Abstract
Child-bearing years are often the most precarious management period in the life of a woman with epilepsy. This article reviews the results of many different studies with findings that enable the healthcare team to make confident decisions and recommendations during these critical periods. Preconceptional planning, effective contraception and folic acid supplementation are important fundamentals in preparation for pregnancy. There is growing evidence to avoid valproic acid use during the child-bearing years. Emerging data on congenital malformations and neurocognitive outcomes are available for some of the second-generation antiepileptic drugs and appear reassuring for lamotrigine and levetiracetam. Also reviewed are the benefits of postpartum drug tapers and favorable breastfeeding facts. Counseling the mother and her family on medication choices enables the healthcare team to implement informed decisions that are beneficial for the mother and child.
Keywords: antiepileptic drugs, epilepsy, pregnancy
The management of epilepsy during pregnancy is challenging and complicated. Epilepsy is the fourth most common neurologic disorder, but one of the most common chronic medical disorders of any kind that requires daily treatment with known teratogens during pregnancy. Over 1 million women with epilepsy in the USA are of reproductive age, and these women give birth to approximately 20,000 infants every year [1]. The majority of patients with epilepsy maintain seizure control during pregnancy, with actual seizure freedom reported in 66% of pregnant women in one large, international pregnancy registry [2]. But while some studies report that 63% of women experience no change in seizure activity, 17% experience an increase, and 16% a decrease in seizure frequency [3]. Seizures pose a risk to the developing fetus, especially if generalized tonic clonic convulsions. They can cause direct injuries from a fall, compromise the blood supply to the fetus, cause postictal hypoxia and lactic acidosis. This argues for stricter vigilance about seizure control during pregnancy than in any other period of a woman’s life. However, the treatment of epilepsy during pregnancy is a double-edged sword, because many of the antiepileptic drugs (AEDs) that most effectively control seizures are also teratogenic to various degrees, posing another obvious risk to the developing fetus. This makes the management of the pregnant patient with epilepsy a unique challenge (Figure 1). With the increasing use of AEDs for various nonepileptic disorders like chronic or neuralgic forms of pain, migraines and mood disorders, it is necessary to understand the best evidence based strategies for using AEDs in pregnant women. This review presents numerous prospective studies, registry data and updated results describing treatment strategies and outcomes for treating epileptologists, general neurologists, internists, family practitioners, obstetricians and pediatricians.
Figure 1. Complex tridirectional interactions and influences.
AED: Antiepileptic drug.
Preconception
As many women are unaware of their pregnancy until after the initial weeks of organogenesis, the management of women with epilepsy (WWE) should include preconceptional counseling beginning with the initial clinic visit and continuing throughout their reproductive years. The discussion about sexual dysfunction, fertility and effective contraception methods are essential during the reproductive years. Careful planning and management, prior to and during pregnancy will minimize the risks to the mother and the fetus. Antiepileptic drug choices should be discussed before conception so that there need not be any major medication changes during pregnancy.
Contraception
Discussion of effective methods of contraception starts with the awareness of patient’s approximate plans for conception/pregnancy. Concomitant use of hormonal contraceptives and AEDs increases the complexity of care from contraception failure and/or possible effects on seizure control. Some studies on animal models also suggest a proconvulsant effect of estrogen and anti-convulsant properties for progesterone, e specially its active metabolite allopregnanolone [4].
Women with epilepsy on various enzyme-inducing AEDs (EIAEDs) face the risk of failure of contraception, as the metabolism of orally administered contraceptive hormones increases and hormone levels fall substantially, permitting ovulation and unplanned pregnancy. Other non-oral forms of hormonal contraception like vaginal ring and patch are also influenced in the same way. EIAEDs include phenytoin, phenobarbital, primidone, carbamazepine, oxcarbazepine (OXC), felbamate and clobazam. Topiramate decreases ethinyl estradiol in a dose-dependent manner (Box 1) [5]. These EIAEDs are classified as a “Category 3: the risks (birth control failure) generally outweigh the benefits” by the CDC Medical Eligibility Criteria for contraception.
Box 1. Antiepileptic drug effects on hormonal contraceptive agents.
Risk of contraception failure (hepatic enzyme inducers)
Phenobarbital
Phenytoin
Carbamazepine
Primidone
Topiramate (>200 mg/day)
Oxcarbazepine
Rufinamide
Felbamate
Clobazam
Low risk of contraception failure
Ethosuximide
Vigabatrin
Gabapentin
Lacosamide
Tiagabine
Levetiracetam
Zonisamide
Pregabalin
Lamotrigine† (modest decrease of progestins)
†Estrogen-related induction of lamotrigine glucuronidation lower lamotrigine drug levels, resulting in increased risk of seizures. Therefore, lamotrigine dose may need to be increased.
Levetiracetam (LEV) and lamotrigine (LTG) for most part are considered as nonenzyme inducing AEDs. Lamotrigine modestly decrease progestin concentration without evidence of ovulation [6]. More importantly concentration of lamotrigine decreases when taken in combination with oral contraceptives, probably related to ethinyl estradiol-induced glucuronidation [7]. With the cessation of oral contraceptives, up to 84% increase in the LTG concentration occurs almost within a week. This suggests that the induction and deinduction of Lamotrigine glucuronidation is faster than the other metabolic pathways [8] and AED dose adjustments should be performed with close monitoring of blood levels.
Other forms of birth control, specifically the long-acting reversible contraceptives (levonorgestrel/etonogestrel implant or intrauterine devices or depot medroxy progesterone acetate) are more appropriate in women on these antiepileptic drugs with interactions, including lamotrigine [9]. Intrauterine contraception (Copper intrauterine device [IUD] or progestin-only levonorgestrel IUD) or other long acting reversible contraceptives (progestin implant or intramuscular medroxyprogesterone acetate) may provide a reliable and easier form of contraception. Intramuscular medroxyprogesterone acetate is considered second tier within this group by some experts due to its side effect profile of weight gain, delayed return to fertility and increased risk of osteopenia, especially if a woman is on an EIAED. The Mirena IUD (impregnated with progesterone) exerts its effect locally on the endometrium and by thickening the cervical mucus, so AEDs are unlikely to impact its efficacy. In addition, estrogen mediated AED clearance and theoretical proconvulsant p roperties of estrogen can be mitigated (Table 1).
Table 1.
2009 UK Medical Eligibility Criteria for Contraceptive Use categories for anticonvulsant therapy and contraception.
| Anticonvulsant | Combined hormonal methods | Progestogen-only pill | Progestogen-only implant | Progestogen-only injectable | Levonorgestrel-releasing intrauterine system | Copper-IUD |
|---|---|---|---|---|---|---|
| EIAEDs | 3† | 3† | 2† | DMPA − 1, NET-EN - 2† | 1 | 1 |
| Lamotrigine | 3 | 1 | 1 | 1 | 1 | 1 |
UK Medical Eligibility Criteria for Contraceptive Use Category 1: A condition for which there is no restriction for the use of the contraceptive method with the condition or in that circumstance.
UK Medical Eligibility Criteria for Contraceptive Use Category 2: A condition where the advantages of using the method generally outweigh the theoretical or proven risks.
UK Medical Eligibility Criteria for Contraceptive Use Category 3: A condition where the theoretical or proven risks generally outweigh the advantages of using the method. The provision of a method requires expert clinical judgement and/or referral to a specialist provider, since use of the method is not usually recommended unless other methods are not available or not acceptable.
UK Medical Eligibility Criteria for Contraceptive Use Category 4: A condition which represents unacceptable health risk if the method is used.
The consistent use of condoms is recommended.
DMPA: Depot medroxyprogesterone acetate; EIAED: Phenytoin, carbamazepine, barbiturates, primidone, topiramate, oxcarbazepine; NET-EN: Norethisterone enanthate. Reproduced with permission from [10].
