Abstract
Background
Sequestration of Plasmodium falciparum–infected erythrocytes (IEs) in the microvasculature contributes to pathogenesis of severe malaria in children. This mechanism is mediated by antigens expressed on the IE surface. However, knowledge of specific targets and functions of antibodies to IE surface antigens that protect against severe malaria is limited.
Methods
Antibodies to IE surface antigens were examined in a case-control study of young children in Papua New Guinea presenting with severe or uncomplicated malaria (n = 448), using isolates with a virulent phenotype associated with severe malaria, and functional opsonic phagocytosis assays. We used genetically modified isolates and recombinant P. falciparum erythrocyte membrane protein 1 (PfEMP1) domains to quantify PfEMP1 as a target of antibodies associated with disease severity.
Results
Antibodies to the IE surface and recombinant PfEMP1 domains were significantly higher in uncomplicated vs severe malaria and were boosted following infection. The use of genetically modified P. falciparum revealed that PfEMP1 was a major target of antibodies and that PfEMP1-specific antibodies were associated with reduced odds of severe malaria. Furthermore, antibodies promoting the opsonic phagocytosis of IEs by monocytes were lower in those with severe malaria.
Conclusions
Findings suggest that PfEMP1 is a dominant target of antibodies associated with reduced risk of severe malaria, and function in part by promoting opsonic phagocytosis.
Keywords: antibodies, immunity, Plasmodium falciparum, PfEMP1, severe malaria
Antibodies to surface antigens of infected erythrocytes are associated with resistance to severe malaria in children, and target PfEMP1 and promote opsonic phagocytosis by monocytes.
(See the Major Article by Rambhatla et al on pages 808–18.)
The global burden of malaria has declined in recent years due to improved access to malaria interventions [1]. However, challenges of resistance to antimalarial drugs have escalated the need for an effective vaccine. The most advanced vaccine, RTS,S, has only approximately 30% efficacy in children [2]. To develop malaria vaccines with increased efficacy, especially against severe malaria (SM), further understanding of the targets of antibody responses that protect against disease is required. Among endemic populations with high transmission levels, SM mainly affects young children [3]. The pathogenesis of SM from Plasmodium falciparum, the major cause of human malaria, is in part due to the sequestration of large numbers of mature P. falciparum–infected erythrocytes (IEs) in the microvasculature of specific organs (reviewed in [4]). The mechanical obstruction of blood flow and associated inflammation contribute to the manifestation of severe disease complications such as cerebral malaria [5–8].
Sequestration is mediated by the specific interaction of P. falciparum erythrocyte membrane protein 1 (PfEMP1), expressed on the IE surface, with receptors on the host endothelium (reviewed in [4]). PfEMP1 is encoded by the var multigene family [9], which can be divided into 3 main groups (A, B, C) and a chimeric group B/A var gene (termed DC8) based on their upstream promoter regions [10]. Transcription of different var gene subgroups has been linked to clinical disease manifestations [11]. Expression of group A var genes has been associated with SM in children from Tanzania and Papua New Guinea (PNG) [12–14]. Group A and B var genes encode PfEMP1 variants involved in key pathogenic features of SM, such as rosetting [15, 16] and adhesion to intercellular adhesion molecule 1 (ICAM-1) on brain endothelium [17]. Despite the high rate of var gene recombination, certain tandem domain arrangements of the extracellular portion of PfEMP1, also known as domain cassettes (DCs), appear to be highly conserved. A subset of group A var genes and the DC8 var gene can bind to endothelial protein C receptor (EPCR) expressed by human brain endothelial cells [18], contributing to the pathogenesis of SM [19]. Severe malaria in children was associated with expression of PfEMP1 variants containing DC8 (Group B/A) and DC13 (group A) domain arrangements [20–22], which bind to EPCR [18, 23, 24]. DC13 PfEMP1 has dual specificity and adheres to EPCR and ICAM-1 on brain endothelial cells [25, 26]. Parasites from cerebral malaria patients were also more likely to bind EPCR and ICAM-1 than those with uncomplicated malaria (UM) [19]. Other parasite proteins identified on the IE surface have also been proposed to play roles in disease pathogenesis, including RIFIN, STEVOR, and SURFIN [27–31].
After repeated exposure to P. falciparum, individuals living in malaria-endemic regions can acquire immunity that protects against severe disease [32–34]. However, targets and mechanisms of immunity to SM are poorly understood. PfEMP1 and other IE surface antigens have been identified as key targets of acquired antibodies (reviewed in [4]). Prior studies using genetically modified P. falciparum with suppressed PfEMP1 expression, and other approaches, demonstrated that PfEMP1 is a dominant IE surface target of naturally acquired antibodies and found that PfEMP1-specific antibodies were associated with protection against uncomplicated pediatric malaria [35–37]. Some studies have found associations between antibodies to recombinant PfEMP1 domains and protection from UM, although findings have not been highly consistent (reviewed in [4]).
Much less is known about responses mediating protection from SM. Studies have suggested that young children tend to first acquire antibodies to PfEMP1, encoded by group A and DC8 var genes, that are associated with severe disease [12, 38], compared to groups B and C; this may contribute to protection from severe disease [39, 40]. In several small studies, it was reported that children with SM had antibodies that recognized DC8 and DC13 PfEMP1 variants [20–22]. Antibodies to IEs can promote opsonic phagocytosis by monocytes. This is thought to play a major role in immunity, but the contribution of opsonic phagocytosis to immunity against SM has not been investigated. Limited data are available on the association between antibodies to PfEMP1 and protection against SM or quantifying PfEMP1 and other IE surface antigens as antibody targets on IEs during SM. Currently, very little is known regarding immunity to SM in non-African populations.
In the present study, we evaluated the acquisition of naturally acquired antibodies to IE surface antigens in a case-control study of children (n = 448) in PNG, presenting with severe or UM. We studied the importance of PfEMP1 and other IE surface antigens as targets of naturally acquired antibodies and related these to protective associations. We compared antibody responses between severe and UM, during acute infection and following convalescence, to evaluate the acquisition of immunity. We used P. falciparum isolates expressing PfEMP1 variants associated with SM to quantify the levels of acquired antibodies. We investigated the significance of PfEMP1 as an antibody target using genetically modified P. falciparum with substantially reduced PfEMP1 expression and using recombinant PfEMP1 domains. Additionally, we evaluated the functional importance of acquired antibodies in their ability to mediate the opsonic phagocytosis of IEs.
