Abstract
Objectives:
A significant body of knowledge implicates menopausal estrogen levels in the pathogenesis of the ommon pelvic floor disorders (PFDs). These health conditions substantially decrease quality of life, increase depression, social isolation, caregiver burden, and economic costs to the individuals and society.
Methods:
This review summarizes the epidemiology of the individual PFDs with particular attention to the understanding of the relationship between each PFD and menopausal estrogen levels, and the gaps in science and clinical care that affect menopausal women. In addition, we review the epidemiology of recurrent urinary tract infection (rUTI)—a condition experienced frequently and disproportionately by menopausal women and hypothesized to be potentiated by menopausal estrogen levels.
Results:
The abundance of estrogen receptors in the urogenital tract explains why the natural reduction of endogenous estrogen, the hallmark of menopause, can cause or potentiate PFDs and rUTIs. A substantial body of epidemiological literature suggests an association between menopause, and PFDs and rUTIs; however, the ability to separate this association from age and other comorbid conditions makes it difficult to draw definitive conclusions on the role of menopause alone in the development and/or progression of PFDs. Similarly, the causative link between the decline in endogenous estrogen levels and the pathogenesis of PFDs and rUTIs has not been well-established.
Conclusions:
Innovative human studies, focused on the independent effects of menopausal estrogen levels, uncoupled from tissue and cellular senescence, are needed.
Keywords: Menopause, Pelvic floor disorders, Pelvic organ prolapse, Recurrent urinary tract infection, Urinary incontinence
Based on the National Institute of Aging statistics, the life expectancy for US women is 81 years and the average age of menopause is 51.1 Thus, one-third of the modern US woman’s life occurs after menopause. As life expectancy continues to increase, the number of women entering menopause—a marker of biological aging in females1—is higher than ever. While women have aspirations and expectations about healthy aging, menopause-related changes in the genitourinary system and pelvic floor often interfere with well-being. Common conditions include pelvic floor disorders (PFDs), which partially overlap with genitourinary syndrome of menopause (GSM), and recurrent urinary tract infections (rUTIs). These health conditions substantially decrease quality of life, increase depression, social isolation, caregiver burden, and economic costs to the individuals and society.
There is clear evidence that estrogen regulates functionally relevant pathways within and across organ systems of vertebrates. This occurs through the use of various receptors and signaling cascades to induce proteomic and genomic responses of the target cells and tissues. Estrogen binds to one of two major classes of estrogen receptors (ERs) present in various tissues. The first class includes nuclear transcription factors— ERα and ERβ—encoded by ESR1 and ESR2 genes in humans.2,3 Estrogen regulates the transcriptional signature of its target tissues via the nuclear pathway activated by binding to ERα and ERβ. The second class of ERs is comprised of G- protein-coupled membrane receptors (GPERs), including GPR30, ER-X, and Gq-mER. The induction of non-nuclear cytoplasmic pathways linked to intracellular signal transduction proteins are mediated through these receptors, resulting in the immediate estrogen effect on the tissues.4
Human studies with direct tissue sampling demonstrate presence of both isoforms of nuclear ERs in the portions of the genitourinary tract that contain squamous epithelium, including vagina, and bladder trigone and proximal urethra exhibiting squamous metaplasia.5 On the contrary, the non-squamous transitional epithelium of the lower urinary tract predominantly expresses ERβ, with ERα expressed to a much lesser degree.5–7 At the time of this writing, there are no published studies that examine the distribution of GPERs in female genitourinary tract. Figure 1 summarized the distribution of estrogen receptions in the pelvic organs and soft tissues of women.
FIG. 1.
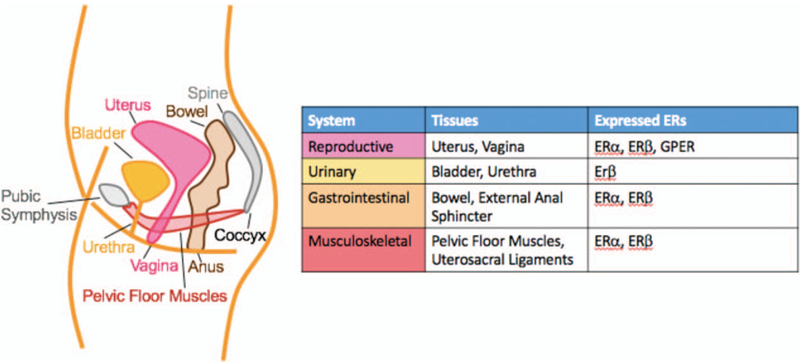
Location of estrogen receptors in the female pelvic organs and soft tissues.
The abundance of ERs in the urogenital tract explains why the natural reduction of endogenous estrogen, the hallmark of menopause, can cause or potentiate PFDs and rUTIs. This review summarizes the epidemiology of the individual PFDs with particular attention to the understanding of the relationship between each PFD and menopausal estrogen levels, and the gaps in science and clinical care that affect menopausal women. In addition, we review the epidemiology of rUTI—a condition experienced frequently and disproportionately by menopausal women and hypothesized to be potentiated by menopausal estrogen levels.
The epidemiology of common pelvic floor disorders
Pelvic floor disorders (PFDs) traditionally refer to irritative lower urinary tract symptoms (LUTS) and/or overactive bladder dry (OAB), urinary (mainly stress, urgency, and mixed) incontinence (UI), pelvic organ prolapse (POP), and anal/fecal incontinence (AI/FI). Epidemiologic studies indicate that approximately one in three to four (25%−37%) community-dwelling women are affected by PFDs, with the highest rates in menopausal women.8,9 The overall prevalence increases with age from 6% in young women between 20 and 29 years, 32% between 50 and 59 years, to 53% in older women aged 80+ years.8 Key risk factors are well-recognized and include increasing parity, vaginal delivery, increasing age, obesity, and hysterectomy.10–12 To date, a significant body of knowledge has accumulated, consistently demonstrating the detrimental quality of life effect of these common conditions. Astoundingly, 69% of hospitalized patients with serious illnesses who were asked to evaluate different health states as compared with death, considered urinary and fecal incontinence “same or worse than death”, which was higher than the number of people who rated “relying on a ventilator to survive.”13
In addition to the negative impact of these conditions on the individuals, PFDs impart a substantial economic and societal burden with over 1.2 million estimated new patient visits in the United States in 2010.14 Recent studies predict that the demand for PFDs care will further increase by 35%, with over 1.6 million visits expected in 2030.15
Despite the high prevalence of PFDs in older women, the independent contribution of menopausal hormonal levels to these conditions remains unclear. The findings of epidemiologic studies of menopausal women are challenged by the common events (exposures) such as childbirth, tissue senescence, and the comorbidities inherent in aging. The few studies specifically addressing the association between changes in hormonal levels and PFDs have not identified menopause as a significant risk factor.12 However, there are several epidemiologic relationships of interest, the biological plausibility of which is strongly supported by the following: female lower genital and urinary tracts arise from the primitive urogenital sinus, which expresses ERs during development16; ER expression is maintained in the adult vagina, urethra, bladder, and surrounding pelvic connective tissues and musculature5,17; and alterations in response to low estrogen levels uncoupled from aging in experimental animal models, mimic changes observed in the genitourinary tract of menopausal women.18
Decline in estrogen and genitourinary syndrome of menopause
A significant body of knowledge implicates menopausal estrogen levels in the pathogenesis of the common symptoms of GSM, which include vaginal dryness and burning; pelvic pain and dyspareunia; and such PFDs as irritative LUTS (urinary urgency, frequency, and dysuria). These symptoms are highly prevalent, affecting up to 80% of menopausal women.19 Beyond several published investigations that utilize direct tissue sampling, the concept that estrogen exerts its action on the human female lower genitourinary tract is mainly extrapolated from the observational clinical studies. These studies have demonstrated that low systemic estrogen levels in menopausal women are associated with gross anatomic changes in the external genitalia and vagina, and increased incidence of irritative genital symptoms and LUTS.
