Abstract
Background
Macrophages are major targets for HIV-1, contribute to viral propagation in vivo, and are instrumental in the pathogenesis of HAND. While it is known that host sex affects HIV-1 viremia and influences the severity of HIV-1-associated neurocognitive disease, a cellular or molecular basis for these findings remains elusive.
Methods
We explored whether sex affects HIV-1 infectivity of primary human macrophages and CD4+ T cells in vitro.
Results
Macrophages derived from female donors were less susceptible to HIV-1 infection than those derived from males. This sex-dependent difference in macrophage infectivity was independent of the requirement for CD4/CCR5-mediated virus entry and was not observed in CD4+ T cells. Investigations into the mechanism governing these sex-dependent differences revealed that the host restriction factor SAMHD1 exists in a hyperphosphorylated, less active state in male-derived macrophages. In addition, the major kinase responsible for SAMHD1 phosphorylation, CDK1, exhibited lower levels of expression in female-derived macrophages in all tested donor pairs. The sex-dependent differences in viral restriction imposed by SAMHD1 were abrogated upon its depletion.
Conclusions
We conclude that SAMHD1 is an essential modulator of infectivity in a sex-dependent manner in macrophages, constituting a novel component of sex differences in innate immune control of HIV-1.
Keywords: HIV-1, macrophage, SAMHD1, sex
SAMHD1 exhibits sex-dependent differences in activity in primary macrophages. We observed increased SAMHD1 activity in macrophages from female donors relative to males, which correlated with and explained the lower infectivity of HIV-1 in female-derived macrophages.
Sex is an important biological variable involved in susceptibility to and natural history of many diseases including human immunodeficiency virus (HIV)-1. Women constitute the majority of adults living with HIV-1, with an estimated 17.8 million women infected, compared with 16.7 million men worldwide according to the 2016 UNAIDS data. However, participants in clinical research exploring HIV-1 pathogenesis and management overwhelmingly skew male. Underrepresentation of female participants in HIV-1 research, paired with the implicit assumption that observations gleaned from overwhelmingly male study populations can be extrapolated to females, compromises both the basic and clinical understanding of the HIV-1 epidemic.
Sex differences in acute HIV-1 infection, progression to acquired immunodeficiency syndrome (AIDS), and development of HIV-associated neurocognitive disease (HAND) have previously been examined. Cross-sectional and longitudinal cohort studies have described sex differences in plasma viral loads and CD4+ T cell counts among recent seroconverters and those with chronic HIV-1 infection alike [1–4]. Multiple lines of evidence have shown that HIV-1-infected women maintain, on average, lower viral loads and higher CD4+ T cell counts than men in the absence of antiretroviral therapy (ART) [5]. Although viral loads in acute infection display sex-dependent variability, progression to AIDS between men and women occurs at similar rates [5]. Indeed, a variety of geographic and socioeconomic issues are critical factors affecting sex-dependent differences, especially in sub-Saharan Africa where rates of new HIV-1 infections are highest. However, little is known about the contribution of human biology to sex disparities [6].
Although CD4+ T cells constitute the major target of HIV-1 and contribute to viral persistence in vivo, mounting evidence supports an important role for myeloid lineage cells in acute and chronic infection [7–12]. Specifically, macrophages are present in a variety of tissues and are enriched in mucosal sites of HIV-1 transmission [13, 14]. In addition, macrophages and other myeloid lineage cells are thought to play a crucial role during chronic infection and contribute to pathogenesis in various anatomical sites, including the brain [15]. Persistent inflammation resulting from virus replication causes HAND, leading to lasting impairments that linger despite long-term ART [16, 17]. In a recent study, HIV infection in macrophages and microglia was shown to directly contribute to overproduction of neurodegenerative β-amyloid protein, providing clues into the molecular mechanisms that contribute to HAND [18]. Despite the depth of knowledge regarding HIV-1 pathogenesis in CD4+ T cells, little is known about the role of macrophages in acute and chronic infection, and even less is known regarding their contribution to sex-based differences [19].
In the present study, we sought to elucidate whether intrinsic differences in macrophage and CD4+ T-cell susceptibility to HIV-1 exist between males and females in vitro. We report that female-derived macrophages more potently resist infection with HIV-1 when compared with male-derived counterparts. We find that the relative capacity of female-derived monocyte-derived macrophages (MDMs) for HIV-1 restriction occurs independent of the virus strain used and is exerted before generation of double-stranded HIV-1 deoxyribonucleic acid (DNA). We show that SAMHD1, a well known anti-HIV-1 restriction factor expressed in immune cells, exhibits sex-dependent regulation in macrophages but not in CD4+ T cells and largely explains the differences in infectivity. Thus, the activity of SAMHD1 and its ability to restrict HIV-1 infection is influenced by sex.
