Abstract
In the U.S., more than 80% of African-American smokers use mentholated cigarettes, compared to less than 30% of Caucasian smokers. The reasons for these differences are not well understood. To determine if genetic variation contributes to mentholated cigarette smoking, we performed an exome-wide association analysis in a multiethnic population-based sample from Dallas, TX (N = 561). Findings were replicated in an independent cohort of African Americans from Washington, DC (N = 741). We identified a haplotype of MRGPRX4 (composed of rs7102322[G], encoding N245S, and rs61733596[G], T43T), that was associated with a 5-to-8 fold increase in the odds of menthol cigarette smoking. The variants are present solely in persons of African ancestry. Functional studies indicated that the variant G protein-coupled receptor encoded by MRGPRX4 displays reduced agonism in both arrestin-based and G protein-based assays, and alteration of agonism by menthol. These data indicate that genetic variation in MRGPRX4 contributes to inter-individual and inter-ethnic differences in the preference for mentholated cigarettes, and that the existence of genetic factors predisposing vulnerable populations to mentholated cigarette smoking can inform tobacco control and public health policies.
Author summary
An exome-wide association study revealed a significant association between menthol cigarette use and coding variants in MRGPRX4, which encodes a G-protein coupled receptor expressed in sensory neurons. The variant haplotype is found only in populations of African ancestry, and encodes a receptor that displays reduced agonism by Nateglinide. Our findings indicate genetic variation contributes to the high rate of menthol cigarette use in African Americans.
Introduction
Cigarette smoking remains a leading cause of preventable disease and mortality in the United States, contributing to >480,000 deaths annually [1]. Although the overall rates of smoking have declined dramatically over the last 50 years [1], the use of mentholated cigarettes has not, and has actually increased in some groups [2, 3]. Menthol is a flavoring additive commonly used in cigarettes and tobacco products. It is thought to reduce the harshness of cigarette smoke due to its cooling and anesthetic properties [4–6]. Menthol cigarettes currently account for about 30% of the cigarette market in the U.S. [7]. Scientific evidence suggests that the use of mentholated cigarettes leads to increased smoking initiation among youth and reduced rates of cessation [8, 9]. This has led the FDA to conclude that menthol cigarettes likely pose a public health risk above that of nonmenthol cigarettes [10, 11].
The prevalence of menthol cigarette smoking varies markedly between demographic groups, and is especially high among young adults and in African Americans [3, 9, 12]. In the U.S., nearly 83% of African-American smokers use menthol cigarettes, compared to 24% of white and 32% of Hispanic smokers. Whether this disparity has a genetic basis, or is attributable solely to social or cultural factors, is not known.
Menthol is known to interact with transient receptor potential (TRP) channels, including TRPM8 [13] and TRPA1 [14]. Although one study found that common variants in TRPA1 were associated with menthol tobacco use among European-American smokers [15], this finding awaits replication. Variations in the TAS2R38 bitter taste receptor gene appear to have a modest effect on smoking and on menthol cigarette use [16–20], but no comprehensive analysis of the role of variation in these and other genes in menthol cigarette smoking has been carried out to date.
To determine whether inherited variations in the protein-coding regions of the genome contribute to menthol cigarette smoking, we performed an exome-wide association study using a population-based cohort of African Americans (AA) and European Americans (EA) from Dallas, Texas. The findings were replicated in a cohort of African-American smokers from Washington, DC.
Results
Study cohort
The discovery cohort included 561 participants (394 AA and 167 EA) from the Dallas Heart Study (DHS) and the Dallas Biobank (Table 1). The average age of participants was 55±11.0 (SD) years, and 60% were women. Nearly 78% of DHS AA and 86% of Biobank AA subjects reported smoking mentholated cigarettes, compared to 33% of European Americans (P<0.001), consistent with national trends [3]. Menthol smokers were younger than non-menthol smokers among African Americans (P<0.05), but there was no difference in age among European-American smokers. The prevalence of menthol smoking was not significantly different between DHS and Biobank AA after adjusting for age (P = 0.59). In the replication cohort (Schroeder), most of the participants (N = 424, 57.2%) were menthol smokers (Table 1). A higher percentage of menthol smokers than non-menthol smokers were female (39.6% versus 24.3%, P<0.001) consistent with previous literature [12]. No differences were found in the mean age of menthol smokers and non-menthol smokers (P = 0.41).
Table 1. Characteristics of study participants according to menthol smoking status.
| Population | Characteristic | Non-Menthol Smokers | Menthol Smokers | P-value |
|---|---|---|---|---|
| DHS AA (N = 261) | N | 58 (22.2) | 203 (77.8) | - |
| Age (yr) | 62.8 ± 9 | 56.4 ± 9.1 | <0.0001 | |
| Female, no. (%) | 34 (58.6) | 124 (61.1) | 0.762 | |
| Biobank AA (N = 133) | N | 19 (14.3) | 114 (85.7) | - |
| Age (yr) | 56.4 ± 11.8 | 47.2 ± 12.5 | 0.0031 | |
| Female, no. (%) | 12 (63.2) | 72 (63.2) | 1 | |
| DHS EA (N = 167) | N | 112 (67.1) | 55 (32.9) | - |
| Age (yr) | 55.9 ± 9.3 | 55.3 ± 10.3 | 0.75 | |
| Female, no. (%) | 59 (52.7) | 33 (60.0) | 0.4105 | |
| Schroeder AA (N = 741) | N | 317 (42.8) | 424 (57.2) | - |
| Age (yr) | 44.6 ± 10.6 | 45.3 ± 10.9 | 0.41 | |
| Female, no. (%) | 77 (24.3) | 168 (39.6) | <0.0001 |
Values are mean ± SD. AA–African American; EA–European American.
P-values were calculated using t-tests (for age) and chi-square tests (for gender).
Identification of MRGPRX4
A total of 52,298 variants were tested for association with menthol cigarette smoking in the Dallas cohort. Genomic control [21] value was acceptable (λgc = 1.05) and QQ-plot of P-values showed no systematic inflation of association results (S1 Fig). No variant met our exome-wide significance threshold (9.6x10-7). We therefore decided to investigate the top variants with a suggestive level of significance (P<1x10-4) in greater detail. A total of three variants reached this level of significance in our exome-wide screen (Table 2), and these were genotyped in an additional cohort of 741 AA smokers from Washington DC (Schroeder cohort). While no association was found with two of the three variants (Table 2), the third variant, rs7102322 in the gene MRGPRX4, was strongly associated with menthol smoking in the replication cohort (P = 2.1x10-6). Meta-analysis of the two samples together revealed an even lower P-value that exceeded criteria for genome-wide significance (P = 1.6x10-8, Table 2).
Table 2. Top association results from exome-wide analysis.
| Discovery cohort (N = 561) |
Replication cohort (N = 741) |
Meta-analysis P-value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chr | GRCh37/hg19 Position |
rs ID | Gene | Ref/Alt | Alt AF | OR (95% CI) | P-value | Alt AF | OR (95% CI) | P-value | |
| 17 | 38156712 | rs4794822 | PSMD3 | G/A | 0.34 | 0.53 (0.39–0.72) | 4.6E-05 | 0.33 | 1.28 (0.99–1.59) | 0.06 | 0.052 |
| 11 | 18195537 | rs7102322 | MRGPRX4 | A/G | 0.06 | 8.55 (2.04–35.9) | 4.8E-05 | 0.05 | 6.3 (2.90–13.4) | 2.1E-06 | 1.6E-08 |
| 3 | 142178144 | rs2229032 | ATR | G/A | 0.10 | 2.81 (1.63–4.86) | 8.6E-05 | 0.08 | 0.96 (0.65–1.43) | 0.83 | 0.37 |
Ref–reference allele, Alt–alternate allele, AF–allele frequency, OR–odds ratio. In the Discovery cohort, the P-values were calculated using logistic regression adjusted for age, gender, and 6 leading principal components of ancestry (based on a likelihood-ratio test). In the replication cohort, P-values were calculated using logistic regression adjusted for age and gender. The combined p-values were calculated using random-effects inverse-variance weighted meta-analysis. Odds ratios were calculated assuming additive genetic model.
