 29, 109]. These similarities could be attributed to herd management and the presence of cats in farms [28]. Sawadogo et al. [109] reported that the infection rate in meat from sheep in the Marrakech region was lower than in other regions. In fact, high temperatures during the summer in Marrakech with an average annual rainfall up to 360 mm could reduce oocyst life span and consequently the prevalence of infection. Determination of T. gondii seroprevalence in goats was conducted only in the Northern Morocco and Middle Atlas regions, revealing a low infection rate (8.5%) [29]. One study was performed to determine the genotypes of T. gondii occurring in Morocco using 15 microsatellite markers, and referred to a human strain of type III genotype [59].
Figure 1.
Prevalence of Toxoplasma gondii infection in Morocco: Animal toxoplasmosis, seroprevalence.
Table 6.
Animal toxoplasmosis prevalence in North African countries.
| Country (author) | Region | Population/sample | Technique | Positive/examined (%) | Reference |
|---|---|---|---|---|---|
| Morocco (Benkirane et al., 1990) | Rabat | Sheep | ELISA | −(30) | [28] |
| Morocco (Belbacha et al., 2004) | Marrakech | Sheep | ELISA | 15/50 (30) | [24] |
| Morocco (Sawadogo et al., 2005) | Marrakech | Sheep | ELISA | 72/261 (27.6) | [109] |
| Morocco (Benkirane et al., 2015) | Northern Morocco and Middle Atlas | Sheep | ELISA | 42/202 (20.8) | [29] |
| Morocco (Benkirane et al., 2015) | Northern Morocco and Middle Atlas | Goats | ELISA | 9/106 (8.5) | [29] |
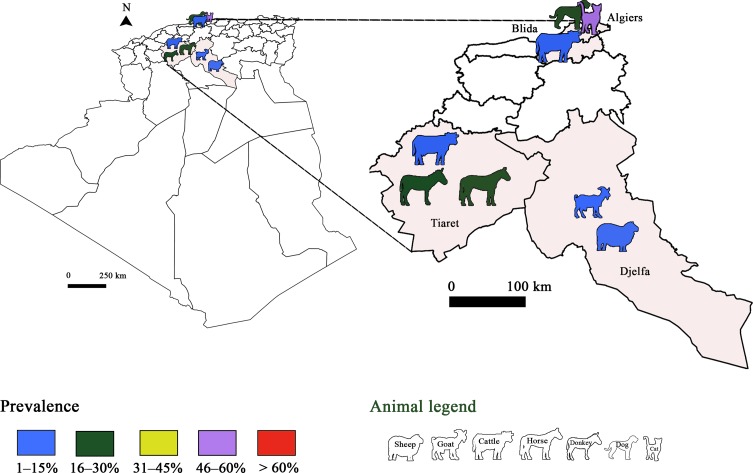
| Algeria (Balozet, 1955) | Algiers | Dogs | CFT | 32/105 (30.5) | [16] |
| Algeria (Dechicha et al., 2015) | Blida | Cattle | IFAT | 13/332 (3.9) | [37] |
| Algeria (Dechicha et al., 2015) | Djelfa | Sheep | IFAT | 32/276 (11.6) | [37] |
| Algeria (Dechicha et al., 2015) | Djelfa | Goats | IFAT | 14/106 (13.2) | [37] |
| Algeria (Abdelhadi et al., 2015) | Tiaret | Cattle | ELISA | 14/92 (15.2) | [2] |
| Algeria (Mohamed–Cherif et al., 2015) | Tiaret | Horses | MAT | 76/293 (25.9) | [94] |
| Algeria (Mohamed–Cherif et al., 2015) | Tiaret | Donkeys | MAT | 9/30 (30) | [94] |
| Algeria (Yekkour et al., 2017) | Algiers | Stray cats | MAT | 48/96 (50) | [129] |
| Algeria (Dahmani et al., 2018) | Western, Eastern and South | Sheep | ELISA | 48/580 (8.3) | [36] |
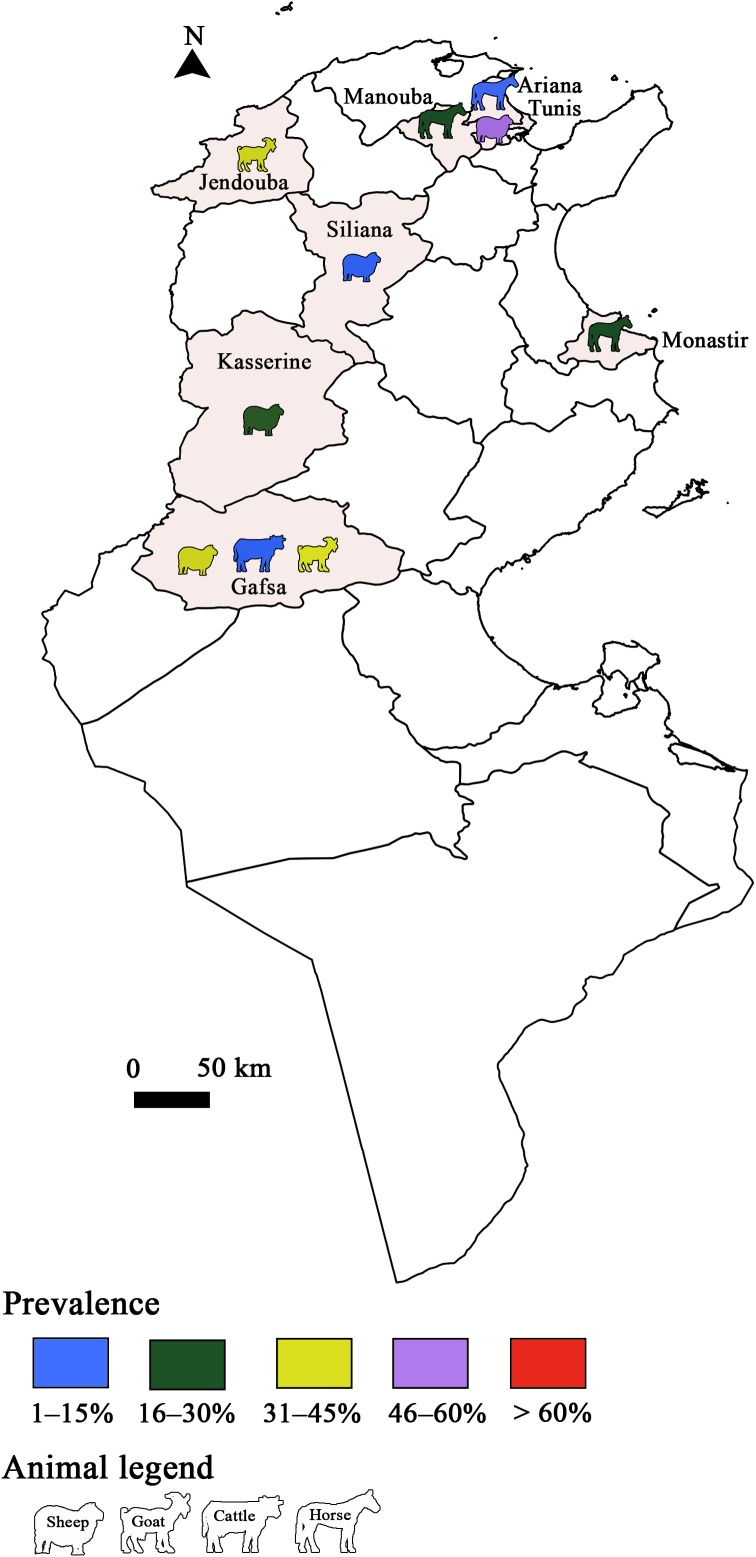
| Tunisia (Ben Rachid and Blaha, 1970) | – | Sheep | SFDT | 169/225 (75.1) | [30] |
| Tunisia (Ben Rachid and Blaha, 1970) | – | Goats | SFDT | 51/85 (60) | [30] |
| Tunisia (Ben Rachid and Blaha, 1970) | – | Cattle | SFDT | 93/250 (37.2) | [30] |
| Tunisia (Ben Rachid and Blaha, 1970) | – | Cattle | SFDT | 16/100 (16) | [30] |
| Tunisia (Ben Rachid and Blaha, 1970) | – | Camels | SFDT | 48/120 (40) | [30] |
| Tunisia (Ben Rachid and Blaha, 1970) | – | Dogs | SFDT | 142/200 (71) | [30] |
| Tunisia (Boughattas et al., 2011) | Sidi Thabet, Monastir and Battan | Horses | MAT | 28/158 (17.7) | [33] |
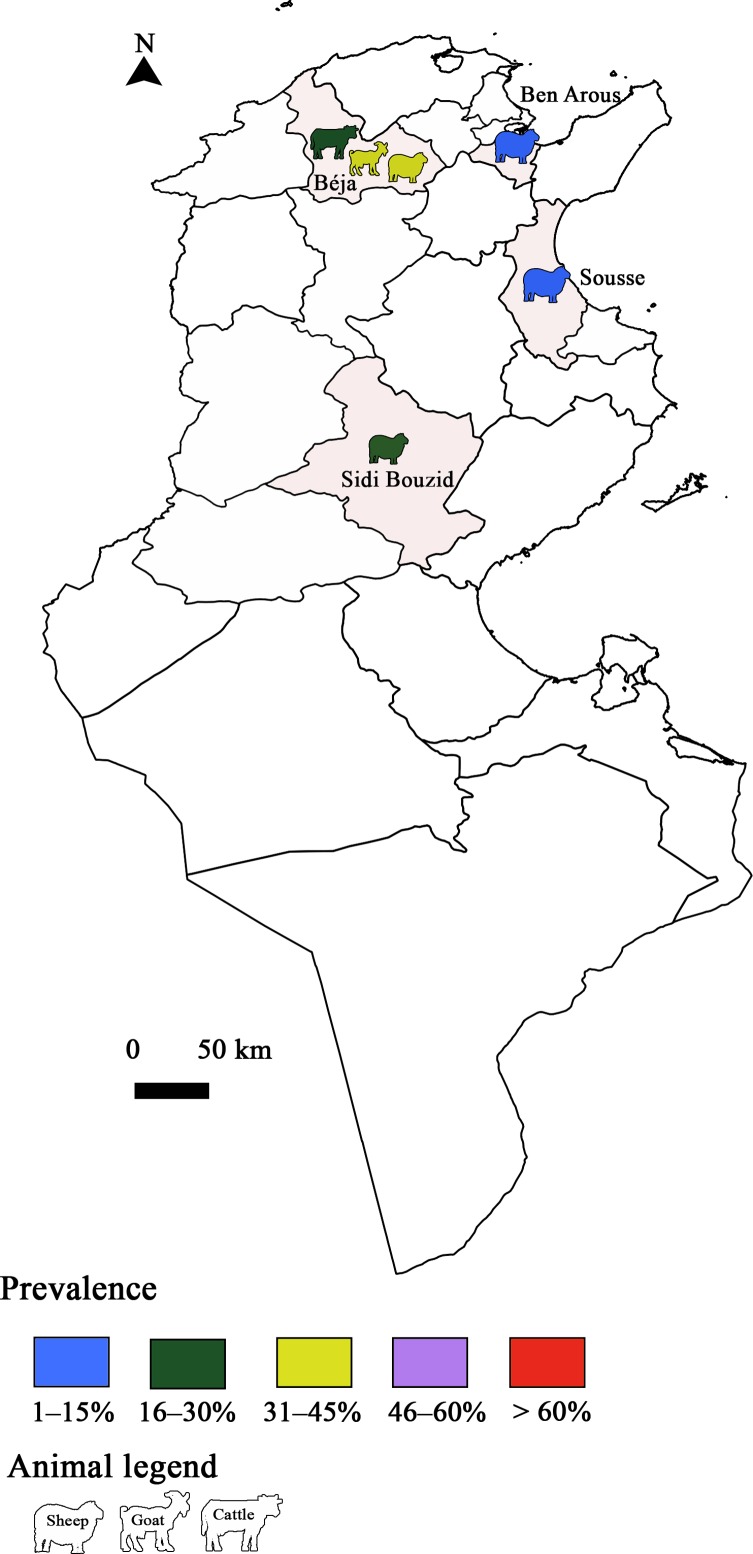
| Tunisia (Gharbi et al., 2013) | Ben Arous | Sheep | PCR | 9/71 (12.7) | [62] |
| Tunisia (Gharbi et al., 2013) | Kasserine | Sheep | ELISA | 35/184 (19) | [62] |
| Tunisia (Gharbi et al., 2013) | Sidi Bouzid | Sheep | PCR | 27/106 (25.5) | [62] |
| Tunisia (Gharbi et al., 2013) | Siliana | Sheep | ELISA | 3/166 (1.8) | [62] |
| Tunisia (Khayeche et al., 2013) | Sousse | Sheep | PCR | 4/70 (5.7) | [79] |
| Tunisia (Boughattas et al., 2014) | Tunis | Young sheep | MAT | 83/217 (38.2) | [34] |
| Tunisia (Boughattas et al., 2014) | Tunis | Adult sheep | MAT | 92/125 (73.6) | [34] |
| Tunisia (Lahmar et al., 2015) | Gafsa | Sheep | MAT | 82/204 (40.2) | [86] |
| Tunisia (Lahmar et al., 2015) | Gafsa | Goats | MAT | 11/32 (34.4) | [86] |
| Tunisia (Lahmar et al., 2015) | Gafsa | Cattle | MAT | 3/25 (12) | [86] |
| Tunisia (Amairia et al., 2016) | Tabarka | Goats | ELISA | 17/34 (50) | [9] |
| Tunisia (Amairia et al., 2016) | Hammam Bourghiba | Goats | ELISA | 7/43 (16.3) | [9] |
| Tunisia (Amairia et al., 2016) | Tabarka | Goats’ milk | PCR | 0/34 | [9] |
| Tunisia (Amairia et al., 2016) | Hammam Bourghiba | Goats’ milk | PCR | 6/43 (13.9) | [9] |
| Tunisia (Rouatbi et al., 2017) | Beja | Sheep | PCR | 48/150 (32) | [107] |
| Tunisia (Rouatbi et al., 2017) | Sidi Bouzid | Sheep | PCR | 54/174 (31) | [107] |
| Tunisia (Amdouni et al., 2017) | Beja | Sheep | PCR | 50/150 (33.3) | [10] |
| Tunisia (Amdouni et al., 2017) | Beja | Cattle | PCR | 29/150 (19.3) | [10] |
| Tunisia (Amdouni et al., 2017) | Beja | Goats | PCR | 39/120 (32.5) | [10] |
| Libya (Azwai et al., 1993) | – | Goats | IHAT | −(50) | [14] |
| Libya (Azwai et al., 1993) | – | Sheep | IHAT | −(26.2) | [14] |
