Abstract
Background
Epstein–Barr virus-encoded LMP1 plays a critical role in the carcinogenesis of nasopharyngeal carcinoma (NPC), but the mechanism remains elusive. We aimed to analyze the expression and clinical pathological significance of provirus integration site for Moloney murine leukemia virus 1 (Pim1) in clinical NPC, and to elucidate the effect of LMP1 on Pim1 expression and its mechanism.
Methods
Immunohistochemical staining was used to detect the expression of Pim1 in clinical NPC tissues and control nasopharyngeal chronic inflammation (NPI) tissues, and the correlation between Pim1 and clinical parameters of NPC patients was analyzed. The LMP1 stable expression cell line CNE1-LMP1-OV was constructed through infecting the well-differentiated nasopharyngeal carcinoma cells CNE1 with LMP1 overexpressing lentivirus. Then the in vivo experiments were conducted.
Results
Among 89 NPC patients, 48 cases (53.93%) were positive for Pim1, while only one case was Pim1 positive in 15 NPI controls (6.67%). Pim1 expression was not correlated with gender, age, smoking status and clinical classification of NPC patients, but positively correlated with T, N and M classification. CNE1-LMP1-OV cell line was successfully established, which displayed a higher cell proliferation ability and Pim1 expression. NF-κB inhibitor PDTC, PKC inhibitor GF109203X and STAT3 inhibitor Stattic significantly attenuated LMP1-induced Pim1 expression, and while AP-1 inhibitor SR11302 showed no inhibitory effect. Interestingly, Pim1 inhibitor quercetagetin significantly inhibited the proliferation of CNE1-LMP1-OV cells.
Conclusion
LMP1 mediates Pim1 expression through NF-κB, PKC and STAT3 signaling, which promotes the proliferation of NPC cells and participate in the clinical progression of NPC.
Keywords: nasopharyngeal carcinoma, Pim1, LMP1, cell proliferation
Introduction
Provirus integration site for Moloney murine leukemia virus 1 (Pim1) is one of the serine/threonine kinases. High Pim1 expression is tightly associated with clinical progression of many human cancers.1–4 To date, Pim1 functions in cell proliferation, migration, apoptosis, cell cycle progression, epithelial–mesenchymal transition (EMT) and synergizes with other chemotherapeutic agents in cancers.5–7 Thus, Pim1 is reported as a novel and potential target for cancer therapy. Increasing data indicate novel Pim1 specific inhibitors may be of interest in cancer therapy.8–10 To further clarify the role and mechanism of Pim1 in human cancers could be beneficial for promoting the translation of Pim1 target for cancer treatment.
Nasopharyngeal carcinoma (NPC) is a kind of regional malignant cancer that is common in Southern China, Southeast Asia and northern Africa. Due to tobacco control, changes in diets and economic development and advancements in diagnostic and radiotherapy techniques, the global trends in incidence and mortality have declined.11 Genetic susceptibility, and dietary and environmental factors such as Epstein–Barr virus (EBV) infection, are common causes of NPC.12 The present authors’ laboratory previously proved that many signaling abruptions were involved in the progression of NPC.13–16 These findings expand our insights into the pathogenesis of NPC. We also have explored the biological role of Pim1 in NPC and found that high expression of Pim1 contributes to the proliferation and migration of NPC cells,17 but we failed to clarify the mechanism of elevated Pim1 expression in NPC.
NPC is an EBV-associated carcinoma, and EBV-encoded LMP1 has been known to have oncogenic properties during type II latent infection in NPC.18 In this study, we hypothesized that LMP1 in NPC cells may regulate Pim1 expression through certain signaling pathways and then participate in NPC progression.
Materials and methods
Patients and ethical statement
Paraffin-embedded specimens were obtained from 104 patients at the Affiliated Gaozhou Hospital of Guangdong Medical University during 2008–2010. Patients had not received any preoperative radiotherapy or chemotherapy. Cases included NPC (n=89; 53 male and 36 female, with a median age of 44 years) and nasopharyngeal chronic inflammation (NPI) (n=15; 10 male and five female, with a median age of 46 years). Clinical data of the NPC patients were reviewed based on the pathological tumor-node-metastasis system (AJCC/UICC 2002). All NPC patients were diagnosed with non-keratinizing carcinoma following histological examination. The use of human tissue samples in this study was approved by the Ethics Council of the Affiliated Gaozhou Hospital of the Guangdong Medical University (Gaozhou, China) for Approval of Research Involving Human Subjects. Written informed consent was obtained from the patients whose tissue specimens were used for this research, and ethical guidelines under the Declaration of Helsinki were followed.
Immunohistochemistry
Immunohistochemistry was performed to test Pim1 protein expression in human NPC specimens by standard protocols as described previously.15,16 Primary antibody for Pim1 was purchased from Cell Signaling (Danvers, MA, USA; 1:50 in dilution). PBS substituted for Pim1 antibody was used as a blank control. Antigenic sites were visualized using PV9000 and DAB kits (Zhongsan Golden Bridge Biotech, Beijing, China). The immunoreactive score (IRS) of Pim1 was calculated as follows: 0, negative; 1, weak; 2, moderate; 3, strong. The percentage of positive cells was scored as 0, no positive cells; 1, 1–10% positive cells; 2, 11–50% positive cells and 3, >51% positive cells. Samples with a total IRS of 0–1 and ≥2 were considered to be (-) and (+) of Pim1 expression.
Cell culture
Well-differentiated human NPC cell line CNE1 (EBV-, presented by the Cancer Institute of Southern Medical University and its use ethically approved by Guangdong Medical University) was maintained in RPMI-1640 medium (HyClone, Beijing, China) supplemented with 10% FBS (HyClone), 100 U/mL penicillin and 100 µg/mL streptomycin. Cells were cultured at 37°C in a humidified atmosphere with 5% CO2. The medium was changed every 2 days. Cells in the logarithmic growth phase were used in related experiments.
