Abstract
Purpose
Childhood development sets the baseline for adult fracture risk. Most studies evaluate development using postero-anterior (pa) DXA areal bone mineral density, bone mineral content and bone mineral apparent density. In a prior analysis, we demonstrated that pa DXA reflects posterior element properties, rather than vertebral body fracture sites, such that loading is associated with subtle differences in vertebral body geometry, not 3D density. The current analysis is restricted to pre-pubertal girls, for a focused exploration of key nutrient intakes and physical activity as factors in dual plane indices of vertebral body geometry, density and strength.
Methods
This cross-sectional analysis used paired pa and supine lateral (lat) lumbar spine DXA scans to assess vertebral body bone mineral apparent density (palatBMAD), axial compressive strength (palatIBS), and fracture risk index (palatFRI). Diet data were collected using the Youth/Adolescent Questionnaire (YAQ, 1995); organized physical activity was recorded via calendar-based form. Pearson correlations and backward stepwise multiple linear regression analyzed associations among key nutrients, physical activity and bone outcomes.
Results
After accounting for activity and key covariates, fiber, unsupplemented vitamin B12, zinc, carbohydrate, vitamin C, unsupplemented magnesium and unsupplemented calcium intake explained significant variance for one or more bone outcomes (p<0.05). After adjustment for influential key nutrients and covariates, activity exposure was associated with paBMD, paBMC, paWIDTH, latBMC, palatCSA, palatIBS and palatFRI benefits (p<0.05).
Conclusions
Physical activity, fiber intake and unsupplemented B12 intake appear to influence vertebral body bone mass, density, geometry and strength in well-nourished pre-pubertal girls; high fiber intakes may adversely affect childhood vertebral body growth.
Keywords: Children, nutrition, exercise, female, bone geometry, bone density
Mini-abstract:
In pre-pubertal girls, nutrient intakes and non-aquatic organized activity were evaluated as factors in vertebral body bone mass, structure and strength. Activity, vitamin B12 and dietary fiber predicted bone outcomes most consistently. Exercise and vitamin B12 appear beneficial, whereas high fiber intake appears to be adverse for vertebral body development.
INTRODUCTION
Osteoporosis is a leading cause of morbidity for post-menopausal women.(1) As peak bone mass correlates negatively with osteoporosis risk, greater childhood and adolescent bone mass accretion reduces lifetime osteoporosis risk.(2) While 60–80% of peak bone mass variance is dependent on genetic factors, modifiable factors such as diet and exercise play important roles in bone development.(3) However, researchers have yet to establish specific, effective diet and exercise prescriptions for optimizing bone development.
Existing evidence indicates that participation in weight-bearing and/or impact-loading activity during growth results in significantly greater bone mineral accrual compared with non-weight-bearing and non-impact activity.(4) Research indicates that calcium and vitamin D are important for bone growth and maintenance in children and adults.(5) However, there have been conflicting reports of bone fragility associated with calcium, vitamin D and dairy intakes (6). Protein, magnesium, phosphorous, potassium, vitamin C, vitamin K, vitamin B12 and zinc are less well-studied, but are thought to be key nutrients for bone health and development.(7–10)
Mechanical loading and diet effects upon bone appear to be site-specific and tissue-specific. (11, 12) Our research group has evaluated loading associations in cortical and cancellous bone tissues, using traditional postero-anterior (pa) DXA at a variety of sites, paired postero-anterior and supine lateral (palat) DXA of the lumbar spine and peripheral quantitative computed tomography (pQCT) scans of the forearm (12–14). Our work indicates that femoral neck loading appears to constrain bone width in favor of greater cortical thickness and density (12); in contrast, at the radius (12) and lumbar spine (14), loading is associated with expanded bone geometry, particularly of the cortex, without tangible increases in cancellous tissue volumetric density (12–14).
Vertebral bodies are the predominant sites for insufficiency fractures of the spine. Although vertebral body fractures are rare during childhood, failure to develop robust bone during youth increases fracture risk in adulthood. Accordingly, adoption of a lifestyle that improves vertebral body bone properties is an important strategy to reduce site-specific fracture risk in adulthood. As vertebral bodies are primarily composed of cancellous tissue, increasing cancellous volumetric density should be an important goal in reducing vertebral fracture risk. Our prior work in a maturationally diverse cohort indicated that gymnastic loading does NOT increase vertebral body volumetric density, as assessed using paired palat DXA scans. (13) Our study demonstrated that prior research on lumbar spine loading adaptation erroneously exaggerated benefits by reporting areal bone mineral density (BMD) and bone mineral apparent density (BMAD) based solely on pa DXA scans(13). pa scans include the cortical posterior elements and also use pa area to calculate “volume”, thereby exaggerating benefits.(13) Our prior analysis led us to question whether: 1) the maturational diversity of our cohort masked vertebral body loading adaptations; 2) evaluating gymnastic loading as a discrete variable concentrated associations within the posterior elements and/or blunted capacity to evaluate loading patterns across the entire sample, and 3) unmeasured nutrient intakes were key factors in vertebral body properties.
Thus, for the current analysis, we evaluated a new range of dietary factors and assessed loading as a continuous variable in a maturationally homogeneous cohort of pre-pubertal girls. We used palat DXA to address the limitations of pa DXA lumbar spine assessment, including fan beam magnification error, failure to account for vertebral depth, and lack of ability to distinguish vertebral body BMC from the posterior elements.(13) We still use DXA, rather than CT, as pa DXA BMD is strongly correlated with fracture risk, and DXA scans can rapidly measure large regions of interest, have high precision and accuracy and generate low radiation exposure.(3, 15, 16)
By pairing pa scans with lat scans, we reduce confounding effects of fan beam magnification error and isolate BMC from the vertebral body, thereby excluding posterior element tissue. (13, 17) Palat DXA scans also provide a three-dimensional lumbar spine geometric assessment, incorporating paWIDTH, latDEPTH, and latHEIGHT. (13, 17) Palat assessments yield more appropriate bone geometry and total bone mineral apparent density (BMAD) than BMAD derived from 2 dimensional pa or lat scans alone.(13) As bone strength is determined by both bone mineral content and structure, palat DXA allows superior estimation of 3D bone structure and strength indices, specific to the vertebral body. (13)
To our knowledge, no studies have evaluated the influence of organized physical activity and diet on pre-pubertal vertebral body properties using palat DXA. The aim of the current analysis is to associate key bone nutrients and physical activity exposure with bone mass, geometry, and strength in pre-pubertal girls, using paired pa and lat DXA scans to generate 3D assessments. We hypothesize that by limiting our sample to pre-pubertal girls, expanding our nutrient assessments and evaluating exercise loading as a continuous variable, we will improve our capacity to explain variability in vertebral body-specific properties. In this manner, we will improve understanding of the roles of diet and exercise in optimal bone development.
METHODS
Study Design
The data used in this cross-sectional, observational study are from an on-going study of bone growth in relation to physical activity, including female gymnasts and non-gymnasts.(13) The study was approved by the Institutional Review Board of SUNY Upstate Medical University, and complies with US bioethical legislation and the ethical standards of the Declaration of Helsinki. Written parental consent and child assent were obtained.