Folic acid supplementation
All women who take AEDs during their reproductive years should take folic acid supplementation, but it is especially important prior to conception. As formation of the neural tube is completed often before the pregnancy is even recognized, AAN guidelines recommend folic acid supplementation to decrease the risk of major congenital malformations [11].
Although the mechanism of depletion of folic acid by newer AEDs is not quite clear, older AEDs like carbamazepine, phenytoin, primi-done and phenobarbital are known to deplete folic acid. Lamotrigine and valproic acid also interfere with folic acid metabolism. Maternal folate levels decrease during pregancy and use of AEDs may enhance this decline. Multiple studies have demonstrated a link between the use of AEDs and serum folate levels in pregnant women with epilepsy, and that folic acid supplementation in women on AEDs resulted in normalization of the serum folate concentration [12]. However, a prospective observational registration and follow-up study from Morrow et al. reported that the increased risk of major congenital malformations (MCMs) in this population may not be a direct antifolate effect given that their study did not demonstrate a protective effect of preconceptual folic acid in women with epilepsy [13]. Despite this unclear evidence in women with epilepsy, supplemental folic acid provides a clear benefit in the general population and is recommended to lower the risk for major congenital malformations. The amount of supplementation that is deemed adequate still remains unclear. In the USA, Canada and the UK, the recommended doses range from 0.4 to 5 mg/day, starting prior to conception, with many experts recommending a daily dose closer to the higher end of the range, especially if on valproic acid or carbamazepine [11,14]. Recent data from the neurodevelopmental effects of antiepileptic drugs (NEAD) study also showed higher intelligence quotients (IQs) in children who were born to mothers on periconceptional folate [15].
Gestational period
Seizure control
The large prospective European and International Registry of Antiepileptic Drugs in Pregnancy (EURAP) study provided reassuring information that the frequency of seizures during pregnancy will not change in most (50–83%) WWE. But approximately 20–33% of patients will have an increase in their seizures and 7–25% have a decrease in seizures [3,15]. In addition, the 2009 AAN Practice Parameter Update also concluded that seizure freedom for at least 9 months prior to pregnancy is associated with a 80–90% likelihood of seizure freedom during pregnancy [16]. Though some older studies reported worsening of seizure control in the first and last trimesters, findings from the EURAP study noted better seizure control in second and third trimesters compared with the first [3]. Similarly, smaller single-site studies noted seizure worsening most prominent during the 4th through 7th months of pregnancy, especially in association with a 35% or greater decrease in AED levels from preconception b aseline levels [17,18].
Prospective data from the Kerala Registry of Epilepsy and Pregnancy cohort in India outlined some predictors that were associated with risk for seizure relapse during pregnancy. These included the type of seizures (partial seizures more than generalized seizures), AED polytherapy (although this may be confounded by more severe disease), and most importantly, the occur-rence of seizures in the prepregnancy month [19].
Nevertheless, a single breakthrough seizure may be disastrous during pregnancy, not only due to the harm to the mother and fetus but also because of its implications on driving, family dynamics and emotional burden. Generalized tonic-clonic convulsions can cause maternal and fetal hypoxia and acidosis, fetal heart rate decelerations, fetal intracranial hemorrhage, miscarriages and stillbirths [1,20]. Effects of nonconvulsive seizures during pregnancy still remain unclear though isolated case reports on complex partial seizures noted prolonged uterine contractions with fetal heart rate deceleration [21,22].
Various physiological changes in sex hormone concentrations, changes in AED metabolism, sleep deprivation and new stresses also affect the seizure frequency. Patients often have misconceptions about the danger of AEDs during pregnancy and thus may abruptly stop them, as soon as they know about their pregnancy. This reemphasizes the role of adequate education of the patient along with her family members regarding the importance of medication compliance and monitoring, the current evidence on safety regarding her AEDs and the risks involved with the occurrence of seizures, especially generalized tonic clonic convulsions.
AED management
Several physiologic changes during pregnancy affect the pharmacokinetics of AEDs. Increased clearance of AEDs occurs with every successive week of gestation due to many factors, including changes in absorption, protein-binding, volume of distribution, increased metabolism through induction of various hepatic pathways and enhanced excretion through kidneys, resulting in wide variations in the drug levels (Box 2) [23]. For optimal seizure control during pregnancy, AED doses should be titrated to an appropriate serum level within the patient’s therapeutic range (therapeutic drug monitoring). This level should be determined during the prepregnancy period.
Box 2. Antiepileptic drug primary routes of elimination.
Hepatic CYP450†
Phenytoin†
Phenobarbital†
Carbamazepine†
Zonisamide
Renal excretion‡
Levetiracetam
Pregabalin
Vigabatrin
Topiramate
Glucuronidation (most affected during pregnancy)‡
Valproate (and beta oxidation)†
Lamotrigine
Oxcarbazepine (MHD)
†Highly protein bound.
‡Main route of metabolism.
MHD: Monohydroxy dervative of oxcarbazepine, the active metabolite of oxcarbazepine.
The 2009 AAN/American Epilepsy Society (AES) Practice Parameter Update concluded the following: pregnancy probably causes an increase in the clearance and a decrease in the concentration of LTG, phenytoin (PHT), and to a lesser extent carbamazepine (CBZ), and possibly decreases the level of LEV and the active OXC metabolite, the monohydroxy derivative [11]. The authors went on to state that monitoring of LTG, PHT and CBZ should be considered during pregnancy and monitoring of LEV and the OXC metabolite may be considered. As OXC quickly undergoes keto-reduction to an active monohydroxy derivative (MHD), the concentration of MHD should be measured rather than the parent drug [24]. Since the writing of this practice parameter update, two small studies reported that there was significant increase in clearance of topiramate during pregnancy, although levels did not clearly correlate with seizure frequency. Thus the authors concluded that following levels during pregnancy might be of value [25,26]. CBZ is another AED that has mixed reports with only modest changes in the clearance of total and free carbamazepine and its active metabolite, carbamazepine-10,11-epoxide and lack of correlation between levels and seizure control [27]. Thus, monthly monitoring may not be necessary for carbamazepine, especially if the patient has relatively easy to control seizures and resources for therapeutic drug monitoring are limited.
Among the newer AEDs, lamotrigine is extremely susceptible to changes in levels during pregnancy. This is because of its primary elimination via hepatic glucuronidation that is directly affected by rising sex steroid hormone levels. Recent data from the EURAP pregnancy registry on a large cohort reported that the pregnancies exposed to lamotrigine were less likely to be seizure-free and were more likely to require an increase in drug load. The mean dose increase was highest for the lamotrigine group at 26% [2]. The Class I data from a prospective, observational study showed that both free and total LTG clearance were increased during all three trimesters, with peaks of increases by 94% (total) and 89% (free) in the third trimester. This study also examined therapeutic drug monitoring and seizure frequency, and empiric postpartum taper to avoid toxicity. The authors reported that increased seizure frequency in the second trimester was associated with a decline in the LTG level to 65% or less of the preconceptional individualized target LTG concentration [17]. A retrospective analysis combining all AEDs also found that seizure worsening in the second trimester of pregnancy was associated with a decline in the AED level to 65% or less of the preconception baseline [18].
A recent study on LTG identified two sub-populations who exhibited different LTG clearances during pregnancy that differed by tenfold [28]. The majority of women (77%) had higher clearance whereas 23% of women had modest clearance of the drug. This information could have therapeutic implications as this influences the titration regimen in a particular group based on their presumed different pharmacogenomic profiles [17,28]. However, future studies are needed to identify the genetic differences to allow preconceptional testing to identify into which sub-population an individual fits.