MATERIALS AND METHODS
Generation of Monocyte-Derived Macrophages and Isolation of CD4+ T Cells
Monocyte-derived macrophages were differentiated from monocytes isolated via CD14 positive selection from peripheral blood mononuclear cells (PBMCs) as previously described [20]. Memory CD4+ T cells were isolated via negative selection from the remaining fraction after CD14+ monocyte isolation (Miltenyi Biotec). This kit depletes naive T cells, CD8 T cells, B cells, natural killer (NK) cells, γ/δ T cells, monocytes, dendritic cells (DCs), granulocytes, platelets, and erythroid cells by incubation with a cocktail of biotynlated antibodies against CD45RA, CD8, CD14, CD16, CD19, CD56, CD36, CD123, anti-T-cell receptor γ/δ, and CD235a, and subsequent removal with antibiotin-coated magnetic beads. Memory CD4+ T cells were cultured for 2–5 days in macrophage growth media before infection. Infection was carried out via spinoculation at 2900 rpm for 2 hours at 37°C.
Viruses and Virus Clones
Replication-defective (HIV-1-ΔEnv-GFP) and replication-competent (HIV-1-NL4-3-BaL-IRES-HSA and HIV-1-NL4-3-AD8) constructs were used in this study. Replication-incompetent HIV-1-ΔEnv-GFP clones were pseudotyped with vesicular stomatitis virus glycoprotein (VSVG) (Supplementary Figure S3). The replication-competent, R5 clone HIV-1-NL4-3-BaL-IRES-HSA was a gift from Michel J. Tremblay (Universite Laval, Quebec City, Quebec). The replication-competent, R5 clone HIV-1-NL4-3-AD8 is a derivative of pNL4-3 (Eric O. Freed, NIH AIDS Reagent Program).
Virus Generation and Infection of Monocyte-Derived Macrophages
Viruses were generated via calcium phosphate-mediated transfection of HEK293T cells. Supernatants were collected 36 hours after transfection and cryopreserved at −80°C until use. Primary MDMs were infected in static culture with 125–250 ng p24 as determined by enzyme-linked immunosorbent assay (ZeptoMetrix) as previously described [20]. To minimize experimental variability, infection of male and female pairs were performed simultaneously using identical virus stocks and reagents.
Flow Cytometric Analysis
Monocyte-derived macrophages were prepared for flow cytometry as previously described [20]. CD14 and CD16 levels were assessed using fluorescein isothiocyanate-conjugated (FITC)-anti-CD14 and allophycocyanin-conjugated (APC)-anti-CD16 followed by flow cytometric analysis in a LSRFORTESSA X-20 (BD Bioscience). To assess infection, HIV-1-ΔEnv-GFP/VSVG-infected cells were analyzed for green fluorescent protein (GFP) gene expression. Cells infected with HIV-1-BaL-IRES-HSA were stained for surface expression of CD24 with APC-anti-CD24 (eBioscience). Cells infected with HIV-1-NL4-3-AD8 were permeabilized and fixed with Cytofix/Cytoperm (BD Biosciences) for 30 minutes at 4°C, then washed with Perm/Wash Buffer (BD Biosciences) and stained with a 1:40 dilution of anti-p24 antibody (Beckman Coulter).
Measurement of Viral Deoxyribonucleic Acid via Gag-Polymearse Chain Reaction
Total genomic DNA was isolated (QIAGEN) from mock-infected or HIV-1-ΔEnv-GFP/VSVG-infected macrophages 24 hours postinfection. Real-time quantitative polymearse chain reaction (PCR) was performed in triplicate 20-μL volumes utilizing 2× PCR Master Mix (Thermo Fisher Scientific) and 250 ng of total DNA input. Samples were analyzed on a Roche LC480 real-time PCR instrument. Primers and probes used were as follows: forward primer (5’ to 3’) CATGTTTTCAGCATTATCAGAAGGA, reverse primer (5’ to 3’) TGCTTGATGTCCCCCCACT, and probe (5’ to 3’) FAM-CCACCCCACAAGATTTAAATACCATGCTTT-BHQ1.
Statistical Analysis
All statistical tests were performed using GraphPad Prism 7 software. Paired samples were analyzed using the Wilcoxon matched-pairs signed-rank test. For cases in which donors could not be analyzed in a pairwise-comparison, unpaired t tests with Welch’s correction were used. P values are indicated in the figures, and the analyses applied are indicated in the figure legends.
Consent, Approval, and Population Selection
Written informed consent was obtained from all donors. The study population comprised healthy donors 18 years and older and included students, staff, and faculty at the University of Utah and greater Salt Lake City. Participants were recruited via direct face-to-face interaction in the School of Medicine to undergo peripheral phlebotomy to obtain human PBMCs according to an approved and active institutional review board (IRB) protocol at the University of Utah (IRB 0067637). Fifty percent of participants in this study identified as female. The mean age of donors was 32.07 ± 9.59 years (age range, 20–63). Self-reported race and ethnicity of donors reflect the demographics of the State of Utah. Eighty-one percent of donors identified as white, 7.1% identified as Asian, 7.1% identified as Hispanic, and 4.8% identified as African American.
