Abstract
Rationale: Frailty represents an increased vulnerability to adverse health outcomes. The frailty phenotype conceptual model (three or more patient attributes of wasting, exhaustion, low activity, slowness, and weakness) is associated with increased morbidity and mortality in geriatric populations.
Objectives: Our objective was to describe the risks associated with frailty in patients with chronic obstructive pulmonary disease.
Methods: Data from the National Emphysema Treatment Trial (NETT) were retrospectively analyzed. The frailty phenotype conceptual model was operationalized as three or more frailty parameters (a body mass index decrease of ≥5% over 12 months, self-reported exhaustion, low 6-minute walk distance, or physical activity or respiratory muscle strength in the lowest quartile). Frail participants were compared with participants with two or fewer frailty parameters. Participants were followed starting 12 months after NETT randomization (to minimize surgical effect) for 24 months. Univariate, multivariate, Kaplan-Meier, and Cox proportional hazard analyses were performed, adjusting for treatment arm, age, modified Medical Research Council dyspnea scale, sex, and baseline forced expiratory volume in 1 second (FEV1). Multiple imputation was used for missing values.
Results: The participants (N = 902) were predominantly white (94.5%) males (59.5%), with a median age of 67 years (interquartile range, 63–70 yr) and a median FEV1% predicted of 26 (interquartile range, 20–33). Six percent of the participants (95% confidence interval [CI], 4.5 to 7.6) were frail. The incidence rate of frailty was 6.4 per 100 person-years. Frail participants reported significantly worse disease-specific and overall quality of life by St. George’s Respiratory Questionnaire total score (mean difference of 11.6; 95% CI, 7.6 to 15.6; P < 0.001), mental composite on Medical Outcomes Survey Short Form-36 (mean difference −6.8; 95% CI, −10.0 to −3.6; P < 0.001), and physical composite scores on Medical Outcomes Survey Short Form-36 (mean difference −16.7; 95% CI, −21.3 to −12.1; P = 0.001). Frail participants had an increased rate of hospitalization (adjusted hazard ratio, 1.6; 95% CI, 1.1 to 2.5; P = 0.02) and an adjusted increase in hospital use of 8.0 days (95% CI, 4.4 to 11.6; P < 0.001) compared with nonfrail participants. Frail participants had a higher mortality rate (adjusted hazard ratio, 1.4; 95% CI, 0.97 to 2.0; P = 0.07).
Conclusions: Among adults with chronic obstructive pulmonary disease, our measure of frailty (modified from the Fried frailty phenotype) was associated with incident and longer-duration hospitalization, and with poor quality of life.
Keywords: frailty, COPD, survival, hospitalizations, quality of life
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death in the world, and is associated with high clinical and financial burdens (1, 2). COPD is a heterogeneous disease; some patients with COPD have certain attributes that others do not (e.g., frequent exacerbations) that influence outcomes (3). Further research into COPD prognostic factors is necessary to improve the care and counseling of patients (4–6).
Frailty is a syndrome of vulnerability due to physiologic dysfunction and decline. It is commonly described as an increased susceptibility to adverse health outcomes. Frailty is associated with increased morbidity and mortality in geriatric and certain chronic-disease populations (7–14). Frailty is often assessed according to the Fried frailty phenotype (7). First described in geriatric individuals, the conceptual model of the frailty phenotype consists of a constellation of three or more frailty parameters (wasting, exhaustion, decreased physical activity, slowness, and weakness) (7). Patients with respiratory impairment are at an increased risk for frailty (15, 16). In fact, the prevalence of frailty by the Fried frailty phenotype in elderly patients with COPD is 10.2%, roughly twice that observed in patients without COPD (16). In patients with COPD, frailty is a risk factor for noncompletion of pulmonary rehabilitation (17). Maddocks and colleagues attributed this risk to hospitalizations and acute exacerbations in frail patients (17). Interestingly, frailty in lung transplant candidates (a significant proportion of whom are patients with COPD) is associated with both pre- and post-transplant mortality (13, 14). Further study is needed to determine the importance of the frailty phenotype in COPD.
Our primary objective was to determine whether the frailty phenotype was associated with mortality in COPD. Our secondary objectives were 1) to determine whether the frailty phenotype was associated with the timing and extent of hospitalizations in COPD, 2) to examine quality of life in the context of frailty, and 3) to describe the prevalence and incidence of frailty in our cohort.
This work was presented in poster form at the annual meeting of the International Society of Heart and Lung Transplantation, Nice, France, 2015 (18).
Methods
Inclusion/Exclusion
This study used data from the previously published NETT study, a large, randomized controlled trial that was conducted over 4.5 years and compared lung volume reduction surgery with medical management in patients with COPD (19, 20). The NETT was conducted in accordance with the amended Declaration of Helsinki. Local institutional review boards approved the NETT protocol and written informed consent was obtained from participants (19, 20). Participants from 17 centers were included if they were nonsmokers (abstinent ≥ 6 mo) with moderate to severe COPD. Subjects were excluded if they had ≥10% weight loss in the prior 90 days or a 6-minute walk distance (6MWD) of ≤140 m after pulmonary rehabilitation (20). Prospective collection of patient-reported outcomes, 6MWD, pulmonary function tests, and Medicare claims data was performed (21). In addition to annual visits, participating sites phoned participants every 3 months to collect exercise and healthcare use data. Our study included NETT participants 12 months after randomization (our study’s baseline to minimize surgical effects) and followed participants an additional 24 months (using the participants’ annual study visits and telephonically collected data). Mortality was recorded as of September 30, 2008.