Establishment and identification of LMP1 stable expression cell line
LMP1 (GenBank: X58140.1) overexpressing lentiviruses were designed and packaged by GeneCopeia (Guangzhou, China). This LMP1 overexpressing lentiviral vectors and negative control (NC) vectors were constructed based on lentiviral vector p-EZ-Lv201 (CMV-ORF-SV40-eGFP-IRES-Puro). CNE1 cells were seeded in 6-well plates overnight, and LMP1 stably expressed and NC lentiviral particles were added at the multiplicity of infection (MOI) of 20. After infection, the cells were cultured in an incubator at 37°C, 5% CO2, and saturated humidity. 12 hours later, complete medium was added allowing cell growth. Medium was changed every 48 hours. At 72 hours post infection, eGFP signals were observed under fluorescence microscope to judge the transfection efficiency. Then cells were screened with 2.0 µg/mL puromycin (Sigma-Aldrich Co., St Louis, MO, USA), and immunoblot was performed to determinate the expression of LMP1.
Drug treatment and grouping
To explore the mechanisms regarding LMP1 mediates Pim1 expression, we treated CNE1-LMP1-OV cells with inhibitors of LMP1 downstream signals. Cells were grouped into dimethyl sulfoxide (DMSO) (complete medium containing 0.1% DMSO, v/v), pyrrolidine dithiocarbamate (PDTC) (complete medium supplemented with 10 µmol/L NF-κB inhibitor, Beyotime Institute of Biotechnology, Haimen, China), GF109203X (complete medium supplemented with 10 µmol/L PKC inhibitor, Selleck, Houston, TX, USA), Stattic (complete medium supplemented with 10 µmol/L STAT3 inhibitor, Selleck) and SR11302 (complete medium supplemented with 10 µmol/L AP-1 inhibitor, ApexBio, Houston, TX, USA). Total protein was extracted after 48 hours, and the expression of Pim1 was detected by immunoblot. In order to clarify the effects of suppression of Pim1 activity on cell proliferation, CNE1-LMP1-OV cells were treated with Pim1 inhibitor quercetagetin19 (Cal Chemical Corp., Coventry, RI, USA), details were included in the following cell counting kit-8 (CCK-8) assay and plate clone formation assay.
RNA isolation, RT and quantitative PCR
Total RNA from cells were extracted with Trizol (Ambion, Foster City, CA, USA). 500 ng total RNA was used to generate cDNA using an RT kit (TaKaRa, Kusatsu, Japan) with an oligo (dT18) primer. Quantitative PCR was conducted using a Light-Cycler480 II instrument (Roche China, Guangzhou, China) as described previously.13,20 Primers (5′–3′) were synthesized by Sangon Biotech (Shanghai, China) as follows: Pim1 (NM_002648.3), 5′-CTTCGGCTCGGTCTACTCAG-3′, 5′-AGTGCCATTAGGCAGCTCTC-3 ′; β-actin (BC002409), 5′-TGACGTGGACATCCGCAAAG-3′, 5′-CTGGAAGGTGGACAGCGAGG-3′. The 20 µL PCR reaction system consisted of 10 µL of mixture containing SYBR, 2 µL of cDNA, 1.6 µL of upstream and downstream primers and 6.4 µL of d2H2O. For PCR analyses, the expression of β-actin served as a loading control. The PCR cycle parameters were performing pre-denaturation at 95°C for 5 minutes, followed by 45 cycles at 95°C for 10 seconds and 60°C for 30 seconds. The 2−ΔΔCt method was used to determine the levels of target gene expression.
Immunoblot
Immunoblot was performed as previously described.20 A total of 50 µg of protein was transferred onto PVDF membranes by electrophoretic transfer following electrophoretic separation by 12% SDS-PAGE. The antibodies used were rabbit-anti human Pim1 (1:1,000; Cell Signaling), rabbit-anti human PCNA (1:1,000; Cell Signaling), mouse-anti LMP1 (1:500; Abcam, Cambridge, UK) and rabbit-anti human β-actin (1:3,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The membranes were incubated overnight at 4°C, washed with tris-buffered saline-Tween (TBST), and incubated with HRP-labeled IgG (1:3,000; ProteinTech, Wuhan, China) for 2 hours at room temperature. After ECL luminescence, the target protein bands were scanned with gel imaging system (Tanon, Shanghai, China).
Immunofluorescence staining
Cells grown on coverslips and NPC specimens were subjected to indirect immunofluorescence to identify the expression and location of Pim1, PCNA and LMP1, which was performed as described previously.21 Images were captured using a laser scanning confocal microscope (TCS SP5 II; Leica Micro-systems, Wetzlar, Germany). For the antigen localization, fluorescein isothiocyanate (FITC)- or tetramethylrhodamine isothiocyanate (TRITC)-coupled IgGs (ProteinTech, Wuhan, China) were used. Nuclei were count stained with DAPI (Sigma-Aldrich).
Cell viability assessed by CCK-8 assay
Proliferation rates were determined using CCK-8 assays as described previously.16 Briefly, CNE1-LMP1-OV cells and CNE1-eGFP cells were seeded in 96-well plates (3,000 cells/per well), cells were treated with DMSO (0.1%, v/v) or 5.5 µmol/L quercetagetin for either 0, 24 or 48 hours, then a 100 µL mixture of CCK-8 reagent (Cat. #C0038, Beyotime Institute of Biotechnology) and RPMI-1640 (v/v=1/10) was added per well. Three hours later, the optical density was measured using a microplate reader (Multiskan MKS, Thermo Fisher Scientific, Waltham, MA, USA) in dual wavelength mode (450/630 nm). Each group was repeated in 6 wells, and data are presented as mean ± SEM of 3 independent experiments.