RESULTS
Sex Influences Macrophage Infection With Human Immunodeficiency Virus-1
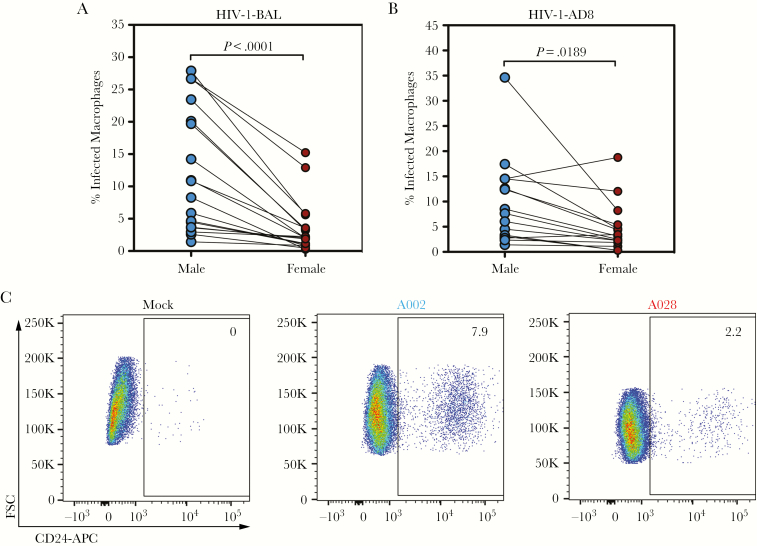
To investigate the role of biological sex in HIV-1 infection, healthy donors were recruited in male/female pairs. Monocyte-derived macrophages generated from these donors were infected with either of 2 replication-competent virus clones, HIV-1-NL-4-3-BaL-IRES-HSA (subsequently referred to as HIV-1-BAL) or HIV-1-NL-4-3-AD8 (subsequently referred to as HIV-1-AD8), both R5-tropic viruses (Supplementary Figure 3). HIV-1-BAL expresses mouse heat-stable antigen (HSA or CD24), and infection was analyzed by the surface expression of HSA on infected cells or by intracellular staining for HIV-1 p24 [21]. HIV-1-AD8 infection is analyzed by staining for HIV-1 p24. Forty-eight hours after infection, paired male/female macrophages were detached and analyzed via flow cytometry for CD24 (HIV-1-BAL) or p24 (HIV-1-BAL and HIV-1-AD8). Although we observed a wide range of donor susceptibility to infection (HIV-1-BAL: mean = 12.11, range = 1.4%–27.9% [males], mean = 3.59, range = 0.3%–15.3% [females]; HIV-1-AD8: mean = 9.29, range = 1.4%–34.7% [males], mean = 4.63, range = 0.30%–18.8% [females]), male-derived macrophages exhibited consistently enhanced infectivity when compared with female-derived macrophages (Figure 1A and B, representative flow cytometry in Figure 1C). These results show that donor sex influences infection of MDMs with 2 replication-competent strains of HIV-1.
Figure 1.
Human immunodeficiency virus (HIV)-1 inefficiently infects female-derived macrophages. (A) Male- (blue) and female-derived (red) monocyte-derived macrophages (MDMs) infected in pairs with HIV-1-bronchoalveolar lavage (BAL). Percentage infected macrophages represents the percentage of CD24+ or p24+ cells of n = 18 males and n = 18 females. (B) Male- and female-derived MDM infected in pairs with HIV-1-AD8. Macrophages were permeabilized and stained for intracellular p24 in n = 16 males and n = 16 females. (C) Representative flow plots of mock-infected and HIV-1-BAL-infected macrophages from A002 (male) and A028 (female). Numbers indicate percentage of cells within the CD24+ gate. P values determined using the Wilcoxon matched-pairs signed-rank test.
Sex-Dependent Differences in Human Immunodeficiency Virus-1 Infection Occur Early, Are Independent of CD4 and CCR5, and Result in Differences in Viral Deoxyribonucleic Acid Accumulation
To determine whether the observed sex-dependent difference in infectivity required multiple rounds of replication, MDMs were generated from 8 healthy donors and infected with HIV-1-BAL. Infection was tracked over a 14-day time course. Differences in infectivity were observed as early as 2 days postinfection, and differences were compounded at the later time points (Supplementary Figure 1A). Therefore, sex differences in infectivity are observed both in single-cycle infections as well as during ongoing replication [22].
