Summary
The ultimate cause of death for most patients with newly diagnosed chronic lymphocytic leukaemia (CLL) and its relationship to co-morbid health conditions is poorly defined. We conducted a prospective cohort study that systematically followed 1143 patients diagnosed with CLL between June 2002 and November 2014. Comorbid health conditions at the time of CLL diagnosis and their relationship to survival and cause of death were evaluated. Collectively, 1061 (93%) patients had at least one co-morbid health condition at the time of CLL diagnosis (median number 3). Despite this, 89% of patients had a low-intermediate Charlson Comorbidity Index score (CCI) at diagnosis. After a median follow-up of 6 years, 225 patients have died. Death was due to CLL progression in 85 (46%) patients, infection in 14 (8%) patients, other cancer in 35 (19%) patients and comorbid health conditions in 50 (27%) patients. Higher CCI score and a greater number of major comorbid health conditions at the time of CLL diagnosis was associated with shorter non-CLL specific survival, but not with shorter CLL-specific survival on multivariate analysis. In conclusion, CLL and CLL-related complications (infections and second cancers) are the overwhelming cause of death in patients with CLL, regardless of CCI score and number of comorbid health conditions at diagnosis.
Keywords: chronic lymphocytic leukaemia, comorbidities, causes of death
Chronic lymphocytic leukaemia (CLL) is a disease of the elderly (median age at diagnosis ~72 years) and typically occurs in individuals with coexistent health problems (Satram-Hoang et al, 2014), which may affect survival (Charlson et al, 1987). Over the last 15 years, a number of genetic, biological and molecular characteristics of CLL B-cells that are associated with survival have been identified (Di Giovanni et al, 1989; Dohner et al, 2000; Rassenti et al, 2008) Although comprehensive approaches to integrate clinical and biological factors into a single risk score have been developed (Wierda et al, 2007; Pflug et al, 2014), they have not yet integrated the impact of comorbidities and competing causes of death. These facts make accurate risk stratification and counselling of newly diagnosed patients challenging.
It is already known that traditional prognostic factors play a more limited role for predicting outcome among patients aged 75 years or older (Shanafelt et al, 2010). However, how to account for the impact of concomitant medical conditions on patient outcome in newly diagnosed CLL patients remains unclear (Thurmes et al, 2008; Goede et al, 2014; Nabhan et al, 2014). Conflicting data are available in the literature as to whether comorbid conditions predict prognosis in newly diagnosed patients with CLL (Thurmes et al, 2008; Reyes et al, 2012), whereas they more clearly play an independent prognostic role at time of first treatment (Goede et al, 2014; Manda et al, 2014).
Here we report the findings of a prospective cohort study that evaluated the cause of death in newly diagnosed patients with CLL and evaluated whether the number of comorbidities predicts the cause of death and survival of newly diagnosed patients.
Methods
Study population
The Molecular Epidemiology Resource (MER) of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (SPORE) was used for this study. The MER is a prospective cohort study of non-Hodgkin lymphoma and CLL outcomes that was initiated in 2002 (Thompson et al, 2011; Maurer et al, 2014). Newly diagnosed patients with CLL who were evaluated at Mayo Clinic, Rochester, MN, within 9 months of CLL diagnosis, were aged 18 years or older, resident in the United States and enrolled in MER between June 2002 and November 2014, were included in this cohort. Exclusion criteria included human immunodeficiency virus infection, non-English speaking, and inability to provide written informed consent. At baseline, patients provided data on personal and family history, comorbidities and functional status. Patients were prospectively and systematically contacted every 6 months during the first 3 years after initial diagnosis to assess vital status, disease progression and new treatments. Patients then continued to be followed until death or last to follow-up. The study was approved by the Institutional Review Board of Mayo Clinic and was conducted in accordance with the principles of the Declaration of Helsinki.
Comorbidity and mortality assessment
Comorbidities diagnosed prior to or simultaneously with CLL diagnosis were recorded at the time of first CLL evaluation at Mayo Clinic. Comorbidities were assigned to one of 15 categories: stroke, cardiovascular disease (including coronary artery disease, peripheral vascular disease, cardiomyopathy, valve heart disease and atrial fibrillation), hypertension, respiratory disease, diabetes mellitus, other endocrinological disease, dyslipidaemia, rheumatological disease, gastrointestinal disease, genitourinary disease, psychiatric disease, history of deep venous thrombosis or pulmonary embolism, alcohol abuse, sexually transmitted disease, and other cancers (excluding non-melanoma skin cancer). For other analyses, baseline comorbidities were further analysed as major comorbid conditions (e.g. cerebrovascular disease, cardiovascular disease, respiratory disease, diabetes mellitus and other cancer). The Charlson Comorbidity Index (CCI) was also calculated for each patient, based on comorbid health conditions present at the time of diagnosis (Deyo et al, 1992). The CCI is an age-weighted prognostic score based on 17 disease categories, which has been validated over the last 25 years and has reliably predicted survival in any patient population (Charlson et al, 1987). A CCI score ≤3 was considered low, 4–5 was moderate, 6–7 was high and ≥8 was very high.