METHODS
A comprehensive description of the methods used in this study is shown in the Supplementary Materials.
Study Population
Samples for antibody measurement were extracted for a frequency-matched case-control study of children presenting with severe or UM in Madang, PNG, from 2006 to 2009 [41]. This case-control study was nested within a cohort study described elsewhere [41]. Blood samples were collected from children (n = 805; age range, 2 months–10 years; Supplementary Table 1) at enrollment (acute infection) and 2 months postinfection (convalescence). A summary of demographic and malariometric characteristics of children presenting with uncomplicated and SM is presented in Table 1.
Table 1.
Distribution of Demographic Characteristics of Study Participants at Enrollment (Acute Infection)
| Variable | Uncomplicated Malaria (n = 213) |
Severe Malaria (n = 235) |
|---|---|---|
| Age, mo, median (25th–75th percentile) | 42 (29–56) | 40 (29–55) |
| Sex, male (%) | 127 (60) | 131 (56) |
| Ethnicity | ||
| Madang | 174 (82) | 178 (76) |
| Madang/Sepik | 16 (8) | 23 (10) |
| Other | 13 (6) | 13 (6) |
| Sepik | 10 (5) | 20 (9) |
Data are presented as No. (%) unless otherwise indicated.
Ethics Statement
Ethics approval was obtained from the PNG Medical Research Advisory Committee, PNG Institute of Medical Research Institutional Review Board, and Alfred Hospital Human Research Ethics Committee. Written informed consent was obtained from all study participants or their legal guardians.
Plasmodium falciparum Culture and Isolates
Plasmodium falciparum isolates were maintained in continuous culture and synchronized as previously described [35, 36]. 3D7vpkd [35, 42] and 1E2 (IT4var19) parasites [20] were generated as previously described.
Measuring Antibodies to the IE Surface by Flow Cytometry
Measuring immunoglobulin G (IgG) binding to the IE surface of pigmented trophozoites was performed with an established flow cytometry–based assay, as described [35].
Measuring Antibodies to Recombinant PfEMP1 Domains by Enzyme-Linked Immunosorbent Assay
Antibodies to recombinant domains of PfEMP1 (DBLα2, CIDRα1, DBLβ12, and DBLγ6) was measured by enzyme-linked immunosorbent assay using established methods [43].
Measuring Opsonic Phagocytosis
The level of opsonic phagocytosis to the IE surface was measured by flow cytometry, as described previously [35, 44].
Statistical Analyses
For the primary analyses, multivariable logistic regression models were used to estimate the associations between total antibody responses and severe P. falciparum malaria, adjusting for age, sex, and ethnicity.
RESULTS
PfEMP1 Is a Dominant Target of Naturally Acquired Antibodies to the IE Surface Among Young Children With Severe or Uncomplicated Malaria
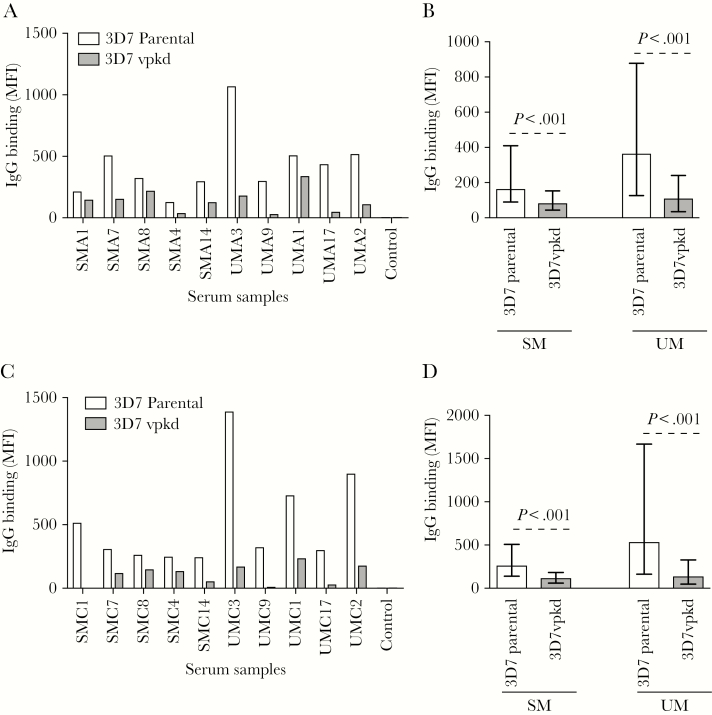
To quantify the role of PfEMP1 as a target of acquired antibodies, we measured antibody reactivity to the IE surface using an established flow cytometry–based assay [35]. We used a 3D7 isolate predominantly expressing PfEMP1 variants associated with virulent phenotypes that contribute to SM [37]. The dominant PfEMP1 expressed by our 3D7 isolate is a group A type (PF11_0521) with a DC13 domain structure that mediates adhesion to endothelial cells [37]. Two other group A var genes were also upregulated (PFD1235w and PFA0015c) [37]. We compared antibody levels of 3D7parental to a transgenic line with inhibited PfEMP1 surface expression due to endogenous var gene suppression (var promoter “knock-down”; 3D7vpkd) [35, 42]; this isolate has greatly reduced PfEMP1 expression, but still expresses other antigens, including RIFIN and STEVOR [35]. We measured the level of IgG binding to the IE surface in serum samples collected at enrollment (acute infection) from children with SM (n = 235) or UM (n = 213). Antibody levels were further measured in serum samples collected 2 months postinfection (following convalescence) from the same children (SM, n = 184; UM, n = 173). All individuals at both time-points showed a marked reduction in IgG binding to 3D7vpkd compared with 3D7parental (Figure 1A and 1C). Overall, during acute infection, IgG binding to 3D7vpkd was substantially reduced by 41.7% and 59.5% compared with 3D7parental, for SM and UM, respectively (Figure 1B, P < .001; Supplementary Figures 1A and 2). Similarly, at convalescence, IgG binding to 3D7vpkd was reduced by 48.6% and 67.2% compared to 3D7parental for SM and UM, respectively (Figure 1D, P < .001; Supplementary Figure 1B). While the antibody reactivity to 3D7vpkd was greatly reduced compared to 3D7parental, in both SM and UM, there was a strong, positive correlation between antibody responses to 3D7parental and 3D7vpkd at acute infection (SM: Spearman rank correlation coefficient, r2 = 0.54, P < .0001; UM: r2 = 0.68, P < .0001) and following convalescence (SM: r2 = 0.56, P < .0001; UM: r2 = 0.65, P < .0001). Our findings suggest that PfEMP1 is a major target of naturally acquired antibodies, consistent with our previous reports (which did not include individuals with SM) [35, 36].