Participants
Participants were recruited from local gymnastics schools, private schools, and athletic groups(13, 18) To avoid the potential confounding influence of variability in sexual hormones on bone mass acquisition, and to examine an isolated maturity stage, only pre-pubertal participants were included in the present analysis (Tanner stages: breast I, pubic I). One participant was excluded as an outlier for vitamin C and vitamin B12 intakes, yielding 50 participants. Racial/ethnic cohort composition is as follows: 1 Native American, 4 Asian/Mixed Race, 0 Black/Mixed Race, 45 white; 0 Hispanic.
Anthropometry
Height and weight were measured contemporaneously with the DXA scans, using a wall-mounted stadiometer (healthometer) and an electronic digital scale (Detecto), respectively. (13, 18) Body Mass Index (BMI, kg/m2) of each participant was calculated. BMI-for-age percentiles were plotted using the Centers for Disease Control and Prevention (CDC) Growth Charts age for Children and adolescents, 2 to 20 years.(19)
In the current analysis, physical maturity has been purposefully limited to pre-pubertal status. During pre-puberty, linear growth dominates and weight and height are tightly coupled (current study r= 0.79, p< 0.0001). Furthermore, in this cohort, lean mass is tightly coupled with both height and weight (r= 0.88, 0.92, p < 0.0001). Thus, collinearity is high for height, weight and lean mass correlations with indices of bone geometry, density and strength. While weight and lean mass explain bone index variance, we prioritize evaluation of loading and nutrition influences after accounting for general skeletal size (height), particularly as height was the strongest correlate with palatBMAD. Thus, we evaluated height as a covariate, rather than weight, to account for body size as a factor in vertebral body properties. Height was excluded from models for dependent variables that are a direct function of height or include height in their calculation (vertebral body height, fracture risk index).
Physical Maturity
Self-assessed Tanner breast and pubic stages were used to determine pre-pubertal status.(20) Line drawings of Tanner’s development stages were presented to the participants, with five stages of sex characteristics (breast and pubic hair development) described in captions. Participants circled the best representations of their own breast and pubic development, with parental assistance as necessary.(12, 13)
Dietary Intake
Participants’ usual dietary intakes were evaluated semi-annually using the self-administered semi-quantitative Youth Adolescent Questionnaire (YAQ: 1995 version), developed for use in children and adolescents by researchers at Harvard University. The questionnaire lists 145 foods and supplements, and standard serving sizes or natural portions of each item.(21) YAQ validity was validated against 24-hour recalls, with the exception of vitamin A, carotene, and alcohol.(21) Intakes were evaluated with and without supplementation of the focal nutrients, using current dietary guidelines for recommended dietary allowances and estimated average requirements/ adequate intakes, as detailed.(22)
YAQ data collected contemporaneous with the DXA scan were used in analyses, as an indication of habitual intakes over the prior year. The following macronutrients were evaluated: total calories, fat, protein, carbohydrate and fiber; regressions evaluating total calories and protein were performed in isolation from other nutrients due to high collinearity. The following micronutrients were evaluated: calcium, magnesium, phosphorus, zinc, vitamin C, vitamin D and vitamin B12 (with and without supplementation). We did not evaluate vitamins A and K in this analysis, due to concerns regarding validity of assessments for both vitamins. As noted above, vitamin A validity was deemed inadequate by the main Harvard research group.(21) Regarding vitamin K, the questionnaire did not specify intakes of live and active cultures via yogurt and other fermented foods (e.g. kefir, specific cheeses, kimchi, sauerkraut, etc.), thereby eliminating our means to evaluate the intakes of increasingly popular sources of vitamin K (K-2: menaquinone).(23) Thus, we had concerns about the quality of resultant vitamin K data and did not evaluate vitamin K in our analyses.
Physical Activity
To account for osteogenic activity exposure, total organized, non-aquatic physical activity was recorded to represent participation for the year prior to baseline and semi-annually thereafter, using a calendar-based form to yield activity-specific participation in organized activities (hours per week, h/wk). Based on recruitment for the longitudinal study, girls were grouped as either gymnasts (annual mean training 6 h/wk) or non-gymnasts. Non-gymnasts were not necessarily sedentary. Hence, instead of focusing on gymnastics, the current study evaluated participants according to their annual mean organized physical activity levels, excluding aquatic activity (mean h/wk). To more accurately evaluate the relationship between activity exposure and bone outcomes, participants’ activity levels were used as a continuous variable, reflecting annual activity exposure for the year prior to the focal DXA scan.
Body Composition, Bone Density and Bone Geometry
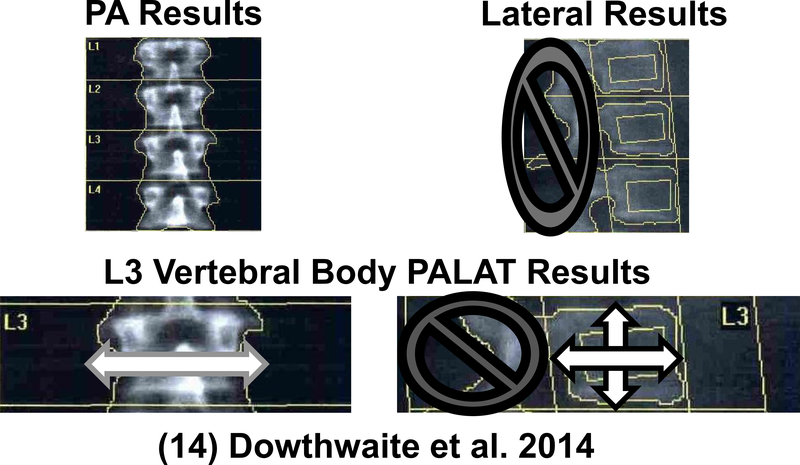
DXA was used to assess bone (Hologic Discovery A, software v.12.7.3, Waltham, MA). At annual DXA measurements, pa and lat lumbar spine DXA scans were performed to obtain paBMD, paBMC, paWIDTH, latHEIGHT and latBMC, using equations published previously (13, 17) [see Figure 1]. All scans were analyzed by a single investigator (JD) using Apex software (Hologic Discovery A, software v.12.7.3, Waltham, MA, USA).
Fig. 1. At annual DXA measurements, pa and lat lumbar spine DXA scans were performed to obtain postero-anterior areal bone mineral density (paBMD), pa bone mineral content (paBMC), paWIDTH, latHEIGHT, latDEPTH and lateral BMC (llatBMC). For additional details on PALAT calculations, see Dowthwaite, Rosenbaum and Scerpella, 2011 [14].
Only data from lumbar vertebra 3 (L3) were analyzed, as L2 and L4 are often overlapped by rib and pelvic bone in lateral scans.(13, 24) Plane-specific bone geometric indices were calculated, including mean paWIDTH (=paAREA/latHEIGHT) and latDEPTH (=latAREA/latHEIGHT). PalatBMAD, palatIBS, and palatFRI were calculated using formulae based on simplified geometric models.(13, 17) Rather than relying upon Hologic standard-width-adjusted BMD and width-adjusted volume, investigator-calculated palatBMAD was used for data analysis. These calculations were performed because the standard Hologic output erroneously uses only lateral bone geometric indices, whereas our methodology incorporates pa bone width to assess 3D geometry.(13)
Data Analysis
Descriptive statistics were computed. Data from dependent variables were normally distributed, with the exception of palatFRI (fracture risk index). Thus, palatFRI was converted to natural logarithms for analysis and back-transformed for presentation of results. Data for many independent variables were non-normally distributed; accordingly, Kruskal-Wallis non-parametric tests were used to evaluate potential bias (gymnasts vs. non-gymnasts). Pearson correlation coefficients were calculated to evaluate the bivariate relationships between dietary intakes and bone measurements.