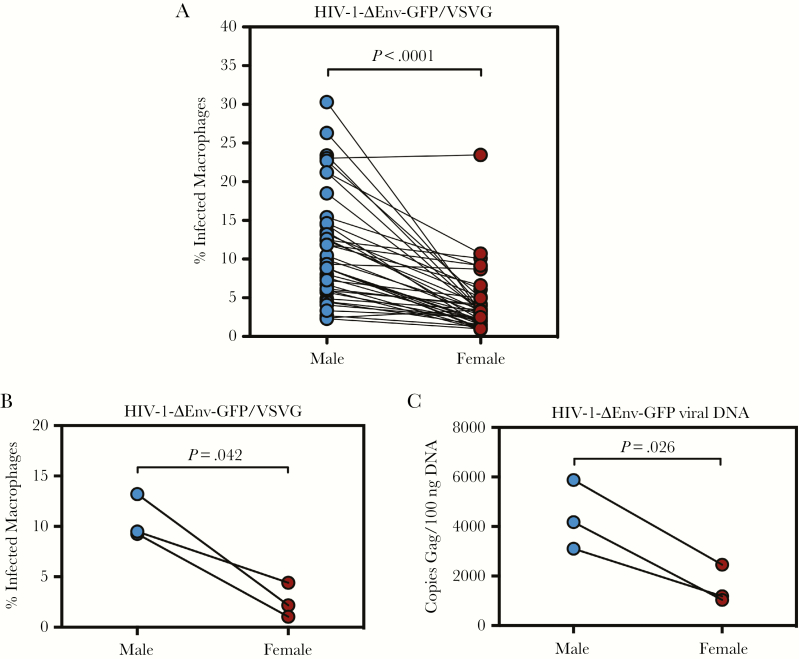
We next sought to ascertain whether viral glycoprotein interaction with CD4 and CCR5 could explain the observed sex-dependent differences in HIV-1 infectivity. To circumvent the requirement for CD4 and CCR5, we generated replication-defective HIV-1 viruses pseudotyped with vesicular stomatitis virus glycoprotein G (HIV-1-ΔEnv-GFP/VSVG). HIV-1-ΔEnv-GFP contains a deletion and frameshift mutation in the envelope glycoprotein gene and is capable of only a single round of infection (Supplementary Figure 3). More importantly, VSVG utilizes the low-density lipoprotein receptor, which shunts viruses towards an endocytic pathway that is independent of gp160-receptor/coreceptor interactions [23]. Infection of paired male and female healthy donor MDMs revealed a wide range of infectivity (mean = 11.06, range = 2.3%–30.3% [males]; mean = 4.22, range = 0.9%–23.5% [females]), with sex-dependent differences that mirrored results obtained during infection with viruses encoding native HIV-1 envelope (Figure 2A). Analysis of the HIV-1 receptor/coreceptors CD4 and CCR5 by flow cytometry revealed no significant differences in cell surface expression, consistent with the notion that the differences in intrinsic susceptibility of male and female MDMs to infection occurs at a receptor-independent, postentry step (Supplementary Figure 1B).
Figure 2.
Sex differences in human immunodeficiency virus (HIV)-1 infection occur during single-round infection and result in differences in viral deoxyribonucleic acid (DNA) accumulation. (A) Monocyte-derived macrophages (MDMs) were infected on day 7 postdifferentiation with HIV-1-ΔEnv-GFP/VSVG and analyzed 48 hours later for reporter gene expression in n = 20 male donors and n = 20 female donors in 42 separate experiments. Values are represented as percentage of GFP+ cells as analyzed by flow cytometry. (B) Monocyte-derived macrophages from 6 healthy donors (3 males and 3 females) were infected with HIV-1-ΔEnv-GFP/VSVG and analyzed 48 hours postinfection for GFP expression. (C) Gag-polymerase chain reaction conducted on genomic DNA isolated from MDM infected in (B). Values indicate copies of gag per 100 ng of genomic DNA input. P values determined using the Wilcoxon matched-pairs signed-rank test.
We next examined total genomic DNA of HIV-1-ΔEnv-GFP/VSVG-infected MDMs to test whether differences in infectivity correlated with differences in viral DNA synthesis. Infections of MDMs from 6 donors were analyzed by flow cytometry for GFP expression (Figure 2B). Gag-PCR revealed significant differences between male- and female-derived macrophages, suggesting that the relative block specific to female-derived macrophages occurs before or at the level of viral DNA synthesis (Figure 2C).
CD4+ T Cells Do Not Display Sex-Dependent Differences in Infectivity
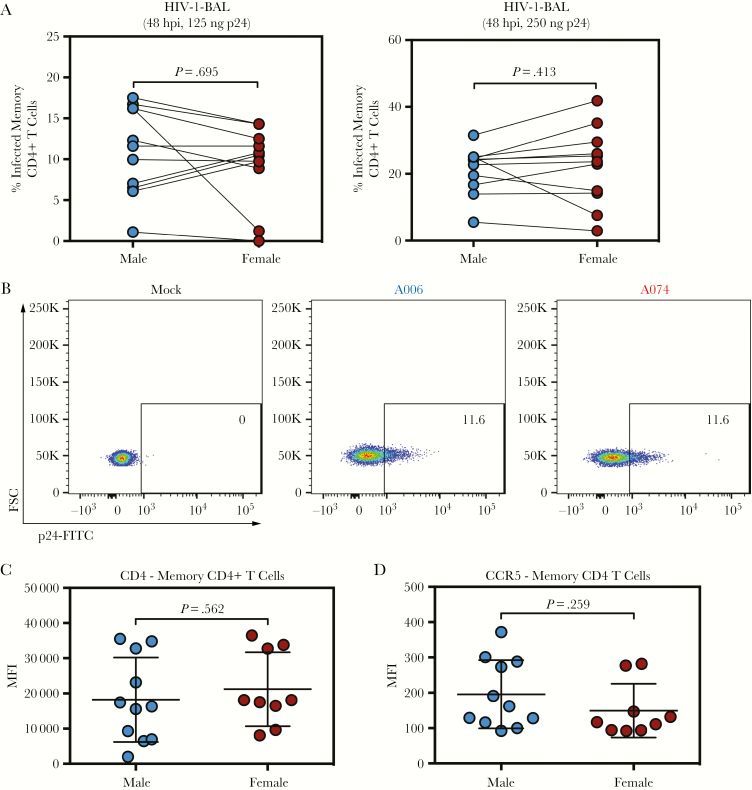
To determine whether the observed differences in infectivity were unique to MDMs or whether this observation could be extended to CD4+ T cells, cells were isolated from male and female donors and infected ex vivo via spinoculation with HIV-1-BAL. Unlike MDMs, memory CD4+ T cells failed to exhibit significant sex differences in infectivity at 48 hours postinfection independent of the amount of virus used (Figure 3A, representative flow cytometry in Figure 3B). Similar to our experiments in MDMs, we observed no differences in the expression of either CD4 or CCR5 (Figure 3C and D). The observation that sex differences do not exist in HIV-1 infection in CD4+ T cells supports a myeloid cell type-specific, sex-dependent difference in infectivity with HIV-1.