LEV levels in pregnancy are also significantly affected, as the primary mode of elimination is excretion through kidneys (66%). Levels are decreased by 38–60% during the 3rd trimester because of increased renal blood flow and glomerular filtration rate [29–31]. Though no prospective systemic studies are currently available to address the direct relation between drop in LEV level and seizure frequency, the retrospective analysis by Reisinger et al. [18] did indicate that seizure was more likely to worsen if the LEV level fell below 65% of the preconception baseline. Therefore, therapeutic drug monitoring during pregnancy should be considered to maintain the individual’s target concentration. Interestingly, a recent analysis from the Australian Register of Antiepileptic Drugs reported that levetiracetam controlled seizures more effectively than lamotrigine and topiramate with results that are comparable to older antiepileptic drugs (OR: 0.87) like carbamazepine and valproic acid [32]. Decisions about the use of serum AED levels and any adjustments in dose were made individually by the patient’s treating physician.
Despite the lack of standard trials on many newer agents, it is reasonable to adjust doses for those that have high renal clearance (pregabalin, vigabatrin) and/or undergo glucuronidation (eslicarbazepine) to avoid lower levels. As the AED concentrations during pregnancy may be affected, not only by the pharmacokinetic changes but also from the possibility of noncompliance, some experts recommend at least monthly monitoring of all AED concentrations [33,34].
Congenital malformations
The teratogenic potential of seizures directly is extremely unlikely, and there are a few clinical studies in the last decade that did not show increased risk of major malformations in women who had generalized seizures during their first trimester [3,35,36]. There are various proposed mechanisms of teratogenicity caused by AEDs. Oxidative stress causing release of free radicals, formation of toxic epoxide intermediates, altered folate metabolism and histone deacetylase inhibition are thought to be some of the mechanisms, though enough evidence does not exist. In addition, not much is known about the mechanisms of teratogenicity of newer AEDs in particular.
Major malformations
MCM is defined as an abnormality of an essential anatomical structure present at birth that interferes significantly with function and/or requires major intervention. The MCMs that are most commonly reported at a higher rate with AED exposure compared with the general population include congenital heart defects, cleft lip/palate, urogenital defects, skeletal defects and neural tube defects (for valproic acid [VPA] and CBZ). The reported MCM rates in the general population vary between 1.6 and 3.2% [37]. As per the results of a meta-analysis from 2004, the risk for MCMs in the offspring of women with untreated epilepsy was not higher than among nonepilepsy controls (odds ratio [OR]: 1.92; 95% CI: 0.92–4.00), but higher in offspring of mothers who received AEDs (OR: 3.26; 95% CI: 2.15–4.93) (Table 2) [38].
Table 2.
Major congenital malformations in infants of women with epilepsy.
| Congenital defects | Infants of women with epilepsy | General population | Timing of malformations (postconceptional age) [39] |
|---|---|---|---|
| Congenital heart defects | 1.5–2% | 0.5% | 42 days (VSD) |
| Cleft lip/palate | 1.4% | 0.15% | 36/47–70 days |
| Neural tube defect | 1–3.8% (VPA) 0.5–1% (CBZ) | 0.06% | 28 days |
| Urogenital defects | 1.7% | 0.7% |
Congenital heart defects can include atrial septal defect, ventricular septal defect, patent ductus arteriosus, coarctation of aorta and tetralogy of fallot.
Urogenital can include glandular hypospadias.
Neural tube defects mostly often include the lower midline defects, spina bifida aperta and myelomeningocele and rarely anencephaly.
CBZ: Carbamazepine; VPA: Valproic acid; VSD: Ventricular septal defect.
AED polytherapy
Several studies show that AED polytherapy regimens substantially increase the risk of congenital malformations [40,41], but only with specific combinations of AEDs. Data from the North American Pregnancy registry showed that the women were only at an increased risk of fetal MCMs when taking lamotrigine or carbamazepine in combination with valproic acid [42] while other polytherapy combinations were safe (Table 3). This urges better preconceptional planning using AED monotherapy or safe regimens of AED polytherapy.
Table 3.
Risk of major congenital malformations in polytherapy with and without valproic acid.
| Lamotrigine | Carbamazepine | |
|---|---|---|
| With valproic acid | 9.1 | 15.4 |
| With other AEDs | 2.9 | 2.5 |
AED: Antiepileptic drug. Data taken from [42].
AED monotherapy
Pregnancy registries from various parts of the world and ongoing prospective studies on WWE provide a significant fund of knowledge about specific AEDs, and thus the ability to lower the teratogenicity risk in WWE. Conclusions about intrauterine first trimester exposure and risk for MCMs through an evidence based approach from the 2009 AAN Practice Parameter Updates on “Management issues for women with epilepsy – focus on pregnancy” [43] are as follows:
It is highly probable that valproic acid exposure poses a higher risk of MCMs than carbamazepine, and possibly higher compared with phenytoin or lamotrigine; compared to untreated WWE, valproic acid probably as part of polytherapy and possibly as monotherapy contributes to the development of MCMs; AED polytherapy as compared with monotherapy regimens probably contributes more to the development of MCMs; carbamazepine probably does not substantially increase the risk of MCMs in the offspring of WWE and there is probably a dose-dependent relationship between valproic acid and lamotrigine and the risk of development of MCMs in the offspring of WWE.
Some specific types of MCM associations are also reported:
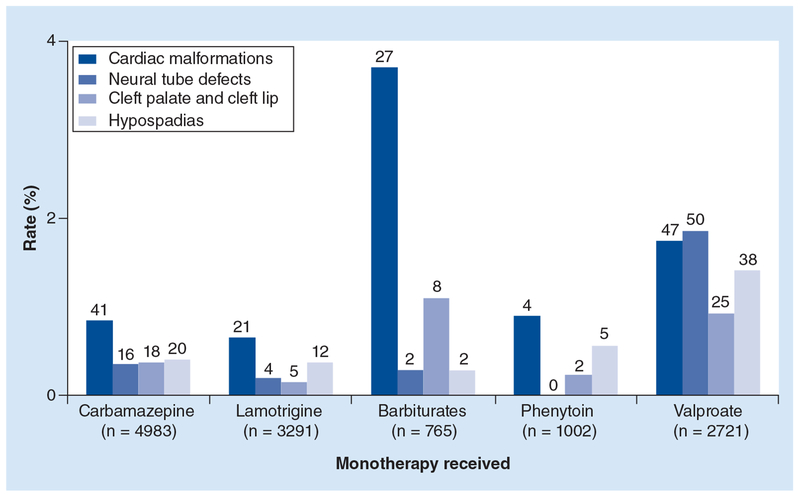
Phenytoin possibly contributes to the risk of cleft palate; carbamazepine possibly contributes to the risk of posterior cleft palate; valproic acid probably contributes to neural tube defects and facial clefts and possibly contributes to hypospadias and phenobarbital exposure in utero possibly contributes to cardiac malformations (Figure 2).
Figure 2. Specific major congenital malformations associated with different antiepileptic drug monotehrapies (combined data from 21 prospective studies).
Reproduced with permission from [44].