Definitions
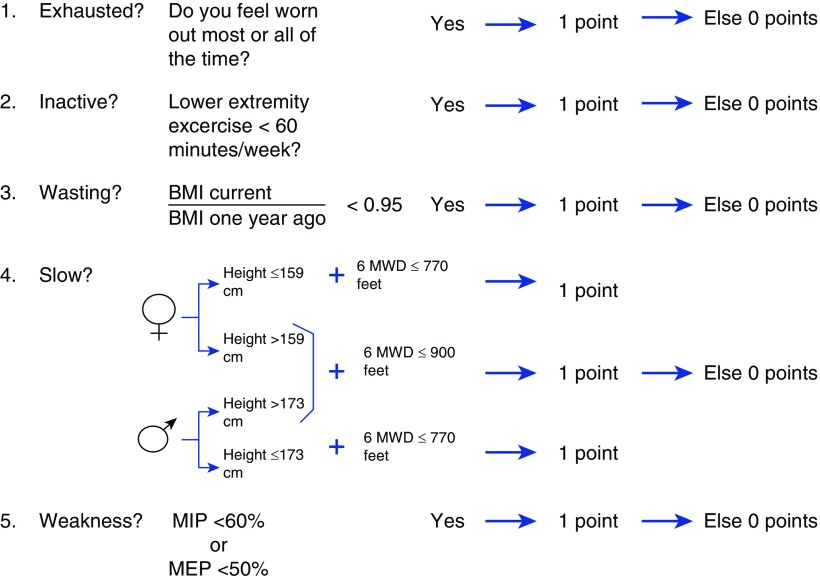
We used the conceptual model of the Fried frailty phenotype to define frailty (7). All baseline parameters were measured 12 months after NETT study enrollment. Participants were considered “frail” if they had three or more of the following frailty parameters: wasting, exhaustion, low physical activity, slowness, and weakness (Figure 1) (7). Participants with one or two parameters present were “prefrail” and those with none were “nonfrail.” Wasting was defined as a decline in measured body mass index (BMI) of ≥5% over the past year, a modification of Fried’s criteria to place the weight loss in the context of the height and baseline weight of the participant. Exhaustion was defined as feeling worn out “all” or “most” of the time in the past 4 weeks, a patient-reported measure of the Medical Outcomes Survey Short Form-36 (SF-36) in lieu of the two exhaustion questions from the Chronic Epidemiologic Studies of Depression Questionnaire (these data were not collected during the NETT study) (22). Low physical activity was defined as <60 minutes of lower-extremity exercise per week (the lower quartile of activity for this cohort), as assessed telephonically during the NETT study. This was in substitution for the short Minnesota Leisure Time Activity Questionnaire used by Fried and colleagues (7). Slowness was defined as a 6MWD ≤ 770 ft if the subject was short (≤173 cm for men; ≤159 cm for women) or ≤900 ft if the subject was tall (>173 cm for men; >159 cm for women) (7). These cutoffs were derived from the lowest quintile for gait speed in men and women used by Fried and colleagues (7). Grip strength was not collected during NETT. Prior studies have demonstrated maximal respiratory pressures correlates with grip strength (r = 0.7) and differs between frail, prefrail, and nonfrail individuals (23). Weakness was defined as a maximal inspiratory pressure (MIP) < 60% or a maximal expiratory pressure (MEP) < 50% predicted, the lower quartile for this cohort (24).
Figure 1.
Frailty phenotype in chronic obstructive pulmonary disease (COPD). “Frail” was defined as the presence of three or more of the following parameters: wasting, exhaustion, low physical activity, slowness, or weakness. Participants with one or two parameters present were “prefrail” and those with none were “nonfrail/normal.” Wasting was defined as a decline in body mass index (BMI) of ≥5% over the past year. Exhaustion was defined as feeling worn out “all” or “most” of the time in the past 4 weeks. Low physical activity was defined as <60 minutes of lower-extremity exercise per week (the lower quartile for this cohort). Slowness was defined using the 6-minute walk distance (6MWD): ≤770 ft if the subject was short (≤173 cm for men; ≤159 cm for women) and ≤900 ft if the subject was tall (>173 cm for men; >159 cm for women). Weakness was defined as a maximal inspiratory pressure (MIP) < 60% or a maximal expiratory pressure (MEP) < 50% predicted (the lower quartile for this cohort).
Predictors and Outcomes
Our primary predictor of interest was the presence or absence of the frailty phenotype as a dichotomous variable comparing frail with nonfrail/prefrail. Our primary outcome of interest was all-cause mortality. Secondary outcomes of interest included time to first hospitalization and the number of hospital days. We also evaluated frailty prevalence and associations with quality of life as measured by St. George’s Respiratory Questionnaire (SGRQ) and the SF-36 (22, 25).
Statistical Analysis
Participant characteristics were summarized using medians and interquartile ranges (IQRs) for continuous variables, and counts and percentages with standard deviations (SDs) for categorical variables. Differences between groups were tested using Wilcoxon rank-sum and chi-square tests as appropriate. Spearman correlation coefficients were used to assess the strength of associations between variables. Changes in frailty over time were determined using the percent frail at each time point and tested using chi-square tests. Person-years of frailty were calculated as the number of frail cases divided by the total time the participants were at risk for frailty. Statistical analyses were performed using SAS software version 9.4 (SAS Institute, Inc.). P values < 0.05 were considered significant.
All of the primary models used frailty as a binary indicator variable that compared the frail group with the group that combined nonfrail and prefrail subjects. All time-related variables were calculated as the time from our study’s baseline (the NETT evaluation 12 months after randomization). Time to death and time to first hospitalization were modeled using Cox proportional hazards models. Kaplan-Meier curves and log-rank tests were also used for these time-to-event endpoints. The number of days in the hospital was modeled using linear multivariable regression models. All of the models were checked to verify that the modeling assumptions were met. We adjusted for components of the ADO index (age, modified Medical Research Council [mMRC] scale, and baseline forced expiratory volume in 1 second [FEV1]) (26) sex, and lung volume reduction surgery. Missing data values were imputed via the Markov chain Monte Carlo method with 25 multiple imputations. All models were validated using bootstrapping. One thousand bootstrapped samples were selected from the original study population. Lasso models were selected from each of the bootstrapped samples, and the percent of time each variable was selected for the model was calculated. Variables that were selected in >70% of the samples were considered stable predictors (27).
