Abstract
Background
Regional anaesthesia may reduce the rate of persistent postoperative pain (PPP), a frequent and debilitating condition. This review was originally published in 2012 and updated in 2017.
Objectives
To compare local anaesthetics and regional anaesthesia versus conventional analgesia for the prevention of PPP beyond three months in adults and children undergoing elective surgery.
Search methods
We searched CENTRAL, MEDLINE, and Embase to December 2016 without any language restriction. We used a combination of free text search and controlled vocabulary search. We limited results to randomized controlled trials (RCTs). We updated this search in December 2017, but these results have not yet been incorporated in the review. We conducted a handsearch in reference lists of included studies, review articles and conference abstracts. We searched the PROSPERO systematic review registry for related systematic reviews.
Selection criteria
We included RCTs comparing local or regional anaesthesia versus conventional analgesia with a pain outcome beyond three months after elective, non‐orthopaedic surgery.
Data collection and analysis
At least two review authors independently assessed trial quality and extracted data and adverse events. We contacted study authors for additional information. We presented outcomes as pooled odds ratios (OR) with 95% confidence intervals (95% CI), based on random‐effects models (inverse variance method). We analysed studies separately by surgical intervention, but pooled outcomes reported at different follow‐up intervals. We compared our results to Bayesian and classical (frequentist) models. We investigated heterogeneity. We assessed the quality of evidence with GRADE.
Main results
In this updated review, we identified 40 new RCTs and seven ongoing studies. In total, we included 63 RCTs in the review, but we were only able to synthesize data on regional anaesthesia for the prevention of PPP beyond three months after surgery from 39 studies, enrolling a total of 3027 participants in our inclusive analysis.
Evidence synthesis of seven RCTs favoured epidural anaesthesia for thoracotomy, suggesting the odds of having PPP three to 18 months following an epidural for thoracotomy were 0.52 compared to not having an epidural (OR 0.52 (95% CI 0.32 to 0.84, 499 participants, moderate‐quality evidence). Simlarly, evidence synthesis of 18 RCTs favoured regional anaesthesia for the prevention of persistent pain three to 12 months after breast cancer surgery with an OR of 0.43 (95% CI 0.28 to 0.68, 1297 participants, low‐quality evidence). Pooling data at three to 8 months after surgery from four RCTs favoured regional anaesthesia after caesarean section with an OR of 0.46, (95% CI 0.28 to 0.78; 551 participants, moderate‐quality evidence). Evidence synthesis of three RCTs investigating continuous infusion with local anaesthetic for the prevention of PPP three to 55 months after iliac crest bone graft harvesting (ICBG) was inconclusive (OR 0.20, 95% CI 0.04 to 1.09; 123 participants, low‐quality evidence). However, evidence synthesis of two RCTs also favoured the infusion of intravenous local anaesthetics for the prevention of PPP three to six months after breast cancer surgery with an OR of 0.24 (95% CI 0.08 to 0.69, 97 participants, moderate‐quality evidence).
We did not synthesize evidence for the surgical subgroups of limb amputation, hernia repair, cardiac surgery and laparotomy. We could not pool evidence for adverse effects because the included studies did not examine them systematically, and reported them sparsely. Clinical heterogeneity, attrition and sparse outcome data hampered evidence synthesis. High risk of bias from missing data and lack of blinding across a number of included studies reduced our confidence in the findings. Thus results must be interpreted with caution.
Authors' conclusions
We conclude that there is moderate‐quality evidence that regional anaesthesia may reduce the risk of developing PPP after three to 18 months after thoracotomy and three to 12 months after caesarean section. There is low‐quality evidence that regional anaesthesia may reduce the risk of developing PPP three to 12 months after breast cancer surgery. There is moderate evidence that intravenous infusion of local anaesthetics may reduce the risk of developing PPP three to six months after breast cancer surgery.
Our conclusions are considerably weakened by the small size and number of studies, by performance bias, null bias, attrition and missing data. Larger, high‐quality studies, including children, are needed. We caution that except for breast surgery, our evidence synthesis is based on only a few small studies. On a cautionary note, we cannot extend our conclusions to other surgical interventions or regional anaesthesia techniques, for example we cannot conclude that paravertebral block reduces the risk of PPP after thoracotomy. There are seven ongoing studies and 12 studies awaiting classification that may change the conclusions of the current review once they are published and incorporated.