The primary role of estrogen in vulvovaginal tissues is to maintain the integrity of the external genitalia; vascularization with the associated vaginal lubrication; and vaginal epithelium and stroma, with the latter comprised of collagen (both fibrillar and nonfibrillar), elastin, and smooth muscle. However, the mechanistic links between menopausal genitourinary symptoms and low estrogen levels are poorly understood. Several published studies demonstrate marked decline or even absence of ESR2 gene expression, which encodes ERβ. However, the expression of ESR1, which encodes ERα, remains unchanged in the full-thickness vaginal tissue samples of menopausal compared with premenopausal women.20,21 Surprisingly, decreased expression of vaginal ERβ in menopausal women does not appear to be affected by exogenous estrogen supplementation.21 This is likely due to the following: ERα is the dominant subtype regulating estrogen-mediated tissue homeostasis in the female reproductive tract; and studies using ERα and ERβ-selective agonists indicate that vaginal estrogen acts predominantly through vaginal ERα.22
The use of both systemic and local vaginal estrogen therapies for the treatment of GSM is widespread, with several studies indicating that topical estrogen application is more efficacious than systemic administration.23 The difference in efficacy by route of administration may be due to the fact that topical estrogens lead to a greater ER increase in vaginal epithelium, compared with systemic preparations.24 However, there is only low-quality evidence supporting the use of vaginal estrogen over placebo for GSM symptoms, as concluded in the most recent Cochrane review of 30 RCTs with over 6,000 women treated for atrophic vaginitis, a variably defined term.25 This finding is consistent with the possibility that the effects of topical estrogens are limited to vaginal epithelium, causing maturation of the basilar cells and epithelial layer thickening, without an impact on vaginal stroma or vascularization.26 Therefore, the therapeutic effect of local vaginal estrogen supplementation subsides once treatment is discontinued. The above notion is supported by comparative studies of topical estrogen versus laser therapy for the treatment of vaginal atrophy. Light Amplification by Stimulated Emission of Radiation, or laser, therapy uses spatially and temporally coherent light to generate a thermal effect in the target tissues, in turn, stimulating resident fibroblasts to synthetize collagen. Collagen remodeling in vaginal stroma induced by Erbium:YAG nonablative laser has been shown to produce a more sustainable relief from such GSM symptoms as dyspareunia, vaginal dryness, and irritation, compared with vaginal estrogen therapy.26 Similarly, comparison of fractional CO2 laser to topical estrogen in a randomized trial of 45 menopausal women demonstrated that sustainable improvement in the vaginal health index (elasticity, fluid volume, pH, epithelial integrity, and moisture) was only present in women treated with CO2 laser.27 Collectively, these findings support a rationale for well-designed experimental studies exploring the effects of concomitant laser and topical estrogen therapies on full-thickness vaginal tissue. Importantly, more data to assess true safety and effectiveness of laser technology, particularly over the long term, are urgently needed.
Critical to such investigations would be selection of the appropriate clinical dose of vaginal estrogen. An elegant study conducted in an ovariectomized rat model, demonstrated that the beneficial changes in vaginal stromal proteins, such as collagens I and III, and also up-regulation of genes important for collagen assembly, observed in response to low-dose local estrogen therapy, were lost with the use of high-dose vaginal estrogen administration.28 In addition, low-dose topical therapy was also superior to the systemic estrogen replacement. However, there is a seemingly counter-intuitive lack of efficacy of vaginal estrogen supplementation, demonstrated by the existing clinical studies. In addition to the ineffectiveness of higher estrogen doses in the rat model of menopause, another possible explanation may be found in the mouse experiments that indicate estrogen is helpful, but not sufficient. These studies suggest that secreted growth factors derived from vaginal epithelium downstream of ERα, such as amphiregulin, are necessary for the full activation of estrogen effects in the vaginal stroma.18 Moreover, topical application of keratinocyte growth factor, a member of the fibroblasts growth factors that maintain the integrity of epithelial tissues, effectively treated vaginal atrophy in the murine model of menopause.29 Experimental studies designed to identify additional growth factors and their role in human vaginal tissue could provide mechanistic data behind a wide range of effects observed in menopausal women in response to topical estrogen therapy and offer a potential additive or alternative strategy for GSM treatment.
Finally, autonomic and sensory neurons that express ERs are another important estrogen target in the female genitourinary tract. Neuronal plasticity of the uterus in response to hormonal alterations has been well established, with the known inverse relationship between estrogen levels and myometrial nerve density. Published studies in experimental models and humans demonstrate that vaginal innervation is also strongly influenced by the hormonal milieu, with altered estrogen levels impacting sympathetic, parasympathetic, and sensory nerves.30 It is therefore plausible that such GSM symptoms as hyperalgesia, dyspareunia, and vaginal dryness, are at least partially due to a higher density of sensory and sympathetic afferents, caused by the decline in estrogen levels. Notably, immunostaining of the biopsied vaginal tissue with the pan-neuronal marker PGP 9.5, demonstrates that the majority of vaginal innervation is present in the stroma, as opposed to epithelium,31 further highlighting the vaginal fibromuscular layer as an important therapeutic target. While the vaginal innervation density decreases with both topical and systemic estrogen therapy, the magnitude of this effect is substantially greater in response to local estrogen supplementation.31 These mechanistic investigations underscore that estrogen directly impacts the target tissues and provide a scientific rationale for the superiority of topical estrogen therapy, as compared with systemic hormonal administration, in alleviating menopausal genitourinary symptoms.
