Abstract
Objectives
Recent interest has been generated about reports of declining incidence in cognitive impairment among more recently born cohorts. At the same time, attained education, which is related to cognition, has increased in recent cohorts of older adults. We examined cohort differences in cognitive function in a nationally representative sample of Americans aged 25 and older followed for 25 years (1986–2011) and considered the extent to which cohort differences in education account for differences.
Method
Data come from the Americans’ Changing Lives Study (N = 3,617). Multiple cohort latent growth models model trajectories of cognition (errors on the Short Portable Mental Status Questionnaire) across four 15-year birth cohorts. Demographic factors, educational attainment, and time-varying health conditions were covariates.
Results
Significant cohort differences were found in the mean number of cognitive errors (e.g., 0.26 more errors at age 65 in cohort born pre-1932 vs cohort born 1947–1961, p < .001). Although demographic and health conditions were associated with level and rate of change in cognitive dysfunction, education solely accounted for cohort differences.
Discussion
Compression of cognitive morbidity is seen among the highly educated, and increasing educational opportunities may be an important strategy for decreasing the risk for cognitive impairment in later life.
Keywords: Cognition, cognitive impairment, cohort differences, education
As life expectancy improves and the global population ages, the number of individuals with dementia and cognitive impairment will rise. Determining individual and population level interventions for cognitive functioning will become increasingly important. In considering cognitive aging, researchers have long been interested in piecing out ontogeny and normative age-related change, from cohort differences due to historical, societal, or cultural impacts. Cohort effects refer to a variety of different factors which may vary among groups given shared historical or societal/cultural experiences. For example, differences in cognition among age groups may be due to advances in education experienced by more recent birth cohorts but not older ones (Salthouse, 2009). A better understanding of cohort differences in cognitive aging may, therefore, have implications for societal changes that could lead to better cognitive functioning, increased productivity, and successful aging (Zelinski & Kennison, 2007).
Cohort Differences in Mean Level and Rate of Change in Cognition
A growing number of recent studies have suggested that the risk for cognitive decline and dementia have decreased among adults born in the second half of the 20th century (Langa et al., 2008a; Schrijvers et al., 2012). The Rotterdam Study remarkably found that a more recent-born cohort had significantly larger brain volumes and less cerebral small vessel disease than an earlier-born cohort (Schrijvers et al., 2012), whereas the Medical Research Council Cognitive Function and Ageing Study found dementia prevalence to be 1.8% lower than expected among a later-born cohort. When examining the mean level of performance on cognitive abilities, later-born cohorts have been found to perform better on cognitive screening tests across the life span relative to earlier-born cohorts. For example, studies dividing their sample into several 20th-century birth cohorts tend to find that later-born cohorts have significantly better average performance in verbal, memory, and spatial abilities, and even perform equivalently to those 9 to 15 years younger on tests of reason, list recall, space, and text recall (Bowles, Grimm, & McArdle, 2005; Finkel, Reynolds, McArdle, & Pedersen, 2007; Schaie, Willis, & Pennak, 2005; Zelinski & Kennison, 2007). (note. All studies cited included samples that were population based however Finkel et al., 2007 contained twin pairs.) On the other hand, it is important to note that some researchers have found declining scores on cognitive tests, particularly verbal ability, among more recent-born cohorts relative to earlier-born cohorts (Alwin, 2009; Alwin, McCammon, Wray, & Rodgers, 2008; Schaie, 2008).
Corresponding to cohort differences in cognition, Salthouse (2006) defines concepts of preserved differentiation and differential preservation. Preserved differentiation would occur whereby cohorts differ in level of performance, but these differences are stable across the life course such that cohorts would have parallel trajectories. Differential preservation occurs as cohort differences vary by level and rate of change. It may be that the factor, or cohort effect, that influences one’s level of performance also affects whether or not that performance is maintained over time. Thus, cognitive function might be preserved better in one cohort relative to another. A study that considers both mean level and rate of change in cognition is important to disentangle the effects of cohort from the effects of maturation.
Several studies have also found cohort differences in the rate of change in cognition with age. Earlier-born cohorts tend to have more rapid age-related declines in such abilities as advanced vocabulary performance, reasoning and verbal meaning, and other primary mental abilities (Schaie et al., 2005). Some distinctions and limitations of the above studies may be drawn, however. First, most of the above studies defined cohorts very broadly, usually defining two. When only a couple of broad cohorts are considered, within-cohort variability may be masked. In addition, further research is needed to determine what factors may be explaining these cohort differences so that appropriate social-cultural or health-related interventions may be forged to improve the cognitive health of future generations.
Cohort Effects: Can Differences Be Explained?
Finding cohort differences in cognitive function suggests that brain development and function may be malleable and responsive to historical changes in social and medical resources. Longitudinal research suggests that the gains in cognitive function among more recent birth cohorts may be due to their greater levels of education and wealth (Brayne et al., 2010; Langa et al., 2008a; Schrijvers et al., 2012). For instance, education and wealth explained 40% of the difference in cognitive impairment prevalence between two birth cohorts in the U.S. Health and Retirement Study (Langa et al., 2008a) potentially due to increases in “cognitive reserve” that result from education’s beneficial impact on the development of neural circuits, which are better able to compensate for age-related inflammatory and vascular changes (Brayne et al., 2010; Ronnlund, Nyberg, Backman, & Nilsson, 2005; Scarmeas & Stern, 2004). Differences in education by cohort may enhance cognitive reserve (Ronnlund et al., 2005; Scarmeas & Stern, 2004) underlying the cohort differences seen in cognitive functioning.
Educational attainment has increased dramatically in the United States over the last century (Alwin & McCammon, 2001; Ronnlund et al., 2005), and educational systems have qualitatively improved (Schaie, 2008). Other changes may also affect education including better access to schooling, increased education and engagement in parents, small family size, and changes in visual modalities (computer, film, television, etc.; Zelinski & Kennison, 2007). Ultimately, advancement in education is believed to partially delay onset of dementia and compresses cognitive morbidity toward the end of life (Brayne et al., 2010).