The rs7102322 variant was seen exclusively in African-American participants (minor allele frequency [MAF] = 8% in the Dallas cohorts, 5% in Schroeder) and was not observed in European Americans (0% in DHS EA). Among the AA participants in the Dallas cohorts, the allele frequency of the variant was five-to-eight fold higher in menthol smokers compared to non-menthol smokers (10.4% vs 1.3%, odds ratio (OR) = 8.5, P = 5.6x10-5 (Table 3). A similar magnitude of difference was seen in the Schroeder cohort (7.0% vs 1.3%, OR = 6.3, P = 2.1x10-6, Table 3). Although limited by low power, our analyses found highly similar differences in the MRGPRX4 allele frequencies between menthol and non-menthol smokers in males and females (S1 Table). To determine whether the lower frequency of the rs7102322 variant in the Schroeder cohort (5%) was influenced by admixture, we estimated the percentage of African and European ancestry in a subset of this cohort. This indicated that Schroeder cohort participants indeed have a higher degree of European admixture compared to West Africans and African Americans from other regions of the U.S. (S2 Fig). Further analyses that included ancestry informative markers and an inferred proportion of African ancestry at this locus maintained strong support for association with menthol smoking (P<1e-5), indicating that the observed association is unlikely to be due to differential admixture (S4 and S5 Tables).
Table 3. Association of MRGPRX4 rs7102322 with menthol smoking in African-American participants.
| rs7102322 genotype | ||||||
|---|---|---|---|---|---|---|
| Population | Smoking status | A/A | A/G | G/G | MAF | P-value |
| DHS | Non-menthol | 57 | 1 | 0 | 0.9% | |
| Menthol | 165 | 34 | 4 | 10.3% | 0.00014 | |
| % Menthol | 74.3% | 97.1% | 100.0% | |||
| Biobank | Non-menthol | 18 | 1 | 0 | 2.6% | |
| Menthol | 90 | 24 | 0 | 10.5% | 0.079 | |
| % Menthol | 83.3% | 96.0% | - | |||
| DHS + Biobank | Non-menthol | 75 | 2 | 0 | 1.3% | |
| Menthol | 255 | 58 | 4 | 10.4% | 5.65E-05 | |
| % Menthol | 77.3% | 96.7% | 100.0% | |||
| Schroeder | Non-menthol | 309 | 8 | 0 | 1.3% | |
| Menthol | 365 | 59 | 0 | 7.0% | 2.06E-06 | |
| % Menthol | 54.1% | 88.1% | - | |||
MAF—minor allele frequency
P-values were calculated using logistic regression adjusted for age and gender. In addition, cohort indicator was included as a covariate in the combined DHS + Biobank analysis.
Characterization of MRGPRX4 variation and function
The MRGPRX4 gene encodes a Mas-related G-protein coupled receptor member X4, which is expressed in nociceptive neurons of the dorsal root ganglia and trigeminal neurons, and may regulate pain and somatosensation [22–24]. The MRGPRX4 rs7102322 variant encodes an asparagine-to-serine substitution at codon 245 (N245S). The residue is conserved in chimpanzees, and resides immediately 5’ to a region highly conserved across primates (Fig 1).
Fig 1. MRGPRX4 multiple sequence alignment.
Bolded letters denote residues that are conserved among primates. Residue 245 is shown in red.
To evaluate whether rs7102322 SNP was in linkage disequilibrium (LD) with another functional variant in the MRGPRX4 locus, we examined data from the 1000 Genomes Project [25]. Consistent with our observations, the rs7102322 variant was observed solely in African-ancestry populations (MAF = 11.5% in Africans and 8% in African Americans in Southwest U.S.). The rs7102322 variant was in LD with the SNP rs61733596[A/G], which encodes a synonymous substitution at codon 43 (T43T) in MRGPRX4. Genotyping the rs61733596 variant in the Schroeder cohort confirmed that this variant is in complete linkage disequilibrium (R2 = 1) with rs7102322.
Sequencing of MRGPRX4
To identify whether additional coding variants in MRPGRX4 were associated with menthol cigarette smoking, we sequenced all exons of MRPGRX4 in a subset of Dallas cohort participants (N = 389, Table 4). This analysis confirmed that the rs7102322 (N245S) variant was in complete LD with rs61733596 (T43T), which showed an equivalent association with menthol smoking (OR = 3.3, P = 0.007). No other coding variant in MRGPRX4 was in linkage disequilibrium with N245S or associated with menthol cigarette smoking in this group.
Table 4. MRGPRX4 variants identified by exome sequencing in Dallas cohort participants (N = 389).
| CHR | GRCh37/hg19 Position |
SNP | Ref/Alt | MAF Menthol (N = 227) |
MAF Non-menthol (N = 162) |
OR (95% CI) | P-value | Variant Effect |
|---|---|---|---|---|---|---|---|---|
| 11 | 18194793 | rs11024530 | G/A | 13.9% | 14.8% | 1.36 (0.86–2.13) | 0.1801 | 5' UTR |
| 11 | 18194827 | rs2468774 | C/G | 26.4% | 26.2% | 1.06 (0.76–1.49) | 0.7227 | F8L |
| 11 | 18194878 | rs2445180 | T/G | 26.4% | 25.6% | 1.09 (0.78–1.53) | 0.6125 | N25K |
| 11 | 18194932 | rs61733596 | A/G | 6.6% | 1.5% | 3.34 (1.25–8.89) | 0.0067 | T43 |
| 11 | 18194944 | rs11024531 | T/A | 14.8% | 15.1% | 1.39 (0.89–2.17) | 0.1440 | V47 |
| 11 | 18194964 | rs1869788 | A/G | 37.7% | 32.7% | 1.16 (0.84–1.58) | 0.3622 | Y54C |
| 11 | 18194996 | rs144828790 | A/C | 0.7% | 0.9% | 0.52 (0.13–2.13) | 0.3710 | I65L |
| 11 | 18195051 | rs2445179 | C/T | 8.6% | 6.2% | 0.97 (0.54–1.73) | 0.9204 | L83S |
| 11 | 18195173 | rs61733597 | G/A | 0.9% | 1.2% | 0.56 (0.13–2.34) | 0.4299 | V124I |
| 11 | 18195227 | rs78287429 | G/A | 9.0% | 8.6% | 0.71 (0.41–1.22) | 0.2181 | V142M |
| 11 | 18195252 | rs73434269 | C/T | 2.2% | 1.9% | 0.81 (0.30–2.18) | 0.6774 | S150F |
| 11 | 18195347 | rs113302139 | G/T | 0.9% | 0.3% | 1.98 (0.22–17.98) | 0.5195 | A182S |
| 11 | 18195348 | rs11024532 | C/T | 10.8% | 17.6% | 0.83 (0.54–1.27) | 0.3925 | A182V |
| 11 | 18195448 | rs4630269 | C/T | 26.2% | 25.9% | 1.07 (0.76–1.50) | 0.7039 | Y215 |
| 11 | 18195537 | rs7102322 | A/G | 6.6% | 1.5% | 3.34 (1.25–8.89) | 0.0067 | N245S |
| 11 | 18195609 | rs146132319 | C/T | 0.4% | 0.6% | 1.36 (0.18–10.23) | 0.7661 | P269L |
P-values were calculated using logistic regression adjusted for age, gender, and self-reported ancestry (African American vs European American). Bolded numbers indicate P<0.05.