Information on cause of death was also collected from electronic records. For patients in whom cause of death could be accurately determined, the cause of death was classified into one of four categories: (i) progressive CLL, (ii) infection, (iii) other cancers or (iv) comorbid health condition (in absence of CLL progression). When the cause of death could not be accurately determined, the cause of death was categorized as ‘unknown’.
Statistical analysis
Descriptive statistics were used to summarize baseline clinical characteristics. The relationship between baseline categorical or continuous variables and ultimate cause of death were compared using the χ2 or Fisher exact tests and the Kruskal–Wallis test, as appropriate. Comorbidity categorical indices were modelled as continuous variables. Survival was modelled using the Kaplan–Meier method using a log-rank test to compare each comorbidity index. Overall survival (OS) was defined as time from diagnosis to death or last follow-up. CLL-specific survival was defined as time from diagnosis to death from a CLL-related cause. CLL progression, infection and second cancer were considered CLL-related causes of death for this analysis. Patients who died due to other causes were treated as a competing risk. Non-CLL specific survival was defined as time from diagnosis to death from a cause unrelated to CLL. Patients who died due to CLL progression, infection, and second cancer were treated as a competing risk at the time of death for this analysis. Patients with unknown cause of death were excluded for both the CLL-specific and non-CLL specific survival analyses. Logistic regression was used to compare comorbidities at baseline by cause of death (i.e., CLL-specific versus non-CLL specific). Survival was displayed using a cumulative incidence function with competing risks for CLL- and non-CLL specific analyses using Gray’s test to compare groups.
Multivariate analyses (MVA) to identify characteristics independently associated with mortality were performed using Cox regression analysis, accounting for competing risks (when appropriate) using Fine-Gray models. Each model contained age (10-year increments), sex and Rai stage; we then added one of the comorbidity measures (e.g. CCI or number of major co-morbidities) to the model. Due to the number of missing values for molecular CLL prognostic factors [e.g. fluorescence in situ hybridization (FISH), IGHV], we were unable to include these factors in the multivariate models. All P-values were 2-sided and considered significant if ≤0·05. Analyses were performed using SAS version 9·4 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics and comorbidities
One thousand and forty-three patients were included in the study. Baseline characteristics are shown in Table I. Median age was 63 years and two-thirds were men. Among those patients with CLL prognostic testing available, 44% had unmutated IGHV and 14% had unfavourable genetic characteristics on FISH analysis (del17p or del11q).
Table I.
Baseline characteristics of CLL patients.
| Patients (N = 1143) | Number (%), median [range] |
|---|---|
| Age at diagnosis (years) | 63.0 [23.7–89.5] |
| Age <60 | 464 (41%) |
| Age 60–69 | 372 (33%) |
| Age 70+ | 307 (27%) |
| Sex | |
| Male | 769 (67) |
| Female | 374 (33) |
| Race | |
| Caucasian | 1066 (98) |
| Non-Caucasian | 27 (2) |
| Missing | 50 |
| ALC (× 109/l) | 9.5 [0.4–958] |
| ALC <25 | 940 (83%) |
| ALC 25–50 | 88 (8%) |
| ALC >50 | 101 (9%) |
| Missing | 14 |
| Haemoglobin (g/l) | 141 [49–179] |
| Missing | 11 |
| Platelet count (× 109/l) | 198 [19–675] |
| Missing | 12 |
| Creatinine clearance (ml/min) | 85.4 [9.5–251.1] |
| Missing | 287 |
| Albumin (g/l) | 37 [11–50] |
| Missing | 246 |
| Total bilirubin (μmol/l) | 0.03 [0.005–1.3] |
| Missing | 240 |
| Rai stage | |
| 0 | 629 (55) |
| I–II | 458 (40) |
| III–IV | 55 (5) |
| Missing | 1 |
| B2M | |
| < 35 mg/l | 845 (81) |
| ≥ 35 mg/l | 194 (19) |
| Missing | 104 |
| CD49d | |
| Negative (<45%) | 707 (69) |
| Positive (≥45%) | 317 (31) |
| Missing | 119 |
| CD49d | |
| Negative (<30%) | 658 (64) |
| Positive (≥30%) | 365 (36) |
| Missing | 120 |
| IGHV | |
| Mutated | 559 (56) |
| Unmutated | 436 (44) |
| Missing | 148 |
| High-Risk FISH | |
| No (negative, del13q, +12) | 903 (86) |
| Yes (del11q, del17p) | 148 (14) |
| Missing/Other | 92 |
ALC, absolute lymphocyte count; B2M, beta-2-microglobulin; FISH, fluorescence in situ hybridization; IGHV, Immunoglobulin heavy chain variable region gene.
