INTRODUCTION
Epilepsy is one of the most common chronic conditions affecting women. Although the prevalence of epilepsy and treatment approaches are similar for women and men, women are more likely to experience seizure patterns related to hormonal cycles and are at risk of reproductive alterations and pregnancy complications.
NEUROSTEROIDS
Neurosteroids are molecules that modulate brain excitability and, therefore, can affect the occurrence of seizures. Estrogen and progesterone, the primary reproductive hormones for women, both affect neuronal excitability. The actions of neurosteroids on neuroexcitability occur via 2 mechanisms. The first is a short latency, nongenomic effect mediated by the neuronal membrane.1,2 The second is a long-latency (hours to days) genomic effect.2,3 Estrogen has been suggested as neuroexcitatory, and some women with epilepsy might be susceptible to its proconvulsant effect.2 Progesterone, on the other hand, promotes neuroinhibition, primarily through the action of its metabolite, allopregnanolone, which acts as a positive allosteric modulator of γ-aminobutyric acid conductance.2,4,5
SEX STEROID HORMONE AXIS
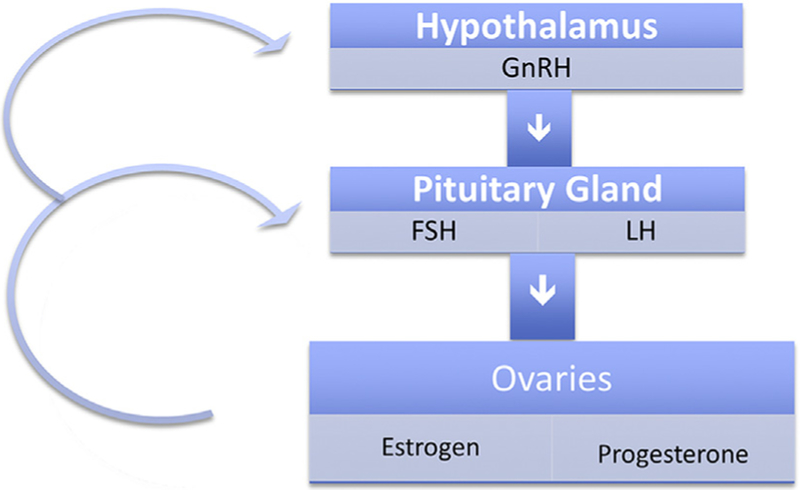
Release of female reproductive steroid hormones is controlled by the hypothalamic-pituitary-ovarian axis through a bidirectional feedback loop, as shown in Fig. 1.6 Gonadotropin-releasing hormone (GnRH) is secreted by the hypothalamus and stimulates release of follicle-stimulating hormone (FSH) by the pituitary. FSH stimulates formation of the ovarian follicles, which secrete estradiol (the main estrogen in women). Midcycle, a surge of luteinizing hormone (LH) induces oocyte maturation, ovulation, and conversion of the follicle into the corpus luteum. This marks the end of the follicular phase and the beginning of the luteal phase. After ovulation, the corpus luteum secretes progesterone. Progesterone inhibits secretion of GnRH, FSH, and LH. If there is no pregnancy, the corpus luteum regresses and production of progesterone and estradiol declines. When progesterone secretion tapers off and GnRH inhibition decreases, the cycle repeats.
Fig. 1.
Hypothalamic-pituitary-ovarian axis.
CATAMENIAL EPILEPSY
The pattern of seizure clustering during certain parts of the menstrual cycle is termed catamenial, derived from the Greek, katamenios, meaning monthly.2 The reported prevalence of catamenial epilepsy depends on the specific definition used during quantification. Menstrual seizure exacerbations have been reported in up to 70% of women with epilepsy, although a more strict definition used by Herzog and colleagues7 in their 1997 study suggested that this pattern was present in approximately one-third of women with epilepsy.
Menstrual Cycle
The average menstrual cycle is 28 (24–35) days, with day 1 the first day of menses and ovulation occurring on day 14 (or day −14 to adjust for cycle lengths other than 28 days, because ovulation always occurs 14 days prior to menses). The follicular phase then occurs during days 1 to 14 and the luteal phase on days 15 to 28 (or days −14 to −1). The hormonal fluctuations most relevant for catamenial seizure exacerbations are the rapid surge of estrogen at day 13, which initiates ovulation, and the rapid decline of progesterone followed by estrogen on days 26 to 28, just before the onset of menses.2
Dysregulated secretion of FSH leads to poor follicular development and, therefore, poor functioning of the corpus luteum. This disorder is known as inadequate luteal phase and is associated with lack of ovulation.2,8 Inadequate development of the corpus luteum leads to low production of progesterone during the luteal phase, whereas estrogen production remains robust.2
Criteria for Diagnosis
The most accepted definition of catamenial epilepsy is based on the work of Herzog and colleagues,7 who statistically derived the patterns of seizure occurrence throughout the menstrual cycle. They described an approximate doubling of seizure frequency during 3 portions of the menstrual cycle: perimenstrual (C1: days −3 to 3) and periovulatory (C2: days 10 to −13) in normal cycles and luteal (C3: days 10 to 3) in inadequate luteal phase cycles.9 In the National Institutes of Health (NIH) Progesterone Treatment Trial, the prevalence of catamenial epilepsy by pattern was 39.8% C1, 33.9% C2, and 47.1% C3.10 Table 1 shows each type with its corresponding hormonal triggers. A catamenial pattern has been reported most frequently with focal-onset seizures, but it can be seen in other types of seizures.
Table 1.