MRGPRX4 N245S mutation attenuates agonist activity
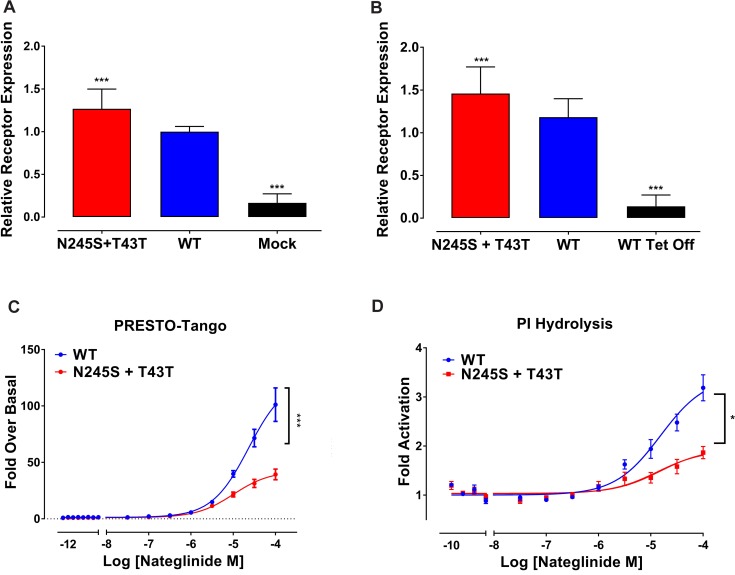
MRGPRX4 is an orphan G protein-coupled receptor (GPCR) expressed in mammalian sensory neurons [22, 23]. Although the endogenous ligand(s) for this receptor are not known, the potassium channel modulator Nateglinide has been identified to be a highly efficacious agonist and was used to demonstrate that this receptor couples predominantly to Gαq [26]. We used a cell-based approach to determine if the N245S variant affected the responsiveness of either MRGPRX4 β-arrestin or Gαq downstream signaling in response to Nateglinide. We first generated FLAG-tagged, codon-optimized wild-type (WT) and N245S variant constructs for the PRESTO-Tango β-arrestin recruitment assay and then generated stable, tetracycline-inducible cell lines for the FLAG-tagged WT MRGPRX4 and the N245S variant. Notably, all N245S variant constructs in this study also included the synonymous variant T43T. To measure membrane expression levels of WT MRGPRX4 and N245S+T43T, we used an established whole-cell ELISA assay [27] in HTLA cells. Using a 1-way ANOVA, we determined that N245S+T43T was expressed slightly but significantly more than the WT receptor in HTLA cells and in the tetracycline-inducible stable cells (Fig 2A and 2B).
Fig 2. MRGPRX4 N245S variant has dampened signaling when compared to WT.
(A) Receptor expression as calculated by whole cell ELISA in HTLA cells transfected with N245S+T43T or WT receptor with mock transfected shown as negative control (n = 2, 64 wells per experiment; y-axis is fold expression normalized to WT). (B) Receptor expression as calculated by whole cell ELISA in tetracycline inducible WT or N245S+T43T cells with non-tetracycline-induced cells shown as negative control (n = 2, 64 wells per experiment; y-axis depicts fold expression normalized to WT). (C) Average concentration response curves for Nateglinide in PRESTO-Tango arrestin assay with WT and N245S+T43T receptors, (n = 4, in quadruplicate; y-axis is in fold response over basal signaling). (D) Average concentration response curves for Nateglinide in PI hydrolysis assay with WT and N245S+T43T tet-inducible cell lines, (n = 3, in duplicate; y-axis is in fold response over basal signaling). * indicates P<0.05, *** indicates P<0.001 as calculated by F-test.
We then examined the effect of the agonist Nateglinide on the recruitment of β-arrestin in the PRESTO-Tango recruitment assay, which provides a quantitative measure of receptor activation and downstream signaling [28, 29]. We found that Nateglinide had equal potency at both N245S+T43T and WT receptors, but the N245S+T43T variant displayed a dramatic reduction of fold activation (42 fold) in β-arrestin recruitment, significantly less than the WT receptor (123.9 fold) (P<0.001, Fig 2C, S6 Table). In an independent quantitative assay, we also examined the effect of the variant on G protein signaling using the G protein-dependent phosphatidylinositol (PI) hydrolysis assay. We observed that the maximal (Emax) PI hydrolysis values following Nateglinide addition were significantly reduced in cells expressing the N245S+T43T variant compared with those expressing the WT receptor (PI Hydrolysis P<0.001, Fig 2D, S6 Table). Together, these data demonstrate that despite an apparent increase in N245S+T43T expression, the variant has significantly reduced arrestin and G protein signaling in comparison to WT.
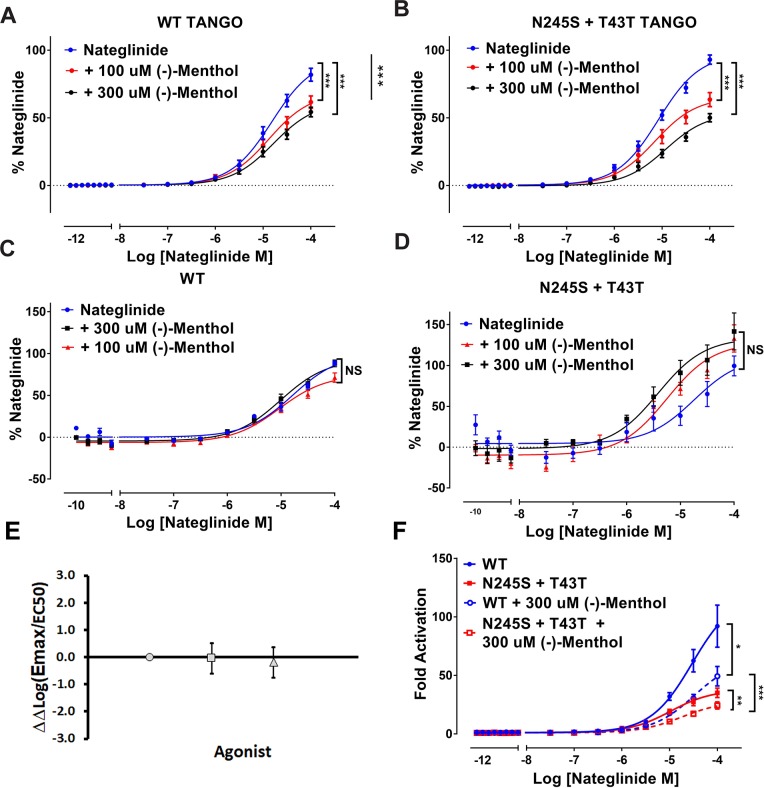
(-)-Menthol suppresses MRGPRX4 agonism
To determine whether (-)-menthol, the additive present in menthol cigarettes, alters the activity of MRGPRX4 WT or the N245S+T43T variant, we added the compound and repeated the functional assays. In the Tango assay, (-)-menthol alone showed no agonist activity at MRGPRX4 (up to 1 mM) (S3 Fig). We then tested whether (-)-menthol altered agonist-induced activity of the WT and N245S+T43T receptors. Increasing concentrations of (-)-menthol were added to each assay together with Nateglinide (Fig 3). We observed that 100 μM and 300 μM (-)-menthol significantly reduced the Emax of the agonist Nateglinide on the WT (P<0.001) and N245S+T43T (P<0.001) in the arrestin pathway (Fig 3A and 3B) but not in the G protein pathway as measured using the PI hydrolysis assay (Fig 3C and 3D). To determine whether (-)-menthol’s modulatory effect differed significantly between WT and N245S+T43T variant, we calculated ΔΔlog(Emax/EC50) [30] for our reference agonist Nateglinide in the presence or absence of 100 and 300 μM (-)-menthol and found that (-)-menthol modulated WT and the variant equivalently (Fig 3E). A comparison of the fold change activation of β-arrestin recruitment revealed that 300 μM (-)-menthol significantly reduces Nateglinide-induced activation of the N245S variant when compared to WT (P<0.001, Fig 3F), similar to the differences in fold change for non-menthol conditions (Fig 2C). To test for non-specific effects of menthol on cells or cell membranes, we tested the effect of menthol on the unrelated D2 dopamine receptor in the PRESTO-Tango assay. This control showed that (-)-menthol had no modulatory effect on this receptor in this assay (S3 Fig, panel C). Similarly, (-)-menthol and Nateglinide had no effect on PI hydrolysis in cells where tetracycline was not added (i.e., with no MRGPRX4 receptor expression) (S3 Fig, panel D).
