Abstract
Transcutaneous cardiac pacing is a temporary method of pacing that may be indicated in patients with severe symptomatic or hemodynamically unstable bradyarrhythmias. It is particularly helpful in patients with reversible or transient conditions, such as digoxin toxicity and atrioventricular block in the setting of inferior wall myocardial infarction, or when transvenous pacing is not immediately available or carries a high risk of complications. Most patients with minimal hemodynamic compromise require a current of 40 to 80 mA; pacing thresholds tend to be higher in patients who have emphysema or pericardial effusion and in those who receive positive pressure ventilation. On electrocardiography, successful capture usually is characterized by a widened QRS complex, followed by a distinct ST segment and broad T wave. The hemodynamic response to pacing also must be confirmed by assessing the patient’s arterial pulse. Proper skin preparation and electrode positioning ensure successful capture in most situations. Adequate sedation and analgesia are essential in ensuring patient comfort.
Transcutaneous pacing was first introduced by Zoll and associates1 in 1956 as a novel method of treating asystole and significant bradyarrhythmias. However, the high current densities that the skin electrodes required to pace the cardiac tissue in those early devices caused painful stimulation of the cutaneous nerves and underlying skeletal muscles.
With the development of transvenous pacing leads later in the 1950s, interest in external pacing waned. Transvenous pacing has served as the mainstay of urgent temporary pacing since then, although the significant time and operator skill needed to implement the technique are less than ideal. As a result, noninvasive pacing reemerged, in the 1980s, as a therapy for bradycardia and asystole.2,3 Technical advances, including large adhesive electrodes and ECG filtering, have largely overcome the early problems of extreme discomfort and interpretation of capture on the ECG.
External noninvasive pacing offers several advantages over invasive pacing. It is widely available on most crash carts, along with defibrillator units. It is easy to perform and requires minimal training and, therefore, may be instituted by physicians, nurses, and paramedics. Because it can be performed quickly, noninvasive pacing can be initiated almost immediately, eliminating the setup and the insertion time of invasive techniques (transvenous and epicardial pacing). Noninvasive pacing carries a much smaller risk of serious complications compared with invasive techniques, and it is more cost-effective.
In this article, we will review the indications and techniques for transcutaneous pacing and how to avoid complications.
INDICATIONS
Transcutaneous cardiac pacing is a temporary method of pacing indicated in various clinical settings, including some cardiac emergencies. In general, external pacing is indicated as a temporary method of pacing in patients with severe symptomatic or hemodynamically unstable bradyarrhythmias, particularly in those who do not respond to pharmacologic therapy (such as atropine).
It is particularly helpful in reversible or transient conditions (such as digoxin toxicity and atrioventricular [AV] block in the setting of inferior wall myocardial infarction [MI]) or when transvenous pacing is not immediately available or carries a high risk of complications.4-8 It is often used as a bridge to temporary transvenous pacing or to a permanent pacemaker, and it should not be relied on if temporary pacing is required for a prolonged period.
The hemodynamically significant bradyarrhythmias that often require temporary pacing include various types of acquired AV block, sinus node dysfunction, and symptomatic bifascicular block (Table 1). In addition, transcutaneous pacing has been used in patients with asystolic cardiac arrest (asystole).9-12
Table 1.
Common indications for transcutaneous pacing
| Severe symptomatic or hemodynamically unstable bradyarrhythmias Acquired AV blocks Complete or third-degree AV block with symptoms (syncope, pre-syncope, dizziness) Complete AV block with asystolic pauses exceeding 3 seconds Complete AV block with escape pacemaker rate < 40 beats/min Symptomatic second-degree AV block (type I or II) Sinus node dysfunction Sinus pause with symptoms of cerebral hypoperfusion (syncope, pre-syncope, dizziness) Chronic sinus node dysfunction with or without symptoms but with escape rates < 40 beats/min Symptomatic sinus bradycardia Symptomatic bifascicular block Bradysystolic cardiac arrest (not routinely recommended) Bradyarrhythmias (as above) when the condition is expected to be transient (digoxin toxicity or AV block in the setting of inferior wall MI) As a standby pacing method when bradyarrhythmias are expected to occur Acute anterior or inferior wall MI Digoxin, ß-blocker, or calcium channel blocker overdose Before surgery in patients with bifascicular block Before cardiac diagnostic studies (electrophysiologic studies or tilt-table testing) Insertion of Swan-Ganz catheter in patients with left bundle branch block Antitachycardia pacing, to suppress ventricular and supraventricular tachycardia |
AV, atrioventricular; MI, myocardial infarction.