Figure 1.
Antibodies to the surface of Plasmodium falciparum–infected erythrocytes (IEs) are directed at PfEMP1. A and C, A representative selection of serum samples tested for antibodies to 3D7parental and 3D7vpkd parasites. Samples were collected from severe malaria (SM) and uncomplicated malaria (UM) at acute infection (A) and following convalescence (C). Samples from nonexposed Melbourne residents were used as a negative control (control). Immunoglobulin G (IgG) binding to 3D7vpkd was substantially reduced in all individuals. There was minimal background reactivity observed among sera from Melbourne residents. IgG binding levels are expressed as geometric mean fluorescence intensity (MFI) for all graphs; assay was performed once; bars represent MFI values of samples tested in singles. B and D, Total IgG binding to the surface of erythrocytes infected with 3D7vpkd was substantially reduced compared to 3D7parental parasites in both SM and UM groups at acute infection (B) and following convalescence (D). Assay was performed once; bars represent median and interquartile ranges of samples that were classified as antibody positive to 3D7parental (B, n = 182/235 for SM and n = 177/213 for UM; D, n = 157/184 for SM and n = 153/173 for UM); P values were calculated using a paired Wilcoxon signed-rank test. Abbreviations: IgG, immunoglobulin G; MFI, mean fluorescence intensity; SM, severe malaria; UM, uncomplicated malaria.
Antibodies to the IE Surface Are Higher in Uncomplicated Malaria
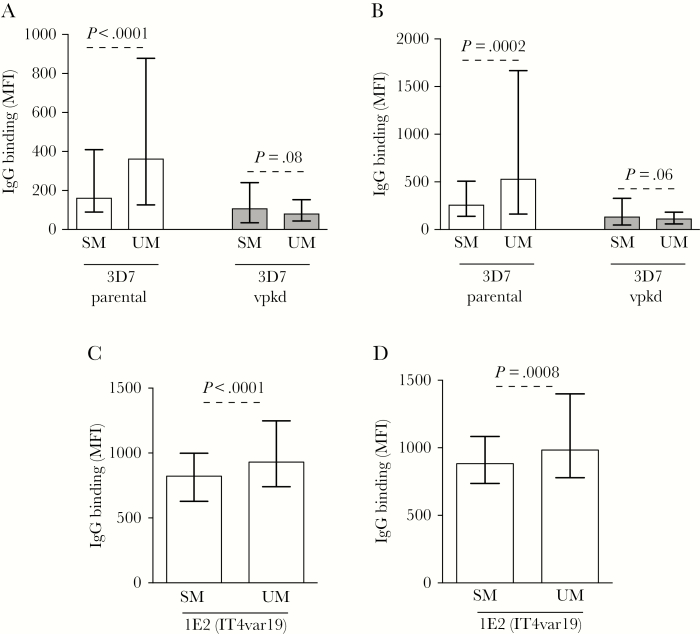
Children with SM and UM had similar age, sex, and ethnicity (characteristics used for matching), but SM children had significantly higher parasitemia, which is a common feature of SM (Table 1). IgG binding to the IE surface of 3D7parental was 57.5% and 64.3% higher in UM compared to SM during acute infection (Figure 2A, P < .0001) and following convalescence (Figure 2B, P = .0002). In contrast, there was a trend of lower IgG binding to 3D7vpkd in UM compared to SM samples, at acute infection (Figure 2A, P = .08) and following convalescence (Figure 2B, P = .06). Children with antibodies to 3D7parental and PfEMP1-specific antibodies (calculated as IgG levels to 3D7parental minus 3D7vpkd) had reduced odds of SM relative to UM, but this was not observed for those with antibodies to 3D7vpkd (Supplementary Table 2). We also measured antibody levels toward the IE surface of a genetically different isolate, 1E2 (IT4var19) [20], which expresses a specific PfEMP1 variant (DC8-type) with a virulent phenotype. Similar to 3D7parental, IgG binding to 1E2 (IT4var19) was higher in UM compared to SM, during acute infection (Figure 2C; 28.6% higher, P < .0001) and following convalescence (Figure 2D; 26.4% higher, P = .0008). Children with antibodies to 1E2 (IT4var19) parasites had reduced odds of SM relative to UM (Supplementary Table 2).
Figure 2.
Antibodies to Plasmodium falciparum–infected erythrocyte (IE) surface antigens are higher among young children presenting with uncomplicated malaria (UM). A and B, The level of immunoglobulin G (IgG) binding to the surface of erythrocytes infected with 3D7parental and 3D7vpkd parasites was higher in samples from UM compared to severe malaria (SM) at both acute infection (A) and convalescence (B). Assay was performed once; bars represent median and interquartile range (IQR) of samples that were classified as antibody positive to 3D7parental (A, n = 182/235 for SM and n = 177/213 for UM; B, n = 157/184 for SM and n = 153/173 for UM); P values were calculated using an unpaired Mann–Whitney test. C and D, The level of IgG binding to the surface of erythrocytes infected with 1E2 (IT4var19) parasites was higher in samples from UM compared to SM at both acute (C) and convalescence (D). Assay was performed once; bars represent median and IQR (C, n = 235 for SM and n = 213 for UM; D, n = 184 for SM and n = 173 for UM); P value was calculated using an unpaired Mann–Whitney test. Abbreviations: IgG, immunoglobulin G; MFI, mean fluorescence intensity; SM, severe malaria; UM, uncomplicated malaria.