| Libya (Azwai et al., 1993) | – | Horses | IHAT | −(4.8) | [14] |
| Libya (Azwai et al., 1993) | An-Najila | Cattle | IHAT | −(27.4) | [14] |
| Libya (Azwai et al., 1993) | Khadra’ | Cattle | IHAT | −(14.3) | [14] |
| Libya (Azwai et al., 1993) | Al Hany | Cattle | IHAT | −(10.6) | [14] |
| Libya (El-Gomati et al., 2010) | Tripoli | Mice | Toxocell latex test | 21/60 (35) | [49] |
| Libya (Al-mabruk et al., 2013) | Western, Central, Eastern and Southern | Sheep | LATa | 4122/5806 (71) | [8] |
| Egypt (Rifaat et al., 1976) | Cairo | Chickens | DTb | 17/85 (20) | [105] |
| Egypt (Rifaat et al., 1976) | Cairo | Rabbits | DT | 49/100 (49) | [105] |
| Egypt (Ibrahim et al., 1997) | Tanta | Sheep | IHAT | 114/258 (44.2) | [72] |
| Egypt (Ibrahim et al., 1997) | Tanta | Sheep | IFAT | 126/258 (48.8) | [72] |
| Egypt (Hilali et al., 1998) | – | Camels | DATc | 29/166 (17.5) | [71] |
| Egypt (El-Ghaysh, 1998) | Monofia | Donkeys | ELISA | 79/121 (65.3) | [48] |
| Egypt (Dubey et al., 2003) | Giza | Free range chickens | MAT | 49/121 (40.5) | [42] |
| Egypt (Ghazy et al., 2007) | – | Horses | ELISA | 160/420 (38.1) | [63] |
| Egypt (Shaapan et al., 2008) | Cairo | Sheep | MAT | 131/300 (43.7) | [112] |
| Egypt (Shaapan et al., 2008) | Cairo | Sheep | ELISA | 125/300 (41.7) | [112] |
| Egypt (Shaapan et al., 2008) | Cairo | Sheep | IFAT | 111/300 (37) | [112] |
| Egypt (Shaapan et al., 2008) | Cairo | Sheep | DT | 102/300 (34) | [112] |
| Egypt (Ibrahim et al., 2009) | Sharkia | Cattle | ELISA | 10/93 (10.7) | [73] |
| Egypt (Haridy et al., 2010) | Cairo | Working donkeys | ELISA | 45/100 (45) | [69] |
| Egypt (Haridy et al., 2010) | Cairo | Donkeys’ milk | ELISA | 7/15 (46.7) | [69] |
| Egypt (Ghoneim et al., 2010) | El Fayoum | Sheep | ELISA | 61/62 (98.4) | [64] |
| Egypt (Ghoneim et al., 2010) | El Fayoum | Sheep | PCR | 42/62 (67.7) | [64] |
| Egypt (Ghoneim et al., 2010) | El Fayoum | Goats | ELISA | 10/24 (41.7) | [64] |
| Egypt (Ghoneim et al., 2010) | El Fayoum | Goats | PCR | 6/24 (25) | [64] |
| Egypt (Harfoush and Tahoon, 2010) | Kafr El-Sheikh | Domestic ducks | IHAT | −(55) | [68] |
| Egypt (Harfoush and Tahoon, 2010) | Kafr El-Sheikh | Free-range chickens | IHAT | −(38.1) | [68] |
| Egypt (Harfoush and Tahoon, 2010) | Kafr El-Sheikh | Turkeys | IHAT | −(29.4) | [68] |
| Egypt (Ashmawy et al., 2011; Harfoush and Tahoon, 2010) | Kafr El-Sheikh | Domestic rabbits | IHAT | −(17.5 to 37.5) | [12, 68] |
| Egypt (Al-Kappany et al., 2011) | Cairo | Feral cats | MAT | 172/180 (95.5) | [7] |
| Egypt (Ashmawy et al., 2011) | Alexandria | Domestic rabbits | IHAT | 9/85 (10.6) | [12] |
| Egypt (Ashmawy et al., 2011) | Behera | Domestic rabbits | IHAT | 6/69 (8.7) | [12] |
| Egypt (Barakat et al., 2012) | Cairo | Chickens | ELISA | −(62.2) | [18] |
| Egypt (Barakat et al., 2012) | Gharbia | Chickens | ELISA | −(82.3) | [18] |
| Egypt (Barakat et al., 2012) | Kafr El sheikh | Chickens | ELISA | −(67.1) | [18] |
| Egypt (Barakat et al., 2012) | Quena | Chickens | ELISA | −(75) | [18] |
| Egypt (Barakat et al., 2012) | Sharkia | Chickens | ELISA | −(59.5) | [18] |
| Egypt (Barakat et al., 2012) | Sohag | Chickens | ELISA | −(50) | [18] |
| Egypt (Behairy et al., 2013) | Giza | Turkeys | MAT | 103/173 (59.5) | [45] |
| Egypt (Behairy et al., 2013) | Giza | Chickens | MAT | 51/108 (47.2) | [45] |
| Egypt (Behairy et al., 2013) | Giza | Ducks | MAT | 24/48 (50) | [45] |
| Egypt (El-Madawy and Metawea, 2013) | Ismailia | Ostriches | ELISA | 15/120 (12.5) | [51] |
| Egypt (El-Madawy and Metawea, 2013) | Ismailia | Ostriches | PCR | 9/120 (7.5) | [51] |
| Egypt (Behairy et al., 2013) | Giza | Dogs | MAT | 50/51 (98) | [45] |
| Egypt (Behairy et al., 2013) | Giza | Hearts of dogs | Bioassay | 22/43 (51.2) | [45] |
| Egypt (Ahmed et al., 2014) | Sharkia | Sheeps’ milk | PCR | 1/50 (2) | [4] |
| Egypt (Ahmed et al., 2014) | Sharkia | Goats’ milk | PCR | 4/50 (8) | [4] |
| Egypt (Ahmed et al., 2014) | Sharkia | Cows’ milk | PCR | 0/50 | [4] |
| Egypt (Mahmoud et al., 2015) | Gharbia | Stray cats | IHAT | 17/92 (18.5) | [90] |
| Egypt (Mahmoud et al., 2015) | Gharbia | Stray cats | IFAT | 19/92 (20.7) | [90] |
| Egypt (Mahmoud et al., 2015) | Gharbia | Owned cats | IHAT | 4/32 (12.5) | [90] |
| Egypt (Mahmoud et al., 2015) | Gharbia | Owned cats | IFAT | 5/32 (15.6) | [90] |
| Egypt (Ibrahim et al., 2017) | Menoufia | Sheep | ELISA | −(51.04) | [74] |
| Egypt (Ibrahim et al., 2017) | Menoufia | Sheep | RT-PCR | −(17.71) | [74] |
| Egypt (Ibrahim et al., 2017) | Gharbia | Sheep | ELISA | −(52.70) | [74] |
| Egypt (Ibrahim et al., 2017) | Gharbia | Sheep | RT-PCR | −(17.57) | [74] |
LAT: Latex Agglutination Test.