Backward, stepwise, multiple linear regressions were used to determine the most influential nutrients and examine associations among key nutrients (total calories and protein evaluated separately, as noted above) and bone measurements, accounting for physical activity, age and body size, as appropriate. For all dependent variables, these analyses ensured statistical consideration of the role of annual mean physical activity, age and/or height, as these factors are known to influence bone status; in cases where these variables were not influential (p > 0.10), they were excluded from models to avoid potential variance inflation. Height was excluded from models for bone outcomes that are a function of height (latHEIGHT, palatFRI). The criterion for independent variable removal from stepwise regression models was p>0.10. Beyond these practices, variance inflation factors were evaluated as an indicator of excessive collinearity (acceptable VIF<5.0). The alpha level was set at p<0.05 for all tests. All data analyses were performed using SPSS for Windows, version 23 (SPSS, Inc., Chicago, IL).
RESULTS
Participant Characteristics
Participants included in the analyses were 7 to 12 years of age. The mean percentile rank for BMI for age was 43.08 (SD=25.34, 4–98). Descriptive characteristics of the subjects are shown in Table 1 (anthropometrics) and Table 2 (intakes). Participants were active in a variety of activities during the year prior to the focal DXA scan, with the most common activities being gymnastics, soccer and dance [Table 3]. There were no significant differences between gymnasts (≥6 h/wk annual mean training for year prior to DXA) and non-gymnasts (all others) for any independent variables other than annual mean h/wk of organized activity participation (GYM > NON, Kruskal-Wallis p<0.001) and vitamin D (GYM < NON, with or without supplementation, Kruskal-Wallis p<0.04). However, there were strong trends for differences in age (GYM> NON, K-W p=0.05) and supplemented intakes for calcium, phosphorus, B12 and zinc (NON > GYM, K-W p<0.09), as well as unsupplemented calcium intake (NON > GYM, K-W p<0.10).
Table 1.
Descriptive characteristics of pre-pubertal girls (n=50; Tanner breast and pubic stage= 1).
| Mean ± SD | Range | |
|---|---|---|
| Age (years) | 9.5 ± 1.2 | 7.8–12.8 |
| Weight (kg) | 29.2 ± 5.4 | 19.8–45.2 |
| Height (standing, cm) | 133.1 ± 8.8 | 116.0–156.5 |
| BMI (weight/height2: kg/m2) | 16.4 ± 1.8 | 13.5–24.8 |
| BMI for age (percentile rank) | 43.1± 25.3 | 4–98 |
| Fat Mass (kg) | 5.8 ± 2.4 | 1.9–16.8 |
| Total Lean Mass (kg) | 20.4 ± 3.9 | 13.8 –33.0 |
| Soft lean tissue Mass (kg) | 19.6 ± 3.7 | 13.3–31.7 |
| Percent Lean Mass (%) | 78.4 ± 5.0 | 59.1–88.7 |
| Percent Fat Mass (%) | 21.7 ± 5.0 | 11.3 – 40.9 |
| Physical Activity (h/wk) | 6.9 ± 5.1 | 0.1–21.5 |
| PABMC (g) | 5.25 ± 1.28 | 3.32–9.58 |
| PABMD (g/cm2) | 0.623 ± .073 | 0.461–0.883 |
| PAWIDTH (cm) | 5.69 ± 0.45 | 4.66–7.20 |
| LATBMC (g) | 3.22 ± 0.76 | 1.95–5.73 |
| LATHEIGHT (cm) | 1.47 ± 0.20 | 1.08–1.93 |
| LATDEPTH (cm) | 3.72 ± 0.25 | 3.07–4.43 |
| PALATBMAD (g/ cm3) | 0.13 ± 0.01 | 0.10–0.15 |
| PALATIBS (g2/cm4) | 0.29 ± 0.07 | 0.17–0.58 |
| FRI | 1.66 ± 0.02 | 1.01 – 2.82 |
PABMC= postero-anterior areal bone mineral content; PABMD= postero-anterior bone mineral density; PAWIDTH= postero-anterior vertebral width; LATBMC: lateral bone mineral content; LATHEIGHT= lateral vertebral height; LATDEPTH= lateral vertebral depth; PALATBMAD= veterbral bone mineral apparent density. PALATIBS= index of structural strength in axial compression; FRI=Fracture risk index.
Table 2.
Dietary intakes and dietary recommendations of 9–13 year old girls (n=50)
| Total Sample Mean±SD | EAR | RDA/AI | |
|---|---|---|---|
| Energy (kcal/d) | 2137±571 | 1800–2200 | |
| Protein (g/d) | 88±28 g/d | 34g/d | |
| Protein (g/kg/d) | 3.8±0.9 g/kg/d | 0.8g/kg/d | |
| Protein (percentage of kcal) | 20±1% | 10–30% | |
| Total fat (g/d) | 72±23 | ||
| Total fat (percentage of kcal) | 32±2% | 25–35% | |
| Carbohydrate (g/d) | 292 ± 77 | 100 | 130 |
| Carbohydrate (percentage of kcal) | 49±4% | 45–65% | |
| Fiber (AOAC) (g/d) | 19±5 | 26 | |
| Calcium (mg/d) | 1289±534 | 1,100 | 1,300 |
| Calcium wo (mg/d) | 1224±531 | ||
| Vitamin D (IU/d) | 531±332 | 400 | 600 |
| Vitamin D wo (IU/d) | 271±167 | ||
| Phosphorus (mg/d) | 1604±517 | 1250 | |
| Phosphorus wo (mg/d) | 1539±512 | ||
| Potassium (mg/d) | 2946±921 | 4500 | |
| Potassium wo (mg/d) | 2946±921 | ||
| Magnesium (mg/d) | 305±83 | 240 | |
| Magnesium wo (mg/d0 | 292±81 | ||
| Vitamin C (mg/d) | 147±70 | 45 | |
| Vitamin C wo (mg/d) | 112±54 | ||
| Zinc (mg/d) | 22±11 | 7 | 8 |
| Zinc wo (mg/d) | 12±4 | ||
| Vitamin B12 (mcg/d) | 9.7±4.5 | 1.8 | |
| Vitamin B12 wo | 6.0±2.7 |
EAR: estimated average requirement; RDA: recommended dietary allowance; AI: adequate intake; Wo: oral intake without supplementation. RDA/AI in excess of mean intakes for the sample are noted in bold italics.
Table 3:
Activity Participation by “Sport”
| Activity | Number of Girls |
|---|---|
| Baseball/Softball | 5 |
| Basketball | 5 |
| Cheerleading | 4 |
| Cross-Country Running | 6 |
| Dance | 12 |
| Downhill Skiing | 5 |
| Gymnastics | 37 |
| Horse riding | 1 |
| Ice Skating | 1 |
| Lacrosse | 5 |
| Martial Arts | 6 |
| Snowboarding | 1 |
| Soccer | 20 |
| Swimming: AQUATIC | 7 |
| Tennis | 1 |
| Trampoline | 2 |
Note: Girls often participate in more than one activity, thus activity participation is not mutually exclusive. For example, Girl #3 participated in gymnastics, soccer, tennis, dance and basketball during the year prior to the focal DXA scan.