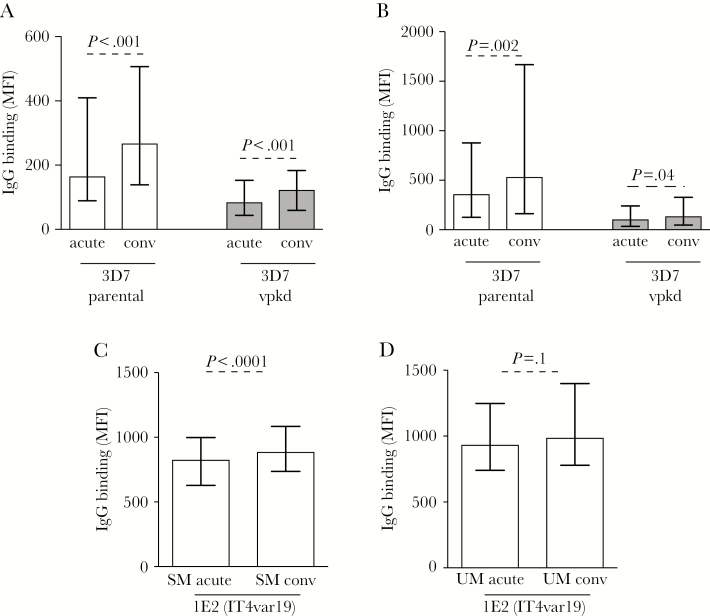
Antibodies to the IE Surface Are Boosted by Infection
Next, we compared antibody responses within individuals using samples that were collected at acute infection and at convalescence to determine antibody boosting. IgG binding to 3D7parental and 3D7vpkd was higher following convalescence for both SM (Figure 3A; 18.4% higher for 3D7parental, 3.84% higher for 3D7vpkd; P < .001; Supplementary Figure 1C) and UM (Figure 3B; 31.4% higher, P = .002 for 3D7parental, 38.4% higher, P = .04 for 3D7vpkd; Supplementary Figure 1D). IgG binding to 1E2 (IT4var19) parasites was also slightly higher during convalescence for SM (Figure 3C; 6.2% higher, P < .0001) and UM (Figure 3D; 3.3% higher, P = .1). Comparing the magnitude of antibody boosting in children (calculated as antibody levels at convalescence minus acute), there was an increase in the magnitude of antibody levels observed for SM and UM, for both 3D7parental and 3D7vpkd parasites (Supplementary Figure 3A). Antibody boosting to 1E2 (IT4var19) parasites was only observed with SM (Supplementary Figure 3B). Greater boosting of antibodies to 3D7 may indicate that this isolate expresses antibody epitopes or antigenic determinants that are more common in our study population than those expressed by 1E2.
Figure 3.
Antibodies to Plasmodium falciparum–infected erythrocyte (IE) surface antigens are higher among convalescent samples. A and B, Total immunoglobulin G (IgG) binding to the surface of erythrocytes infected with 3D7vpkd was substantially reduced compared to 3D7parental parasites at acute and convalescence for severe malaria (SM; A) and uncomplicated malaria (UM; B). Assay was performed once; bars represent median and interquartile range (IQR) of samples that were classified as antibody positive to 3D7parental (A, n = 182/235 for acute, n = 157/184 for convalescence; B, n = 177/213 for acute, n = 153/173 for convalescence); P values were calculated using a paired Wilcoxon signed-rank test. C and D, The level of IgG binding to the surface of erythrocytes infected with 1E2 (IT4var19) parasites was higher in convalescence compared to acute samples for SM (C) and UM (D). Assay was performed once; bars represent median and IQR (C, n = 235 for acute, n = 184 for convalescence; D, n = 213 for acute, n = 173 for convalescence); P values were calculated using a paired Wilcoxon signed-rank test. Abbreviations: IgG, immunoglobulin G; conv, convalescence; MFI, mean fluorescence intensity; SM, severe malaria; UM, uncomplicated malaria.
No significant correlation was observed between antibodies to 3D7parental and 3D7vpkd for SM and UM, at acute infection and convalescence (Supplementary Table 3). However, there was a moderate, positive correlation between antibodies to 1E2 (IT4var19) parasites for SM and UM, at acute infection and convalescence (Supplementary Table 3). There was a weak, positive correlation between antibodies to 3D7parental and 1E2 (IT4var19) for SM measured at acute infection (Supplementary Table 4), but no correlation was observed for SM at convalescence. Similarly, no correlation was observed for antibodies to 3D7vpkd and 1E2 (IT4var19) parasites for SM at acute infection or convalescence (Supplementary Table 4). In UM, there was a strong, positive correlation between antibodies to 3D7parental and 1E2 (IT4var19), and a moderate, positive correlation between 3D7vpkd and 1E2 (IT4var19), at acute infection and convalescence (Supplementary Table 4).