Patterns of catamenial epilepsy
| Catamenial Pattern | Menstrual Cycle Days | Menstrual Cycle Phase | Hormonal Changes |
|---|---|---|---|
| C1 | −3 to 3 | Perimenstrually | ↓ Progesterone |
| C2 | 10 to −13 | Periovulatory | ↑ Estrogen |
| C3 | 10 to 3 | Inadequate luteal phase | ↑ Estrogen/progesterone ratio |
Progesterone Therapy
Cyclic progesterone therapy supplements progesterone during the luteal phase and withdraws it gradually premenstrually, as shown in Box 1. The NIH Progesterone Trial Treatment was a randomized, placebo-controlled, double-blind, clinical trial of progesterone in the treatment of intractable seizures in women with and without catamenial epilepsy.11 Treatment consisted of baseline optimal AED therapy plus adjunctive natural progesterone lozenges or matching placebo. The findings of this trial showed that cyclic progesterone is comparable to placebo in the treatment of intractable seizures in women with focal-onset epilepsy.11 A prespecified secondary analysis, however, identified a subset of women with perimenstrual seizure exacerbation (C1) of greater than or equal to 3-fold who were responsive to progesterone treatment. The detection of a greater than or equal to 3-fold level of perimenstrual seizure exacerbation might suggest a favorable response to treatment with adjunctive cyclic progesterone.10 Cyclic progesterone supplement may have greater efficacy where progesterone withdrawal (C1) is implicated.10 Potential adverse effects of natural progesterone lozenges include sedation, depression, asthenia, weight gain, irregular vaginal bleeding, and constipation.10
Box 1. Cyclic progesterone treatment.
Natural progesterone lozenges (200 mg)
Instructions (day 1 = 1st day of menses)
1 Lozenge TID, days 14 to 25
½ Lozenge TID, days 26 to 27
¼ Lozenge TID, day 28
Stop day 29
Medroxyprogesterone acetate, a synthetic progesterone compound administered via intramuscular injection, may lower seizure frequency when it is given in sufficient dosage to induce amenorrhea.12 Although it and other synthetic progestins are not metabolized to the inhibitory neurosteroid allopregnanolone, the benefit is thought to be due to eliminating the fluctuation of endogenous sex steroid hormones. In 1 open-label study of 14 women with refractory focal-onset seizures and normal ovulatory cycles, medroxyprogesterone administration in doses large enough to induce amenorrhea resulted in a 39% seizure reduction.12 Side effects include those encountered with natural progesterone as well as hot flashes and a delay in return of regular ovulatory cycles and fertility. Long-term hypoestrogenic effects on cardiovascular status, bone density, and emotional health need to be considered with chronic use. Oral synthetic progestins administered cyclically or continuously have not proved effective therapy for seizures.12,13
Other Treatments
Acetazolamide has been used to treat catamenial epilepsy, but it has not been assessed in randomized trials. Effectiveness at doses of 250 mg to 500 mg daily administered for 3 to 7 days, starting approximately 3 days before menses, has been reported.14 Clobazam has been formally studied for the treatment of catamenial epilepsy. In a double-blind, placebo-controlled, crossover study, clobazam was associated with better control than placebo. Complete control was seen in most patients during a 10-day treatment phase beginning 7 to 2 days before the menstrual flow adjusted for each patient so that treatment would begin 2 to 4 days before the time during which the exacerbation of epilepsy usually occurred.15 A temporary increase in the dose of a patient’s usual AEDs at specific times during the menstrual cycle is another reasonable, empirical approach, although phenytoin should not be increased due to the risk of toxic effects associated with its nonlinear kinetics.2
REPRODUCTIVE AND SEXUAL DYSFUNCTION
Increased rates of infertility have been reported in some studies of women with epilepsy, but other studies have reported normal fertility. Some of the postulated mechanisms for reduced fertility are related to the effect of epilepsy on reproductive hormones. Regions of the limbic cortex, in particular the amygdala, have reciprocal connections with the hypothalamus and can modulate the hypothalamic-pituitary-ovarian axis.2 GnRH is produced by a population of cells in the preoptic area of the hypothalamus, which is vulnerable to injury by seizures. Dysfunction of GnRH cells is followed by the abnormal release of FSH and LH.2 This phenomenon is known as hypogonadotropic hypogonadism.
EFFECT OF ANTIEPILEPTIC DRUGS ON REPRODUCTIVE HORMONES
Enzyme-inducing AEDs alter hepatic metabolism and decrease concentrations of reproductive hormones. They also induce production of sex hormone–binding globulin, which reduces concentrations of free reproductive hormones in serum.2 Lofgren and colleagues16 reported low total testosterone concentrations and free androgen indices in men with epilepsy taking carbamazepine or oxcarbazepine. Low total testosterone and free androgen levels may result in decreased sex drive.
Some AEDs directly alter hormonal production. For example, valproate has been reported to induce androgen synthesis in the ovaries.2,17 This drug is associated with increased testosterone concentrations, anovulation, and polycystic ovarian syndrome.