Furthermore, although the majority of girls (n =37) participated in gymnastics the year prior to the DXA scan, most of them did not achieve an annual mean training level of greater than 6 hours per week for the year prior to the focal DXA scan (the threshold for “gymnast status” in our prior palat lumbar spine DXA analysis).
Dietary Intakes
Focal daily mean intakes of energy, macronutrients, and micronutrients are shown in Table 2. The mean intakes of the focal nutrients that met or exceeded the Dietary Reference Intakes were as follows: carbohydrate, protein, phosphorus, magnesium, vitamin A, vitamin C, zinc, and vitamin B12. The mean intakes of fiber, calcium, vitamin D, and potassium were below the Recommended Dietary Allowance (RDA). The biggest nutrient of concern was potassium, since only 8% of the participants met the RDA for this nutrient. For all other nutrients of interest excluding potassium, 70% of subjects met the Estimated Average Requirement (EAR) with supplementation; 18% met the EAR without supplementation. For all nutrients other than potassium, fifty percent of the subjects exceeded the RDA including supplements, whereas only 2% of the subjects met or exceeded the RDA without supplementation. In the present study, 58% of participants reported taking multivitamin/mineral (MVMM) supplements, with 38% of participants taking ≥ 3 MVMM pills per week.
Physical Activity
On average, annual mean non-aquatic organized activity exposure was 6.9 ± 5.1 h/wk. In total, only 30% of subjects met the physical activity recommendations of ≥ 1 hour per day (on average, approximated as at least 7 h/wk). Of these subjects, >85% did not participate in organized aquatic activity, and no subject participated in aquatic activity for an annual mean of >1 h/wk. This proportion (30%) was identical when aquatic activity was included, but increased to approximately 50% when school-based physical education of 1.41 h/wk was entered (45 min × 2.5 days/wk × 9/12 months). Organized non-aquatic physical activity levels were variable, ranging from 0 to 21.5 h/wk. Organized non-aquatic physical activity exposure was not significantly correlated with any nutrient intake, although there was a trend toward negative correlation with supplemented vitamin D intake (Pearson r= −0.27, p= 0.06).
Bone Outcome Regression Results
Organized activity was included in all models (Table 4), explaining 3% (latBMC) to 19% (lnpalatFRI) of variance (p<0.05), other than models for lat depth and palatBMAD (exclusion criterion p = 0.10). Age was included in all models, explaining 3% to 29% of variance (p≤0.06), other than models for paBMD, latBMC, latDEPTH, palatBMAD, palatIBS (exclusion p= 0.10). Height was purposefully excluded from models for latHEIGHT and lnpalatFRI, because these variables are a function of subject height and/or include height in their calculation. Otherwise, height was included in all models, explaining 17% to 50% of variance (p≤0.001). Fiber (negative) and unsupplemented B12 (positive) were the most common nutrients included in models. Carbohydrate, unsupplemented minerals (zinc, magnesium, calcium) and supplemented vitamin C were also included in one or more models. Variance inflation factors were <5.0 for all included variables in all models, indicating that appropriate levels of collinearity were not exceeded. The exception was palatBMAD, for which no effective regression model was developed due to low proportion of explained variance and high collinearity among included variables (VIF=6.9 to 26.6). Upon evaluation of simple correlations within the palatBMAD regression analysis results, we noted trends that indicate small to medium effect sizes for a negative correlation with fiber (r= −0.18, p = 0.10) and for positive correlations with age, height, activity and unsupplemented B12 (r=+0.18 to +0.23, p ≤ 0.11).
Table 4.
Regression results
| Bone Outcome | Model Adj. r2 | Activity (h/wk) | Age (yrs) | Height (cm) | Fiber | Vit B12 No sup | Zn No sup | Carb | Vit C | Mg No sup |
|---|---|---|---|---|---|---|---|---|---|---|
| PA | 0.43c | 0.004a | --- | c | −0.005a | 0.01b | −0.007a | 0.000a | --- | --- |
| BMD (g/cm2) | (0.001, 0.007) | --- | (−0.009,−0.001) | (0.003,0.02) | (−0.02, 0.000) | (0.000, 0.001) | --- | --- | ||
| +0.28 | --- | +0.41 | −0.25 | +0.30 | −0.22 | +0.25 | --- | --- | ||
| PA | 0.64c | 0.08b | (0.06) | c | −0.06b | 0.12b | --- | --- | --- | --- |
| BMC (g) | (0.03, 0.13) | (−0.11, −0.02) | (0.03, 0.21) | --- | --- | --- | --- | |||
| +0.26 | −0.17 | +0.60 | −0.25 | +0.24 | --- | --- | --- | --- | ||
| LAT | 0.63c | 0.03a | --- | c | −0.03a | 0.07b | --- | --- | --- | --- |
| BMC (g) | (0.000, 0.05) | --- | (−0.06, −0.005) | (0.02, 0.12) | --- | --- | --- | --- | ||
| +0.18 | --- | +0.71 | −0.20 | +0.24 | --- | --- | --- | --- | ||
| LAT | 0.19c | --- | --- | c | --- | --- | --- | --- | --- | --- |
| DEPTH(cm) | --- | --- | --- | --- | --- | --- | --- | --- | ||
| --- | --- | +0.46 | --- | --- | --- | --- | --- | --- | ||
| LAT | 0.35c | −0.01b | c | XXXX | (0.09) | --- | --- | --- | --- | --- |
| HEIGHT(cm) | (−0.02, −0.005) | XXXX | --- | --- | --- | --- | --- | |||
| −0.34 | +0.54 | XXXX | −0.20 | --- | --- | --- | --- | --- | ||
| PA | 0.26b | 0.03a | a | c | --- | --- | --- | 0.003a | (0.06) | (0.06) |
| WIDTH (cm) | (0.006, 0.06) | --- | --- | --- | (0.000, 0.007) | |||||
| +0.30 | −0.26 | +0.44 | --- | --- | --- | +0.27 | −0.23 | −0.24 | ||
| PALAT | 0.21b | 0.17a | a | c | --- | --- | --- | --- | --- | --- |
| CSA (cm2) | (0.02, 0.31) | --- | --- | --- | --- | --- | --- | |||
| +0.29 | −0.27 | +0.48 | --- | --- | --- | --- | --- | --- | ||
| PALAT | 0.36c | 0.004b | --- | c | (0.09) | 0.008b | --- | --- | --- | --- |
| IBS(g2/cm4) | (0.001, 0.007) | --- | (0.002, 0.01) | --- | --- | --- | --- | |||
| +0.33 | --- | +0.43 | −0.20 | +0.31 | --- | --- | --- | --- | ||
| PALAT | 0.25c | −0.02c | c | XXXX | --- | --- | --- | --- | 0.001a | --- |
| lnFRI | (−0.03, −0.009) | XXXX | --- | --- | --- | --- | (0.000, 0.002) | --- | ||
| −0.44 | +0.42 | XXXX | --- | --- | --- | --- | +0.27 | --- | ||
For all models, focal nutritional variables, age, height and organized physical activity dose were initially entered for backward stepwise analysis.
Exceptions were: LATHEIGHT and lnFRI, for which height was not entered, as those variables are a function of total body height.
Due to high collinearity with other variables, calories and protein were run in separate regressions from other dietary variables; they were excluded from all models.
Model adjusted r2 is reported for the final model, as determined by backward stepwise regression.
Significance (beta t) and semi-partial correlation coefficients are reported for all included variables.