Figure 3.
CD4+ T cells do not exhibit sex-dependent differences in infectivity. (A) Memory CD4+ T cells isolated from healthy donors and infected with 125 or 250 ng of human immunodeficiency virus (HIV)-1-bronchoalveolar lavage (BAL) and analyzed at 48 hours postinfection in n = 11 male and n = 11 female donors. Values represent %p24+ cells as analyzed by flow cytometry. P values were determined using the Wilcoxon matched-pairs signed-rank test. (B) Representative flow cytometry plots of HIV-1-BAL-infected memory CD4+ T cells from male (A006) and female (A001) donors 48 hours postinfection. (C) Mean fluorescence intensity (MFI) of CD4 in n = 11 male-derived and n = 9 female-derived memory CD4+ T cells. (D) Mean fluorescence intensity of CCR5 in n = 11 male-derived and n = 9 female-derived memory CD4+ T cells. (C and D) P values were determined using an unpaired t test with Welch’s correction. Horizontal lines represent the mean ± standard error of the mean. FITC, fluorescein isothiocyanate-conjugated; FSC, forward scatter.
SAMHD1 Is Differentially Regulated in Male and Female Macrophages
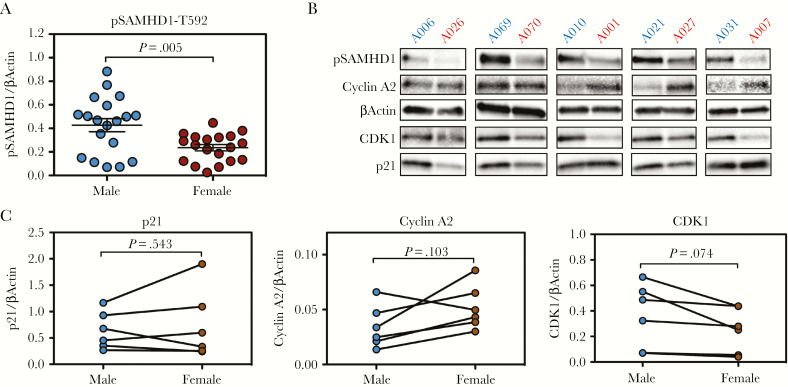
SAMHD1 has been shown to restrict HIV-1 at a preintegration step through inhibition of reverse transcription, a blockade that is imposed potently in macrophages [24–26]. SAMHD1 is a deoxynucleoside triphosphate (dNTP) triphosphohydrolase, and its activity as an HIV-1 restriction factor is inhibited by threonine-592 (T592) phosphorylation, which prevents dNTPase activity by destabilizing the tetramer required for its catalytic activity [27–29]. We have previously shown that donor-to-donor differences in HIV-1 restriction in macrophages are determined by SAMHD1 activity, as measured by T592 phosphorylation (pSAMHD1-T592), and not by the abundance of total protein [20]. Therefore, we tested phosphorylation of SAMHD1 in male- and female-derived macrophages. We found that levels of the inactive enzyme (pSAMHD1-T592) were present at a significantly higher degree in male-derived MDMs (Figure 4A, representative Western blots in Figure 4B).
Figure 4.
SAMHD1 is hyperphosphorylated in male-derived macrophages. (A) Western blot quantification of pSAMHD1-threonine-592 ([T592] inactive form) in n = 19 male and n = 19 female individual donors. P values were determined using the unpaired t test with Welch’s correction. Horizontal lines represent the mean ± standard error of the mean. (B) Western blot analysis of 5 male/female donor pairs analyzed for pSAMHD1, cyclin A2, βActin, CDK1, and p21. (C) Quantification of Western blot (densitometry) images from (B) for p21, cyclin A2, and CDK1. P values were determined using the Wilcoxon matched-pairs signed-rank test.
SAMHD1 is phosphorylated by a number of cyclin-dependent kinases (CDKs) including CDK1, CDK2, CDK4, and CDK6 [27, 30]. These CDKs coordinate with cyclins to exert SAMHD1 phosphorylation to inactivate the enzyme during cell division, providing the cell with adequate levels of dNTPs required for cellular DNA synthesis [27]. CDK1 and Cyclin A2 constitute the predominant CDK-cyclin pair responsible for SAMHD1 phosphorylation in macrophages [20, 27]. An additional layer of SAMHD1 regulation occurs via p21, a cell-cycle-related protein that leads to SAMHD1 inactivation indirectly via inhibition of CDKs [31]. We thus sought to ascertain whether differences in pSAMHD1-T592 could be explained by the expression of these SAMHD1-regulating proteins. Although we observed no significant differences in the expression of p21 or Cyclin A2, CDK1 expression appeared to exhibit differences in expression between male- and female-derived macrophages, although these were not statistically significant (P = .074) (Figure 4C). Further analysis of macrophage subsets via CD14 and CD16 expression did not reveal significant differences in the activation status of monocytes or the macrophages derived thereof (Supplementary Figure 2A and 2B).
