Abstract
Objectives
People’s preferences for temporal sequences of events have implications for life-long health and well-being. Prior research suggests that other aspects of intertemporal choice vary by age, but evidence for age differences in sequence-preferences is limited and inconclusive. In response, the present research examined age differences in sequence-preferences for real outcomes administered in a controlled laboratory setting.
Methods
A pilot study examined sequence-preferences for aversive electrodermal shocks in 30 younger and 30 older adults. The main study examined sequence-preferences for electrodermal shocks, physical effort, and monetary gambles in an adult life-span sample (N = 120). It also examined emotional and physiological responses to sequences as well as underlying mechanisms including time perception and emotion-regulation.
Results
There were no significant age differences in sequence-preferences in either of the studies, and there were no age differences in responses to sequences in the main study. Instead, there was a domain effect with participants preferring decreasing sequences for shocks and mixed sequences for effort and money.
Discussion
After considering potential methodological limitations, theoretical contributions and implications for real-life decisions are discussed.
Keywords: Decision making, Intertemporal choice, Sequence-preferences, Time discounting
Many important choices require people to sort a given set of events into a preferred temporal order. Examples range from mundane everyday decisions to momentous life-changing choices and include arranging different tasks over a workday, scheduling exercise sessions during a busy week, selecting long-term treatment regimens for chronic health conditions, or managing financial resources over the course of one’s retirement. The resulting temporal sequences have implications for productivity, health, wealth, and happiness. However, prior research on sequence-preferences has focused almost exclusively on younger samples (e.g., Ariely & Carmon, 2000; Chapman, 2000; Loewenstein & Prelec, 1993) and the few studies including older adults are inconclusive (Drolet, Lau-Gesk, & Scott, 2011; Löckenhoff, Reed, & Maresca, 2012). The present research begins to address this research gap by examining preferences for sequences of real outcomes in adults of different ages. To provide the rationale for this approach, we first discuss relevant theoretical perspectives. We then review existing research on age differences in sequence-preferences and other forms of intertemporal choice and finally describe the design and hypotheses of the present research.
Theoretical Frameworks
Sequence-preferences are a subtype of intertemporal choice in which decision makers are presented with predetermined sets of outcomes and asked to decide about the relative order in which they would prefer to experience the outcomes. Prior work in this area (Frederick & Loewenstein, 2008; Loewenstein & Prelec, 1993) differentiates among improving sequences (i.e., saving the most positive or least intense stimuli for last), worsening sequences (i.e., leaving the most negative or most intense stimuli for last), and mixed sequences (i.e., interleaving stimuli of different valence and intensity). Research on younger adults has documented a preference for improving sequences across a wide variety of outcomes ranging from monetary payouts and pain to annoying sounds, restaurant meals, and performance-based tasks (e.g., Ariely & Carmon, 2000; Frederick & Loewenstein, 2008; Loewenstein & Prelec, 1993). There are theoretical reasons to expect that sequence preferences vary by age, but specific conceptual frameworks—drawing on age differences in time perception on the one hand and age differences in emotion-regulatory strategies on the other hand—differ in the proposed direction of such effects.
Prior considerations of age differences in intertemporal choice have focused on “temporal discounting,” the tendency to devalue distant gains and losses relative to more immediate ones (Frederick, Loewenstein, & O’Donoghue, 2002). Evidence suggests that the tendency to discount future monetary gains decreases with age (e.g., Eppinger, Nystrom, & Cohen, 2012; Löckenhoff, O’Donoghue, & Dunning, 2011; for a review see Löckenhoff & Rutt, 2015). In other words, younger adults are more likely than older adults to prefer smaller but sooner monetary gains over larger delayed gains. Theoretical explanations of such effects (Löckenhoff & Rutt, 2015; Löckenhoff & Rutt, 2017) have pointed to age-related limitations in future horizons and corresponding increases in the subjective speed of time. This may lead older adults to perceive future events as more proximal and thus more relevant and salient (John & Lang, 2015). In addition, affective forecasting, the ability to anticipate one’s future emotional states, is well-preserved and may even improve in later life as life experiences accumulate (Samanez-Larkin, 2013; Scheibe, Mata, & Carstensen, 2010). This may result in a more vivid representation of delayed experiences and therefore a reduced tendency toward temporal discounting (Mata et al., 2011). Extending such reasoning to sequence-preferences, one might expect that older adults show an even stronger preference for improving sequences than younger adults, because they are less likely to devalue positive events that occur later in the sequence. However, it is not clear whether age differences in temporal discounting generalize beyond the monetary domain to consumable or experiential outcomes (Jimura et al., 2011; Löckenhoff et al., 2016; Seaman et al., 2016). Further, in contrast to temporal discounting paradigms, the chosen order in sequence paradigms does not affect the size of the cumulative outcomes. Thus, there is no inherent benefit to postponing positive outcomes.
A second theoretical perspective on age differences in sequence choices draws on contemporary theories of emotional aging. Specifically, it has been postulated that resources for effortful emotion-regulation decline with age and that physiological recovery from sustained negative affect is more taxing for older as compared to younger adults (Charles, 2010; Urry & Gross, 2010). As a result, older adults are thought to leverage experienced-based insights and engage in prospective emotion-regulation to avoid negative experiences before they occur (Urry & Gross, 2010). Although improving sequences may be appealing to younger adults because they “save the best for last,” such sequences also entail a prolonged period of unfavorable experiences at the beginning, and this may be challenging for older adults, especially when outcomes are emotionally salient or physiologically taxing. From this point of view, one would expect that older adults prefer mixed sequences in which extreme stimuli are interspersed with mild and moderate stimuli to provide sufficient time for physiological recovery.
Prior Empirical Evidence
To the best of our knowledge, only two prior studies have directly examined age differences in sequence-preferences, and their results are contradictory. Drolet and colleagues (2011) explored hypothetical choices about the order in which to consume three tapas dishes of varying quality. They found that older adults were more likely to prefer improving sequences than younger adults. Löckenhoff and colleagues (2012), in contrast, implemented real choices involving exposure to emotional photos. Participants were asked in which order they preferred to view a series of 30 photos with positive, negative, and neutral emotional valence. Older adults were less likely to favor improving sequences than younger adults.