Results
Demographics
Acknowledging that frail patients are less likely to provide Month 12 data than nonfrail patients due to early deaths and drop outs, we performed a landmark analysis comparing frail and nonfrail patients 12 months after NETT randomization. Of the original 1,218 NETT participants, 902 had data 12 months after randomization, allowing for inclusion. Included participants did not differ from excluded subjects by age or BMI, but did have small, but statistically significantly better, mMRC (mean difference 0.1 ± 0.05) and FEV1% predicted (mean difference 1.4% ± 0.41%) values. Because a low 6MWD after pulmonary rehabilitation was an exclusion criterion, it was not surprising that excluded patients also had lower 6MWD values (mean difference 38 ± 6 m). No data were missing for age, sex, or treatment allocation arm. mMRC scores were missing for 36 participants (4%), and FEV1 was missing for 113 patients (13%). Baseline characteristics are included in Table 1. The participants were predominantly white (94.5%), male (59.5%), and married (65.2%), with a median age of 67 (IQR, 63–70) and a median FEV1% predicted of 26 (IQR, 20–33). Comparisons of baseline characteristics by frailty categorization are provided in Table 1. Frail participants were more likely to be in the nonsurgical arm of the study (P = 0.02) and had less education (P = 0.02). Over the course of our study, 298 of the 902 participants died and 304 hospitalizations occurred.
Table 1.
Participant characteristics
| Frail | Prefrail or Normal | |
|---|---|---|
| Treatment, n (%) | ||
| Lung volume reduction therapy | 20 (35.1) | 428 (51.6) |
| Medical therapy | 37 (64.9) | 401 (48.4) |
| Age, years median (IQR) | 69 (64–71) | 67 (63–70) |
| Male, n (%) | 34 (59.6) | 529 (59.7) |
| Race, n (%) | ||
| White (not Hispanic) | 52 (91.2) | 785 (94.7) |
| African American (not Hispanic) | 3 (5.3) | 30 (3.6) |
| Hispanic | 1 (1.8) | 4 (0.5) |
| Asian or Pacific Islander | 0 (0) | 8 (1.0) |
| Other | 1 (1.8) | 2 (0.2) |
| Marital status, n (%) | ||
| Single, never married | 0 (0.0) | 25 (3.0) |
| Separated | 0 (0.0) | 15 (1.8) |
| Divorced or annulled | 8 (14.0) | 119 (14.4) |
| Widowed | 8 (14.0) | 132 (15.9) |
| Married | 41 (71.9) | 538 (64.9) |
| Education, n (%) | ||
| Did not complete high school | 19 (33.3) | 153 (18.5) |
| Completed high school | 11 (19.3) | 272 (32.8) |
| Some college or post high school | 20 (35.1) | 280 (33.8) |
| Bachelor degree or higher | 7 (12.3) | 124 (15.0) |
| Economic status, n (%) | ||
| <$15,000 | 10 (17.5) | 145 (17.7) |
| $15,000–$29,999 | 24 (42.1) | 293 (35.7) |
| $30,000–$49,999 | 14 (24.6) | 219 (26.7) |
| ≥$50,000 | 9 (15.8) | 163 (19.9) |
| Questionnaires | ||
| St. George’s Respiratory Questionnaire composite, median (IQR) | 60.3 (52.8–69.1) | 48.7 (35.7–60.2) |
| SF-36, physical functioning median (IQR) | 15 (5–30) | 30 (15–50) |
| SF-36, physical composite, median (IQR) | 25.1 (21.3–31.6) | 30.1 (23.9–38.8) |
| SF-36, mental composite, median (IQR) | 46.0 (37.2–56.3) | 57.3 (47.4–61.7) |
| Pulmonary function testing | ||
| Forced expiratory volume in 1 s, % predicted, median (IQR) mMRC, median (IQR) | 23 (18.5–29.0) | 26 (20–34) |
| 4 (3–4) | 3 (2–4) | |
| Total lung capacity, % predicted, median IQR | 122 (106–134) | 119 (107–130) |
| Laboratory testing | ||
| Albumin abnormal | 1.8% | 5.2% |
| White blood cell count, median (IQR) | 8.5 (6.4–10.1) | 8.0 (6.6–9.9) |
Definition of abbreviations: IQR = interquartile range; SF-36 = Medical Outcomes Short Form-36; mMRC = modified Medical Research Council.
The data show the number of participants, with percentage in parentheses, unless the medians and IQRs are indicated.
Prevalence and Incidence
The prevalence of frailty was 6% (95% confidence interval [CI], 4.5–7.6%). This reflects the percentage of participants who were frail 12 months after NETT randomization, our study’s baseline. At this baseline, the majority of participants were prefrail (n = 547), 282 were nonfrail, 57 were frail, and 16 did not have available frailty data. Of the frail participants, 31 had wasting, 37 were slow, 28 were exhausted, 31 had decreased physical activity, 25 had a low MIP, and 47 had a low MEP.
Interestingly, the number and percentage of frail participants did not vary much between baseline (6%), 12 months (7%), and 24 months of follow-up (4%). The incidence rate (the percentage of patients who were not frail at one evaluation but were frail at the next evaluation) for new frailty phenotype in 1 year was 5.9%, with an incident rate per 100 person-years of 6.4 (95% CI, 5.5–7.4). Of those who were nonfrail at baseline, 50% stayed nonfrail, 43% became prefrail, 2% became frail, and 6% died (with 28 lost to follow-up). Of those who were prefrail at baseline, 23% improved to nonfrail in 12 months, 63% remained prefrail, 9% became frail, and 5% died (43 lost to follow-up). This gives an incidence of frailty in the prefrail category of 9% (95% CI, 6–11%) per year, or greater than four times that observed in nonfrail participants (2%; 95% CI, 1–5%). Of those who were frail at baseline, 8% died and 14% remained frail. Of the others, 64% became prefrail and 14% became normal (with 7 lost to follow-up).
