Abstract
Objectives:
The aim of this study was to examine puffing behavior and topography over 24 hours among regular electronic cigarette (e-cigarette) users.
Methods:
Twenty-four adult e-cigarette users (15 male) vaped their personal e-cigarettes ad-lib over the course of 24 hours. Participants took each puff via calibrated CReSS pocket topography monitors. We analyzed: number of puffs per day per session, mean puff volume, mean puff flow rate, mean duration of puff, and mean interval between puffs.
Results:
Over 24 hours participants took on average 156.2±10.3 puffs, clustered in 10.2±7.9 puffs per puffing session with an average puff interval of 15.4±22.0 sec. A single puff lasted on average 3.0±1.2 sec, had a volume of 73.4±51.5 ml, and was taken with the average flow rate of 24.7±10.2 ml/sec.
Conclusions:
There is substantial variability among e-cigarette users in the way they puff on these devices. When e-cigarette aerosol is generated for laboratory studies, there is a need for validated puffing protocols that not only reflects the most-common pattern but also intensive puffing behaviors observed among some e-cigarette users.
Keywords: electronic cigarette, e-cigarette, puffing topography, puffing behavior
INTRODUCTION
Electronic cigarettes (e-cigarettes) come in a variety of styles with unique product characteristics, making it difficult to standardize, test and regulate these products.1 For regulatory purposes and for protection of public and individual health, it is essential to assess health effects of these devices.2 Although epidemiological and clinical studies will provide accurate assessment of relative and absolute risk of e-cigarette use, laboratory studies that look at the e-cigarette aerosol constituents and its toxicity are faster and more cost-effective research tools. Such laboratory studies often require generation of aerosol from e-cigarettes that can be further tested for chemical composition as well as in-vitro and in-vivo toxicity. For accurate assessment of the e-cigarette safety in laboratory settings, it is important that aerosol from these devices is generated in a similar way that users operate their device in real-life.
Previous studies have examined puff topography among e-cigarette users3,4. However, these studies have either only used first generation devices (i.e. rechargeable e-cigarettes)3, or did not examine all puff characteristics (e.g. puff volume)4. We have previously shown that smokers that switched from tobacco cigarettes to e-cigarettes modify their puffing behavior by taking longer and slower puffs.5 In the current study, we aimed to evaluate daily pattern of e-cigarette use (number of puffs taken over 24-hours as well as puffing frequency) and puffing topography among daily e-cigarette users in their natural setting to further understand users’ behaviors. We also aimed to establish a puffing protocol that would reflect naturalistic behaviors among e-cigarette users and that can be implemented for generation of e-cigarette aerosol for analytical purposes.
METHODS
Study Design
This longitudinal observational study required participants to attach the CReSS pocket device (Borgwalt, Germany) to their personal e-cigarettes during each puffing session for a single 24-hour period. No additional intervention or control group was used.
Participants
Participants were recruited from the Silesia region in Poland using advertisement in vape shops, Facebook, and e-cigarette forums as well as direct e-mail to vapers. Participants were eligible for the study if they met the following criteria: (1) 18 years of age or older, (2) daily e-cigarette users, and (3) used at least 1 ml of e-liquid daily. Interested participants were excluded if: (1) they could not refrain from smoking tobacco cigarettes over 24 hours during the study, (2) used e-cigarette with 0 mg/ml nicotine, (3) reported serious respiratory health problems (e.g., asthma, COPD), and (4) rejected to use e-cigarette with provided smoking topography device. All dual users were requested not to smoke tobacco cigarettes during data collection.
Study Protocol
Participants were asked to bring their personal e-cigarette and e-liquids with usual nicotine concentrations for a baseline assessment visit. Prior to data collection through the CReSS pocket device, participants completed a 26-item questionnaire to collect baseline information on their demographics, smoking and vaping history. Upon completion of the study survey, each participant was given a CReSS pocket monitor to attach to their e-cigarettes with every use for 24 hours. Each participant was given explicit instruction to attach their e-cigarette to the puffing monitor when they wanted to begin puffing, and to remove it once they completed each vaping session. These actions were considered one vaping session. Participants were asked to start using their vaping device with the puffing monitor at 6:00 am or when they woke up in the morning of the first day and use the puffing monitor every time they vape through 6:00 am the following morning.
