Abstract
With the excellent solubility, mobility, bioaccumulation and carcinogenesis, hexavalent chromium Cr (VI), widely exists in various industrial effluents such as chrome plating, metal finishing, pigments, and tanning. Cr (VI) is one of the toxic metal pollutants among all the heavy metals. Therefore, the purpose of this work was to convert highly water-soluble Cr (VI) into Cr (III) species using electrocoagulation (EC) process. The Box–Behnken design (BBD) as was applied to investigate the effects of major operating variables and optimization conditions. The predicted values of responses obtained using the model is agreed well with the experimental data. This work demonstrated that the Cr (VI) is entirely converted into Cr (III) in solid-phases in electrocoagulation process. It was also found that reduction increased with current density that suggesting that the reduction efficiency is closely related to the generation of floc.
Keywords: Cr (VI), Cr (III), EC, BBD, Fe, Al
Specifications table
| Subject area | Wastewater treatment |
| More specific subject area | Chemical engineering |
| Type of data | Table and Figure |
| How data was acquired | UV–vis Double Beam Spectrophotometer. (Hitachi U-2900, India) |
| Data format | Raw, analyzed |
| Experimental factor | Box–Behnken design matrix was used to investigate the effects of major operating variables using Al and Fe electrodes in batch EC process. |
| Experimental features | Removal of Cr (VI) by EC |
| Data source location | Guru Gobind Singh Indraprastha University, Dwarka New Delhi, India |
| Data accessibility | The data are available with this article |
Value of the data
-
•
Now day׳s the regulation of Cr (VI) in drinking water have spurred strong interests due its adverse effect of Cr(VI) on human as well ecosystem.
-
•
The acquired data will be advantageous for the scientific community wanting to scale up and design an electrocoagulation process for removal of Cr (VI).
-
•
Based on the dataset, electrocoagulation is considered as a promising treatment technology for wastewater such as electroplating, metal finishing, and tanning etc.
-
•
The proposed design correlations may prove to be a useful tool in designing pilot and commercial plants for Cr (VI) removal.
1. Data
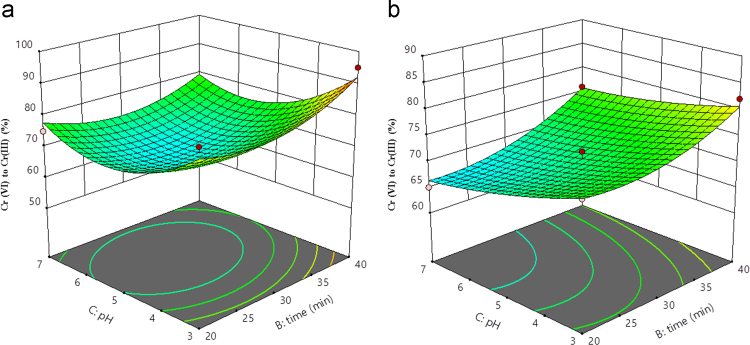
This dataset contains 3 Tables and 5 Figures that represent statistical optimization of electrocoagulation process for reduction of Cr (VI) to Cr (III) from synthetic wastewater in batch mode of operation using BBD. A total 15 number of batch experiments including three centre points were carried out in triplicates using statistically deigned experiments. The results are shown in Table 1, Table 2, Table 3a and 3b. The suitability of the selected model to provide adequate approximation of the real system is also confirmed by the diagnostic plots. Such plots include normal probability plots, residuals versus predicted and the predicted versus actual value plot (See Figs. 1 and 2). The 3D graphs were plotted to identify the optimized reaction conditions and to understand the individual effects of pH, voltage and time for efficient conversion of Cr (VI) to Cr (III) (See Fig. 3, Fig. 4, Fig. 5).
Table 1.
Factor and levels of experiment through BBD.
| Factors | Levels | ||
|---|---|---|---|
| Voltage (V) | 5 | 10 | 15 |
| Time (min) | 20 | 30 | 40 |
| pH | 3 | 5 | 7 |
Table 2.