However, in randomized clinical trials, patients with out-of-hospital asystolic cardiac arrest in whom early external pacing was established by paramedics had no significant improvement in survival, compared with controls, despite modest initial success in achieving pacing.13-17
Similar results were found using transcutaneous pacing in patients with in-hospital asystolic cardiac arrest, 18 as well as in patients arriving at the emergency department with asystolic arrest.19 Pacing is not routinely recommended in such patients, in view of poor overall outcome.
Transcutaneous pacing has been widely used as a standby pacing method9 when bradyarrhythmias are expected to occur, as in acute MI,20 in digoxin overdose, before surgery in patients with bifascicular block,21-23 and before cardiac diagnostic studies that carry a high risk of hemodynamically significant bradyarrhythmias.
The American College of Cardiology and the American Heart Association have issued guidelines regarding the use of external pacing in the setting of acute MI (Table 2).20 The widespread use of early reperfusion strategies with thrombolytics and primary angioplasty has reduced the need for temporary or permanent pacing in this setting. In patients with acute MI who receive thrombolysis, anticoagulants, and/or antiplatelet agents, transcutaneous pacing is an invaluable method of pacing, considering the risk of bleeding complications associated with intravenous pacemaker insertion.
Table 2.
ACC/AHA recommendations for placement of transcutaneous patches and active (demand) transcutaneous pacing in patients with acute MI20
| Class I |
| Sinus bradycardia (rate < 50 beats/min) with signs of hypotension (systolic blood pressure < 80 mm Hg) that is unresponsive to drug therapy Mobitz type II second-degree AV block Third-degree heart block Bilateral BBB (alternating BBB, or RBBB and alternating LAFB or LPFB) irrespective of time of onset Newly acquired or age-indeterminate LBBB, RBBB with LAFB, or RBBB with LPFB RBBB or LBBB with first-degree AV block |
| Class IIa |
| Stable bradycardia (systolic blood pressure > 90 mm Fig, no hemodynamic compromise or compromise responsive to initial drug therapy) Newly acquired or age-indeterminate RBBB |
| Class IIb |
| Newly acquired or age-indeterminate first-degree AV block |
| Class III |
| Uncomplicated acute MI without evidence of conduction system disease |
ACC, American College of Cardiology; AHA, American Heart Association; MI, myocardial infarction; AV atrioventricular; BBB, bundle branch block; RBBB, right bundle branch block; LAFB, left anterior fascicular block; LPFB, left posterior fascicular block; LBBB, left bundle branch block.
Finally, external cardiac pacing methods have been used to suppress ventricular and supraventricular tachyarrhythmias refractory to medical treatment by means of over-drive pacing.24-27 Antitachycardia pacing is complicated and carries the risk of transforming the tachycardia into more malignant arrhythmia.
CONTRAINDICATIONS
As noted above, transcutaneous pacing should not be relied on if temporary pacing is required for a prolonged period. In patients with severe hypothermia, cardiac pacing is relatively contraindicated. In these patients, bradycardia may be a physiologic phenomenon resulting from decreased metabolic rate.27 Also, as body temperature drops, the ventricles become more prone to fibrillation and more resistant to defibrillation.28
Pacing is relatively contraindicated in patients with asystolic cardiac arrest, especially if the resuscitation efforts were delayed for more than 20 minutes, because of the poor resuscitation outcome in these patients.12-19
TECHNIQUE
Explaining to the patient—if the clinical situation permits—the purpose of the procedure and the equipment used is crucial, because the procedure may cause significant discomfort and pectoral muscle twitching from the high voltage required for external pacing.