Plate clone formation assay
CNE1-LMP1-OV cells were seeded onto 6-well plates with 500 cells/well. After one night recovery, cells were administrated with medium enriched in DMSO (0.1%, v/v) and quercetagetin (5.5 µmol/L). The medium was changed every two days; 10 days later, PBS-washed cells were fixed with 4% formalin following stained with 0.1% crystal violet. The clone formation efficiency was calculated. Each group was repeated in 3 wells.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA). Correlation analysis of Pim1 expression and related parameters in clinical specimens was performed using the chi-squared test. In vivo experimental data are expressed as mean ± SEM. Differences between two groups were analyzed using Student’s t-test. For comparisons between multiple groups, ANOVAs were used followed by the Student–Newman–Keuls test. P<0.05 was considered to be statistically significant.
Results
Immunohistochemical results and correlation analysis in clinical specimens
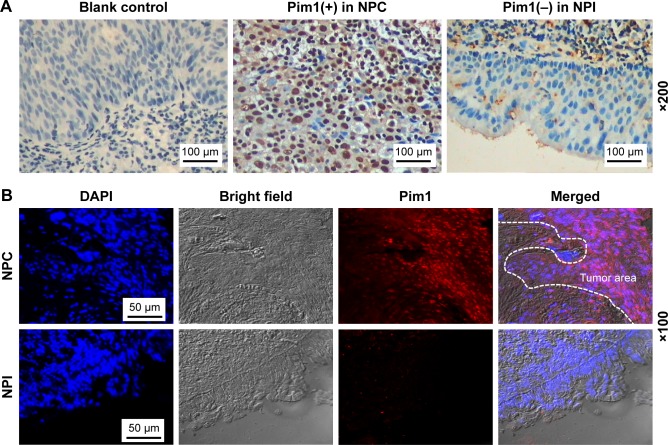
Among 89 cases of NPC, 48 cases (53.93%) were Pim1 positive, while only one case (6.67%, 1/15) was Pim1 positive in NPI (P<0.001). Further hierarchical studies showed that positive expression of Pim1 was not associated with age, gender, smoking condition or clinical classification of NPC patients, but was positively correlated with T classification, N classification and M classification (all P<0.05). Regarding the intracellular localization, we found Pim1 protein was expressed in both nucleus and cytoplasm and mainly expressed in the nucleus. We selected some samples for immunofluorescence qualitative and localization detection, and found that the tumor area in the clinical NPC samples showed obvious nucleus positive for Pim1, while it was absent in epithelial cells in the control groups (Figure 1, Table 1).
Figure 1.
Pim1 was upregulated in NPC clinical specimens.
Notes: (A) Pim1 immunohistochemistry. Representative images of Pim1 expression in clinical specimens. PBS substituted for primary antibody was served as blank control, nuclei were counterstained with hematoxylin. Bars, 100 µm. (B) Representative immunofluorescence images of Pim1 in NPC and NPI clinical specimens. Nuclei were counterstained with DAPI, and antigens for Pim1 were colorized with rhodamine-coupled IgGs. Bars, 50 µm.
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; NPC, nasopharyngeal carcinoma; NPI, nasopharyngeal chronic inflammation.
Table 1.
The expression of Pim1 protein in clinical specimens and its correlation to NPC patients’ clinical parameters
| Clinical parameters | n | Pim1 | P-value | |
|---|---|---|---|---|
| + | − | |||
| Histological type | ||||
| NPC | 89 | 48 | 41 | <0.001* |
| NPI | 15 | 1 | 14 | |
| Gender | ||||
| Male | 53 | 29 | 24 | 0.603 |
| Female | 36 | 18 | 18 | |
| Age | ||||
| ≥50 | 44 | 24 | 20 | 0.746 |
| <50 | 45 | 23 | 22 | |
| Smoking | ||||
| Yes | 38 | 21 | 17 | 0.689 |
| No | 51 | 26 | 25 | |
| Clinical classification | ||||
| I–II | 17 | 7 | 10 | 0.285 |
| III–IV | 72 | 40 | 32 | |
| T classification | ||||
| T1–T2 | 34 | 12 | 22 | 0.030* |
| T3–T4 | 55 | 32 | 23 | |
| N classification | ||||
| N0–N1 | 50 | 21 | 29 | 0.021* |
| N2–N3 | 39 | 27 | 12 | |
| M classification | ||||
| M0 | 72 | 34 | 38 | 0.034* |
| M1 | 17 | 13 | 4 | |
Note:
Significance as indicated.
Abbreviations: NPC, nasopharyngeal carcinoma; NPI, nasopharyngeal chronic inflammation.