EFFECT OF REPRODUCTIVE HORMONES ON ANTIEPILEPTIC DRUGS
On the other hand, levels of AEDs may fluctuate during the menstrual cycle due to hormonal effects. High levels of circulating estrogen in the luteal phase could theoretically induce hepatic isoenzymes used for AED metabolism, especially for glucuronidation, and thereby lower the level of circulating AED premenstrually; 1 study suggested minor changes in lamotrigine and valproate levels but findings were not significant.18
CONTRACEPTION
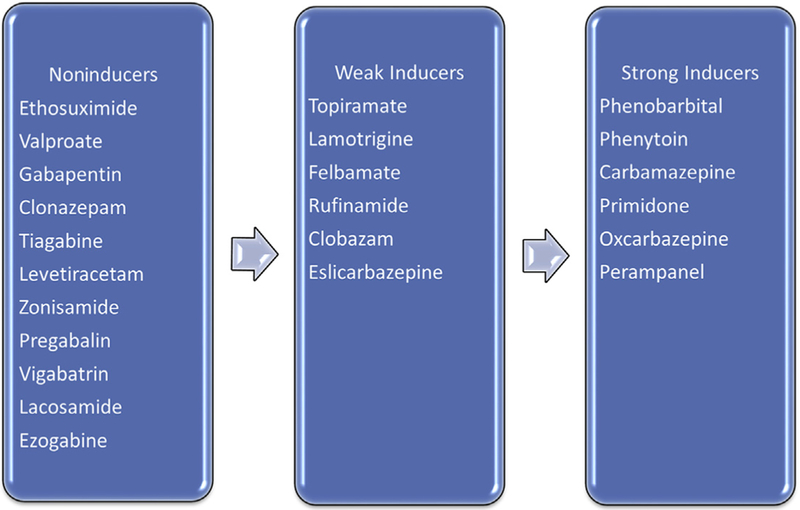
Pharmacokinetics of AEDs and hormonal contraceptives can interact bidirectionally, potentially increasing the elimination rate of either drug. The most widely used contraceptives are combined oral contraceptive (COC) formulations containing an estrogen and a progestin compound, but other hormonal contraceptives include progestin-only pills (POPs), patches, and vaginal rings, which work by secretion of an estrogen and progestin into the blood circulation, subcutaneous progestin implants, and intramuscular progestin injections. AEDs with a potential for hepatic enzyme induction— included in Fig. 2—may accelerate the hepatic metabolism of COCs and other hormonal contraceptives. The non–enzyme-inducing AEDs do not influence hormonal contraceptive metabolism and, therefore, can be administered without risk of contraceptive failure.
Fig. 2.
Degree of induction of sex steroid hormones metabolism.
Combined OCs can increase the elimination of AEDs that are metabolized by glucuronidation. Among AEDs, this metabolic pathway has been studied most intensively for lamotrigine. The metabolism of lamotrigine is accelerated approximately 50% by cotreatment with COCs.19 The clearance of valproic acid is also increased with COCs.18 This information applies to other hormonal forms of birth control, including the vaginal ring and patch.
Only sparse data are available about possible interactions between AEDs and POPs. Enzyme-inducing AEDs increase the metabolism of progestins as well as estrogens; thus, POPs are not adequately efficacious when taken with enzyme-inducing AEDs. For women taking enzyme-inducing AEDs, an intrauterine device (IUD) is an excellent choice, and, given the safety and high contraceptive efficacy, an IUD is a favorable option for all women with epilepsy of reproductive age.20 The levonorgestrel IUD prevents pregnancy by local hormonally mediated changes and is unlikely to be impacted by enzyme-inducing AEDs. Intramuscular medroxyprogesterone acetate is another long-acting reversible contraceptive that is likely adequate with coadministration of enzyme-inducing AEDs, because the concentration of progestin is high enough that efficacy is maintained but is often not considered a first-line option due to its side effects.
PREGNANCY
Epilepsy is the most common neurologic disorder that requires continuous treatment during pregnancy, and AEDs are one of the most frequent teratogen exposures.20 Approximately 1.5 million women with epilepsy are of childbearing age in the United States, and 3 to 5 births per 1000 will be to women with epilepsy.21 In a large observational study of women with epilepsy, approximately 52% of patients had seizures during pregnancy.22 AED treatment during pregnancy is a balancing act between teratogenic risks to the fetus and maintaining maternal seizure control.
Risk of Congenital Malformations with Antiepileptic Drug Use
Major congenital malformations (MCMs) are defined as birth defects that are life threatening, have a major impact on the person, and/or require surgical treatment. The reported MCM rates in the general population vary between 1.6% and 3.2%, and women with epilepsy not receiving AEDs show similar rates.20 The average MCM rates among all AED exposures vary between 3.1% and 9%, approximately 2-fold to 3-fold higher than in the general population.23
Minor anomalies are defined as structural deviations from the norm that do not constitute a health threat. Minor anomalies affect 6% to 20% of infants born to women with epilepsy, approximately 2.5-fold the rate of the general population.20 Some examples of minor anomalies are ocular hypterteleroism, micrognathia, syndactyly, and polydactyly.
Antiepileptic Drug Monotherapies
A high risk of MCMs with first-trimester valproate exposure has been found in several large pregnancy registries.24–27 The relative risk of MCMs with first-trimester exposure to valproate is approximately 3-fold, and the absolute risk is 6% to 9%.28 For individual MCMs, many are elevated for valproate and include spina bifida. A publication from the North American AED Pregnancy Registry (NAAPR) revealed that phenobarbital also has a significantly increased risk of MCMs, most often cardiac defects.29 If possible, both valproate and phenobarbital should be avoided in women of child-bearing potential. There is a dose-associated risk for valproate and, in women who need these AEDs for seizure control and whose seizures have failed to respond adequately to all other AEDs, the smallest dose possible should be used.
In a 2010 study using the European Surveillance of Congenital Anomalies antiepileptic study database and incorporating other published reports, the findings suggested an association between spina bifida and in utero carbamazepine exposure compared with no exposure (odds ratio 2.6; 95% CI, 1.2–5.3).30 The risk with carbamazepine, however, was approximately 80% less than with valproate.
Another important recent finding is the association of facial clefts with in utero topiramate exposure. The rate of oral clefts with first-trimester exposure to topiramate was 1.4% in the NAAPR, which is an approximately 10-fold increase compared with the control population prevalence of 0.11%.29 As a point of comparison, in this report, oral cleft prevalence was 0.5% with lamotrigine, carbamazepine, and phenytoin; 1.2% with valproate; and 4% with phenobarbital.29 This increased risk was also found in other large birth defect registries in the United States and in the UK Epilepsy and Pregnancy Register.28,31 Emerging findings also suggest an association between topiramate use and hypospadias in exposed offspring.32
In the same NAAPR publication, the risk of MCMs with first-trimester exposures to lamotrigine, levetiracetam, carbamazepine, or phenytoin was between 2% and 2.5% for each individual drug.29 Although there were not enough exposures to oxcarbaze-pine, gabapentin, or zonisamide in this study to determine precise estimates, the upper limits of the 95% CIs were low.28 Table 2 shows the risk of MCMs with exposure to each specific AED monotherapy during the first trimester, and Table 3 summarizes the relative risk of MCMs with exposure to a specific AED monotherapy during the first trimester compared with unexposed and lamotrigine groups.29
Table 2.