The causes of these contradictory findings are difficult to discern because the studies varied in multiple aspects including outcome domain, sequence length, and whether outcomes were hypothetical or real. Conceivably, Drolet et al.’s hypothetical and abstract descriptions of sequence choices may have activated age-related variations in time horizons, whereas Löckenhoff et al.’s emotionally salient stimuli and real outcomes may have activated age-related variations in emotion-regulatory preferences. This could account for the observed differences in age effects between the studies. However, the relative role of such mechanisms cannot be determined without the implementation of real and emotionally salient outcomes, a comprehensive assessment of covariates, and the examination of both sequence-preferences and responses to sequences within the same study.
The Present Research
To address these concerns, the present research aimed to (a) quantify adult age differences in sequence-preferences across multiple domains of real and emotionally salient outcomes, (b) examine age differences in responses to the selected sequences, and (c) consider theoretically implicated covariates.
Based on age differences in emotion-regulatory strategies and resources, we expected that, consistent with Löckenhoff et al. (2012), younger adults would show a preference for improving sequences, whereas older adults would prefer mixed sequences which intersperse different types of stimuli and thus limit extended exposure to intense or aversive experiences. To rule out potential age differences in the ability to track the constructed sequences, we provided clear visual feedback and elicited sequence-preferences via both free- and forced-choice procedures.
To examine the consistency of effects across domains, we compared sequence-preferences for monetary outcomes, sustained effort, and aversive physical outcomes. These domains were chosen because they vary in emotional valence, salience, and motivational aspects, and because choices made across these domains activate overlapping but dissociable neural systems (Berns et al., 2006; Knutson et al., 1999; Treadway et al., 2009). Also, in contrast to monetary outcomes, sustained effort and physical outcomes are not fungible and elicit immediate physical sensations. Finally, although limited to a laboratory setting, the domains of monetary, effortful, and aversive physical stimuli map loosely onto real-life choices in the financial, work, and health domains.
To assess responses to the sequences, we adopted a multimodal approach combining measures of concurrent and retrospective self-reported affect with indicators of psychophysiological activation (i.e., heart rate and skin conductance). This approach extends beyond earlier work on emotional aging, which mostly relies on self-reported affect (Samanez-Larkin & Carstensen, 2011) and it is in line with current research trends acknowledging the multilayered nature of affective responses (Scheibe & Carstensen, 2010).
A range of theoretically implicated covariates were included. To examine the potential role of age differences in time perception, we examined future time perspective and subjective position in the life span. To examine the potential role of age difference in emotion-regulatory resources and strategies, we examined ambient mood, self-reported coping mechanisms, and subjective mental/physical health. The evaluation of temporal sequences may also be influenced by age differences in cognition and personality (Davis, Patte, Tweed, & Curtis, 2007; Isen, 2001). We therefore included brief screening measures for these variables.
Method
Pilot Study
A pilot study was conducted to explore the feasibility of using electrodermal shocks in a sequencing task and to provide initial evidence of age effects in sequence choices when outcomes are real and negative in valence. This study asked younger and older adults (n = 60) to indicate in which order they preferred to receive six blocks of electrodermal shocks ranging from 10% to 90% in intensity. Findings indicated that it was feasible to implement electrodermal shocks as outcomes in a sequencing task and that the task could be safely used with an older sample. There were no significant age differences in sequence preferences, t(58) = .34, p > .70, Cohen’s d = 0.1, and analyses revealed a JZS Bayes factor of 3.63 in support of the null hypothesis (r-scale = .707; Rouder, Speckman, Sun, Morey, & Iverson, 2009). Thus, given the present results, it is 3.63 times more likely that age differences in this task do not exist than that such differences do exist. See Supplement A for a complete description of the pilot study.
Participants
Participants in the main study (N = 120, aged 22–84) were recruited from participant databases and public postings and compensated $60. To ensure the safe use of the electrodermal stimuli, we screened out individuals with heart or lung conditions, diabetes, neurological disorders, wearable medical devices, skin conditions or allergies, and pain/stiffness in the upper body, as well as individuals taking daily pain medication or scoring below 24 on the Mini Mental State dementia screen (Folstein, Folstein, & McHugh, 1975).
Table 1 (left columns) shows descriptive data and associations with age for demographics and selected covariates (Supplement B shows the age distribution, Supplement C shows an expanded version of the table including all covariates).
Table 1.
Selected Demographic and Background Variables and Their Associations With Age and Sequence-preferences
| M (SD)/% | r age | r electro | r effort | r money | |
|---|---|---|---|---|---|
| Age | 49.28 (18.02) | .05 | .07 | −.11 | |
| % White | 77% | .33* | .06 | .06 | −.09 |
| Affect slider – valence | 70.49 (19.37) | .03 | −.16 | −.19* | −.09 |
| Affect slider – arousal | 27.56 (24.35) | .18* | .11 | −.06 | −.13 |
| AVI – low arousal | 3.13 (0.88) | −.27** | −.04 | −.09 | −.13 |
| AVI – low arousal negative | 1.83 (0.82) | −.32** | .10 | .05 | −.09 |
| AVI – high arousal | 1.64 (0.62) | .07 | .04 | −.18* | −.16 |
| PCI – Avoidance coping | 2.77 (0.67) | .22** | .00 | .12 | .04 |
| Future time perspective | 48.53 (12.63) | −.53** | −.04 | −.01 | .01 |
| Subjective life position | 47.63 (14.09) | .48** | −.10 | −.09 | −.10 |
| FFI – Agreeableness | 3.03 (0.23) | .27* | −.08 | .03 | −.08 |
| SF 12 – Mental health | 50.11 (8.78) | .28** | −.04 | .07 | .01 |
| SF 12 – Physical health | 52.03 (7.42) | −.31** | .00 | .10 | .10 |
| Digit span | 6.13 (1.89) | −.33** | −.04 | .02 | .08 |
| Digit symbol | 54.87 (13.54) | −.56** | −.24** | −.26** | .02 |
| n-back | 0.86 (0.12) | −.22* | −.15 | −.13 | −.03 |
| Vocabulary | 17.94 (4.44) | .44** | −.05 | .05 | −.07 |
| Numeracy | 59.44 (33.52) | −.21* | .03 | .05 | .04 |
| Effort (minimum threshold) | 10.83 (5.52) | .02 | −.06 | −.27** | −.24** |
| Effort (maximum threshold) | 25.25 (5.05) | −.33** | .01 | −.07 | .11 |
Note: rage,relectro,reffort,rmoney, refer to correlations with age and sequence-trend scores for electrodermal shocks, physical effort, and monetary payouts. The point-biserial correlation is shown for race, all others are Pearson correlations. The table only shows variables with significant associations to age or sequence-preferences. See Supplement C for the full table. AVI = Affect Valuation Index; FFI = Five-Factor Inventory; PCI = Proactive Coping Inventory.