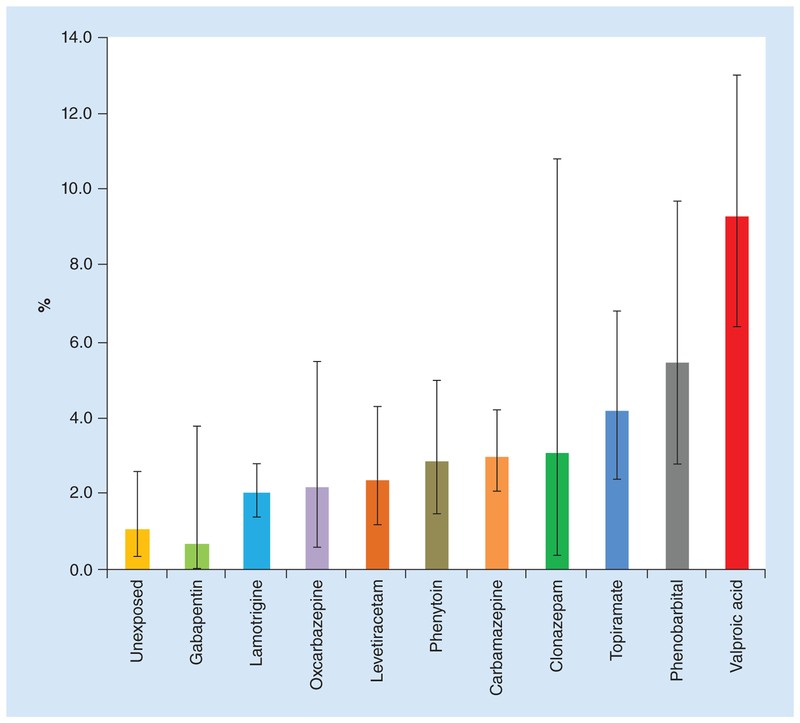
Since these practice parameter updates, major epilepsy pregnancy registries and several prospective studies looked at various monotherapy and polytherapy combinations. These studies consistently showed higher risk of MCMs with valproic acid (Figure 3) [45–48]. Wlodarczyk et al. provide a detailed review of animal and human studies, including historical and updated i nformation, about each of the AEDs [12].
Figure 3. Number (%) and 95% CIs of major congenital malformations identified among infants exposed to a specific AED in monotherapy during the first trimester and among the internal comparison group of unexposed infants: North American Antiepileptic Drug Pregnancy Registry 1997–2011.
Data taken from [45].
Lamotrigine has been the most widely studied with promising findings in various international registries for about 20 years. Among 1558 firsttrimester monotherapy exposures, 35 infants were noted to have MCMs (2.2%; 95% CI: 1.6–3.1%) [49]. These rates appear similar to the estimates from general population-based cohorts. Though the MCM rates were significantly higher at 10.7% among lamotrigine/valproate polytherapy exposure group, it was 2.8% among infants exposed to lamotrigine polytherapy without valproate. There is no specific MCM pattern consistently observed so far, although the North American AED Pregnancy Registry has reported higher rates for oral clefts, and further monitoring is ongoing through various registries and surveillance networks.
Data regarding MCMs related to topiramate exposure during pregnancy are available through the UK Epilepsy and Pregnancy Registry that had 203 pregnancies with 178 live births [50]. The reported incidence was 9.0%, and 4.8% on monotherapy. MCMs like oral clefts (2.2%) and hypospadias (5.1%) were reported. In spite of the sample size being small and wide confidence intervals, caution should be noted with usage of topiramate as this incidence is 11 times the background rate. This risk has been replicated even in other studies [51]. Based on North American Pregnancy Registry data, Hernandez-Diaz et al. reported a higher MCM rate of 4.2% (15 of 359) related to topiramate use, compared with that of reference population [45].
The results from the UK and Ireland epilepsy and pregnancy registry provide reassuring findings on first trimester exposure of levetiracetam, which is especially helpful given that it is a commonly prescribed AED including in women of child-bearing age. As a monotherapy agent, the MCM rate was 0.70%; CI: 0.19–2.51%. Polytherapy use of levetiracetam with lamotrigine had MCM rate of 1.77%; which is considerably less than combinations with valproate (6.90%) or carbamazepine (9.38%) [52].
Recent data from other pregnancy registries also noted that the malformation risk is dose-dependent. Investigators from the EURAP analyzed MCM rates by dose at the time of conception. Doses later in pregnancy were not accounted for in this analysis. The MCM rates were lowest with lamotrigine at less than 300 mg per day (2.0%; 95% CI: 1.19–3.24) and carbamazepine at less than 400 mg per day (3.4%; 95% CI: 1.11–7.71). Compared with lamotrigine monotherapy at doses less than 300 mg per day, risks of malformation were higher with carbamazepine at doses greater than 400 mg per day and significantly higher with valproic acid and phenobarbital at all doses (Table 4) [36]. Therefore, reduction of the dose prior to conception while maintaining seizure control can further reduce the risk of structural teratogenicity. Determining the women’s individual target concentration preconception can also be a valuable tool for therapeutic drug monitoring during pregnancy.
Table 4.
Dose-dependent malformation rates of various antiepileptic drug monotherapies.
| AED (number exposed) | Dose range (mg/day) | Malformation rate (95% CI) |
|---|---|---|
| Carbamazepine (n =1402) | <400 | 3.4% (1.11–7.71) |
| ≥400 <1000 | 5.3% (4.07–6.89) | |
| ≥1000 | 8.7% (5.24–13.39) | |
| Lamotrigine (n =1280) | <300 | 2.0% (1.19–3.24) |
| ≥300 | 4.5% (2.77–6.87) | |
| Phenobarbital (n = 217) | <150 | 5.4% (2.51–10.04) |
| ≥150 | 13.7% (5.70–26.26) | |
| Valproic acid (n =1010) | <700 | 5.6% (3.60–8.17) |
| ≥700 <1500 | 10.4% (7.83%–13.50) | |
| ≥1500 | 24.2% (16.19–33.89) |
Reproduced with permission from [36].
A population-based cohort study from Denmark on newer generation AEDs showed that among 1532 infants exposed to lamotrigine (n = 1019), OXC (n = 393), topiramate (n = 108), gabapentin (n = 59) or levetiracetam (n = 58) during the first trimester, 49/3.2% were diagnosed with a major birth defect compared with 19,911/2.4% of the 836,263 who were not exposed to an AED (95% CI: 0.72–1.36) [53]. However, this study did not distinguish poly-therapy from monotherapy. In a small prospective comparative cohort study involving gabapentin exposed pregnancies, the rates of major malformations appeared to be similar in both exposed and unexposed groups [54].
It is important to note that reproductive safety information is still lacking for many newer AEDs (often referred to as third-generation AEDs), and many of the second-generation AEDs do not yet have long-term neurodevelopmental data (see below), although prospective studies are underway. AEDs without reproductive safety information should be avoided when possible in women with epilepsy during pregnancy, unless they are the only medications that provided s eizure control for that individual.
Surveillance for birth defects
A multidisciplinary approach is essential for women on AEDs during pregnancy, and if possible, during preconceptional planning. Many women with epilepsy on AEDs will be referred to a perinatologist/maternal fetal medicine specialist in addition to her general obstetrician, but this varies greatly by region of practice. The important principle is that she be offered prenatal testing including a detailed structural ultrasound. Many regions have genetic counselors that are part of this multidisciplinary team, either early in the process or if a malformation is suspected. It is important to rule out other potential contributing factors such as genetic. If a malformation is identified, this not only allows the woman and her family to make decisions about termination, but also to provide in utero and neonatal treatment options if the pregnancy will continue. Additionally serial surveillance may be considered for fetal growth under the guidance of an obstetrician.
Peripartum period
Obstetrical complications
The 2009 AAN/AES Practice Parameters concluded that the risk of cesarean delivery or late pregnancy bleeding is probably not increased in WWE taking AEDs and there is no exaggerated risk of premature contractions/labor or delivery [16]. A later population-based cohort study from Norway reported increased risk of mild pre-eclampsia, gestational hypertension, vaginal bleeding late in pregnancy and premature delivery (<34 weeks) [55]. A British prospective observational study showed similarly increased rates of obstetric complications [56]. WWE should be counseled about the possibility of pregnancy complications early in the pregnancy. Current ongoing prospective studies should help clarify and quantify any increased obstetric risks in WWE, with considerations of seizure types and frequencies and AED types and doses.
Labor & delivery management
Though the incidence of breakthrough seizures is not particularly high during delivery, risk may be increased especially in patients with primary generalized epilepsy [57]. Women should be advised to take their AEDs without fail, as triggers such as sleep deprivation and stress lower the seizure threshold. If there is concern for nausea or vomiting or even prolonged labor, intravenous formulations should be substituted.