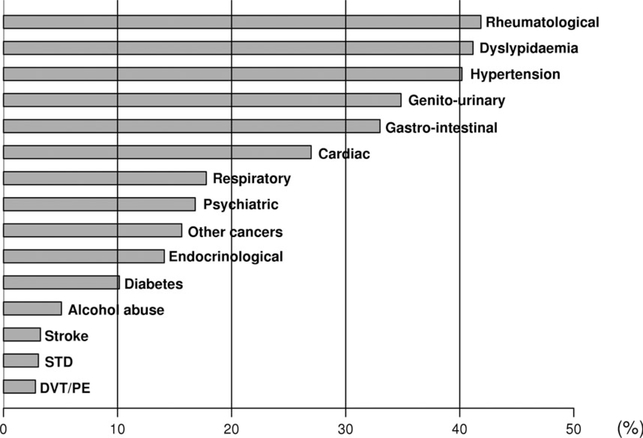
The prevalence of various comorbid health conditions present at the time of CLL diagnosis are shown in Fig 1. Rheumatological diseases (primarily osteoarthritis, 42% of patients), dyslipidaemia (41%) and hypertension (40%) were the three most common comorbid health conditions present at the time of CLL diagnosis. The CCI score was low (0–3) at the time of CLL diagnosis in 831 (73%) patients, intermediate (4–5) in 185 (16%), high (6–7) in 76 (7%) and very high (8+) in 51 (4%). While 548 (48%) patients had no major comorbidities at time of CLL diagnosis, 394 (34%) had one major comorbidity and 201 (18%) had two or more major comorbidities.
Fig 1.
Baseline comorbid health conditions in 1143 CLL patients. DVT, deep venous thrombosis; PE, pulmonary embolism; STD, sexually transmitted disease. ‘Other cancers’ did not include non-melanoma skin cancers.
Cause of death
After a median follow-up of 6 years, 225 patients had died. The cause of death could be accurately determined in 184 (82%) of these patients: CLL progression in 85 (46%) patients, infection in 14 (8%), other cancer in 35 (19%) and comorbid health conditions in 50 (27%). The relationship between causes of death and baseline characteristics is shown in Table II. Patients with unmutated IGHV had a greater likelihood of experiencing a CLL-related death (odds ratio [OR] 219, 95% confidence interval [CI] 106–454; P = 004). No other CLL-related prognostic parameters had a statistically significant association with a CLL-related cause of death, although power was limited for some comparisons (e.g., high-risk FISH). With respect to comorbid health conditions at the time of CLL diagnosis, patients with a history of stroke (OR 574, 95% CI 102–3238; P = 0048), history of cardiac disease (OR 202, 95% CI 104–393; P = 004), higher CCI (OR 146, 95% CI 107–198; P = 002) and higher number of major comorbidities (OR 173, 95% CI 113–265; P = 001) had a higher likelihood of dying due to non-CLL related causes. No other co-morbid health conditions had a statistically significant association with a non-CLL related cause of death on univariate analysis.
Table II.
Causes of mortality by baseline characteristics and comorbidities*
| Cause of death |
|||
|---|---|---|---|
| CLL-related (n = 134) | Unrelated to CLL (n = 50) | P-value | |
| Age (years) | 68 [39–87] | 71 [44–89] | 0.06 |
| Age at diagnosis (years) | |||
| Age <60 | 29 (22) | 8 (16) | 0.30 |
| Age 60–69 | 47 (35) | 14 (28) | |
| Age 70+ | 58 (43) | 28 (56) | |
| Sex | |||
| Males | 103 (77) | 41 (82 | 0.45 |
| Females | 31 (23) | 41 (82) 9 (18) | |
| Laboratory parameters at diagnosis | |||
| ALC at diagnosis (× 109/l) | 10.1 [0.4–958] | 8.2 [1.0–115] | 0.52 |
| Creatinine clearance (ml/min) | 80 [10–199] | 74 [21–143] | 0.16 |
| Albumin (g/l) | 37 [11–49] | 36 [28–47] | 0.32 |
| Total bilirubin (lmol/l) | 0.03 [0.01–1.3] | 0.03 [0.02–0.1] | 0.93 |
| B2M | |||
| ≥35 mg/l | 45 (41) | 17 (39) | 0.76 |