Plain language summary
Local and regional anaesthesia at the time of surgery to prevent longer‐term persistent pain after surgery
Review question
We set out to determine if the use of local anaesthetics (numbing medicine) at the time of surgery reduces the risk of having pain that persists for three months and more after surgery. The comparison was with pain killers alone, such as opioids and non‐steroidal anti‐inflammatory drugs.
Background
Pain that persists long after surgery is called persistent postoperative pain (PPP), and is not uncommon. Tissue damage and nerve injury can change pain pathways and sensibility to pain so that pain persists for months. A person may also feel pain more intensely or with a stimulus that normally is not perceived as pain. These changes can be permanent. Applying local anaesthetics close to nerves, bundles of nerves, or nerve roots in the central nervous system, as with an epidural, can interrupt the conduction of pain impulses from the surgical site to the central nervous system. Effective treatment of acute pain may prevent PPP. Wound infiltration uses a specially designed tube with multiple holes that is placed inside the wound to deliver the local anaesthetic.
Study characteristics
The evidence is current to December 2016. We found 63 randomized controlled trials (RCTs) with participants undergoing open chest, heart, breast, abdominal, vascular, gynaecological and other surgery, but not orthopaedic surgery. RCTs are studies where people are allocated by chance to one or the other of different treatments being studied. The studies included only adults, and were mostly conducted in Europe and North America, with some from China, Egypt and Brazil. The types of surgery included surgery with a high event rate of persistent pain after surgery, such as breast surgery, limb amputation and opening the chest, and surgery with a lower risk but high numbers of procedures, such as caesarean section.
We were able to pool results from 39 RCTs enrolling a total of 3027 participants for our inclusive analysis. Follow‐up was for 1293 participants at three months, 1365 participants at six months, 326 participants at 12 months, and 43 participants at 20 or more months after surgery. The RCTs did not report surgical and anaesthetic complications consistently and little information was available on these. The studies were mostly funded by the institutions conducting the studies.
Key results
Regional anaesthesia reduced the number of people who experienced persistent pain after undergoing non‐orthopaedic surgery. For open chest surgery, giving an epidural halved the odds of a person having persistent postoperative pain at three to 18 months after surgery (7 RCTs, 499 participants, moderate‐quality evidence). Seven people needed to be treated in this way for one to benefit.
For the prevention of persistent pain three to 12 months after breast cancer surgery, seven people needed regional anaesthesia for one to benefit (18 RCTs, 1297 participants, low‐quality evidence). Infusion of local anaesthetic into a vein was shown to reduce the risk of persistent pain three to six months after breast surgery (2 RCTs, 97 participants, moderate‐quality evidence), with three people needing to be treated for one to benefit. Regional anaesthesia reduced the odds by more than half of a woman experiencing persistent pain after caesarean section (4 RCTs, 551 participants, moderate‐quality evidence). The number of women treated for one to benefit was 19.
Continuous local anaesthetic infusion of the site where bone tissue was obtained from the hip bone did not clearly reduce the number of people with persistent pain at three to 55 months (3 RCTs, 123 participants, low‐quality evidence).
We could not synthesize evidence for limb amputation, hernia repair, cardiac or abdominal surgery because of differences in how treatment was given or how results were reported.
Quality of the evidence
We found consistent evidence supporting the use of regional anaesthesia in adults to prevent persistent pain after a number of types of surgery. However, we observed variations in the effect sizes, and at different times after surgery. Some studies could not be blinded to the treatment received and our results are affected by the small number of studies and participants, and the loss to follow‐up of participants over time. The evidence was therefore of low or moderate quality.
Summary of findings
Background
Description of the condition
Pain arising from a surgical intervention and persisting beyond three months is termed persistent postoperative pain (PPP) (Kehlet 2006). PPP continues to be frequent and is sometimes severe, but often neglected (Bayman 2014; Gewandter 2015; Kehlet 2006; Perkins 2000). The risk of developing PPP varies from 5% after minor surgery to 50% for phantom limb pain or postmastectomy pain syndrome (Jung 2003; Perkins 2000). Young age, the surgical procedure and perioperative pain predict PPP, while genetic risk factors remain unknown (Lewis 2015; Montes 2015). PPP may be only mild or it may be severely disabling (Kehlet 2006). Even the relatively low risk (about 10%) of developing PPP after caesarean section is a major concern due to the frequency of caesarean sections (Sng 2009). Most clinical studies focus on acute postoperative pain, and few address the preventive effects of regional anaesthesia on PPP (MacRae 2001; MacRae 2008). Recent reviews deplored the poor quality of available studies and documented the high event rate after a variety of surgical interventions, from hernia repair to breast surgery (MacRae 2001; MacRae 2008). Our current review focuses on the ability of local anaesthetics or regional anaesthesia to reduce the risk of PPP.