BBD matrix with experimental and predicted result.
| Run no. | Voltage (V) | Time (min) | pH |
CRE (%) |
|||
|---|---|---|---|---|---|---|---|
| Fe (Exp) | Fe (Pre) | Al (Exp) | Al (Pre) | ||||
| 1 | 5 | 40 | 5 | 63.00 | 64.50 | 70.00 | 71.13 |
| 2 | 5 | 30 | 7 | 66.00 | 62.88 | 60.00 | 59.00 |
| 3 | 10 | 30 | 5 | 68.00 | 68.33 | 70.00 | 70.67 |
| 4 | 15 | 30 | 3 | 90.00 | 93.13 | 80.00 | 81.00 |
| 5 | 5 | 20 | 5 | 58.00 | 58.25 | 64.00 | 63.63 |
| 6 | 10 | 30 | 5 | 67.00 | 68.33 | 72.00 | 70.67 |
| 7 | 15 | 30 | 7 | 80.00 | 78.63 | 82.00 | 81.75 |
| 8 | 10 | 40 | 3 | 95.00 | 92.13 | 82.00 | 80.63 |
| 9 | 10 | 20 | 7 | 75.00 | 77.88 | 65.00 | 66.38 |
| 10 | 15 | 40 | 5 | 85.00 | 84.75 | 87.00 | 87.38 |
| 11 | 10 | 30 | 5 | 70.00 | 68.33 | 70.00 | 70.67 |
| 12 | 10 | 20 | 3 | 85.00 | 83.38 | 74.00 | 74.13 |
| 13 | 10 | 40 | 7 | 78.00 | 79.63 | 76.00 | 75.88 |
| 14 | 15 | 20 | 5 | 82.00 | 80.50 | 80.00 | 78.88 |
| 15 | 5 | 30 | 3 | 65.00 | 66.38 | 72.00 | 72.25 |
Table 3a.
ANOVA analysis for Al electrode.
| Source | Sum of squares | df | Mean square | F-value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 1605.32 | 9 | 178.37 | 16.39 | 0.0033 | Significant |
| A-voltage | 903.12 | 1 | 903.12 | 82.98 | 0.0003 | |
| B-time | 55.12 | 1 | 55.12 | 5.07 | 0.0742 | |
| C-pH | 162.00 | 1 | 162.00 | 14.89 | 0.0119 | |
| AB | 1.0000 | 1 | 1.0000 | 0.0919 | 0.7740 | |
| AC | 30.25 | 1 | 30.25 | 2.78 | 0.1564 | |
| BC | 12.25 | 1 | 12.25 | 1.13 | 0.3373 | |
| A² | 17.33 | 1 | 17.33 | 1.59 | 0.2626 | |
| B² | 125.64 | 1 | 125.64 | 11.54 | 0.0193 | |
| C² | 304.64 | 1 | 304.64 | 27.99 | 0.0032 | |
| Residual | 54.42 | 5 | 10.88 | |||
| Lack of Fit | 49.75 | 3 | 16.58 | 7.11 | 0.1258 | Not significant |
| Pure Error | 4.67 | 2 | 2.33 | |||
| Cor Total | 1659.73 | 14 | ||||
| Std. Dev. | 3.30 | R2 | 0.9672 | |||
| Mean | 75.13 | Adj R2 | 0.9082 | |||
| C.V.% | 4.39 | Adeq. Precision | 12.9473 | |||
Table 3b.
ANOVA analysis for Fe electrode.
| Source | Sum of squares | df | Mean square | F-value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 792.18 | 9 | 88.02 | 38.55 | 0.0004 | Significant |
| A-voltage | 496.12 | 1 | 496.12 | 217.28 | <0.0001 | |
| B-time | 128.00 | 1 | 128.00 | 56.06 | 0.0007 | |
| C-pH | 78.13 | 1 | 78.13 | 34.22 | 0.0021 | |
| AB | 0.2500 | 1 | 0.2500 | 0.1095 | 0.7541 | |
| AC | 49.00 | 1 | 49.00 | 21.46 | 0.0057 | |
| BC | 2.25 | 1 | 2.25 | 0.9854 | 0.3664 | |
| A² | 13.56 | 1 | 13.56 | 5.94 | 0.0588 | |
| B² | 26.26 | 1 | 26.26 | 11.50 | 0.0194 | |
| C² | 3.10 | 1 | 3.10 | 1.36 | 0.2963 | |
| Residual | 11.42 | 5 | 2.28 | |||
| Lack of Fit | 8.75 | 3 | 2.92 | 2.19 | 0.3290 | Not significant |
| Pure Error | 2.67 | 2 | 1.33 | |||
| Cor Total | 803.60 | 14 | ||||
| Std. Dev. | 1.51 | R2 | 0.9858 | |||
| Mean | 73.60 | Adjusted R2 | 0.9602 | |||
| C.V.% | 2.05 | Adeq Precision | 22.9984 | |||
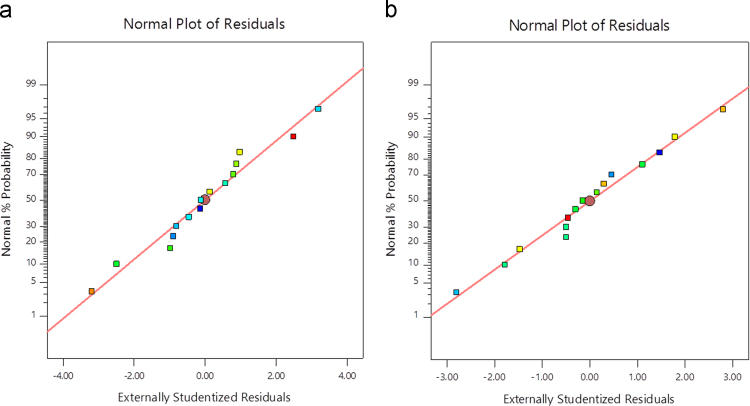
Fig. 1.