Fig 3. (-)-Menthol reduces MRGPRX4 WT and N245S+T43T arrestin signaling.
(A),(B) Average concentration response curves for Nateglinide in MRGPRX4-WT-Tango (A) or MRGPRX4-N245S+T43T-Tango (B) following 100 μM or 300 μM (-)-menthol addition, (n = 3, in triplicate, y-axis is % Nateglinide). (C),(D) Average concentration response curves for Nateglinide-induced PI hydrolysis in MRGPRX4-WT (C) or MRGPRX4- N245S+T43T (D) tetracycline inducible cells following 100 μM or 300 μM (-)-menthol addition, (n = 3, in duplicate, y-axis is % Nateglinide). (E) Plot showing the effect of agonist treatments (x-axis) Nateglinide (gray circles), Nateglinide + 100 μM (-)-menthol (gray squares) and Nateglinide + 300 μM (-)-menthol (gray triangles) between WT and N245S+T43T variant shown as ΔΔLog(Emax/EC50) values (y-axis). Y vales > 0 indicate increased effect for agonist at WT and values < 0 indicate increased effect for agonist at N+T variant. Bars depict 95% confidence intervals. (F) Average concentration response curves for fold change activation with Nateglinide in MRGPRX4-WT-Tango or MRGPRX4-N245S+T43T-Tango following 300 μM or 300 μM (-)-menthol addition, (n = 3, in quadruplicate). For all: NS = not significant, * p<0.05, ** p <0.01, and *** p<0.001 as calculated by F-test.
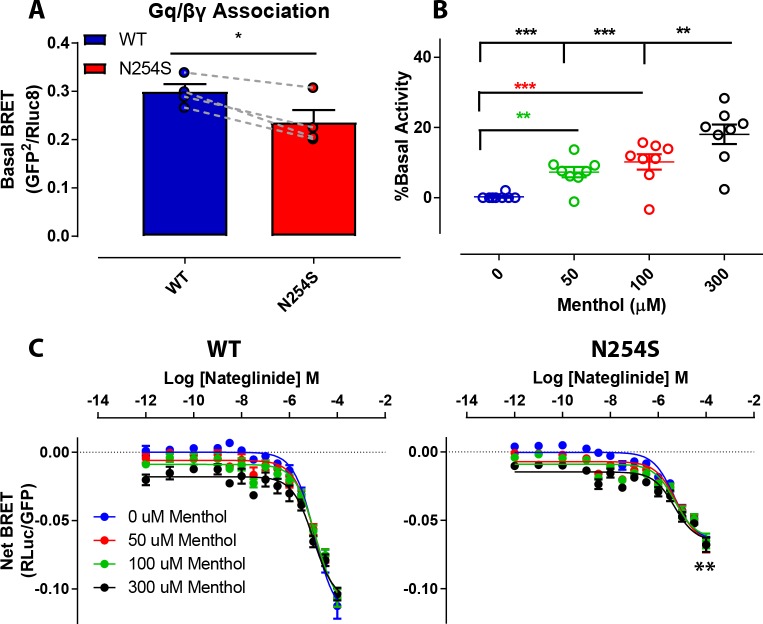
Menthol affects agonism of the MRGPRX4 N245S receptor
We also performed further studies using bioluminescence resonance energy transfer (BRET) as another measure of MRGPRX4 activity. Basal BRET in the Gq/βγ dissociation assay (an index of constitutive receptor activity) was reduced at MRGPRX4 N245S relative to WT (Fig 4A, 0.24 ± 0.025 vs 0.3 ± 0.015, t(3) = 4.986; p = 0.0155). Conversely, while basal activity of both WT and MRGPRX4 N245S was found to be increased by menthol (F(3,18) = 32.89, p < 0.0001), there was no effect of or interaction with genotype. Increasing amounts of menthol resulted in significantly elevated activity (Fig 4B, Linear effect, F(1,28) = 42.83; p < 0.0001) at all increments except between 50 and 100 μM (Fig 4B). Under agonist stimulation conditions, the probe Nateglinide showed an approximate 2-fold greater potency at MRGPRX4 N245S compared to WT (Table 5, EC50 values of 10.49 ± 0.61 μM vs 5.25 ± 0.25 μM). This effect was significant (F(1,18) = 7.44, p = 0.0343) accounting for 43.21% of the variance between the two populations. No effect of menthol on potency nor interaction with genotype were indicated. Similarly, efficacy of the probe Nateglinide (Emax) was not affected by menthol at any concentration (Fig 4C), though Emax as calculated by net BRET was reduced at MRGPRX4 N245S vs WT (-0.067 ± 0.001 vs -0.113 ± 0.006, F(1,6) = 15.51, p = 0.0076).
Fig 4. Bioluminesence resonance energy transfer (BRET) analysis of MRGPRX4 activity.
(A). Differences in basal BRET activity of the WT and variant (N245S) MRGPRX4 receptors. (B). The effect of menthol on the basal activity of MRGPRX4. (C). The effects of varying concentrations of menthol on MRGPRX4 agonism by Nateglinide.
Table 5. BRET measure of the effect of menthol on agonist potency.
| μM Menthol | |||||
|---|---|---|---|---|---|
| EC50 | μM | 0 | 50 | 100 | 300 |
| WT | 10.49 ± 0.69 | 14.23 ± 0.72 | 7.99 ± 1.01 | 11.71 ± 1.04 | 8.04 ± 1.91 |
| N245S | 5.25 ± 0.2548 | 2.01 ± 0.29 | 6.20 ± 0.74 | 7.60 ± 1.20 | 5.17 ± 0.99 |
| EMax | Net BRET | ||||
| WT | -0.113 ± 0.006 | -0.092 ± 0.002 | -0.131 ± 0.009 | -0.109 ± 0.001 | -0.120 ± 0.004 |
| N245S | -0.067 ± 0.001 | -0.063 ± 0.001 | -0.055 ± 0.001 | -0.060 ± 0.001 | -0.091 ± 0.001 |
MRGPRX4 is expressed in the relevant sensory dorsal root ganglia
To provide further evidence for a role of MRGPRX4 in somatosensation, we used RT-PCR with RNA obtained from human thoracic dorsal root ganglia (DRG). The DRG serves to relay sensory information to the central nervous system, with the thoracic DRGs receiving sensory input from the lungs and airway. Using RT-PCR primers covering the length of MRGPRX4, following by sequencing of the RT-PCR products to ensure they originated from MRGPRX4 rather than any of the other closely related MRGPRX genes, we found clear expression in this tissue (S4 Fig), consistent with a role for MRGPRX4 in somatosensation in tissues exposed to cigarette smoke.
Discussion
We have identified a variant haplotype of MRGPRX4 that is associated with increased prevalence of menthol cigarette smoking. This variant is found solely in individuals of African ancestry, and increases the odds of menthol use 5-to-8 fold among cigarette smokers. Cell-based assays of MRGPRX4 receptor function identified menthol as a novel negative modulator for this receptor, acting to reduce the responsiveness of this G protein-coupled receptor to its only known agonist at the WT and African-specific coding variant further.
While our understanding of MRGPRX4 gene function is limited, the members of this gene family are expressed in primary sensory neurons and are believed to be involved in somatosensation and nociception, including pruritus [22–24]. Although the natural ligand(s) for MRGPRX4 have not yet been identified, it is of interest that the MRGPRX4 agonist Nateglinide, a drug used to treat Type 2 diabetes, has been reported to have pruritus as a side effect [31]. Together, this suggests that menthol may act outside of the taste sensory system and may exert an anesthetic effect, which is further enhanced by the African-specific form of this receptor, which has dampened signaling capacity.