Attachment
Before applying the patches, the skin site should be cleansed with soap and water; alcohol and other flammable liquids should be avoided. In patients with excessive body hair, trimming rather than shaving is preferred to avoid tiny skin nicks that can increase pain and skin irritation.
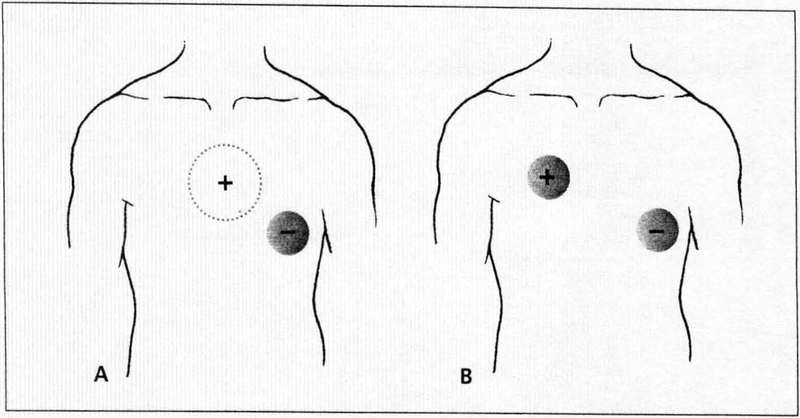
Most defibrillator units are equipped with transcutaneous pacing capability. Two pacing electrodes are applied to the patient’s thorax (Figure 1). The negative electrode is placed on the anterior chest wall, centered on the apex, or at the lead V3 position along the left sternal border. The positive electrode is placed on the patient’s back behind the negative electrode, to the left of the thoracic spine between the spine and the left scapular bone.4,5 In an extremely stiff unconscious patient, it is sometimes difficult to properly place the positive electrode on the patient’s back; in such cases, the positive electrode may be placed on the right side of the chest at the lead V1 position.
Figure 1.
Electrode positions in transcutaneous pacing are shown here. Most commonly, the negative electrode is placed on the anterior chest wall, centered on the apex, or at the lead V3 position along the left sternal border. The positive electrode is placed on the patient’s back between the spine and left scapula, directly posterior to the anterior electrode (A). In some patients, an alternative is to place the positive electrode on the right side of the chest at the lead V1 position and the negative electrode on the cardiac apex (B).
Settings
The electrodes are then connected to the pacing unit. Most manufacturers use a color-coded system to label the connecting cables. Red is usually used for the positive (posterior) electrode and black for the negative (anterior) electrode. Some manufacturers allow for adjustable pacing stimuli pulse width in their units. Increasing the pulse width can reduce the pacing threshold, which allows for a higher rate of success in achieving reliable and tolerable pacing.
All transcutaneous pacing units allow for an adjustable pacing rate (beats per minute) and ventricular output (mA), which is the electrical stimulus produced by the pulse generator and is intended to trigger a ventricular depolarization. Unlike the case with transvenous and permanent pacing devices, which allow for an adjustable sensitivity threshold to intrinsic ventricular and/or ventricular activities, in transcutaneous pacing units, the sensing threshold of the intrinsic ventricular electrical activity is not adjustable.
Transcutaneous pacing units allow for pacing in demand mode, in which the pacemaker generates electrical stimuli to pace the ventricles when the heart rate falls below the pacing rate. The device can also be used for overdrive pacing by selecting a pacing rate higher than the intrinsic heart rate and delivering it as a “burst” of pacing impulses at varying intervals. Up to 15 pacing impulses per burst can be delivered each time. The overdrive pacing technique is used in an attempt to terminate the reentry circuit of supraventricular and ventricular tachyarrhythmias.