| <35 mg/l | 64 (59) | 27 (61) | 0.76 |
| Missing | 25 | 6 | |
| CLL characteristics at diagnosis | |||
| Rai stage | |||
| 0 | 53 (40) | 24 (48) | 0.16 |
| I–II | 62 (47) | 24 (48) | |
| III–IV | 18 (14) | 2 (4) | |
| Missing | 1 | 0 | |
| CD49d | |||
| Positive (≥45%) | 51 (48) | 14 (34) | 0.13 |
| Negative (<45%) | 55 (52) | 27 (66) | |
| Missing | 28 | 9 | |
| CD49d | |||
| Positive (≥30%) | 58 (55) | 17 (41) | 0.15 |
| Negative (<30%) | 48 (45) | 24 (59) | |
| Missing | 28 | 9 | |
| IGHV | |||
| Unmutated | 78 (72) | 23 (53) | 0.034 |
| Mutated | 31 (28) | 20 (47) | |
| Missing | 25 | 7 | |
| High-Risk FISH | |||
| No (negative, del13q, +12) | 76 (66) | 37 (80) | 0.06 |
| Yes (del11q, del17p) | 40 (34) | 9 (20) | |
| Missing/Other | 18 | 4 | |
| Comorbid conditions at diagnosis | |||
| Other cancer† | |||
| Yes | 32 (24) | 10 (20) | 0.57 |
| No | 99 (76) | 39 (80) | |
| Missing | 3 | 1 | |
| Stroke | |||
| Yes | 2 (1) | 4 (8) | 0.047 |
| No | 132 (99) | 46 (92) | |
| Cardiac disease | |||
| Yes | 42 (31) | 24 (48) | 0.036 |
| No | 92 (69) | 26 (52) | |
| Hypertension | |||
| Yes | 58 (43) | 29 (58) | 0.08 |
| No | 76 (57) | 21 (42) | |
| Respiratory | |||
| Yes | 28 (21) | 14 (28) | 0.31 |
| No | 106 (79) | 36 (72) | |
| Endocrinological | |||
| Yes | 22 (16) | 6 (12) | 0.46 |
| No | 112 (84) | 44 (88) | |
| Diabetes | |||
| Yes | 15 (11) | 11 (22) | 0.06 |
| No | 119 (89) | 39 (78) | |
| Dyslipidaemia | |||
| Yes | 47 (35) | 25 (50) | 0.07 |
| No | 87 (65) | 25 (50) | |
| Rheumatological | |||
| Yes | 56 (42) | 16 (32) | 0.23 |
| No | 78 (58) | 34 (68) | |
| Gastrointestinal | |||
| Yes | 47 (35) | 13 (74) | 0.24 |
| No | 87 (65) | 37 (74) | |
| Genitourinary | |||
| Yes | 53 (40) | 23 (46) | 0.43 |
| No | 81 (60) | 27 (54) | |
| Psychiatric | |||
| Yes | 17 (13) | 5 (10) | 0.62 |
| No | 117 (87) | 45 (90) | |
| DVT/PE | |||
| Yes | 2 (1) | 3 (6) | 0.12 |
| No | 132 (99) | 47 (94) | |
| Alcohol abuse | |||
| Yes | 8 (6) | 3 (6) | 1.00 |
| No | 126 (94) | 47 (94) | |
| STD | |||
| Yes | 5 (4) | 1 (2) | 1.00 |
| No | 129 (96) | 49 (98) | |
| CCI (median, range) | 3 (0–17) | 4 (0–12) | 0.019 |
| CCI score | |||
| 0–3 | 86 (64) | 20 (40) | 0.006 |
| 4–5 | 20 (15) | 16 (32) | |
| 6–7 | 19 (14) | 6 (12) | |
| 8+ | 9 (7) | 8 (16) | |
| Major CM(median, range) | 1 (0–4) | 1 (0–4) | 0.011 |
| Major CM | |||
| 0 | 56 (42) | 11 (22) | 0.032 |
| 1 | 48 (36) | 21 (42) | |
| 2+ | 30 (22) | 18 (36) | |
ALC, absolute lymphocyte count; B2M, beta-2-microglobulin; CCI, Charlson Comorbidity Index; CLL, chronic lymphocytic leukaemia; CM, comorbidities; DVT, deep venous thrombosis; FISH, fluorescence in situ hybridization; IGHV, Immunoglobulin heavy chain variable region gene; PE, pulmonary embolism; STD, sexually transmitted disease.
Deaths due to infection and other cancers are categorized as CLLrelated death. Deaths due to unknown cause are not included.
‘Other cancers’ did not include non-melanoma skin cancers.