DT: Dye Test.
DAT: Direct Agglutination Test.
Toxoplasma gondii infection in Algeria
The first paper studying T. gondii in Algeria was published in 1955 by Balozet [16]. It was a serological investigation (complement-fixation test) confirming the presence of T. gondii antibodies in 10% and more than 30% in humans and dogs, respectively. Schneider et al. [110] found higher infection rates in patients (53.2%) by the indirect immunofluorescence test. All other published papers concerned seroprevalence in pregnant women. Serological surveys revealed small variations in infection rates: Messerer et al. [93] found the same results in Annaba (47.8%), whereas Berredjem et al. [31] found lower seroprevalence (39.8%). The combination between serological and molecular tools for congenital toxoplasmosis diagnosis was only studied by Berredjem et al. [31]. The results of this study should be interpreted with caution since using PCR for the detection of the parasite’s DNA in peripheral blood samples has low sensitivity and is indicated only in certain cases of immunocompromized patients [5]. Consumption of undercooked meat and the presence of cats in the household were the major risk factors associated with T. gondii infection [31]. Genotype II was isolated from a congenital toxoplasmosis case and human strains [15].
Only serological studies have been performed to detect T. gondii in animals in Algeria (Fig. 2). Seroprevalence rates of T. gondii in sheep were low (8.28%) in Eastern and South Algeria, and in Djelfa locality (11.6%) using ELISA and immunofluorescent antibody tests (IFAT), respectively [36, 37]. Through these studies, three risk factors were associated with T. gondii infection, namely season, origin of animals, and absence of abortion history. Concerning the season, summer, spring and autumn were characterized as more suitable periods for oocyst survival. The authors also suggested that the high relative humidity that typifies Northern Algeria (Center, Eastern and Western) enhances oocyst viability since infection rates were higher than in the south. The presence of T. gondii antibodies in goats has only been investigated using IFA testing in a study carried out by Dechicha et al. [37]. In the same study, the seroprevalence of T. gondii in cattle was lower than that reported for sheep and goats. This emphasises the lower susceptibility of cattle to T. gondii compared to small ruminants. Other studies were conducted in cats, horses and donkeys. The seroprevalence in cats was comparable to that in humans, and the two species shared the same genotype, i.e. genotype II [93, 129].
Figure 2.
Prevalence of Toxoplasma gondii infection in Algeria: Animal toxoplasmosis, seroprevalence.
Toxoplasma gondii infection in Tunisia
The first study concerning human infection was published in 1970 followed by many other studies in humans [80, 81], or studies of T. gondii as a parasite of food origin [82]. In the first survey, conducted by Ben Rachid and Blaha [30] using the Sabin-Feldman Dye Test (SFDT), results showed that the overall seroprevalence of T. gondii infection increased with age. This pattern was later confirmed in a larger survey including 142 individuals from the northern parts of the country using ELISA and IFAT [35]. This epidemiological profile suggests that even though infection is a frequent event in early childhood, women of childbearing age remain susceptible to toxoplasmosis. Other surveys focused on pregnant women and congenital toxoplasmosis [19, 25, 114]. The seroprevalence rates range from 39.3% in the southern regions to 47.7% in the northern regions using ELISA [26, 56, 111]. Most of the Tunisian authors suspected consumption of undercooked meat and unwashed vegetables as the two main contamination routes [56]. Infection was present in regions where agriculture was the predominant activity and sheep meat was the most consumed meat [30]. Toxoplasmosis is regularly diagnosed in immunocompromized patients in Tunisia. Before highly active antiretroviral therapy (HAART), cerebral toxoplasmosis was reported as one of the most prevalent infections in patients with HIV [111].
Toxoplasma gondii infection has also been studied in animals and several studies were conducted in sheep (Figs. 3 and 4). Sero-surveys found a maximum infection rate of 73.6% using a modified agglutination test (MAT) [34, 62, 86]. Using molecular tools, Boughattas et al. [34] found the highest infection rate in ewe tissues (50%) in Tunis city. Consequently, the authors considered sheep meat as a major risk factor of T. gondii transmission through meat consumption. Three studies determined the molecular prevalence of T. gondii in apex heart samples from sheep, and the infection rates ranged between 5.7% and 25.5% [62, 79] in the first two studies. The lowest rate was reported by Khayeche et al. [79] in the third study, where apex hearts samples were collected from slaughtered sheep during the Muslim feast of Eid Al-Adha. This could be explained by the very low age of these animals. In fact, the majority of households (92.9%) slaughtered a sheep aged less than 1 year. This study highlighted that the majority of meat handlers did not respect hygiene rules, since 91% of them did not wash their hands after handling and before preparing or consuming food. In addition, the presence of factors that increase the risk of toxoplasmosis such as cat feces or eating raw meat during Eid Al-Adha was detected in 14% of the households.
Figure 3.
Prevalence of Toxoplasma gondii infection in Tunisia: Animal toxoplasmosis, seroprevalence.
Figure 4.
Prevalence of Toxoplasma gondii infection in Tunisia: Animal toxoplasmosis, molecular prevalence.
Along with sheep, T. gondii infection was studied in other species such as goats and horses [10, 33]. To assess the risk of toxoplasmosis transmission through contaminated food, one study was conducted in goat milk samples, reporting a molecular infection rate of 7.8% [9]. Detecting parasite DNA in milk does not mean that the parasite is alive; further studies are needed to determine parasite viability. Additionally, it has been confirmed experimentally that tachyzoites survive in goat’s milk for three to seven days at +4 °C [116]. Moreover, Tenter et al. [120] confirmed that unpasteurized goat’s milk is an important source of human toxoplasma infection.
Toxoplasma gondii infection in Libya
The first serological survey on human infection was carried out by Khadre and El Nageh [78] in Tripoli. In the same locality (Tripoli), Gashout et al. [61] reported an infection rate of 38.5% in women with spontaneous abortions. Positive results were also detected in 47.7% of pregnant women in Benghazi (by indirect hemagglutination antibody testing (IHAT)), with the highest rate observed among the older age group (63.3%) [77]. Lower seroprevalence in pregnant women was found in the Alkhoms district, using ELISA (39.3%) [60]. The authors of this study emphasized an association between T. gondii prevalence and several risk factors, including age (age group), living area (rural and urban), diet (consumption of lamb meat), drinking water source, and contact with cats. Recently, T. gondii DNA was detected in 9.9% of the umbilical cord of neonates, indicating a high level of congenital toxoplasmosis [67]. In 2014, the prevalence of T. gondii infection in psychiatric patients in Tripoli was estimated and this prevalence was significantly higher than in the control group. The authors explain this as a causal relationship between toxoplasmosis infection and psychiatric diseases [53]. Nevertheless, this result needs to be investigated further to explain the relationship between toxoplasmosis and psychiatric diseases.
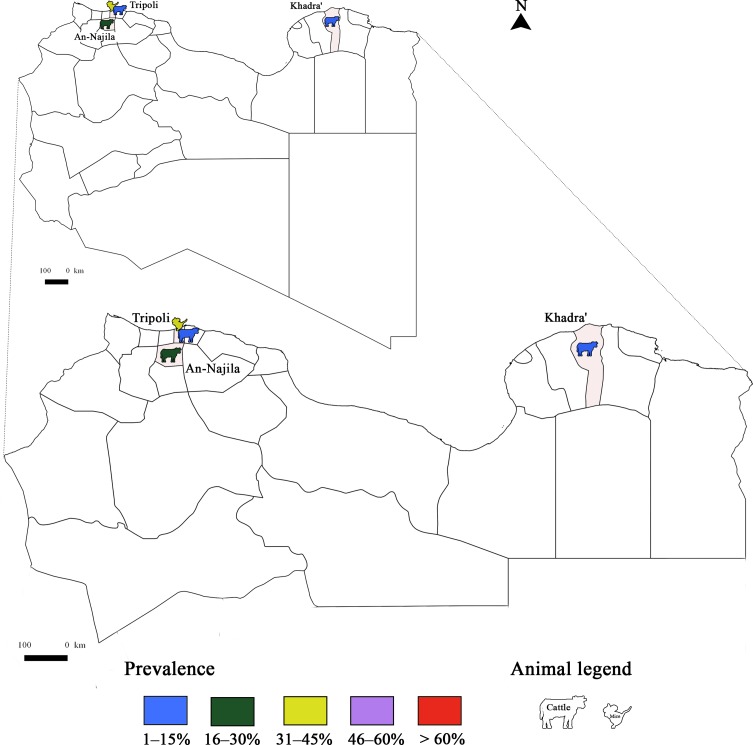
To the best of our knowledge, there are few studies in Libya related to T. gondii infection in animal species (Fig. 5). Indirect hemagglutination testing (IHAT) was used to serologically determine the prevalence of the infection in cattle, sheep, goats and horses sampled from different parts of Libya by Azawi et al. [14]. A higher seroprevalence (76.6%) was reported in sheep from the western region compared to the central region using latex agglutination testing (LAT). The presence of cats and the specific climate in each region were the two determinants of T. gondii infection distribution. Associations with different risk factors were studied, showing a significant correlation with the age group, abortion in sheep, and the management system (extensive and intensive) [8].