Risk of major congenital malformations with exposure to a specific antiepileptic drug monotherapy during the first trimester as reported by the North American AED Pregnancy Registry, 1997–2011
| Antiepileptic Drug Monotherapy | Major Congenital Malformations, No. (%) and 95% Cl |
|---|---|
| Unexposed (n = 442) | 5 (1.1), 0.37–2.6 |
| Lamotrigine (n = l562) | 31 (2.0), 1.4–2.8 |
| Carbamazepine (n = l033) | 31 (3.0), 2.1–4.2 |
| Phenytoin (n = 416) | 12 (2.9), 1.5–5.0 |
| Levetiracetam (n = 450) | 11 (2.4), 1.2–4.3 |
| Topiramate (n = 359) | 15 (4.2), 2.4–6.8 |
| Valproate (n = 323) | 30 (9.3), 6.4–13.0 |
| Phenobarbital (n = 199) | 11 (5.5), 2.8–9.7 |
| Oxcarbazepine (n = 182) | 4 (2.2), 0.6–5.5 |
| Gabapentin (n = 145) | 1 (0.7), 0.02–3.8 |
| Zonisamide (n = 90) | 0 (0), 0.0–3.3 |
| Clonazepam (n = 64) | 2 (3.1), 0.4–10.8 |
Table 3.
Relative risk of major congenital malformations with exposure to a specific antiepileptic drug monotherapy during the first trimester, compared with unexposed and lamotrigine groups, as reported by the North American AED Pregnancy Registry, 1997–2011
| Antiepileptic Drug Monotherapy | Unexposed Reference Relative Risk, 95% Cl | Exposed Reference Relative Risk, 95% Cl |
|---|---|---|
| Lamotrigine (n = l562) | 1.8, 0.7–4.6 | Reference |
| Carbamazepine (n = 1033) | 2.7, 1.0–7.0 | 1.5, 0.9–2.5 |
| Phenytoin (n = 416) | 2.6, 0.9–7.4 | 1.5, 0.7–2.9 |
| Levetiracetam (n = 450) | 2.2, 0.8–6.4 | 1.2, 0.6–2.5 |
| Topiramate (n = 359) | 3.8, 1.4–1.06 | 2.2, 1.2–4.0 |
| Valproate (n = 323) | 9.0, 3.4–23.3 | 5.1, 3.0–8.5 |
| Phenobarbital (n = 199) | 5.1, 1.8–14.9 | 2.9, 1.4–5.8 |
| Oxcarbazepine (n = 182) | 2.0, 0.5–7.4 | 1.1, 0.4–3.1 |
| Gabapentin (n = 145) | 0.6, 0.07–5.2 | 0.3, 0.05–2.5 |
| Zonisamide (n = 90) | N/A | N/A |
| Clonazepam (n = 64) | 2.8, 0.5–4.8 | 1.6, 0.4–6.8 |
Unexposed Reference = Relative risk of major congenital malformations with exposure to each specific antiepileptic drug monotherapy during the first trimester, compared with unexposed subjects or subjects not exposed to antiepileptic drug(s).
Exposed reference = Relative risk of major congenital malformations with exposure to each specific antiepileptic drug monotherapy during the first trimester, compared with subjects exposed to lamotrigine.
Abbreviation: N/A, not available, relative risk not available due to insufficient data.
Polytherapy
MCM rates have been reported higher for AED polytherapy compared with monotherapy regimens,33 leading to the American Academy of Neurology and American Epilepsy Society (AAN-AES) Practice Parameter recommendation that AED monotherapy is preferred to polytherapy during pregnancy, and monotherapy should be achieved during the preconception phase.33 Since this Practice Parameter, however, new data suggest that this could be an oversimplification. Data from the NAAPR suggest that not all AED polytherapy combinations are the same. Both lamotrigine and carbamazepine had relatively low MCM rates in polytherapy regimens that did not contain valproate. The MCM rate for lamotrigine was 2.9% with any AED other than valproate but 9.1% for lamotrigine combined with valproate. Similarly, the MCM rate for carbamazepine was 2.5% with any AED other than valproate, but 15.4% for carbamazepine combined with valproate.
Cognitive and Behavioral Teratogenesis of Antiepileptic Drugs
Studies investigating cognitive outcomes in children of women with epilepsy suggest an increased risk of mental impairment.33 Verbal scores on neuropsychometric measures may be selectively more involved.34 Although a variety of factors contribute to the cognitive problems of children of women with epilepsy, AEDs seem to play a major role. AED-induced functional and anatomic defects may involve different mechanisms because anatomic risks are related to first-trimester exposure, and functional deficits may be related primarily to third-trimester exposure or at least exposure throughout the entire pregnancy. AEDs that are known to induce apoptosis, such as valproate, seem to affect children’s neurodevelopment in a more severe fashion.35,36 The results of the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study revealed that fetal valproate exposure has dose-dependent associations with reduced cognitive abilities across a range of domains, including low IQ, and these seem to persist at least until the age of 6.37 Some studies have shown neurodevelopmental deficiencies, including low verbal intelligence, associated with the use of phenobarbital and possibly phenytoin.38–42 So far, most of the investigations available suggest that fetal exposures to lamotrigine or levetiracetam are safer with regard to cognition compared with other AEDs.37,43,44 Studies on carbamazepine exposure show consistent results that support normal neurodevelopment, leading to an AAN-AES practice parameter that concludes this AED probably does not increase poor cognitive outcome compared with unexposed controls.32,33,36 Overall, children exposed to polytherapy prenatally seem to have worse cognitive and behavioral outcomes than children exposed to monotherapy.45–47 There is an increased risk of neurodevelopmental deficits when polytherapy involves the use of valproate versus other agents.48 In addition, the Liverpool and Manchester Neurodevelopment Group reported findings suggesting that valproate may be associated with autism spectrum disorder. In their small sample, 6.3% of the children exposed to valproate monotherapy had clinically diagnosed autism spectrum disorder.49 This is 7-fold higher than the control group and more than 10-fold higher than the reported incidence in the general population. Additional evidence comes from a study by Christensen and colleagues,50 in which the absolute risk of autism spectrum disorder among 432 children exposed to valproate was4.15% (95% CI, 2.20–7.81), and the absolute risk of childhood autism was 2.95% (95% CI, 1.42–6.11).