Yet, as educational systems have changed over the last century, so have gender and racial trends in educational attainment. Thus, as racial and gender disparities exist in cognitive function (Diaz-Venegas, Downer, Langa, & Wong, 2016), adjusting for changes in education is key. In the second half of the 20th century, for example, college attendance and completion rates grew rapidly among women, surpassing men (Flashman, 2013; Peter & Horn, 2005). Educational disparities have also been declining among ethnic and racial minorities (Kao & Thompson, 2003). Therefore, separating out the effect of education from gender and race is important.
At the same time, population-level changes in vascular risk factors and treatments, such as the decline in smoking rates and increased use of antihypertensive and lipid-lowering medications, may be contributing to better cognitive performance in more recent-born cohorts (Langa & Larson, 2014; Schrijvers et al., 2012; Verhaegen, Borchelt, & Smith, 2003). Although rates of cardiovascular diseases have been declining over the past 30 years (Setoguchi et al., 2008), increases in obesity, diabetes, and stroke survival may reverse this positive trend of better cognitive functioning in more recent birth cohorts (Langa & Larson, 2014; Mirowsky, 2011).
We used 25 years of data from the nationally representative Americans’ Changing Lives (ACL) Study to test for cohort differences in cognitive dysfunction across four 15-year birth cohorts covering the entire adult life course. We expand upon prior work that considers only broad age cohorts, which may mask important within-cohort variability. We first examine if and where there are cohort group differences in level and rate of change in cognitive function over adulthood. We next consider the extent to which previously proposed explanations for cohort differences (education, gender, race, circulatory diseases, diabetes, and obesity) account for any cohort group differences seen. Having a better understanding of changes in population level factors as they explain cohort group differences in cognitive dysfunction will foster a better understanding of the societal and cultural interventions that may be most effective in prolonging successful cognitive aging.
Method
Data
The ACL Study (House, Kessler, & Herzog, 1990; House, Lantz, & Herd, 2005) is a cohort longitudinal study based on a stratified, multistage area probability sample of noninstitutionalized adults aged 25 and older, living in the coterminous United States, and followed over a 25-year period. African Americans and adults aged 60 and older each were oversampled. The first wave of the survey was conducted in-person in 1986 with 3,617 adults (68% sample response rate for individuals). Surviving respondents were re-interviewed by telephone in 1989 (N = 2,867, 83% of survivors), in 1994 (N = 2,562, 83% of survivors), in 2001/2002 (N = 1,787, 74% of survivors), and again in 2011/2012 (N = 1,427, 81% of survivors).
Of the 3,617 total respondents, 1,071 (30%) completed all five waves, whereas 1,361 (38%) completed all possible waves prior to their death. Of those who dropped out (N = 654, 18%), 264 (7%) completed only the first wave, 128 (3%) the first and second wave, 159 (5%) the first through third wave, and 103 (3%) all but the fifth wave. The remaining 531 (15%) had intermittent patterns of response. Nonresponse weights were computed based on the probability of response at a given wave, considering predictors of response including, gender, age, education, race/ethnicity, and baseline measures of marital status, geographic region of residence, income, employment status, measures of social integration, depression, health status, extroversion, self-efficacy, and other factors, and multiplied together to give the nonresponse weights used in the analysis. These sampling weights for nonresponse, coupled with a poststratification adjustment to the 1986 Census estimates of the U.S. population aged 25 years and older, make the ACL sample representative of the age, gender, and race distribution of the U.S. population living in the United States in 1986, and except for differences due to post-1986 immigration and out-migration, representative of this cohort as they aged over this 25-year period (House et al., 2005). The ACL Study has been reviewed and approved annually by the University of Michigan’s Institutional Review Board.
Measures
Cognitive dysfunction was measured using an abbreviated version of the Short Portable Mental Status Questionnaire (SPMSQ), which has been shown to be a valid and reliable instrument for differentiating between cognitively intact individuals and those with some degree of impairment and to have acceptable sensitivity (92.3–100) and specificity (83.5–100) for cognitive screening (Lin et al., 2013; Pfeiffer, 1975). Items were assessed at each wave and included two orientation items for recall of the current day and date (day of the week, day, month, and year), the names of the current and past president, and a serial 3 subtraction test. For the serial subtraction test, respondents were asked to subtract the number 3 from 20 for a total of six subtractions. We created an indicator of cognitive dysfunction by summing the number of errors to all five items (where each incorrect subtraction in the serial 3 subtraction test was counted as an error), generating an index from 0 to 10, with a higher score indicating greater cognitive dysfunction. The SPMSQ was only given to nonproxy respondents (Wave 3 had 164 proxies, Wave 4 had 95, and Wave 5 had 108). The reliability of the measure at each wave, calculated by subtracting the error variance from the observed variance based on the unconditional quadratic growth model and expressed as a proportion of the total observed variance, ranges from 0.76 to 0.98.
Four 15-year birth cohort groups were based on age at study entry (1986): cohort 1 = over 70 (born before 1917); cohort 2 = age 55–69 (born 1917–1931); cohort 3 = age 40–54 (born 1932–1946); cohort 4 = age 25–39 (born 1947–1961). (note. A 10-year six cohort group model was also tested providing a similar pattern of results, thus for parsimony the four cohort group model is presented.) Other covariates included attained education (categorized as less than high school, high school diploma but not college degree, and college degree or higher), gender, and racial/ethnic minority. We included time-varying indicators of medically diagnosed diabetes and cardiovascular diseases (heart disease, hypertension, and stroke) self-reported by the respondent in the past 12 months. We also accounted for time-varying obesity based on a body mass index (BMI = weight in kilograms/[height in meters]2) of 30 or greater.