Frailty Phenotype and Prefrailty
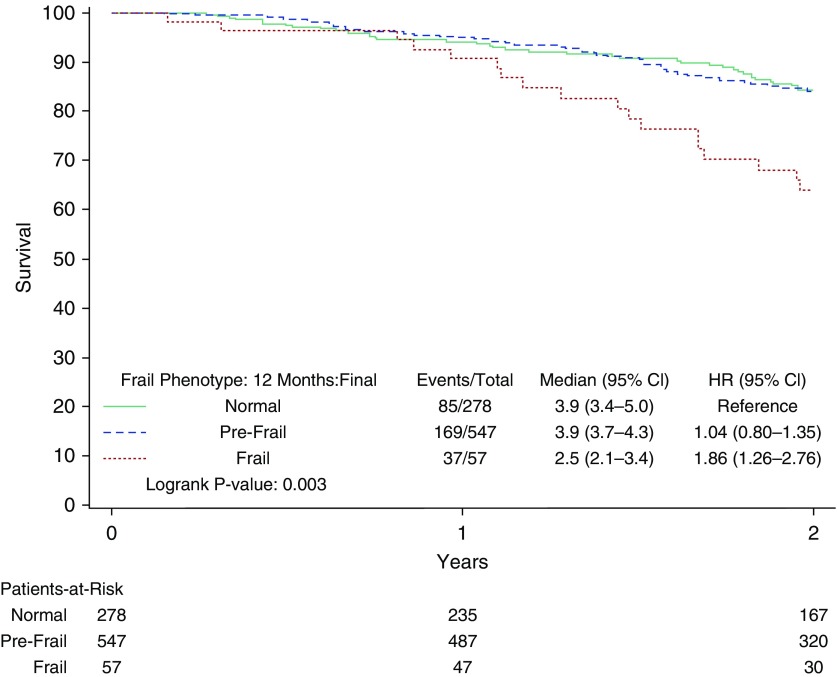
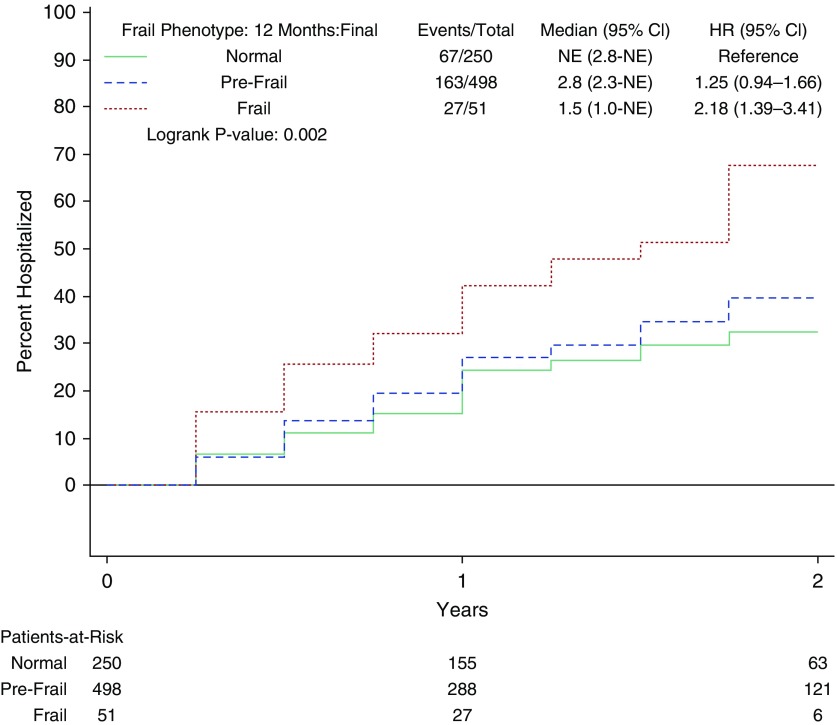
Frail participants had significantly worse survival by Kaplan-Meier survival analysis (see Figure 2). The 2-year event rate for mortality was 36% (29.2 per 100 person-years) for frail participants versus 16% for prefrail or nonfrail participants (13.4 per 100 person-years; see Table 2). After adjustment, frail participants had a hazard ratio (HR) of 1.4 (95% CI, 0.97–2.0; P = 0.07) for mortality. In addition, the frailty phenotype was associated with an 8.0-day increase in hospital length of stay (CI, 4.4–11.6 d; P < 0.0001) and increased incidence of hospitalization (adjusted HR [aHR], 1.6; CI, 1.1–2.5; P = 0.02) after adjustment (Figure 3).
Figure 2.
Kaplan-Meier curves for time to death stratified by the frailty phenotype. The curves demonstrate survival of normal (solid green line), prefrail (dashed blue line), and frail (dotted red line) participants. CI = confidence interval; HR = hazard ratio.
Table 2.
Associations of frailty with mortality and hospitalization in this cohort
| Frailty Phenotype Score 0–5 (Continuous Variable) | Frail (Score ≥ 3) | Prefrail (Score 1–2) | Nonfrail (Score 0) | |
|---|---|---|---|---|
| Mortality | ||||
| Number of subjects | 882 | 57 | 547 | 278 |
| Number of deaths | 291 | 37 | 169 | 85 |
| Rate per 100 person-years | 29.2 | 13.4 | 13.4 | |
| Two-year event rate (95% CI) | 36% (24–51) | 16% (13–20) | 16% (11–21) | |
| Crude hazard ratio (95% CI)* | 1.2 (1.1–1.4) | 1.9 (1.3–2.8) | 1.0 (0.8–1.4) | — |
| Adjusted hazard ratio (95% CI)* | 1.1 (0.9–1.2) | 1.5 (0.96–2.4) | 1.0 (0.7–1.3) | — |
| Hospitalizations | ||||
| Number of subjects | 799 | 51 | 498 | 250 |
| Number of hospitalizations | 257 | 27 | 163 | 67 |
| Rate per 100 person-years | 47.4 | 27.0 | 21.4 | |
| Two-year event rate (95% CI) | 68% (50–84) | 40% (35–45) | 32% (26–40) | |
| Crude hazard ratio (95% CI)* | 1.3 (1.2–1.5) | 2.2 (1.4–3.4) | 1.3 (0.9–1.7) | — |
| Adjusted hazard ratio (95% CI)* | 1.2 (1.1–1.4) | 1.8 (1.1–2.9) | 1.1 (0.8–1.5) | — |
Definition of abbreviation: CI = confidence interval.
Hazard ratios are calculated compared with the nonfrail group.
Figure 3.
Cumulative incidence of time to first hospitalization stratified by the presence or absence of the frailty phenotype. The curves demonstrate time to first hospitalization for normal (solid green line), prefrail (dashed blue line), and frail (dotted red line) participants. CI = confidence interval; HR = hazard ratio; NE = not evaluable.
A post hoc sensitivity analysis was performed using the frailty phenotype score as a continuous variable, with minimal impact on the results. A one-point increase in the frailty phenotype score resulted in an estimated increase of 1.7 days in the hospital (95% CI, 0.7–2.7 d; P < 0.0001), an aHR of 1.2 (95% CI, 1.1–1.4; P < 0.01) for time to hospitalization, and an aHR of 1.1 (95% CI, 0.9–1.2; P = 0.25) for survival. Prefrail (as an additional indicator variable in the adjusted models) was not significantly related to days in the hospital, time to hospitalization, or survival time (see Table 2).