The MRGPRX4 variant associated with menthol cigarette smoking is relatively uncommon, with a MAF 8% in African Americans. Therefore, this variant alone cannot account for all of the difference in menthol cigarette smoking prevalence between African Americans and other ethnic groups. Thus, it is likely that other factors contribute to these differences. Surprisingly, we did not observe any consistent association at loci previously reported to be associated with menthol cigarette smoking (such as TRPA1 and TAS2R38), or the gene encoding the TRPM8 channel, which has been shown to be the target for menthol action in the somatosensory system. This may be due to the small size of our discovery cohort, which was powered to discover only large effect sizes (OR >2–3). The TRPA1 variants previously linked to menthol smoking had more modest effect sizes (odds ratios 1.3–1.4); thus, our study may have had insufficient power to detect their effects. We also genotyped the TRPA1 SNPs in our Schroeder cohort (N = 741) and could not replicate these associations, suggesting that factors other than power may be responsible for the difference in the results. The previously described association of TRPA1 variants with menthol smoking was restricted to heavy smokers, and was not observed in lighter smokers. Although our discovery cohort likely included a substantial proportion of light smokers, our replication cohort included mostly heavy smokers, which suggests that the lack of association in TRPA1 is unlikely to be explained entirely by the difference in phenotype.
Another possibility is that menthol smoking preferences are regulated by TRPA1 or TRPM8 non-coding variants that were not captured by the Exome chip or whole-exome sequencing. However, we have previously sequenced the exons and adjacent intronic regions of TRPM8 and TRPA1 in the Schroeder and Dallas cohorts and could not find variants with a consistent association with menthol smoking. Nevertheless, these genes remain plausible candidates and further studies, including larger samples of precisely phenotyped individuals, are warranted.
There are currently no crystal structures of the MRGPRX4 receptor available to conclusively determine the location and role of the N245S variant uncovered in this study. Based on a sequence alignment of MRGPRX4 with a published computational model of the related receptor MRGPRX2 [32], N245 (D in MRGPRX2) appears to be located in the third extracellular loop (EL3) of the receptor. Here, N245S reduces Nateglinide-induced agonism in both arrestin and G protein signaling pathways despite an apparent increase in membrane expression. EL3 has been demonstrated in the serotonin receptor 5HT2B to be involved in sterics of ligand binding and the kinetics of ligand and receptor interactions [28]. Thus, it is possible that the N245S variant changes the steric or kinetic properties of Nateglinide binding that influence arrestin and G protein signaling, though further studies will be needed to dissect the mechanism of this effect.
The strengths of our study are the use of a population-based sample including both African-American and European-American smokers, and a replication in a large independent cohort of African Americans. Unlike previous studies that looked at candidate polymorphisms, we performed a hypothesis-free exome-wide screen that provided broad and dense coverage of variation in the coding regions of the genome.
One limitation of our study, as mentioned above, is the relatively small size of our discovery cohort, which provided adequate power to discover only variants with large effect sizes (OR>2–3), and may have missed other genetic variants with lower allele frequencies or smaller effect sizes. Nevertheless, our approach represents an unbiased investigation into the genetic determinants of menthol cigarette smoking in a multiethnic cohort. Another potential limitation is that our data on menthol cigarette use was based on self-report, thus some individuals may have been misclassified with regard to their phenotype. However, our estimates of prevalence of menthol cigarette smoking among ethnic groups were consistent with national estimates, suggesting that misclassification error, if present, is likely small. Likewise, if such misclassification in the Schroeder population led to an overestimate of the association between MRGPRX4 and menthol smoking due to hidden population substructure, this is likely to be small because we found minimal evidence for heterogeneity within this group by large-scale SNP genotyping. Finally, not all participants responded to our questionnaire, thus results may not generalize to other populations. However, responders were similar to non-responders in terms of age and ethnicity (see Materials and Methods).
Menthol is known to exert its effects through transient receptor potential (TRP) channels TRPM8 [33, 34], and to a lesser extent TRPA1 [14]. TRPM8 is also known to mediate menthol-induced analgesia [35–37], and studies have shown that even low levels of menthol in tobacco, below those required to produce mint-like taste or aroma in tobacco, can activate TRPM8 [38]. Although our study was underpowered to detect variants with small effects, we found no evidence of association between variants at the TRPM8 and TRPA1 loci and menthol use, suggesting that variation in these menthol receptors is not a major contributor to the differential use of menthol cigarettes among African Americans.
Menthol cigarettes have been identified as a major threat to public health that have a disproportionate effect on ethnic minorities [39]. Our data suggest that ancestry-specific variants in genes involved in nociception contribute to both inter-individual and inter-ethnic differences in menthol cigarette smoking. The existence of population-specific genetic variants presents a new risk factor for menthol cigarette use, and suggests that the existence of this risk factor can inform health policies and tobacco regulatory actions designed to reduce health disparities in the United States.
Materials and methods
Ethics statement
Participants gave written informed consent under protocols approved by the IRB of the University of Texas Southwestern Medical Center (protocol #STU 112013–048), and the Western IRB (protocol #20131296). The overall study was carried out under protocol #01-DC-0230 and was approved by National Institutes of Health Combined Neurosciences IRB.
Study populations
Discovery cohort
For the primary analysis, we enrolled participants from two population-based cohorts: the Dallas Heart Study (DHS) and the Dallas Biobank. DHS is a multiethnic population-based probability sample of Dallas County, Texas, with intentional oversampling of African Americans [40]. All participants completed a detailed survey and underwent a physical examination, including collection of blood samples for laboratory and genetic analyses. Race and ethnicity were self-assigned according to categories used in the US Census. Smoking status was obtained from the questionnaire. A total of 2267 individuals (including 1217 African Americans self-identified as non-Hispanic black, 720 European Americans self-identified as non-Hispanic white, and 289 Hispanics) identified themselves as current of former smokers and were eligible for the present study. Due to the small number of Hispanic smokers, we limited our investigation to African-American and European-American participants. The Dallas Biobank is a repository of DNA and plasma samples from individuals ascertained at various locations in north-central Texas. Smoking status was obtained via questionnaire. African-American individuals who identified themselves as smokers (N = 2601) were eligible for the present study. All participants were ≥18 years of age.
Menthol data collection
All eligible participants were invited to complete a survey regarding mentholated tobacco use, via telephone and/or email. The survey was approved by the IRB of the University of Texas Southwestern Medical Center under protocol number STU 112013–048. A total of 677 individuals (including 438 DHS and 239 Biobank participants) completed the questionnaire. Responders were more often female compared to non-responders (58.7% versus 49.0% in DHS, and 67.4% versus 52.9% in Biobank, P<0.001 for both), but were not significantly different from non-responders in age (mean age 50.2 ± 11.3 (SD) versus 49.8 ± 9.6 years in DHS, P = 0.45; and 43.6 ± 13.6 versus 43.6 ± 13.0 years in Biobank, P = 0.93) or ethnic composition (63% versus 61% were African American in DHS, P = 0.39; 100% were African American in Biobank). Of the subjects who had completed the questionnaire, 561 (428 from DHS and 133 from Biobank) had exome-wide genotype data available (see below) and were included in our discovery cohort.
Replication cohort
To confirm the findings in the Dallas cohorts, we recruited participants from the Schroeder population, comprising a total of 741 Washington DC resident smokers who were enrolled through the DC Tobacco Quitline (DCQL) [41]. All subjects were self-described African Americans, aged ≥18 years. General variables, such as gender, cigarettes per day (CPD), marital status and education level were obtained from all subjects. Data on mentholated cigarette use were obtained via a questionnaire and from the Wisconsin Inventory of Smoking Dependence Motives (WISDM) [42].
Genotyping
In the Dallas cohorts, genomic DNA was extracted from circulating leukocytes. A total of 4,591 DHS participants and 4,975 African-American participants from the Biobank were previously genotyped using Illumina Infinium HumanExome BeadChip v12.1, which captured >200K markers, including protein-altering variants (>90%), disease-associated variants from previously published genome-wide association studies, ancestry-informative markers, and other variants. Genotypes were called using Illumina GenomeStudio software. Samples were excluded if they met the following criteria: genotype call rate <99%, duplicate sample, discordant duplicate pair, or genotyped gender did match the stated gender. Variants were excluded based on a call rate of <99% or a deviation from Hardy-Weinberg equilibrium in African Americans with P <0.0001. Of the individuals who were successfully genotyped, 561 had data on menthol cigarette smoking and were included in the present study. After quality filtering, 116,212 autosomal variants were polymorphic in our study sample. Due to the relatively small sample size, we removed variants with MAF<1% (<10 carriers). After exclusions, 52,298 autosomal variants were available for analysis.