SAMHD1 Is Required for Sex-Dependent Differences in Human Immunodeficiency Virus Infection of Macrophages
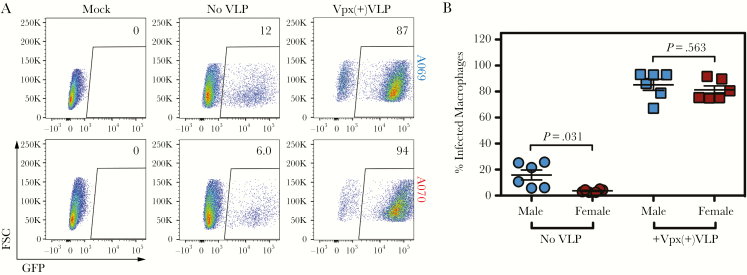
In view of the above results, we hypothesized that enhanced activity of SAMHD1 was responsible for the relative restriction observed in female-derived macrophages. We have previously shown that infectivity in MDMs is determined by activity of SAMHD1 and not total protein expression [20]. To confirm that SAMHD1 is responsible for the differences observed, we utilized the HIV-2 accessory protein Vpx. Vpx targets SAMHD1 for ubiquitination, leading to proteasome-directed proteolysis in the setting of HIV-2 infection [24–26, 32, 33]. Although HIV-1 does not encode Vpx, and therefore does not induce SAMHD1 degradation, it can be complemented with Vpx through virus-like particle (VLP)-mediated delivery to enhance HIV-1 infection of myeloid cells [26, 34]. In agreement with our experiment utilizing excess deoxynucleosides to overcome the SAMHD1-mediated restriction, pretreatment of cells with Vpx(+)VLPs completely reversed the restrictive capacity of MDMs (Figure 5A, representative flow cytometry). More importantly, Vpx(+)VLPs extinguished differences in infectivity between male- and female-derived MDMs (Figure 5B). Furthermore, addition of excess deoxynucleosides eliminated these sex-dependent differences, supporting a role for enhanced SAMHD1-mediated dNTPase activity in female-derived macrophages (data not shown). These experiments confirm that SAMHD1 is the key antiviral restriction factor responsible for sex-dependent differences in MDM infectivity with HIV-1, and that its activity is responsible for the restrictive capacity of female-derived MDMs relative to male-derived MDMs.
Figure 5.
Sex differences in monocyte-derived macrophage (MDM) infectivity are overcome via Vpx-mediated SAMHD1 degradation. (A) Representative flow plots of MDM mock-infected or infected with HIV-1-ΔEnv-GFP/VSVG ± 24 hours pretreatment with Vpx(+)VLPs. (B) Summary of n = 6 male and n = 6 female individual donors infected ± Vpx(+)VLPs. P values determined using the Wilcoxon matched-pairs signed-rank test. Horizontal lines represent the mean ± standard error of the mean. FSC, forward scatter; GFP, green fluorescent protein; VLP, virus-like particle.
DISCUSSION
SAMHD1 is among the most important restriction factors determining the low permissivity to HIV-1 infection of nondividing cells [24–26, 35, 36]. Activated, cycling CD4+ T cells that maintain SAMHD1 in a phosphorylated and inactive state are exquisitely sensitive to infection with HIV-1, whereas cells that express high levels of active SAMHD1 such as macrophages resist infection [24–26, 37]. An important mechanism of SAMHD1 regulation is T592 phosphorylation, a modification carried out by a number of cyclin-CDK pairs [27–29]. Threonine-592 phosphorylation catalyzed by these cyclin-CDK units destabilizes the SAMHD1 tetramer, which is required for its dNTPase and anti-HIV-1 activity [28, 29]. In activated CD4+ T cells, SAMHD1 phosphorylation is robustly enhanced to satisfy the cell’s requirement for dNTPs in DNA synthesis [27]. SAMHD1 phosphorylation in macrophages and other noncycling cells is similarly under dynamic control and is dependent on cues that range from growth factor signaling to pathogen recognition [20, 27, 38]. Although it has been shown that Toll-like receptor (TLR)7-dependent sex differences exist in DC susceptibility and response to HIV-1, differential regulation of SAMHD1 between males and females has not, to our knowledge, been previously observed. In this report, we identify SAMHD1 regulation as a novel molecular mechanism underlying baseline sex differences in infectivity of human macrophages.