Other Birth Outcomes
Chen and colleagues51 reported that seizures in women with epilepsy during pregnancy were associated with a 1.5-fold increase for preterm delivery or infants being small for gestational age. A secondary analysis of the neonatal outcomes from the NEAD cohort reported that adverse neonatal outcome risks may differ between the AEDs; the odds ratio for infants being born small for gestational age was higher for the valproate and carbamazepine groups, and reduced 1-minute Apgar scores occurred more frequently in the phenytoin and valproate groups.52 More recently, findings from the NAAPR suggested an association between in utero exposure to zonisamide and topiramate and a decrease in mean birth weight and length.53 Prenatal exposure to topiramate or zonisamide was associated with mean lower birth weights of 221 g and 202 g, respectively, and a mean lesser neonatal length of 1 cm compared with lamotrigine exposure (P<.1). The prevalence of small for gestational age was 6.8% for lamotrigine, 17.9% for topiramate (RR 2.4; 95% CI, 1.8–3.3), and 12.2% for zonisamide (RR 1.6; 95% CI 0.9%–2.8%). Similar results were found when a group of 457 unexposed neonates was used as a reference.
Folic Acid Use
Folic acid supplementation in women in the general population during pregnancy has been associated with a risk reduction in the occurrence of congenital malformations, including neural tube defects.54 In addition, the NEAD study showed higher mean IQs in the children of mothers who took periconceptional folic acid supplementation in addition to folic acid later during pregnancy.37 Although in the past some organizations advocated that women on treatment with AEDs associated with a higher risk of neural tube defects took a folic acid dose of 4 mg per day, the effectiveness of this dose versus lower doses in the absence of a family history of neural tube defects has not been established.
Antiepileptic Drug Metabolism and Management During Pregnancy
Many of the AEDs undergo enhanced clearance during pregnancy and the serum concentrations need to be monitored. Per the 2009 AAN-AES Practice Parameter, it seems reasonable to individualize this monitoring for each patient with the aim of maintaining a level near the preconceptional level, presumably at which the women with epilepsy were doing well with seizure control.20 A proposed approach is to check AED serum levels monthly and adjust the dose to compensate for significant declines. Studies have indicated that a decrease in the concentration to 65% or less of an individual’s target concentration is associated with increased risk of seizure worsening.55,56 Postpartum, the AED should be gradually tapered to the preconception dose to avoid supratherapeutic levels and toxicity. Different approaches may be used to accomplish this taper adapted to the particular AED. The postpartum pharmacokinetic changes and clinical consequences are best documented for lamotrigine, and the adjustment in dose should begin within 3 days postpartum with return to preconception dose or slightly higher to accommodate sleep-deprivation effects within 10 to 21 days postpartum.56 Postpartum pharmacokinetic changes with AEDs that are metabolized primarily by the cytochrome p450 system likely occur more gradually.57
A range of physiologic changes during gestation may alter the pharmacokinetics of AEDs.58 Increased volume of distribution may lead to reduced AED serum concentrations. Increased renal blood flow and glomerular filtration rate may reduce the serum concentrations of AEDs predominantly eliminated via the kidneys. Reduced serum albumin concentrations may affect AED protein binding and plasma clearance. Increased estrogen levels lead to accelerated drug glucuronidation.59 In addition, the activity of some cytochrome P450 enzymes is increased.60 Lamotrigine, one of the most commonly used AEDs during pregnancy, is extensively metabolized by glucuronidation. Its clearance in the last trimester of pregnancy increases to an average of double compared with baseline, with a reduction in the dose-normalized serum concentration by 40% to 60%.61–65 A more in-depth pharmacokinetic modeling study of lamotrigine demonstrated, however, that 77% of women had a greater than 3-fold increase in clearance during pregnancy whereas 23% had only a minimal increase, and the investigators postulated that the subpopulations may be due to pharmacogenetic differences.56 The interindividual variability is another argument for the use of therapeutic drug monitoring during pregnancy.
Oxcarbazepine is metabolized to the pharmacologically active monohydroxycarbazepine, which is eliminated as a glucoronide. In 2 small studies, the serum concentrations of monohydroxycarbazepine were at least 36% lower during pregnancy compared with prepregnancy or postpregnancy values.66,67 One-third of an oral dose of levetiracetam is metabolized in blood by hydrolysis and two-thirds are usually found unchanged in the urine. The clearance of levetiracetam increases significantly during pregnancy. Case series have demonstrated reduced serum concentration as low as 50% of baseline.68,69
BREASTFEEDING
Estimates of AED exposure from breast milk suggest that it is low for many AEDs.70 Data on AED serum levels in the breastfeeding child are sparse, however, except for lamotrigine. In a study of 30 mother-child pairs, infant plasma concentrations were 18.3% of maternal plasma concentrations.71 No adverse effects of AED exposure via breast-milk were observed at age 6 in the NEAD study. In addition, breastfed children in this study exhibited a higher IQ and enhanced verbal abilities.72 As a general rule, breast-feeding is recommended to mothers with epilepsy based on the known positive effects of breastfeeding and lack of scientific evidence to support the theoretic risks.