Establishment of LMP1 stably overexpressing NPC cell line
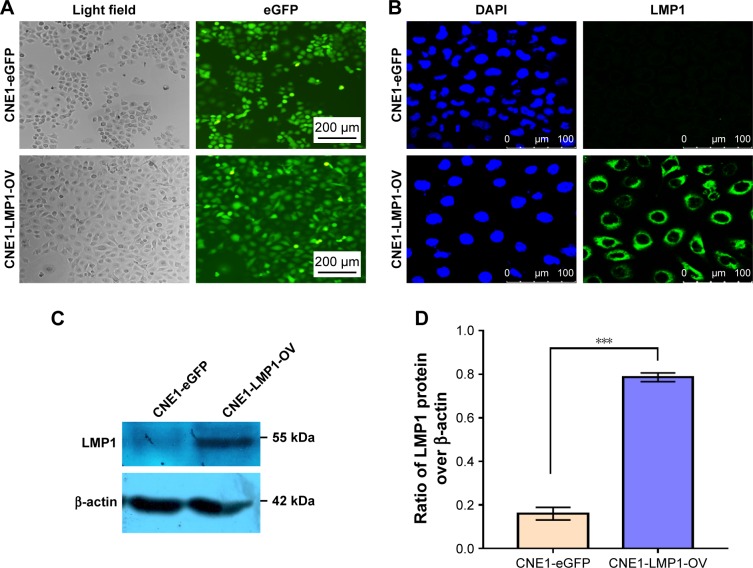
Given that NPC is an EBV-associated cancer, we hypothesized that EBV infection may mediate Pim1 expression through its encoded LMP1. To this end, we infected LMP1 overexpressing lentivirus and NC lentivirus into CNE1 cells, respectively, and obtained stable expression cell lines by puromycin stress. As results, the eGFP signal generated by lentivirus-infected CNE1 cells was observed under fluorescence microscopy (Figure 2A). Further immunofluorescence (Figure 2B) and immunoblot results (Figure 2C and D) confirmed the successful detection of LMP1 in the experimental group. Thus, this successful establishment of LMP1-overexpressing cell line laid a foundation for further analysis of the effects and mechanisms of LMP1-regulated Pim1 expression.
Figure 2.
Establishment of LMP1 overexpressing cell line.
Notes: (A) Efficiency of lentiviral infection in CNE1 cells. LMP1 overexpressing lentiviral particles and control lentiviral particles infected the CNE1 cells at MOI of 20, 3 days post infection, and the eGFP signal was observed under a fluorescence microscope. Bars, 200 µm. (B) Detection of LMP1 in lentivirus-infected CNE1 cells by immunofluorescence staining. Nuclei were counterstained with DAPI, and antigens for LMP1 were colorized with FITC-coupled IgGs. Bars, 100 µm. (C) Immunoblotting of LMP1 protein in established cell lines. (D) Semiquantitative results of LMP1. The experiment was repeated three times (n=3). ***P<0.001.
Abbreviation: DAPI, 4′,6-diamidino-2-phenylindole.
LMP1 promotes proliferation of NPC cells
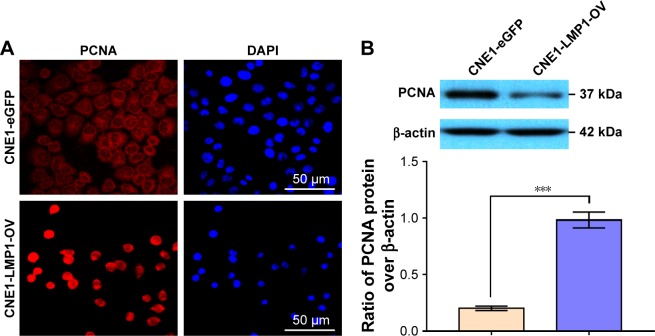
LMP1 has functions such as promoting cell proliferation, inhibiting apoptosis and inducing EMT in cells. As shown in Figure 3, by immunofluorescence and immunoblot, we confirmed that overexpression of LMP1 significantly induced the expression of PCNA, the marker for cell proliferation, in NPC cells. The immunofluorescence signal indicated that the expression of PCNA in the highly differentiated CNE1 cells before LMP1 transfection was weak and mostly cytoplasmic expression, while the expression of PCNA was enhanced after LMP1 infection and the nuclear localization was dominant.
Figure 3.
LMP1 induces PCNA expression in NPC cells.
Notes: (A) Detection of PCNA in lentivirus-infected CNE1 cells by immunofluorescence staining. Nuclei were counterstained with DAPI, and antigens for PCNA were colorized with TRITC-coupled IgGs. Bars, 50 µm. (B) Immunoblotting of PCNA in established cell line. The experiment was repeated three times (n=3) and the representative bands are shown. ***P<0.001.
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; PCNA, proliferating cell nuclear antigen; TRITC, tetramethylrhodamine isothiocyanate.
LMP1-induced Pim1 expression mediates NPC cell proliferation
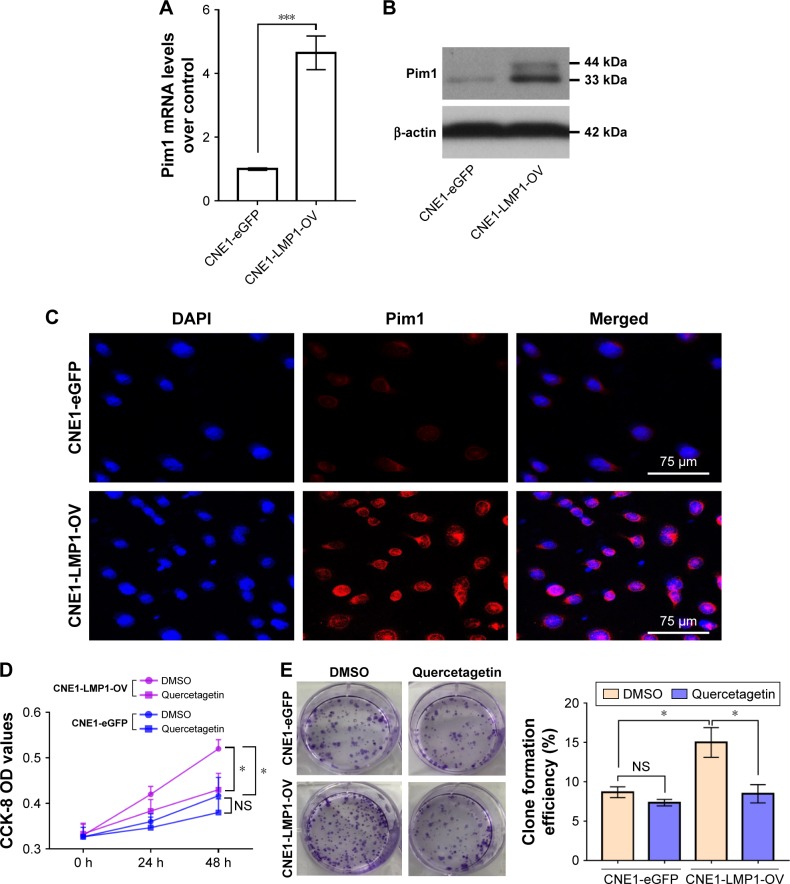
Based on the successful construction of LMP1 stably expressing cells, we further analyzed the effect of LMP1 on Pim1 expression. The results confirmed that LMP1 positively regulates Pim1 expression at both transcriptional and translational levels (Figure 4A–C). The Pim1 gene expresses two proteins, namely a larger 44 kDa protein and a smaller 33 kDa one, due to its two selective stop codons.22 We first found that LMP1 in NPC mainly induced the expression of the 33 kDa small Pim1 protein (Figure 4B). Subsequently, the LMP1 stably expressed CNE1 cells and control cells were treated with Pim1 specific activity inhibitor quer-cetagetin,17 and we found that suppression of Pim1 activity by quercetagetin significantly attenuated the proliferative activity of LMP1-expressing cells but not the control cells (Figure 4D and E).