Puffing Monitoring
Puffing topography was measured for the cohort with a CReSS pocket device. A smoking machine was used to calibrate the CReSS pocket device before being used by individual participants as follows: 1. an e-cigarette was connected to the CReSS device and then the CReSS was connected to the smoking machine, 2. a wide range of puffing regiments were utilized (puff volume in a range of 20 to 150 ml and puff duration in a range of 1 to 10 secs), 3. the calibration factor used in the CReSS device was adjusted to have the reading ± 10% of the smoking machine puffing profile. E-cigarettes were connected to the puffing topography device using silicon tube adapters. After participants returned puffing monitors, the collected data was downloaded into Cresshost version 1.1.17 software (Plowshare Technologies, USA), de-identified, and coded for later analysis. Measurement variables from the device included: time of each puff, number of puffs, number of puffing sessions, number of puffs in every session, puff volume, average puff flow, peak puff flow, duration of puff, intervals between puffs, and time of puff peak.
Statistical Analysis
We calculated average number of puffs, puff volume, flow, peak flow, duration, intervals and time of peak for each puffing session. Next, we calculated average number of puffs, puff volume, flow, peak flow, duration, intervals and time of peak for each participant. Finally, we calculated average number of puffs, puff volume, flow, peak flow, duration, intervals and time of peak from all participants to determine average puffing behavior among all participants. Total number of puffs were calculated by taking the summed number of puffs per session for each participant and averaging all participants. Statistical comparisons were performed with IBM SPSS Version 23 software.
RESULTS
Characteristics of Study Participants
We recruited 25 e-cigarette users and 24 subjects completed the study. One participant was excluded from the data analysis due to CReSS pocket device malfunction that led to miscalculated data. The average age of participants was 28.0 and most participants were between the ages of 21–27 (n=17; 71%). Most participants disclosed that they still occasionally smoked tobacco cigarettes in addition to their e-cigarettes (dual users; n=16, 67%) with all participants reported regular smoking in their lifetime. Those who disclosed their dual use of electronic and tobacco cigarettes also reported smoking between 0–2 tobacco cigarettes on average per day (Supplementary Table 1).
Participants had less than 1 month, (n=4, 17%), 1–6 months (n=8, 33%), 6–12 months (n=3, 13%) and 1 year or more (n=9, 33%) experience with e-cigarettes. The most common reason for buying an e-cigarette was to quit smoking (n=10, 42%) followed by being cheaper or less harmful than cigarettes (n=6, 25%), then as a gift or for friends/family (n=1, 4%). All participants used a refillable eGO e-cigarette with battery voltage setting from 3.2V to-4.8Vand a variety of flavors of e-liquid. Manually operated eGO e-cigarettes that requires the user to press a button in order to activate the e-cigarette were the most popular among the cohort (n=23, 96%). Within this cohort, 7–12 mg/ml was the most popular nicotine concentration (n=10, 42%), with other participants using 1–6 mg/ml (n=4, 17%), 13–18 mg/ml (n=6, 25%) and 19–24 mg/ml (n=4, 17%). Most users reported refilling their e-cigarette every day (n=10, 42%), others refilled every 2–3 days (n=7, 29%, every 7–14 days (n=5, 21%) and every 1–2 or 3–4 days (both n=1, 4%). The most popular e-cigarette flavors used in this study were fruit (e.g., cherry) followed by tobacco (e.g., Marlboro).
Before participants began using their e-cigarettes, 9 participants (38%) smoked between 0 and 10 CPD, 10 (42%) smoked between 11 and 20 CPD, and 5 (21%) smoked over 20 CPD. At the time of the study, 12 of the participants (50%) reported that they were not trying to quit smoking and 18 (75%) intended to reduce their CPD (Supplementary Table 1).