Significance is denoted as follows:
p<0.05
p<0.01
p<0.001
For strong non-significant trends, p is listed in superscript parentheses.
Other than for age and height, β (95% Confidence Interval) is reported for each significant independent variable.
Dependent variables are as follows: PABMD= postero-anterior areal bone mineral density; PABMC= PA bone mineral content; LATBMC= lateral BMC; LATDEPTH= lateral vertebral depth (out of PA scan plane); LATHEIGHT= lateral vertebral body height; PAWIDTH= PA width; PALATCSA= “3D” bone cross-sectional area (coronal plane); PALATIBS= “3D” index of structural strength in axial compression; lnFRI= natural logarithm of fracture risk index.
Due to low explanatory value (model adj. r2= 0.07) and high collinearity (variance inflation factors=6.9 to 26.6), the PALAT bone mineral apparent density model was discarded.
DISCUSSION
To our knowledge, this is the first study to evaluate the relationship between diet, physical activity, and three-dimensional bone outcomes, as measured by paired palat lumbar spine DXA scans, in pre-pubertal girls. Positive associations were detected between physical activity exposure and multiple bone outcomes, including paBMC, paBMD, paWIDTH, palatCSA and palatIBS; a beneficial negative association was observed between physical activity and palatFRI. The negative association of activity with latHEIGHT explains part of the association between higher activity and lower palatFRI. These activity associations were significant after adjusting for age and/or height and all nutrients evaluated, as appropriate. We did not detect a significant independent influence of any variable on palatBMAD, suggesting that lumbar spine cancellous bone adaptation to mechanical loading is geometric in nature, not densitometric, and that cancellous bone density is not responsive to variability in nutrient intakes over the observed range. Overall, the results support the importance of physical activity to lumbar vertebral body BMC, BMD, width and strength indices, suggesting that adaptation occurs via greater width at the expense of vertebral height. The most consistent influential nutrients appeared to be vitamin B12 and fiber, with high fiber intakes appearing to exert a detrimental influence on several vertebral body properties and B12 positively influencing multiple bone outcomes.
Expanded bone geometry has been correlated with greater organized activity exposure by other researchers using pa DXA.(25) In the current analysis, we have provided the first report of a significant positive association between physical activity exposure as a continuous variable and latBMC. LatBMC is thought to be superior to paBMC for evaluation of vertebral body properties, because lateral DXA scans allow isolation of the vertebral body (main osteoporotic fracture site) from the posterior elements.(13) Notably, the present study detected significant positive associations between activity exposure dose as a continuous variable, and L3 vertebral body bone mass, after accounting for the statistical effects of multiple key nutrient intakes. This novel finding indicates possible dose-dependent benefits of weight-bearing activity in girls prior to puberty that are NOT a function of the posterior elements.
Bone geometry is an important determining factor for bone strength and fracture risk, in addition to and separate from bone mass and density.(26, 27) We detected a significant positive association between L3 width and physical activity exposure, consistent with other studies.(26, 27) Corroborating our prior findings in a more heterogeneous sample, we detected associations between loading during growth and advantages in both paWIDTH and palatCSA.(13) No significant associations were found between activity exposure as a continuous variable and latDEPTH or palatBMAD. As in our heterogeneous maturational analysis, we detected a negative association between loading exposure and vertebral body height, indicating possible prioritization of bone deposition to increase bone width in the lateral plane (paWIDTH), at the expense of growth in height. In the current analysis, we limited our sample to girls who have yet to be exposed to increased pubertal estrogen levels, and we accounted for influential variability in key nutrient intakes.
In our prior work, specific to gymnastic loading, it was postulated that latDEPTH expansion may be diminished by tensile resistance from posterior elements and ligamentous connections, while vertebral paWIDTH expansion may be required due to the relative absence of medial-lateral accessory support against loading.(13) The current analysis corroborates this observed positive association between activity-related loading exposure and paWIDTH, in what appears to be a dose-dependent manner, across a variety of activity types.
In the present study, no significant association was found between activity and palatBMAD. Our palatBMAD regression model demonstrated limited explanatory value (adjusted model r2= 0.07), with excessive variance inflation factors (6.9–26.6). When we attempted to reduce variance inflation by excluding magnesium, all predictors were excluded from the model. Looking at simple correlations, the most promising correlates were fiber (r= −0.18, p= 0.20) and unsupplemented B12 (r= +0.19, p= 0.19). Overall, in this sample, palatBMAD was not significantly correlated with any of the independent variables evaluated, including age, height, physical activity, or nutrient intake. It is possible that we would detect significant associations with fiber and B12 intakes in a larger sample. Alternatively, BMAD (or vBMD) may not be responsive to focal dietary variables or loading exposures. Overall, the lack of significant associations with palatBMAD is similar to findings from our previous work.(13) It is possible that lumbar spine BMAD and vBMD reflect trabecular microarchitectural properties that are primarily determined by genetic factors, or that they are only subtly modified by environmental conditions that do not stray into pathological territory (e.g. malnutrition, anorexia, severe illness/disability, bed rest).
We identified significant positive associations between physical activity and PALATIBS, and negative associations between activity and FRI. In contrast with studies that reported higher fracture risk in children with higher physical activity,(28) we did not evaluate actual fracture incidence.
Fiber intake was negatively correlated with multiple bone outcomes (paBMD, paBMC, latBMC, latHEIGHT, palatIBS). These findings are consistent with other research evaluating fiber intake and lumbar spine BMD in female adolescent athletes.(29) One potential mechanism for an inverse correlation between dietary fiber intake and bone properties is that fiber reduces the digestibility of food and the absorption of nutrients by increasing the bulk of intestinal contents and shortening gastrointestinal transit time; thus, with high fiber intakes, calcium and other minerals have less time to be absorbed.(30, 31) This mechanism is supported by the findings of Wolf et al., in which dietary fiber served as an independent predictor of calcium absorption efficiency (high fiber was correlated with low calcium absorption).(30)
An alternative explanation for our observed negative correlation between fiber and bone outcomes is that fiber may affect female reproductive hormones, which are, in turn, associated with bone metabolic function. Dietary fiber can bind to sex hormones- particularly estrogen; this binding reduces estrogen reabsorption and promotes estrogen excretion.(32) In a prospective cohort study of 259 healthy pre-menopausal women, Gaskins and colleagues provided evidence to support this mechanism: high dietary fiber intake was positively correlated with risk of anovulation and inversely associated with serum concentrations of estradiol, progesterone, luteinizing hormone, and follicle stimulating hormone. (33)
In our analyses, unsupplemented vitamin B12 intake was positively correlated with multiple bone outcomes. A possible mechanism for this association is that inadequate B12 may reduce osteoblast activity, as evidenced by supplementation-related increases in osteocalcin and bone-specific alkaline phosphatase levels among B12-deficient patients, but not among B12-replete subjects.(10, 34) Unfortunately, the majority of relevant research has been performed in populations at high risk for vitamin B12 deficiency or high risk of osteoporosis;(9, 10, 34) accordingly, the role of vitamin B12 in bone health among healthy adolescents is not well-established. In a cross-sectional study evaluating serum B12 levels as a factor in adolescent BMD and BMC, serum B12 was significantly lower among subjects with low BMD or BMC than in subjects with normal BMD or BMC.(35) Similar associations have also been observed in elderly women.(36)
In addition to potential direct effects on bone mineral density and content, vitamin B12 may also indirectly affect bone fracture risk via homocysteine.(9, 10) High serum homocysteine levels may weaken bone by interfering with collagen cross-linking, thus increasing the risk of bone fracture.(9, 10, 34) Vitamin B12 is one of the key determinants of serum homocysteine concentrations, and multiple studies have indicated that elevated homocysteine is an important risk factor for bone fracture.(34, 36, 37) Future research is needed to evaluate associations among serum B12, serum homocysteine and bone properties in adolescents.
