In our original paper, we demonstrated, using a novel lineage tracing reporter system, that the epithelial-to-mesenchymal transition (EMT) is not necessary for metastasis but contributes to chemoresistance1. In the accompanying Comment2, Ye et al. begin by advocating for hypothetical definitions of EMT3, including concepts such as “various versions of EMT”, “certain versions of EMT” and “partial” EMT. In our view, these concepts are not well-defined by molecular characterization, nor is there direct evidence of their requirement in metastasis; thus we disagree that they can be used to interpret our findings. Ye et al.2 also suggest that Fsp1–Cre or Vim–CreER are inadequate in reporting EMT, and that miR-200 overexpression may not have inhibited EMT. Here, we wish to provide clarity regarding the tools we have used in our study.
Vim and Fsp1 promoters were selected as they are sentinels of EMT activation. They are commonly accepted mesenchymal markers, which have been employed in many published reports4,5. Ye et al.2 provided immunofluorescence images to indicate a lack of Fsp1 and vimentin in Snail+ or Zeb1+ cytokeratin+ ‘EMT’ cells. While the sensitivity and specificity of the antibodies used may contribute to the interpretation of immunofluorescence results, it is important to also recognize that the expression of one EMT transcription factor is not sufficient to conclude that EMT—a process characterized by extensive morphologic and phenotypic changes—has occurred. In addition, a recent report6 noted vimentin upregulation (>6-fold) in Snail+ cells (figure 3e in ref. 6), in agreement with our data, although we think it is unclear whether the Snail–YFP+ cells detected in the primary tumours are the true initiators of metastasis in vivo.
Ye et al.2 referenced a study showing that genetic deletion of Snail impaired lung metastasis7. However, a more recent report showed that the major impact of Snai1 knockout was the delayed onset of primary tumour growth8. Snail was highly expressed in preneoplastic lesions, and worked as a p53 suppressor that directly impacted cell proliferation in tumour-initiating cells. In pancreatic cancer models, Zheng et al. found that knockout of Twist or Snail had no effect on metastasis9. These inconsistent results further justify our decision to utilize downstream markers of EMT for our study.
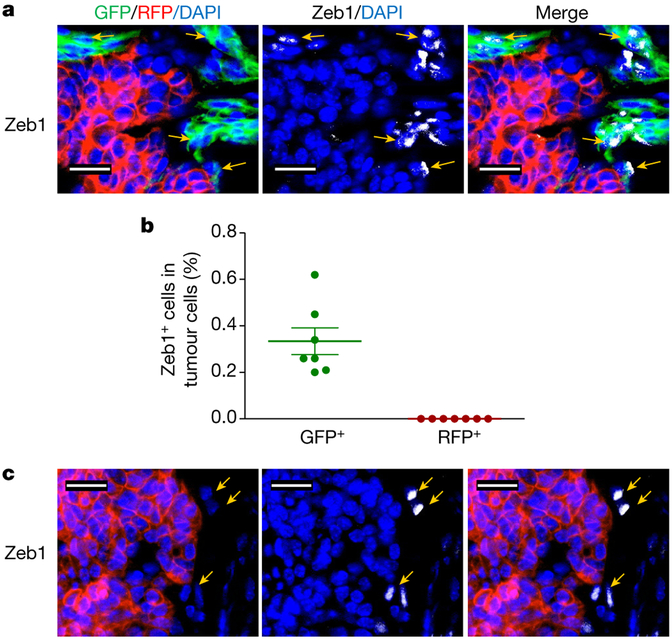
We extensively characterized the fidelity and efficiency of both Fsp1–Cre and Vim–CreER in reporting the EMT lineage in primary tumours, circulation and early metastatic lungs1. The activation of Fsp1–Cre, which led to the switch from RFP to GFP, was associated with a broad panel of EMT marker changes in the tumour cells both in vitro and in vivo. This includes analysis of the EMT transcription factor Zeb1. Quantitative immunofluorescence of the orthotopic tumours revealed Zeb1 expression in GFP+ cells, and not in RFP+ cells (Fig. 1); reinforcing the precision of our EMT lineage tracing model wherein no EMT cells were detected in the RFP+ population.