Overall survival
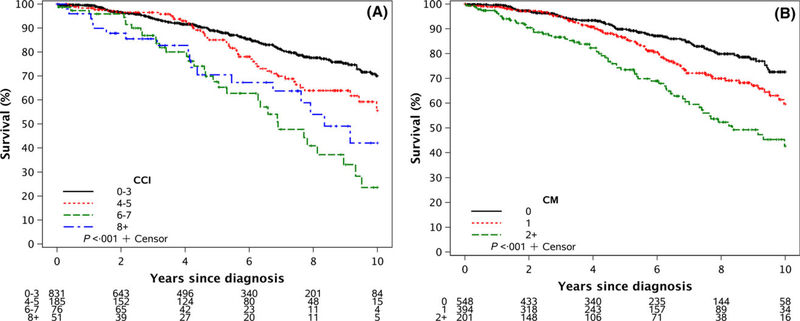
After a median follow-up of 6 years, the median OS had not been reached. On univariate analysis, both a higher CCI score (Fig 2A) and a higher number of major comorbidities at diagnosis (Fig 2B) were significantly associated with a shorter OS (both P < 0001). On MVA, after adjusting for age, sex and Rai stage, a higher CCI score appeared to correlate with OS although this difference was not statistically significant (Table IIIA; HR = 114; 95% CI 098–132; P = 010). A greater number of major comorbidities at diagnosis were significantly associated with OS in the MVA (Table III; HR = 127; 95% CI 106–152; P = 0009).
Fig 2.
Overall survival. (A) Overall survival by Charlson Comorbidity Index (CCI) score at the time of CLL diagnosis. (B) Overall survival by number of major comorbidities (CM) at time of CLL diagnosis. Events included all types of death, both CLL-related, non-CLL related, and of unknown cause.
Table III.
Multivariate analysis of mortality.
| Overall mortality |
CLL-specific mortality |
Non-CLL specific mortality |
||||
|---|---|---|---|---|---|---|
| HR [95% CI] | P-value | HR [95% CI] | P-value | HR [95% CI] | P-value | |
| (A) Including Charlson Comorbidity Index. | ||||||
| Age (10 years) | 1.82 [1.56–2.11] | <0.001 | 1.67 [1.37–2.05] | <0.001 | 1.81 [1.29–2.54] | <0.001 |
| Males | 1.68 [1.22–2.31] | 0.001 | 1.47 [0.98–2.20] | 0.06 | 2.00 [0.96–4.18] | 0.06 |
| Rai categories | 2.21 [1.78–2.74] | <0.001 | 2.52 [1.91–3.33] | <0.001 | 1.38 [0.90–2.12] | 0.14 |
| CCI categories | 1.14 [0.98–1.32] | 0.10 | 1.00 [0.80–1.25] | 0.99 | 1.37 [1.02–1.82] | 0.035 |
| (B) Including number of major comorbidities. | ||||||
| Age (10 years) | 1.81 [1.58–2.09] | <0.001 | 1.66 [1.38–1.99] | <0.001 | 1.90 [1.38–2.63] | <0.001 |
| Males | 1.58 [1.15–2.19] | 0.005 | 1.45 [0.97–2.18] | 0.07 | 1.74 [0.82–3.69] | 0.15 |
| Rai categories | 2.21 [1.78–2.74] | <0.001 | 2.52 [1.91–3.34] | <0.001 | 1.31 [0.86–2.00] | 0.21 |
| Major CM | 1.27 [1.06–1.52] | 0.009 | 1.03 [0.82–1.30] | 0.79 | 1.61 [1.11–2.33] | 0.012 |
95% CI, 95% confidence interval; CCI, Charlson Comorbidity Index; CLL, chronic lymphocytic leukaemia; CM, comorbidities; HR, hazard ratio.
CLL-specific and non-CLL specific mortality
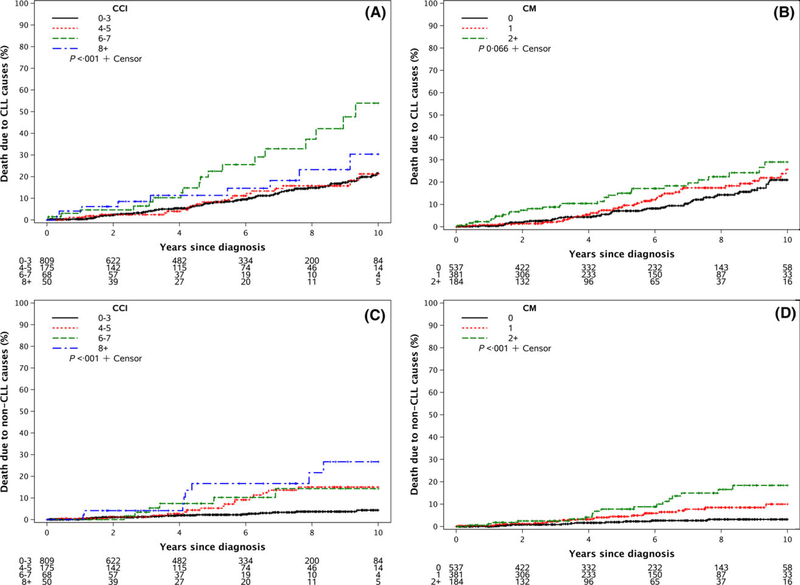
Median CLL-specific and non-CLL specific mortality have not been reached with current follow-up. On univariate analysis, higher CCI score was associated with a higher CLL-specific mortality (Fig 3A; P < 0001). A greater number of major comorbidities at diagnosis had a non-significant trend toward higher CLL-specific mortality (Fig 3B; P = 0066). Both a higher CCI score and higher number of major comorbidities were associated with higher non-CLL specific mortality (Figs 3C and D, respectively; P < 0001 for both).