Decline in estrogen and lower urinary tract symptoms
Lower urinary tract symptoms (LUTS) is a broad umbrella term that includes GSM symptoms such as urinary urgency, frequency, and lower urinary tract pain, as well as urgency urinary incontinence (UUI) and voiding dysfunction. As a group, LUTS are highly prevalent, affecting nearly one in three women.32 LUTS are associated with substantial negative impact on quality of life, lower work productivity, poor sleep quality, decreased sexual satisfaction, and depression.33–35 Despite high prevalence and negative impact, few (<25%) women seek care.36 Low treatment rates are related to multiple barriers to care, including persistent social stigma, perception that symptoms are “normal” part of aging, lack of access to trained providers, or knowledge of available treatment options. Notwithstanding low levels of care seeking, the economic burden of LUTS is high, with estimated total national costs of UUI of $76.2 billion in 2015 and expected costs of $82.6 billion in 2020.37 A recent systematic review of OAB-specific costs estimated a range of 656 to 860 USD per-patient annually, 47% to 117% higher than per-patient without OAB.38
As described earlier, the presence of ERs throughout the lower urinary tract is consistent with the likelihood that estrogen modulates urinary tract functions. Characteristic histologic and biomechanical changes in the bladder and urethra are known to occur in the setting of menopausal estrogen levels. These changes include: urethral shortening, thinning of urethral mucosa, decreased urinary sphincter contractility, and reduced bladder compliance. Furthermore, a 2014 systematic review of 44 individual clinical studies concluded that all available vaginal estrogen preparations reduce LUTS.
Mechanistic studies also provide compelling evidence that low estrogen levels in animal models of menopause cause amplification of basal and stretch-induced acetylcholine release in the bladder, accompanied by increased urinary frequency and decreased voided volume. Importantly, the exaggerated acetylcholine release was reversed by exogenous estrogen replacement, supporting the role of estrogen in cholinergic signaling modulation.39 This interesting finding likely explains the results of a several small randomized trials, suggesting the clinical efficacy of topical estrogen may be comparable with OAB medications in the anticholinergic class. A small trial compared vaginal estrogen versus anticholinergic medication (oxybutynin) in 59 menopausal women with OAB- dry (no UI); the efficacy of topical estrogen in reducing the number of daily voids and improving quality of life did not differ from that of oxybutynin.40 In another small randomized trial, vaginal estrogen was compared with tolterodine. Consistently, vaginal estrogen proved to be similarly effective in improving OAB symptoms.41 However, the combination of tolterodine with topical estrogen has not shown any additive effect in reducing irritative LUTS or UUI, beyond the effect seen with tolterodine alone.42 Taken altogether, the existing data suggest that estrogen regulates cholinergic signaling, but that its modulation is parallel rather than synergistic with that of the drugs that block muscarinic receptors in the bladder, such as oxybutynin and tolterodine.
Urinary incontinence (UI), a component of LUTS, is the most common PFD affecting women across the lifespan, with increased prevalence with aging. UI usually manifests as urinary leakage with physical exertion (stress urinary incontinence [SUI]), leakage associated with a strong desire or uncontrolled bladder contraction (UUI), or a combination of the two (mixed urinary incontinence [MUI]). Less common causes of UI include overflow or functional incontinence, fistulae, or congenital urinary tract anomalies. Thus, UI is a symptom resulting from a variety of anatomic and neurologic conditions often with no single predisposing factor.
The urinary continence mechanism depends on normal bladder capacity and compliance, urethral support by the vagina and pelvic floor muscles, and the intact function of the urethral sphincter complex. The sphincteric complex includes striated and smooth muscles; a vascular plexus; submucosa; and urothelium.43 It is generally accepted that SUI stems from a loss of urethral support, weakness of the striated urethral sphincter, and/or inadequate urethral coaptation during abdominal pressure increases.44–46 SUI predominates in younger and middle-age women, while UUI and MUI are more common in older women with an increasing prevalence with each decade.32 UUI is attributed to disruption in the normal neuromuscular regulation. Disruptions can involve inappropriate sensory and/or motor signaling at any point from the lower urinary tract and the nervous system, including the spinal cord and pontine micturition center. Abnormal signals during bladder filling can be associated with inappropriate bladder emptying.
Nearly a third of women report significant UI and up to 60% report at least some (any) UI. The prevalence and degree of UI vary widely depending on the definition used and the population studied.47 However, regardless of definition, UI prevalence increases with age, affecting approximately 10% to 20% of community dwelling women and up to 77% of women residing in nursing homes.9,47,48 Epidemiologic cross-sectional and longitudinal studies suggest a spike in prevalence around the time of menopause, with 70% of women connecting the onset of UI to their final menstrual period.49
Estrogen receptor expression in the bladder and urethra is similar in women in different estrogenic states,5 suggesting that the female lower urinary tract is receptive to the actions of endogenous, and also exogenous estrogens. Support for the epidemiologic association between menopause and UI, and the use of estrogen supplementation comes from the mechanistic investigations that perturb the independent effects of altered estrogen levels on urinary continence in experimental animal models and in vitro studies of human tissue. In the rat model of estrogen deprivation, achieved via ovariectomy, decreased urethral closure pressure and the accompanying sneeze induced SUI was observed in 60% of animals 6 weeks after ovariectomy.50 In the same study, exogenous estrogen administration decreased the incidence of SUI by 35%, highlighting the important role of estrogen in urinary continence mechanism. Urodynamic evaluation of menopausal women with UI demonstrate that local estrogen therapy increases urethral closure pressure (a measure associated with urethral health), possibly via its effects on the urethral vascular network,23 and inhibits filling phase bladder contractions.51 However, the molecular mechanisms by which estrogen controls urethral sphincter function and detrusor excitability are not well understood. Critical targets of nongenomic estrogen-mediated effects on smooth muscle in various organ systems are ion channels, including voltage-gated and Ca2+-activated (BK) potassium channels.52,53 BK channels have been identified as the most prominent potassium channels in the bladder. The fundamental role of estrogen control of the BK channels in regulating detrusor contractility was demonstrated in a recent study using human detrusor samples, isolated from full-thickness bladder biopsies of 27 male and female patient-participants without UI. The investigators explicitly demonstrated that 17β-estradiol reduced spontaneous phasic detrusor contractions through direct nongenomic activation of BK channels by increasing the BK channel open probability.54 Overall, an accumulating body of knowledge indicates that the role estrogen plays in ion channel regulation in the urinary tract could serve as a fruitful target for novel UUI treatments.