The contribution of interferons (IFNs) to HIV-1 restriction is well established, and multiple downstream mechanisms that block infection from entry to release have been studied and reviewed at length [39]. An important consequence of IFN signaling is the dephosphorylation and activation of SAMHD1, which presents a potent barrier to infection that can be overcome by SAMHD1 proteolysis or small interfering ribonucleic acid-mediated silencing [20]. Given that a sex-dependent difference has been observed in IFN production in plasmacytoid DCs in response to TLR signaling, it is formally possible that baseline differences in IFN production contribute to differences in MDM infection with HIV-1 [40]. A type I IFN, IFN epsilon, is constitutively expressed in cells of the female reproductive tract, and its expression is highly induced upon steroid hormone treatment [41]. The fact that women are disproportionately affected by autoimmune disorders, particularly interferonopathies such as systemic lupus erythematosus, warrants further investigation into differences in immunologic pathways that govern both susceptibility to viral infections and chronic inflammatory diseases. We have previously reported a role for IFN-induced CDK1 downregulation as a crucial pathway responsible for SAMHD1 dephosphorylation in MDMs [20]. Intriguingly, we have shown that the resulting SAMHD1 activation is a vital component of the anti-HIV-1 IFN response. Although we did not address the possibility that baseline IFN expression may be an important factor in our observations, future studies will be conducted to explore this possibility.
CONCLUSIONS
Although this study is limited by small sample size and the use of cells from healthy, uninfected individuals, the results underscore the importance of sex as a biological variable and the need to include participants of both sexes in clinical and basic HIV-1 research. We establish for the first time SAMHD1 and its regulation as a critical component of sex differences in the control of HIV-1 infection, which may inform future investigations into natural immunity to HIV-1 and predisposition to conditions such as HAND.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We express our sincere gratitude to the study volunteers for their continued participation in this work and ongoing translational research. We also wish to express our gratitude to Dr Angela Presson for her invaluable assistance with statistical analysis.
Author contributions. The authors contributed to the following: conceptualization - M. A. S. and V. P.; methodology - M. A. S. and V. P.; investigation - M. A. S. and V. P.; writing, original draft - M. A. S. and V. P.; writing, review and editing - M. A. S., V. P., A. M. S., and A. B.; funding acquisition - M. A. S. and V. P.; and supervision - V. P., A. M. S., and A. B.
Financial support. This work was funded in part by National Institutes of Health (NIH) grants R21 AI122377-01 (NIAID, National Institutes of Allergy and Infectious Diseases; Funder ID 100000060) (to V. P.), R01 HL126547 (NHLBI, National Heart, Lung, and Blood Institute; Funder ID 100000050) (to A. M. S.), and 5T32DK007115-40 (NIDDK, National Institutes of Diabetes and Digestive and Kidney Diseases; Funder ID 100000062) (to M. A. S). A. M. S. is funded by a Doris Duke Charitable Foundation Clinical Scientist Development Award (CSDA201612). V. P. is funded by grant UM1-AI126620 (NIAID; Funder ID 100000060) (BEAT-HIV Delaney Collaboratory). A. B. is funded by grant UM1-AI126617 (NIAID; Funder ID 100000060) (BELIEVE Delaney Collaboratory).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med 2001; 344:720–5. [DOI] [PubMed] [Google Scholar]
- 2. Sterling TR, Lyles CM, Vlahov D, Astemborski J, Margolick JB, Quinn TC. Sex differences in longitudinal human immunodeficiency virus type 1 RNA levels among seroconverters. J Infect Dis 1999; 180:666–72. [DOI] [PubMed] [Google Scholar]
- 3. Touloumi G, Pantazis N, Babiker AG, et al. Differences in HIV RNA levels before the initiation of antiretroviral therapy among 1864 individuals with known HIV-1 seroconversion dates. AIDS 2004; 18:1697–705. [DOI] [PubMed] [Google Scholar]
- 4. Gandhi M, Bacchetti P, Miotti P, Quinn TC, Veronese F, Greenblatt RM. Does patient sex affect human immunodeficiency virus levels?Clin Infect Dis 2002; 35:313–22. [DOI] [PubMed] [Google Scholar]
- 5. Farzadegan H, Hoover DR, Astemborski J, et al. Sex differences in HIV-1 viral load and progression to AIDS. Lancet 1998; 352:1510–4. [DOI] [PubMed] [Google Scholar]
- 6. Meditz AL, MaWhinney S, Allshouse A, et al. Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. J Infect Dis 2011; 203: 442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997; 278:1295–300. [DOI] [PubMed] [Google Scholar]
- 8. Chun TW, Stuyver L, Mizell SB, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A 1997; 94:13193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong JK, Hezareh M, Günthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 1997; 278:1291–5. [DOI] [PubMed] [Google Scholar]