Pain pathways, and hence pain perception, can be modulated, sensitized and permanently altered (Woolf 2000). Persistent pain, postoperative hyperalgesia and allodynia (Kehlet 2006), after surgery are the consequence of neuronal plasticity, that is permanent synaptic neuronal changes in the peripheral and central nervous system in response to tissue trauma and nerve injury; where hyperalgesia refers to pain felt more intensely and allodynia describes a painful sensation after a stimulus that normally is not perceived as pain (Wilder‐Smith 2006).
Description of the intervention
Before or after surgery, local anaesthetics may be applied locally to interrupt the conduction of pain impulses from the site of injury to the central nervous system. If local anaesthetics are applied locally at the site of surgery this is called local anaesthesia. If local aesthetics are applied close to nerves, but at a distance from the surgical site, this is called regional anaesthesia. Sometimes, local aesthetics are also applied intravenously. All three modes of administration of local aesthetics may prevent the central sensitization described in the Description of the condition. Epidural and spinal anaesthesia act at the nerve roots while nerve blocks, plexus anaesthesia and wound infiltration inhibit peripheral nerves. By blocking sympathetic nerves, local anaesthetics may also have desirable effects on bowel motility or unwanted effects on blood pressure. Systemically (for example intravenously) administered local anaesthetics might also exert beneficial effects including preventing PPP, hyperalgesia and allodynia (Duarte 2005; Herroeder 2007; Lavand'homme 2005; Strichartz 2008; Vigneault 2011). As in our previous review, in this update we also focused on the pre‐emptive (Kissin 1996), use of local anaesthetics with or without opioids or other adjuvants intravenously or in regional anaesthesia.
The local and regional anaesthesia techniques described above can be used as an alternative or in addition to conventional pain control. Opioids like morphine, non‐steroidal anti‐inflammatory drugs (NSAIDs) such as ibuprofen, and other analgesics like paracetamol (acetaminophen in the USA) are the most frequently used conventional pain killers. They are administered systemically and, therefore, often cause systemic side effects that limit their use, like the nausea and constipation caused by opioids or kidney damage as a result of use of NSAIDs. We have provided an explanation of regional anaesthesia and conventional analgesia in Appendix 1.
How the intervention might work
We hypothesize that preventing pain transmission using local or regional anaesthesia during or soon after surgery, or both, reduces the risk of PPP (Atchabahian 2015b; Woolf 1993). Local anaesthetics applied close to the nerves will block pain perception and prevent the central sensitization in the spinal cord that leads to hyperalgesia and PPP (Kehlet 2006) (see: Description of the condition). However, systemic toxicity of local anaesthetics is well described (Brown 1995), either as a side effect after absorption or when given intravenously (Herroeder 2007; Strichartz 2008). Anti‐hyperalgesic effects of systemic lidocaine persist days beyond drug delivery and cannot be explained by sodium channel blockage. The actual mechanism remains elusive (Strichartz 2008).
Our review focused on preventive analgesia. We defined preventive analgesia as antinociception with local anaesthetics or regional anaesthesia to reduce the risk of PPP regardless of the timing of the intervention in relation to surgery (Kissin 2000). We did not study if local anaesthetics or regional anaesthesia were more effective if applied before, during or after surgery (Bong 2005; Lavand'homme 2011).