Circadian Puffing Behavior among E-cigarette Users
Over the course of 24 hours of data collection from 6:00 am through 6:00 am following day, participants took on average 156.2±10.3 puffs from their e-cigarette devices (Figure 1). The cumulative number of puffs over 24 hours varied from 44 to 345. The average number of puffs per hour changed during a day from 2.0±5.2 (6:00 am) to 14.4±14.9 (11:00 am). In addition to a peak in the number of puffs taken per hour at 11:00 am; a second peak of 13.1±3.4 puffs was observed at 6:00 pm (Figure 1). We observed that subjects clustered the puffs in the puffing sessions and had on average 15.3±8.0 (from 3 to 30) sessions a day with 10.2±7.9 puffs per session (from 1 to 40). Within-subject average number of puffs per puffing session ranged from 3.3±1.4 to 26.2±9.5 (Supplementary Table 2).
Figure 1.
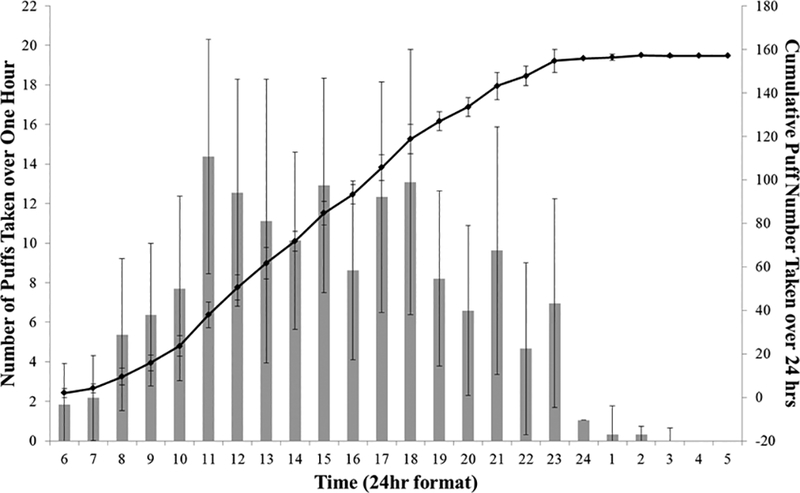
Average (left; bars) and cumulative (right, solid line) number of puffs taken by study participants over 24 hrs. Error bars shows 95% confidence intervals.
Puffing Topography among E-cigarette Users
On average, subjects took 10.2±7.9 puffs per puffing session with an inter-puff interval of 15.4±22.0 sec. The average puff volume of a single puff was 73.4±51.5ml and the average puff duration was 3.0±1.2 sec. The average flow rate was 24.7±10.2 ml/sec and the average peak flow rate was 34.8±16.7 ml/sec, observed at 1.1±0.7 sec after puff initiation (Table 1).
Table 1.
Average puffing topography among 24 regular e-cigarette users measured over 24 hours (mean ± SD).
| Puffing Topography Measure | Mean±SD |
|---|---|
| Total Number of Puffs over 24 hrs | 156.2±95.3 |
| Total Puff Volume over 24 hrs | 854.4±544.5 ml |
| Number of Puffing Session | 15.3±8.0 |
| Number of Puffs Per Single Session | 10.2±7.9 |
| Single Puff Volume | 73.9±51.5 ml |
| Single Puff Duration | 3.0±1.2 sec |
| Interval Between Single Puffs | 15.4±22.0 sec |
| Average Puff Flow Rate | 24.7±10.2 ml/sec |
| Peak Puff Flow Rate | 34.8±16.7 ml/sec |
| Time of Peak Flow | 1.1±0.7 sec |
Puffing topography was highly variable among all subjects. Average interval time between puffs among individual subjects ranged from 4.9 to 99.9 sec and the average duration of puffs for individual subjects varied from 0.7 to 4.7 sec. For example, subject 23 (male; 37 yo) took puffs that had a volume varying from 82.1 to 373.2 ml and the puff duration varied from 2.7 to 10.3 sec; subject 14 (female; 21 yo) took puffs with a volume that varied from 49.4 to 89.0 ml and the puff duration varied from 2.9 to 4.4 sec. Detailed topography data for each subject can be found in Supplementary Table 2.