Figure 1 |. Zeb1 expression in tri-PyMT primary tumours.
a, b, Primary tumours of the orthotopic tri-PyMT model were stained with an anti-Zeb1 antibody. Representative images show that Zeb1 signals are only detected in GFP+ cells, not in RFP+ cells (as indicated by arrows in a, and quantified in b). c, Zeb1 is also expressed by stromal cells, which do not exhibit any GFP or RFP.
Ye et al.2 question the recombination efficiency of Vim–CreER. However, we reported the co-localization of vimentin with GFP+, but not RFP+ tumour cells in the Vim–CreER model (extended data fig. 6a in ref. 1), indicating the presence of GFP+ EMT cells in the primary tumours. The GFP+ EMT cells constitute 4.46 ± 1.0% of the total primary tumour cells in the Vim–CreER model1. Importantly, none of the metastases we observed were derived from these GFP+ EMT cells.
They also suggest that in the miR-200 studies, suppression of EMT was incomplete, citing a <10% reduction in N-cadherin and approximate twofold reduction in Twist. However, in extended data fig. 7d of ref. 1, we showed that E-cadherin and occludin increased 8–10-fold and vimentin and Fsp1 decreased by 5–20-fold. Notably, we reported the statistically significant suppression of the transcription factors defined as ‘EMT master regulators’ Zeb1/2 (7.1- and 5.2-fold reduction, respectively) and Snail1 and Snail2 (4.2- and 7.8- fold reduction, respectively). Yet, this had no impact on metastasis formation in our model. Ye et al.2 also postulated that the metastasis-promoting function of miR-200 would negate the anti-metastatic effects of EMT suppression. In our view, this runs counter to the theory that EMT is vital for metastasis. If the suppression of EMT is able to be compensated for by a process, “that goes beyond [miR-200’s] regulation of E-cadherin and epithelial phenotype”10, then EMT is not required for metastasis; in agreement with our findings.
Based on our EMT lineage tracing models, EMT is not required to generate distant metastasis. However, we confirmed a role for EMT in metastatic recurrence in the context of chemotherapy, consistent with findings in a model of pancreatic cancer9. We believe that future studies employing innovative technologies will continue to investigate the role of EMT; however, alternative modes of tumour dissemination such as collective or cluster-based migration and/or invasion11,12 should be considered.
This Reply was prepared by the authors who made major concept contributions to the original paper1, while other authors who made technical and material contributions did not participate.
Methods
Primary tumours from mice (the orthotopic tri-PyMT model) were collected and fixed in 4% paraformaldehyde overnight, followed by 30% sucrose for 2 days. Tissues were then embedded in Tissue-Tek O.C.T. and serial sections (10 μm, at least 10 sections) were prepared with a cryostat (Leica Biosystems) at —30 °C. Sections were blocked in PBS with 0.25% Triton X-100 and 2% FBS, and incubated with primary antibody against Zeb1 at 4 °C overnight, followed by anti-rabbit secondary antibody conjugated to Alexa Fluor 647. Cell nuclei were counterstained with DAPI (5 μg ml−1) in PBST. GFP and RFP signals were detected by inherent cell fluorescence. Images were acquired using a computerized Zeiss fluorescent microscope (Axiovert 200M, Carl Zeiss). Tumour cells from ten random sections of three individual primary tumours were used for quantification.
Footnotes
Data availability. All data are available from the corresponding author upon reasonable request.
References
- 1.Fischer KR et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527, 472–476 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye X et al. Upholding a role for EMT in pancreatic cancer metastasis. Nature 547, 10.1038/nature22816 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nieto MA, Huang RY, Jackson RA & Thiery JP EMT: 2016. Cell 166, 21–45 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Kalluri R & Weinberg RA The basics of epithelial-mesenchymal transition. J. Clin. Invest 119, 1420–1428 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhim AD et al. EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–361 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye X et al. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature 525, 256–260 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran HD et al. Transient SNAIL1 expression is necessary for metastatic competence in breast cancer. Cancer Res. 74, 6330–6340 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni T et al. Snail1-dependent p53 repression regulates expansion and activity of tumour-initiating cells in breast cancer. Nat. Cell Biol 18, 1221–1232 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng X et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527, 525–530 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korpal M et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat. Med 17, 1101–1108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung KJ et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl Acad. Sci. USA 113, E854–E863 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung KJ, Gabrielson E, Werb Z & Ewald AJ Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 155, 1639–1651 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]