Why it is important to do this review
PPP is frequent and difficult to treat (Kehlet 2006). Hence prevention of PPP is paramount (Gewandter 2015). We are interested in investigating whether local anaesthetics or regional anaesthesia prevent PPP several months after surgery. Clinical trials report conflicting results. For example, epidural anaesthesia may reduce the risk of PPP after thoracotomy (Ju 2008; Lu 2008; Senturk 2002), but these effects have not been consistently reproduced (Ochroch 2006). Our previous review and evidence synthesis (Andreae 2012), favoured regional anaesthesia for PPP after breast cancer surgery and thoracotomy; but these inferences were based on a few small studies and plagued by unit‐of‐analysis issues. Also we found that pertinent studies reported repeated outcomes at different and disparate follow‐up intervals (Andreae 2012). We did not find enough studies to allow us to make inferences for other surgical subgroups. No other meta‐analysis is presently available on the effect of local or regional anaesthesia on PPP six to 12 months after surgery. A systematic review by Ong focused mostly on immediate postoperative pain control and the timing of regional anaesthesia (Ong 2005); some have questioned his results and methods (Møiniche 2002). Existing narrative reviews of regional anaesthesia for PPP have not attempted evidence synthesis (MacRae 2001; MacRae 2008). Terkawi 2015a sought to synthesize the evidence on paravertebral block for the prevention of PPP, but found the outcome reporting of available randomized controlled trials (RCTs) disparate and hence evidence synthesis difficult.
Objectives
To compare local anaesthetics and regional anaesthesia versus conventional analgesia for the prevention of PPP beyond three months in adults and children undergoing elective surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included studies with a randomized, controlled design. We also included single‐blinded studies because regional anaesthesia causes numbness of the affected body part and, therefore, neither participant nor anaesthesia provider can be reliably blinded to the intervention. However, blinding of the outcome observer was a prerequisite for inclusion in this review.
Types of participants
We included studies in adults and children undergoing elective surgical procedures, encompassing general, thoracic, abdominal, vascular, gynaecological and other surgery. This included the main groups of surgery with a high event rate of persistent pain after surgery, such as breast surgery, limb amputation and thoracotomy, but also groups with a lower baseline risk but high surgical volume, such as caesarean section.
We excluded studies in participants undergoing orthopaedic procedures as they are covered by another Cochrane Review (Atchabahian 2015a).
Types of interventions
We included studies comparing local anaesthetics or regional anaesthesia versus conventional pain control (Appendix 1).
Interventions
We included studies comparing local anaesthetics and regional anaesthesia versus conventional pain control.
We defined local anaesthetics as any pharmacological agents acting on the sodium channel to block nerve conduction (Movassaghian 2013; Rodriguez‐Navarro 2011).
The inclusion criteria for the intervention groups were as follows.
Studies administering local anaesthetics or regional anaesthesia, including:
studies that employed local anaesthetics or regional anaesthesia for any length of time during the perioperative period;
studies that employed local anaesthetics by any route (Appendix 1);
studies that may also have employed adjuvants or opioids, either locally or systemically, in any one group.
The exclusion criteria for the intervention groups were:
studies that only compared different regional anaesthesia techniques or varying dose regimens of local anaesthetics during the same perioperative time span;
studies using local anaesthetics for other than anaesthetic or analgesic purposes (for example as anti‐arrhythmics).
The inclusion criteria for the comparator groups were:
studies that used conventional postoperative pain control (Appendix 1).
Types of outcome measures
We studied primary and secondary outcomes as follows.
Primary outcomes
Our primary outcome was persistent postoperative pain (PPP) at three or more months after surgery.
We defined PPP as new pain, (which did not exist before the operation), but lasting beyond three months after surgery. We defined our primary outcome of interest as a dichotomous contrast, namely the presence versus absence of pain elicited at that clinical encounter. We accepted the dichotomous pain outcomes as reported in the studies, mostly contrasting pain versus no pain, even though definitions varied at times. Use of pain medication is by some assessed as a dichotomous outcome (no pain medication versus pain medication) or as an ordinal outcome (no pain medication versus non‐opioid pain medication versus opioid pain medication) (Lavand'homme 2005). Some primary study authors define the presence or absence of pain in their study as pain exceeding a given threshold on a continuous pain scale, analogous to responder analysis. We accepted the thresholds used by the study authors, though they sometimes employed different scales or instruments. This responder analysis (Andreae 2015c; Dworkin 2009a), also employed during our previous version of this review (Andreae 2015), counts the number of people with an outcome above a defined threshold. Responder analysis informed our approach to missing data imputation (Andreae 2013b), as detailed below (Dealing with missing data). We discussed responder analysis and the heterogeneity of outcome reporting in greater detail in (Overall completeness and applicability of evidence). Studies elicited the presence of pain at different follow‐up intervals beyond our cut‐off of three months and we discuss the two approaches we took (inclusive versus classical analyses) to address this heterogeneity in Data synthesis.
We also assessed differences in scores based on validated pain scales, such as the visual analogue scale (VAS); the verbal rating score; or the McGill pain questionnaire (Dworkin 2009b).