After the pacing unit is turned on, the pacing parameters (pacing rate and ventricular output) are determined. The pacing rate is usually set between 70 and 80 beats per minute to simulate the normal beating heart rate. Depending on the clinical setting and the hemodynamic status of the patient, the ventricular output is set by 2 different methods:
In conscious bradycardic patients, or when used prophylactically, pacing is begun in the demand mode at a rate slightly faster than the native rhythm and at minimal output (5 to 10 mA) and then increased gradually. Small increments of 5 to 10 mA at a time are usually made to the current output until cardiac capture is demonstrated, or until intolerable discomfort develops. The lowest output at which capture is achieved is termed “the pacing threshold.” The final output setting should be 5 to 10 mA above the pacing threshold to maintain reliable pacing.
In patients with bradycardic arrest or in unconscious patients, it is recommended to turn the stimulating current to maximal output (200 mA) to ensure prompt ventricular capture. Once capture is achieved, the current output may be decreased gradually until loss of capture occurs. The lowest output at which capture is maintained determines the pacing threshold. Once capture is lost, the current output is increased promptly to a level 5 to 10 mA above the pacing threshold to maintain adequate pacing.
Transcutaneous pacing can be used as a standby measure when hemodynamically significant bradyarrhythmias are anticipated. In this scenario, the operator should first document that capture is possible by initiating a brief period of pacing at a rate slightly faster than the patient’s intrinsic rate. The device may then be operated on standby mode, which keeps the pacing parameters’ (pacing rate and ventricular output) setting readily available for future use. This allows the operator to resume pacing promptly if significant bradyarrhythmias occur.4
Most patients require a current in the range of 20 to 140 mA.9 Pacing threshold tends to be lower in relatively healthy persons or in patients with minimal hemodynamic compromise. In these settings, the threshold is usually in the range of 40 to 80 mA.9,28-30 No clear correlation has been established between pacing threshold and patient age, weight, body size, chest diameter, or cause of heart disease.29 However, thresholds are usually elevated following thoracic surgery. Pacing thresholds also tend to be higher in patients with emphysema or pericardial effusion and in those who receive positive pressure ventilation because of increased chest cavity impedance from air or fluids.
In some new devices, the pulse width (pulse duration) is adjustable. Using a wider pulse duration can reduce the output (mA) needed to achieve proper capture, which can potentially minimize the pain and discomfort of skeletal muscle stimulation. The pulse width in transcutaneous devices is approximately 20 to 30 milliseconds long. In comparison, the pulse width of permanent pacemaker devices is about 0.5 millisecond.
ASSESSMENT OF SUCCESSFUL PACING
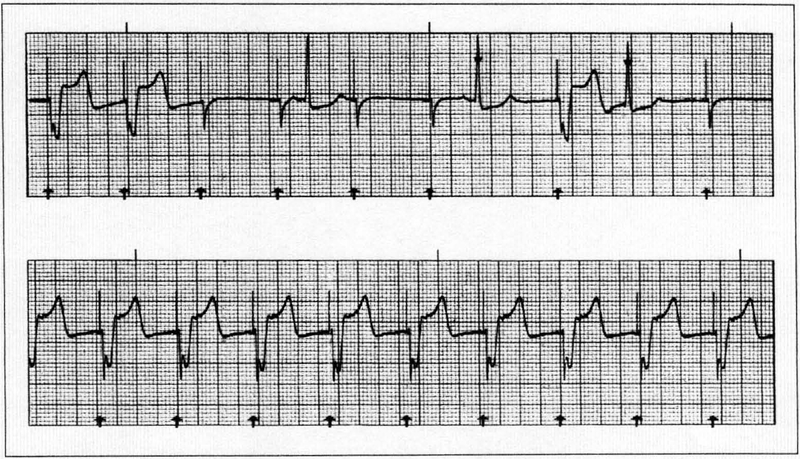
Electrical capture is assessed by inspection of the ECG tracing on the pacing unit monitor. Successful capture is usually characterized by a widened QRS complex, followed by a distinct ST segment and broad T wave (Figure 2). It is easy to mistake the wide, slurred afterpotential following an external pacing spike for electrical capture. The only sure sign of electrical capture is the presence of a consistent ST segment and T wave after each pacer spike.