MENOPAUSE
Increased rates of premature menopause characterized by amenorrhea and ovarian failure have been reported in women with focal-onset epilepsy of temporal lobe origin.73 In a 1986 survey of women with epilepsy by Herzog and colleagues,73 2 of 50 women had an onset of menopause before age 40, which is much higher than the expected rate of less than 1% in the general population. In a study by Harden and colleagues74 (2003), the median age of menopause was 47 years among women with epilepsy compared with 51.4 years in the background population, and seizure frequency or lifetime number of seizures was associated with the timing of cessation of reproductive cycling.74 Hormone replacement therapy is associated with a dose-related increase in seizure frequency in postmenopausal women with epilepsy.75
SUMMARY
Optimal treatment of women with epilepsy requires an understanding of the complex interactions of sex steroid hormones with epilepsy and AEDs, and the potential risks of AEDs during pregnancy. Informed treatment recommendations provide improved seizure control with fewer adverse effects for women with epilepsy and their offspring.
KEY POINTS.
Women with epilepsy may experience seizure patterns related to the menstrual cycle and are at risk of reproductive abnormalities and pregnancy complications.
There are bidirectional pharmacokinetic interactions between antiepileptic drugs (AEDs) and hormonal contraceptives, which may result in reduced efficacy of either one.
AED treatment during pregnancy is a balancing act between teratogenic risks to the fetus and maintaining maternal seizure control.
Folic acid supplementation prior to and during pregnancy has been associated with a risk reduction in the occurrence of congenital malformations and cognitive teratogenesis.
Estimates of AED exposure from breast milk suggest that it is low for many AEDs.
Acknowledgments
Preparation of this article was supported in part by NIH NINDS #2U01-NS038455 (N.J. Vélez-Ruiz and P.B. Pennell).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest that are directly relevant to the content of this review.
REFERENCES
- 1.Gu Q, Moss RL. 17 beta-Estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci 1996;16(11):3620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harden CL, Pennell PB. Neuroendocrine considerations in the treatment of men and women with epilepsy. Lancet Neurol 2013;12(1):72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiland NG. Estradiol selectively regulates agonist binding sites on the N-methyl-D-aspartate receptor complex in the CA1 region of the hippocampus. Endocrinology 1992;131(2):662–8. [DOI] [PubMed] [Google Scholar]
- 4.Reddy DS, Rogawski MA. Neurosteroids - endogenous regulators of seizure susceptibility and role in the treatment of epilepsy In: Noebels JL, Avoli M, Rogawski MA, et al. , Jasper’s basic mechanisms of the epilepsies. 4th edition New York: Oxford University Press; 2012. p. 984–97. [Google Scholar]
- 5.Majewska MD, Harrison NL, Schwartz RD, et al. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 1986;232(4753): 1004–7. [DOI] [PubMed] [Google Scholar]
- 6.Pennell PB. Hormonal aspects of epilepsy. Neurol Clin 2009;27(4):941–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia 1997;38(10):1082–8. [DOI] [PubMed] [Google Scholar]
- 8.Jones GS. Luteal phase defect: a review of pathophysiology. Curr Opin Obstet Gynecol 1991;3(5):641–8. [PubMed] [Google Scholar]
- 9.Herzog AG. Catamenial epilepsy: definition, prevalence pathophysiology and treatment. Seizure 2008;17(2):151–9. [DOI] [PubMed] [Google Scholar]
- 10.Herzog AG. Catamenial epilepsy: update on prevalence, pathophysiology and treatment from the findings of the NIH progesterone treatment trial. Seizure 2015;28:18–25. [DOI] [PubMed] [Google Scholar]
- 11.Herzog AG, Fowler KM, Smithson SD, et al. Progesterone vs placebo therapy for women with epilepsy: a randomized clinical trial. Neurology 2012;78(24): 1959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattson RH, Cramer JA, Caldwell BV, et al. Treatment of seizures with medroxyprogesterone acetate: preliminary report. Neurology 1984;34(9):1255–8. [DOI] [PubMed] [Google Scholar]
- 13.Dana-Haeri J, Richens A. Effect of norethisterone on seizures associated with menstruation. Epilepsia 1983;24(3):377–81. [DOI] [PubMed] [Google Scholar]
- 14.Ansell B, Clarke E. Acetazolamide in treatment of epilepsy. Br Med J 1956; 1(4968):650–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feely M, Calvert R, Gibson J. Clobazam in catamenial epilepsy. A model for evaluating anticonvulsants. Lancet 1982;2(8289):71–3. [DOI] [PubMed] [Google Scholar]
- 16.Lofgren E, Tapanainen JS, Koivunen R, et al. Effects of carbamazepine and oxcarbazepine on the reproductive endocrine function in women with epilepsy. Epilepsia 2006;47(9):1441–6. [DOI] [PubMed] [Google Scholar]
- 17.Nelson-DeGrave VL, Wickenheisser JK, Cockrell JE, et al. Valproate potentiates androgen biosynthesis in human ovarian theca cells. Endocrinology 2004; 145(2):799–808. [DOI] [PubMed] [Google Scholar]
- 18.Herzog AG, Blum AS, Farina EL, et al. Valproate and lamotrigine level variation with menstrual cycle phase and oral contraceptive use. Neurology 2009;72(10): 911–4. [DOI] [PubMed] [Google Scholar]
- 19.Christensen J, Petrenaite V, Atterman J, et al. Oral contraceptives induce lamotrigine metabolism: evidence from a double-blind, placebo-controlled trial. Epilepsia 2007;48(3):484–9. [DOI] [PubMed] [Google Scholar]