Secondary outcomes
Our secondary outcomes were as follows.
Allodynia and hyperalgesia
Use of pain medication
Adverse effects of techniques and agents used
Acceptable continuous measures for allodynia or hyperalgesia may, for example, be the area of punctuate allodynia or hyperalgesia measured with von Frey hair (Lavand'homme 2005).
For adverse events we accepted any definition by the authors of the primary studies, who in the previous version of this review (Andreae 2012), sparsely reported on adverse events and most anecdotally or in narrative form. We discuss in Overall completeness and applicability of evidence, that registries are better suited to assess adverse events after regional anaesthesia given their rare occurrences.
Search methods for identification of studies
We performed an electronic search of common databases and handsearched reference lists of relevant studies and conference abstracts.
Electronic searches
In December 2016 we searched for studies on local anaesthetics or regional analgesia for the prevention of PPP in the Evidence‐Based Medicine Reviews (EBMR) via OVID‐Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 12), Ovid MEDLINE (1946 to December 2016), and Ovid Embase (1980 to December 2016).
We performed an additional search in December 2017 and added the results to Studies awaiting classification to be incorporated into the next update of this review.
We limited the results in MEDLINE using the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision), as described in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). As there is, as yet, no Cochrane Highly Sensitivity Search Strategy for Embase, we limited the results in Embase using a filter we found at the University of Alberta library, based on a trial done in MEDLINE (Glanville 2006; University of Alberta Library Guide 2014).
We combined a free text search with a controlled vocabulary search, covering from the inception of the database to the present.
We searched for studies using local or regional anaesthesia for painful postsurgical conditions with an outcome follow‐up of weeks or months. Our MEDLINE, Embase and CENTRAL search terms are reproduced in the appendices (see:Appendix 2; Appendix 3; Appendix 4).
We did not impose a language restriction.
Searching other resources
We conducted a handsearch of the reference lists of included studies, review articles and other identified relevant studies for additional citations, and in the conference abstracts of the International Anesthesia Research Society (IARS) and the European Society of Regional Anaesthesia (ESRA) for 2005 through to 2007.
Because the yield of the handsearch was very low, we did not update this search in 2015.
We followed links for related articles in Pubmed Central. We searched the PROSPERO systematic review registry (Booth 2012), for related systematic reviews, which might list relevant studies.
Data collection and analysis
We present a diagram illustrating the process of the searches and selection and we followed the recommendations of the QUORUM and PRISMA statements (Moher 1999; Moher 2010; Figure 1).
1.
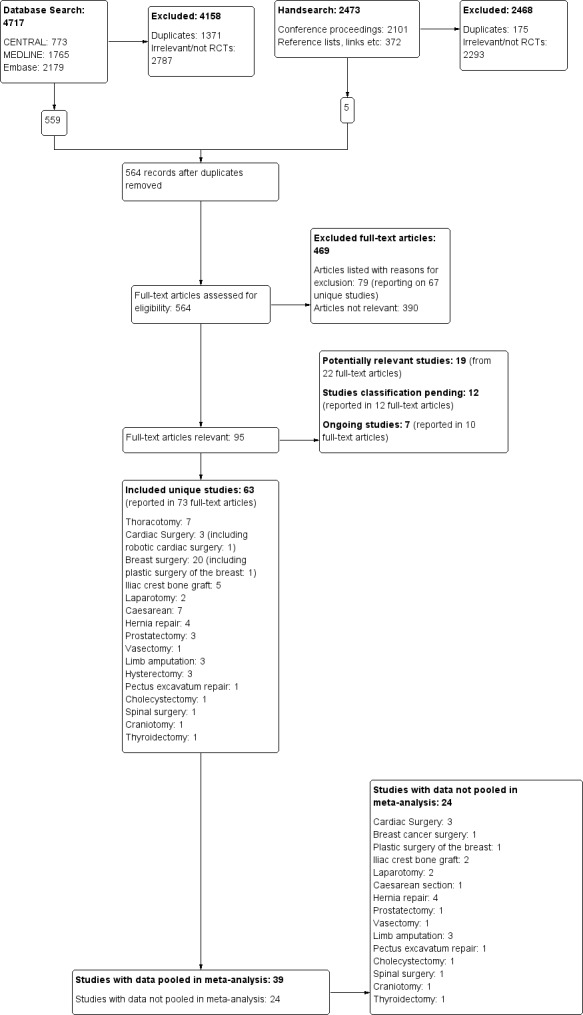
The study flow diagram documents the search and selection process. We included 63 studies. We were able to pool data from 39 of the 63 included studies in our inclusive analysis; data from 24 studies were not available or otherwise could not be pooled (Appendix 11).