Generalized seizures should be treated with parenteral lorazepam or diazepam. Close monitoring should be performed to recognize potential adverse effects related to benzodiazepines on the neonate (respiratory depression or depressed heart rate) or the mother (apnea especially if benzodiazepines are given in large doses). Other general precautions like oxygen supplementation, or placing the patient on her left side, help to increase uterine blood flow and decrease maternal aspiration [58].
Postpartum period
Antiepileptic drug dose adjustments
Most of the antiepileptic drug levels gradually increase after delivery and plateau by 10 weeks postpartum. One of the studies on lamotrigine reported that the LTG levels returned to preconception values within 3 weeks postpartum [28]. This knowledge allows the physician to plan the down-titration strategy and avoid toxicity [17]. An empiric postpartum taper for LTG was also proposed by Pennell et al. Authors suggested a steady decrease in dosing at postpartum days 3, 7 and 10, with return to preconception dose or preconception dose plus 50 mg to help counteract the effects of sleep-deprivation. The exact time course is not as well established for the other AEDs as with LTG, but AED doses should be adjusted during the postpartum period as early as 3–14 days. Women should be monitored closely for clinical signs of drug toxicity or breakthrough seizures while making these rapid medication adjustments. Some women prefer maintaining higher levels to minimize seizure risk secondary to postpartum fatigue and stress, and correlating dose with AED levels can help with decision-making. However, in the early postpartum period, AED levels need to be interpreted with the caution that the patient is likely not in steady-state with rapid pharmacokinetic changes ongoing and there can be a delay from the clinical laboratory in obtaining the result.
Breast feeding
Despite various concerns about exposure of AEDs through breastmilk, most studies indicate that infants can be successfully breastfed without complications, and the benefits are believed to outweigh the risks. The concentrations of the different AEDs in breast milk are considerably less than those in maternal serum (Table 5). Davanzo et al. outlined drug specific infant monitoring, mostly for sedation, with reassuring safety data [59]. Continuous breast feeding may interfere with the rest and sleep of the patient, so women should be encouraged to pump the milk or substitute a one or more night time feeds with formula, so that she can get few hours of uninterrupted sleep.
Table 5.
Antiepileptic drug exposure through breast milk.
| AED | Breast milk/maternal concentration | Adult half-life (h) | Neonate half-life (h) |
|---|---|---|---|
| CBZ | 0.36–0.41 | 8–25 | 8–36 |
| PHT | 0.06–0.19 | 12–15 | 15–105 |
| PB | 0.36–0.46 | 75–125 | 100–500 |
| ESX | 0.86–1.36 | 32–60 | 32–38 |
| PRM | 0.72 | 4–12 | 7–60 |
| VPA | 0.01–0.1 | 6–20 | 30–60 |
| LTG | 0.5–0.77 | 30 | – |
| ZNS | 0.41–0.93 | 63 | 61–109 |
| TPM | 0.86 | 21 | 24 |
| GBP | 0.7–1.3 | 7–9 | 14 |
| OXC | 0.5–0.65 | 19.3 | 17–22 |
| LEV | 0.8–1.3 | 6–8 | 16–18 |
AED: Antiepileptic drug; CBZ: Carbamazepine; ESX: Ethosuxemide; GBP: Gabapentin; LEV: Levetiracetam; LTG: Lamotrigine; OXC: Oxcarbazepine; PB: Phenobarbital; PHT: Phenytoin; PRM: Primidone; TPM: Topiramate; VPA: Valproic acid; ZNS: Zonisamide. Reproduced with permission from [61].
Most recent data from the multicenter, prospective cohort in the NEAD study, which included mothers on PHT, LTG, VPA or CBZ monotherapy, demonstrated improved neurodevelopmental outcomes in the children who were breastfed compared with those who were not. Meador et al. compared the IQ until age-6 between children who breastfed (43%) to those who did not, finding that adjusted IQ was actually higher by 4 points and verbal ability was greater in children who were breastfed. This again supports the recommendation to breastfeed despite continued exposure to AEDs via breast milk [62].
Neonatal complications
Results from studies vary as to whether there is an increased risk for fetal loss or stillbirth in pregnant women with epilepsy compared with the general population [1]. Prematurity (with an OR of approximately 2.5–2.8), low birth weight (LBW <2500 g), small for gestational age (SGA <10th percentile) and small head circumference do appear to be more common in infants born to WWE [63,64]. The 2009 AAN/AES Practice Parameter guidelines concluded that infants of WWEs are twice as likely to be small for gestational age, and have a 1-min APGAR (appearance, pulse, grimace, activity and respiration) score of <7 and that there is possibly a substantially increased risk of premature contractions and labor and delivery for women with epilepsy who smoke in particular [43].
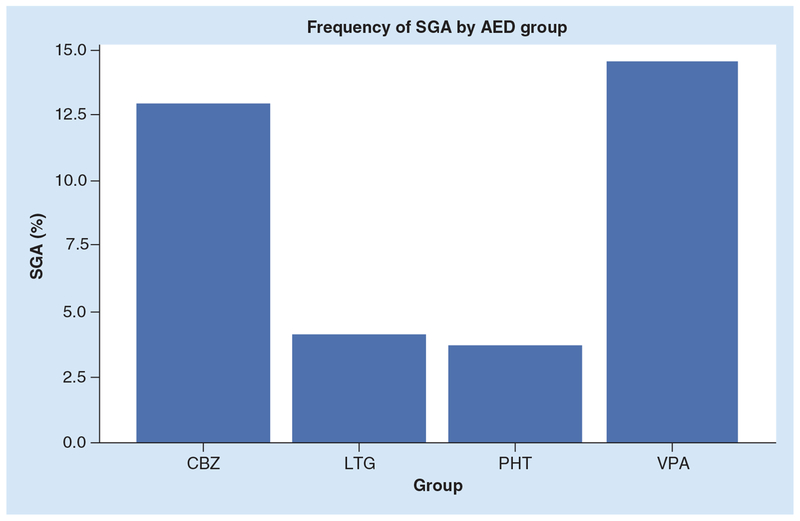
These complications have been attributed to both seizures during pregnancy and AED use. The data from the NEAD cohort indicated that VPA and CBZ are associated with higher risk for SGA compared with PHT and LTG. Transiently reduced 1-min Apgar scores were also noted to occur more frequently in the PHT and VPA groups (Figure 4) [65]. Evidence of early micro-cephaly in CBZ group followed by VPA group was also noted though the clinical implications were unclear, given that they catch up with the controls. Recent follow-up data from the North American Antiepileptic Drug Pregnancy Registry showed that the women on topiramate or zonisamide monotherapy had offspring with lesser mean birth weight and length. The prevalence of SGA was higher in topiramate (17.9%) and zonisamide (12.2%) groups in comparison with LTG (6.8%) [66]. In these studies, though maternal smoking was found to be associated with higher prevalence of these neonatal complications, the findings of higher rates of SGA persisted even after controlling for maternal smoking.
Figure 4. Differential antiepileptic drug effects on SGA in the NEAD cohort.
Odds ratio for SGA was higher for VPA versus PHT (7.25; 95% CI: 1.30–40.28), VPA versus LTG (4.09; 95% CI: 1.11–14.96), and CBZ versus PHT (5.58; 95% CI: 1.06–29.34).
AED: Antiepileptic drug; CBZ: Carbamazepine; LTG: Lamotrigine; PHT: Phenytoin; SGA: Small for gestational age; VPA: Valproic acid.
Reprinted with permission from [65].
Further studies have also shown that the off-springs of WWE are at increased risk of preterm birth, LBW and SGA, independent of AED intake, especially in association with seizure frequency [67]. Fujii et al., in a recent prospective study on gabapentin exposed pregnancies, showed a higher rate of preterm births (p = 0.019) and low birth weight (p = 0.033) in the exposed group. Increased risk of neonatal intensive care unit observation/treatment was also noted in exposed group (38%) compared with 2.9% in the comparison group (p < 0.001). But these results were among patients who were on gabapentin for pain and psychiatric reasons and only a small proportion of them had epilepsy (34%) [54].