Quality of Life
Frail patients reported a worse quality of life. The total SGRQ score for frail patients was 60.3, with higher scores indicating more impairment (25). The mean difference was 11.6 (95% CI, 7.6–15.6; P < 0.0001)—nearly three times the reported minimum clinically important difference for SGRQ of 4 (28). Likewise, frail patients reported consistently lower scores (indicating worse functioning) than the other patients for SF-36 physical functioning (mean difference −16.7; 95% CI, −21.3 to −12.1; P < 0.0001) and physical composite (mean difference −5.5; 95% CI, −7.6 to −3.4; P = 0.0001) scores. When the frailty phenotype score was used as a continuous variable, the Spearman correlation coefficients were −0.26 and −0.29 for SF-36 physical composite and physical function scores, respectively (P < 0.001). Frail participants also reported a lower (worse) SF-36 mental composite score (mean difference −6.8; 95% CI, −10.0 to −3.6; P < 0.0001) (22).
Discussion
Given the association of frailty with important health outcomes in geriatric patients and those with other chronic diseases, we postulated that frailty would be an important predictor in patients with COPD (7, 14, 29–32). Based on the initial work of Fried and colleagues and subsequent frailty studies, we used the conceptual paradigm of the frailty phenotype to measure frailty in COPD and assess relevant outcomes.
We found the prevalence of frailty in COPD to be 6%. This is lower than the 10.2% frailty prevalence (by the Fried frailty phenotype) in elderly patients with COPD (median age 75 yr) described by Lahousse and colleagues (16). Our lower prevalence likely reflects the different frailty measurements used, the younger cohort used in the current study, and the rigorous screening process of NETT (whereby subjects with baseline weight loss, pulmonary rehabilitation noncompleters, or those with a low 6MWD despite rehabilitation were excluded). The persistence of an association of frailty with increased hospitalization despite this screening and pulmonary rehabilitation, we believe, strengthens the importance of the association. It is similar to previous findings in lung-transplant studies, which showed that heavily scrutinized and selected candidates, who often underwent pretransplant pulmonary rehabilitation, still demonstrated an increased risk of pre- and post-transplant mortality when they were frail (13, 14). In our study, prefrail patients had a greater than fourfold increased risk of developing frailty. Prior studies of the pathogenesis of frailty support this finding. Frailty is often described as a “cycle of decline,” in which impairment in one parameter, such as low physical activity, leads to declines in other frailty parameters (33).
We have demonstrated a possible association of the frailty phenotype with increased mortality in patients with COPD as frail patients. The “frail” designation had an HR of 1.4 in this cohort, after controlling for ADO index, sex, and treatment allocation arm (P = 0.07). This expands upon the work of Vaz Fragoso and colleagues, who identified an increased risk of mortality in patients with both respiratory impairment by spirometry and frailty compared with those without either (15). This finding is also consistent with findings in frail lung-transplant candidates. Singer and colleagues demonstrated that frail candidates are at increased risk of a composite endpoint of death or delisting (14). In addition, Wilson and colleagues demonstrated that pretransplant frailty is associated with increased post-transplant mortality (13).
Mittal and colleagues previously showed that frail pulmonary rehabilitation attendees (both with and without COPD) were more likely to self-report a hospitalization in the previous 12 months than nonfrail patients (34). Furthermore, Maddocks and colleagues demonstrated that frail patients with COPD were less likely to complete pulmonary rehabilitation, in part due to hospitalizations (17). Our work confirms and expands upon these prior findings. We have also demonstrated a significant association of the frailty phenotype with decreased time to first hospitalization (aHR, 1.6). In addition, frail patients with COPD had a median increase of 8 days in the hospital.
COPD Phenotype
Recent work in COPD has focused on describing clusters of patients with similar attributes that are associated with prognosis and important clinical outcomes termed phenotypes (3). Previous works have described the emphysema-hyperinflation, COPD-asthma overlap, and frequent-exacerbator COPD phenotypes (35). The recognition of such phenotypes allows clinical screening for a unique population at risk and allows for tailored medical regimens. We believe that the frailty phenotype similarly identifies a unique population at risk for increased hospitalizations, death, and poor quality of life. Based on the preliminary work by Maddocks and colleagues, pulmonary rehabilitation may offer unique benefits to this population (17). Although pulmonary rehabilitation is already recommended for symptomatic COPD, further studies to clarify whether pulmonary rehabilitation significantly improves frailty and frailty parameters are desired. An exercise intervention may be the best intervention to reverse the frailty syndrome. This notion is supported by preliminary studies in geriatric individuals and patients with COPD that demonstrated improvement of frailty parameters after exercise intervention (36, 37).
Implications for Clinical Practice
We believe this study has immediate implications for clinical practice. Given the use of multicenter recruitment and the large number of participants in this study, we expect our findings to be widely generalizable to patients with moderate to severe COPD. Even after adjustment for known prognostic factors, participants with a frailty phenotype had a decreased time to first hospitalization and an increased duration of hospitalization by a median of 8 days, suggesting a large burden of morbidity and costs associated with frailty. Assessing the frailty phenotype will assist clinicians to counsel their patients about the risks and prognosis associated with frailty. In addition, emerging literature in geriatrics suggests that exercise-based frailty interventions may improve frailty (37). Furthermore, Altenburg and colleagues have demonstrated that patients with advanced COPD and lower exercise capacity and strength at baseline have greater improvement after pulmonary rehabilitation (36). Finally, although it was powered as a prevalence study, the work by Maddocks and colleagues suggested that completion of pulmonary rehabilitation is beneficial for frail patients with COPD (17). Further studies are needed to determine whether the benefits of such interventions are sustained, and whether relapses of frailty can be treated with similar interventions.
Currently, measurement of the frailty phenotype is not the standard of care. However, with a simple stepwise approach, frailty assessments should only minimally increase clinic visit times and testing.
Implications for Future Research
This study has described the importance of the frailty phenotype in COPD. A further understanding of how the COPD frailty phenotype can be modified or treated, and whether modification improves hospitalization, mortality, and quality of life outcomes are key topics for future research.