Figure 5.
Prevalence of Toxoplasma gondii infection in Libya: Animal toxoplasmosis seroprevalence.
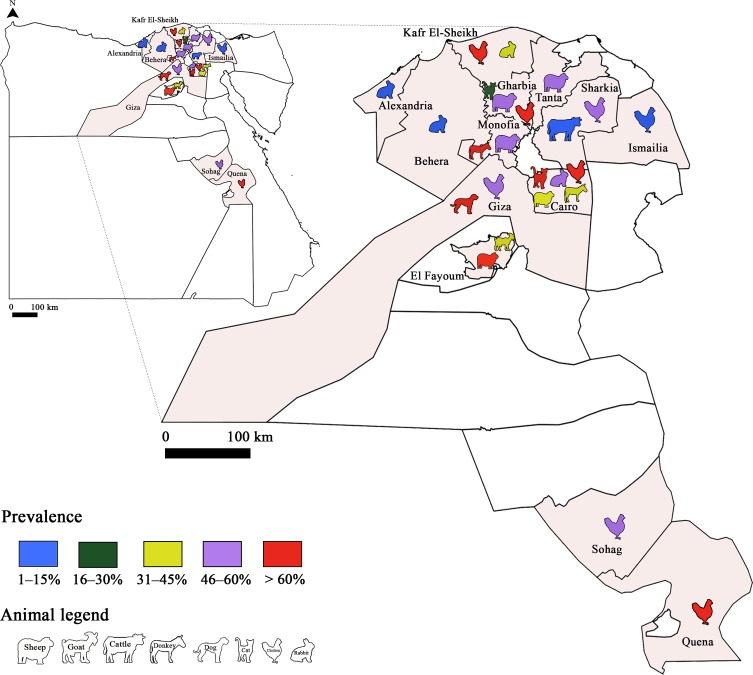
Toxoplasma gondii infection in Egypt
Toxoplasmosis was declared for the first time as a “new disease” in Egypt in 1952 [108]. Egyptian studies on T. gondii infection exceed 200 articles and clearly show it is widespread in the country. Nevertheless, many studies were not taken into consideration to respect the imposed criteria in this review. The seroprevalence of T. gondii in humans is high, reaching 59.6% [54, 72, 130], and both T. gondii genotypes I and II were reported in Egyptian patients [3]. T. gondii DNA was detected in several population groups: in children and their mothers (43.7% and 37.5%, respectively), and in inhabitants and workers [13, 72, 130]. Pregnant women, being the main category at risk, had high T. gondii infection rates [47, 50, 73, 76]. The seroprevalence of T. gondii in Egyptian cats reached 97% [6, 7]. Since the majority of cats were seropositive, the soil is suspected to be heavily contaminated with oocytes. This supports the idea that contact with cats might be the main risk factor for toxoplasmosis transmission in Egypt.
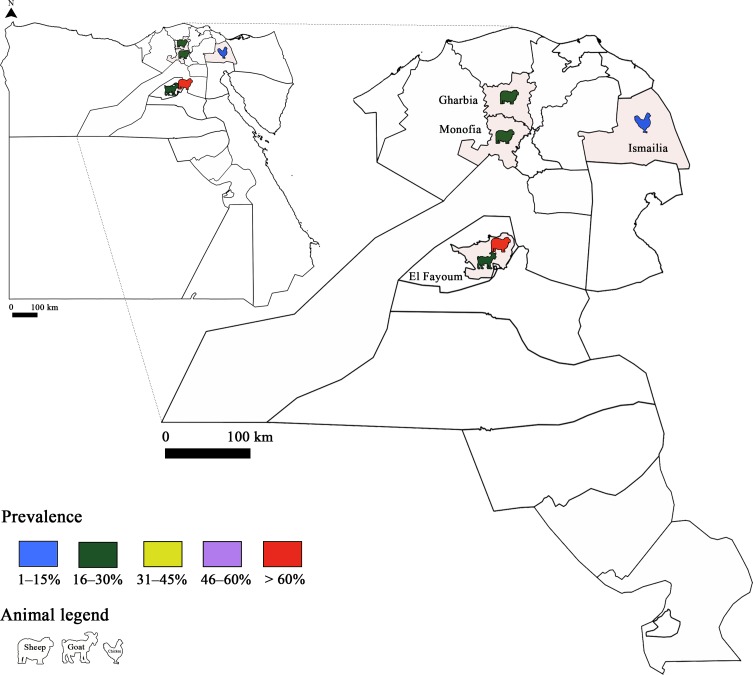
In small ruminants, high prevalence of infection was reported, reaching 98.4% and 41.7% by serological tools, and 67.6% and 25% by molecular methods in sheep and goat samples, respectively [64] (Figs. 6 and 7). The infection rate with T. gondii in sheep has shown a downward trend in recent studies, at 17.65% [74]. This could be explained by the sensitivity of the tests used, such as real-time PCR. Also, a combination of techniques (serological and molecular methods) increases sensitivity and specificity. In Egyptian cattle, T. gondii prevalence was comparably low. Toxoplasma gondii in chickens, ostriches and ducks was also reported [18, 42, 51]. Isolates of T. gondii from chicken samples belonged to genotypes II and III, while in ducks, the genotype was type III. Several studies were performed in camels, horses, donkeys and stray dogs [45, 63, 69, 71]. Recently, a prevalence study in milk samples from cows, sheep and goats showed rates of 0%, 2% and 8%, respectively [4].
Figure 6.
Prevalence of Toxoplasma gondii infection in Egypt: Animal toxoplasmosis, seroprevalence.
Figure 7.
Prevalence of Toxoplasma gondii infection in Egypt: Animal toxoplasmosis, molecular prevalence.
Discussion: overall analyses of the surveys carried out in North Africa
Toxoplasmosis is a widespread zoonosis in North African countries in both humans and animals. This zoonosis was studied in five North African countries (Morocco, Algeria, Tunisia, Libya and Egypt), demonstrating that the epidemiological cycle of this protozoan is very well maintained in this region.
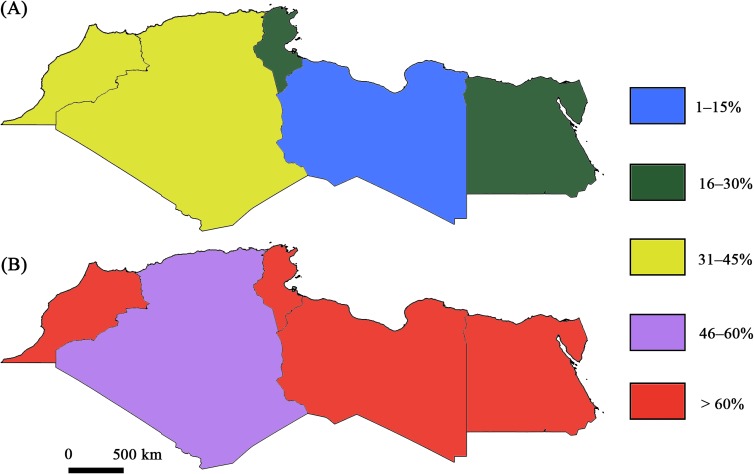
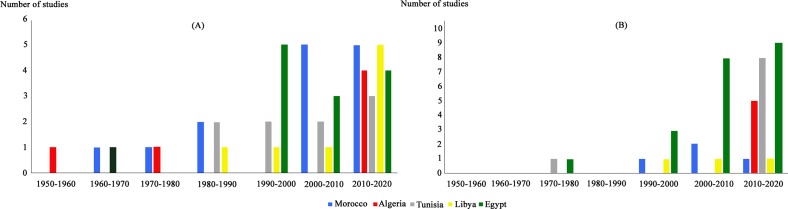
The number of studies dealing with T. gondii infection in humans was almost equal in the five countries (Fig. 8). For animal infections, more studies were performed in Egypt (Fig. 9). For human and animal infections and at the region level, a gap of knowledge was recorded during the period between 1960 and 1970. An increase in the number of studies was recorded in the period between 2000 and 2018, with a marked predominance of studies conducted in Egypt (Fig. 10). This is may be due to the recognition that toxoplasmosis is a significant public health challenge and to public awareness. In Morocco for example, awareness about the risk of this zoonosis is increasing thanks to new initiatives and research established by the Ministry of Health [46].
Figure 8.
Overall status of Toxoplasma gondii seroprevalence in humans in North African countries: (A) Minimum infection rates; (B) Maximum infection rates.
Figure 9.
Overall number of studies dealing with human and animal toxoplasmosis in five North African countries (Morocco, Algeria, Tunisia, Libya and Egypt).
Figure 10.
Distribution of studies dealing with human and animal toxoplasmosis in five North African countries over time (Morocco, Algeria, Tunisia, Libya and Egypt).
Morocco’s health policy, like in Tunisia and Algeria, is influenced by French approaches that represent a strong benchmark for countries planning to set up a national program against toxoplasmosis [46]. In fact, France reported a decrease in the prevalence and severity of toxoplasmosis after the implementation of mandatory gestational screening, with standardized screening and treatment protocols in addition to public awareness.
To detect T. gondii infection, many techniques were used and varied widely between the five countries. In general, there was a lack of diversity in techniques and only serological methods were used to detect human and animal infection. This could be explained by the lack of molecular diagnostic tools especially in state laboratories, and the limited information regarding contributing risk factors [46].
For human infection, there was broad diversity in sampling in the five countries. Only in Egypt, a variety of animal species was screened for the presence of T. gondii (turkeys, ducks, chicken, ostriches, sheep, goats, etc.). This is a concern since T. gondii infection is widespread in food animals consumed in North Africa, especially chicken, camel, sheep, and goat meat [123]. In both human and animal T. gondii infection, there was heterogeneity in the molecular and serological prevalences estimated in the five North African countries, which is correlated with the techniques used. In fact, serological methods appear to lack sensitivity and specificity, even though the qualitative detection of antibodies remains a standard tool. At the same time, there are differences within the serological techniques. Moreover, Dubey et al. [39] found that the diagnostic performance of a MAT was higher than that of ELISA.