Neurodevelopmental outcome
A variety of behavioral and cognitive disabilities and developmental delays are reported in varying frequencies in children of WWE. The incidence and severity of these complications is determined by various factors like seizure frequency (>5 convulsive seizures [68]), other malformations, decreased maternal education, impaired maternal-child relations and the use of specific AEDs and polytherapy combinations [69,70]. Earlier studies investigating cognitive outcome in children of WWE report an increased risk of mental deficiency and lower IQ scores, affecting 1.4–6% of children of WWE, compared with 1% of controls. In comparison to children of WWE not taking AEDs, the odds ratios for additional educational needs were 1.49 for all children exposed to AEDs in utero and 3.4 for children exposed to VPA monotherapy [71]. Verbal scores on neuropsychometric measures may be selectively more involved [72] especially in patients on VPA and in certain polytherapy exposures.
Summary of 2009 AAN/AES Practice Parameter Update findings on Neurodevelopmental Outcomes [16]:
Cognition is probably not reduced in children of untreated WWE;
CBZ probably does not increase poor cognitive outcomes compared with unexposed controls;
Monotherapy exposure to VPA probably reduces cognitive outcomes. Monotherapy exposure to PHT or PB possibly reduces c ognitive outcomes;
AED polytherapy exposure probably reduces cognitive outcomes as compared with AED monotherapy.
A population-based, prospective Norwegian Mother and Child Cohort Study in 2009 showed that the children exposed in utero to AEDs had impairments in their fine motor skills and social skills at 6 months [63]. In addition, normal development was noted in children of WWE who did not use AEDs and children of fathers with epilepsy. But the limitations of this study include small sample size with very small individual AED groups, lack of use of diagnostic testing and criteria and lack of screening for intellectual disabilities in the children or even in the mothers. Thus other studies are needed to confirm these findings.
Since 2009 further multicenter prospective studies provided additional information on in utero exposure of AEDs and cognitive outcomes. The NEAD study was a prospective, observational, multicenter study across 25 centers in the USA and parts of UK [43]. They reported cognitive outcomes on 309 mother/child pairs exposed to AED monotherapy: CBZ, LTG, PHT and VPA. The primary outcome was IQ at 6 years, adjusted for maternal IQ, AED type, AED standardized dose, gestational age and use of periconceptional folate. Results indicated that the VPA group had statistically significant lower IQ scores compared with those taking the other three AEDs at 3 and 6 years follow-up, with a more prominent decline in verbal scores (Table 6). The effects on IQ along with verbal ability, nonverbal ability, memory and executive function are also observed to be dose dependent, although no particular dose of VPA could be deemed as a safe range. Use of periconceptional folate along with any AED interestingly showed better mean IQ scores within the same AED group, thus re-emphasizing the early s upplementation of folic acid.
Table 6.
Antiepileptic drug exposure and neurocognitive outcome at 6 years of age in 311 children.
| AED | Children (n) | Mean IQ (verbal worse than nonverbal) |
|---|---|---|
| Valproic acid | 62 | 97 |
| Carbamazepine | 93 | 105 |
| Lamotrigine | 100 | 108 |
| Phenytoin | 56 | 108 |
AED: Antiepileptic drug. Data taken from [60].
Another recent report from Liverpool and Manchester Neurodevelopment Group showed a similarly increased risk of neurodevelopmental disorders on VPA as monotherapy and poly-therapy compared with controls [73]. Autistic spectrum disorder was more frequent in VPA exposures compared with carbamazepine or lamotrigine exposed children. A population-based study from Denmark also demonstrated significantly increased risk of autism spectrum disorder and childhood autism in the offspring, even after adjusting for maternal epilepsy (4.15% in VPA exposed vs 2.95% unexposed group) [74].
With the increasing use of levetiracetam, an initial observational study (2011) from Liverpool and Manchester Neurodevelopment Group and the United Kingdom Epilepsy and Pregnancy Registry reported favorable early cognitive and overall developmental outcomes of children <24 months of age compared with controls [75]. More recent studies from the same group reported neurodevelopmental outcomes of children at 36–54 months of age, who were exposed in utero to LEV compared with control children and children exposed in utero to VPA. After adjusting for confounding variables and employing standard mental and language development scales, children exposed to VPA scored lower on measures of gross motor skills, comprehension language abilities and expressive language abilities compared with children exposed in utero to LEV. Children exposed to LEV in utero did not differ from the unexposed control children and there was no detectable dose effect [76].
Conclusion
Knowledge about various anti AEDs and their complexinteractions especially during pregnancy with changes in sex hormones, help the healthcare professionals, guide patients to make informed treatment decisions with adequate prepregnancy planning. Increasing fund of knowledge on safety, efficacy and tolerability of AEDs during pregnancy and breast feeding, helps to provide optimal treatments for better seizure control and improved pregnancy outcomes for the mother and the child (Box 3).
Box 3. Management of the reproductive-age woman with epilepsy.
Preconception
Adequate seizure control
Determine need for AED
If AED needed, determine if correct AED
Discontinue VPA, if possible
Folic acid and prenatal vitamin supplementation
Baseline individualized therapeutic AED serum levels
Pregnancy
AED serum levels every month
Emphasize medication compliance with necessary dose adjustments
Fetal anatomy ultrasound scans
Discuss peripartum and postpartum issues
- Peripartum management:
- Continue medications
- Lorazepam as needed for acute seizure control
Postpartum
Lower AED in first few weeks to months postpartum, depending on AED
Encourage breast feeding
Sleep hygiene, discuss about help from family
Counseling about depression especially if there is a previous history
AED levels at 6–12 weeks postpartum
Safety with the newborn
Postpartum contraception
AED: Antiepileptic drug; VPA: Valproic acid.
Future perspective
The breadth and depth of data have grown exponentially over the past two decades about pregnancy outcomes in women with epilepsy, mostly procured from large AED pregnancy registries scattered around the world and from more refined, smaller prospective, observational studies of the neurodevelopment of their children. However, consistent and conclusive findings are available for only a small number of AEDs. Continued research is needed to obtain detailed data about the other AEDs, as well as the many polytherapy combinations that are commonly used in practice. Additionally, more refined knowledge is needed regarding the factors that contribute to changes in seizure control during pregnancy and postpartum, type and relative risks of obstetrical complications, type and relative risks of neonatal complications and the neurodevelopmental consequences of all of these occurrences that can affect fetal and neonatal brain development. The elements that contribute to all of these risks need to be evaluated in order to discover modifiable factors that can lower these risks, and these likely include genetic, pharmacokinetic/pharmacodynamic, neuroendocrine, stress and sleep factors, among others. The information available about the management of women with epilepsy during the child-bearing years has increased dramatically, allowing the clinician to make evidence-based decisions, but these resources should continue to grow along with research in this important area of study. The ultimate goal is to reduce the maternal and child risks to a level as close as p ossible to that of the general population.
EXECUTIVE SUMMARY.
Counsel patients and families regarding the importance of planned pregnancy and suggest appropriate contraception method until then.
Because many pregnancies are unplanned, use of supplemental folic acid is recommended during child-bearing years, regardless of the antiepileptic drug (AED) choice and current stated family planning.
Consultation with a specialist should be encouraged when planning pregnancy, for helping the family make informed decisions.
Avoid use of valproic acid in reproductive years, unless all other AEDs have failed. Many studies from various parts of the world have consistently demonstrated increased rates of structural teratogenicity and impaired neurodevelopmental outcomes, including autism.