In the Schroeder population, DNA was collected using Oragene saliva collection kits and extracted according to the manufacturer’s protocol (Genotek Inc., Kanata, Ontario, Canada). Variants identified in the Dallas cohort were assayed by Sanger sequencing, using a dedicated set of primers (S2 Table). DNA chromatograms were analyzed and checked individually in order to evaluate the presence of calling errors with the Lasergene suite (DNASTAR, Madison, Wisconsin). In addition, 24 menthol smokers and 24 non-menthol smokers were randomly chosen and genotyped using the Illumina HumanOmni1 Chip that assayed 1,140,419 SNPs genome-wide to estimate ancestry levels. Variants were excluded based on a call rate of <99% or a deviation from Hardy-Weinberg equilibrium with P <0.0001. High-quality variants were further pruned for linkage disequilibrium (r2<0.1). Ancestry was inferred using ADMIXTURE software v.1.3.0 [43].
Whole-exome sequencing
Exons of MRGPRX4 were sequenced in a subset of Dallas participants (N = 389) using whole-exome sequencing, as part of a related investigation into the role of genetic variation in smoking behaviors. Sample preparation and whole-exome sequencing were performed at the McDermott Center Next-Generation Sequencing core. Three micrograms of genomic DNA was sonicated using a Covaris S2 ultrasonicator (Covaris, Woburn, MA), purified, and assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). DNA was end-repaired, and 3′ ends were adenylated and barcoded with truncated adapters. PCR amplified libraries were purified with AmpureXP beads (New England Biolabs, Ipswich, MA) and assayed using an Agilent 2100 Bioanalyzer. A 750 ng aliquot of the fragment library was concentrated by vacuum to 3.5 μL and hybridized and captured with a SureSelect Human All Exon V4 kit (Agilent Technologies, Santa Clara, CA). Following hybridization, the captured library was amplified and index tags were added to the adapters. DNA was again purified with AmpureXP beads, and fragment sizes were assayed using the Agilent 2100 Bioanalyzer. Paired-end sequencing (150 basepairs) was performed using an Illumina Hiseq 2500. We achieved sufficient coverage depth to provide mean coverage of >115x for targeted bases, with over 94% of target bases covered at least 20x in 95% of the samples (>91% of target bases covered ≥20x in >99% of the samples).
Reads were aligned to the human reference genome build GRCh37 using the Burrows-Wheeler Alignment Tool [44], and variants were called using the Genome Analysis Toolkit (GATK) HaplotypeCaller [45]. Only high-quality variants (GQ >80% and allele depth >20x) were retained for analysis. Variants were annotated using SnpEff [46].
Exome-wide association analysis
In the Dallas cohorts, ancestry was inferred using principal component analysis implemented in EIGENSTRAT [47]. Exome-wide association analysis was performed using PLINK v1.90p [48]. Significance was determined based on a likelihood-ratio test [49], using an R [50] plug-in function for logistic regression. An additive genetic model was assumed, and the analysis was adjusted for age, gender, and 6 leading principal components of ancestry. All variants reaching a significance threshold P<1x10-4 in the discovery cohort were tested for replication in the validation cohort. The analysis was performed in PLINK, with adjustment for age and gender. The association results were combined using random-effects inverse-variance weighted meta-analysis.
Dorsal root ganglia expression
Fresh frozen human dorsal root ganglia was obtained from the National Disease Research Interchange (https://ndriresource.org) under protocol DDRD6 001 001. Following tissue preparation on a Covaris CPO2/S2, RNA was purified using RNeasy Quick start (Qiagen) according to the manufacturer’s instructions and including a DNase digestion. The resulting total RNA was used as template for cDNA synthesis using Superscript IV First-Strand Synthesis System (Invitrogen) with the Oligo d(T)20 primers. Subsequent PCR primers (S8 Table) were used to generate PCR products that were analyzed on a 1% agarose gel (S4 Fig), followed by dideoxy- Sanger sequencing to confirm the RT-PCR products originated from MRGPRX4, rather than the other, closely related MRGPRX gene family members.
Functional studies
Cells and reagents
HTLA cells were a gift from Dr. Richard Axel (Columbia University) and were maintained at low passage batches in DMEM (Corning) containing 10% FBS, 2 μg/mL puromycin and 100 μg/mL hygromycin B in a humidified atmosphere at 37°C with 5% CO2. Inducible MRGPRX4 and MRGPRX4 mutant stable cell lines were generated according to manufacturer’s instructions from the FLP-IN/T-REX HEK-293 cells (Invitrogen, Cat# R78007) and were certified as HEK and mycoplasma-free by Invitrogen.
Constructs
MRGPRX4-Tango codon-optimized plasmids were made as previously described [26]. The MRGPRX4 receptor was then subcloned into the FLP-IN construct without the Tango C-terminus. Mutant MRGPRX4-N245S, MRGPRX4-T43T, and MRGPRX4-N245S+T43T constructs were generated by site-directed mutagenesis PCR using PrimeStar (Takara). Mutations were confirmed by Sanger sequencing using primers V2tail forward, CMVforward BGHR, and TEV tail reverse primers.
Whole cell ELISA
To compare surface expression of the FLAG-tagged MRGPRX4 receptor and the MRGPRX4 mutant N245S, we performed immunohistochemistry on whole, unpermeabilized cells plated in 384-well plates as previously described [27]. Briefly, transfected cells or stably expressing MRGPRX4 cells were seeded at a density of 10,000 cells/well in poly-lysine-coated 384 well plates. After 16–18 hours, cells were fixed with 20 ul/well of 4% paraformaldehyde solution for 10 minutes at room temperature. Cells were then washed twice with 40 ul/well of phosphate-buffered saline (PBS), pH 7.4. Cells were then blocked with 5% normal goat serum in PBS for 30 minutes at room temperature. After removing blocking solution, 20 ul/well of anti-FLAG–horseradish peroxidase–conjugated antibody (Sigma-Aldrich, diluted 1/10,000) was added and incubated at room temperature for 1 hour. Following antibody incubation, cells were washed 2x with 80 ul/well of PBS. Then, 20 ul/well of SuperSignal Enzyme-Linked Immunosorbent Assay Pico Substrate (Sigma-Aldrich) was added and the resulting luminescence was measured using a MicroBeta Trilux luminescence counter. Replicates were averaged by taking fold change compared to WT receptor expression. Each experiment was performed with 64 wells per receptor.
Presto-tango β-arrestin assay
In brief, HTLA cells were seeded at 50% confluency and transfected with MRGPRX4-Tango constructs using the calcium phosphate method [51]. The following day, transfected cells were transferred to glass-bottomed, poly-L-lysine-coated white 384-well plates at a density of 20,000 cells/well in DMEM (Corning) supplemented with 1% dialyzed FBS and 100 IU/mL penicillin and 100 μg/ml streptomycin. The next day, cells were treated with concentration response curves in triplicate with MRGPRX4 agonist Nateglinide in the presence or absence of various concentrations of (-)-Menthol and incubated for 18–24 hours. All chemical compounds were diluted in drug buffer (1X HBSS with 20 mM HEPES and 0.3% Bovine Serum Albumin, pH 7.4). After drug incubation, medium was removed, 20 μL/well of Bright Glo (Promega) (diluted 20-fold) was added, and luminescence was measured on a TriLux luminescence counter. Replicates were averaged using normalized data (fold change over basal).