*p < .05, **p < .01.
Materials
Electrodermal stimuli
Electrodermal stimuli were administered to the inner wrist of the nondominant hand. Upward-stepwise calibration was used to obtain the perceptual threshold and maximum bearable intensity. Stimuli were set at 10%–30%–50%–70%–90% on a scale from 1 (perceptual threshold) to 100 (maximum bearable). For details on apparatus and safety procedures, see Supplement A and Löckenhoff et al. (2016).
Sustained effort stimuli
Sustained effort stimuli were adapted from the Effort Expenditure for Rewards Task (EEfRT, Treadway et al., 2009). Over a 5-s interval, participants were asked to make repeated button presses on the zero key of the keyboard pin pad using the small finger of their dominant hand.
Stimuli were calibrated by asking participants to tap the key at a regular, comfortable rate (i.e., spontaneous motor tempo, Baudouin, Vanneste, & Isingrini, 2004) and to tap as fast as possible (i.e., maximum tempo). Stimuli were set at 10%–30%–50%–70%–90% on a scale from 1 (spontaneous tempo) to 100 (maximum tempo).
Monetary stimuli
Monetary stimuli consisted of monetary gambles with a constant likelihood of 50% for gain versus loss. Outcome levels were set at $1–$3–$5–$7–$9. Participants were given an initial endowment of $5 to which any gains and losses were applied. Any resulting earnings were paid out at the end of the study. (Note that in contrast to risky choice paradigms, participants could not accept/reject or influence the probabilities of the gambles. They merely decided in which sequence they would witness the gambles and their outcomes.)
Sequence selection task
Participants received 3 stimuli at each of 5 intensity levels for a total of 15 stimuli per domain.
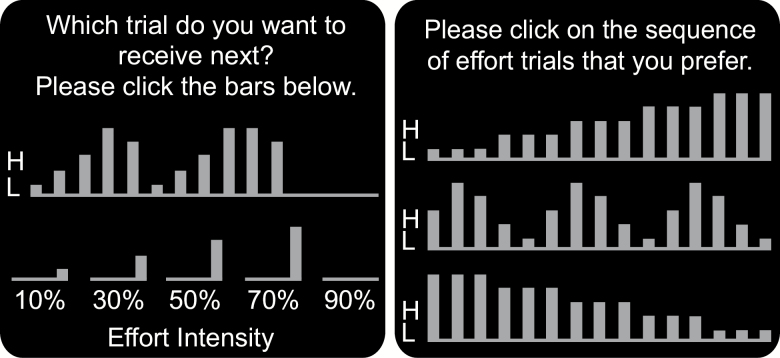
In the free-choice task (Figure 1, left), participants constructed the sequence one trial at a time. The chosen sequence was shown at the top and remaining stimuli were shown at the bottom. Participants clicked on stimuli at the bottom to be added to the sequence until all stimuli had been placed. The labels at the bottom showed stimulus intensities (in percentages) for the effort and electrodermal stimuli and the amount of money at stake (in $) for the monetary stimuli. To capture individual variations in sequence-preferences, we calculated sequence-trend scores (Löckenhoff et al., 2012), based on Spearman’s rank-order correlations between trial (1–15) and stimulus intensity (10%–90%) within each individual. The resulting scores range from −1 (monotonously decreasing) to 1 (monotonously increasing) with neutral scores indicating a preference for mixed sequences.
Figure 1.
Sequence selection screens (samples show effort domain). Bar size indicated stimulus magnitude. Free-choice (left): The upper portion of the screen showed the already selected stimuli, the lower portion showed the remaining stimuli for each magnitude. Forced-choice (right): Participants chose one of the three options.
A forced-choice task (Figure 1, right) was administered immediately after the free-choice task, asking participants to select among an increasing, mixed, and decreasing sequence. Forced-choice responses were coded as −1 for decreasing intensity, 0 for mixed, and 1 for increasing intensity.