- Sufficient data are now available for some AEDs, demonstrating a relatively modest increase in the risk of major congenital malformations (MCMs) compared with the general population:
- Lamotrigine and levetiracetam have abundant evidence on relative safety during pregnancy with low MCM rates, as well as favorable neurocognitive and behavioral profiles (up to 6 years of age for lamotrigine and 3 years of age for levetiracetam).
AEDs without reproductive safety information should be avoided when possible in women with epilepsy during pregnancy, unless they are the only medications that provided seizure control for that individual.
Maintaining adequate seizure control during pregnancy is extremely important for improved maternal and fetal outcomes.
With the knowledge of preconceptional therapeutic drug levels, the benefits of obtaining frequent AED levels throughout pregnancy (therapeutic drug monitoring) outweigh the risks for most AEDs. In addition, these risk profiles may change as the new data come out.
Monontherapy may be better than polytherapy, or at least certain combinations, with regard to risk for MCMs as well as risk for neurodevelopmental effects. These risks may also be dose-dependent for some AEDs.
Breast feeding should be presented as a favorable option for women on antiepileptic drugs.
Financial & competing interests disclosure
PB Pennell has received salary support for research from the Epilepsy Foundation, the Epilepsy Therapy Project, and the NIH. This manuscript in part was supported by funding NIH (NINDS and NICHD) 2U01NS038455 (PBP). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:• of interest; •• of considerable interest
- 1.Kaplan PW, Norwitz ER, Ben-Menachem E et al. Obstetric risks for women with epilepsy during pregnancy. Epilepsy Behav. 11(3), 283–291 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Battino D, Tomson T, Bonizzoni E et al. Seizure control and treatment changes in pregnancy: observations from the EURAP epilepsy pregnancy registry. Epilepsia 54(9), 1621–1627 (2013). [DOI] [PubMed] [Google Scholar]
- 3.EURAP Study Group. Seizure control and treatment in pregnancy observations from the EURAP epilepsy pregnancy registry. Neurology 66(3), 354–360 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Reddy DS, Rogawski MA. Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics 6(2), 392–401 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenfeld WE, Doose DR, Walker SA, Nayak RK. Effect of topiramate on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in patients with epilepsy. Epilepsia 38, 317–323 (1997). [DOI] [PubMed] [Google Scholar]
- 6.Sidhu J, Job S, Singh S, Philipson R. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br. J. Clin. Pharmacol 61(2), 191–199 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herzog AG, Blum AS, Farina EL et al. Valproate and lamotrigine level variation with menstrual cycle phase and oral contraceptive use. Neurology 72(10), 911–914 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Pennell PB. Hormones, seizures, and lamotrigine: oh, my! Epilepsy Curr. 8(1), 8–10 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaffield ME, Culwell KR, Lee CR. The use of hormonal contraception among women taking anticonvulsant therapy. Contraception 83(1), 16–29 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Faculty of Sexual and Reproductive Health Care. http://www.fsrh.org
- 11.Harden CL, Pennell PB, Koppel BS et al. Practice parameter update: management issues for women with epilepsy – focus on pregnancy (an evidence-based review): vitamin K, folic acid, blood levels, and breastfeeding: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 73(2), 142–149 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Rigorous evidence-based review.
- 12.Wlodarczyk BJ, Palacios AM, George TM, Finnell RH. Antiepileptic drugs and pregnancy outcomes. Am. J. Med. Genet . A 158A(8), 2071–2090 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrow JI, Hunt SJ, Russell AJ et al. Folic acid use and major congenital malformations in offspring of women with epilepsy: a prospective study from the UK Epilepsy and Pregnancy Register. J. Neurol. Neurosurg. Psychiatry 80(5), 506–511 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Wilson RD, Davies G, Desilets V et al. The use of folic acid for the prevention of neural tube defects and other congenital anomalies. J. Obstet. Gynaecol. Can 25(11), 959–973 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Pennell PB. EURAP outcomes for seizure control during pregnancy: useful and encouraging data. Epilepsy Curr. 6(6), 186–188 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harden CL, Hopp J, Ting TY et al. Practice parameter update: management issues for women with epilepsy – focus on pregnancy (an evidence-based review): obstetrical complications and change in seizure frequency: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 73(2), 126–132 (2009).•• Rigorous evidence based review.
- 17.Pennell PB, Peng L, Newport DJ et al. Lamotrigine in pregnancy: clearance, therapeutic drug monitoring, and seizure frequency. Neurology 70(22 Pt 2), 2130–2136 (2008).• Class I evidence for therapeutic drug monitoring for lamotrigine during pregnancy.
- 18.Reisinger TL, Newman M, Loring DW, Pennell PB, Meador KJ. Antiepileptic drug clearance and seizure frequency during pregnancy in women with epilepsy. Epilepsy Behav. 29(1), 13–18 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas SV, Syam U, Devi JS. Predictors of seizures during pregnancy in women with epilepsy. Epilepsia 53(5), e85–88 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Hiilesmaa VK, Bardy A, Teramo K. Obstetric outcome in women with epilepsy. Am. J. Obstet. Gynecol 152(5), 499–504 (1985). [DOI] [PubMed] [Google Scholar]
- 21.Sahoo S, Klein P. Maternal complex partial seizure associated with fetal distress. Arch. Neurol 62(8), 1304–1305 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Nei M, Daly S, Liporace J. A maternal complex partial seizure in labor can affect fetal heart rate. Neurology 51(3), 904–906 (1998). [DOI] [PubMed] [Google Scholar]
- 23.Johannessen Landmark C, Johannessen SI, Tomson T. Host factors affecting antiepileptic drug delivery-pharmacokinetic variability. Adv. Drug Deliv. Rev 64(10), 896–910 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Petrenaite V, Sabers A, Hansen-Schwartz J. Seizure deterioration in women treated with oxcarbazepine during pregnancy. Epilepsy Res. 84(2–3), 245–249 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Ohman I, Sabers A, De Flon P, Luef G, Tomson T. Pharmacokinetics of topiramate during pregnancy. Epilepsy Res. 87(2–3), 124–129 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Westin AA, Nakken KO, Johannessen SI, Reimers A, Lillestolen KM, Brodtkorb E. Serum concentration/dose ratio of topiramate during pregnancy. Epilepsia 50(3), 480–485 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Johnson EL, Stowe ZN, Ritchie JC et al. Carbamazepine clearance and seizure stability during pregnancy. Epilepsy Behav. 33, 49–53 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polepally AR, Pennell PB, Brundage RC et al. Model-based lamotrigine clearance changes during pregnancy: clinical implication. Ann. Clin. Transl. Neurol 1(2), 99–106 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomson T, Battino D. Pharmacokinetics and therapeutic drug monitoring of newer antiepileptic drugs during pregnancy and the puerperium. Clin. Pharmacokinet 46(3), 209–219 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Tomson T, Landmark CJ, Battino D. Antiepileptic drug treatment in pregnancy: changes in drug disposition and their clinical implications. Epilepsia 54(3), 405–414 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Fraile IP, Cid AO, Juste AO, Modrego PJ. Levetiracetam plasma level monitoring during pregnancy, delivery, and postpartum: clinical and outcome implications. Epilepsy Behav. 15(3), 372–375 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Vajda FJ, O’brien T, Lander C, Graham J, Eadie M. The efficacy of the newer antiepileptic drugs in controlling seizures in pregnancy. Epilepsia 55(8), 1229–1234 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Yerby MS. Quality of life, epilepsy advances, and the evolving role of anticonvulsants in women with epilepsy. Neurology 55(5), 21–31 (2000). [PubMed] [Google Scholar]
- 34.Pennell PB, Hovinga CA. Antiepileptic drug therapy in pregnancy I: gestation-induced effects on AED pharmacokinetics. Int. Rev. Neurobiol 83, 227–240 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Kaaja E, Kaaja R, Hiilesmaa V. Major malformations in offspring of women with epilepsy. Neurology 60(4), 575–579 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Tomson T, Battino D, Bonizzoni E et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol. 10(7), 609–617 (2011).•• Dose dependent MCM risk is discussed among 4 commonly prescribed AEDs.