There are major discrepancies regarding the role of estrogen administration for UI treatment in the existing literature. Despite the epidemiological and experimental findings described above, there are conflicting results in published clinical studies that assess the role of exogenous estrogen supplementation in treating UI. While subjective improvement in UI is demonstrated in some studies,55 other clinical investigations demonstrate lack of efficacy of estrogen therapy for the treatment of UI.56,57 Moreover, a recent Cochrane review concluded that while local estrogen supplementation is not beneficial long term, systemic estrogen therapy is, in fact, detrimental, resulting in worsening UI rates, compared with placebo.58 Mechanisms that account for this seeming incongruity are not understood. Interestingly, in early 2018, a team of neuroscientists identified a novel population of estrogen- sensitive neurons in the Barrington’s nucleus, or pontine micturition center, that express ERα, using neurotransmitter identity and anatomical connections to the lower urinary tract in the murine model.59 Targeted manipulation of these ERα+ neurons via optogenetics revealed that their main function is to relax the urethral striated sphincter and facilitate micturition. These intriguing results may help explain why systemic estrogen, which potentiates supraspinal neuronal control of urethral relaxation, actually causes clinical worsening of UI in menopausal women.
Decline in estrogen and pelvic organ prolapse
Pelvic organ prolapse occurs when the native support of the vagina and uterus weakens, resulting in the descent of the pelvic organs through the vaginal outlet.
Symptoms of POP typically include a sensation of vaginal bulge, vaginal pressure, and heaviness, and sometimes difficult urinary or bowel evacuation. Objective evidence of POP is found in up to 65% of older women; however, rates of symptomatic POP (generally defined as a bulge sensation) are far lower at 10% or less.9,60 As with UI, the prevalence increases with vaginal parity (the leading risk factor) in addition to aging, obesity, and prior hysterectomy.10,61 The majority of the associated economic burden is related to the surgical management of POP, which occurs in approximately 11% of women by 80 years of age, with costs reported between $ 1 and 1.5 billion in USD over 20 years ago.61,62
Estrogen receptors have been identified in all major structural components that provide support to the pelvic organs, including uterosacral ligaments, vagina and its supportive tissue complex, and pelvic floor muscles.63–65 The total collagen content and ratios of specific collagen isoforms predominantly account for the biomechanical properties of pelvic connective tissues. Studies examining the vaginal supportive tissues demonstrate a dramatic (75%) decrease in collagen I, the major determinant of tissue’s tensile strength, in menopausal compared with premenopausal women.66 Also, systemic estrogen therapy results in restoration of collagen I levels to premenopausal state.66 In the follow-up in vitro study, using primary cell culture derived from the paravaginal connective tissues, the same group of investigators determined that the amount of active matrix metallopeptidase 13 (MMP13)—a key collagenase responsible for the degradation of fibrillar collagens—was decreased in the presence of estradiol.67 These findings provide one of the mechanisms by which estrogen may maintain integrity of the pelvic connective tissues. Studies that compare the uterosacral ligaments of women with and without POP are limited. However, a study conducted in Cynomolgus monkey model of menopause, demonstrated an increase in the uterosacral ligament’s tensile strength in response to the systemic hormonal replacement (estrogen + progesterone).68
The results of clinical studies, focused on the effects of menopause on the pelvic floor muscles, are conflicting. While studies indicate that menopausal state, separate from chronological age, is not associated with pelvic floor muscle dysfunction,69 others suggest that pelvic floor muscles’ responsiveness to strength training is diminished in older menopausal, relative to younger premenopausal women.70 In summary, menopausal status has not been established as an independent predictor of POP, despite multiple clinical studies.71
Although estrogen supplementation has not been established as an effective preventative or therapeutic measurer for POP, vaginal estrogen is often used to reduce side effects associated with conservative treatments (pessaries) and surgically implanted materials. Pessaries support prolapsed vaginal tissue to reduce symptoms of bothersome bulge. This nonsurgical approach rarely has serious complications and is the first-line therapy for symptomatic POP. However, pessary use can cause vaginal discharge and friable vaginal ulcerations, leading to pessary discontinuation.72 A retrospective cohort study of 134 women treated with pessaries for at least 3 months demonstrated that women using vaginal estrogen were more likely to continue pessary use.73 While topical estrogen did not impact the rate of vaginal ulcerations and related vaginal bleeding, women who used vaginal estrogen were less likely to experience bothersome vaginal discharge compared with those who did not.73
Recent studies in animals and humans have examined the impact of pre and postoperative use of vaginal estrogen on vaginal tissue characteristics. A limited randomized trial of estrogen versus placebo cream demonstrated that preoperative vaginal estrogen application increased synthesis of mature collagen, decreased MMP-12 activity, and increased epithelial and muscularis thickness.74 These histologic changes are hypothesized to improve the tissue quality at the time of surgical repair and pelvic connective tissue integrity in general. Postoperative administration of topical estrogen has been shown to decrease granulation tissue and objective signs of vaginal atrophy.74 However, a recent mechanistic study conducted in the rat model indicates that while application of vaginal estrogen after a full-thickness vaginal incision promotes healing of the epithelium, regeneration of the stromal layer is impeded by topical estrogen.75 Stromal tissue is responsible for vaginal tensile strength; thus the above experimental data underscore the importance of investigating the impact of postoperative estrogen supplementation on prolapse recurrence. Scientific progress is needed in this important area of clinical care to establish a causative link between menopausal decline in estrogen levels and POP; and to test the hypothesis that the histomorphometric properties of vaginal epithelium are appropriate surrogate marker for the biomechanical integrity of vaginal stroma and supportive tissue complex.
Decline in estrogen and accidental bowel leakage (anal/fecal incontinence)
Accidental bowel leakage (ABL), also referred to as anal/ fecal incontinence (AI/FI), is the complaint of involuntary loss of gas and/or solid or liquid stool per rectum. Similar to other PFDs, the prevalence of AI/FI depends heavily on the definition used (only solid/liquid vs solid/liquid/gas) and the population studied. The prevalence of ABL has been reported in up to 24% of community-dwelling women and as high as 42% in nursing home settings.76–79 Up to 40% of women with ABL report severe negative impact on quality of life, and the presence of ABL in association with UI further worsens QOL over the impact of UI alone.80,81 ABL is an uncommonly discussed condition, with most women suffering in silence and fewer than 30% seeking care for their condition.80 There is a paucity of data on the economic impact of ABL, although this remains an important area of epidemiologic research.
The mechanism of fecal continence includes normal rectal capacity and compliance, intact rectal sensation, and functional internal and external anal sphincters. Risk factors of increasing age, presence of diarrhea, and comorbid conditions such as diabetes have been implicated, but little to no data regarding the role of menopause exist.9 Analogous to other pelvic tissues, ERs have been identified within the anal sphinchter complex.82 However, a small randomized trial comparing vaginal estrogen versus placebo in 36 menopausal women with FI, found no difference between the groups with respect to subjective improvement assessed by Wexner score—a validated condition-specific questionnaire.83 Furthermore, the fact that prevalence rates between men and women are quite similar suggests that hormones may not play a substantial role in this devastating PFD.