Sequence administration
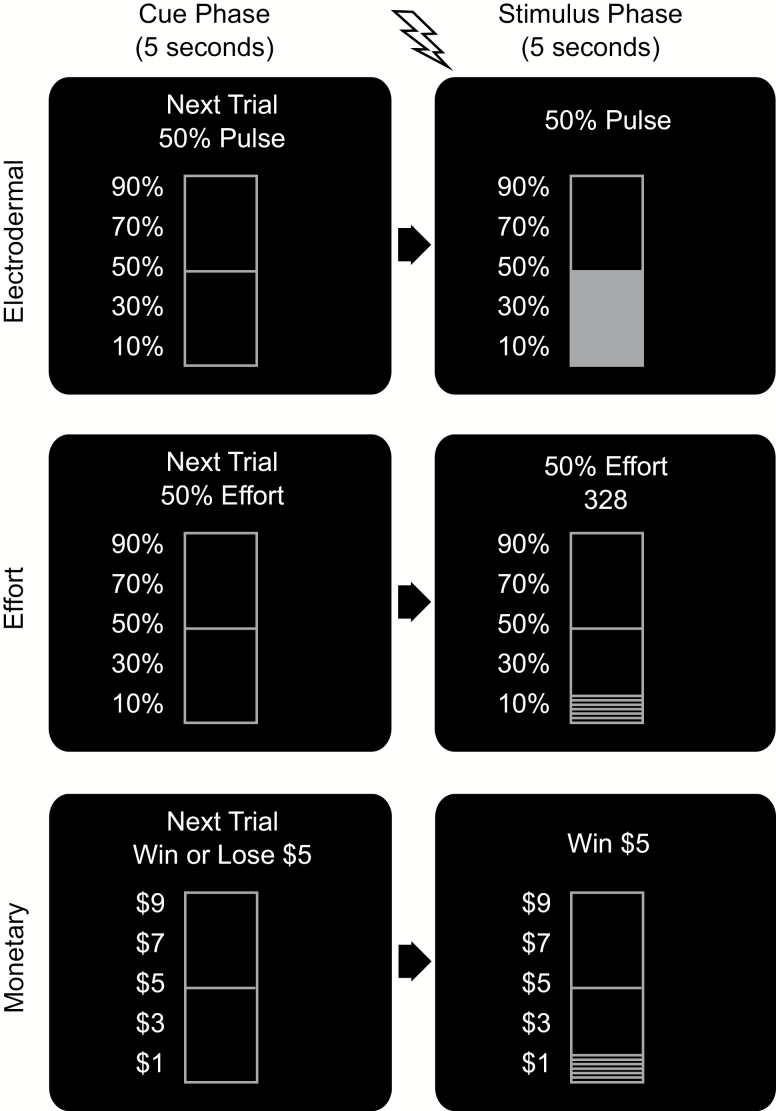
To limit the number of possible sequences and thus facilitate analyses, we implemented the sequences chosen in the forced-choice task. Participants received the 15 trials in each domain in the order they had selected. Figure 2 shows sample trials.
Figure 2.
Sequence administration screens (samples show 50%/$5). For the electrodermal domain, a 500 ms stimulus was administered at the beginning of the stimulus phase. For the effort domain, the bar filled up gradually with each keystroke. For the monetary domain, the bar filled up gradually from the bottom for wins and emptied out from the top for losses.
During a cue phase, participants viewed a 5-s cue showing the words “Next Trial” along with the appropriate stimulus information (e.g., “50%,” “$5) and a bar graph with a line visualizing stimulus magnitude. Next, there was a 5-s stimulus phase.
For electrodermal stimuli, participants received a 500 ms stimulus at the beginning of this phase. With stimulus onset, the screen changed to a filled-in bar of the corresponding intensity that remained visible for 5 s after stimulus onset.
For effort stimuli, participants saw a countdown timer at the top of the screen. The bar below filled in gradually with each keystroke. This screen remained visible for 5 s regardless of whether or not participants had reached the target level at the end.
For monetary stimuli, the top of the screen showed the outcome of the trial (e.g., “win $5”). For winning trials, the bar gradually filled up and for losing trials the bar gradually emptied out over the course of 5 s.
A 10-s inter-trial-interval consisted of a simple fixation cross.
Measures
Demographics included age, gender, race, ethnicity, and education level (1 = some high school to 8 = graduate degree).
Affect sliders assessed variations in current affect over the course of the study. Participants adjusted virtual sliders on two visual analog scales from “very negative” to “very positive” and from “not aroused at all” to “very aroused” (Nielsen, Knutson, & Carstensen, 2008).
Ambient mood was assessed with the “actual affect” version of the Affect Valuation Index (AVI, Tsai, Knutson, & Fung, 2006).
Emotion-regulatory and coping strategies were assessed with the Emotion-Regulation Questionnaire (ERP) (Gross & John, 2003) and the Proactive Coping Inventory (PCI) (Greenglass, Schwarzer, Jaubiec, Fiksenbaum, & Taubert, 1999).
Subjective time perceptions included global time horizons (Future Time Perspective Scale; Carstensen & Lang, 1996) and subjective position in the life span (asking participants to indicate their current position on a line marked “birth” on the left and “death” on the right, Hancock, 2010).
Cognitive assessments included a phone version of the Mini Mental State to screen for dementia (Folstein et al., 1975), the vocabulary portion of the Nelson-Denny Reading Test (Brown, Fishco, & Hanna, 1993), the Digit-Span and Digit-Symbol subtests of the Wechsler Adult Intelligence Scale (Wechsler, 1981), a letter-based version of the n-back task (Ragland et al., 2002), and numeracy (Schwartz, Woloshin, Black, & Welch, 1997).
Personality was assessed with the NEO Five-Factor Inventory (NEO-FFI, Costa & McCrae, 1992).
Mental/physical health were assessed with the SF-12 (Ware, Snow, Kosinski, & Gandek, 1993).
Procedure
Eligibility criteria were screened by phone. Upon arrival, participants provided consent, completed demographics, and rated current affect (using the affect sliders) and ambient mood (using the AVI). Next, electrodes for physiological recording were connected, followed by a battery of background measures (emotion-regulatory and coping strategies, subjective time perceptions, cognitive abilities, personality traits, and mental/physical health). This was followed by a 2-min physiological baseline (see Supplement D for physiological methods and results).
Next, participants were connected to the electrodes for electrodermal stimulation and completed the calibration procedures and the free- and forced-choice sequence-selection for each of the three stimulus domains in counterbalanced order. This was followed by another physiological baseline.
Participants then completed the sequence administration phase. Within each domain, stimuli were presented in the specific sequence chosen during the forced-choice task. After every three trials, participants rated their current affect using the affect sliders. Afterwards, participants completed a third physiological baseline.
Finally, participants retrospectively rated their responses to the sequence administration on the affect sliders (both average and peak affect). After debriefing, participants received their payment including any earnings from the monetary condition. Procedures were IRB approved and there were no complaints or adverse outcomes.
Results
Descriptive Analyses
Table 1 (left columns) reports descriptive statistics and correlations with age for each of the demographics and covariates as well as the calibration thresholds for electrodermal and effort stimuli.