Normality probability plot (a) Al (b) Fe.
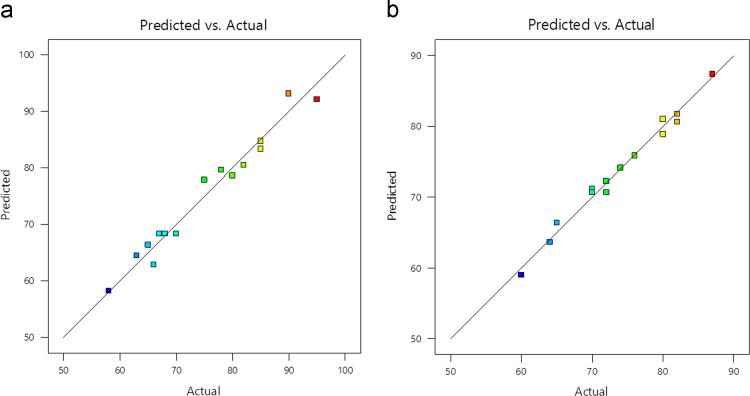
Fig. 2.
Predicted vs actual (a) Al (b) Fe.
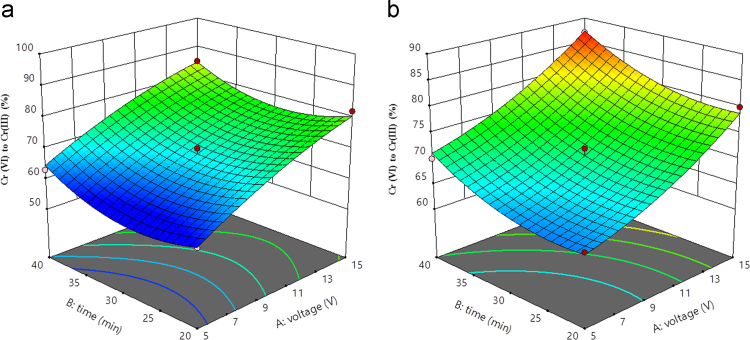
Fig. 3.
3D plot of time and voltage (a) Al (b) Fe.
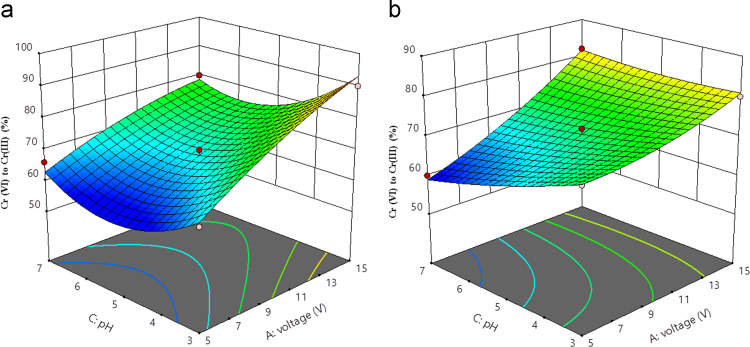
Fig. 4.
3D plot of pH and voltage (a) Al (b) Fe.
Fig. 5.
3D plot of pH and time (a) Al (b) Fe.
2. Experimental design, materials, and methods
2.1. Sample preparation
Stock solution (100 mg/L) of Cr (VI) was prepared by dissolving of potassium dichromate (Qualigens, India) in distilled water. Sodium hydroxide (Qualigens, India) and sulphuric acid (Qualigens, India) were used to adjust the pH of the solution. Potassium permanganate (Qualigens, India), sodium azide (Qualigens, India) and 1,5-diphenylcarbazide (Qualigens, India) were used for analysis of the chromium present in synthetic solution [1], [2].