- 20.Pennell PB. Pregnancy, epilepsy, and women’s issues. Continuum (Minneap Minn) 2013;19(3 Epilepsy):697–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harden CL, Pennell PB, Koppel BS, et al. Practice parameter update: management issues for women with epilepsy–focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 2009;73(2):142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas SV, Syam U, Devi JS. Predictors of seizures during pregnancy in women with epilepsy. Epilepsia 2012;53(5):e85–8. [DOI] [PubMed] [Google Scholar]
- 23.Pennell PB. Antiepileptic drugs during pregnancy: what is known and which AEDs seem to be safest? Epilepsia 2008;49(Suppl 9):43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrow J, Russell A, Guthrie E, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry 2006;77(2):193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wide K, Winbladh B, Kallen B. Major malformations in infants exposed to antiepileptic drugs in utero, with emphasis on carbamazepine and valproic acid: a nation-wide, population-based register study. Acta Paediatr 2004;93(2):174–6. [DOI] [PubMed] [Google Scholar]
- 26.Artama M, Auvinen A, Raudaskoski T, et al. Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology 2005;64(11): 1874–8. [DOI] [PubMed] [Google Scholar]
- 27.Campbell E, Kennedy F, Russell A, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and Ireland Epilepsy and Pregnancy Registers. J Neurol Neurosurg Psychiatry 2014; 85(9):1029–34. [DOI] [PubMed] [Google Scholar]
- 28.Harden CL. Pregnancy and epilepsy. Continuum (Minneap Minn) 2014;20(1 Neurology of Pregnancy):60–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez-Diaz S, Smith CR, Shen A, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology 2012;78(21):1692–9. [DOI] [PubMed] [Google Scholar]
- 30.Jentink J, Dolk H, Loane MA, et al. Intrauterine exposure to carbamazepine and specific congenital malformations: systematic review and case-control study. BMJ 2010;341:c6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margulis AV, Mitchell AA, Gilboa SM, et al. Use of topiramate in pregnancy and risk of oral clefts. Am J Obstet Gynecol 2012;207(5):405.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vajda FJ, O’Brien TJ, Graham J, et al. Associations between particular types of fetal malformation and antiepileptic drug exposure in utero. Acta Neurol Scand 2013;128(4):228–34. [DOI] [PubMed] [Google Scholar]
- 33.Harden CL, Meador KJ, Pennell PB, et al. Management issues for women with epilepsy-focus on pregnancy (an evidence-based review): II. Teratogenesis and perinatal outcomes: report of the quality standards subcommittee and therapeutics and technology subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia 2009;50(5):1237–46. [DOI] [PubMed] [Google Scholar]
- 34.Meador KJ, Baker GA, Browning N, et al. Foetal antiepileptic drug exposure and verbal versus non-verbal abilities at three years of age. Brain 2011;134(Pt 2): 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A 2002;99(23): 15089–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bittigau P, Sifringer M, Ikonomidou C. Antiepileptic drugs and apoptosis in the developing brain. Ann N Y Acad Sci 2003;993:103–14 [discussion: 123–4]. [DOI] [PubMed] [Google Scholar]
- 37.Meador KJ, Baker GA, Browning N, et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol 2013;12(3):244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farwell JR, Lee YJ, Hirtz DG, et al. Phenobarbital for febrile seizures–effects on intelligence and on seizure recurrence. N Engl J Med 1990;322(6):364–9. [DOI] [PubMed] [Google Scholar]
- 39.Reinisch JM, Sanders SA, Mortensen EL, et al. In utero exposure to phenobarbital and intelligence deficits in adult men. JAMA 1995;274(19):1518–25. [PubMed] [Google Scholar]
- 40.Sulzbacher S, Farwell JR, Temkin N, et al. Late cognitive effects of early treatment with phenobarbital. Clin Pediatr (Phila) 1999;38(7):387–94. [DOI] [PubMed] [Google Scholar]
- 41.Vanoverloop D, Schnell RR, Harvey EA, et al. The effects of prenatal exposure to phenytoin and other anticonvulsants on intellectual function at 4 to 8 years of age. Neurotoxicol Teratol 1992;14(5):329–35. [DOI] [PubMed] [Google Scholar]
- 42.Scolnik D, Nulman I, Rovet J, et al. Neurodevelopment of children exposed in utero to phenytoin and carbamazepine monotherapy. JAMA 1994;271(10): 767–70. [PubMed] [Google Scholar]
- 43.Shallcross R, Bromley RL, Cheyne CP, et al. In utero exposure to levetiracetam vs valproate: development and language at 3 years of age. Neurology 2014;82(3): 213–21. [DOI] [PubMed] [Google Scholar]
- 44.Shallcross R, Bromley RL, Irwin B, et al. Child development following in utero exposure: levetiracetam vs sodium valproate. Neurology 2011;76(4):383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forsberg L, Wide K, Kallen B. School performance at age 16 in children exposed to antiepileptic drugs in utero–a population-based study. Epilepsia 2011;52(2): 364–9. [DOI] [PubMed] [Google Scholar]
- 46.Koch S, Titze K, Zimmermann RB, et al. Long-term neuropsychological consequences of maternal epilepsy and anticonvulsant treatment during pregnancy for school-age children and adolescents. Epilepsia 1999;40(9):1237–43. [DOI] [PubMed] [Google Scholar]
- 47.Losche G, Steinhausen HC, Koch S, et al. The psychological development of children of epileptic parents. II. The differential impact of intrauterine exposure to anti-convulsant drugs and further influential factors. Acta Paediatr 1994;83(9):961–6. [DOI] [PubMed] [Google Scholar]