Age was not significantly associated with gender or education, but—consistent with the local population—older participants were more likely to be White. Regarding ambient mood, age was associated with lower arousal on the affect slider and the AVI. Further, age was associated with higher avoidance coping, shorter time horizons, more advanced life position, higher agreeableness, better mental health, but worse physical health. Regarding cognition, age was positively associated with vocabulary but negatively associated with all other aspects of cognitive functioning. Finally, age was not significantly associated with the thresholds for the electrodermal task, but the maximum speed in the effort task was negatively associated with age (ps < .05).
Sequence-preferences
Domain effects
For the free-choice task, an ANOVA with domain as the within-subject variable and sequence-trend as the dependent variable found a significant domain effect, F(2, 238) = 12. 36, p < .001, F0E72p = .01, with post-hoc tests indicating that sequence-trend scores were more negative in the electrodermal condition (M = −0.22, SD = 0.68), than in the effort condition (M = .01, SD = 0.74), and the monetary condition (M = 0.13, SD = 0.73, ps < .05). Thus, participants preferred sequences of decreasing intensity in the electrodermal domain but more mixed sequences in the other domains.
For the forced-choice task, similar patterns emerged (Table 2). The mixed sequence was the most frequent response in each domain. However, for electrodermal stimuli, twice as many participants preferred the decreasing sequence as compared to the increasing sequence. For the monetary and effort domain, there was no significant preference for a specific sequence type.
Table 2.
Sequence-preferences for the Forced-choice Task
| Stimulus type | Selected sequence | χ2 | p | ||
|---|---|---|---|---|---|
| Decreasing intensity | Mixed | Increasing intensity | |||
| Electrodermal | 35% | 48% | 17% | 18.20 | <.001 |
| Effort | 35% | 40% | 25% | 4.20 | .12 |
| Monetary | 30% | 39% | 31% | 1.85 | .40 |
Note: χ2 tests examine deviations from equal distribution across sequence types within each domain.
To examine associations between forced- and free-choice we conducted ANOVAs with forced-choice (decreasing, mixed, increasing) as the independent variable and free-choice sequence-trends as the dependent variable. As expected, free-choice sequence-trends were smallest for the decreasing condition, intermediate for the mixed condition, and largest for the increasing condition (across domains, Fs > 17.70, ps < .001, η2p > .23).
Free-choice sequence-trends also showed some associations across domains, but effects were only moderate in size (rmonetary-electrodermal =.31; rmonetary-effort = .39; relectrodermal-effort = .44; ps < .001). Thus, we examined age effects separately for each domain.
Age effects
In the free-choice task, age was not significantly correlated with sequence-trends for any of the domains (Table 1, top row). Also, regression analyses including both the centered age variable and a quadratic age term did not find any evidence of quadratic age effects (|βs| < 0.14, ps > .13). JZS Bayes factor analyses (r-scale = .707; Rouder et al., 2009) were all in favor of the null hypothesis (electrodermal = 4.51, effort = 3.99; monetary = 2.83). Thus, given the present results, it is 2.83 (monetary) to 4.51 (electrodermal) times more likely that age differences in sequence preferences do not exist than that such age differences do exist.
In the forced-choice task, we computed one-way ANOVAS comparing the average age of respondents that preferred a given sequence type. There was no evidence of age effects in any of the domains (ps > .25).
Covariates
To control for age differences in covariates, we conducted regression analyses for the free-choice task and ANCOVAs for the forced-choice task including age along with the variables showing significant associations with age (Table 1, first column). Because of concerns about collinearity, potential covariates were included one at a time. No significant age differences in sequence-preferences emerged in any of these analyses (all ps > .8).
In general, sequence-trend scores showed very few associations with the covariates (Table 1, right columns). Adopting a more conservative significance level of p less than .01 to account for multiple comparisons, sequence-trend scores were not significantly associated with any of the demographic and self-report variables. The only somewhat consistent associations were found for two measures of motor performance: Higher psychomotor speed (Digit Symbol) was associated with a greater preference for decreasing sequences in effort and electrodermal stimuli, and higher spontaneous motor tempo (i.e., minimum threshold for effort) was associated with a greater preference for decreasing sequences in effort and monetary stimuli.
Affective Responses
Concurrent affect
Valence and arousal were assessed every three trials resulting in 5 data points per domain. To examine variations by age and sequence type, we conducted ANCOVAs with selected sequence type (decreasing, mixed, increasing) as a between-subjects factor, sequence position (1–5) as a within-subjects factor, age as a covariate, and affect ratings as the dependent variable. Separate analyses were performed for valence and arousal and for each domain. Greenhouse-Geisser corrections addressed sphericity deviations.
There were no significant main or interaction effects of age and no significant main effects of sequence type or sequence position (ps > .05). However, we found significant sequence type by sequence position interactions for electrodermal stimuli with regard to arousal, F(3.60, 208.59) = 7.89, p < .001, η2p = .12, and for effort with regard to both arousal, F(4.92, 285.51) = 2.62, p < .05, η2p = .04, and valence, F(4.96, 287.93) = 2.46, p < .05, η2p = .04. Within-subjects contrasts indicated that the linear trend of affect over the course of the sequence varied by sequence type. Increasing sequences were associated with increasing arousal in the electrodermal and effort domain and increasingly positive affect in the effort domain. Decreasing sequences were associated with decreasing arousal in both domains and increasingly negative affect in the effort domain. Mixed sequences were associated with stable affect in both domains.
Retrospective affect
To examine whether retrospective ratings of average and peak affect differed by age or selected sequence, we computed ANCOVAs with selected sequence as a between-subjects factor, age as a continuous covariate, and retrospective affect ratings as the dependent variables. Separate analyses were performed for average versus peak ratings, valence versus arousal, and outcome domains. The effects of sequence type and age did not reach statistical significance (ps > .05) indicating retrospective affect ratings did not vary based on respondents’ age or the sequence they had selected.
Discussion
To the best of our knowledge, the present research is the first to examine age differences in sequence-preferences across multiple domains of real outcomes which are set to occur in the immediate future. In addition, we assessed affective and physiological responses to sequence administration and controlled for a range of theoretically implicated covariates. We found that sequence preferences did not vary by age, and this pattern was consistent across the pilot study and the main study and across the three outcome domains. Responses to sequences also did not vary by age—neither for self-reported affect nor for physiological responses (see Supplement D).