Figure 4.
LMP1-induced Pim1 expression promotes NPC cell proliferation.
Notes: (A) Enhanced Pim1 mRNA levels in LMP1 stably expressing cells. ***P<0.001. The experiment was repeated three times (n=3). (B) LMP1 mainly upregulates the 33 kDa Pim1 protein but not the 44 kDa protein in NPC cells. The experiment was repeated three times (n=3) and the representative bands are shown. (C) Expression and cellular location of Pim1 protein in NPC cells. Antigens for Pim1 were colorized with TRITC-coupled IgGs. Bars, 75 µm. (D) Cell viability of LMP1-overexpressing cells and control cells treated with Pim1 inhibitor quercetagetin, assessed by CCK-8 assay. *P<0.05. Each group was repeated in six wells (n=6). (E) Clone formation efficiency of quercetagetin-treated cells. *P<0.05. Each group was repeated in three wells (n=3).
Abbreviations: CCK-8, cell counting kit-8; DAPI, 4′,6-diamidino-2-phenylindole; DMSO, dimethyl sulfoxide; NS, no significance; TRITC, tetramethylrhodamine isothiocyanate.
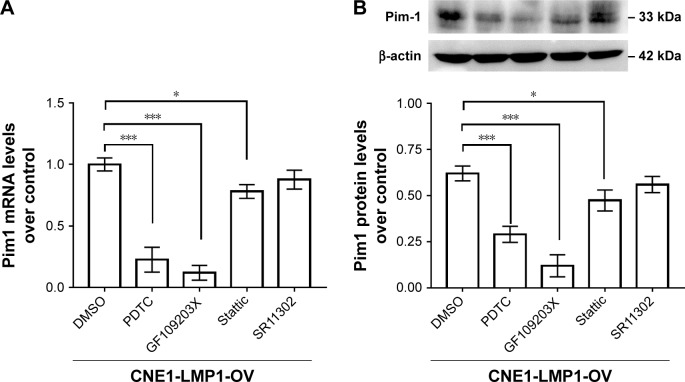
The mechanism of LMP1 inducing Pim1 expression
LMP1 stably expressed CNE1 cells were treated with classical inhibitors of LMP1 downstream signaling including NF-κB, PKC, STAT3 and AP-1, respectively. Quantitative PCR and immunoblot results confirmed that compared with DMSO control groups, NF-κB inhibitors PDTC and PKC inhibitor GF109203X significantly attenuated the expression of Pim1, and the STAT3 inhibitor Stattic attenuated the expression of Pim1 to a certain extent, while the AP-1 inhibitor SR11302 had no effects on the expression of Pim1 in NPC cells (Figure 5). Details of the mechanisms of LMP1-induced Pim1 expression in NPC cells are summarized in Figure 6.
Figure 5.
Signaling involved in LMP1-induced Pim1 expression in NPC cells.
Notes: (A) Pim1 mRNA levels were detected in LMP1 downstream signaling inhibitors including PDTC, GF109203X, Stattic and SR11302. *P<0.05, ***P<0.001; PDTC, NF-κB inhibitor; GF109203X, PKC inhibitor; Stattic, STAT3 inhibitor; SR11302, AP-1 inhibitor. The experiment was repeated three times (n=3). (B) Pim1 protein levels were detected in LMP1 downstream signaling inhibitors including PDTC, GF109203X, Stattic and SR11302. *P<0.05, ***P<0.001. The experiment was repeated three times (n=3) and the representative bands are shown.
Abbreviations: DMSO, dimethyl sulfoxide; PDTC, pyrrolidine dithiocarbamate.