Statistical Analyses
We used a multiple cohort latent growth model to analyze trajectories of cognitive dysfunction by cohort groups over time (Muthen & Muthen, 2000). This approach extends beyond the examination of differences in mean levels of cognitive dysfunction by considering cohort differences in trajectories of cognitive dysfunction. Since the dependent variable was a count of the number of errors in the cognitive tests, we used a negative binomial regression model, which accounts for overdispersion in the count outcome. We used an accelerated longitudinal design within the growth curve framework by using age (not year of observation) as the indicator of time, creating a synthetic cohort from ages 25 through 100 (Raudenbush & Chan, 1992). This is an age-based model such that age and age-squared are used as the loadings for the slope and quadratic terms, respectively. To facilitate parameter interpretation, age was centered at the midpoint of adulthood (setting age 55 to 0).
We estimated models in a sequential fashion by first constraining all cohort-specific growth parameters to be equal and specifying the shape of the growth. Subsequent models tested for differences in intercepts and slopes across the different birth cohorts by successively freeing growth parameters. Covariates were then sequentially introduced to the model to explain any cohort group differences in trajectories. Nested models were compared according to three goodness-of-fit indices: (a) change in the log likelihood (adjusted by a scaling correction factor for nonnormal outcomes), which follows a chi-square distribution with degrees of freedom (df) equal to the difference in the number of parameters tested between models; (b) change in the Akaike Information Criterion (AIC); and (c) change in the Bayesian Information Criterion (BIC), which makes an adjustment for model parsimony (Raftery, 1995). All models were estimated in Mplus Version 7.4 using full information maximum likelihood (FIML). An advantage of this modeling approach is that it allows for respondents with as little as one observation to enter the model. Additionally, by including variables related to attrition (age, education, health conditions), maximum likelihood produces unbiased coefficients under the assumption that the attrition process is conditional on observed variables in our models. We found little difference between respondents and nonrespondents on observed variables, suggesting that a missing completely at random assumption (MCAR) is reasonable for these data. This is consistent with other work examining the MCAR assumption in the first three waves of the ACL data, which found support for the hypothesis that the data in ACL are missing completely at random (Alwin, 2007). All analyses were weighted, with robust standard errors to account for the sampling design.
Results
Table 1 shows the characteristics of the sample at baseline (1986), stratified by birth cohort group. Educational attainment increased monotonically across the birth cohorts, with more recent cohorts much more likely to finish high school or college. Individuals in the more recent cohort groups were also more likely to be male and from a minority race/ethnicity compared with those from the earlier-born cohorts.
Table 1.
Descriptive Statistics (Weighted Percent or Mean [± SD]) for Study Sample Characteristics by Birth Cohort Group, Americans’ Changing Lives Study (1986–2011)
| Full sample baseline (N = 3,617) | 1916 or earlier (N = 774) | 1917–1931 (N = 1,122) | 1932–1946 (N = 648) | 1947–1961 (N = 1,073) | |
|---|---|---|---|---|---|
| Gender (%) | |||||
| Female | 52.9 | 61.4 | 54.5 | 53.0 | 49.6 |
| Male | 47.1 | 38.6 | 45.5 | 47.0 | 50.4 |
| Race/ethnicity (%) | |||||
| Minority | 20.8 | 12.6 | 15.6 | 22.7 | 24.6 |
| White | 79.2 | 87.4 | 84.4 | 77.3 | 75.4 |
| Education (%) | |||||
| Less than high school | 25.6 | 54.0 | 38.9 | 22.3 | 12.5 |
| High school diploma | 54.7 | 37.9 | 50.4 | 58.1 | 59.7 |
| College degree | 19.7 | 8.1 | 10.6 | 19.5 | 27.8 |
| Number of errors on cognitive tests (range 0–10) (mean, SD) | |||||
| Baseline/Wave 1 (1986) | 0.9 (1.5) | 1.5 (1.6) | 1.1 (1.4) | 0.9 (1.6) | 0.7 (1.4) |
| Wave 2 (1989) | 0.5 (1.4) | 1.2 (1.6) | 0.7 (1.3) | 0.5 (1.5) | 0.4 (1.2) |
| Wave 3 (1994) | 0.9 (1.7) | 1.8 (1.8) | 1.1 (1.5) | 1.0 (1.9) | 0.7 (1.6) |
| Wave 4 (2001) | 0.6 (1.5) | 1.7 (1.7) | 0.9 (1.4) | 0.7 (1.7) | 0.5 (1.4) |
| Wave 5 (2011) | 0.8 (1.5) | 0.8 (0.8) | 1.3 (1.6) | 1.0 (2.1) | 0.6 (1.5) |
| Cardiovascular diseases (%) | |||||
| Baseline/Wave 1 (1986) | 23.5 | 50.8 | 42.1 | 17.7 | 9.7 |
| Wave 2 (1989) | 24.7 | 48.7 | 44.8 | 21.4 | 11.0 |
| Wave 3 (1994) | 28.6 | 51.3 | 50.0 | 29.5 | 14.2 |
| Wave 4 (2001) | 40.1 | 56.3 | 57.4 | 48.0 | 28.5 |
| Wave 5 (2011) | 50.4 | 54.6 | 65.9 | 60.7 | 42.4 |
| Diabetes (%) | |||||
| Baseline/Wave 1 (1986) | 5.8 | 14.1 | 10.3 | 4.7 | 1.9 |
| Wave 2 (1989) | 6.3 | 12.5 | 13.5 | 4.5 | 2.2 |
| Wave 3 (1994) | 6.8 | 12.5 | 13.9 | 6.5 | 2.7 |
| Wave 4 (2001) | 10.5 | 7.9 | 18.1 | 13.9 | 6.1 |
| Wave 5 (2011) | 17.7 | 0.0 | 19.8 | 23.4 | 14.4 |
| Obesity (%) | |||||
| Baseline/Wave 1 (1986) | 14.4 | 11.5 | 18.2 | 17.1 | 11.9 |
| Wave 2 (1989) | 16.8 | 12.1 | 17.8 | 20.3 | 15.4 |
| Wave 3 (1994) | 20.4 | 12.1 | 18.4 | 27.5 | 18.5 |
| Wave 4 (2001) | 26.3 | 7.6 | 16.9 | 34.0 | 26.5 |
| Wave 5 (2011) | 30.4 | 16.0 | 12.9 | 32.7 | 32.4 |
Note: Birth cohort 1 (before 1917) sample size: (1986) = 774, (1989) = 546, (1994) = 398, (2002) = 128, (2012) = 15; birth cohort 2 (1917–1931) sample size: (1986) = 1,122, (1989) = 925, (1994) = 806, (2002) = 517, (2012) = 247; birth cohort 3 (1932–1946) sample size: (1986) = 648, (1989) = 540, (1994) = 521, (2002) = 430, (2012) = 392; birth cohort 4 (1947–1961) sample size: (1986) = 1,073, (1989) = 856, (1994) = 835, (2002) = 712, (2012) = 773.