Figure 2.
Assessment of capture requires the use of electrocardiography. The top panel shows no consistent capture. The underlying rhythm is sinus bradycardia. Note the lack of appropriate capture following pacing stimuli (3, 4, 5, 6, and 8). These are followed by brief narrow spikes with no distinct ST segment or T wave, which should not be mistaken for ventricular capture. The bottom panel shows ventricular capture (pacing above threshold). Pacing spikes are followed by ventricular depolarization, which is characterized by a broad QRS complex followed by a distinct ST segment and/or T wave.
The hemodynamic response to pacing also must be confirmed by assessing the patient’s arterial pulse—manually or by using automated blood pressure cuff, pulse oximetry, arterial waveform, or arterial catheter. The pulse rate obtained should match the pacing rate indicated on the generator monitor. Manual count of the pulse rate should be assessed at the right carotid or right femoral artery to avoid confusion between the jerking muscle contractions caused by the pacer and arterial pulse wave. A significantly lower pulse rate than the pacing rate demonstrated on the pacing unit monitor may indicate failure to capture. It is recommended that capture be confirmed on a standard 12-lead ECG as well.
Success rates in achieving ventricular capture varies widely depending on the setting in which transcutaneous pacing is used. Success rates appear to be highest when transcutaneous pacing is used prophylactically or early (within 5 minutes of asystolic cardiac arrest). In these settings, success rates may exceed 90%.9,10 Zoll and associates10 reported 78% success rates in diverse clinical situations. Time to initiation of pacing largely determines the success rate.
COMPLICATIONS
Capture should be evaluated periodically, since pacing threshold may increase with prolonged pacing. Also, periodic skin evaluation with repositioning of the electrodes is suggested, especially in unconscious patients, to minimize the possibility of serious skin burns. In conscious patients, generous analgesia (morphine sulfate or meperidine) and sedation (midazolam) should be used, and the level of patient comfort should be assessed periodically.
Failure to capture
With prolonged pacing, changes in pacing thresholds can lead to capture failure. Failure to capture may have a variety of other causes as well, most of which can be avoided or corrected by careful attention to the patient and the device.
Suboptimal electrode placement is a common cause of failure to capture. Repositioning the electrodes, avoiding a bony structure (spine, scapula, or sternum) between the electrodes and the heart, usually solves this problem. Placing the negative electrode posteriorly also results in failure to capture. This can be simply corrected by placing the electrode anteriorly over the cardiac apex or at the lead V3 position.
Poor skin-electrode contact from sweat, hair, or skin debris can result in failure to capture or a high pacing threshold. Thorough cleaning and drying of the skin and hair trimming help avoid this problem. Also, special attention should be paid to faulty electrical connections or generator battery depletion.
Increased chest impedance from fluids (pericardial effusion) or intrathoracic air (pneumothorax, positive pressure ventilation, or recent thoracic operation) may make transcutaneous pacing very problematic. Pericardiocentesis, relieving the pneumothorax with chest tube, or deceasing positive pressure ventilation can help restore effective transcutaneous pacing. Myocardial ischemia or metabolic derangement (acidosis, hypoxia) raises the pacing threshold and may prevent capture. Correcting any such underlying condition is the mainstay of management.
Increasing the pulse width of the pacing stimuli, if the pacing unit allows for such adjustment, can reduce a high pacing threshold of any cause.
Painful transcutaneous pacing
Pain can result from a number of factors. A conductive foreign body, sweat, or salt deposits on the skin beneath the electrode can result in painful stimulation, which can be resolved simply by removing the foreign body and prevented by first cleaning the skin properly. Painful pacing also can result from placing the electrode over skin abrasions. Hair trimming, rather than shaving, is the best way to avoid inducing any skin abrasions.