The limited number of significant associations among diet variables and bone outcomes may be a result of adequate energy and intakes for most nutrients. The participants in our study met the DRIs for energy and most macronutrients, as well as most of the key bone nutrients, even without taking dietary supplements into account. In addition to optimal oral intake, 59% of the participants also reported taking MVMM supplements, which is higher than the average intake of dietary supplements (31%) in U.S. children.(38) In a study by Dwyer et al., dietary supplementation was higher among individuals reporting very good health, and physical activity level and healthy dietary habits were positively associated with supplement use.(38)
For calcium and vitamin D, although the mean intakes for our subjects were slightly below the RDA, girls did meet the EARs,(39) and their intakes were higher than the average intakes of U.S. girls aged 9–13 years of age, respectively.(40) The current study did not detect associations between calcium or vitamin D and bone outcomes. In contrast, past intervention studies reported positive associations between high dietary or supplementary calcium and vitamin D and bone mass accrual in pre-pubertal children.(41, 42) In those studies, the baseline average intakes of these nutrients were below recommendations, and sunlight exposure was likely to be low based on the latitude where the population resided (Finland).(41, 42) Positive relationships between key bone nutrients and bone outcomes may not be as pronounced in well-nourished children, compared to those with nutrient deficits.
The current study has several strengths. The participants were a relatively homogeneous group based on physical maturity and geographical location, which alleviates confounding factors. In particular, the pre-pubertal status of all the participants minimized the influence of variable hormonal exposure. Furthermore, the current study investigated associations of dietary intake in relation to lumbar spine bone mass, geometry, and strength, while accounting for physical activity. When evaluating the association between activity exposure dose and lumbar spine bone outcomes, the statistical effect of each focal nutrient was taken into account. Therefore, the findings reflect diet and physical activity as factors in bone outcomes among well-nourished children, allowing comparison of diet and activity explanatory value.
Methodologically, the paired scans provided 3D metrics for more appropriate evaluation of vertebral body-specific bone mineral content, density, geometry, and strength. The YAQ questionnaire allowed us to assess the intakes of each focal nutrient through diet and supplementation. This detail has only been evaluated by a few past studies investigating the relationship between dietary intake and bone outcomes.(43, 44) The assessment of both dietary and supplemental intake provided us the advantage of comparing the influence of nutrients from different sources on bone outcomes in children. In this scenario, “non-supplemented” indicators of vitamin intake were more influential as factors in bone outcomes than supplement-based indices. This suggests that “whole food” based intakes of may be the most important nutritional factors to consider in pediatric bone health.
This study is not without limitations. First, the study did not take genetics into account. Second, we did not evaluate change in the participants’ bone mass over time. Third, the exclusion of pubertal and mature subjects from the parent study led to a relatively small sample size, which reduced statistical power. In addition, due to the secondary nature of this analysis, the participants were not recruited based on nutrition-related objectives but were recruited based on age and activity patterns. In this cohort, we observed a broad range of physical activity doses (0 – 21.5 h/wk) and energy intakes (1149.24 – 3550.13 kcal). It is possible that inadequate calorie intake for energy expenditure may have played a role in bone properties, potentially dampening favorable associations between loading and bone properties. Future research should be designed to more specifically evaluate energy balance, loading and nutrient intakes as factors in PALAT bone properties.
Furthermore, to evaluate vertebral body properties, one should ideally assess multiple vertebrae. In this cohort, we found that PABMD was positively correlated for L3 versus L1-L4 (r= +0.63, p< 0.001). We are not able to evaluate the extent to which intervertebral discrepancies in PABMD are a function of vertebral body properties (relevant to our analyses) versus posterior element variation (irrelevant to our analyses). Accordingly, future research should be conducted using quantitative computed tomography to evaluate lifestyle variables as factors in vertebral body specific properties for multiple vertebrae.
Finally, we evaluated a large number of dietary variables, although these were less than 10% of the variables provided as YAQ output (>230 nutrient variables). Based on chance alone, we would expect 5% of nutritional variables evaluated to correlate with bone outcomes (1/21). After accounting for activity and age or height, as appropriate, we detected significant bone associations for 7/21 or 33% of diet variables evaluated, thereby exceeding the number of significant factors predicted due to chance alone. As we evaluated multiple bone outcomes, the representation of individual variables across variables of each type (bone mass, geometric and “hybrid” indices) suggests that fiber and B12 are most likely to represent influential factors in development of vertebral body bone mass, geometry, density and strength. Further research is necessary to see if these nutrients are consistently influential across the entire pediatric growth curve, across the skeleton and in other populations.
Our study participants appear to be guided and supported to develop healthy eating habits and participate in extracurricular exercise. Associations between nutrient intake and bone outcomes in well-nourished children might not be as obvious as in children with variable levels of nutrient insufficiency. In addition, only 30% of the participants’ average weekly hours of physical activity met the recommendation of 60 minutes/day, with or without aquatic activity included.(45) The high observed coefficient of variation for physical activity (CV=74%) indicates high variability in exercise exposure among subjects. Our findings may indicate that physical activity plays a stronger role in bone outcomes than diet in well-nourished children. Specifically, if exercise loading trumps nutrient intake for associations with bone outcomes, then the trend for a negative correlation between vitamin D intake and activity exposure may have masked the influence of vitamin D on bone. Alternatively, it may be easier to detect the influence of physical activity when participants range widely from inadequate to very high exercise exposure; nutritional variability may have been inadequate to detect significant dietary correlations for many key variables.
CONCLUSION
We identified significant dose-related associations for physical activity, fiber intake and unsupplemented vitamin B12 intake as factors in a variety of bone outcomes in this cohort of well-nourished and active pre-pubertal girls. Physical activity appears to play a stronger, more consistent role in influencing bone outcomes than nutrient intakes in this context. Higher levels of physical activity were significantly associated with higher third lumbar vertebral body bone mass, areal density, vertebral width and calculated bone strength in axial compression, as well as lower vertebral height and fracture risk index. We did not observe significant relationships between physical activity and vertebral depth, volume or paired scan bone mineral apparent density. Failure to observe associations between assessed lifestyle factors and apparent density calls into question decades of PA areal density findings that have been interpreted to suggest that vertebral body trabecular density may be improved via diet and exercise. Vitamin B12 intake was positively associated with multiple bone parameters, while fiber intake was negatively associated with bone outcomes. The observed negative correlations between fiber intake and bone properties are of concern, as they suggest that recommendations for “high fiber diets” may be carried too far, compromising bone health for growing girls. Future studies investigating the influence of genetics on bone outcomes are warranted. Furthermore, studies with a more diverse cohort composition in terms of race, ethnicity, nutrient intake, and socio-economic status are also recommended.
ACKNOWLEDGMENTS
We acknowledge the assistance of the following individuals with data collection: Cathy Riley, Eileen Burd, Amy Allen, Tina Craig and Kristy Kmack. This research was supported by NIAMS (R01 AR54145) and bridge funding from the University of Wisconsin (Department of Orthopedics and Rehabilitation; School of Medicine and Public Health).