In many studies conducted in the five countries of North Africa, age was a major risk factor. Higher levels of positive results were found in older animals. This is consistent with many studies conducted in France and Iran for example [44, 66]. The higher prevalence in adult compared to younger animals may be explained by the longer period of exposure [123].
Farm management is also a risk factor. For example, in Algeria, sheep are reared in extensive systems and fed on fresh bulk feed or pasture, which are a greater risk as sources of contamination [36].
Generally, in North Africa, trends indicate that production systems have become more intensive: agricultural by-products, non-conventional sources of feed, and commercial concentrates are increasingly used. The use of concentrates represents a risk factor since contaminated grain could be responsible for the rapid spread of infection in a flock [101]. Widespread oocyst contamination in the environment is also due to fecal contamination of soil and groundwater by either domestic or feral cats [123].
Since animals play an important role in the transmission of T. gondii to humans via meat or milk consumption, or by the prominent role of cats in the contamination of the environment by oocysts, studying prevalence rates of animal toxoplasmosis will be helpful to estimate the rate of human toxoplasmosis [66].
In the five North African countries studied, little is known about the epidemiology of toxoplasmosis in wild animals. In fact, there is no information about T. gondii prevalence in wild felids.
Concerning the evolutionary history of Toxoplasma, the presence of T. gondii in Africa could be due to the spread of this parasite from the Americas to Asia via the Bering Strait. It is believed that this parasite entered Africa around 1.5 million years ago [20]. Only genotypes II and III have been identified in North Africa among the three archetypal lineage types I, II, and III. Apart from their presence in North Africa, types II and III are the main lineages in the Middle East, Europe, and North America [59].
The similarities in T. gondii infection patterns between the populations of the Mediterranean basin could be explained by human travel and trade within these regions [59, 91]. Moreover, the emergence of clonal lineages II and III coincided with the advent of agriculture 10,000 years ago and cat domestication in the eastern Mediterranean basin [21, 118, 124].
In North African countries, no specific national programs against toxoplasmosis are currently in place. The major tool for avoiding congenital T. gondii infections and their complications is prevention. The preventive measures depend strongly on the knowledge of women about toxoplasmosis. However, this remains a major problem in the North African context since, within the same country, there are considerable differences in the socioeconomic status of women.
Even though serological screening for the infection is highly recommended during the first antenatal care visit, an analysis of the current situation indicates that a control program for human toxoplasmosis is lacking and pregnant women are not sufficiently aware of all the infection routes.
Conclusion
Toxoplasmosis represents a significant health threat to both humans and livestock, inducing high morbidity and economic losses. While the occurrence of T. gondii is fairly well documented in most countries, little information is available to quantify the resulting impact for the livestock sector and for public health. Having better impact data would make it easier to convince decision makers to invest in toxoplasmosis control and prevention. In addition, more in-depth epidemiological studies are needed to inform the design of regional strategies and to guide implementation of control programs involving both the medical and veterinary sectors.
Given the involvement of the environment in the transmission cycle, attention should also be given to environmental sampling in order to develop adequate transmission models between animals, the environment and people, providing the basis for a real One Health approach in the control of toxoplasmosis.
Acknowledgments
This work was supported by the Laboratoire d’Épidémiologie des Infections Enzootiques des Herbivores en Tunisie : Application à la Lutte (Ministère de l’Enseignement Supérieur et de la Recherche Scientifique, Tunisia). This study was also partly supported by the CGIAR Research Program on Livestock (CRP Livestock).
Cite this article as: Rouatbi M, Amairia S, Amdouni Y, Boussaadoun MA, Ayadi O, Al-Hosary AA, Rekik M, Ben Abdallah RB, Aoun K, Darghouth MA, Wieland B & Gharbi M. 2019. Toxoplasma gondii infection and toxoplasmosis in North Africa: a review. Parasite 26, 6.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Addebbous A, Adarmouch L, Tali A, Laboudi M, Amine M, Aajly L, Rhajaoui M, Chabaa L, Zougaghi L. 2012. IgG anti-Toxoplasma antibodies among asymptomatic HIV-infected patients in Marrakesh-Morocco. Acta Tropica, 123, 49–52. [DOI] [PubMed] [Google Scholar]
- 2.Abdelhadi FZ, Abdelhadi SA, Niar A, Benallou B, Meliani S, Smail NL, Mahmoud D. 2015. Abortions in cattle on the level of Tiaret Area (Algeria). Global Veterinaria, 14, 638–645. [Google Scholar]
- 3.Abdel-Hameed DM, Hassanein OMA. 2008. Genotyping of Toxoplasma gondii strains from female patients with toxoplasmosis. Journal of the Egyptian Society of Parasitology, 38, 511–520. [PubMed] [Google Scholar]
- 4.Ahmed HA, Shafik SM, Ali MEM, Elghamry ST, Ahmed AA. 2014. Molecular detection of Toxoplasma gondii DNA in milk and risk factors analysis of seroprevalence in pregnant women at Sharkia, Egypt. Veterinary World, 7, 594–600. [Google Scholar]
- 5.Ajzenberg D, Lamaury I, Demar M, Vautrin C, Cabié A, Simon S, Nicolas M, Desbois-Nogard N, Boukhari R, Riahi H, Dardé ML, Massip P, Dupon M, Preux PM, Labrunie A, Boncoeur MP. 2016. Performance testing of PCR assay in blood samples for the diagnosis of toxoplasmic encephalitis in AIDS patients from the French departments of America and genetic diversity of Toxoplasma gondii: A prospective and multicentric study. PLoS Neglected Tropical Diseases, 10(6), e0004790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Kappany YM, Rajendran C, Ferreira LR, Kwok OCH, Abu-Elwafa SA, Hilali M, Dubey JP. 2010. High prevalence of toxoplasmosis in cats from Egypt: isolation of viable Toxoplasma gondii, tissue distribution, and isolate designation. Journal of Parasitology, 96, 1115–1118. [DOI] [PubMed] [Google Scholar]
- 7.Al-Kappany YM, Lappin MR, Kwok OCH, Abu-Elwafa SA, Hilali M, Dubey JP. 2011. Seroprevalence of Toxoplasma gondii and concurrent Bartonella spp., feline immunodeficiency virus, feline leukemia virus, and Dirofilaria immitis infections in Egyptian cats. Journal of Parasitology, 97, 256–258. [DOI] [PubMed] [Google Scholar]
- 8.Al-mabruk AA, Somia RA, El-Buni AA, Annajar BB, Elsaid MMA. 2013. Seroprevalence of Toxoplasma gondii antibodies in sheep from Libya. International Journal of Advanced Research, 1, 148–154. [Google Scholar]
- 9.Amairia S, Rouatbi M, Rjeibi MR, Nouasri H, Sassi L, Mhadhbi M, Gharbi M. 2016. Molecular prevalence of Toxoplasma gondii DNA in goats’ milk and seroprevalence in Northwest Tunisia. Veterinary Medicine and Science, 2, 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amdouni Y, Rjeibi MR, Rouatbi M, Amairia S, Awadi S, Gharbi M. 2017. Molecular detection of Toxoplasma gondii infection in slaughtered ruminants (sheep, goats and cattle) in Northwest Tunisia. Meat Science, 133, 180–184. [DOI] [PubMed] [Google Scholar]
- 11.Amrei MA, Al-Hamshary AM, Fotoh OA, Abdel-Rahman S. 1999. Studies on prenatal infections in children with unknown cause of mental retardation and examination of their mothers. Journal of the Egyptian Society of Parasitology, 29, 59–67. [PubMed] [Google Scholar]
- 12.Ashmawy KI, Abuakkada SS, Awad AM. 2011. Seroprevalence of antibodies to Encephalitozoon cuniculi and Toxoplasma gondii in farmed domestic rabbits in Egypt. Zoonoses Public Health, 58, 357–364. [DOI] [PubMed] [Google Scholar]
- 13.Azab ME, Kamel AM, Makled KM, Khattab H, El-Zayyat EA, Abo-Amer EA, Samy G. 1992. Naturally occurring Toxoplasma antibodies in serum and milk of lactating women. Journal of the Egyptian Society of Parasitology, 22, 561–568. [PubMed] [Google Scholar]
- 14.Azwai SM, El-Gammoudi FT, Gameel SEAM. 1993. A serological survey of toxoplasmosis in some animal species in Libya. Alex. J. Vet. Sci., 9(3), 133–135. [Google Scholar]
- 15.Bachi F, Yebbous-Bensaid SA, Gourbdji E, Dardé ML. 2018. First molecular characterization of two Algerian strains of Toxoplasma gondii isolated from two congenital toxoplasmosis case patients. Médecine et Maladies Infectieuses, 48, 78–80. [DOI] [PubMed] [Google Scholar]