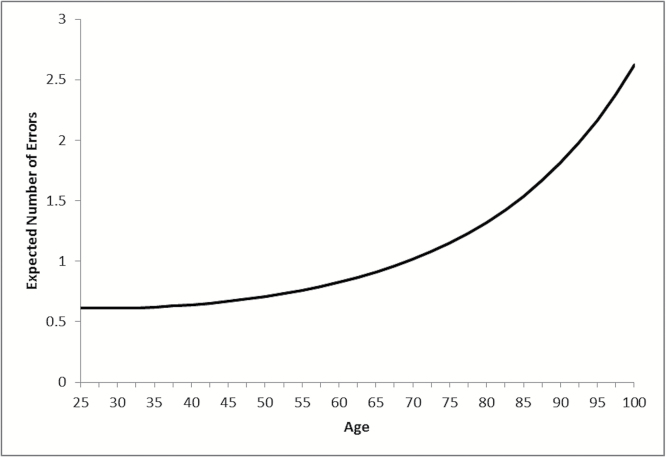
Results for the negative binomial regression growth model, constraining all intercepts and slopes (and associated variances and covariances) to be equal across birth cohorts, are illustrated in Figure 1. A quadratic model for age provided an improvement in fit over the linear model (based on the chi-square difference test, AIC and BIC, see Supplementary Table A). The expected number of cognitive errors predicted from this model reflects the population average trajectory of cognitive dysfunction over adulthood, with fewer cognitive errors in early adulthood (less than 1), but an exponential increase in errors with age, reaching close to 2 errors on average by age 90.
Figure 1.
Expected number of errors on cognitive tests over adulthood: Americans’ Changing Lives Study 1986–2011.
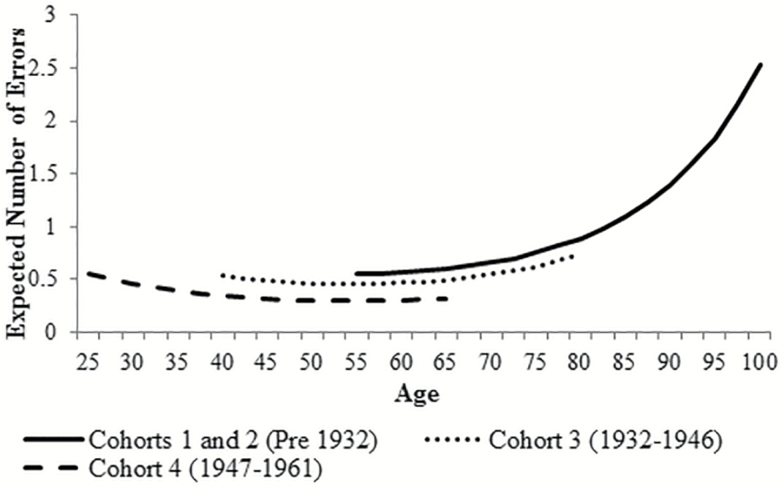
In an iterative series of models, we relaxed the equality constraints on the intercept and slopes (and cohort-specific variance components) for each cohort in turn to identify if significant differences in cognition trajectories existed across birth cohorts. We found that the best fitting model was one with equal slopes across cohorts (Model A, Table 2). Birth cohorts 1 and 2 were also found to have equal intercepts indicating no cohort differences for adults born prior to 1932. However, at any given age, significant differences in levels (intercept) of cognitive errors were found for those born 1932–1946 (cohort 3) and born 1947–1961 (cohort 4) (chi-square difference test = 29.69 [2df], p < .001) (Supplementary Table A [Models B–D]). Predicted values are shown in Figure 2.
Table 2.