- 48.Nadebaum C, Anderson V, Vajda F, et al. The Australian brain and cognition and antiepileptic drugs study: IQ in school-aged children exposed to sodium valproate and polytherapy. J Int Neuropsychol Soc 2011;17(1):133–42. [DOI] [PubMed] [Google Scholar]
- 49.Bromley RL, Mawer GE, Briggs M, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry 2013;84(6):637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christensen J, Gronborg TK, Sorensen MJ, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 2013;309(16): 1696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen YH, Chiou HY, Lin HC, et al. Affect of seizures during gestation on pregnancy outcomes in women with epilepsy. Arch Neurol 2009;66(8):979–84. [DOI] [PubMed] [Google Scholar]
- 52.Pennell PB, Klein AM, Browning N, et al. Differential effects of antiepileptic drugs on neonatal outcomes. Epilepsy Behav 2012;24(4):449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hernandez-Diaz S, Mittendorf R, Smith CR, et al. Association between topiramate and zonisamide use during pregnancy and low birth weight. Obstet Gynecol 2014;123(1):21–8. [DOI] [PubMed] [Google Scholar]
- 54.Czeizel AE. Periconceptional folic acid and multivitamin supplementation for the prevention of neural tube defects and other congenital abnormalities. Birth Defects Res A Clin Mol Teratol 2009;85(4):260–8. [DOI] [PubMed] [Google Scholar]
- 55.Reisinger TL, Newman M, Loring DW, et al. Antiepileptic drug clearance and seizure frequency during pregnancy in women with epilepsy. Epilepsy Behav 2013;29(1):13–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polepally AR, Pennell PB, Brundage RC, et al. Model-based lamotrigine clearance changes during pregnancy: clinical implication. Ann Clin Transl Neurol 2014;1(2):99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pennell PB, Hovinga CA. Antiepileptic drug therapy in pregnancy I: gestation-induced effects on AED pharmacokinetics. Int Rev Neurobiol 2008;83:227–40. [DOI] [PubMed] [Google Scholar]
- 58.Brodtkorb E, Reimers A. Seizure control and pharmacokinetics of antiepileptic drugs in pregnant women with epilepsy. Seizure 2008;17(2):160–5. [DOI] [PubMed] [Google Scholar]
- 59.Reimers A, Brodtkorb E. Second-generation antiepileptic drugs and pregnancy: a guide for clinicians. Expert Rev Neurother 2012;12(6):707–17. [DOI] [PubMed] [Google Scholar]
- 60.Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet 2005;44(10):989–1008. [DOI] [PubMed] [Google Scholar]
- 61.de Haan GJ, Edelbroek P, Segers J, et al. Gestation-induced changes in lamotrigine pharmacokinetics: a monotherapy study. Neurology 2004;63(3):571–3. [DOI] [PubMed] [Google Scholar]
- 62.Ohman I, Vitols S, Tomson T. Lamotrigine in pregnancy: pharmacokinetics during delivery, in the neonate, and during lactation. Epilepsia 2000;41(6):709–13. [DOI] [PubMed] [Google Scholar]
- 63.Pennell PB, Newport DJ, Stowe ZN, et al. The impact of pregnancy and childbirth on the metabolism of lamotrigine. Neurology 2004;62(2):292–5. [DOI] [PubMed] [Google Scholar]
- 64.Petrenaite V, Sabers A, Hansen-Schwartz J. Individual changes in lamotrigine plasma concentrations during pregnancy. Epilepsy Res 2005;65(3):185–8. [DOI] [PubMed] [Google Scholar]
- 65.Tran TA, Leppik IE, Blesi K, et al. Lamotrigine clearance during pregnancy. Neurology 2002;59(2):251–5. [DOI] [PubMed] [Google Scholar]
- 66.Mazzucchelli I, Onat FY, Ozkara C, et al. Changes in the disposition of oxcarbazepine and its metabolites during pregnancy and the puerperium. Epilepsia 2006;47(3):504–9. [DOI] [PubMed] [Google Scholar]
- 67.Christensen J, Sabers A, Sidenius P. Oxcarbazepine concentrations during pregnancy: a retrospective study in patients with epilepsy. Neurology 2006;67(8):1497–9. [DOI] [PubMed] [Google Scholar]
- 68.Tomson T, Palm R, Kallen K, et al. Pharmacokinetics of levetiracetam during pregnancy, delivery, in the neonatal period, and lactation. Epilepsia 2007;48(6): 1111–6. [DOI] [PubMed] [Google Scholar]
- 69.Westin AA, Reimers A, Helde G, et al. Serum concentration/dose ratio of levetiracetam before, during and after pregnancy. Seizure 2008;17(2):192–8. [DOI] [PubMed] [Google Scholar]
- 70.Hovinga CA, Pennell PB. Antiepileptic drug therapy in pregnancy II: fetal and neonatal exposure. Int Rev Neurobiol 2008;83:241–58. [DOI] [PubMed] [Google Scholar]
- 71.Newport DJ, Pennell PB, Calamaras MR, et al. Lamotrigine in breast milk and nursing infants: determination of exposure. Pediatrics 2008;122(1):e223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meador KJ, Baker GA, Browning N, et al. Breastfeeding in children of women taking antiepileptic drugs: cognitive outcomes at age 6 years. JAMA Pediatr 2014; 168(8):729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herzog AG, Seibel MM, Schomer DL, et al. Reproductive endocrine disorders in women with partial seizures of temporal lobe origin. Arch Neurol 1986;43(4): 341–6. [DOI] [PubMed] [Google Scholar]
- 74.Harden CL, Koppel BS, Herzog AG, et al. Seizure frequency is associated with age at menopause in women with epilepsy. Neurology 2003;61(4):451–5. [DOI] [PubMed] [Google Scholar]
- 75.Harden CL, Herzog AG, Nikolov BG, et al. Hormone replacement therapy in women with epilepsy: a randomized, double-blind, placebo-controlled study. Epilepsia 2006;47(9):1447–51. [DOI] [PubMed] [Google Scholar]