Antibodies to Specific Recombinant 1E2 (IT4var19) PfEMP1 Domains Are Higher in Uncomplicated Malaria
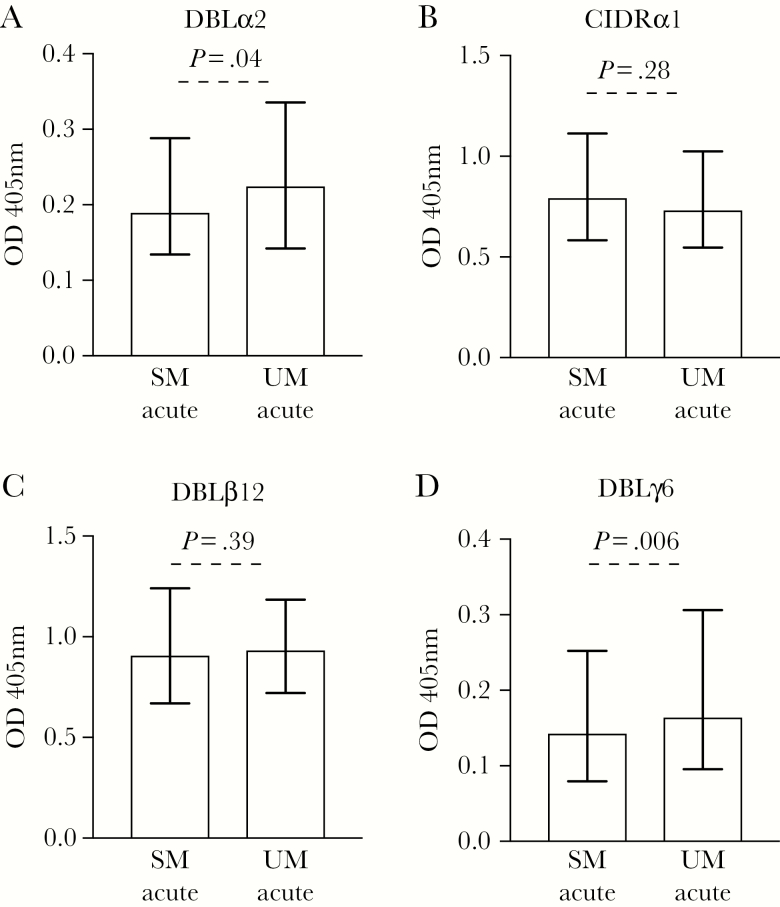
We tested serum samples for antibodies to 4 recombinant domains of the PfEMP1 variant encoded by 1E2 (IT4var19) parasites (DBLα2, CIDRα1, DBLβ12, and DBLγ6) to further evaluate the significance of PfEMP1 as a target of acquired antibodies and examine whether responses to specific PfEMP1 domains may be important in protection from severe disease. During acute infection, the level of IgG binding was significantly higher in UM compared to SM for DBLα2 (Figure 4A; 17.4% higher, P = .04) and DBLγ6 (Figure 4D; 17.6% higher, P = .006). This was not observed for CIDRα1 (Figure 4B; 7.6% lower, P = .28) and DBLβ12 (Figure 4C; 3.2% higher, P = .39). Children with antibodies to DBLα2 and DBLγ6 had reduced odds of SM relative to UM (Supplementary Table 5). Antibodies to recombinant PfEMP1 domains were correlated, suggesting coacquisition (Supplementary Table 6). There was a strong, positive correlation observed between antibody responses to 1E2 (IT4var19) parasites and the recombinant PfEMP1 domains DBLα2 and DBLγ6, but not CIDRα1 or DBLβ12, at acute infection for SM and UM (Supplementary Table 7).
Figure 4.
Comparing the levels of antibodies to recombinant antigens of PfEMP1 in samples at acute infection. Total immunoglobulin G (IgG) binding to the recombinant antigens of PfEMP1 1E2 (IT4var19) is presented as DBLα2 (A), CIDRα1 (B), DBLβ12 (C), and DBLγ6 (D). Significantly higher antibody levels to uncomplicated malaria (UM) compared to severe malaria (SM) was only observed for DBLα2 (A) and DBLγ6 (D). Antibody levels are expressed as optical density values measured at 405 nm. Assay was performed once; bars represent median and interquartile range of samples tested in duplicate (n = 235 for SM and n = 213 for UM); P values were calculated using an unpaired Mann–Whitney test. Abbreviations: OD, optical density; SM, severe malaria; UM, uncomplicated malaria.
Antibodies That Mediate the Opsonic Phagocytosis of IEs Are Higher in Uncomplicated Malaria and Target PfEMP1
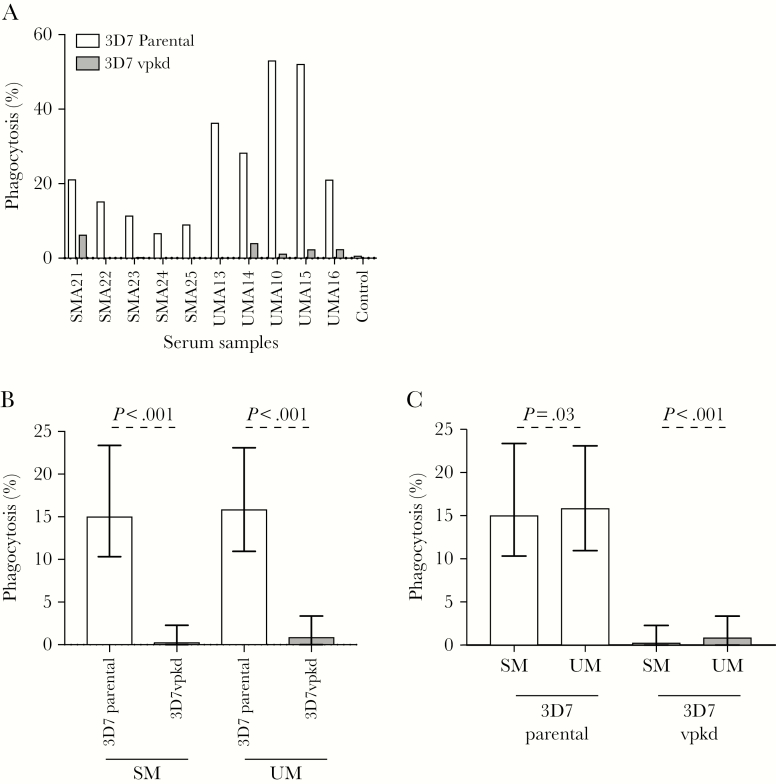
To quantify the functional capacity of antibodies targeting IE surface antigens, SM and UM samples at acute infection were tested in an established opsonic phagocytosis assay using undifferentiated THP1 monocytic cells [35, 44]. The majority of individuals showed a marked reduction in phagocytosis activity with 3D7vpkd compared to 3D7parental (Figure 5A). Overall, the level opsonic phagocytosis activity was markedly reduced in 3D7vpkd for both SM and UM samples (Figure 5B; P < .001), indicating that PfEMP1 is a major target of functional antibodies that promote IE phagocytosis. Opsonic phagocytosis activity was higher in UM compared to SM for both 3D7parental (Figure 5C; 24% higher, P = .03) and 3D7vpkd (Figure 5C; 27% higher, P < .001). Children with antibodies that promote opsonic phagocytosis of 3D7parental, 3D7vpkd, and 3D7-PfEMP1 had reduced odds of SM relative to UM (Supplementary Table 2). The level of total IgG binding to the IE surface and opsonic phagocytosis activity was not strongly correlated for SM or UM samples for 3D7parental and 3D7vpkd (Supplementary Figure 4), suggesting that total IgG levels may not be a good measure of antibody function.
Figure 5.