Multiple Cohort Negative Binomial Growth Model: Explaining Cohort Differences in Trajectories of Cognitive Function (Number of Errors) Over Adulthood (ACL 1986–2011)
| Multiple cohort model Model A | + Education Model B | + Gender & race Model C | + CV diseases Model D | + Diabetes Model E | + Obesity Model F | |
|---|---|---|---|---|---|---|
| Intercept | ||||||
| Birth cohort 1 (pre-1917) | −0.593*** | −1.785*** | −1.831*** | −1.839*** | −1.842*** | −1.850*** |
| Birth cohort 2 (1917–1931) | −0.593*** | −1.785*** | −1.831*** | −1.839*** | −1.842*** | −1.850*** |
| Birth cohort 3 (1932–1946) | −0.781*** | −1.654*** | −1.781*** | −1.783*** | −1.783*** | −1.772*** |
| Birth cohort 4 (1947–1961) | −1.218*** | −1.876*** | −2.024*** | −2.024*** | −2.023*** | −1.990*** |
| <HS education | 1.889*** | 1.707*** | 1.703*** | 1.696*** | 1.697*** | |
| HS education | 0.796*** | 0.755*** | 0.754*** | 0.752*** | 0.751*** | |
| Female | 0.101 | 0.100 | 0.097 | 0.095 | ||
| Racial/ethnic minority | 0.654*** | 0.652*** | 0.649*** | 0.656*** | ||
| CV diseases | 0.024 | 0.006 | 0.002 | |||
| Diabetes | 0.138 | 0.130 | ||||
| Obesity | 0.191* | |||||
| Slope (age) | 0.009 | 0.059*** | 0.014 | 0.015 | 0.014 | 0.024 |
| <HS education | 0.003 | −0.009 | −0.009 | −0.011 | −0.012 | |
| HS education | −0.035 | −0.038 | −0.039 | −0.040 | −0.039 | |
| Female | 0.067* | 0.066* | 0.068* | 0.068* | ||
| Racial/ethnic minority | 0.060* | 0.059* | 0.056* | 0.058* | ||
| CV diseases | −0.001 | −0.004 | 0.001 | |||
| Diabetes | 0.040 | 0.033 | ||||
| Obesity | −0.180* | |||||
| Slope (quadratic) | 0.073*** | 0.110*** | 0.112*** | 0.112*** | 0.111*** | 0.108*** |
| <HS education | −0.075*** | −0.065** | −0.065** | −0.065** | −0.066** | |
| HS education | −0.038*** | −0.035 | −0.035 | −0.035 | −0.035 | |
| Female | −0.005 | −0.005 | −0.004 | −0.003 | ||
| Racial/ethnic minority | −0.023 | −0.023 | −0.023 | −0.025 | ||
| CV diseases | .418*** | 0.340** | 0.324** | |||
| Diabetes | 0.518*** | 0.508*** | ||||
| Obesity | 0.078 | |||||
| Fit statistics | ||||||
| BIC | 38,454.05 | 37,822.29 | 37,704.97 | 79,829.25 | 79,804.64 | 79,813.65 |
| AIC | 38,336.37 | 37,667.45 | 37,512.97 | 79,463.84 | 79,420.65 | 79,411.08 |
Note: ACL = Americans’ Changing Lives; AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion; CV = cardiovascular, HS = high school.
*p < .05. **p < .01. ***p < .001 (two-tailed tests).
Figure 2.
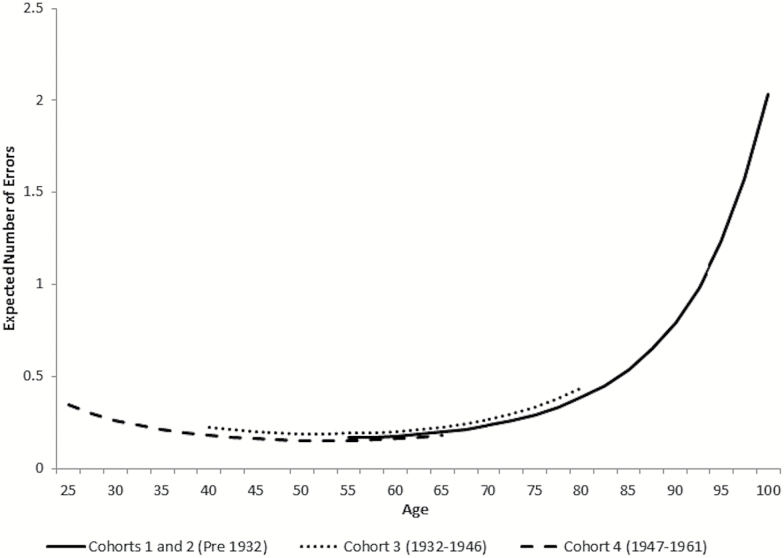
We then added covariates to the model (education, demographics, cardiovascular conditions, diabetes, and obesity) to try to explain the differences in levels of cognitive dysfunction across cohorts. Model B (Table 2) regresses the cohort-specific intercepts and slopes on education. After modeling the effects of education, the cohort-specific intercepts are almost identical and not statistically different (chi-square = 5.28 [5df], p > .05). Thus, differences in education across cohorts effectively explain the observed cohort group differences in mean levels of cognitive dysfunction across the life span. Adults with less than a college education have a significantly higher number of cognitive errors at age 55 than the college educated (intercept beta = 1.89, Model B, Table 2). Figure 3 shows the expected cognitive dysfunction trajectories among those with college education.
Figure 3.
Expected number of errors on cognitive tests over adulthood by birth cohort: ACL (1986–2011). Adjusting for education (plotting expected number of errors among college educated) (Table 2, Model B). ACL = Americans’ Changing Lives.
Model C (Table 2) adjusts for gender and race/ethnicity. Birth cohort intercepts changed little, indicating that the effects of education in explaining cohort differences in levels of cognitive dysfunction over adulthood are not a function of gender or racial/ethnic differences in educational attainment across cohorts. However, racial/ethnic minorities do have significantly higher levels of cognitive dysfunction than whites (beta = 0.654, Model C, Table 2) and both women and racial ethnic minorities have more rapid increases (steeper linear slopes) in the number of cognitive errors over mid- to late-adulthood than men and whites.
Adjusting for time-varying health conditions did little to shift the mean number of errors (intercept) across cohorts (Models D through F, Table 2). But adults who were obese at any given wave had a significantly greater number of cognitive errors at age 55 (intercept beta = 0.191, Model F, Table 2), and those with medically diagnosed cardiovascular conditions or diabetes had more rapid rates of cognitive decline (increasing number of cognitive errors with time) in their later years (Models D and E). Obesity was associated with a somewhat slower rate of increase in cognitive dysfunction (Model F, linear slope), due to the higher starting point (intercept) at age 55. No additional cohort group differences were explained by these covariates over and above the effects of education.
Discussion
In a nationally representative sample of U.S. adults followed for 25 years, we found that individuals from more recent birth cohorts had significantly less cognitive dysfunction over adulthood compared with adults from earlier birth cohorts, thus supporting Salthouse’s (2006) concept of preserved differentiation. Our results compare similarly to studies examining cohort differences on tests of reason, spatial abilities, and recall (Bowles et al., 2005; Finkel et al., 2007; Schaie et al., 2005; Zelinski & Kennison, 2007), yet contrasts with some work on verbal ability which shows declines among more recent-born cohorts relative to earlier-born cohorts (Alwin, 2009; Alwin et al., 2008; Schaie, 2008). This may reflect a distinction between fluid and crystallized abilities, with our measure of recall and subtraction items aligning more with fluid abilities (Horn & Cattell, 1967). Although temporal trends in cardiovascular diseases, diabetes, and obesity were all hypothesized to contribute to cohort group differences in cognition, we found that educational attainment alone fully explained these cohort group differences in level of cognition dysfunction across adulthood.