- 16.Balozet L. 1955. Enquête sérologique sur la toxoplasmose de l’homme et du chien dans la région d’Alger. Archives de l’Institut Pasteur d’Alger, 33, 78–83. [PubMed] [Google Scholar]
- 17.Barkat A, Kabiri M, Tligui H, Bouazzaoui LN. 2010. Seroprevalence of toxoplasmosis in Morocco. Pediatric Research, 68, 486–487. [Google Scholar]
- 18.Barakat AM, Salem LMA, El-Newishy AMA, Shaapan RM, El-Mahllawy EK. 2012. Zoonotic chicken toxoplasmosis in some Egyptians governorates. Pakistan Journal of Biological Sciences, 15, 821–826. [DOI] [PubMed] [Google Scholar]
- 19.Bchir A, Jebara H, Soltani M, Ennigrou S, Kheder M, Brahim H, Jeddi M. 1992. Séroépidémiologie de la toxoplasmose chez les femmes enceintes dans la région de Monastir (Tunisie). Médecine et Maladies Infectieuses, 22, 951–953. [Google Scholar]
- 20.Bertranpetit E, Jombart T, Paradis E, Pena H, Dubey J, Su C, Mercier A, Devillard S, Ajzenberg D. 2017. Phylogeography of Toxoplasma gondii points to a South American origin. Infection, Genetics and Evolution, 48, 150–155. [DOI] [PubMed] [Google Scholar]
- 21.Boyle JP, Rajasekar B, Saeij JPJ, Ajioka JW, Berriman M, Paulsen I, Roos DS, Sibley LD, White MW, Boothroyd JC. 2006. Just one cross appears capable of dramatically altering the population biology of a eukaryotic pathogen like Toxoplasma gondii. Proceedings of the National Academy of Sciences of the United States of America, 103, 10514–10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buxton D, Thomson K, Maley S, Wright S, Bos HJ. 1991. Vaccination of sheep with a live incomplete strain (S48) of Toxoplasma gondii and their immunity to challenge when pregnant. Veterinary Record, 129, 89–93. [DOI] [PubMed] [Google Scholar]
- 23.Buxton D, Finlayson J. 1986. Experimental infection of pregnant sheep with Toxoplasma gondii: pathological and immunological observations on the placenta and foetus. Journal of Comparative Pathology, 96, 319–333. [DOI] [PubMed] [Google Scholar]
- 24.Belbacha I, Hafid J, Tran Manh Sung R, Flori P, Raberin H, Aboufatima R, Regragui A, Dalal A, Chait A. 2004. Toxoplasma gondii: level of carriage in sheep of Marrakech region (Mnabha). Schweizer Archiv für Tierheilkunde, 146, 561–564. [DOI] [PubMed] [Google Scholar]
- 25.Ben Abdallah R, Aoun K, Siala E, Souissi O, Maatoug R, Hlioui S, Bouratbine A. 2008. Congenital toxoplasmosis: clinical and biological analysis of 11 cases in Tunisia. Archives de Pédiatrie, 16, 118–121. [DOI] [PubMed] [Google Scholar]
- 26.Ben Abdallah R, Siala E, Bouafsoun A, Maatoug R, Souissi O, Aoun K, Bouratbine A. 2013. Toxoplasmosis mother-to-child screening: study of cases followed in the Pasteur Institute of Tunis (2007–2010). Bulletin de la Société de Pathologie Exotique, 106, 108–112. [DOI] [PubMed] [Google Scholar]
- 27.Ben Ayed Nouira N, Hafsia S, Khaled S, Zhioua F, Ferchiou M, Jedoui A, Meriah S, Ben Rachid MS. 1994. Incidence of toxoplasmosis during pregnancy and risk of fetal infection. Tunisie Médicale, 72, 487–491. [PubMed] [Google Scholar]
- 28.Benkirane A, Jabli N, Rodolakis A. 1990. Fréquence d’avortement et séroprévalence des principales maladies infectieuses abortives ovines dans la région de Rabat (Maroc). Annales de Recherches Vétérinaires, 21(4), 267–273. [PubMed] [Google Scholar]
- 29.Benkirane A, Essamkaoui S, El Idrissi A, Lucchese L, Natale A. 2015. Indagine sierologica sulle più comuni cause di aborto infettivo nei piccoli ruminanti in Marocco. Veterinaria Italiana, 51, 25–30. [DOI] [PubMed] [Google Scholar]
- 30.Ben Rachid MS, Blaha R. 1970. Human and animal toxoplasmosis in Tunisia. Tunisie Médicale, 48, 101–110. [PubMed] [Google Scholar]
- 31.Berredjem H, Aouras H, Benlaifa M, Becheker I, Djebar MR. 2017. Contribution of IgG avidity and PCR for the early diagnosis of toxoplasmosis in pregnant women from the North-Eastern region of Algeria. African Health Sciences, 17, 647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biava MF, Jana M, El Mansouri A, Percebois G. 1983. Etude séro-épidémiologique de la toxoplasmose à Marrakech (Maroc). Médecine et Maladies infectieuses, 13, 503–506. [Google Scholar]
- 33.Boughattas S, Bergaoui R, Essid R, Aoun K, Bouratbine A. 2011. Seroprevalence of Toxoplasma gondii infection among horses in Tunisia. Parasites & Vectors, 4, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boughattas S, Ayari K, Sa T, Aoun K, Bouratbine A. 2014. Survey of the parasite Toxoplasma gondii in human consumed ovine meat in Tunis City. PLoS One, 9, e85044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouratbine A, Siala E, Chahed MK, Aoun K, Ben Ismail R. 2001. Sero-epidemiologic profile of toxoplasmosis in northern Tunisia. Parasite, 8, 61–66. [DOI] [PubMed] [Google Scholar]
- 36.Dahmani A, Harhoura K, Aissi M, Zenia S, Hamriouri B, Guechi N, Ait Athmane M, Kadour R. 2018. The zoonotic protozoan of sheep carcasses in the north of Algeria: A case of ovine toxoplasmosis. Journal of the Hellenic Veterinary Medical Society, 69, 1004–1012. [Google Scholar]
- 37.Dechicha AS, Bachi F, Gharbi I, Gourbdji E, Ammi DB, Errahmani MB, Guetarni D. 2015. Sero-epidemiological survey on toxoplasmosis in cattle, sheep and goats in Algeria. African Journal of Agricultural Research, 10, 2113–2119. [Google Scholar]
- 38.do Nascimento LC, Pena HFJ, Leite Filho RV, Argenta FF, Alves BF, Oliveira S, Gennari SM, Driemeier D. 2017. Rare case of acute toxoplasmosis in a domestic rabbit (Oryctolagus cuniculus) in Brazil associated with the type Br III Brazilian clonal lineage of Toxoplasma gondii. Parasitology Research, 116, 2873–2876. [DOI] [PubMed] [Google Scholar]
- 39.Dubey JP, Lappin MR, Thulliez P. 1995. Long-term antibody responses of cats fed Toxoplasma gondii tissue cysts. Journal of Parasitology, 81, 887–893. [PubMed] [Google Scholar]
- 40.Dubey JP. 2009. History of the discovery of the life cycle of Toxoplasma gondii. International Journal of Parasitology, 39, 877–882. [DOI] [PubMed] [Google Scholar]
- 41.Dubey JP. 2010. Toxoplasmosis of animals and humans, 2nd edn CRC Press Inc, Boca Raton, New York. [Google Scholar]
- 42.Dubey JP, Graham DH, Dahl E, Hilali M, El-Ghaysh A, Sreekumar C, Kwok OCH, Shen SK, Lehmann T. 2003. Isolation and molecular characterization of Toxoplasma gondii from chickens and ducks from Egypt. Veterinary Parasitology, 114, 89–95. [DOI] [PubMed] [Google Scholar]
- 43.Dubey JP, Lindsay DS, Speer CA. 1998. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clinical Microbiology Reviews, 11, 267–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dumètre A, Ajzenberg D, Rozette L, Mercier A, Dardé ML. 2006. Toxoplasma gondii infection in sheep from Haute-Vienne, France, seroprevalence and isolate genotyping by microsatellite analysis. Veterinary Parasitology, 142, 376–379. [DOI] [PubMed] [Google Scholar]
- 45.Behairy AM, Choudhary S, Ferreira LR, Kwok OCH, Hilali M, Su C, Dubey JP. 2013. Genetic characterization of viable Toxoplasma gondii isolates from stray dogs from Giza, Egypt. Veterinary Parasitology, 193, 25–29. [DOI] [PubMed] [Google Scholar]
- 46.El Bissati K, Levigne P, Lykins J, Adlaoui EB, Barkat A, Berraho A, Laboudi M, El Mansouri B, Ibrahimi A, Rhajaoui M, Quinn F, Murugesan M, Seghrouchni F, Enrique Gómez-Marín J, Peyron F, McLeod R. 2012. Global initiative for congenital toxoplasmosis: an observational and international comparative clinical analysis. Emerging Microbes and Infections, 7, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El Deeb HK, Salah-Eldin H, Khodeer S, Allah AA. 2012. Prevalence of Toxoplasma gondii infection in antenatal population in Menoufia governorate, Egypt. Acta Tropica, 124, 185–191. [DOI] [PubMed] [Google Scholar]
- 48.El-Ghaysh A. 1998. Seroprevalence of Toxoplasma gondii in Egyptian donkeys using ELISA. Veterinary Parasitology, 80, 71–73. [DOI] [PubMed] [Google Scholar]
- 49.El-Gomati KM, Rashed AM, El Naas AS, Elsaid MMA, EL-Attar SR. 2010. A study on Toxoplasma gondii in mice (Mus musculus) in Tripoli - Libya. African Journal of Biology, 6, 123–126. [Google Scholar]
- 50.El-Gozamy BR, Mohamed SA, Mansour HAM. 2009. Toxoplasmosis among pregnant women in Qualyobia Governorate, Egypt. Journal of the Egyptian Society of Parasitology, 39, 389–401. [PubMed] [Google Scholar]
- 51.El-Madawy SR, Metawea FY. 2013. Serological assays and PCR for detection of Toxoplasma gondii infection in an ostrich farm at Ismailia Province, Egypt. IOSR Journal of Agriculture and Veterinary Science, 2, 56–60. [Google Scholar]
- 52.El Mansouri B, Rhajaoui M, Sebti F, Amarir F, Laboudi M, Bchitou R, Hamad M, Lyagoubi M. 2007. Seroprevalence of toxoplasmosis in pregnant women in Rabat, Morocco. Bulletin de la Société de Pathologie Exotique, 100, 289–290. [PubMed] [Google Scholar]
- 53.Elsaid MMA, Azbedah AG, Dia Eddin E EL-Alem, Alkout A. 2014. The prevalence of Toxoplasma gondii infection in psychiatric patients in Tripoli, Libya. Journal of American Science, 10, 135–140. [Google Scholar]