Opsonic phagocytosis of Plasmodium falciparum–infected erythrocytes (IEs) by undifferentiated THP-1 monocytes. A, Representative selection of serum samples collected during acute infection was tested for opsonic phagocytosis activity to 3D7parental and 3D7vpkd parasites. Assay was performed once (n = 235 for severe malaria [SM]; n = 213 for uncomplicated malaria [UM]); bars represent the mean level of phagocytosis as a percentage of positive control. B, Opsonic phagocytosis activity of serum antibodies was markedly reduced with 3D7vpkd parasites compared to 3D7parental, for SM and UM. Bars represent the median and interquartile range (IQR) of samples that were classified as antibody positive to 3D7parental (n = 80/235 for SM; n = 96/213 for UM); P values were calculated using a paired Wilcoxon signed-rank test. C, There was a higher level of opsonic phagocytosis activity with samples from UM compared to SM. Bars represent the median and IQR of samples that were classified as opsonic phagocytosis activity that is positive to 3D7parental (n = 80/235 for SM; n = 96/213 for UM); P value was calculated using an unpaired Mann–Whitney test. Abbreviations: SM, severe malaria; UM, uncomplicated malaria.
DISCUSSION
In this study, we found that children with UM had significantly higher antibodies to IE surface antigens and PfEMP1 specifically. We demonstrated this by quantifying antibodies with 2 different IE isolates that express virulent PfEMP1 types associated with SM pathogenesis, alongside genetically modified P. falciparum with suppressed PfEMP1 expression and using recombinant PfEMP1 domains. We demonstrated that PfEMP1 is a major target of naturally acquired antibodies to the IE surface in these children; importantly, PfEMP1-specific antibodies, quantified using native proteins expressed on the IE surface and recombinant antigens, were higher in those with UM, and associated with reduced odds of SM. This suggests that PfEMP1-specific antibodies play a role in protection from SM. Furthermore, antibodies to IE surface antigens were boosted following either severe or UM. Antibodies promoting opsonic phagocytosis were higher in children with UM and associated with protection from SM, suggesting that functional antibodies have important roles in immunity from SM. Together, our results suggest the importance of antibodies to the IE surface, predominantly PfEMP1, in contributing to protective immunity against SM in young children.
The overall level of IgG binding to the IE surface was higher in children with UM, compared to SM, for IEs of 3D7 and 1E2 (IT4var19). This difference was observed in samples collected at acute infection and following convalescence. The 3D7 and 1E2 (IT4var19) isolates were used because they are known to express virulent PfEMP1 types associated with SM pathogenesis. The transcription level of both group A and DC8 EPCR-binding var genes is increased in SM infections [18, 22, 45–47]. The dominant PfEMP1 expressed by our 3D7 isolate is a group A type (PF11_0521) that has a DC13 domain structure that mediates adhesion to endothelial cells [37], and 2 other group A var genes were also upregulated (PFD1235w and PFA0015c) [37]. The 1E2 (IT4var19) parasite line expresses a specific DC8 PfEMP1 that was upregulated when parasites were selected for adhesion to brain endothelial cells [20]. The PfEMP1 variant expressed by the 1E2 (IT4var19) parasites has a DC8 arrangement associated with severe disease. Our findings suggest that higher levels of antibodies to group A and DC8 PfEMP1 contribute to protection from SM. Future studies in additional populations might enable the identification of antibody thresholds for protection against SM. In our studies we considered all children meeting the criteria of SM in one group and did not perform analyses of subgroups different SM syndromes, which could be considered in future studies.
Comparing antibody responses between 3D7parental and 3D7vpkd IEs allowed quantification of PfEMP1-specific antibodies. Overall IgG binding to the IE surface of 3D7vpkd was markedly reduced compared to 3D7parental, indicating that the majority of acquired antibodies to the IE surface are targeting PfEMP1. The decrease in IgG binding to 3D7vpkd was consistently observed with samples from children presenting with SM and UM, and among acute convalescent samples. This finding suggests that naturally acquired antibodies to the IE surface are predominantly PfEMP1-specific, which is supported by our previous data in PNG [36, 37] and Africa [35]. Low levels of antibodies to 3D7vpkd (which still expresses RIFIN, STEVOR, and other antigens [35]) suggest that other IE surface antigens play a minor role as antibody targets. Of note, antibodies specific to PfEMP1 were significantly higher among children with UM and associated with reduced odds of SM. In contrast, there was no association between antibodies to 3D7vpkd and odds of SM. Our data suggest that PfEMP1 is a major target of antibodies associated with protection from SM, whereas antibodies to non-PfEMP1 antigens represent a less important component of protective immunity. Further investigation of other antigens is warranted in future studies.
Antibodies to recombinant 1E2 (IT4var19) PfEMP1 domains were also significantly higher in UM, further supporting the contribution of PfEMP1 antibodies in immunity to SM. Interestingly, only antibodies to 2 domains (DBLα2 and DBLγ6) were significantly associated with disease severity, suggesting these may be more important contributors of protective immunity. Collectively, our findings suggest that PfEMP1-specific antibodies protect against SM in PNG children. Published work has suggested the importance of other CIDR domains in immunity against SM [48, 49], indicating that more detailed analyses are required to assess the relative contribution of specific DC8 PfEMP1 domains in protective immunity. A recent study in children in Mali (n = 78 severe, n = 73 uncomplicated cases) evaluated antibodies to PfEMP1 fragments using a microarray approach [50]. Antibodies were higher to recombinant PfEMP1 fragments in UM vs SM, but antibodies to the intact IE surface or antibody function were not evaluated. A potential limitation of the microarray approach is the use of protein fragments generated in an Escherichia coli cell-free translation system; therefore, correct folding of PfEMP1 domains may be unlikely to occur, which may be important for antibody binding.
Antibodies to 3D7parental and 3D7vpkd were higher following convalescence; this was seen in uncomplicated and SM, suggesting that naturally acquired antibodies to IE surface antigens are boosted upon infection. Interestingly, antibody levels to 1E2 (IT4var19) IEs were higher at convalescence for SM only. This might reflect the expression of PfEMP1 types that are antigenically similar to the 1E2 (IT4var19) PfEMP1 in SM, but this expression may be rare in UM. Complementary research conducted with samples from this same clinical study profiled antibodies using a recombinant PfEMP1 domain array. They found that SM resulted in the induction of antibodies to EPCR-binding CIDRα1 domains of PfEMP1 [51].