Strengths and Limitations
The strengths of this study include multicenter recruitment, a large sample size, and the rigorous prospective methodology of the NETT database.
This study has several limitations. Although we used the previously described frailty phenotype paradigm from the geriatric literature (wasting, slowness, low physical activity, weakness, and exhaustion) (7), we also used practical clinical measures that were available in the NETT database and are likely available in clinical settings. For example, in lieu of gait speed, we used the 6MWD to measure slowness. We believe this is justifiable because gait speed is strongly correlated with the 6MWD (r = 0.77–0.80); however, further comparisons may be needed to avoid misclassifications (38). In lieu of grip strength, which was unavailable in the NETT database, we used respiratory muscle strength. Respiratory muscle strength is strongly correlated with grip strength (r = 0.7) and has been demonstrated to differ significantly between frail and nonfrail subjects (23). Respiratory muscle strength is also more likely to be clinically available than grip strength. However, respiratory muscle strength has the disadvantage that it may also be affected by worsening pulmonary disease. Finally, we did not use the Minnesota Leisure Time Activity Questionnaire, as used in the original geriatric cohort by Fried and colleagues, and instead used self-reported lower-extremity physical activity (7). However, Baldwin and colleagues demonstrated that the Minnesota Leisure Time Activity Questionnaire had a substantial floor effect in a population with respiratory disease (39). Although it was done for pragmatic reasons of data availability, translating the Fried frailty phenotype into clinically available measures might be seen as a strength of this study.
Our study was conducted in a population of patients with COPD who were prescreened for inclusion in a clinical trial. The influence of comorbidities on frailty was not explored. The selection criteria excluded patients with unintentional weight loss or a low 6MWD after pulmonary rehabilitation. Although our cohort began 1 year later, frailty prevalence may have been underestimated.
Missing data is another limitation. Sixteen participants lacked data on all frailty data points. In addition, the 6MWD was only measured at our study’s baseline (12 months after randomization) and 1 year later. However, we found that the overall 6MWD declined by only 60 ft, or 4%, from the initial measurement to the follow-up measurement 12 months later. If we were to assume another 4% decline in the 6MWD in the subsequent 12 months, we may have underestimated the number of frail participants at our 24-month follow-up by four subjects, or 0.4%. Given the small effect on frailty categorization, we did not believe it was necessary to use an imputed value for the 24-month 6MWD. Finally, there was an 8.6% loss to follow-up between our baseline and 12-month follow-up data.
Additionally, the NETT study involved a largely white population, and it is unknown whether our work can be extrapolated to nonwhite patients.
Conclusions
Frailty is a state of increased susceptibility to adverse health outcomes. In this study, frailty was associated with decreased time to first hospitalization and increased days in the hospital compared with subjects who were not frail. In addition, frail patients reported a worse quality of life.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the NETT investigators for the use of the NETT database: A. P. Fishman, B. A. Bozzarello, A. Al-Amin, M. Katz, C. Wheeler, E. Baker, P. Barnard, J. Carter, S. Chatziioannou, K. Conejo-Gonzales, J. Haddad, D. Hicks, N. Kleiman, M. Milburn-Barnes, C. Nguyen, M. Reardon, J. Reeves-Viets, S. Sax, A. Sharafkhaneh, C. Young, R. Espada, R. Butanda, K. Dubose, M. Ellisor, P. Fox, K. Hale, E. Hood, A. Jahn, S. Jhingran, K. King, C. Miller, I. Nizami, T. Officer, J. Ricketts, J. Rodarte, R. Teague, K. Williams, J. Reilly, D. Sugarbaker, C. Fanning, S. Body, S. Duffy, V. Formanek, A. Fuhlbrigge, P. Hartigan, S. Hooper, A. Hunsaker, F. Jacobson, M. Moy, S. Peterson, R. Russell, D. Saunders, S. Swanson, R. McKenna, Z. Mohsenifar, C. Geaga, M. Biring, S. Clark, R. Frantz, P. Julien, M. Lewis, J. Minkoff-Rau, V. Yegyan, M. Joyner, M. De-Camp, J. Stoller, Y. Meli, J. Apostolakis, D. Atwell, J. Chapman, P. DeVilliers, R. Dweik, E. Kraenzler, R. Lann, N. Kurokawa, S. Marlow, K. McCarthy, P. McCreight, A. Mehta, M. Meziane, O. Minai, P. O’Donovan, M. Steiger, K. White, J. Maurer, C. Hearn, S. Lubell, R. Schilz, T. Durr, M. Ginsburg, B. Thomashow, P. Jellen, J. Austin, M. Bartels, Y. Berkman, P. Berkoski, F. Brogan, A. Chong, G. DeMercado, A. DiMango, B. Kachulis, A. Khan, B. Mets, M. O’Shea, G. Pearson,J. Pfeffer, L. Rossoff, S. Scharf, M. Shiau, P. Simonelli, K. Stavrolakes, D. Tsang, D. Vilotijevic, C. Yip, M. Mantinaos, M. McKeon, N. MacIntyre, R. D. Davis, J. Howe, R. E. Coleman, R. Crouch, D. Greene, K. Grichnik, D. Harpole, A.Krichman, B. Lawlor, H. McAdams, J. Plankeel, S. Rinaldo-Gallo, J. Smith, M. Stafford-Smith, V. Tapson, M. Steele, J. Norten, J. Utz, C. Deschamps, K. Mieras, M. Abel, M. Allen, D. Andrist, G. Aughenbaugh, S. Bendel, E. Edell, M. Edgar, B. Edwards, B. Elliot, J. Garrett, D. Gillespie, J. Gurney, B. Hammel, K. Hanson, L. Hanson, G. Harms, J. Hart, T. Hartman, R. Hyatt, E. Jensen, N. Jenson, S. Kalra, P. Karsell, D. Midthun, C. Mottram, S. Swensen, A.-M. Sykes, K. Taylor, N. Torres, R. Hubmayr, D. Miller, S. Bartling, K. Bradt, B. Make, M. Pomerantz, M. Gilmartin, J. Canterbury, M. Carlos, P. Dibbern, E. Fernandez, L. Geyman, C. Hudson, D. Lynch, J. Newell, R. Quaife, J. Propst, C. Raymond, J. Whalen-Price, K. Winner, M. Zamora, R. Cherniack, P. Diaz, P. Ross, T. Bees, H. Awad, J. Drake, C. Emery, M. Gerhardt, M. Kelsey, M. King, D. Rittinger, M. Rittinger, K. Naunheim, F. Alvarez, J. Osterloh, S. Borosh, W. Chamberlain, S. Frese, A. Hibbit, M.E. Kleinhenz, G. Ruppel, C. Stolar, J. Willey, C. Keller, G. Criner, S. Furukawa, A.M. Kuzma, R. Barnette, N. Brister, K.Carney, W. Chatila, F. Cordova, G. D’Alonzo, M. Keresztury, K. Kirsch, C. Kwak, K. Lautensack, M. Lorenzon, U. Martin, P. Rising, S. Schartel, J. Travaline, G. Vance, P. Boiselle, G. O’Brien, A. Ries, R. Kaplan, C. Ramirez, D. Frankville, P. Friedman, J. Harrell, J. Johnson, D. Kapelanski, D. Kupferberg, C. Larsen, T. Limberg, M. Magliocca, F. J. Papatheofanis, D. Sassi-Dambron, M. Weeks, M. Krasna, H. Fessler, I. Moskowitz, T. Gilbert, J. Orens, S. Scharf, D. Shade, S. Siegelman, K. Silver, C. Weir, C. White, F. Martinez, M. Iannettoni, C. Meldrum, W. Bria, K. Campbell, P. Christensen, K. Flaherty, S. Gay, P. Gill, P. Kazanjian, E. Kazerooni, V. Knieper, T. Ojo, L. Poole, L. Quint, P. Rysso, T. Sisson, M. True, B. Woodcock, L. Zaremba, L. Kaiser, J. Hansen-Flaschen, M. L. Geraghty, A. Alavi, T. Alcorn, J. Aronchick, S. Aukberg, B. Benedict, S. Craemer, R. Daniele, J. Edelman, W. Gefter, L. Kotler-Klein, R. Kotloff, D. Lipson, W. Miller Jr., R. O’Connell, S. Opelman, W. Russell, H. Sheaffer, R. Simcox, S. Snedeker, J. Stone-Wynne, G. Tino, P. Wahl, J. Walter, P. Ward, D. Zisman, J. Mendez, A. Wurster, F. Sciurba, J. Luketich, C. Witt, G. Ayres, M. Donahoe, C. Fuhrman, R. Hoffman, J. Lacomis, J. Sexton, W. Slivka, D. Strollo, E. Sullivan, T. Simon, C. Wrona, G. Bauldoff, M. Brown, E. George, R. Keenan, T. Kopp, L. Silfies, J. Benditt, D. Wood, M. Snyder, K. Anable, N. Battaglia, L. Boitano, A. Bowdle, L. Chan, C. Chwalik, B. Culver, T. Gillespy, D. Godwin, J. Hoffman, A. Ibrahim, D. Lockhart, S. Marglin, K. Martay, P. McDowell, D. Oxorn, L. Roessler, M. Toshima, S. Golden, L. Bosco, Y.-P. Chiang, C. Clancy, H. Handelsman, S. Sheingold, T. Carino, J. Chin, J. Farrell, K. McVearry, A. Norris, S. Shirey, C. Sikora, S. Piantadosi, J. Tonascia, P. Belt, K. Collins, B. Collison, J. Dodge, M. Donithan, V. Edmonds, J. Fuller, J. Harle, R. Jackson, H. Koppelman, S. Lee, C. Levine, H. Livingston, J. Meinert, J. Meyers, D. Nowakowski, K. Owens, S. Qi, M. Smith, B. Simon, P. Smith, A. Sternberg, M. Van Natta, L. Wilson, R. Wise, R. M. Kaplan, J. S. Schwartz, Y-P. Chiang, M. C. Fahs, A. M. Fendrick, A. J. Moskowitz, D. Pathak, S. Ramsey, S. Sheingold, A. L. Shroyer, J. Wagner, R. Yusen, S. Ramsey, R. Etzioni, S. Sullivan, D. Wood, T. Schroeder, R. Smith, K. Berry, N. Myers, E. Hoffman, J. Cook-Granroth, A. Delsing, J. Guo, G. McLennan, B. Mullan, C. Piker, J. Reinhardt, J. Sieren, W. Stanford, J.A. Waldhausen, G. Bernard, D. DeMets, M. Ferguson, E. Hoover, R. Levine, D. Mahler, A. J. McSweeny, J. Wiener-Kronish, O. D. Williams, M. Younes, G. Criner, C. Soltoff, G. Weinmann, J. Deshler, D. Follmann, J. Kiley, and M. Wu.
A complete listing of the NETT Research Group members is provided in the online supplement.
Supported by a Mayo Clinic Department of Medicine Research Career Development Award (PI: C.C.K.) and the National Institutes of Health (K23 HL128859, PI: C.C.K.; and 5R01HL094680-05, PI: R.P.B.). The National Emphysema Treatment Trial was supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119), the Centers for Medicare and Medicaid Services, and the Agency for Healthcare Research and Quality.