Fig 3.
CLL-specific and non-CLL specific mortality. (A) CLL-specific-mortality by Charlson Comorbidity Index (CCI) score at time of CLL diagnosis. (B) CLL-specific-mortality by number of major comorbidities (CM) at time of CLL diagnosis. (C) Non-CLL specific mortality by Charlson Comorbidity Index score at time of CLL diagnosis. (D) Non-CLL specific mortality by number of major comorbidities at time of CLL diagnosis.
After adjusting for age, sex and Rai stage, MVA identified no statistically significant associations between CCI score or the number of major comorbidities at the time of diagnosis and CLL-specific mortality (Tables IIIA,B). In contrast, both higher CCI score (Table IIIA; HR = 137; 95% CI 102–182; P = 0035) and higher number of major comorbidities (Table III; HR = 161; 95% CI 111–233; P = 0012) were significantly associated with increased non-CLL specific mortality.
Finally, when the analyses were repeated either (i) censoring deaths of unknown cause or (ii) assigning patients with deaths of unknown cause to one of the death groups (CLL-related and unrelated), no significant difference in the above findings were observed.
Discussion
Limited information is available regarding cause of death in patients with CLL and its relationship to baseline comorbidities. The dogma is generally that patients with CLL are elderly, frequently have comorbid health conditions, and are likely to ultimately die from causes unrelated to CLL. Here we report one of the few studies to evaluate cause of death in a cohort of newly diagnosed patients with CLL followed prospectively and to evaluate their relationship with comorbidity.
Although comorbidities were common, a majority of patients died directly from CLL (46%) or infection (8%). An additional ~20% died of other cancers that may be at least indirectly related to CLL given the increased risk of non-hematologic cancer associated with this condition. The CCI and the number of comorbidities at diagnosis were associated with survival in MVA and appeared to mediate this effect through an influence on non-CLL specific mortality, but not on CLL-specific mortality. These results indicating that most patients with CLL die directly of CLL or CLL related complications challenge many traditionally held paradigms.
Predicting the most likely cause of death following diagnosis becomes crucial for the management of these patients, particularly when therapy is needed. Currently, many patients are excluded from standard therapy or clinical trials because of concomitant comorbid conditions. Our results, however, demonstrate that CLL and its complications remain the main cause of death in these patients, and that comorbidities don’t affect CLL-specific survival. These results highlight the importance of effective CLL therapy regardless of the presence of concomitant health conditions. In addition, it prompts the need for new therapies that can be employed in elderly patients with comorbid conditions. Ultimately, this may translate into longer survival and lower healthcare-associated costs.
In our study, despite a younger median age than typically reported in CLL (63 vs. 72 years), comorbid health conditions were very common at time of CLL diagnosis, with rheumatological diseases, hypertension and dyslipidaemia each being observed in 40% or more of patients. Their high incidence in this elderly population may just be secondary to aging. However, several studies have shown a significant association between CLL and chronic illnesses, such as renal disease (Strati et al, 2015), dyslipidaemia (Mulas et al, 2011) and hypovitaminosis D (Shanafelt et al, 2011). While both direct organ infiltration and microenvironment activity may be contributing factors to some of these other health conditions, the specific mechanisms underlying such associations remain unclear.
Despite the high frequency of comorbidities at time of CLL diagnosis, the majority of patients showed a low-intermediate CCI and had fewer than two major comorbidities, pointing toward a relatively low burden of comorbid health conditions in our cohort. While a high CCI at time of CLL diagnosis may predict poor survival, there is limited evidence regarding the effects of multiple comorbidities on treatment outcomes (Fried et al, 2014). In fact, although data is limited, some studies suggest CLL and related complications are the cause of death for about 50% of patients with this disease (Thurmes et al, 2008; Goede et al, 2014; Nabhan et al, 2014; Satram-Hoang et al, 2014). In our study, we found an even higher rate, in which 73% died of CLL progression or CLL related complications, such as infections or other cancers (Tsimberidou et al, 2009; Royle et al, 2011; Solomon et al, 2013). Of interest, this is a cohort of patients diagnosed in the chemoimmunotherapy era; while the latter remains the standard of care for fit previously untreated patients, ongoing changes in therapy standards, with the potential introduction of newer biological agents as frontline regimens, may alter this scenario in the near future.