Antibodies to IE surface antigens are believed to function, in part, by opsonizing IEs for clearance by phagocytes. Thus, we measured opsonic phagocytosis activity using undifferentiated THP-1 monocytes [35, 44]. Opsonizing antibodies were significantly higher in UM, suggesting a role for this mechanism in immunity. However, the modest extent of the difference suggests that other mechanisms are likely to play a role in immunity, such as inhibition of vascular adhesion, recruitment of complement, or interactions with other immune cells; these aspects need investigating in future studies. We showed that the level of opsonic phagocytosis activity was markedly reduced in 3D7vpkd compared to 3D7parental, further suggesting the importance of PfEMP1 as a major target of functional antibodies.
In conclusion, our study demonstrated a likely role of acquired antibodies to IE surface antigens in mediating protection against SM in young children from PNG. We showed that PfEMP1 is a dominant target of naturally acquired antibodies to the IE surface and a target of functional antibodies that promote opsonic phagocytosis of IEs. Furthermore, PfEMP1-specific antibodies were associated with protection against SM in children, whereas antibodies to other IE surface antigens were not. These findings significantly contribute to understanding malaria immunity and pathogenesis, and have implications for developing therapeutics or vaccines for preventing SM.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank all of the study participants and their parents and staff involved in the study from PNG Institute of Medical Research, Madang.
Financial support. Funding was provided by the National Health and Medical Research Council of Australia (program grant and senior research fellowship to J. G. Beeson; senior research fellowship to J. A. S.; Early Career Fellowship and Career Development award to M. J. B.) and the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant number 5R01AI114766-04 to J. D. S.). J. G. B., F. J. F., I. M., S. J. R., and J. A. S. were supported in part by the Australian Centre for Research Excellence in Malaria Elimination funded by the National Health and Medical Research Council (NHMRC). The authors gratefully acknowledge support for the Burnet Institute from the Victorian Operational Infrastructure Support Program and the NHMRC Independent Research Institutes Infrastructure Scheme.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. World malaria report 2015 summary. Geneva, Switzerland: WHO, 2016:1–32. [Google Scholar]
- 2. RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015; 386:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Okiro EA, Al-Taiar A, Reyburn H, Idro R, Berkley JA, Snow RW. Age patterns of severe paediatric malaria and their relationship to Plasmodium falciparum transmission intensity. Malar J 2009; 8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan JA, Fowkes FJ, Beeson JG. Surface antigens of Plasmodium falciparum-infected erythrocytes as immune targets and malaria vaccine candidates. Cell Mol Life Sci 2014; 71:3633–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol 1985; 119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 6. Aikawa M. Human cerebral malaria. Am J Trop Med Hyg 1988; 39:3–10. [DOI] [PubMed] [Google Scholar]
- 7. Pongponratn E, Riganti M, Punpoowong B, Aikawa M. Microvascular sequestration of parasitized erythrocytes in human falciparum malaria: a pathological study. Am J Trop Med Hyg 1991; 44:168–75. [DOI] [PubMed] [Google Scholar]
- 8. Abdi AI, Fegan G, Muthui M, et al. Plasmodium falciparum antigenic variation: relationships between widespread endothelial activation, parasite PfEMP1 expression and severe malaria. BMC Infect Dis 2014; 14:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Su XZ, Heatwole VM, Wertheimer SP, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 1995; 82:89–100. [DOI] [PubMed] [Google Scholar]
- 10. Lavstsen T, Salanti A, Jensen AT, Arnot DE, Theander TG. Sub-grouping of Plasmodium falciparum 3D7 var genes based on sequence analysis of coding and non-coding regions. Malar J 2003; 2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tonkin-Hill GQ, Trianty L, Noviyanti R, et al. The Plasmodium falciparum transcriptome in severe malaria reveals altered expression of genes involved in important processes including surface antigen-encoding var genes. PLoS Biol 2018; 16:e2004328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jensen AT, Magistrado P, Sharp S, et al. Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes. J Exp Med 2004; 199:1179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rottmann M, Lavstsen T, Mugasa JP, et al. Differential expression of var gene groups is associated with morbidity caused by Plasmodium falciparum infection in Tanzanian children. Infect Immun 2006; 74:3904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaestli M, Cockburn IA, Cortés A, Baea K, Rowe JA, Beck HP. Virulence of malaria is associated with differential expression of Plasmodium falciparum var gene subgroups in a case-control study. J Infect Dis 2006; 193:1567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Falk N, Kaestli M, Qi W, et al. Analysis of Plasmodium falciparum var genes expressed in children from Papua New Guinea. J Infect Dis 2009; 200:347–56. [DOI] [PubMed] [Google Scholar]
- 16. Ghumra A, Semblat JP, Ataide R, et al. Induction of strain-transcending antibodies against group A PfEMP1 surface antigens from virulent malaria parasites. PLoS Pathog 2012; 8:e1002665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Turner GD, Morrison H, Jones M, et al. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol 1994; 145:1057–69. [PMC free article] [PubMed] [Google Scholar]
- 18. Turner L, Lavstsen T, Berger SS, et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 2013; 498:502–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tuikue Ndam N, Moussiliou A, Lavstsen T, et al. Parasites causing cerebral falciparum malaria bind multiple endothelial receptors and express EPCR and ICAM-1-binding PfEMP1. J Infect Dis 2017; 215:1918–25. [DOI] [PubMed] [Google Scholar]