Inositol phosphate hydrolysis
MRGPRX4 tetracycline-inducible cells were maintained in DMEM containing 10% FBS, 100 μg/mL hygromycin B, and 15 μg/mL blasticidin. For the assay, cells were seeded into poly-L-lysine coated, glass-bottom 96 well plates at a density of 50,000 cells/well in inositol-free DMEM (Caisson labs) containing 1 μCi/well of 3H-myo-inositol (Perkin Elmer), 1 μg/mL tetracycline, and 100 IU/mL penicillin and 100 μg/ml streptomycin and incubated 16–18 hours in a humidified environment at 37°C with 5% CO2. Following tetracycline induction and labeling, medium was removed and 200 μl/well of inositol-free DMEM (Caisson labs) was added per well. Then, 25 μl of 10X concentrated (-)-menthol diluted in drug buffer (1X HBSS with 20 mM HEPES and 0.3% bovine serum albumin, pH 7.4) was added followed by 25 μl of 10X concentrated Nateglinide in concentration response curve. Cells were incubated with drug for 1 hour in a humidified environment at 37°C with 5% CO2. At exactly 15 minutes before lysing, 10 μl/well of 26X concentrated LiCl (15 mM final concentration) was added. After drug and LiCl incubation, 40 μl/well of 50 mM formic acid was added and cells were incubated at 4°C overnight. The next day, 10 μl of lysate was transferred from each well into 96-well flexi-plates (Perkin Elmer) and combined with 75 μl/well of 0.2 mg/well YSI RNA Binding Beads (Perkin Elmer). Lysate-bead mixture was incubated on a shaker at room temperature, protected from light, for 1 hour. Before reading on a TriLux beta counter, plates were centrifuged at 1000 x g for 2 minutes. Replicates were averaged using normalized data (fold change over basal).
BRET assay
Using Transit-2020, HEK293T cells were transfected with 1.5 μg Gαq-rLuc8, 1.5 μg γ1-GFP, 1.5 μg Gβ1 and 1.5 μg of either MRGPRX4 WT or MRGPRX4 N245S. The next day, cells were plated in 1% dFBS/DMEM at a cell density of 40–50,000 cells/well in white 96-well clear-bottom plates. On the third day, media was aspirated from the wells and cells were washed with 60 μL assay buffer (1x Hanks Buffered Saline Solution, 20 mM HEPES, pH 7.4). After the wash was aspirated, 60 μL of assay buffer was added to each well followed by 10 μL of 50 μM coelenterazine 400a. After 5 minutes, drugs were added and the plate was read on an LB940 Mithras using 405/515 emission filters at 1 second signal integration. Data were reported as the ratio of GFP-signal to luciferase signal.
Statistical analysis
Baseline characteristics of participants were compared using t-tests or analysis of variance for continuous variables and chi-square tests for categorical variables. Analyses were performed using R v3.2.1 statistical analysis software [50]. Presto-tango assay data were analyzed in GraphPad Prism V6.07 using an F-test for each EC50 and Emax parameters. ΔΔlog(Emax/EC50) plots were calculated as described in Kenakin et al [30]. BRET assay data were analyzed in Graphpad Prism 7.0 using non-linear regression models. Net BRET was calculated by subtracting values from the no-drug condition. Data comparing effects of menthol were analyzed as two-way repeated measures ANOVA (n = 4 biological replicates, 2 technical replicates for each condition per plate). Post-hoc analyses were performed using a Tukey correction for multiple comparisons.
Supporting information
(PDF)
The figure shows that Schroeder subjects (SCHR) show a greater degree of European admixture (CEU, Utah Residents with Northern and Western Ancestry), relative to West African populations (YRI) and African American from Southwest US (ASW).
(PDF)
(A),(B) Average concentration response curves for Nateglinide or (-)-menthol in agonist mode for MRGPRX4-WT-Tango or MRGPRX4- N245S+T43T-Tango (n = 2, in triplicate, y-axis is % Nateglinide). (C) Average concentration response curves for the dopamine D2-receptor agonist quinpirole in D2-Tango 100 μM or 300 μM (-)-menthol addition, (n = 3, in triplicate, y-axis is % Quinpirole). (D) Average concentration response curves for Nateglinide-induced PI hydrolysis in MRGPRX4-WT tetracycline inducible cells without tetracycline addition (i.e., no receptor expression) following 100 μM or 300 μM (-)-menthol addition, (n = 3, in triplicate, y-axis is relative luminescent counts (RLU).
(TIF)
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Part 1. Comparison of WT versus variant values. Part 2. Comparison of WT versus variant menthol response.
(DOCX)
(DOCX)
Acknowledgments
We thank the Dallas Heart Study Investigators Teresa Eversole and Kate Wilkinson for help with designing the survey and collecting menthol smoking data, McDermott Center Sequencing and Bioinformatics Cores (especially Vanessa Schmid and Chao Xing) for DNA sequencing and bioinformatic analysis, Joanne Gutierrez of the NIDCD for DNA sequencing, and Hong Gao of NYU for figure preparation. We thank the National Disease Research Interchange, Philadelphia, PA for provision of human dorsal root ganglia. We thank Jonathan C. Cohen, Helen H. Hobbs, Elliott M. Ross, and Alexander Chesler for helpful discussions, and the research subjects for their participation.
Data Availability
All relevant data are within the paper and Supporting information files.
Funding Statement
This work was supported by the National Institute on Deafness and Other Communication Disorders (https://www.nidcd.nih.gov) awards NIHOD2013427 under subcontract HHSN263201300011C (J.K.) and Z1A-000046-16 (D.D., D.R., E.S.), by National Center for Advancing Translational Sciences/NIH (https://ncats.nih.gov) under award Number UL1TR001105 (J.K.), by National Institute of Mental Health Award U01MH104974 (B.R., K.L.), the Michael Hooker Distinguished Professorship (B.R.), by a Pharmaceutical Research and Manufacturer’s Association (https://www.phrma.org) Predoctoral fellowship (K.L.), and by National Institute of Neurological Disorders and Stroke (https://www.ninds.nih.gov) award F31 NS093917 (R.O.). Additional support was provided by the National Institutes of Health, Office of the Director and National Institute on Drug Abuse (https://www.drugabuse.gov) Grant RC1-DA028710 (T.K.), and by the U.S. Food and Drug Administration https://www.fda.gov) through funds obtained under the Family Smoking Prevention and Tobacco Control Act (D.D., D.R.). The content was not reviewed by the Food and Drug Administration, but underwent the standard manuscript clearance process for scientific papers published from the NIH intramural research program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta (GA)2014.
- 2.Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. The NSDUH Report: Recent Trends in Menthol Cigarette Use. Rockville, MD; 2011 November 18, 2011.
- 3.Substance Abuse and Mental Health Services Administration, Office of Applied Studies. The NSDUH Report: Use of Menthol Cigarettes. Rockville, MD.; 2009 November 19, 2009.
- 4.Ferris Wayne G, Connolly GN. Application, function, and effects of menthol in cigarettes: a survey of tobacco industry documents. Nicotine Tob Res. 2004;6 Suppl 1:S43–54. [DOI] [PubMed] [Google Scholar]
- 5.Willis DN, Liu B, Ha MA, Jordt SE, Morris JB. Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. FASEB J. 2011;25(12):4434–44. 10.1096/fj.11-188383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson SJ. Marketing of menthol cigarettes and consumer perceptions: a review of tobacco industry documents. Tob Control. 2011;20 Suppl 2:ii20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Federal Trade Commission. Federal Trade Commission Cigarette Report for 2014. Washington, DC2016.
- 8.Kreslake JM, Wayne GF, Alpert HR, Koh HK, Connolly GN. Tobacco industry control of menthol in cigarettes and targeting of adolescents and young adults. Am J Public Health. 2008;98(9):1685–92. 10.2105/AJPH.2007.125542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tobacco Products Scientific Advisory Committee. Menthol Cigarettes and Public Health: Review of the Scientific Evidence and Recommendations Rockville, MD: Center for Tobacco Products, Food and Drug Administration; 2011. [Google Scholar]
- 10.Mitka M. FDA might consider restrictions on menthol cigarettes. JAMA. 2013;310(8):784 10.1001/jama.2013.276273 [DOI] [PubMed] [Google Scholar]
- 11.Food and Drug Administration. Preliminary Scientific Evaluation of the Possible Public Health Effects of Menthol Versus Nonmenthol Cigarettes. Washington, DC2013.