Painful or intolerable pacing also may be the result of patient anxiety or apprehension. Proper sedation and analgesia using benzodiazepines and narcotics, respectively, are essential. Increasing the pulse width of the pacing stimuli can reduce the pacing threshold and subsequently the pain associated with pacing.
Despite all of the measures mentioned above, tolerable effective capture is difficult to achieve and sustain in approximately 10% of cases.9,10 Transvenous or epicardial (transthoracic) pacing may be the only option in such situations.
Patients who are conscious or who regain consciousness during transcutaneous pacing may experience discomfort because of muscle contractions. With higher current output levels, the patient may experience strong, painful knocks on the chest. Generous analgesia and sedation may be necessary to make this discomfort more tolerable until transvenous pacing is instituted. Significant discomfort is less common with newer pacemakers and electrode designs. Up to two thirds of patients describe their experience with transcutaneous pacing as tolerable.10,29
Other complications
Symptoms such as coughing and hiccups may occur as a result of diaphragm pacing. Repositioning the pacing electrodes away from the diaphragm may help resolve this.
Skin burns, including third-degree burns, have been reported in children in whom improper or prolonged pacing has been used.31,32 Frequent skin inspection, with electrode repositioning as necessary, can minimize this complication.
In patients with preserved atrial rhythm, transcutaneous pacing may lead to ventricular pacing that is dissociated from the atrial rhythm (AV dissociation). Also, retrograde atrial activation may follow ventricular depolarization. Less commonly, a simultaneous atrial and ventricular activation may occur.
In all of these scenarios, atrial contractions occur, intermittently or persistently, during ventricular systole when AV valves are closed—a condition similar to “pacemaker syndrome,” which is well described in patients with an implantable ventricular pacing device. This unfavorable complication of transcutaneous pacing diminishes the atrial contribution in ventricular filling and consequently causes a drop in cardiac output and may result in pulmonary congestion. On physical examination, jugular venous congestion with cannon waves may be seen.
CLINICAL CONCLUSIONS:
Transcutaneous pacing can be a lifesaving tool
1 Transcutaneous pacing is a temporary method of cardiac pacing in patients with severe symptomatic bradyarrhythmias caused by high-grade atrioventricular block, sinus node dysfunction, or bradycardic arrest. It has been widely used as a standby pacing method when bradyarrhythmias are expected to occur, as in acute myocardial infarction, in digoxin overdose, and before surgery in patients with bifascicular block.
2 Transcutaneous pacing should not be used if temporary pacing is required for a prolonged period, but it can serve as a bridge to transvenous pacing. It is relatively contraindicated in patients with hypothermia or asystolic cardiac arrest, especially if the resuscitation efforts were delayed for more than 20 minutes.
3 Successful capture must be confirmed by electrocardiography. The hemodynamic response to pacing also must be confirmed by assessing the patient’s arterial pulse, which should match the pacing rate indicated on the generator monitor. An arterial pulse that is significantly lower than that on the monitor may indicate failure to capture.
4 Suboptimal electrode placement is a common cause of failure to capture. The problem usually can be solved by repositioning the electrodes—avoiding a bony structure (spine, scapular, or sternum) between the electrodes and the heart.
5 Failure to capture also can be caused by posterior placement of the negative electrode. This can be corrected by placing the electrode anteriorly over the cardiac apex or at the lead V3 position.
6 Pain resulting from muscle contraction is the most common side effect of transcutaneous pacing. Technologic advances in the newer pacing devices, meticulous attention to electrode placement, and proper sedation and analgesia usually make patients more comfortable.
Contributor Information
Dr RAMI DOUKKY, Rush Medical College and the division of adult cardiology at Cook County Hospital in Chicago.
Dr RAED BARGOUT, Rush Medical College and the division of adult cardiology at Cook County Hospital in Chicago.
Dr RUSSELL F. KELLY, Rush Medical College and the division of adult cardiology at Cook County Hospital in Chicago.