Not surprisingly, the presence of unmutated IGHV was significantly associated with CLL-related mortality, with 77% of unmutated patients dying of CLL-related causes vs. 61% of IGHV mutated patients, as previously observed (Wierda et al, 2007; Fried et al, 2014; Pflug et al, 2014; Kutsch et al, 2015). The presence of stroke or cardiac disease at time of CLL diagnosis was associated in our study with a higher rate of non-CLL related mortality, despite appropriate secondary prevention. In fact, 33% of patients with history of stroke had a CLL-related death versus 74% of patients without it, and 64% of patients with a cardiac disease history died of CLL-related causes versus 78% of patients without it. However, beyond these few associations, CLL progression and complications remained the main cause of death, irrespective of baseline characteristics and comorbidities.
In our study, while associated with a shorter non-CLL specific survival, a higher CCI and a higher number of major comorbidities did not associate with a shorter CLL-specific survival on MVA. Our group had already demonstrated that baseline comorbidities are less relevant than age and Rai stage in predicting survival at time of CLL diagnosis (Thurmes et al, 2008). A more recent study, however, showed that the presence of comorbidities at time of first treatment is an independent prognostic factor for survival (Goede et al, 2014), highlighting the prognostic role played by chronic illnesses when CLL requires therapy. The lack of association between baseline comorbidities and CLL-specific mortality highlights the predominant roles played by CLL progression and complications in the survival of CLL patients. This is a relevant point, as comorbidities can limit therapeutic options for patients with CLL and frequently represent exclusion criteria in clinical trials. As a consequence, both less toxic agents and clinical trials evaluating treatments that are designed to be tolerated by patients who do not meet traditional clinical trial eligibility criteria are needed.
Our study has some limitations. This is a single centre study, employing 2 specific scales to assess comorbidities, and causes of death could not effectively be collected for all patients. In addition, the investigated cohort was younger than the average CLL population, potentially affecting the observed results.
Continued efforts are needed to determine the optimal approach to assess co-morbidity and functional status in patients with CLL, and integrate these measures with established prognostic tools at different time-points in the course of the disease including at the time of and the time of first treatment.
Acknowledgments
This study was funded by the Cancer Center Grant P30 CA015083.
Footnotes
Conflict-of-Interest
The authors declare no conflicts of interest.
References
- Charlson ME, Pompei P, Ales KL & MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases, 40, 373–383. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Cherkin DC & Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology, 45, 613–619. [DOI] [PubMed] [Google Scholar]
- Di Giovanni S, Valentini G, Carducci P & Giallonardo P (1989) Beta-2-microglobulin is a reliable tumor marker in chronic lymphocytic leukemia. Acta haematologica, 81, 181–185. [DOI] [PubMed] [Google Scholar]
- Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, Dohner K, Bentz M & Lichter P (2000) Genomic aberrations and survival in chronic lymphocytic leukemia. The New England journal of medicine, 343, 1910–1916. [DOI] [PubMed] [Google Scholar]
- Esenwa C & Gutierrez J (2015) Secondary stroke prevention: challenges and solutions. Vascular health and risk management, 11, 437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried TR, O’Leary J, Towle V, Goldstein MK, Trentelange M & Martin DK (2014) The effects of comorbidity on the benefits and harms of treatment for chronic disease: a systematic review. PLoS ONE, 9, e112593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goede V, Cramer P, Busch R, Bergmann M, Stauch M, Hopfinger G, Stilgenbauer S, Dohner H, Westermann A, Wendtner CM, Eichhorst B & Hallek M (2014) Interactions between comorbidity and treatment of chronic lymphocytic leukemia: results of German Chronic Lymphocytic Leukemia Study Group trials. Haematologica, 99, 1095–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsch N, Bahlo J, Byrd JC, Dohner H, Eichhorst B, Else M, Geisler C, Grever MR, Lepretre S, Bergman M, Neuberg DS, Oscier D, Rosenquist R, Robak T, Shanafelt TD, Stilgenbauer S & Hallek MJ (2015) The international Prognostic Index for patients with CLL (CLL-IPI): an international meta-analysis. Journal of Clinical Oncology, 33, 779–790. [Google Scholar]
- Manda S, James S, Wang R, Krishnan R & Danilov AV (2014) Impact of comorbidities on treatment outcomes in chronic lymphocytic leukemia: a retrospective analysis. Blood, 124, 1312.25006122 [Google Scholar]