- 12.Caraballo RS, Asman K. Epidemiology of menthol cigarette use in the United States. Tob Induc Dis. 2011;9 Suppl 1:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448(7150):204–8. 10.1038/nature05910 [DOI] [PubMed] [Google Scholar]
- 14.Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, et al. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27(37):9874–84. 10.1523/JNEUROSCI.2221-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uhl GR, Walther D, Behm FM, Rose JE. Menthol preference among smokers: association with TRPA1 variants. Nicotine Tob Res. 2011;13(12):1311–5. 10.1093/ntr/ntr119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannon DS, Baker TB, Piper ME, Scholand MB, Lawrence DL, Drayna DT, et al. Associations between phenylthiocarbamide gene polymorphisms and cigarette smoking. Nicotine Tob Res. 2005;7(6):853–8. 10.1080/14622200500330209 [DOI] [PubMed] [Google Scholar]
- 17.Mangold JE, Payne TJ, Ma JZ, Chen G, Li MD. Bitter taste receptor gene polymorphisms are an important factor in the development of nicotine dependence in African Americans. J Med Genet. 2008;45(9):578–82. 10.1136/jmg.2008.057844 [DOI] [PubMed] [Google Scholar]
- 18.Risso DS, Kozlitina J, Sainz E, Gutierrez J, Wooding S, Getachew B, et al. Genetic Variation in the TAS2R38 Bitter Taste Receptor and Smoking Behaviors. PLoS One. 2016;11(10):e0164157 10.1371/journal.pone.0164157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oncken C, Feinn R, Covault J, Duffy V, Dornelas E, Kranzler HR, et al. Genetic Vulnerability to Menthol Cigarette Preference in Women. Nicotine Tob Res. 2015;17(12):1416–20. 10.1093/ntr/ntv042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Risso D, Sainz E, Gutierrez J, Kirchner T, Niaura R, Drayna D. Association of TAS2R38 Haplotypes and Menthol Cigarette Preference in an African American Cohort. Nicotine Tob Res. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devlin B, Roeder K. Genomic control for association studies. Biometrics. 1999;55(4):997–1004. [DOI] [PubMed] [Google Scholar]
- 22.Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106(5):619–32. [DOI] [PubMed] [Google Scholar]
- 23.Choi SS, Lahn BT. Adaptive evolution of MRG, a neuron-specific gene family implicated in nociception. Genome Res. 2003;13(10):2252–9. 10.1101/gr.1431603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zylka MJ, Dong X, Southwell AL, Anderson DJ. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc Natl Acad Sci U S A. 2003;100(17):10043–8. 10.1073/pnas.1732949100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroeze WK, Sassano MF, Huang XP, Lansu K, McCorvy JD, Giguere PM, et al. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat Struct Mol Biol. 2015;22(5):362–9. 10.1038/nsmb.3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato S, Huang XP, Kroeze WK, Roth BL. Discovery and Characterization of Novel GPR39 Agonists Allosterically Modulated by Zinc. Mol Pharmacol. 2016;90(6):726–37. 10.1124/mol.116.106112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wacker D, Wang S, McCorvy JD, Betz RM, Venkatakrishnan AJ, Levit A, et al. Crystal Structure of an LSD-Bound Human Serotonin Receptor. Cell. 2017;168(3):377–89 e12. 10.1016/j.cell.2016.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature. 2016;537(7619):185–90. 10.1038/nature19112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenakin T, Watson C, Muniz-Medina V, Christopoulos A, Novick S. A simple method for quantifying functional selectivity and agonist bias. ACS Chem Neurosci. 2012;3(3):193–203. 10.1021/cn200111m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nateglinide Side Effects [Available from: https://www.drugs.com/sfx/nateglinide-side-effects.html.
- 32.Lansu K, Karpiak J, Liu J, Huang XP, McCorvy JD, Kroeze WK, et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat Chem Biol. 2017;13(5):529–36. 10.1038/nchembio.2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52–8. 10.1038/nature719 [DOI] [PubMed] [Google Scholar]
- 34.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108(5):705–15. [DOI] [PubMed] [Google Scholar]
- 35.Zygmunt PM, Hogestatt ED. Trpa1. Handb Exp Pharmacol. 2014;222:583–630. 10.1007/978-3-642-54215-2_23 [DOI] [PubMed] [Google Scholar]
- 36.Buday T, Brozmanova M, Biringerova Z, Gavliakova S, Poliacek I, Calkovsky V, et al. Modulation of cough response by sensory inputs from the nose—role of trigeminal TRPA1 versus TRPM8 channels. Cough. 2012;8(1):11 10.1186/1745-9974-8-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu B, Fan L, Balakrishna S, Sui A, Morris JB, Jordt SE. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain. 2013;154(10):2169–77. 10.1016/j.pain.2013.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paschke M, Tkachenko A, Ackermann K, Hutzler C, Henkler F, Luch A. Activation of the cold-receptor TRPM8 by low levels of menthol in tobacco products. Toxicol Lett. 2017;271:50–7. 10.1016/j.toxlet.2017.02.020 [DOI] [PubMed] [Google Scholar]
- 39.Benowitz NL, Samet JM. The threat of menthol cigarettes to U.S. public health. N Engl J Med. 2011;364(23):2179–81. 10.1056/NEJMp1103610 [DOI] [PubMed] [Google Scholar]
- 40.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93(12):1473–80. 10.1016/j.amjcard.2004.02.058 [DOI] [PubMed] [Google Scholar]
- 41.Kirchner TR, Cantrell J, Anesetti-Rothermel A, Ganz O, Vallone DM, Abrams DB. Geospatial exposure to point-of-sale tobacco: real-time craving and smoking-cessation outcomes. Am J Prev Med. 2013;45(4):379–85. 10.1016/j.amepre.2013.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith SS, Piper ME, Bolt DM, Fiore MC, Wetter DW, Cinciripini PM, et al. Development of the Brief Wisconsin Inventory of Smoking Dependence Motives. Nicotine Tob Res. 2010;12(5):489–99. 10.1093/ntr/ntq032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19(9):1655–64. 10.1101/gr.094052.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–60. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–303. 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cingolani P, Platts A, Wang le L, Coon M, Nguyen T, Wang L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin). 2012;6(2):80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–9. 10.1038/ng1847 [DOI] [PubMed] [Google Scholar]
- 48.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7 10.1186/s13742-015-0047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xing G, Lin CY, Wooding SP, Xing C. Blindly using Wald's test can miss rare disease-causal variants in case-control association studies. Ann Hum Genet. 2012;76(2):168–77. 10.1111/j.1469-1809.2011.00700.x [DOI] [PubMed] [Google Scholar]
- 50.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria. 2015.
- 51.Jordan M, Schallhorn A, Wurm FM. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 1996;24(4):596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
The figure shows that Schroeder subjects (SCHR) show a greater degree of European admixture (CEU, Utah Residents with Northern and Western Ancestry), relative to West African populations (YRI) and African American from Southwest US (ASW).
(PDF)
(A),(B) Average concentration response curves for Nateglinide or (-)-menthol in agonist mode for MRGPRX4-WT-Tango or MRGPRX4- N245S+T43T-Tango (n = 2, in triplicate, y-axis is % Nateglinide). (C) Average concentration response curves for the dopamine D2-receptor agonist quinpirole in D2-Tango 100 μM or 300 μM (-)-menthol addition, (n = 3, in triplicate, y-axis is % Quinpirole). (D) Average concentration response curves for Nateglinide-induced PI hydrolysis in MRGPRX4-WT tetracycline inducible cells without tetracycline addition (i.e., no receptor expression) following 100 μM or 300 μM (-)-menthol addition, (n = 3, in triplicate, y-axis is relative luminescent counts (RLU).
(TIF)
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Part 1. Comparison of WT versus variant values. Part 2. Comparison of WT versus variant menthol response.
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and Supporting information files.