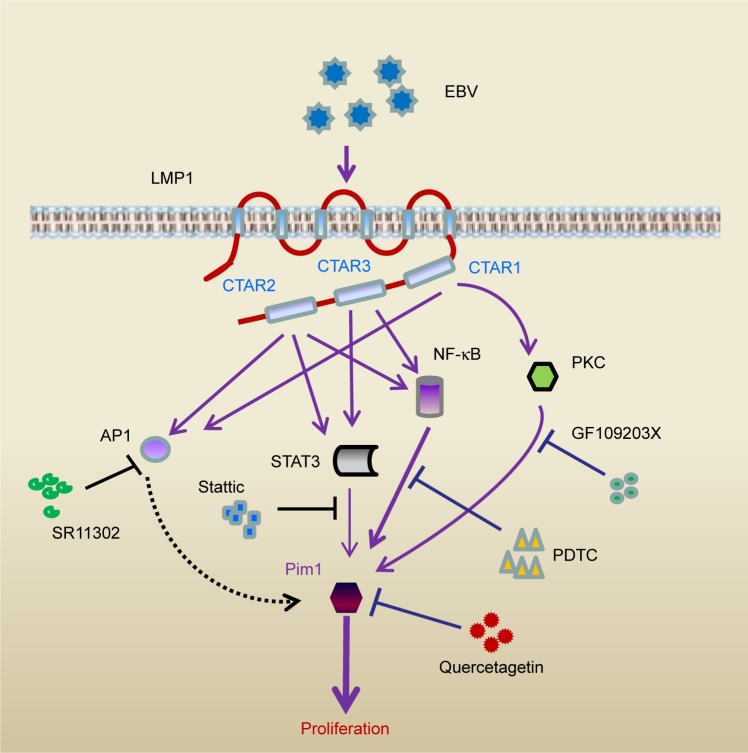
Figure 6.
Schematic illustration of the mechanism of LMP1/Pim1 signaling–promoted proliferation in NPC cells.
Notes: Based on results from the literature29 and this study, the mechanism of LMP1-induced Pim1 expression regulating the proliferation of NPC cells is illustrated. EBV encodes transmembrane protein LMP1 in NPC cells, through its C-terminal activation regions 1, 2 and 3 (CTAR1, CTAR2 and CTAR3), LMP1 activates NF-κB, Jak/STAT3, PKC and AP1 signaling. Inhibition of NF-κB, PKC and Jak/STAT3 signaling by its specific blocker attenuates LMP1-enhanced Pim1 expression, while inhibition of AP1 shows no effect on Pim1 expression. Consequently, this upregulated Pim1 promotes NPC cell proliferation, while inhibition Pim1 activity by quercetagetin suppresses cell proliferation.
Abbreviations: AP-1, activator protein 1; CTAR, C-terminal activation regions; EBV, Epstein–Barr virus; NF-κB, nuclear factor κB; NPC, nasopharyngeal carcinoma; PDTC, pyrrolidine dithiocarbamate; PKC, protein kinase C; STAT3, signal transducer and activator of transcription 3.
Discussion
We have previously reported that Pim1 is differentially expressed in various differentiation statuses of NPC cell lines, and inhibition of Pim1 by quercetagetin significantly attenuated high Pim1-expressing cell proliferation.17 In this study, we aimed to further clarify the role of Pim1 in clinical NPC progression and its mediated mechanism. To this end, through analysis of Pim1 protein expression in clinical NPC specimens, we found that Pim1 expression rate was significantly higher than that in control NPI specimens, suggesting a positive role of Pim1 in NPC biology. Subsequently, we found that Pim1 expression was positively related to NPC patients’ T classification, N classification and M classification, which further indicated overexpression of Pim1 contributes to NPC clinical progression. Regarding Pim1 expression in cancers, increasing data have proved that high Pim1 expression plays a pivotal role in progression of cancers including hematological malignancy,4,23 epithelial cell-derived tumors1,2,7 and mesenchymal neoplasms.24,25 Our current investigation provides additional evidence about Pim1 expression in NPC, a head and neck tumor with unique regional and ethnic distribution differences.
As mentioned above, NPC is an EBV-associated malignancy. During the process of latent infection, three EBV-encoded proteins, namely Epstein–Barr nuclear antigen 1 (EBNA1), LMP1 and LMP2 were expressed, while LMP1 is deemed to be the central player in the development of NPC.26 Therefore, LMP1 reserves therapeutic interests in NPC.27,28 Based on this background, we tried to clarify whether the LMP1/Pim1 signal axis exists in the NPC. Thus, we generated LMP1 stably-expressing CNE1 cell line by infecting the EBV negative, well-differentiated CNE1 cells. Initial results indicated this cell line was successfully established, and forced expression of exogenous LMP1 led to significantly Pim1 upregulation both at the transcriptional and translational levels. This causal relationship suggests the presence of the LMP1/Pim1 signal axis in the NPC.
How does LMP1 mediate Pim1 expression in NPC? To answer this question, we adopted various inhibitors for LMP1 downstream signals, including NF-κB, PKC, Jak/STAT3 and AP-1.29 The results showed that NF-κB inhibitor PDTC, PKC inhibitor GF109203X and STAT3 inhibitor Stattic displayed significantly suppressive effects, while AP-1 inhibitor SR11302 showed no effects on Pim1 expression. Thus, LMP1 may mediate Pim1 expression in NPC cells, mainly through NF-κB, PKC and Jak/STAT3 signaling. The mechanisms were illustrated in Figure 6. This finding broadens our insights on molecular mechanisms about LMP1 in NPC carcinogenesis.
As illustrated by the relationship between Pim1 expression and NPC patient’s clinical parameters, Pim1 expression showed no association with NPC patients’ age, gender, smoking status and clinical classification; however, it was positively related to NPC patients’ T, N and M classification. These epidemiological correlation analysis results indicated that Pim1 expression plays a critical role in NPC clinical progression due to cell proliferation and metastasis. We currently focused on cell proliferation. Consistent with our expectations, we found that LMP1-induced increase in Pim1 expression indeed promoted proliferation of NPC cells. Our results were consistent with other lab findings that proved high Pim1 expression enhanced cancerous cell growth.1,30–32
So far, Pim1 has attracted much interest in cancer target therapy. Many new generations of Pim1 inhibitors have been designed and tested.8,9 Flavonol quercetagetin (3,3′,4′,5,6,7-hydroxyflavone) was a moderately potent and selective inhibitor of Pim1 kinase.19 The mechanism of quercetagetin anti-Pim1 is due to the suppression of Pim1 activity but not its expression, which could be assessed by the changes in downstream gene expression or phosphorylation modification state.19,20,33 Our previous report17 and another laboratory33 proved that quercetagetin effectively inhibited cell proliferation. Future investigations using novel and selective Pim1 inhibitors would strengthen the basis for clinical application of Pim1 inhibitors in NPC adjuvant therapy.