With regard to covariates, theoretical considerations had led us to explore the potential role of age differences in time perspective and emotion regulatory mechanisms. For time perception, we found familiar age-related limitations in future horizons and remaining time left in life. For emotional experience and regulation, we found that older adults reported better mental health, higher avoidance coping, as well as higher arousal at baseline. We also found the anticipated age differences in physiological resources, both in terms of lower self-reported physical health and in terms of lower tonic skin conductance responses. However, none of these variables were associated with sequence trends. Instead, the most consistent associations between sequence trends and covariates were found for measures of spontaneous motor tempo and perceptual speed with faster pace corresponding to a preference for decreasing intensities. This harkens back to historical interest in “personal tempo” and its associations with working habits and impulsivity (Rimoldi, 1951; Takala & Partanen, 1964), but it could also point towards a role of fluid intelligence (Craik & Salthouse, 2008). These possibilities warrant a more thorough exploration in future research.
Beyond examining age differences, the present findings add to the general literature on sequence-preferences. First, even though sequence-trend scores were correlated across domains, we also found significant domain effects. Participants preferred decreasing intensities for electrodermal stimuli but more mixed trajectories for physical effort and monetary payouts. As noted by Frederick and Loewenstein (2008), sequence-preferences may be influenced by multiple motives. For example, preferences for electrodermal stimuli may have been driven by efforts to manage anticipatory dread, which would favor decreasing intensities, whereas preferences for effort and monetary payouts may have been driven by the simple heuristic of distributing events evenly over time. To shed more light on such patterns, future studies should systematically manipulate specific motives and the subjective cost of each stimulus type.
Although null findings play a critical role in scientific progress (Franco, Malhotra, & Simonovits, 2014), it is important to consider methodological limitations that may account for the lack of age differences. All available indicators suggested that the experimental tasks functioned as intended. Sequence-preferences were consistent for the free and forced-choice version of the task and moderately associated across outcome domains. This speaks to the reliability of the sequence trend scores, although future studies should bolster this evidence by obtaining test–retest reliability. Affective responses to sequence administration also followed the expected patterns. For electrodermal stimuli and effort, trajectories of self-reported affect matched the selected sequences (e.g., sequences with increasing intensity resulted in increasing arousal). Surprisingly, increasing sequences for the effort task were also associated with increasingly positive affect. Anecdotal reports gathered at the end of the study indicated that some participants enjoyed the challenge of “beating the clock” on this task. For the monetary domain, no association between the selected sequence and affective responses was observed—most likely because participants paid more attention to whether they had won or lost a given gamble than to the amount of money at stake. Physiological responses (Supplement D) also showed the expected effects. For physiological baselines, there was a significant drop in spontaneous skin conductance responses (SCRs) at the end of the study. Thus, the experimental tasks did in fact impose a physiological load on participants. Further, patterns of SCRs during sequence administration indicated that the electrodermal stimuli elicited more responses and more intense responses than the effort and monetary task. Within each domain, more intense stimuli elicited more intense SCRs.
Of course, other methodological limitations remain. One obvious concern is sample size. The main study (n = 120) was well powered (.96) to detect moderately sized effects (r > .3), but the sample size may not have been sufficient to detect smaller but nonetheless meaningful age effects. Note, however, that the direction of age differences in sequence-preferences was inconsistent across domains and that observed effect sizes were very small (average |r| = .08). Further, the average JZS Bayes factor across samples and domains was 3.74. According common conventions (Lee & Wagenmakers, 2013) this would be considered “substantial” evidence in favor of the null hypothesis.
Another concern is that the older participants may not have been representative because the use of electrodermal shocks required stringent health-based exclusions. This could have attracted uncharacteristically healthy older adults with better homeostatic capability and therefore less incentive to spread out arousing stimuli. However, we found familiar age decrements in self-rated physical health, fluid cognition, psychomotor speed, and tonic skin conductance. This speaks against the notion that we recruited an overly healthy sample. Nonetheless, the present findings should be backed up by future studies using other forms of aversive stimuli (e.g., annoying sounds) that do not require health-based exclusions.
Characteristics of the experimental paradigm may have contributed to the lack of age effects as well. The sequences comprised only 15 items lasting for a total of 5 min per domain. This may not have been long enough to require the strategic management of emotion-regulation over time. Note that Löckenhoff et al. (2012), who found an age-related preference for mixed sequences, included twice as many items, whereas Drolet et al. (2011), who found an age-related preference for improving sequences, included only three items. Thus, future studies should systematically manipulate sequence length and implement outcomes that span longer time frames (i.e., days to weeks) to examine if age effects emerge when there is a long-term drain on resources.
Of course, it is also possible that age differences in sequence-preferences are domain dependent, and we may have inadvertently selected domains that are not susceptible to age effects. Research on temporal discounting, a related form of intertemporal choice, provides some hints that age differences vary by outcome domain. Jimura et al. (2011), for example, found age differences in the discounting of hypothetical monetary payouts, but not in the discounting of real liquid rewards. Similarly, Seaman et al. (2016) found that age differences in discounting varied across the monetary, health, and social domain. However, in the present study, even the monetary domain did not show any significant age effects.
Although various methodological concerns remain to be addressed in future studies, our findings raise the possibility that preferences for sequences may in fact remain stable with age. Previous studies showing evidence for age differences in sequence-preferences (Drolet et al., 2011; Löckenhoff et al., 2012) may have been ambiguous in their question framing or the depiction of outcomes (Frederick & Loewenstein, 2008). Given well-documented age differences in framing effects (Kim, Goldstein, Hasher, & Zacks, 2005; Mikels & Reed, 2009), younger and older adults may have differentially interpreted such ambiguities resulting in spurious age effects. The present studies, in contrast, offered a clear visual depiction of the sequences and provided multiple opportunities to sample the stimuli. This may have reduced noise and framing effects and thus provided a clearer view of age-related stability in sequence choices.