Menopause and urinary tract infections
Urinary tract infection represents a leading cause of bacterial infections in women, across the lifespan.84 In women, UTI is nearly always treated with antibiotics. These infections have a significant negative impact on health-related QOL and high impact on health care, with an estimated annual social cost of 1.6 billion dollars and 11.3 million prescriptions.85–87 The growing concerns about collateral effects and antibiotics resistance are propelling research into new therapies and strategies for prevention.
Recurrent UTIs (rUTI), defined as three or more UTIs in 12 months or two UTIs in 6 months, occurs commonly after menopause. While up to 15% of women over 60 will develop frequent UTIs,85 this percentage increases to 20% for women aged >65 years. Approximately 25% to 50% of women aged >80 years have detectable bacteruria on the standard urine culture.
The presumed etiology of rUTI relates to the reservoir of potential uropathogens within the gut; these microbes increase vaginal colonization with enteric bacteria, increasing UTI risk. The pathogenesis of rUTIs in menopausal women may, in part, be related to the microbial changes in vagina associated with menopause. Lower systemic and local estrogen levels raise the vaginal pH and decrease the lactobacillus dominant vaginal flora typical for premenopausal women. Together, these factors increase the chance that microbes with uropathogenic capability (ie, Escherichia coli and Enterococcus) establish residence in the vagina.
Differentiation of the bladder urothelium is thought to be critical in defense against uropathogens as only terminally differentiated cells express receptors mediating bacteria-induced apoptosis and shedding. Consistent with the known effects on the vagina, estrogen promotes proliferation of epithelial cells of the lower urinary tract.88 Estrogen also induces expression of tight junction proteins, namely E- cadherins in bladder epithelial cells.89 Expresson of E-cadherin is reduced in patients with recurrent UTI consistent with the idea of disruption of the urothelial cell barrier. Estrogen is also known to increase expression of antimicrobial peptides including hBD1–3, ribonuclease 7, and psoriasin by bladder urothelial cells.89 Urothelial cells present in mid-stream voided specimens from menopausal women collected before and after treatment with topical estrogen demonstrate a significant increase in antimicrobial peptide expression after even short periods (2 weeks) of estrogen treatment. Levels of these peptides are also increased in premenopausal, compared with menopausal, women consistent with estrogen modulation of expression. In addition to the favorable effects of estrogen discussed earlier in this review, topical vaginal estrogen also appears to induce favorable microbial changes in the vagina. Topical vaginal estrogen has been shown to clinically reduce rUTI episodes.90
Recently, the discovery of the female urobiome has raised the possibility of alternatives to antibiotic treatment that modulate the urinary microbiome. Despite sharing many bacterial genera, the urinary microbiome is unique and independent of the vaginal microbiome. Early urobiome studies compared the microbiota of urine collected by transurethral catheter with voided specimens, to ensure purity of the sampling technique and ultimately verifying that there is a resident community of living microbes within the human adult bladder. Urinary microbiota from healthy asymptomatic women often include genera that also inhabit the vagina including Bifidobacterium, Enterococcus, Actinomyces, Prevotella, and Atopobium.91–93 Many shared bacterial genera are present, however, in different quantities and distributions, consistent with the divergent environmental niches of the bladder and vagina. The communications and signaling mechanisms between these two microbial niches requires further study.
Ongoing studies will help clarify parameters of the normal female urobiome. While current evidence suggests that the urinary microbiome shares a number of genera with other known human microbiomes, the urobiome has two distinct features: decreased overall bacterial load; and decreased bacterial diversity. On average, the urinary microbiome consists of <104 CFU/mL total bacteria in comparison to the colon which contains 1011 to 1012 CFU/g with a median bacteria load of 85 CFU/mL. There is also less diversity in the urinary microbiota compared with other characterized microbiomes including skin and mouth; however, the bacterial abundance and diversity is similar to other low abundance microbial niches including eye.91 Due to the low bacterial abundance and decreased diversity, the urobiome is potentially more sensitive to disruption by antibiotics or infection.
Alterations in the female urobiome have be associated with clinical disorders. The best studied clinical phenomenon to date associating alterations in the urinary microbiome with clinical pathology is UUI, a common problem for menopausal women, as discussed earlier in this review. The urinary microbiome in women with and without UUI was directly compared using both an enhanced urine culture technique (expanded quantitative urine culture [EQUC]) and 16s rRNA sequencing.94 These two techniques demonstrate statistically significant changes in frequency and abundance of urinary bacteria, and also differences in species richness between cohorts. Further studies also relate hormonal status and BMI to urobiome characteristics, with estrogen use and higher BMIs associated with increased urobiome diversity.95
Topical vaginal estrogen may benefit the urobiome; studies are underway to investigate whether, similar to vaginal response to vaginal estrogen, the bladder develops a more favorable (lactobacillus-dominant) microbial community that is better able to defend against UTI. Given these important effects on the lower urinary tract, the relationship of the urobiome, UTI, rUTI, and estrogen (especially topical vaginal estrogen) will require further study to optimize therapeutic potential.
CONCLUSIONS
The etiology of PFDs is related to decline in structural, functional, and regenerative capabilities of the integral components of the female pelvic floor and continence mechanisms. A substantial body of epidemiological literature suggests an association between menopause, and PFDs and rUTIs; however, the inability to separate this association from aging and comorbid conditions makes it difficult to draw definitive conclusions on the role of menopause alone in the development and/or progression of PFDs. Similarly, the causative link between the decline in endogenous estrogen and the pathogenesis of PFDs and rUTIs has not been well-established. As nearly all human studies are limited by the collinearity of menopause and aging, investigations conducted in various experimental models are essential to decipher the mechanisms by which target tissues are affected by variable systemic and local estrogen levels. The existing disconnect between the basic science and clinical observations suggests that estrogen is important, but may not be sufficient for the desired therapeutic effects. Innovative human studies, focused on the independent effects of menopausal estrogen levels, uncoupled from tissue and cellular senescence, are needed. Such investigations have a high potential for bridging the laboratory and clinical findings and for providing a scientific rationale for estrogen replacement regiments ± adjuvant therapies aimed at counteracting PFDs and rUTIs, which significantly interfere with well-being of menopausal women.
Acknowledgments
Funding/support: None reported.