Some limitations exist in this study. 1) Due to the low detectability of LMP1 protein in clinical NPC samples, we have not successfully shown the relation of Pim1 to LMP1 in clinical NPC specimens. 2) We only focused on cell proliferation in this study; other cell biological functions such as apoptosis, migration should be considered. 3) The current change in phenotypes of Pim1-induced cells was based on overexpression of LMP1 through the CNE1-LMP1-OV cell line, which could potentially give rise to phenotypes that are not biologically relevant. Therefore, it is more convincing to test LMP1-positive cells from tumor tissues of clinical NPC patients. 4) In vivo animal experiments would make our current conclusion more solid.
Conclusion
High Pim1 expression was positively related to NPC patients’ clinical progression, and LMP1 mediated Pim1 mainly through NF-κB, PKC and STAT3 signaling. This LMP1-induced cell proliferation could be attenuated by the Pim1 inhibitor. Our current study provided novel mechanism of LMP1-mediated NPC cell progression and supported Pim1 as a potential target in NPC treatment.
Acknowledgments
The authors would like to thank Wei-quan Wu (Affiliated Hospital of Guangdong Medical University) for technical support in confocal microscope scanning. We thank pathologist Han-guo Jiang and Ai-hua Luo for their assistant in the clinical specimen diagnosis, and we thank S. Win, PhD, from Liwen-Bianji, Edanz Editing China for editing the English text of a draft of this manuscript. This work was supported by a grant of the YangFan Plan of Guangdong Province (4YF16007G).
Footnotes
Author contributions
Ran-ran Ding, Jian-ling Yuan, Ya-nan Jia, Xiao-min Liao, Si-si Wang, Zhong-ming Shao and Mu-yin Feng performed the experiments. Wei Jie and Zhi-hua Shen conceived of and coordinated the study. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zhang M, Liu T, Sun H, et al. PIM1 supports human colorectal cancer growth during glucose deprivation by enhancing the Warburg effect. Cancer Sci. 2018;109(5):1468–1479. doi: 10.1111/cas.13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung CO, Wong CC, Fan DN, et al. PIM1 regulates glycolysis and promotes tumor progression in hepatocellular carcinoma. Oncotarget. 2015;6(13):10880–10892. doi: 10.18632/oncotarget.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herzog S, Fink MA, Weitmann K, et al. PIM1 kinase is upregulated in glioblastoma multiforme and mediates tumor cell survival. Neuro Oncol. 2015;17(2):223–242. doi: 10.1093/neuonc/nou216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brault L, Menter T, Obermann EC, et al. PIM kinases are progression markers and emerging therapeutic targets in diffuse large B-cell lymphoma. Br J Cancer. 2012;107(3):491–500. doi: 10.1038/bjc.2012.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merkel AL, Meggers E, Ocker M. PIM1 kinase as a target for cancer therapy. Expert Opin Investig Drugs. 2012;21(4):425–436. doi: 10.1517/13543784.2012.668527. [DOI] [PubMed] [Google Scholar]
- 6.Holder SL, Abdulkadir SA. PIM1 kinase as a target in prostate cancer: roles in tumorigenesis, castration resistance, and docetaxel resistance. Curr Cancer Drug Targets. 2014;14(2):105–114. doi: 10.2174/1568009613666131126113854. [DOI] [PubMed] [Google Scholar]
- 7.Zhao B, Liu L, Mao J, Zhang Z, Wang Q, Li Q. PIM1 mediates epithelial-mesenchymal transition by targeting Smads and c-myc in the nucleus and potentiates clear-cell renal-cell carcinoma oncogenesis. Cell Death Dis. 2018;9(3):307. doi: 10.1038/s41419-018-0348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arunesh GM, Shanthi E, Krishna MH, Sooriya Kumar J, Viswanadhan VN. Small molecule inhibitors of PIM1 kinase: July 2009 to February 2013 patent update. Expert Opin Ther Pat. 2014;24(1):5–17. doi: 10.1517/13543776.2014.848196. [DOI] [PubMed] [Google Scholar]
- 9.Oyallon B, Brachet-Botineau M, Logé C, et al. Structure-based design of novel quinoxaline-2-carboxylic acids and analogues as Pim-1 inhibitors. Eur J Med Chem. 2018;154:101–109. doi: 10.1016/j.ejmech.2018.04.056. [DOI] [PubMed] [Google Scholar]
- 10.Horiuchi D, Camarda R, Zhou AY, et al. PIM1 kinase inhibition as a targeted therapy against triple-negative breast tumors with elevated MYC expression. Nat Med. 2016;22(11):1321–1329. doi: 10.1038/nm.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang LL, Chen WQ, Xue WQ, et al. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett. 2016;374(1):22–30. doi: 10.1016/j.canlet.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 12.Wu L, Li C, Pan L. Nasopharyngeal carcinoma: a review of current updates. Exp Ther Med. 2018;15(4):3687–3692. doi: 10.3892/etm.2018.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Shen Z, Wang K, et al. High FMNL3 expression promotes nasopharyngeal carcinoma cell metastasis: role in TGF-β1-induced epithelia-to-mesenchymal transition. Sci Rep. 2017;7:42507. doi: 10.1038/srep42507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang H, Gao M, Shen Z, et al. Blocking PI3K/Akt signaling attenuates metastasis of nasopharyngeal carcinoma cells through induction of mesenchymal-epithelial reverting transition. Oncol Rep. 2014;32(2):559–566. doi: 10.3892/or.2014.3220. [DOI] [PubMed] [Google Scholar]