- 10. Honeycutt JB, Thayer WO, Baker CE, et al. HIV persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy. Nat Med 2017; 23:638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Honeycutt JB, Wahl A, Baker C, et al. Macrophages sustain HIV replication in vivo independently of T cells. J Clin Invest 2016; 126:1353–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gama L, Abreu CM, Shirk EN, et al. Reactivation of simian immunodeficiency virus reservoirs in the brain of virally suppressed macaques. AIDS 2017; 31:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sattentau QJ, Stevenson M. Macrophages and HIV-1: an unhealthy constellation. Cell Host Microbe 2016; 19:304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koppensteiner H, Brack-Werner R, Schindler M. Macrophages and their relevance in human immunodeficiency virus type I infection. Retrovirology 2012; 9:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rodrigues V, Ruffin N, San-Roman M, Benaroch P. Myeloid cell interaction with HIV: a complex relationship. Front Immunol 2017; 8:1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maschke M, Kastrup O, Esser S, Ross B, Hengge U, Hufnagel A. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART). J Neurol Neurosurg Psychiatry 2000; 69:376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heaton RK, Clifford DB, Franklin DR Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chai Q, Jovasevic V, Malikov V, et al. HIV-1 counteracts an innate restriction by amyloid precursor protein resulting in neurodegeneration. Nat Commun 2017; 8:1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Macedo AB, Resop RS, Martins LJ, et al. Influence of biological sex, age, and HIV status in an in vitro primary cell model of HIV latency using a CXCR4 tropic virus. AIDS Res Hum Retroviruses 2018; 34:769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szaniawski MA, Spivak AM, Cox JE, et al. SAMHD1 phosphorylation coordinates the anti-HIV-1 response by diverse interferons and tyrosine kinase inhibition. MBio 2018; 9:pii:e00819-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Imbeault M, Lodge R, Ouellet M, Tremblay MJ. Efficient magnetic bead-based separation of HIV-1-infected cells using an improved reporter virus system reveals that p53 up-regulation occurs exclusively in the virus-expressing cell population. Virology 2009; 393:160–7. [DOI] [PubMed] [Google Scholar]
- 22. Spivak AM, Rabi SA, McMahon MA, et al. Short communication: dynamic constraints on the second phase compartment of HIV-infected cells. AIDS Res Hum Retroviruses 2011; 27:759–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Finkelshtein D, Werman A, Novick D, Barak S, Rubinstein M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc Natl Acad Sci U S A 2013; 110:7306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lahouassa H, Daddacha W, Hofmann H, et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol 2012; 13:223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laguette N, Sobhian B, Casartelli N, et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 2011; 474:654–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hrecka K, Hao C, Gierszewska M, et al. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 2011; 474:658–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cribier A, Descours B, Valadão AL, Laguette N, Benkirane M. Phosphorylation of SAMHD1 by cyclin A2/CDK1 regulates its restriction activity toward HIV-1. Cell Rep 2013; 3:1036–43. [DOI] [PubMed] [Google Scholar]
- 28. Jang S, Zhou X, Ahn J. Substrate specificity of SAMHD1 triphosphohydrolase activity is controlled by deoxyribonucleoside triphosphates and phosphorylation at Thr592. Biochemistry 2016; 55:5635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yan J, Kaur S, DeLucia M, et al. Tetramerization of SAMHD1 is required for biological activity and inhibition of HIV infection. J Biol Chem 2013; 288:10406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pauls E, Badia R, Torres-Torronteras J, et al. Palbociclib, a selective inhibitor of cyclin-dependent kinase4/6, blocks HIV-1 reverse transcription through the control of sterile α motif and HD domain-containing protein-1 (SAMHD1) activity. AIDS 2014; 28:2213–22. [DOI] [PubMed] [Google Scholar]
- 31. Pauls E, Ruiz A, Riveira-Muñoz E, et al. p21 regulates the HIV-1 restriction factor SAMHD1. Proc Natl Acad Sci U S A 2014; 111:E1322–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berger G, Goujon C, Darlix JL, Cimarelli A. SIVMAC Vpx improves the transduction of dendritic cells with nonintegrative HIV-1-derived vectors. Gene Ther 2009; 16:159–63. [DOI] [PubMed] [Google Scholar]
- 33. Ahn J, Hao C, Yan J, et al. HIV/simian immunodeficiency virus (SIV) accessory virulence factor Vpx loads the host cell restriction factor SAMHD1 onto the E3 ubiquitin ligase complex CRL4DCAF1. J Biol Chem 2012; 287:12550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goujon C, Jarrosson-Wuillème L, Bernaud J, Rigal D, Darlix JL, Cimarelli A. With a little help from a friend: increasing HIV transduction of monocyte-derived dendritic cells with virion-like particles of SIV(MAC). Gene Ther 2006; 13:991–4. [DOI] [PubMed] [Google Scholar]
- 35. Descours B, Cribier A, Chable-Bessia C, et al. SAMHD1 restricts HIV-1 reverse transcription in quiescent CD4(+) T-cells. Retrovirology 2012; 9:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cenker JJ, Stultz RD, McDonald D. Brain microglial cells are highly susceptible to HIV-1 infection and spread. AIDS Res Hum Retroviruses 2017; 33:1155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pan X, Baldauf HM, Keppler OT, Fackler OT. Restrictions to HIV-1 replication in resting CD4+ T lymphocytes. Cell Res 2013; 23:876–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Badia R, Pujantell M, Riveira-Muñoz E, et al. The G1/S specific cyclin D2 is a regulator of HIV-1 restriction in non-proliferating cells. PLoS Pathog 2016; 12:e1005829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang B, Kang W, Zuo J, Kang W, Sun Y. The significance of type-I interferons in the pathogenesis and therapy of human immunodeficiency virus 1 infection. Front Immunol 2017; 8:1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Meier A, Chang JJ, Chan ES, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 2009; 15:955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fung KY, Mangan NE, Cumming H, et al. Interferon-ε protects the female reproductive tract from viral and bacterial infection. Science 2013; 339:1088–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.