Author Contributions: C.C.K.: Project conception and hypothesis, designed the data analysis, wrote the first draft of the manuscript, revised the manuscript, and approved final version to be published. P.J.N.: Assisted with design of data analysis, wrote and revised statistical portion of the manuscript and approved final version to be published. N.K.L.: Assisted with design of data analysis, revised the manuscript, and approved the final version to be published. R.A.W. and F.C.S.: Revised the manuscript and approved the final version to be published. R.P.B.: Assisted with project conception and hypothesis, assisted with design of data analysis, revised the manuscript, and approved the final version to be published. C.C.K. and R.P.B. had full access to the data and takes responsibility for the integrity of the data and the accuracy of the data analysis, and assumes full responsibility for the integrity of the submission as a whole.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: the NETT Research Group
References
- 1.Guarascio AJ, Ray SM, Finch CK, Self TH. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013;5:235–245. doi: 10.2147/CEOR.S34321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Top 10 causes of death; 2014 [accessed 2015 July 8]. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/
- 3.Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182:598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 5.Schünemann HJ, Dorn J, Grant BJ, Winkelstein W, Jr, Trevisan M. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest. 2000;118:656–664. doi: 10.1378/chest.118.3.656. [DOI] [PubMed] [Google Scholar]
- 6.Puhan MA, Hansel NN, Sobradillo P, Enright P, Lange P, Hickson D, et al. International COPD Cohorts Collaboration Working Group. Large-scale international validation of the ADO index in subjects with COPD: an individual subject data analysis of 10 cohorts. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-002152. pii: e002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 8.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 9.Boyd CM, Xue Q-L, Simpson CF, Guralnik JM, Fried LP. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am J Med. 2005;118:1225–1231. doi: 10.1016/j.amjmed.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 10.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Dunlay SM, Park SJ, Joyce LD, Daly RC, Stulak JM, McNallan SM, et al. Frailty and outcomes after implantation of left ventricular assist device as destination therapy. J Heart Lung Transplant. 2014;33:359–365. doi: 10.1016/j.healun.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson ME, Vakil AP, Kandel P, Undavalli C, Dunlay SM, Kennedy CC. Pretransplant frailty is associated with decreased survival after lung transplantation. J Heart Lung Transplant. 2016;35:173–178. doi: 10.1016/j.healun.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Singer JP, Diamond JM, Gries CJ, McDonnough J, Blanc PD, Shah R, et al. Frailty phenotypes, disability, and outcomes in adult candidates for lung transplantation. Am J Respir Crit Care Med. 2015;192:1325–1334. doi: 10.1164/rccm.201506-1150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaz Fragoso CA, Enright PL, McAvay G, Van Ness PH, Gill TM. Frailty and respiratory impairment in older persons. Am J Med. 2012;125:79–86. doi: 10.1016/j.amjmed.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lahousse L, Ziere G, Verlinden VJ, Zillikens MC, Uitterlinden AG, Rivadeneira F, et al. Risk of frailty in elderly with COPD: a population-based study. J Gerontol A Biol Sci Med Sci. 2016;71:689–695. doi: 10.1093/gerona/glv154. [DOI] [PubMed] [Google Scholar]
- 17.Maddocks M, Kon SSC, Canavan JL, Jones SE, Nolan CM, Labey A, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71:988–995. doi: 10.1136/thoraxjnl-2016-208460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy CC, Novotny PJ, LeBrasseur NK, Wise RA, Sciurba FC, Benzo RP. Frailty is associated with increased mortality and hospitalizations in COPD. J Heart Lung Transplant. 2015;34:S246–S247. [Google Scholar]
- 19.The National Emphysema Treatment Trial Research Group. Rationale and design of The National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. Chest. 1999;116:1750–1761. doi: 10.1378/chest.116.6.1750. [DOI] [PubMed] [Google Scholar]
- 20.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al. National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 21.Ramsey SD, Sullivan SD, Kaplan RM, Wood DE, Chiang YP, Wagner JL NETT Research Group. Economic analysis of lung volume reduction surgery as part of the National Emphysema Treatment Trial. Ann Thorac Surg. 2001;71:995–1002. doi: 10.1016/s0003-4975(00)02283-9. [DOI] [PubMed] [Google Scholar]
- 22.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 23.Pegorari MS, Ruas G, Patrizzi LJ. Relationship between frailty and respiratory function in the community-dwelling elderly. Braz J Phys Ther. 2013;17:9–16. doi: 10.1590/s1413-35552012005000065. [DOI] [PubMed] [Google Scholar]
- 24.Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis. 1969;99:696–702. doi: 10.1164/arrd.1969.99.5.696. [DOI] [PubMed] [Google Scholar]
- 25.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 26.Puhan MA, Garcia-Aymerich J, Frey M, ter Riet G, Antó JM, Agustí AG, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet. 2009;374:704–711. doi: 10.1016/S0140-6736(09)61301-5. [DOI] [PubMed] [Google Scholar]
- 27.Efron B. The jackknife, the bootstrap, and other resampling plans. Philadelphia: Society for Industrial and Applied Mathematics; 1982. [Google Scholar]
- 28.Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2:75–79. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 29.Macklai NS, Spagnoli J, Junod J, Santos-Eggimann B. Prospective association of the SHARE-operationalized frailty phenotype with adverse health outcomes: evidence from 60+ community-dwelling Europeans living in 11 countries. BMC Geriatr. 2013;13:3. doi: 10.1186/1471-2318-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garonzik-Wang JM, Govindan P, Grinnan JW, Liu M, Ali HM, Chakraborty A, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012;147:190–193. doi: 10.1001/archsurg.2011.1229. [DOI] [PubMed] [Google Scholar]
- 31.Althoff KN, Jacobson LP, Cranston RD, Detels R, Phair JP, Li X, et al. Multicenter AIDS Cohort Study (MACS) Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci. 2014;69:189–198. doi: 10.1093/gerona/glt148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14:1870–1879. doi: 10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue QL, Bandeen-Roche K, Varadhan R, Zhou J, Fried LP. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2008;63:984–990. doi: 10.1093/gerona/63.9.984. [DOI] [PubMed] [Google Scholar]
- 34.Mittal N, Raj R, Islam EA, Nugent K. The frequency of frailty in ambulatory patients with chronic lung diseases. J Prim Care Community Health. 2016;7:10–15. doi: 10.1177/2150131915603202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miravitlles M, Calle M, Soler-Cataluña JJ. Clinical phenotypes of COPD: identification, definition and implications for guidelines. Arch Bronconeumol. 2012;48:86–98. doi: 10.1016/j.arbres.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Altenburg WA, de Greef MHG, ten Hacken NHT, Wempe JB. A better response in exercise capacity after pulmonary rehabilitation in more severe COPD patients. Respir Med. 2012;106:694–700. doi: 10.1016/j.rmed.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Cameron ID, Fairhall N, Langron C, Lockwood K, Monaghan N, Aggar C, et al. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med. 2013;11:65–75. doi: 10.1186/1741-7015-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karpman C, DePew ZS, LeBrasseur NK, Novotny PJ, Benzo RP. Determinants of gait speed in COPD. Chest. 2014;146:104–110. doi: 10.1378/chest.13-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baldwin MR, Singer JP, Huang D, Sell J, Gonzalez WC, Pollack LR, et al. Refining low physical activity measurement improves frailty assessment in advanced lung disease and survivors of critical illness. Ann Am Thorac Soc. 2017;14:1270–1279. doi: 10.1513/AnnalsATS.201612-1008OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.