- 15.Shen Z, Zeng Y, Guo J, et al. Over-expression of the special AT rich sequence binding protein 1 (SATB1) promotes the progression of nasopharyngeal carcinoma: association with EBV LMP-1 expression. J Transl Med. 2013;11:217. doi: 10.1186/1479-5876-11-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen Z, Jiang X, Zeng C, et al. High expression of ubiquitin-conjugating enzyme 2C (UBE2C) correlates with nasopharyngeal carcinoma progression. BMC Cancer. 2013;13:192. doi: 10.1186/1471-2407-13-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jie W, He QY, Luo BT, et al. Inhibition of Pim-1 attenuates the proliferation and migration in nasopharyngeal carcinoma cells. Asian Pac J Trop Med. 2012;5(8):645–650. doi: 10.1016/S1995-7645(12)60132-1. [DOI] [PubMed] [Google Scholar]
- 18.Yoshizaki T, Kondo S, Endo K, et al. Modulation of the tumor micro-environment by Epstein-Barr virus latent membrane protein 1 in nasopharyngeal carcinoma. Cancer Sci. 2018;109(2):272–278. doi: 10.1111/cas.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holder S, Zemskova M, Zhang C, et al. Characterization of a potent and selective small-molecule inhibitor of the PIM1 kinase. Mol Cancer Ther. 2007;6(1):163–172. doi: 10.1158/1535-7163.MCT-06-0397. [DOI] [PubMed] [Google Scholar]
- 20.Wang K, Deng X, Shen Z, et al. High glucose promotes vascular smooth muscle cell proliferation by upregulating proto-oncogene serine/threonine-protein kinase Pim-1 expression. Oncotarget. 2017;8(51):88320–88331. doi: 10.18632/oncotarget.19368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang K, Ding R, Ha Y, et al. Hypoxia-stressed cardiomyocytes promote early cardiac differentiation of cardiac stem cells through HIF-1α/Jagged1/Notch1 signaling. Acta Pharm Sin B. 2018;8(5):795–804. doi: 10.1016/j.apsb.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saris CJ, Domen J, Berns A. The Pim-1 oncogene encodes two related protein-serine/threonine kinases by alternative initiation at AUG and CUG. Embo J. 1991;10(3):655–664. doi: 10.1002/j.1460-2075.1991.tb07994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarado Y, Giles FJ, Swords RT. The PIM kinases in hematological cancers. Expert Rev Hematol. 2012;5(1):81–96. doi: 10.1586/ehm.11.69. [DOI] [PubMed] [Google Scholar]
- 24.Nga ME, Swe NN, Chen KT, et al. Pim-1 kinase expression in adipocytic neoplasms: diagnostic and biological implications. Int J Exp Pathol. 2010;91(1):34–43. doi: 10.1111/j.1365-2613.2009.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao Y, Feng Y, Shen J, et al. Clinical and biological significance of PIM1 kinase in osteosarcoma. J Orthop Res. 2016;34(7):1185–1194. doi: 10.1002/jor.23134. [DOI] [PubMed] [Google Scholar]
- 26.Yoshizaki T, Kondo S, Wakisaka N, et al. Pathogenic role of Epstein-Barr virus latent membrane protein-1 in the development of nasopharyngeal carcinoma. Cancer Lett. 2013;337(1):1–7. doi: 10.1016/j.canlet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Tao Y, Shi Y, Jia J, Jiang Y, Yang L, Cao Y. Novel roles and therapeutic targets of Epstein-Barr virus-encoded latent membrane protein 1-induced oncogenesis in nasopharyngeal carcinoma. Expert Rev Mol Med. 2015;17:e15. doi: 10.1017/erm.2015.13. [DOI] [PubMed] [Google Scholar]
- 28.Hannigan A, Wilson JB. Evaluation of LMP1 of Epstein-Barr virus as a therapeutic target by its inhibition. Mol Cancer. 2010;9:184. doi: 10.1186/1476-4598-9-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng H, Li LL, Hu DS, Deng XY, Cao Y. Role of Epstein-Barr virus encoded latent membrane protein 1 in the carcinogenesis of nasopharyngeal carcinoma. Cell Mol Immunol. 2007;4(3):185–196. [PubMed] [Google Scholar]
- 30.Mawas AS, Amatya VJ, Suzuki R, Kushitani K, Mohi El-Din MM, Takeshima Y. PIM1 knockdown inhibits cell proliferation and invasion of mesothelioma cells. Int J Oncol. 2017;50(3):1029–1034. doi: 10.3892/ijo.2017.3863. [DOI] [PubMed] [Google Scholar]
- 31.Brasó-Maristany F, Filosto S, Catchpole S, et al. PIM1 kinase regulates cell death, tumor growth and chemotherapy response in triple-negative breast cancer. Nat Med. 2016;22(11):1303–1313. doi: 10.1038/nm.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li JQ, Yang X, Zhou XM. PIM1 gene silencing inhibits proliferation and promotes apoptosis of human esophageal cancer cell line Eca-109. Cancer Biomark. 2017;18(2):149–154. doi: 10.3233/CBM-160038. [DOI] [PubMed] [Google Scholar]
- 33.Willert M, Augstein A, Poitz DM, Schmeisser A, Strasser RH, Braun-Dullaeus RC. Transcriptional regulation of Pim-1 kinase in vascular smooth muscle cells and its role for proliferation. Basic Res Cardiol. 2010;105(2):267–277. doi: 10.1007/s00395-009-0055-x. [DOI] [PubMed] [Google Scholar]