- Maurer MJ, Ghesquieres H, Jais JP, Witzig TE, Haioun C, Thompson CA, Delarue R, Micallef IN, Peyrade F, Macon WR, Molina TJ, Ketterer N, Syrbu SI, Fitoussi O, Kurtin PJ, Allmer C, Nicolas-Virelizier E, Slager SL, Habermann TM, Link BK, Salles G, Tilly H & Cerhan JR (2014) Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-Cell lymphoma treated with immunochemotherapy. Journal of Clinical Oncology, 32, 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulas MF, Abete C, Pulisci D, Pani A, Massidda B, Dessi S & Mandas A (2011) Cholesterol esters as growth regulators of lymphocytic leukaemia cells. Cell proliferation, 44, 360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabhan C, Aschebrook-Kilfoy B, Chiu BC, Smith SM, Shanafelt TD, Evens AM & Kay NE (2014) The impact of race, ethnicity, age and sex on clinical outcome in chronic lymphocytic leukemia: a comprehensive Surveillance, Epidemiology, and End Results analysis in the modern era. Leukemia & Lymphoma, 55, 2778–2784. [DOI] [PubMed] [Google Scholar]
- Pflug N, Bahlo J, Shanafelt TD, Eichhorst BF, Bergmann MA, Elter T, Bauer K, Malchau G, Rabe KG, Stilgenbauer S, Dohner H, Jager U, Eckart MJ, Hopfinger G, Busch R, Fink AM, Wendtner CM, Fischer K, Kay NE & Hallek M (2014) Development of a comprehensive prognostic index for patients with chronic lymphocytic leukemia. Blood, 124, 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassenti LZ, Jain S, Keating MJ, Wierda WG, Grever MR, Byrd JC, Kay NE, Brown JR, Gribben JG, Neuberg DS, He F, Greaves AW, Rai KR & Kipps TJ (2008) Relative value of ZAP-70, CD38, and immunoglobulin mutation status in predicting aggressive disease in chronic lymphocytic leukemia. Blood, 112, 1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes C, Satram-Hoang S, Hoang K, Momin F, Guduru SR & Skettino S (2012) What is the impact of comorbidity burden on treatment patterns and outcomes in elderly chronic lymphocytic leukemia patients? Blood, 120, 758. [Google Scholar]
- Royle JA, Baade PD, Joske D, Girschik J & Fritschi L (2011) Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population-based study. British journal of cancer, 105, 1076–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satram-Hoang S, Reyes C, Hoang KQ, Momin F & Skettino S (2014) Treatment practice in the elderly patient with chronic lymphocytic leukemia-analysis of the combined SEER and Medicare database. Annals of Hematology, 93, 1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanafelt TD, Rabe KG, Kay NE, Zent CS, Jelinek DF, Reinalda MS, Schwager SM, Bowen DA, Slager SL, Hanson CA & Call TG (2010) Age at diagnosis and the utility of prognostic testing in patients with chronic lymphocytic leukemia. Cancer, 116, 4777–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanafelt TD, Drake MT, Maurer MJ, Allmer C, Rabe KG, Slager SL, Weiner GJ, Call TG, Link BK, Zent CS, Kay NE, Hanson CA, Witzig TE & Cerhan JR (2011) Vitamin D insufficiency and prognosis in chronic lymphocytic leukemia. Blood, 117, 1492–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon BM, Rabe KG, Slager SL, Brewer JD, Cerhan JR & Shanafelt TD (2013) Overall and cancer-specific survival of patients with breast, colon, kidney, and lung cancers with and without chronic lymphocytic leukemia: a SEER population-based study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 31, 930–937. [DOI] [PubMed] [Google Scholar]
- Strati P, Nasr SH, Leung N, Hanson CA, Chaffee KG, Schwager SM, Achenbach SJ, Call TG, Parikh SA, Ding W, Kay NE & Shanafelt TD (2015) Renal complications in chronic lymphocytic leukemia and monoclonal B-cell lymphocytosis: mayo clinic experience. Haematologica, 100, 1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CA, Maurer MJ, Cerhan JR, Katzmann JA, Ansell SM, Habermann TM, Macon WR, Weiner GJ, Link BK & Witzig TE (2011) Elevated serum free light chains are associated with inferior event free and overall survival in Hodgkin lymphoma. American Journal of Hematology, 86, 998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmes P, Call T, Slager S, Zent C, Jenkins G, Schwager S, Bowen D, Kay N & Shanafelt T (2008) Comorbid conditions and survival in unselected, newly diagnosed patients with chronic lymphocytic leukemia. Leukemia & Lymphoma, 49, 49–56. [DOI] [PubMed] [Google Scholar]
- Tsimberidou AM, Wen S, McLaughlin P, O’Brien S, Wierda WG, Lerner S, Strom S, Freireich EJ, Medeiros LJ, Kantarjian HM & Keating MJ (2009) Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 27, 904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierda WG, O’Brien S, Wang X, Faderl S, Ferrajoli A, Do KA, Cortes J, Thomas D, Garcia-Manero G, Koller C, Beran M, Giles F, Ravandi F, Lerner S, Kantarjian H & Keating M (2007) Prognostic nomogram and index for overall survival in previously untreated patients with chronic lymphocytic leukemia. Blood, 109, 4679–4685. [DOI] [PubMed] [Google Scholar]