Dr JAMES E. CALVIN, Rush-Presbyterian-St Luke’s Medical Center in Chicago.
REFERENCES
- 1.Zoll PM, Linethal AJ, Norman LR. Treatment of unexpected cardiac arrest by external electric stimulation of the heart. N Engl J Med. 1956;254:541–546. [DOI] [PubMed] [Google Scholar]
- 2.Syverud SA, Hedges JR, Dalsey WC, et al. Hemodynamics of transcutaneous cardiac pacing. Am J Emerg Med. 1986;4:17–20. [DOI] [PubMed] [Google Scholar]
- 3.Dalsey WC, Syverud SA, Trott A. Transcutaneous cardiac pacing. J Emerg Med. 1984;1:201–205. [DOI] [PubMed] [Google Scholar]
- 4.Ellenbogen KA. Cardiac Pacing. 2nd ed. Cambridge, Mass: Blackwell Science; 1996: 168–173. [Google Scholar]
- 5.Cummins RO. Textbook of Advanced Cardiac Life Support. Dallas: American Heart Association; 1997:chap 5, 1–8. [Google Scholar]
- 6.Gregoratos G, Cheitlin MD. Conill A, et al. ACC/AHA Guidelines tor Implantation of Cardiac Pacemakers and Antiarrhythmic Devices: Executive Summary—a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Pacemaker Implantation). Circulation. 1998;97:1325–1335. [DOI] [PubMed] [Google Scholar]
- 7.Hedges JR, Feero S, Shultz B, et al. Prehospital transcutaneous cardiac pacing for symptomatic bradycardia. Pacing Clin Electrophysiol: PACE. 1991; 14:1473–1478. [DOI] [PubMed] [Google Scholar]
- 8.Syverud SA. Cardiac pacing Emerg Med Clin North Am. 1988;6:197–215. [PubMed] [Google Scholar]
- 9.Falk RH, Zoll PM, Zoll RH, Safety and efficacy of noninvasive cardiac pacing: a preliminary report. N Engl J Med. 1983;309:1166–1168. [DOI] [PubMed] [Google Scholar]
- 10.Zoll PM, Zoll RH, Falk RH, et al. External noninvasive temporary cardiac pacing: clinical trials. Circulation. 1985;71:937–944. [DOI] [PubMed] [Google Scholar]
- 11.Altamura G, Toscano S, Lo Bianco F, et al. Emergency cardiac pacing for severe bradycardia. Pacing Clin Electrophysiol: PACE. 1990, 13:2038–2043, [DOI] [PubMed] [Google Scholar]
- 12.Syverud SA, Dalsey WC, Hedges JR. Transcutaneous cardiac pacing for early bradyasystolic cardiac arrest. Ann Emerg Med. 1986;15:121–124. [DOI] [PubMed] [Google Scholar]
- 13.Olson CM, Jastremski MS, Smith RW, et al. External cardiac pacing for out-of-hospital bradysystolic arrest. Am J Emerg Med. 1985;3:129–131. [DOI] [PubMed] [Google Scholar]
- 14.Falk RH, Jacobs L, Sinclair A, Madigan-McNeil C. External noninvasive cardiac pacing in out-of-hospital cardiac arrest. Crit Care Med 1983;11:779–782. [DOI] [PubMed] [Google Scholar]
- 15.Eitel DR, Guzzardi LJ, Stein SE, et al. Noninvasive transcutaneous cardiac pacing in prehospital cardiac arrest. Ann Emerg Med. 1987;16:531–534. [DOI] [PubMed] [Google Scholar]
- 16.Barthell E, Troiano P, Olson D, et al. Prehospital external cardiac pacing: a prospective, controlled clinical trial. Ann Emerg Med. 1988;17:1221–1226. [DOI] [PubMed] [Google Scholar]
- 17.Cummins RO, Graves JR, Larsen MP, et al. Out-of-hospital transcutaneous pacing by emergency medical technicians in patients with asystolic cardiac arrest. N Engl J Med. 1993;328:1377–1382. [DOI] [PubMed] [Google Scholar]