If corroborated by future studies, the present null results could suggest that sequence-preferences are developed relatively early in life and—like certain aspects of personality traits—remain relatively stable thereafter. Longitudinal studies would be needed to systematically explore this possibility. Perhaps more importantly, even though preferences for sequences do not differ much by age, the adaptive value of different types of sequences could change across the life span. For instance, the strategy of tackling the greatest challenges right away may have benefits for younger adults because it allows them to get things done quickly, but maintaining this approach into old age may be a liability if it depletes homeostatic potential to a point that is hard to recover from. Similarly, younger adults may be able to get away with postponing tasks until the last minute and then work nonstop to catch up in time, but this may not be an option for older adults who may find it harder to muster the necessary resources for such intense efforts. These effects could be particularly salient when sequences are not self-selected as in the present studies but imposed by environmental contingencies. To explore this possibility, future studies should examine what happens when participants of different ages are randomly assigned to different types of sequences.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences online.
Funding
This research was supported by National Institute on Aging Grant R21AG043741 to C. E. Löckenhoff. G. R. Samanez-Larkin was supported by National Institute on Aging Pathway to Independence Award R00-AG042596.
Conflict of Interest
None declared
Supplementary Material
Acknowledgments
The authors thank the members of the Cornell Healthy Aging Laboratory and lab managers Katya Swarts, Kyrsten Costlow, and Casey Gallagher for their help with data collection.
References
- Ariely D., & Carmon Z (2000). Gestalt characteristics of experiences. The defining features of summarised events. Journal of Behavioural Decision Making, 13, 191–201. doi:10.1002/(SICI)1099-0771(200004/06)13:2<191::AID-BDM330>3.0.CO;2-A. [Google Scholar]
- Baudouin A. Vanneste S. & Isingrini M (2004). Age-related cognitive slowing: The role of spontaneous tempo and processing speed. Experimental Aging Research, 30, 225–239. doi:10.1080/03610730490447831 [DOI] [PubMed] [Google Scholar]
- Berns G. S. Chappelow J. Cekic M. Zink C. F. Pagnoni G. & Martin-Skurski M. E (2006). Neurobiological substrates of dread. Science, 312, 754–758. doi:10.1126/science.1123721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. A., Fishco V. V., & Hanna G (1993). Nelson-Denny Reading Test: Manual for scoring and interpretation.Rolling Meadows, IL: Riverside Publishing. [Google Scholar]
- Carstensen L. L., & Lang F. R (1996). Future time perspective scale.Stanford, CA: Stanford University. [Google Scholar]
- Chapman G. B. (2000). Preferences for improving and declining sequences of health outcomes. Journal of Behavioral Decision Making, 13, 203–218. doi:10.1002/(sici)1099-0771(200004/06)13:2<203::aid-bdm317>3.0.co;2-s [Google Scholar]
- Charles S. T. (2010). Strength and vulnerability integration: A model of emotional well-being across adulthood. Psychological Bulletin, 136, 1068–1091. doi:10.1037/a0021232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa P. T., & McCrae R. R (1992). Professional manual: Revised NEO Five-Factor Inventory.Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Craik F. I. M., & Salthouse T. A (2008). The handbook of aging and cognition (3rd ed.). New York, NY: Psychology Press. doi:10.1002/acp.1505 [Google Scholar]
- Davis C., Patte K., Tweed S., & Curtis C (2007). Personality traits associated with decision-making deficits. Personality and Individual Differences, 42, 279–290. doi:10.1016/j.paid.2006.07.006 [Google Scholar]
- Drolet A., Lau-Gesk L., & Scott C (2011). The influence of aging on preferences for seqeunces of mixed affective events. Journal of Behavioral Decision Making, 24, 293–314. doi:10.1002/bdm.695 [Google Scholar]
- Eppinger B. Nystrom L. E. & Cohen J. D (2012). Reduced sensitivity to immediate reward during decision-making in older than younger adults. Plos One, 7, e36953. doi:10.1371/journal.pone.0036953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M. F. Folstein S. E. & McHugh P. R (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Franco A. Malhotra N. & Simonovits G (2014). Social science. Publication bias in the social sciences: Unlocking the file drawer. Science (New York, N.Y.), 345, 1502–1505. doi:10.1126/science.1255484 [DOI] [PubMed] [Google Scholar]
- Frederick S., & Loewenstein G (2008). Conflicting motives in evaluations of sequences. Journal of Risk and Uncertainty, 37, 221–235. doi:10.1007/s11166-008-9051-z [Google Scholar]
- Frederick S., Loewenstein G., & O’Donoghue T (2002). Time discounting and time preference: A critical review. Journal of Economic Literature, 40, 351–401. doi:10.1257/002205102320161311 [Google Scholar]
- Greenglass E. R., Schwarzer R., Jaubiec D., Fiksenbaum L., & Taubert S (1999). The Proactive Coping Inventory (PCI): A multidimensional research instrument. Berlin: Free University of Berlin. [Google Scholar]
- Gross J. J. & John O. P (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85, 348–362. doi:10.1037/0022-3514.85.2.348 [DOI] [PubMed] [Google Scholar]
- Hancock P. A. (2010). The effect of age and sex on the perception of time in life. The American Journal of Psychology, 123, 1–13. doi:10.5406/amerjpsyc.123.1.0001 [DOI] [PubMed] [Google Scholar]
- Isen A. M. (2001). An influence of positive affect on decision making in complex situations: Theoretical issues with practical implications. Journal of Consumer Psychology, 11, 75–85. doi:10.1207/S15327663JCP1102_01 [Google Scholar]
- Jimura K. Myerson J. Hilgard J. Keighley J. Braver T. S. & Green L (2011). Domain independence and stability in young and older adults’ discounting of delayed rewards. Behavioural Processes, 87, 253–259. doi:10.1016/j.beproc.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John D. & Lang F. R (2015). Subjective acceleration of time experience in everyday life across adulthood. Developmental Psychology, 51, 1824–1839. doi:10.1037/dev0000059 [DOI] [PubMed] [Google Scholar]