Abbreviations:
- DXA
dual energy X-ray absorptiometry
- PA
postero-anterior scan
- lat
supine lateral scan
- palat
paired pa and lat scan calculated results
- paBMD
postero-anterior areal bone mineral density
- paBMC
pa bone mineral content
- latBMC
lateral BMC
- latDEPTH
lateral vertebral depth (out of pa scan plane)
- latHEIGHT
lateral vertebral body height
- paWIDTH
PA width
- palatCSA
“3D” bone cross-sectional area (coronal plane)
- palatIBS
“3D” index of structural strength in axial compression
- palatBMAD
“3D” vertebral body bone mineral apparent density
- palatFRI
palat fracture risk index
Footnotes
Jie Ren, Lynn Brann, Kay Bruening, Tamara Scerpella and Jodi Dowthwaite declare that they have no conflicts of interest to disclose.
Contributor Information
Jie Ren., Nutrition Science and Dietetics Program. Syracuse University. Address: 1645 Belleville Way #B, Sunnyvale, CA. 94087. Telephone number: (804)-432-2582. jieren510@gmail.com.
Lynn S. Brann., Nutrition Science and Dietetics Program. Syracuse University. Address: 550L White Hall, Syracuse, NY 13244. Telephone number: (315) 443-4805. Fax number: 315-443-9807 lbrann@syr.edu.
Kay S. Bruening., Nutrition Science and Dietetics Program. Syracuse University. Address: 550L White Hall, Syracuse, NY 13244. Telephone number: (315) 443-9326. Fax number: 315-443-9807 ksbrueni@syr.edu.
Tamara A. Scerpella., Department of Orthopedics and Rehabilitation. University of Wisconsin-Madison. Address: 1685 Highland Ave., 6th floor, Madison, WI 53705. Telephone number: (608) 263-5636. scerpella@ortho.wisc.edu.
Jodi N. Dowthwaite., Department of Orthopedic Surgery, Upstate Medical University. Address: 750 East Adams Street, Syracuse, NY 13210. Telephone number: (315) 464-9981. Fax number: (315) 464-6638. dowthwaj@upstate.edu.
REFERENCES:
- 1.Lippuner K, Johansson H, Kanis JA, Rizzoli R. Remaining lifetime and absolute 10-year probabilities of osteoporotic fracture in swiss men and women. Osteoporos Int. 2009. July;20(7):1131–40. [DOI] [PubMed] [Google Scholar]
- 2.Huncharek M, Muscat J, Kupelnick B. Impact of dairy products and dietary calcium on bone-mineral content in children: Results of a meta-analysis. Bone. 2008. August;43(2):312–21. [DOI] [PubMed] [Google Scholar]
- 3.Rizzoli R, Bianchi ML, Garabedian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010. February;46(2):294–305. [DOI] [PubMed] [Google Scholar]
- 4.Greene DA, Naughton GA, Bradshaw E, Moresi M, Ducher G. Mechanical loading with or without weight-bearing activity: Influence on bone strength index in elite female adolescent athletes engaged in water polo, gymnastics, and track-and-field. J Bone Miner Metab. 2012. September;30(5):580–7. [DOI] [PubMed] [Google Scholar]
- 5.Levis S, Lagari VS. The role of diet in osteoporosis prevention and management. Curr Osteoporos Rep. 2012. December;10(4):296–302. [DOI] [PubMed] [Google Scholar]
- 6.Handel MN, Heitmann BL, Abrahamsen B. Nutrient and food intakes in early life and risk of childhood fractures: A systematic review and meta-analysis. Am J Clin Nutr. 2015. November;102(5):1182–95. [DOI] [PubMed] [Google Scholar]
- 7.Laudermilk MJ, Manore MM, Thomson CA, Houtkooper LB, Farr JN, Going SB. Vitamin C and zinc intakes are related to bone macroarchitectural structure and strength in prepubescent girls. Calcif Tissue Int. 2012. December;91(6):430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergman C, Gray-Scott D, Chen JJ, Meacham S. What is next for the dietary reference intakes for bone metabolism related nutrients beyond calcium: Phosphorus, magnesium, vitamin D, and fluoride? Crit Rev Food Sci Nutr. 2009. February;49(2):136–44. [DOI] [PubMed] [Google Scholar]
- 9.van Wijngaarden JP, Doets EL, Szczecinska A, Souverein OW, Duffy ME, Dullemeijer C, Cavelaars AE, Pietruszka B, Van’t Veer P, Brzozowska A, Dhonukshe-Rutten RA, de Groot CP. Vitamin B12, folate, homocysteine, and bone health in adults and elderly people: A systematic review with meta-analyses. J Nutr Metab. 2013;2013:486186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fratoni V, Brandi ML. B vitamins, homocysteine and bone health. Nutrients. 2015. March 30;7(4):2176–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowthwaite JN, Scerpella TA. Skeletal geometry and indices of bone strength in artistic gymnasts. J Musculoskelet Neuronal Interact. 2009. Oct-Dec;9(4):198–214. [PMC free article] [PubMed] [Google Scholar]
- 12.Dowthwaite JN, Rosenbaum PF, Scerpella TA. Site-specific advantages in skeletal geometry and strength at the proximal femur and forearm in young female gymnasts. Bone. 2012. May;50(5):1173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowthwaite JN, Scerpella TA. Distal radius geometry and skeletal strength indices after peripubertal artistic gymnastics. Osteoporos Int. 2011. January;22(1):207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowthwaite JN, Rosenbaum PF, Scerpella TA. Mechanical loading during growth is associated with plane-specific differences in vertebral geometry: A cross-sectional analysis comparing artistic gymnasts vs. non-gymnasts. Bone. 2011. November;49(5):1046–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowthwaite JN, Flowers PP, Scerpella TA. Agreement between pQCT- and DXA-derived indices of bone geometry, density, and theoretical strength in females of varying age, maturity, and physical activity. J Bone Miner Res. 2011. June;26(6):1349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990. June;51(6):1106–12. [DOI] [PubMed] [Google Scholar]
- 17.Leonard MB, Shults J, Zemel BS. DXA estimates of vertebral volumetric bone mineral density in children: Potential advantages of paired posteroanterior and lateral scans. J Clin Densitom. 2006. Jul-Sep;9(3):265–73. [DOI] [PubMed] [Google Scholar]
- 18.Scerpella TA, Davenport M, Morganti CM, Kanaley JA, Johnson LM. Dose related association of impact activity and bone mineral density in pre-pubertal girls. Calcif Tissue Int. 2003. January;72(1):24–31. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Clinical Growth Charts. 2 to 20 years: Girls Body mass index-forage percentiles. [Internet]. Centers for Disease Control and Prevention Published May 2000; Revised October 16, 2000. [revised CDC Growth Charts; cited Accessed May 10, 2014]. Available from: http://www.cdc.gov/growthcharts/data/set1clinical/cj41l024.pdf English. [Google Scholar]
- 20.Duke PM, Litt IF, Gross RT. Adolescents’ self-assessment of sexual maturation. Pediatrics. 1980. December;66(6):918–20. [PubMed] [Google Scholar]