DISCUSSION
This study aimed to ascertain how e-cigarette users operated their e-cigarettes throughout the day. A total of 366 sessions and 3,749 puffs for a cohort of 24 subjects were analyzed to examine the puffing characteristics and behaviors that people exhibit when using their e-cigarette over the course of 24 hours. Amount of use (total number of puffs) varied drastically among participants over 24 hours. The largest number of puffs taken throughout the day was determined to be at around 11am. We hypothesize that this peak in use was a result of two factors: 1) For participants that woke up before 10 am (54%), this was a break time when they could use these products; 2) For subjects who took their first puff from 10–11 am (33%), this might have been the time they woke up. This high number of puffs per hour continued until 6pm. The puffing topography among study participants was consistent in some characteristics (i.e. time of peak use) while varied drastically between others (i.e. number of puffs).
There were several limitations in this study, including broad inclusion criteria and a relatively small sample size. A limited sample size did not allow us to preform additional detailed analysis of the potential effects of product type, nicotine strength, flavorings and other product attributes on puffing topography. Additionally, users were allowed to select their own brand of e-cigarette, e-liquid, and nicotine concentration resulting in all of the participants in this cohort using similar devices (eGO). Therefore, these results may not be representative of other product types. Although we propose a single puffing protocol based on average parameters, taking into account the high variability between and within subjects, there may be more than one user dependent puffing protocol for e-cigarettes. For example their might be a need for an intensive puff protocol for users that self-titrate using number of puffs and puff volume. Overall, future research with larger sample sizes, longer time frames, and a control group will be needed to take an in depth look at puffing characteristics among e-cigarette users.
IMPLICATION FOR TOBACCO REGULATION
Currently, there is lack of a standardized laboratory puffing protocol that reflects real-life behavior of e-cigarette users. We have measured circadian puffing behaviors and topography among 24 daily e-cigarette users We have proposed a puffing protocol reflecting a typical users behavior: 75 ml puff volume, 3 sec duration, 25 ml/sec flow rate, 15 sec interval between puffs, 10 puffs per cluster and 17 puff clusters per study. Such protocol can be implemented in laboratory settings to generate e-cigarette aerosol for analytical and toxicity testing.
Supplementary Material
Supplemental Table 1. Demographics of study participants, history of smoking and e-cigarettes use (n=24).
Supplemental Table 2. Puffing topography among 24 regular e-cigarette users measured over 24 hours (mean±SD).
Footnotes
HUMAN SUBJECTS APPROVAL
The study protocol was reviewed and approved by the IRB at the Medical University of Silesia in Katowice, Poland. and the. All subjects provided a written consent form to participate in the study.
REFERENCES
- 1.Brandon TH, Goniewicz ML, Hanna NH, et al. Electronic nicotine delivery systems: a policy statement from the American Association for Cancer Research and the American Society of Clinical Oncology. Journal of Clinical Oncology. 2015;33(8):952–963. [DOI] [PubMed] [Google Scholar]
- 2.Trtchounian A, Talbot P. Electronic nicotine delivery systems: is there a need for regulation? Tobacco control. 2010:tc. 2010.037259. [DOI] [PubMed] [Google Scholar]
- 3.Robinson R, Hensel E, Morabito P, Roundtree K. Electronic cigarette topography in the natural environment. PloS one. 2015;10(6):e0129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dautzenberg B, Bricard D. Real-time characterization of e-cigarettes use: the 1 million puffs study. J Addict Res Ther. 2015;6(2). [Google Scholar]
- 5.Lee YH, Gawron M, Goniewicz ML. Changes in puffing behavior among smokers who switched from tobacco to electronic cigarettes. Addictive behaviors. 2015;48:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Demographics of study participants, history of smoking and e-cigarettes use (n=24).
Supplemental Table 2. Puffing topography among 24 regular e-cigarette users measured over 24 hours (mean±SD).