2.2. Analytical methods
A 95 mL of Cr (VI) solution tested in a 100 mL volumetric flask. The pH of sample was maintained less than 2 by adding 2 drops of concentrated H2SO4 then 2 drops of phosphoric acid (H3PO4) were added. Then 2 mL of 1,5 Diphenylcarbazide (DPC) added to the solution and mixed thoroughly then leave for 5–10 min for full-color development. After full color development an appropriate amount of the solution (4 mL) was taken into 3 mm quartz cell and measured its absorbance at 540 nm using UV–vis Double Beam (Hitachi U-2900, India) spectrophotometer [3].
2.3. Experimental setup and procedure
The electrolytic cell consists of a glass beaker of 400 mL capacity. Aluminum and iron sheets were used as electrodes. The electrode distance between anode and cathode was maintained constant of 1.5 cm during electrolysis. A direct current was supplied by a DC power source (Science tech 4074, India, 0–5 A and 0–30 V). Agitation was provided to maintain uniform concentration inside the cell using (SPINOT 02, India). A stock solution Cr(VI) was prepared by dissolving an appropriate amount of potassium dichromate (Qualigens, India) in distilled water. All the experiments were carried out under potentiostatic conditions at room temperature. The pH of the solution was adjusted using either dilute HCl or NaOH. After each experiment the samples were collected and analyzed for Cr (VI) using 1,5-diphenylcarbazide (DPC) method.
The Cr (VI) to Cr (III) reduction percentage from synthetic solution was calculated using the following equation reported by [4], [5]:
| (1) |
where Co and Ci are initial and final concentrations of Cr (VI) in mg/L respectively. The reproducibility in the experimental results was found to be ±3%.
2.4. Statistical methods and data analysis
Box–Behnken design was established with the help of the Design Expert 11 software for statistical design of experiment and data analysis. The three significant process variables considered in this study were: Voltage (A), time (B) and pH (C) as shown in Table 1. The total number of experiments in this study was 15 including three center points were carried out in triplicates for the estimation of error. The observed and predicted results for each set of reaction parameters are given in Table 2. A quadratic polynomial equation using Design Expert software was fitted to the experimental data obtained according to the Box–Behnken design. Normality plot have been illustrated in Fig. 1 for aluminum (Al) and iron electrode (Fe) electrodes. Fig. 1 shows the normality assumption is clearly satisfied reduction of 95% which is close to result obtained by EC experiments given as straight line [6], [7]. The actual and the predicted results by EC process using aluminum and iron electrode is shown in Fig. 2. Actual values are the measured response data for a particular run, and the predicted values are evaluated from the model and generated by using the approximating function. It is seen in Fig. 2 that the data points lie close to the diagonal line and the developed model is adequate for the prediction of each response. ANOVA studies presented in Tables 3a and 3b. 3D plots (Fig. 3, Fig. 4, Fig. 5) suggested time and current as the dominant process parameters for reduction of Cr (VI) to Cr (III).
Acknowledgments
All authors wish to acknowledge the financial support made by Guru Gobind Singh Indraprastha University, India Faculty Research Grant project (GGSIPU/DRC/Ph.D/Adm./2016/1588).
Footnotes
Transparency data associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2018.12.054.
Transparency document. Supplementary material
Supplementary material
.
References
- 1.Verma S.K., Khandegar V., Saroha A.K. Removal of chromium from electroplating industry effluent using electrocoagulation. J. Hazard. Toxic Radioact. Waste. 2013;17:146–152. [Google Scholar]
- 2.Tyagi U., Khandegar V. Biosorption potential of Vetiveria zizanioides for the removal of chromium(VI) from synthetic wastewater. J. Hazard. Toxic Radioact. Waste. 2018;22:1–8. [Google Scholar]
- 3.APHA, Standard Methods for the Examination of Water and Waste Water, 1995. [DOI] [PMC free article] [PubMed]
- 4.Golder A.K., Chanda A.K., Samanta A.N., Ray S. Removal of hexavalent chromium by electrochemical reduction-precipitation: investigation of process performance and reaction stoichiometry. Sep. Purif. Technol. 2011;76:345–350. [Google Scholar]
- 5.Bhatti M.S., Reddy A.S., Thukral A.K. Electrocoagulation removal of Cr(VI) from simulated wastewater using response surface methodology. J. Hazard. Mater. 2009;172:839–846. doi: 10.1016/j.jhazmat.2009.07.072. [DOI] [PubMed] [Google Scholar]
- 6.Aleboyeh A., Daneshvar N., Kasiri M.B. Optimization of C.I. Acid Red 14 azo dye removal by electrocoagulation batch process with response surface methodology. Chem. Eng. Process. Process Intensif. 2008;47:827–832. [Google Scholar]
- 7.Ghanim A.N. Optimization of pollutants removal from textile wastewater by electrocoagulation through RSM. J. Babylon. Univ. 2014;22 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material