- 37.Honein MA, Paulozzi LJ, Cragan JD, Correa A. Evaluation of selected characteristics of pregnancy drug registries. Teratology 60, 356–364 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Fried S, Kozer E, Nulman I, Einarson TR, Koren G. Malformation rates in children of women with untreated epilepsy: a meta-analysis. Drug Saf. 27(3), 197–202 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Moore KL. The Developing Human: Clinically Oriented Embryology. WB Saunders, PA, USA: (1988). [Google Scholar]
- 40.Meador KJ, Pennell PB, Harden CL et al. Pregnancy registries in epilepsy: a consensus statement on health outcomes. Neurology 71(14), 1109–1117 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Pennell PB. Antiepileptic drugs during pregnancy: what is known and which AEDs seem to be safest? Epilepsia 49(Suppl. 9), 43–55 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmes LB, Mittendorf R, Shen A, Smith CR, Hernandez-Diaz S. Fetal effects of anticonvulsant polytherapies: different risks from different drug combinations. Arch. Neurol 68(10), 1275–1281 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Harden CL, Meador KJ, Pennell PB et al. Practice parameter update: management issues for women with epilepsy – focus on pregnancy (an evidence-based review): teratogenesis and perinatal outcomes: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 73(2), 133–141 (2009).•• Rigorous evidence-based review.
- 44.Tomson T, Battino D. Teratogenic effects of antiepileptic drugs. Lancet Neurol. 11(9), 803–813 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Hernandez-Diaz S, Smith CR, Shen A et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology 78(21), 1692–1699 (2012).•• Provides data regarding relative risk for MCMs among all AED monotherapies.
- 46.Werler MM, Ahrens KA, Bosco JL et al. Use of antiepileptic medications in pregnancy in relation to risks of birth defects. Ann. Epidemiol 21(11), 842–850 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jentink J, Loane MA, Dolk H et al. Valproic acid monotherapy in pregnancy and major congenital malformations. N. Engl. J. Med 362(23), 2185–2193 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Pennell PB. Too complicated or so simple: AED type and AED dose matter for pregnancy. Epilepsy Curr. 12(2), 63–65 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cunnington MC, Weil JG, Messenheimer JA, Ferber S, Yerby M, Tennis P. Final results from 18 years of the international lamotrigine pregnancy registry. Neurology 76(21), 1817–1823 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Hunt S, Russell A, Smithson WH et al. Topiramate in pregnancy: preliminary experience from the UK Epilepsy and Pregnancy Register. Neurology 71(4), 272–276 (2008). [DOI] [PubMed] [Google Scholar]
- 51.Margulis AV, Mitchell AA, Gilboa SM et al. Use of topiramate in pregnancy and risk of oral clefts. Am. J. Obstet. Gynecol 207(5), 405 e401–e407 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mawhinney E, Craig J, Morrow J et al. Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology 80(4), 400–405 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Molgaard-Nielsen D, Hviid A. Newer-generation antiepileptic drugs and the risk of major birth defects. JAMA 305(19), 1996–2002 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Fujii H, Goel A, Bernard N et al. Pregnancy outcomes following gabapentin use: results of a prospective comparative cohort study. Neurology 80(17), 1565–1570 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borthen I, Eide MG, Veiby G, Daltveit AK, Gilhus NE. Complications during pregnancy in women with epilepsy: population-based cohort study. BJOG 116(13), 1736–1742 (2009). [DOI] [PubMed] [Google Scholar]
- 56.Mawer G, Briggs M, Baker GA et al. Pregnancy with epilepsy: obstetric and neonatal outcome of a controlled study. Seizure 19(2), 112–119 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katz JM, Devinsky O. Primary generalized epilepsy: a risk factor for seizures in labor and delivery? Seizure 12(4), 217–219 (2003). [DOI] [PubMed] [Google Scholar]
- 58.ACOG educational bulletin. Seizure disorders in pregnancy. Number 231, December 1996. Committee on Educational Bulletins of the American College of Obstetricians and Gynecologists. Int. J. Gynaecol. Obstet 56(3), 279–286 (1997). [PubMed] [Google Scholar]
- 59.Davanzo R, Dal Bo S, Bua J, Copertino M, Zanelli E, Matarazzo L. Antiepileptic drugs and breastfeeding. Ital. J. Pediatr 39, 50(2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meador KJ, Baker GA, Browning N et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 12(3), 244–252 (2013).•• Well designed prospective study accounting for key co-variants demonstrating poor cognitive outcomes related to VPA use and the benefit of folic acid supplementation in periconceptional period.
- 61.Hovinga CA, Pennell PB. Antiepileptic drug therapy in pregnancy II: fetal and neonatal exposure. Int. Rev. Neurobiol 83, 241–258 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Meador KJ, Baker GA, Browning N et al. Breastfeeding in children of women taking antiepileptic drugs: cognitive outcomes at age 6 years. JAMA Pediatr. 168(8), 729–736 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Veiby G, Daltveit AK, Engelsen BA, Gilhus NE. Pregnancy, delivery, and outcome for the child in maternal epilepsy. Epilepsia 50(9), 2130–2139 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Pennell PB. Pregnancy in women who have epilepsy. Neurol. Clin 22(4), 799–820 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Pennell PB, Klein AM, Browning N et al. Differential effects of antiepileptic drugs on neonatal outcomes. Epilepsy Behav. 24(4), 449–456 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hernandez-Diaz S, Mittendorf R, Smith CR, Hauser WA, Yerby M, Holmes LB. Association between topiramate and zonisamide use during pregnancy and low birth weight. Obstet. Gynecol. 123(1), 21–28 (2014).• In view of wide spectrum of usage of topiramate in epilepsy, migraine and other off label reasons, this article highlights the risks of MCM and SGA.
- 67.Chen YH, Chiou HY, Lin HC, Lin HL. Affect of seizures during gestation on pregnancy outcomes in women with epilepsy. Arch. Neurol 66(8), 979–984 (2009). [DOI] [PubMed] [Google Scholar]
- 68.Adab N, Kini U, Vinten J et al. The longer term outcome of children born to mothers with epilepsy. J. Neurol. Neurosurg. Psychiatry 75(11), 1575–1583 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adab N, Tudur SC, Vinten J, Williamson P, Winterbottom J. Common antiepileptic drugs in pregnancy in women with epilepsy . Cochrane Database Syst. Rev 3, CD004848(2004). [DOI] [PubMed] [Google Scholar]
- 70.Meador KJ. Neurodevelopmental effects of antiepileptic drugs. Curr. Neurol Neurosci. Rep 2(4), 373–378 (2002). [DOI] [PubMed] [Google Scholar]
- 71.Adab N, Jacoby A, Smith D, Chadwick D. Additional educational needs in children born to mothers with epilepsy. J. Neurol. Neurosurg. Psychiatry 70, 15–21 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meador KJ, Zupanc ML. Neurodevelopmental outcomes of children born to mothers with epilepsy. Cleve. Clin. J. Med 71(Suppl. 2), S38–S40 (2004). [DOI] [PubMed] [Google Scholar]
- 73.Bromley RL, Mawer GE, Briggs M et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J. Neurol. Neurosurg. Psychiatry 84(6), 637–643 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Christensen J, Gronborg TK, Sorensen MJ et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 309(16), 1696–1703 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shallcross R, Bromley RL, Irwin B, Bonnett LJ, Morrow J, Baker GA. Child development following in utero exposure: levetiracetam vs sodium valproate. Neurology 76(4), 383–389 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shallcross R, Bromley RL, Cheyne CP et al. In utero exposure to levetiracetam vs valproate: development and language at 3 years of age. Neurology 82(3), 213–221 (2014). [DOI] [PubMed] [Google Scholar]