- 20. Avril M, Tripathi AK, Brazier AJ, et al. A restricted subset of var genes mediates adherence of Plasmodium falciparum-infected erythrocytes to brain endothelial cells. Proc Natl Acad Sci U S A 2012; 109:E1782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Claessens A, Adams Y, Ghumra A, et al. A subset of group A-like var genes encodes the malaria parasite ligands for binding to human brain endothelial cells. Proc Natl Acad Sci U S A 2012; 109:E1772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lavstsen T, Turner L, Saguti F, et al. Plasmodium falciparum erythrocyte membrane protein 1 domain cassettes 8 and 13 are associated with severe malaria in children. Proc Natl Acad Sci U S A 2012; 109:E1791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lau CK, Turner L, Jespersen JS, et al. Structural conservation despite huge sequence diversity allows EPCR binding by the PfEMP1 family implicated in severe childhood malaria. Cell Host Microbe 2015; 17:118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Avril M, Brazier AJ, Melcher M, Sampath S, Smith JD. DC8 and DC13 var genes associated with severe malaria bind avidly to diverse endothelial cells. PLoS Pathog 2013; 9:e1003430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lennartz F, Adams Y, Bengtsson A, et al. Structure-guided identification of a family of dual receptor-binding PfEMP1 that is associated with cerebral malaria. Cell Host Microbe 2017; 21:403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Avril M, Bernabeu M, Benjamin M, Brazier AJ, Smith JD. Interaction between endothelial protein C receptor and intercellular adhesion molecule 1 to mediate binding of Plasmodium falciparum-infected erythrocytes to endothelial cells. MBio 2016; 7. doi:10.1128/mBio.00615-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nilsson Bark SK, Ahmad R, Dantzler K, et al. Quantitative proteomic profiling reveals novel Plasmodium falciparum surface antigens and possible vaccine candidates. Mol Cell Proteomics 2018; 17:43–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saito F, Hirayasu K, Satoh T, et al. Immune evasion of Plasmodium falciparum by RIFIN via inhibitory receptors. Nature 2017; 552:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kyes SA, Rowe JA, Kriek N, Newbold CI. Rifins: a second family of clonally variant proteins expressed on the surface of red cells infected with Plasmodium falciparum. Proc Natl Acad Sci U S A 1999; 96:9333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaviratne M, Khan SM, Jarra W, Preiser PR. Small variant STEVOR antigen is uniquely located within Maurer’s clefts in Plasmodium falciparum-infected red blood cells. Eukaryot Cell 2002; 1:926–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Winter G, Kawai S, Haeggström M, et al. SURFIN is a polymorphic antigen expressed on Plasmodium falciparum merozoites and infected erythrocytes. J Exp Med 2005; 201:1853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nielsen MA, Staalsoe T, Kurtzhals JA, et al. Plasmodium falciparum variant surface antigen expression varies between isolates causing severe and nonsevere malaria and is modified by acquired immunity. J Immunol 2002; 168:3444–50. [DOI] [PubMed] [Google Scholar]
- 33. Griffin JT, Hollingsworth TD, Reyburn H, Drakeley CJ, Riley EM, Ghani AC. Gradual acquisition of immunity to severe malaria with increasing exposure. Proc Biol Sci 2015; 282:20142657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gupta S, Snow RW, Donnelly CA, Marsh K, Newbold C. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med 1999; 5:340–3. [DOI] [PubMed] [Google Scholar]
- 35. Chan JA, Howell KB, Reiling L, et al. Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity. J Clin Invest 2012; 122:3227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chan JA, Howell KB, Langer C, et al. A single point in protein trafficking by Plasmodium falciparum determines the expression of major antigens on the surface of infected erythrocytes targeted by human antibodies. Cell Mol Life Sci 2016; 73:4141–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chan JA, Stanisic DI, Duffy MF, et al. Patterns of protective associations differ for antibodies to P. falciparum-infected erythrocytes and merozoites in immunity against malaria in children. Eur J Immunol 2017; 47:2124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Turner L, Lavstsen T, Mmbando BP, et al. IgG antibodies to endothelial protein C receptor-binding cysteine-rich interdomain region domains of Plasmodium falciparum erythrocyte membrane protein 1 are acquired early in life in individuals exposed to malaria. Infect Immun 2015; 83:3096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cham GK, Turner L, Lusingu J, et al. Sequential, ordered acquisition of antibodies to Plasmodium falciparum erythrocyte membrane protein 1 domains. J Immunol 2009; 183:3356–63. [DOI] [PubMed] [Google Scholar]
- 40. Duffy MF, Noviyanti R, Tsuboi T, et al. Differences in PfEMP1s recognized by antibodies from patients with uncomplicated or severe malaria. Malar J 2016; 15:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Manning L, Laman M, Law I, et al. Features and prognosis of severe malaria caused by Plasmodium falciparum, Plasmodium vivax and mixed Plasmodium species in Papua New Guinean children. PLoS One 2011; 6:e29203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Voss TS, Healer J, Marty AJ, et al. A var gene promoter controls allelic exclusion of virulence genes in Plasmodium falciparum malaria. Nature 2006; 439:1004–8. [DOI] [PubMed] [Google Scholar]
- 43. Reiling L, Richards JS, Fowkes FJ, et al. Evidence that the erythrocyte invasion ligand PfRh2 is a target of protective immunity against Plasmodium falciparum malaria. J Immunol 2010; 185:6157–67. [DOI] [PubMed] [Google Scholar]
- 44. Ataíde R, Hasang W, Wilson DW, et al. Using an improved phagocytosis assay to evaluate the effect of HIV on specific antibodies to pregnancy-associated malaria. PLoS One 2010; 5:e10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jespersen JS, Wang CW, Mkumbaye SI, et al. Plasmodium falciparum var genes expressed in children with severe malaria encode CIDRα1 domains. EMBO Mol Med 2016; 8:839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mkumbaye SI, Wang CW, Lyimo E, et al. The severity of Plasmodium falciparum infection is associated with transcript kevels of var genes encoding endothelial protein C receptor-binding P. falciparum erythrocyte membrane protein 1. Infect Immun 2017; 85. doi:10.1128/IAI.00841-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shabani E, Hanisch B, Opoka RO, Lavstsen T, John CC. Plasmodium falciparum EPCR-binding PfEMP1 expression increases with malaria disease severity and is elevated in retinopathy negative cerebral malaria. BMC Med 2017; 15:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nunes-Silva S, Dechavanne S, Moussiliou A, et al. Beninese children with cerebral malaria do not develop humoral immunity against the IT4-VAR19-DC8 PfEMP1 variant linked to EPCR and brain endothelial binding. Malar J 2015; 14:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Abdi AI, Hodgson SH, Muthui MK, et al. Plasmodium falciparum malaria parasite var gene expression is modified by host antibodies: longitudinal evidence from controlled infections of Kenyan adults with varying natural exposure. BMC Infect Dis 2017; 17:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Travassos MA, Niangaly A, Bailey JA, et al. Children with cerebral malaria or severe malarial anaemia lack immunity to distinct variant surface antigen subsets. Sci Rep 2018:8:6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rambhatla J, et al. Acquisition of antibodies against Endothelial Protein C Receptor-binding domains of P. falciparum erythrocyte membrane protein 1 in children with severe malaria. In press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.