In particular, we see compression of morbidity among the highly educated. As Figure 3 shows, those with a college education maintain high levels of cognitive functioning for most of their life, with cognitive dysfunction only increasing rapidly past age 80, suggesting greater cognitive reserve, or greater ability to compensate for decline (Scarmeas & Stern, 2004). Thus, if all individuals had a college education, the differences in intercept, or mean level of cognitive dysfunction, would be minimized and morbidity would be compressed. Although a number of prior studies have found educational attainment to be associated with better cognitive performance or reduced dementia incidence (e.g., Brayne et al., 2010; Langa et al., 2008b; Schaie, 2013), we add to this literature by showing that educational attainment solely explained cohort group differences in mean level of cognitive dysfunction in a nationally representative sample of the U.S. population followed over 25 years.
Gender, race, circulatory diseases, diabetes, and obesity, all factors hypothesized to contribute to declining incidence of cognitive dysfunction with subsequent birth cohorts, did not add any explanatory power beyond that of education. However, they were associated with higher levels of and steeper increases in cognitive dysfunction among individuals. Racial/ethnic minorities had higher levels and steeper increases in cognitive dysfunction than whites, and women had more rapid increases than men. Individuals with cardiovascular diseases and diabetes had increased rates of decline in cognition throughout adulthood. Further, at any given time, individuals who were obese had a higher number of cognitive errors. In sum, common and increasingly prevalent health conditions such as diabetes and obesity, vascular risk factors including circulatory diseases, and minority race and female gender do affect both risk for higher number of cognitive errors and increasing risk over time. However, differences in the mean number of errors by birth cohort are effectively explained by differences in educational attainment.
In line with our current findings that accounting for educational attainment reduced mean differences in cognitive dysfunction by cohort, Brayne et al. (2010) found that increased educational attainment was associated with larger brain weight and reduced dementia risk. Furthermore, Meara, Richards, and Cutler (2008) found that increases in life expectancy were almost exclusive to those with higher educational attainment. Though trends have leveled off in recent years, Bound, Geronimus, Rodriguez, and Waidmann (2015) suggest that as educational attainment had increased dramatically over the past century, those with less than a high school education have over time become an increasingly disadvantaged group in relation to poor health outcomes. Therefore, continued social and governmental efforts to strengthen education and increase college degree attainment have the potential to reduce morbidity and lessen the cognitive dysfunction burden of future generations of adults.
Although the strengths of our study include a nationally representative sample spanning the full adult life span followed for 25 years with repeated measures of cognitive function at five different waves of testing, several limitations should be considered. The abbreviated SPMSQ was a reliable and valid cognitive screening instrument when included in the ACL Study in 1986, but is not as detailed as many measures of cognitive function (including verbal and spatial abilities, processing speed) in use today. The SPMSQ is mainly sensitive to advanced cognitive decline and we are not able to make specific inferences about the prevalence of diagnosed dementia using this measure. However, the abbreviated SPMSQ does capture orientation and working memory, two elements believed to represent some of the earliest signs of cognitive loss (Ashford, Kolm, Colliver, Bekian, & Hsu, 1989; Herzog & Wallace, 1997). Further, the trajectory of cognitive dysfunction and decline in the ACL data using this measure is similar to that found in studies using other more detailed cognitive tests (Bowles et al., 2005; Finkel et al., 2007; Schaie et al., 2005; Zelinski & Kennison, 2007), though we extend this work by covering the full adult life span. We also ran an item response theory (IRT) analysis with the cognitive measure to examine discrimination which showed item difficulty was highest for the orientation items (day of the week, date), but the discrimination was highest for the items asking about the current and past president. Thus, at an average level of cognitive function, items asking about current and past president are better at differentiating cognitive dysfunction more than the other items. In final regards to the SPMSQ, although the observed cohort group differences are small, recent work by Wu et al. (2014) found that even mild impairment represented by 3 to 4 errors on the full SPMSQ was associated with increased risk for mortality. This suggests that even minor differences in SPMSQ scores across cohort groups may have important implications for health and cognition in later life. Despite the limitations, we were able to analyze more cohorts and find distinct cohort group differences by using this measure across the adult life span in a representative population-based sample over a long follow-up.
To address the confounding of age and cohort, ideally we would have separate cohorts followed from the same age; however, it is rare to find nationally representative data with this structure. The ACL data allow us to examine birth cohorts followed for 25 years with partial overlap in the ages of observation for each birth cohort, allowing us to investigate cohort differences in cognition for adults at a given age who are observed at different periods of time. In any age-period-cohort model, age is confounded by cohort. We focus on age and cohort which are conceptually and empirically distinct, reflecting the principle of “cohort differences in aging” as advocated by Riley (1987). Further, the 3- to 10-year intervals between cognitive assessments in the ACL Study may miss more acute changes in cognitive dysfunction unfolding more quickly, and future studies may consider shorter time periods between measurements to better reflect true rate of change in functioning. However, our accelerated longitudinal design effectively imputes cognitive dysfunction across all ages, assuming that respondents are similar, conditional on the covariates (Raudenbush & Chan, 1992). Retest effects may also affect cognitive trajectories over time such that memory of cognitive screening procedures may improve scores in the waves following baseline, offsetting the effects of aging over time (Salthouse, 2009). As a result, we may be underestimating increases in cognitive dysfunction; however, these effects would not be expected to vary by cohort group.