Financial disclosures/conflicts of interest: Dr Alperin reports stipends from Renovia, Inc., for serving on the Medical Advisory Board and has received the following grants: NIH/NICHD R01 HD092515 and NIH/ NIA R03 AG050951. Drs Burnett and Lukacz report no disclosures. Dr Brubaker reports editorial stipends from JAMA, Female Pelvic Medicine and Reconstructive Surgery, and Up to Date.
REFERENCES
- 1.Schoenaker DA, Jackson CA, Rowlands JV, Mishra GD. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. Int J Epidemiol 2014;43:1542–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett 1996;392:49–53. [DOI] [PubMed] [Google Scholar]
- 3.Geer LY, Marchler-Bauer A, Geer RC, et al. The NCBI BioSystems database. Nucleic Acids Res 2010;38:D492–D496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 2001;276:36869–36872. [DOI] [PubMed] [Google Scholar]
- 5.Blakeman PJ, Hilton P, Bulmer JN. Oestrogen and progesterone receptor expression in the female lower urinary tract, with reference to oestrogen status. BJU Int 2000;86:32–38. [DOI] [PubMed] [Google Scholar]
- 6.Tincello DG, Taylor AH, Spurling SM, Bell SC. Receptor isoforms that mediate estrogen and progestagen action in the female lower urinary tract. J Urol 2009;181:1474–1482. [DOI] [PubMed] [Google Scholar]
- 7.Teng J, Wang ZY, Jarrard DF, Bjorling DE. Roles of estrogen receptor alpha and beta in modulating urothelial cell proliferation. Endocr Relat Cancer 2008;15:351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu JM, Vaughan CP, Goode PS, et al. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet Gynecol 2014;123:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrence JM, Lukacz ES, Nager CW, Hsu JW, Luber KM. Prevalence and co-occurrence of pelvic floor disorders in community-dwelling women. Obstet Gynecol 2008;111:678–685. [DOI] [PubMed] [Google Scholar]
- 10.Lukacz ES, Lawrence JM, Contreras R, Nager CW, Luber KM. Parity, mode of delivery, and pelvic floor disorders. Obstet Gynecol 2006;107:1253–1260. [DOI] [PubMed] [Google Scholar]
- 11.Handa VL, Blomquist JL, Knoepp LR, Hoskey KA, McDermott KC, Muñoz A. Pelvic floor disorders 5–10 years after vaginal or cesarean childbirth. Obstet Gynecol 2011;118:777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quiroz LH, White DE, Juarez D, Shobeiri SA. Age effects on pelvic floor symptoms in a cohort of nulliparous patients. Female Pelvic Med Reconstr Surg 2012;18:325–328. [DOI] [PubMed] [Google Scholar]
- 13.Rubin EB, Buehler AE, Halpern SD. States worse than death among hospitalized patients with serious illnesses. JAMA Intern Med 2016;176:1557–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sung VW, Washington B, Raker CA. Costs of ambulatory care related to female pelvic floor disorders in the United States. Am J Obstet Gynecol 2010;202:483.e1–483.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirby AC, Luber KM, Menefee SA. An update on the current and future demand for care of pelvic floor disorders in the United States. Am J Obstet Gynecol 2013;209:584.e1–584.e5. [DOI] [PubMed] [Google Scholar]
- 16.Taguchi O, Cunha GR, Robboy SJ. Expression of nuclear estrogen- binding sites within developing human fetal vagina and urogenital sinus. Am J Anat 1986;177:473–480. [DOI] [PubMed] [Google Scholar]
- 17.Iosif CS, Batra S, Ek A, Astedt B. Estrogen receptors in the human female lower uninary tract. Am J Obstet Gynecol 1981;141:817–820. [DOI] [PubMed] [Google Scholar]
- 18.Miyagawa S, Iguchi T. Epithelial estrogen receptor 1 intrinsically mediates squamous differentiation in the mouse vagina. Proc Natl Acad Sci USA 2015;112:12986–12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palma F, Volpe A, Villa P, Cagnacci A; Writing Group of AGATA Study. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: the AGATA study. Maturitas 2016;83:40–44. [DOI] [PubMed] [Google Scholar]
- 20.Chen GD, Oliver RH, Leung BS, Lin LY, Yeh J. Estrogen receptor alpha and beta expression in the vaginal walls and uterosacral ligaments of premenopausal and postmenopausal women. Fertil Steril 1999;71: 1099–1102. [DOI] [PubMed] [Google Scholar]
- 21.Gebhart JB, Rickard DJ, Barrett TJ, et al. Expression of estrogen receptor isoforms alpha and beta messenger RNA in vaginal tissue of premenopausal and postmenopausal women. Am J Obstet Gynecol 2001;185: 1325–1330[discussion 1330–1331]. [DOI] [PubMed] [Google Scholar]
- 22.Stauffer SR, Coletta CJ, Tedesco R, et al. Pyrazole ligands: structure- affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem 2000;43:4934–4947. [DOI] [PubMed] [Google Scholar]
- 23.Long CY, Liu CM, Hsu SC, Wu CH, Wang CL, Tsai EM. A randomized comparative study of the effects of oral and topical estrogen therapy on the vaginal vascularization and sexual function in hysterectomized postmenopausal women. Menopause 2006;13:737–743. [DOI] [PubMed] [Google Scholar]
- 24.Sawczuk B, Gołe˛biewska M, Mazurek A, Chyczewski L. Immunohisto- chemical evaluation of oestrogen receptors alpha and beta in epithelium of the vaginal mucous membrane in women after oestrogen therapy. Prz Menopauzalny 2017;16:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev 2016;Cd001500. [DOI] [PMC free article] [PubMed]
- 26.Gaspar A, Brandi H, Gomez V, Luque D. Efficacy of Erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med 2017;49:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause 2018;25:21–28. [DOI] [PubMed] [Google Scholar]
- 28.Montoya TI, Maldonado PA, Acevedo JF, Word RA. Effect of vaginal or systemic estrogen on dynamics of collagen assembly in the rat vaginal wall. Biol Reprod 2015;92:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ceccarelli S, D’Amici S, Vescarelli E, et al. Topical KGF treatment as a therapeutic strategy for vaginal atrophy in a model of ovariectomized mice. J Cell Mol Med 2014;18:1895–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monica Brauer M, Smith PG. Estrogen and female reproductive tract innervation: cellular and molecular mechanisms of autonomic neuro plasticity. Auton Neurosci 2015;187:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griebling TL, Liao Z, Smith PG. Systemic and topical hormone therapies reduce vaginal innervation density in postmenopausal women. Menopause 2012;19:630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minassian VA, Bazi T, Stewart WF. Clinical epidemiological insights into urinary incontinence. Int Urogynecol J 2017;28:687–696. [DOI] [PubMed] [Google Scholar]