- 21.Rockett HR, Breitenbach M, Frazier AL, Witschi J, Wolf AM, Field AE, Colditz GA. Validation of a youth/adolescent food frequency questionnaire. Prev Med. 1997. Nov-Dec;26(6):808–16. [DOI] [PubMed] [Google Scholar]
- 22.Dietary Reference Intakes (DRIs): Estimated Average Requirements Food and Nutrition Board, Institute of Medicine, National Academies. [Internet]. Institute of Medicine; cited Accessed 7/5/16]. Available from: http://nationalacademies.org/hmd/~/media/Files/Activity%20Files/Nutrition/DRIs/1_%20EARs.pdf [Google Scholar]
- 23.Walther B, Karl JP, Booth SL, Boyaval P. Menaquinones, bacteria, and the food supply: The relevance of dairy and fermented food products to vitamin K requirements. Adv Nutr. 2013. July 1;4(4):463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rupich RC, Griffin MG, Pacifici R, Avioli LV, Susman N. Lateral dual-energy radiography: Artifact error from rib and pelvic bone. J Bone Miner Res. 1992. January;7(1):97–101. [DOI] [PubMed] [Google Scholar]
- 25.Valdimarsson O, Linden C, Johnell O, Gardsell P, Karlsson MK. Daily physical education in the school curriculum in prepubertal girls during 1 year is followed by an increase in bone mineral accrual and bone width--data from the prospective controlled malmo pediatric osteoporosis prevention study. Calcif Tissue Int. 2006. February;78(2):65–71. [DOI] [PubMed] [Google Scholar]
- 26.Duan Y, Parfitt A, Seeman E. Vertebral bone mass, size, and volumetric density in women with spinal fractures. J Bone Miner Res. 1999. October;14(10):1796–802. [DOI] [PubMed] [Google Scholar]
- 27.Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK. Bone loss and bone size after menopause. N Engl J Med. 2003. July 24;349(4):327–34. [DOI] [PubMed] [Google Scholar]
- 28.Lofgren B, Detter F, Dencker M, Stenevi-Lundgren S, Nilsson JA, Karlsson MK. Influence of a 3-year exercise intervention program on fracture risk, bone mass, and bone size in prepubertal children. J Bone Miner Res. 2011. August;26(8):1740–7. [DOI] [PubMed] [Google Scholar]
- 29.Barron E, Cano Sokoloff N, Maffazioli GD, Ackerman KE, Woolley R, Holmes TM, Anderson EJ, Misra M. Diets high in fiber and vegetable protein are associated with low lumbar bone mineral density in young athletes with oligoamenorrhea. J Acad Nutr Diet. 2016. March;116(3):481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolf RL, Cauley JA, Baker CE, Ferrell RE, Charron M, Caggiula AW, Salamone LM, Heaney RP, Kuller LH. Factors associated with calcium absorption efficiency in pre- and perimenopausal women. Am J Clin Nutr. 2000. August;72(2):466–71. [DOI] [PubMed] [Google Scholar]
- 31.Baer DJ, Rumpler WV, Miles CW, Fahey GC,Jr. Dietary fiber decreases the metabolizable energy content and nutrient digestibility of mixed diets fed to humans. J Nutr. 1997. April;127(4):579–86. [DOI] [PubMed] [Google Scholar]
- 32.Diet Adlercreutz H., breast cancer, and sex hormone metabolism. Ann N Y Acad Sci. 1990;595:281–90. [DOI] [PubMed] [Google Scholar]
- 33.Gaskins AJ, Mumford SL, Zhang C, Wactawski-Wende J, Hovey KM, Whitcomb BW, Howards PP, Perkins NJ, Yeung E, Schisterman EF, BioCycle Study Group. Effect of daily fiber intake on reproductive function: The BioCycle study. Am J Clin Nutr. 2009. October;90(4):1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carmel R, Lau KH, Baylink DJ, Saxena S, Singer FR. Cobalamin and osteoblast-specific proteins. N Engl J Med. 1988. July 14;319(2):70–5. [DOI] [PubMed] [Google Scholar]
- 35.Dhonukshe-Rutten RA, van Dusseldorp M, Schneede J, de Groot LC, van Staveren WA. Low bone mineral density and bone mineral content are associated with low cobalamin status in adolescents. Eur J Nutr. 2005. September;44(6):341–7. [DOI] [PubMed] [Google Scholar]
- 36.Dhonukshe-Rutten RA, Lips M, de Jong N, Chin A Paw MJ, Hiddink GJ, van Dusseldorp M, De Groot LC, van Staveren WA. Vitamin B-12 status is associated with bone mineral content and bone mineral density in frail elderly women but not in men. J Nutr. 2003. March;133(3):801–7. [DOI] [PubMed] [Google Scholar]
- 37.McLean RR, Jacques PF, Selhub J, Tucker KL, Samelson EJ, Broe KE, Hannan MT, Cupples LA, Kiel DP. Homocysteine as a predictive factor for hip fracture in older persons. N Engl J Med. 2004. May 13;350(20):2042–9. [DOI] [PubMed] [Google Scholar]
- 38.Dwyer J, Nahin RL, Rogers GT, Barnes PM, Jacques PM, Sempos CT, Bailey R. Prevalence and predictors of children’s dietary supplement use: The 2007 national health interview survey. Am J Clin Nutr. 2013. June;97(6):1331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Institutes of Health. Health Information. Vitamin D fact sheet for health professionals. [Internet]. National Institutes of Health; Reviewed November 10, 2014. [revised NIH website.; cited Accessed May 10, 2014]. Available from: http://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional [Google Scholar]
- 40.Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, Sempos CT, Picciano MF. Estimation of total usual calcium and vitamin D intakes in the united states. J Nutr. 2010. April;140(4):817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng S, Tylavsky F, Kroger H, Karkkainen M, Lyytikainen A, Koistinen A, Mahonen A, Alen M, Halleen J, Vaananen K, Lamberg-Allardt C. Association of low 25-hydroxyvitamin D concentrations with elevated parathyroid hormone concentrations and low cortical bone density in early pubertal and prepubertal finnish girls. Am J Clin Nutr. 2003. September;78(3):485–92. [DOI] [PubMed] [Google Scholar]
- 42.Cheng S, Lyytikainen A, Kroger H, Lamberg-Allardt C, Alen M, Koistinen A, Wang QJ, Suuriniemi M, Suominen H, Mahonen A, Nicholson PH, Ivaska KK, Korpela R, Ohlsson C, Vaananen KH, Tylavsky F. Effects of calcium, dairy product, and vitamin D supplementation on bone mass accrual and body composition in 10–12-y-old girls: A 2-y randomized trial. Am J Clin Nutr. 2005. November;82(5):1115,26; [DOI] [PubMed] [Google Scholar]
- 43.Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: A review of controlled trials. Bone. 2007. January;40(1):14–27. [DOI] [PubMed] [Google Scholar]
- 44.Merrilees MJ, Smart EJ, Gilchrist NL, Frampton C, Turner JG, Hooke E, March RL, Maguire P. Effects of diary food supplements on bone mineral density in teenage girls. Eur J Nutr. 2000. December;39(6):256–62. [DOI] [PubMed] [Google Scholar]
- 45.2008 Physical Activity Guidelines for Americans [Internet]. Office of Disease Prevention and Health Promotion October 18, 2016. [revised 2008 Physical Activity Guidelines; cited Accessed October 18, 2016]. Available from: https://health.gov/paguidelines/guidelines/summary.aspx