- 18.Knowlton AA, Falk RH. External cardiac pacing during in-hospital cardiac arrest. Am J Cardiol. 1986;57:1295–1298. [DOI] [PubMed] [Google Scholar]
- 19.Dasely WC, Syverud SA, Hedges JR. Emergency department use of transcutaneous pacing for cardiac arrests. Crit Care Med. 1985;13:399–401. [DOI] [PubMed] [Google Scholar]
- 20.Ryan TJ, Antman EM, Brooks NH, et al. 1999 update: ACC/AHA Guidelines for the Management of Patients With Acute Myocardial Infarction: Executive Summary and Recommendations: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction). Circulation 1999;100:1016–1030. [DOI] [PubMed] [Google Scholar]
- 21.Gauss A, Hubner C, Meierhenrich R, et al. Perioperative transcutaneous pacemaker in patients with chronic bifascicular block or left bundle branch block and additional first-degree atrioventricular block. Acta Anaesthesiol Scand. 1999;43:731–736. [DOI] [PubMed] [Google Scholar]
- 22.Gauss A, Hubner C, Radermacher P, et al. Perioperative risk of bradyarrhythmias in patients with asymptomatic chronic bifascicular block or left bundle branch block: does an additional first-degree atrioventricular block make any difference? Anesthesiology. 1998; 88:679–687. [DOI] [PubMed] [Google Scholar]
- 23.Gould L, Gopalaswamy C, Chandy F, et al. Prophylactic preoperative insertion of pacemakers in patients with bifascicular block. N Y State J Med. 1983;83:1041–1043. [PubMed] [Google Scholar]
- 24.Estes NA III, Deering TF, Manolis AS, et al. External cardiac programmed stimulation for noninvasive termination of sustained supraventricular and ventricular tachycardia. Am J Cardiol 1989;63:177–183. [DOI] [PubMed] [Google Scholar]
- 25.Rosenthal ME, Samato NJ, Marchlinski FE, Josephson ME. Noninvasive cardiac pacing for termination of sustained, uniform ventricular tachycardia. Am J Cardiol. 1986;58:561–562. [DOI] [PubMed] [Google Scholar]
- 26.Altamura G, Bianconi L, Boccadamo R, Pistolese M. Treatment of ventricular and supraventricular tachyarrhythmias by transcutaneous cardiac pacing. Pacing Clin Electrophysiol: PACE. 1989;12:331–338. [DOI] [PubMed] [Google Scholar]
- 27.Best R, Syverud S, Nowak RM. Trauma and hypothermia. Am J Emerg Med. 1985;3:48–55. [DOI] [PubMed] [Google Scholar]
- 28.Zoll PM, Zoll RH, Belgard AH. External noninvasive electric stimulation of the heart. Crit Care Med. 1981;9:393–394. [DOI] [PubMed] [Google Scholar]
- 29.Falk RH, Ngai ST, Kumanki DJ, Rubinstein JA. Cardiac activation during external cardiac pacing. Pacing Clin Electrophysiol: PACE. 1987;10:503–506. [DOI] [PubMed] [Google Scholar]
- 30.Klein LS, Miles WM, Heger JJ, Zipes DP. Transcutaneous pacing: patient tolerance, strength-interval relations and feasibility for programmed electrical stimulation. Am J Cardiol. 1988;62:1126–1129. [DOI] [PubMed] [Google Scholar]
- 31.Pride HB, McKinley DF. Third-degree burns from the use of an external cardiac pacing device. Crit Care Med. 1990;18:572–573. [DOI] [PubMed] [Google Scholar]
- 32.Walund DC, Lynn AM, Hall DG. A third-degree burn associated with external cardiac pacing in a five-year-old boy. J Thorac Cardiovasc Surg. 1992;104:1754–1755. [PubMed] [Google Scholar]