- 54.Elsheikha HM, Aboul-Dahab MAO, Abdel Maboud AI, El-Sherbini ET. 2009. Prevalence and risk factors of Toxoplasma gondii antibodies in asymptomatic Egyptian blood donors. Journal of the Egyptian Society of Parasitology, 39, 351–361. [PubMed] [Google Scholar]
- 55.Endlicher W. 2000. Mittelmeerländer, in Regionale Klimatologie: Die Alte Welt: Europa. Weischet W, Endlicher W, Editors. Teubner: Afrika, Asien: p. 153–182. [Google Scholar]
- 56.Fakhfakh N, Kallel K, Ennigro S, Kaouech E, Belhadj S, Chaker E. 2013. Risk factors for Toxoplasma gondii and immune status of pregnant women: cause and effect? Tunisie Médicale, 91, 188–190. [PubMed] [Google Scholar]
- 57.FAO. 2013. Bureau sous-régional de la FAO pour l’Afrique du Nord, Les maladies animales transfrontalières : les maladies à fort impact socioéconomique. Lettre d’Information, N°3, , 3ème trimestre Available at http://www.onu-tn.org/uploads/documents/13939229080.pdf [Google Scholar]
- 58.FAO. 2016. Livestock contribution to food security in the near East and North Africa. 33 rd session. Rome, Italy, 9–13 May. [Google Scholar]
- 59.Galal L, Ajzenberg D, Hamidović A, Durieux MF, Dardé ML, Mercier A. 2018. Toxoplasma and Africa: One parasite, two opposite population structures. Trends in Parasitology, 34, 140–154. [DOI] [PubMed] [Google Scholar]
- 60.Gamal MAB, Jaroud RB. 2015. Seroprevalence study of IgG antibodies to Toxoplasma, and risk factors for Toxoplasma infestation among pregnant women in Alkhoms state, Libya. Lebda Medical Journal, 1, 15–19. [Google Scholar]
- 61.Gashout A, Amro A, Erhuma M, Al-Dwibe H, Elmaihub E, Babba H, Nattah N, Abudher A. 2016. Molecular diagnosis of Toxoplasma gondii infection in Libya. BMC Infectious Diseases, 16, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gharbi M, Zribi L, Jedidi M, Chakkhari H, Hamdi S, R’hayem S, Zribi N, Souli M, Darghouth MA. 2013. Prevalence of Toxoplasma gondii infection in Tunisian sheep. Bulletin de la Société de Pathologie Exotique, 106, 184–187. [DOI] [PubMed] [Google Scholar]
- 63.Ghazy AA, Shaapan RM, Abdel-Rahman EH. 2007. Comparative serological diagnosis of toxoplasmosis in horses using locally isolated Toxoplasma gondii. Veterinary Parasitology, 145, 31–36. [DOI] [PubMed] [Google Scholar]
- 64.Ghoneim NH, Shalaby SI, Hassanain NA, Zeedan GSG, Soliman YA, Abdalhamed AM. 2010. Comparative study between serological and molecular methods for diagnosis of toxoplasmosis in women and small ruminants in Egypt. Foodborne Pathogens Disease, 7, 17–22. [DOI] [PubMed] [Google Scholar]
- 65.Guessous-Idrissi N, Lahlou D, Sefiani R, Benmira A. 1984. La toxoplasmose et la rubéole chez la femme marocaine : résultats d’une enquête sérologique. Pathologie Biologie, 32, 761–765. [PubMed] [Google Scholar]
- 66.Guo M, Dubey JP, Hill D, Buchanan RL, Gamble HR, Jones JL, Pradhan AK. 2015. Prevalence and risk factors for Toxoplasma gondii infection in meat animals and meat products destined for human consumption. Journal of Food Products, 78, 457–476. [DOI] [PubMed] [Google Scholar]
- 67.Haq SZ, Abushahama MS, Gerwash O, Hughes JM, Wright EA, Elmahaishi MS, Lun ZR, Thomasson D, Hide G. 2016. High frequency detection of Toxoplasma gondii DNA in human neonatal tissue from Libya. Transactions of the Royal Society of Tropical Medicine and Hygiene, 110, 551–557. [DOI] [PubMed] [Google Scholar]
- 68.Harfoush M, Tahoon AEN. 2010. Seroprevalence of Toxoplasma gondii antibodies in domestic ducks, free-range chickens, turkeys and rabbits in Kafr El-Sheikh Governorate Egypt. Journal of the Egyptian Society of Parasitology, 40, 295–302. [PubMed] [Google Scholar]
- 69.Haridy FM, Saleh NMK, Khalil HHM, Morsy TA. 2010. Anti-Toxoplasma gondii antibodies in working donkeys and donkey’s milk in greater Cairo, Egypt. Journal of the Egyptian Society of Parasitology, 40, 459–464. [PubMed] [Google Scholar]
- 70.Hayde M, Pollak A. 2000. Clinical picture: neonatal signs and symptoms, in Congenital toxoplasmosis: scientific background, clinical management and control. Ambroise-Thomas P, Petersen E, Editors. Springer-Verlag France: Paris: p. 153–164. [Google Scholar]
- 71.Hilali M, Romand S, Thulliez P, Kwok OCH, Dubey JP. 1998. Prevalence of Neospora caninum and Toxoplasma gondii antibodies in sera from camels from Egypt. Veterinary Parasitology, 75, 269–271. [DOI] [PubMed] [Google Scholar]
- 72.Ibrahim BB, Salama MM, Gawish NI, Haridy FM. 1997. Serological and histopathological studies on Toxoplasma gondii among the workers and the slaughtered animals in Tanta Abattoir, Gharbia Governorate. Journal of the Egyptian Society of Parasitology, 27, 273–278. [PubMed] [Google Scholar]
- 73.Ibrahim HM, Huang P, Salem TA, Talaat RM, Nasr MI, Xuan X, Nishikawa Y. 2009. Prevalence of Neospora caninum and Toxoplasma gondii antibodies in Northern Egypt. American Journal of Tropical Medicine and Hygiene, 80, 263–267. [PubMed] [Google Scholar]
- 74.Ibrahim HM, Mohamed AH, El-Sharaawy AA, El-Shqanqery HE. 2017. Molecular and serological prevalence of Toxoplasma gondii in pregnant women and sheep in Egypt. Asian Pacific Journal of Tropical Medicine, 10, 996–1001. [DOI] [PubMed] [Google Scholar]
- 75.Jemni L, Faurant C, Heyer F, Bchir A, Braham MS, Ayachi S, Djaidane A, Bouzakoura C, Lapierre J. 1985. Seroepidemiologic study of toxoplasmosis in the school population of Sousse. Bulletin de la Société de Pathologie Exotique, 78, 810–814. [PubMed] [Google Scholar]
- 76.Kamal AM, Ahmed AK, Abdellatif MZM, Tawfik M, Hassan EE. 2015. Seropositivity of toxoplasmosis in pregnant women by ELISA at Minia University Hospital. Egypt. Korean Journal of Parasitology, 53, 605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kassem HH, Morsy TA. 1991. The prevalence of anti-Toxoplasma antibodies among pregnant women in Benghazi, (S.P.L.A.J.) Libya. Journal of the Egyptian Society of Parasitology, 21, 69–74. [PubMed] [Google Scholar]
- 78.Khadre MA, El Nageh MM. 1987. Serological survey for toxoplasmosis in Tripoli, S.P.L.A.J. Libya. Transactions of the Royal Society of Tropical Medicine and Hygiene, 81, 761–763. [DOI] [PubMed] [Google Scholar]
- 79.Khayeche M, Mhadhbi M, Gharbi M, Nasfi I, Darghouth MA. 2013. Detection of Toxoplasma gondii infection of sheep slaughtered in the governorate of Sousse on the occasion of the Muslim sacrifice feast (Eid Al-Adha) and analysis of risk factors. Bulletin de la Société de Pathologie Exotique. DOI: https://doi.org/10.1007/s13149-014-0325-6. [DOI] [PubMed] [Google Scholar]
- 80.Kennou MF, Bayar N, Rekhis M. 1978. Serodiagnosis of toxoplasmosis in man. Archives de l’Institut Pasteur de Tunis, 55, 1–7. [PubMed] [Google Scholar]
- 81.Kennou MF. 1982. Epidemiology of toxoplasmosis in pregnant Tunisian women. Archives de l’Institut Pasteur de Tunis, 59, 205–211. [PubMed] [Google Scholar]
- 82.Kennou MF. 1983. Parasitoses of food origin in Tunisia. Archives de l’Institut Pasteur de Tunis, 60, 393–407. [PubMed] [Google Scholar]
- 83.Laboudi M. 2017. Review of toxoplasmosis in Morocco: seroprevalence and risk factors for Toxoplasma infection among pregnant women and HIV-infected patients. Panafrican Medical Journal, 27, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Laboudi M, El Mansouri B, Rhajaoui M. 2014. The role of the parity and the age in acquisition of toxoplasmosis among pregnant women in Rabat. International Journal of Innovation and Applied Studies, 6, 488–492. [Google Scholar]
- 85.Laboudi M, El Mansouri B, Sebti F, Amarir F, Coppieters Y, Rhajaoui M. 2009. Risk factors of a positive serological test for toxoplasmosis in a pregnant woman in Morocco. Parasite, 16, 71–72. [DOI] [PubMed] [Google Scholar]
- 86.Lahmar I, Lachkhem A, Slama D, Sakly W, Haouas N, Gorcii M, Pfaff AW, Candolfi E, Babba H. 2015. Prevalence of toxoplasmosis in sheep, goats and cattle in Southern Tunisia. Bacteriology & Parasitology, 6, 245. [Google Scholar]
- 87.Le Viguelloux J, Epardeau B. 1969. Indirect immunofluorescent reactions in epidemiological studies. A preliminary study on toxoplasmosis. Médecine Tropicale, 29, 76–83. [PubMed] [Google Scholar]
- 88.Lionello P, Malanotte P, Boscolo R. 2006. Mediterranean climate variability. Elsevier: Amsterdam. [Google Scholar]
- 89.Livingston SE. 2009. The nutrition and natural history of the Serval (Felis serval) and Caracal (Caracal caracal). Veterinary Clinics of North America: Exotic Animal Practice, 12, 327–334. [DOI] [PubMed] [Google Scholar]
- 90.Mahmoud H, Saedi Dezaki E, Soleimani S, Baneshi MR, Kheirandish F, Ezatpour B, Zia-Ali N. 2015. Seroprevalence and risk factors of Toxoplasma gondii infection among healthy blood donors in southeast of Iran. Parasite Immunology, 37, 362–367. [DOI] [PubMed] [Google Scholar]