- 33.Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol 2003;20:327–336. [DOI] [PubMed] [Google Scholar]
- 34.Sexton CC, Coyne KS, Vats V, Kopp ZS, Irwin DE, Wagner TH. Impact of overactive bladder on work productivity in the United States: results from EpiLUTS. Am J Manag Care 2009;15 (4 Suppl):S98–S107. [PubMed] [Google Scholar]
- 35.Kupelian V, Wei JT, O’Leary MP, et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med 2006;166:2381–2387. [DOI] [PubMed] [Google Scholar]
- 36.Minassian VA, Yan X, Lichtenfeld MJ, Sun H, Stewart WF. The iceberg of health care utilization in women with urinary incontinence. Int Urogynecol J 2012;23:1087–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coyne KS, Wein A, Nicholson S, Kvasz M, Chen CI, Milsom I. Economic burden of urgency urinary incontinence in the United States: a systematic review. J Manag Care Pharm 2014;20:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Powell LC, Szabo SM, Walker D, Gooch K. The economic burden of overactive bladder in the United States: a systematic literature review. Neurourol Urodyn 2018;37:1241–1249. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida J, Aikawa K, Yoshimura Y, Shishido K, Yanagida T, Yamaguchi O. The effects of ovariectomy and estrogen replacement on acetylcholine release from nerve fibres and passive stretch-induced acetylcholine release in female rat bladder. Neurourol Urodyn 2007;26:1050–1055. [DOI] [PubMed] [Google Scholar]
- 40.Nelken RS, Ozel BZ, Leegant AR, Felix JC, Mishell DR Jr. Randomized trial of estradiol vaginal ring versus oral oxybutynin for the treatment of overactive bladder. Menopause 2011;18:962–966. [DOI] [PubMed] [Google Scholar]
- 41.Ellington DR, Szychowski JM, Malek JM, Gerten KA, Burgio KL, Richter HE. Combined tolterodine and vaginal estradiol cream for overactive bladder symptoms after randomized single-therapy treatment. Female Pelvic Med Reconstr Surg 2016;22:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tseng LH, Wang AC, Chang YL, Soong YK, Lloyd LK, Ko YJ. Randomized comparison of tolterodine with vaginal estrogen cream versus tolterodine alone for the treatment of postmenopausal women with overactive bladder syndrome. Neurourol Urodyn 2009;28:47–51. [DOI] [PubMed] [Google Scholar]
- 43.DeLancey JO. Anatomy and physiology of urinary continence. Clin Obstet Gynecol 1990;33:298–307. [DOI] [PubMed] [Google Scholar]
- 44.DeLancey JO. Structural support of the urethra as it relates to stress urinary incontinence: the Hammock hypothesis. Am J Obstet Gynecol 1994;170:1713–1720[discussion 1720–1723]. [DOI] [PubMed] [Google Scholar]
- 45.Delancey JO. Why do women have stress urinary incontinence? Neurourol Urodyn 2010;29 (Suppl 1):S13–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petros PE, Woodman PJ. The integral theory of continence. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:35–40. [DOI] [PubMed] [Google Scholar]
- 47.Minassian VA, Stewart WF, Wood GC. Urinary incontinence in women: variation in prevalence estimates and risk factors. Obstet Gynecol 2008;111 (2 Pt 1):324–331. [DOI] [PubMed] [Google Scholar]
- 48.Offermans MP, Du Moulin MF, Hamers JP, Dassen T, Halfens RJ. Prevalence of urinary incontinence and associated risk factors in nursing home residents: a systematic review. Neurourol Urodyn 2009;28:288–294. [DOI] [PubMed] [Google Scholar]
- 49.Robinson D, Cardozo L. Estrogens and the lower urinary tract. Neurourol Urodyn 2011;30:754–757. [DOI] [PubMed] [Google Scholar]
- 50.Kitta T, Haworth-Ward DJ, Miyazato M, et al. Effects of ovariectomy and estrogen replacement on the urethral continence reflex during sneezing in rats. J Urol 2011;186:1517–1523. [DOI] [PubMed] [Google Scholar]
- 51.Bhatia NN, Bergman A, Karram MM. Effects of estrogen on urethral function in women with urinary incontinence. Am J Obstet Gynecol 1989;160:176–181. [DOI] [PubMed] [Google Scholar]
- 52.Druzin M, Malinina E, Grimsholm O, Johansson S. Mechanism of estradiol-induced block of voltage-gated K+ currents in rat medial preoptic neurons. PLoS One 2011;6:e20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White RE, Han G, Maunz M, et al. Endothelium-independent effect of estrogen on Ca(2+)-activated K(+) channels in human coronary artery smooth muscle cells. Cardiovasc Res 2002;53:650–661. [DOI] [PubMed] [Google Scholar]
- 54.Hristov KL, Parajuli SP, Provence A, Rovner ES, Petkov GV. Non-genomic modulation of the large conductance voltage- and Ca(2+)- activated K(+) channels by estrogen: a novel regulatory mechanism in human detrusor smooth muscle. Physiol Rep 2017;5:doi: 10.14814/phy2.13351. [DOI] [PMC free article] [PubMed]
- 55.Fantl JA, Cardozo L, McClish DK. Estrogen therapy in the management of urinary incontinence in postmenopausal women: a meta-analysis. First report of the Hormones and Urogenital Therapy Committee. Obstet Gynecol 1994;83:12–18. [PubMed] [Google Scholar]
- 56.Grady D, Brown JS, Vittinghoff E, Applegate W, Varner E, Snyder T; HERS Research Group. Postmenopausal hormones and incontinence: the Heart and Estrogen/Progestin Replacement Study. Obstet Gynecol 2001;97:116–120. [DOI] [PubMed] [Google Scholar]
- 57.Hendrix SL, Cochrane BB, Nygaard IE, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA 2005;293:935–948. [DOI] [PubMed] [Google Scholar]
- 58.Cody JD, Jacobs ML, Richardson K, Moehrer B, Hextall A. Oestrogen therapy for urinary incontinence in post-menopausal women. Cochrane Database Syst Rev 2012;10:Cd001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keller JA, Chen J, Simpson S, et al. Voluntary urination control by brainstem neurons that relax the urethral sphincter. Nat Neurosci 2018;21:1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nygaard I, Bradley C, Brandt D; Women’s Health Initiative. Pelvic organ prolapse in older women: prevalence and risk factors. Obstet Gynecol 2004;104:489–497. [DOI] [PubMed] [Google Scholar]
- 61.Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E, Brown JS. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol 2001;98:646–651. [DOI] [PubMed] [Google Scholar]