- Kim S. Goldstein D. Hasher L. & Zacks R. T (2005). Framing effects in younger and older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 60, P215–P218. doi:10.1093/geronb/60.4.P215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Kaiser E., Momenan R., Walker J., Westdorp A., & Hommer D (1999). Anticipation of monetary incentives evokes brain activity: An FMRI replication and extension. Society for Neuroscience Abstracts, 25, 2147. [Google Scholar]
- Lee M. D., & Wagenmakers E.-J (2013). Bayesian cognitive modeling: A practical course. New York, NY: Cambridge University Press. doi:10.1017/CBO9781139087759 [Google Scholar]
- Löckenhoff C. E. O’Donoghue T. & Dunning D (2011). Age differences in temporal discounting: The role of dispositional affect and anticipated emotions. Psychology and Aging, 26, 274–284. doi:10.1037/a0023280 [DOI] [PubMed] [Google Scholar]
- Löckenhoff C. E., & Rutt J. L (2015). Age differences in time perception and their implications for decision making across the life span. In T. Hess J. Strough, & C. E. Löckenhoff (Eds.). Aging and decision making: Empirical and applied perspectives. London, UK: Academic Press/Elsevier. [Google Scholar]
- Löckenhoff C. E. Rutt J. L. Samanez-Larkin G. R. O’Donoghue T. Reyna V. F. & Ganzel B (2016). Dread sensitivity in decisions about real and imagined electrical shocks does not vary by age. Psychology and Aging, 31, 890–901. doi:10.1037/pag0000136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löckenhoff C. E. & Rutt J. L (2017). Age differences in self-continuity: Converging evidence and directions for future research. The Gerontologist, 57, 396–408. doi:10.1093/geront/gnx010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löckenhoff C. E. Reed A. E. & Maresca S. N (2012). Who saves the best for last? Age differences in preferences for affective sequences. Psychology and Aging, 27, 840–848. doi:10.1016/B978-0-12-417148-0.00011-X [DOI] [PubMed] [Google Scholar]
- Loewenstein G. F., & Prelec D (1993). Preferences for sequences of outcomes. Psychological Review, 100, 91–108. doi:10.1037/0033-295X.100.1.91 [Google Scholar]
- Mikels J. A. & Reed A. E (2009). Monetary losses do not loom large in later life: Age differences in the framing effect. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 64, 457–460. doi:10.1093/geronb/gbp043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata R. Josef A. K. Samanez-Larkin G. R., & Hertwig R (2011). Age differences in risky choice: A meta-analysis. Annals of the New York Academy of Sciences, 1235(1), 18–29. doi:10.1111/j.1749-6632.2011.06200.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen L. Knutson B. & Carstensen L. L (2008). Affect dynamics, affective forecasting, and aging. Emotion, 8, 318–330. doi:10.1037/1528-3542.8.3.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland J. D., Turetsky B. I., Gur R. C., Gunning-Dixon F., Turner T., Schroeder L.,…, Gur R. E. (2002). Working memory for complex figures: An fMRI comparison of letter and fractal n-back tasks. Neuropsychology, 16, 370–379. doi:10.1037//0894-4105.16.3.370 [PMC free article] [PubMed] [Google Scholar]
- Rimoldi H. J. A. (1951). Personal tempo. The Journal of Abnormal and Social Psychology, 46, 283–303. doi:10.1037/h0057479 [DOI] [PubMed] [Google Scholar]
- Rouder J. N. Speckman P. L. Sun D. Morey R. D. & Iverson G (2009). Bayesian t tests for accepting and rejecting the null hypothesis. Psychonomic Bulletin & Review, 16, 225–237. doi:10.3758/PBR.16.2.225 [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin G. R. (2013). Financial decision making and the aging brain. APS Observer, 26, 30–33. [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin G. R., & Carstensen L (2011). Socioemotional functioning and the aging brain. In J. Decety & J. T. Cacioppo (Eds.), The Oxford handbook of social neuroscience (pp. 507–521). New York: Oxford University Press. doi:10.1093/oxfordhb/9780195342161.013.0034 [Google Scholar]
- Scheibe S., & Carstensen L. L (2010). Emotional aging: Recent findings and future trends. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 65, 135–144. doi:10.1093/geronb/gbp132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe S., Mata R., & Carstensen L. L (2010). Age differences in affective forecasting and experienced emotion surrounding the 2008 US presidential election. Cognition and Emotion, 25, 1029–1044. doi:10.1037/a0025655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L. M. Woloshin S. Black W. C. & Welch H. G (1997). The role of numeracy in understanding the benefit of screening mammography. Annals of Internal Medicine, 127, 966–972. doi:10.7326/0003-4819-127-11-199712010-00003 [DOI] [PubMed] [Google Scholar]
- Seaman K. L. Gorlick M. A. Vekaria K. M. Hsu M. Zald D. H. & Samanez-Larkin G. R (2016). Adult age differences in decision making across domains: Increased discounting of social and health-related rewards. Psychology and Aging, 31, 737–746. doi:10.1037/pag0000131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takala M., & Partanen N (1964). Psychomotor expression and personality study: III. The problem of ‘personal tempo.’. Scandinavian Journal of Psychology, 5, 161–170. doi:10.1111/j.1467–9450.1964.tb01423.x [Google Scholar]
- Treadway M. T. Buckholtz J. W. Schwartzman A. N. Lambert W. E. & Zald D. H (2009). Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. Plos One, 4, e6598. doi:10.1371/journal.pone.0006598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J. L. Knutson B. & Fung H. H (2006). Cultural variation in affect valuation. Journal of Personality and Social Psychology, 90, 288–307. doi:10.1037/0022-3514.90.2.288 [DOI] [PubMed] [Google Scholar]
- Urry H. L., & Gross J. J (2010). Emotion regulation in older age. Current Directions in Psychological Science, 19, 352–357. doi:10.1177/0963721410388395 [Google Scholar]
- Ware J. E., Snow K. K., Kosinski M., & Gandek B (1993). SF-36 Health Survey - manual and interpretation guide.Boston: The Health Institute. [Google Scholar]
- Wechsler D. (1981). Wechsler Adult Intelligence Scale – Revised.San Antonio, TX: Psychological Corporation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.