- 91.Mercier A, Ajzenberga D, Devillard S, Demar MP, de Thoisy B, Bonnabau H, Collinet F, Boukhari R, Blanchet D, Simon S, Carme B, Dardé ML. 2011. Human impact on genetic diversity of Toxoplasma gondii: Example of the anthropized environment from French Guiana. Infection, Genetics and Evolution, 11(6), 1378–1387. DOI: 10.1016/j.meegid.2011.05.003 [DOI] [PubMed] [Google Scholar]
- 92.Messedi-Triki S, Memmi A, Ben Rachid MS. 1982. Aspects épidémiologiques de la toxoplasmose en Tunisie. Le Pharmacien du Maghreb, 2, 105–108. [Google Scholar]
- 93.Messerer L, Bouzbid S, Gourbdji E, Mansouri R, Bachi F. 2014. Séroprévalence de la toxoplasmose chez les femmes enceintes dans la wilaya d’Annaba, Algérie. Revue d’Épidémiologie et de Santé Publique, 62, 160–165. [DOI] [PubMed] [Google Scholar]
- 94.Mohamed-Cherif A, Ait-Oudhia K, Khelef D. 2015. Detection of anti-Toxoplasma gondii antibodies among horses (Equus caballus) and donkeys (Equus asinus) in Tiaret province, northwestern Algeria. Revue de Médecine Vétérinaire, 166, 271–274. [Google Scholar]
- 95.Mukherjee S, Krishnan A, Tamma K, Home C, Navya R, Joseph S, Das A, Ramakrishnan U. 2010. Ecology driving genetic variation: A comparative phylogeography of Jungle Cat (Felis chaus) and Leopard Cat (Prionailurus bengalensis) in India. PLoS One, 29(5), e13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nejmi S, Alami S. 1973. Étude immunologique de la toxoplasmose dans la population marocaine par la réaction d’immunofluorescence indirecte (1026 sérums). Maroc Médical, 572, 561–568. [Google Scholar]
- 97.OIE. 2012. Organisation Mondiale de la Santé Animale, Santé animale mondiale. Volume 1, vol. 1 & 2: ISBN 978-92-9044-901-0, ISBN 978-92-9044-902-7, ISSN p. 1017-3102. [Google Scholar]
- 98.Pappas G, Roussos N, Falagas ME. 2009. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. International Journal of Parasitology, 39, 1385–1394. [DOI] [PubMed] [Google Scholar]
- 99.Pardini L, Bernstein M, Carral LA, Kaufer FJ, Dellarupe A, Gos ML, Campero LM, Moré G, Messina MT, Schneider MV, Freuler CB, Durlach RA, Unzaga JM, Venturini MC. 2018. Congenital human toxoplasmosis caused by non-clonal Toxoplasma gondii genotypes in Argentina. Parasitology International, 68, 48–52. [DOI] [PubMed] [Google Scholar]
- 100.Pas A, Dubey JP. 2008. Fatal toxoplasmose in sand cats (Felis margarita). Journal of Zoo and Wildlife Medicine, 39, 362–369. [DOI] [PubMed] [Google Scholar]
- 101.Plant JW, Richardson N, Moyle GG. 1974. Toxoplasma infection and abortion in sheep associated with feeding of grain contaminated with cat faeces. Australian Veterinary Journal, 50, 19–21. [DOI] [PubMed] [Google Scholar]
- 102.Rajendran C, Su C, Dubey JP. 2012. Molecular genotyping of Toxoplasma gondii from Central and South America revealed high diversity within and between populations. Infection, Genetics and Evolution, 12, 359–368. [DOI] [PubMed] [Google Scholar]
- 103.Remington JS, Desmonts G. 1990. Toxoplasmosis, in Infectious diseases of the fetus and newborn infant, vol 3, Remington JS, Klein JO, Editors. WB Saunders: Philadelphia, PA: pp. 89–195. [Google Scholar]
- 104.Remington JS, McLeod R, Desmonts G. 1995. Toxoplasmosis, in Infectious diseases of the fetus and newborn infant, vol 4, Remington JS, Klein JO, Editors. WB Saunders: Philadelphia, PA: p. 140–267. [Google Scholar]
- 105.Rifaat MA, Salem SA, Sadek MS, Azab ME, Abdel-Ghaffar FM, Baki MH. 1976. Toxoplasmosis: serological surveys in farm and domestic animals in Egypt (preliminary report). Journal of Egyptian Public Health Association, 51, 258–275. [PubMed] [Google Scholar]
- 106.Robert-Gangneux F, Dardé ML. 2012. Epidemiology of and diagnostic strategies for toxoplasmosis. Clinical Microbiology Reviews, 25, 264–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rouatbi M, Amdouni Y, Amairia S, Rjeibi MR, Sammoudi S, Rekik M, Gharbi M. 2017. Molecular detection and phylogenetic analyses of Toxoplasma gondii from naturally infected sheep in Northern and Central Tunisia. Veterinary Medicine and Science, 3, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ruge H. 1952. Toxoplasmosis; a new disease. Journal of the Egyptian Veterinary Medical Association, 35, 337–338. [PubMed] [Google Scholar]
- 109.Sawadogo P, Hafid J, Bellete B, Sung RTM, Chakdi M, Flori P, Raberin H, Hamouni IB, Chait A, Dalal A. 2005. Seroprevalence of T. gondii in sheep from Marrakech, Morocco. Veterinary Parasitology, 130, 89–92. [DOI] [PubMed] [Google Scholar]
- 110.Schneider R, Tabet-Derraz O, Dedet JP, Belkaid M, Lamri I. 1977. Serodiagnosis of 2,438 cases of toxoplasmosis by immunofluorescence at the Pasteur Institute of Algeria. Epidemiological and clinical corollaries. Archives de l’Institut Pasteur d’Algérie, 52, 95–104. [PubMed] [Google Scholar]
- 111.Sellami H, Amri H, Cheikhrouhou F, Sellami A, Makni F, Trabelsi H, Trabelsi K, Guermazi M, Ayadi A. 2010. Toxoplasmosis in Sfax, Tunisia. Bulletin de la Société de Pathologie Exotique, 103, 37–40. [DOI] [PubMed] [Google Scholar]
- 112.Shaapan RM, El-Nawawi FA, Tawfik MA. 2008. Sensitivity and specificity of various serological tests for the detection of Toxoplasma gondii infection in naturally infected sheep. Veterinary Parasitology, 153, 359–362. [DOI] [PubMed] [Google Scholar]
- 113.Shalaka NS, Garred NA, Zeglam HT, Awasi SA, Abukathir LA, Altagdi ME, Rayes AA. 2015. Clinical profile and factors associated with mortality in hospitalized patients with HIV/AIDS: a retrospective analysis from Tripoli Medical Centre, Libya, 2013. La Revue de Santé de la Méditerranée Orientale, 21, 635–646. [DOI] [PubMed] [Google Scholar]
- 114.Siala E, Aoun K, Chahed MK, Bouratbine A. 2006. Difficulty in dating primary infections by Toxoplasma gondii in pregnant women in Tunisia. Tunisie Médicale, 84, 85–87. [PubMed] [Google Scholar]
- 115.Siala E, Ben Abdallah R, Laouiti F, Maatoug R, Souissi O, Aoun K, Bouratbine A. 2014. Toxoplasmic infections in pregnancy: about 94 cases diagnosed at the Pasteur Institute of Tunis. Gynécologie, Obstétrique & Fertilité, 42, 312–316. [DOI] [PubMed] [Google Scholar]
- 116.Spišák F, Reiterová K, Špilovská S, Dubinský P. 2010. Prevalence estimation and genotypization of Toxoplasma gondii in goats. Biologia, 65, 670–674. [Google Scholar]
- 117.Splendore A. 1908. Un nuovo protozoa parassita de’conigli. Incontrato nelle lesioni anatomiche d’une malattia che ricorda in molti punti il Kala-azar dell’uomo. Nota preliminare pel. Rev. Soc. Scient. Sao Paulo, 3, 109–112. [Google Scholar]
- 118.Su C, Evans D, Cole RH, Kissinger JC, Ajioka JW, Sibley LD. 2003. Recent expansion of Toxoplasma through enhanced oral transmission. Science, 299, 414–416. [DOI] [PubMed] [Google Scholar]
- 119.Sukthana Y. 2006. Toxoplasmosis: beyond animals to humans. Trends in Parasitology, 22, 137–142. [DOI] [PubMed] [Google Scholar]
- 120.Tenter AM, Heckeroth AR, Weiss LM. 2000. Toxoplasma gondii: from animals to humans. International Journal of Parasitology, 30, 1217–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tlamcani Z, Yahyaoui G, Mahmoud M. 2017. Prevalence of immunity to toxoplasmosis among pregnant women in University Hospital center Hassan II of Fez city (Morocco). Acta Medica International, 4, 43–45. [Google Scholar]
- 122.Uhlíková M, Hübner J. 1973. Congenital transmission of toxoplasmosis in domestic rabbits. Folia Parasitologica (Praha), 20, 285–291. [PubMed] [Google Scholar]
- 123.Tonouhewa ABN, Akpo Y, Sessou P, Adoligbe C, Yessinou E, Hounmanou YG, Assogba MN, Youssao I, Farougou S. 2017. Toxoplasma gondii infection in meat animals from Africa: Systematic review and meta-analysis of sero-epidemiological studies. Veterinary World, 10, 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vigne JD, Guilaine J, Debue K, Haye L, Gérard P. 2004. Early taming of the cat in Cyprus. Science, 80(304), 259. [DOI] [PubMed] [Google Scholar]
- 125.Uhl S. 2001. North African cuisine, in Food Ingredients Online, Available on https://www.foodingredientsonline.com/doc/north-african-cuisine-0001. Consulted [18/11/2018]. [Google Scholar]
- 126.White MW, Radke JR, Radke JB. 2014. Toxoplasma development – turn the switch on or off? Cellular Microbiology, 16, 466–472. [DOI] [PubMed] [Google Scholar]
- 127.Worldometers, Available on http://www.worldometers.info/world-population/libya-population/. Consulted [06/12/2017]. [Google Scholar]
- 128.Yan C, Liang LJ, Zheng KY. 2016. Impact of environmental factors on the emergence, transmission and distribution of Toxoplasma gondii. Parasite and Vectors, 9, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yekkour F, Aubert D, Mercier A, Murat JB, Khames M, Nguewa P, Ait- Oudhia K, Villena I, Bouchene Z. 2017. First genetic characterization of Toxoplasma gondii in stray cats from Algeria. Veterinary Parasitology, 239, 31–36. [DOI] [PubMed] [Google Scholar]
- 130.Youssef ME. 1993. Profile of toxoplasmosis in two different localities in Dakahlia Governorate. Journal of the Egyptian Society of Parasitology, 23, 423–430. [PubMed] [Google Scholar]