Attrition and losses due to mortality are inevitable in long-term panel studies, and ACL is no exception. Fully 38% of respondents died over the 25-year period, and survey nonresponse occurred in varying degrees for about 30% of our participants. For respondents in the early birth cohorts (who were observed only in the latter part of the life course), cognitive dysfunction may be underestimated if those who died had worse cognitive function than those who survived. On the other hand, cognitive function in the later-born cohorts (observed in early adulthood and mildlife) may be overestimated (artificially generating cohort differences) if those with poor cognitive function were more likely to die. However, this is unlikely to be the case since we found no evidence among these recent cohorts that death at any given wave was related to the cognitive dysfunction score at the preceding wave (i.e., odds ratios [ORs] for follow-up based on cognition score at previous wave were not significantly different from 1.0 [OR range 0.98–1.20]). Moreover, the use of nonresponse weights helps to minimize bias due to nonresponse in this panel data.
In conclusion, we find that all birth cohorts showed a similar curvilinear increase in cognitive dysfunction with age; cognitive impairment remains relatively flat through most of adulthood and rises more sharply in late life. Birth cohorts did, however, show distinct differences in the mean level (intercept) in number of cognitive errors. Earlier-born cohorts have a higher average number of expected cognitive errors than do later-born cohorts. Educational attainment, however, explained these cohort group differences with other health and demographic variables adding little additional explanatory power. Future work may consider how cognitive disparities vary between cohorts among different levels of educational attainment. In sum, although health factors are certainly not to be ignored, it is important that the growing body of evidence regarding educational attainment and cognitive health is incorporated into social and policy-based change. If changes in early-life educational attainment can reduce the mean level of cognitive dysfunction over time, the potential for successful aging among future generations is a hopeful prospect.
Supplementary Material
Supplementary data are available at The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences online.
Funding
This work was supported by the National Institute on Aging (R01AG018418); the Department of Veterans Affairs (VA IIR 10-176; to K. Zivin); and the National Institute of Mental Health (T32 MH073553; to A. Leggett’s postdoctoral fellowship funding).
Supplementary Material
References
- Alwin D. F. (2007). Margins of error: A study of reliability in survey measurement. Hoboken, NJ: John Wiley & Sons, Inc. doi:10.1002/9780470146316 [Google Scholar]
- Alwin D. F. (2009). History, cohorts, and patterns of cognitive aging. In Bosworth H. B., Hertzog C. (Eds.), Aging and cognition: Research methodologies and empirical advances (pp. 9–28). Washington, DC: American Psychological Association. [Google Scholar]
- Alwin D. F., & McCammon R. J (2001). Aging, cohorts, and verbal ability. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 56, S151–S161. doi:10.1093/geronb/56.3.S151 [DOI] [PubMed] [Google Scholar]
- Alwin D. F. McCammon R. Wray L. A., & Rodgers W. L (2008). Population processes and cognitive aging. In Hofer S. M., Alwin D. F. (Eds.), Handbook of cognitive aging: Interdisciplinary perspectives (pp. 69–89). Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Ashford J. W. Kolm P. Colliver J. A. Bekian C., & Hsu L. N (1989). Alzheimer patient evaluation and the mini-mental state: Item characteristic curve analysis. Journal of Gerontology, 44, P139–P146. doi:10.1093/geronj/44.5.P139 [DOI] [PubMed] [Google Scholar]
- Bound J. Geronimus A. T. Rodriguez J. M., & Waidmann T. A (2015). Measuring recent apparent declines in longevity: The role of increasing educational attainment. Health Affairs (Millwood), 34, 2167–2173. doi:10.1377/hlthaff.2015.0481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles R. P. Grimm K. J., & McArdle J. J (2005). A structural factor analysis of vocabulary knowledge and relations to age. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 60, P234–P241. doi:10.1093/geronb/60.5.P234 [DOI] [PubMed] [Google Scholar]
- Brayne C. Ince P. G. Keage H. A. D. McKeith I. G. Matthews F. E. Polvikoski T., & Sulkava R (2010). Education, the brain and dementia: Neuroprotection or compensation?: EClipSE Collaborative Members. Brain, 133, 2210–2216. doi:10.1093/brain/awq185 [DOI] [PubMed] [Google Scholar]
- Diaz-Venegas C. Downer B. Langa K. M., & Wong R (2016). Racial and ethnic differences in cognitive function among older adults in the USA. International Journal of Geriatric Psychiatry, 31, 1004–1012. doi:10.1002/gps.4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D. Reynolds C. A. McArdle J. J., & Pedersen N. L (2007). Cohort differences in trajectories of cognitive aging. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 62, P286–P294. doi:10.1093/geronb/62.5.P286 [DOI] [PubMed] [Google Scholar]
- Flashman J. (2013). A cohort perspective on gender gaps in college attendance and completion. Research in Higher Education, 54, 545–570. doi:10.1007/s11162-013-9285-8 [Google Scholar]
- Herzog A. R., & Wallace R. B (1997). Measures of cognitive functioning in the AHEAD Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 52, 37–48. doi:10.1093/geronb/52B.Special_Issue.37 [DOI] [PubMed] [Google Scholar]
- Horn J. L., & Cattell R. B (1967). Age differences in fluid and crystallized intelligence. Acta Psychologica, 26, 107–129. doi:10.1016/0001-6918(67)90011-X [DOI] [PubMed] [Google Scholar]
- House J. S. Kessler R. C., & Herzog A. R (1990). Age, socioeconomic status, and health. The Milbank Quarterly, 68, 383–411. doi:10.2307/3350111 [PubMed] [Google Scholar]
- House J. S. Lantz P. M., & Herd P (2005). Continuity and change in the social stratification of aging and health over the life course: Evidence from a nationally representative longitudinal study from 1986 to 2001/2002 (Americans’ Changing Lives Study). The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 60, S15–S26. doi:10.1093/geronb/60.Special_Issue_2.S15 [DOI] [PubMed] [Google Scholar]