- 62.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 1997;89:501–506. [DOI] [PubMed] [Google Scholar]
- 63.Smith P Estrogens and the urogenital tract. Studies on steroid hormone receptors and a clinical study on a new estradiol-releasing vaginal ring. Acta Obstet Gynecol Scand Suppl 1993;157:1–26. [PubMed] [Google Scholar]
- 64.Smith P, Heimer G, Norgren A, Ulmsten U. Steroid hormone receptors in pelvic muscles and ligaments in women. Gynecol Obstet Invest 1990;30:27–30. [DOI] [PubMed] [Google Scholar]
- 65.Copas P, Bukovsky A, Asbury B, Elder RF, Caudle MR. Estrogen, progesterone, and androgen receptor expression in levator ani muscle and fascia. J Womens Health Gend Based Med 2001;10:785–795. [DOI] [PubMed] [Google Scholar]
- 66.Moalli PA, Talarico LC, Sung VW, et al. Impact of menopause on collagen subtypes in the arcus tendineous fasciae pelvis. Am J Obstet Gynecol 2004;190:620–627. [DOI] [PubMed] [Google Scholar]
- 67.Zong W, Meyn LA, Moalli PA. The amount and activity of active matrix metalloproteinase 13 is suppressed by estradiol and progesterone in human pelvic floor fibroblasts. Biol Reprod 2009;80:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vardy MD, Gardner TR, Cosman F, et al. The effects of hormone replacement on the biomechanical properties of the uterosacral and round ligaments in the monkey model. Am J Obstet Gynecol 2005;192:1741–1751. [DOI] [PubMed] [Google Scholar]
- 69.Trutnovsky G, Guzman-Rojas R, Martin A, Dietz HP. Pelvic floor dysfunction: does menopause duration matter? Maturitas 2013;76:134–138. [DOI] [PubMed] [Google Scholar]
- 70.Tosun ÖÇ, Mutlu EK, Tosun G, et al. Do stages of menopause affect the outcomes of pelvic floor muscle training? Menopause 2015;22:175–184. [DOI] [PubMed] [Google Scholar]
- 71.Swift S, Woodman P, O’Boyle A, et al. Pelvic Organ Support Study (POSST): the distribution, clinical definition, and epidemiologic condition of pelvic organ support defects. Am J Obstet Gynecol 2005;192:795–806. [DOI] [PubMed] [Google Scholar]
- 72.Cundiff GW, Weidner AC, Visco AG, Bump RC, Addison WA. A survey of pessary use by members of the American Urogynecologic Society. Obstet Gynecol 2000;95 (6 Pt 1):931–935. [DOI] [PubMed] [Google Scholar]
- 73.Dessie SG, Armstrong K, Modest AM, Hacker MR, Hota LS. Effect of vaginal estrogen on pessary use. Int Urogynecol J 2016;27: 1423–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rahn DD, Ward RM, Sanses TV, et al. Vaginal estrogen use in post- menopausal women with pelvic floor disorders: systematic review and practice guidelines. Int Urogynecol J 2015;26:3–13. [DOI] [PubMed] [Google Scholar]
- 75.Ripperda CM, Maldonado PA, Acevedo JF, et al. Vaginal estrogen: a dual-edged sword in postoperative healing of the vaginal wall. Menopause 2017;24:838–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown HW, Wexner SD, Segall MM, Brezoczky KL, Lukacz ES. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int J Clin Pract 2012;66:1101–1108. [DOI] [PubMed] [Google Scholar]
- 77.Melville JL, Fan MY, Newton K, Fenner D. Fecal incontinence in US women: a population-based study. Am J Obstet Gynecol 2005;193:2071–2076. [DOI] [PubMed] [Google Scholar]
- 78.Saga S, Vinsnes AG, Mørkved S, Norton C, Seim A. Prevalence and correlates of fecal incontinence among nursing home residents: a population-based cross-sectional study. BMC Geriatr 2013;13:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Macmillan AK, Merrie AE, Marshall RJ, Parry BR. The prevalence of fecal incontinence in community-dwelling adults: a systematic review of the literature. Dis Colon Rectum 2004;47:1341–1349. [DOI] [PubMed] [Google Scholar]
- 80.Brown HW, Wexner SD, Segall MM, Brezoczky KL, Lukacz ES. Quality of life impact in women with accidental bowel leakage. Int J Clin Pract 2012;66:1109–1116. [DOI] [PubMed] [Google Scholar]
- 81.Fialkow MF, Melville JL, Lentz GM, Miller EA, Miller J, Fenner DE. The functional and psychosocial impact of fecal incontinence on women with urinary incontinence. Am J Obstet Gynecol 2003;189:127–129. [DOI] [PubMed] [Google Scholar]
- 82.Oettling G, Franz HB. Mapping of androgen, estrogen and progesterone receptors in the anal continence organ. Eur J Obstet Gynecol Reprod Biol 1998;77:211–216. [DOI] [PubMed] [Google Scholar]
- 83.Pinedo G, García E, Zárate AJ, et al. Are topical oestrogens useful in faecal incontinence? Double-blind randomized trial. Colorectal Dis 2009;11:390–393. [DOI] [PubMed] [Google Scholar]
- 84.Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. Vital Health Stat 2011;13:1–38. [PubMed] [Google Scholar]
- 85.Keating KN, Perfetto EM, Subedi P. Economic burden of uncomplicated urinary tract infections: direct, indirect and intangible costs. Expert Rev Pharmacoecon Outcomes Res 2005;5:457–466. [DOI] [PubMed] [Google Scholar]
- 86.Bermingham SL, Ashe JF. Systematic review of the impact of urinary tract infections on health-related quality of life. BJU Int 2012;110 (11 Pt C):E830–E836. [DOI] [PubMed] [Google Scholar]
- 87.Foxman B, Barlow R, D’Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol 2000;10:509–515. [DOI] [PubMed] [Google Scholar]
- 88.Blakeman PJ, Hilton P, Bulmer JN. Cellular proliferation in the female lower urinary tract with reference to oestrogen status. Bjog 2001;108:813–816. [DOI] [PubMed] [Google Scholar]
- 89.Lüthje P, Brauner H, Ramos NL, et al. Estrogen supports urothelial defense mechanisms. Sci Transl Med 2013;5:190ra80. [DOI] [PubMed] [Google Scholar]
- 90.Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med 1993;329:753–756. [DOI] [PubMed] [Google Scholar]
- 91.Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol 2014;52:871–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wolfe AJ, Toh E, Shibata N, et al. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol 2012;50:1376–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fouts DE, Pieper R, Szpakowski S, et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J Transl Med 2012;10:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. MBio 2014;5:e01283–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thomas-White KJ, Kliethermes S, Rickey L, et al. Evaluation of the urinary microbiota of women with uncomplicated stress urinary incontinence. Am J Obstet Gynecol 2017;216:55 e1–55 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]