- Kao G., & Thompson J. S (2003). Racial and ethnic stratification in educational achievement and attainment. Annual Review of Sociology, 29, 417–442. doi:10.2307/30036974 [Google Scholar]
- Langa K. M., & Larson E. B (2014). Education, brain health, and improving life opportunities for women. Journal of the Economics of Ageing, 4, 56–58. doi:10.1016/j.jeoa.2014.08.001 [Google Scholar]
- Langa K. M. Larson E. B. Karlawish J. H. Cutler D. M. Kabeto M. U. Kim S. Y., & Rosen A. B (2008. a). Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity?Alzheimer’s & Dementia, 4, 134–144. doi:10.1016/j.jalz.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa K. M. Larson E. B. Karlawish J. H. Cutler D. M. Kabeto M. U. Kim S. Y., & Rosen A. B (2008. b). Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity?Alzheimer’s & Dementia, 4, 134–144. doi:10.1016/j.jalz.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. S. O’Connor E. Rossom R. C. Perdue L. A. Burda B. U. Thompson M., & Eckstrom E (2013). Screening for cognitive impairment in older adults: An evidence update for the U.S. Preventive Services Task Force (Vol. 107). Rockville, MD: Agency for Healthcare Research and Quality (US). [PubMed] [Google Scholar]
- Meara E. R. Richards S., & Cutler D. M (2008). The gap gets bigger: Changes in mortality and life expectancy, by education, 1981-2000. Health Affairs, 27, 350–360. doi:10.1377/hlthaff.27.2.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirowsky J. (2011). Cognitive decline and the default American lifestyle. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66, i50–i58. doi:10.1093/geronb/gbq070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen B., & Muthen L. K (2000). Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes. Alcoholism, Clinical and Experimental Research, 24, 882–891. doi:10.1111/j.1530-0277.2000.tb02070.x [PubMed] [Google Scholar]
- Peter K., & Horn L (2005). Gender differences in participation and completion of undergraduate education and how they have changed over time. Postsecondary Education Descriptive Analysis Reports. NCES 2005-169. Washington, DC: US Department of Education. [Google Scholar]
- Pfeiffer E. (1975). A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. Journal of the American Geriatrics Society, 23, 433–441. doi:10. 1111/j.1532-5415.1975.tb00927.x [DOI] [PubMed] [Google Scholar]
- Raftery A. E. (1995). Bayesian model selection in social research. Sociological Methodology, 25, 111–164. doi:10.2307/271063 [Google Scholar]
- Raudenbush S. W., & Chan W.-S (1992). Growth curve analysis in accelerated longitudinal designs. Journal of Research in Crime and Delinquency, 29, 387–411. doi:10.1177/0022427892029004001 [Google Scholar]
- Riley M. W. (1987). On the significance of age in sociology. American Sociological Review, 52, 1–14. doi:10.2307/2095388 [Google Scholar]
- Ronnlund M. Nyberg L. Backman L., & Nilsson L. G (2005). Stability, growth, and decline in adult life span development of declarative memory: Cross-sectional and longitudinal data from a population-based study. Psychology and aging, 20, 3–18. doi:10.1037/0882-7974.20.1.3 [DOI] [PubMed] [Google Scholar]
- Salthouse T. A. (2006). Mental exercise and mental aging: Evaluating the validity of the “use it or lose it” hypothesis. Perspectives on Psychological Science, 1, 68–87. doi:10.1111/j.1745-6916.2006.00005.x [DOI] [PubMed] [Google Scholar]
- Salthouse T. A. (2009). When does age-related cognitive decline begin?Neurobiology of Aging, 30, 507–514. doi:10.1016/j.neurobiolaging.2008.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N., & Stern Y (2004). Cognitive reserve: Implications for diagnosis and prevention of Alzheimer’s disease. Current Neurology and Neuroscience Reports, 4, 374–380. doi:10.1007/s11910-004-0084-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie K. W. (2008). Historical processes and patterns of cognitive aging. In Hofer S., Alwin D. F. (Eds.), Handbook of cognitive aging: Interdisciplinary perspectives (pp. 368–384). Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Schaie K. W. (2013). Developmental influences on adult intelligence: The Seattle Longitudinal Study (2nd ed.). New York, NY: Oxford University Press. doi:10.1093/acprof:oso/9780195156737.001.0001 [Google Scholar]
- Schaie K. W. Willis S. L., & Pennak S (2005). An historical framework for cohort differences in intelligence. Research in Human Development, 2, 43–67. doi:10.1080/15427609.2005.9683344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers E. M. C. Verhaaren B. F. J. Koudstaal P. J. Hofman A. Ikram M. A., & Breteler M. M. B (2012). Is dementia incidence declining?: Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology, 78, 1456–1463. doi:10.1212/WNL.0b013e3182553be6 [DOI] [PubMed] [Google Scholar]
- Setoguchi S. Glynn R. J. Avorn J. Mittleman M. A. Levin R., & Winkelmayer W. C (2008). Improvements in long-term mortality after myocardial infarction and increased use of cardiovascular drugs after discharge: A 10-year trend analysis. Journal of the American College of Cardiology, 51, 1247–1254. doi:10.1016/j.jacc.2007.10.063 [DOI] [PubMed] [Google Scholar]
- Verhaegen P. Borchelt M., & Smith J (2003). Relation between cardiovascular and metabolic disease and cognition in very old age: Cross-sectional and longitudinal findings from the berlin aging study. Health psychology, 22, 559–569. doi:10.1037/0278-6133.22.6.559 [DOI] [PubMed] [Google Scholar]
- Wu C.-Y. Chou Y.-C. Huang N. Chou Y.-J. Hu H.-Y., & Li C.-P (2014). Cognitive impairment assessed at annual geriatric health examinations predicts mortality among the elderly. Preventive Medicine, 67, 28–34. doi:10.1016/j.ypmed.2014.06.027 [DOI] [PubMed] [Google Scholar]
- Zelinski E. M., & Kennison R. F (2007). Not your parents’ test scores: Cohort reduces psychometric aging effects. Psychology and Aging, 22, 546–557. doi:10.1037/0882-7974.22.3.546 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.