Selection of studies
We completed screening and data extraction using DistillerSR, a web‐based systematic review software.
The review authors (EJW, MSC, JLL, JYC, DAA and MHA) screened the citations and abstracts of all publications obtained by the search strategies. To avoid location bias, all articles detected by our search, (but not available via online subscription of our institutions) were requested through interlibrary loans. For studies that appeared to be eligible RCTs, we obtained and inspected the full articles to assess their relevance based on the preplanned criteria for inclusion. We noted the reasons for study exclusion and inserted them into the Characteristics of excluded studies table.
Data extraction and management
We developed a standard data collection form within DistillerSR based on a template provided by Cochrane Anaesthesia, Critical and Emergency Care (ACE) for the first version of this review (Andreae 2012). We recorded details of study design, participant characteristics, interventions and outcome measures. We performed a pilot run and revised our data sheet accordingly, published as an appendix in our previous review (Andreae 2012). For this review update, at least two review authors independently collected and extracted data (EJW, JLL, MSC, JYC, MHA and DAA), using the DistillerSR software, based on the previously used data extraction form (Andreae 2012). EJW, JLL, MSC, MHA and DAA checked and entered the data into Review Manager 5 (RevMan 5) (RevMan 2014), computer software.
We extracted the following primary outcome data on pain: any patient‐reported chronic pain outcome (dichotomous, continuous or multidimensional instrument) at three months or beyond after surgery.
Where dichotomous data on persistent postoperative pain were not reported, we attempted to obtain these from the study authors. If unavailable, we used continuous pain assessment and outcome measures (for example the VAS or the Numerical Rating Scale (NRS)) or complex instruments to evaluate chronic pain (for example the Brief Pain Inventory (BPI)).
We extracted the following secondary outcomes, where provided: allodynia and hyperalgesia, use of pain medication.
We also extracted the following data: exclusion criteria; comorbidity; regional anaesthesia technique and local anaesthetic used; quality assurance of the intervention; quality of pain control; assessment of hyperalgesia and allodynia; use of adjuvants; and surgery performed. We extracted data on adverse effects and attrition.
Assessment of risk of bias in included studies
For each report, at least two of the review authors (EJW, MSC, JLL, JYC, MHA and DAA) independently evaluated each report meeting the inclusion criteria. We contacted study authors for missing information regarding their methods. We graded study quality in a 'Risk of bias' table on the basis of a checklist of design components. This comprised randomization, concealed allocation, observer blinding, and intention‐to‐treat analysis. We extracted information on conflicts of interest and funding (see: Characteristics of included studies). We achieved consensus by informal discussion. We judged risk of bias to be unclear, high or low (Higgins 2011a).
In regional anaesthesia interventions, blinding of participants and anaesthesia providers can be difficult and hence this criterion received less weight in the evaluation of performance bias, but not with regard to detection bias. We listed excluded studies with detailed reasons (see: Characteristics of excluded studies).
If the randomization and allocation process was open to substantial bias, for example pseudo‐randomization, we did not include the study data in the data analysis.
Null bias
In response to the first version of this review (Andreae 2013b), clinicians expressed concern about null bias. Null bias might cause studies to underestimate the benefit of regional anaesthesia for the prevention of persistent pain after surgery, if the regional anaesthesia interventions were not effectively delivered (Higgins 2011a; Woods 1995). Indeed, a number of included studies reported no improved pain control in the immediate postoperative period in the experimental (regional anaesthesia) group, as evidenced by inconsequential differences in pain scores between groups perioperatively, or similar requirements of rescue analgesic medications between groups in the immediate postoperative period (Barkhuysen 2010; Baudry 2008; Bollag 2012; Can 2013; Choi 2016; Fassoulaki 2000; Ibarra 2011; Ju 2008; Karmakar 2014; Katz 1996; Lam 2015; Lee 2013; Liu 2015; Loane 2012; McKeen 2014; Micha 2012; Purwar 2015; Singh 2013; Smaldone 2010; Terkawi 2015b; Vrooman 2015; Xu 2017; Zhou 2016). Two review authors therefore extracted information on null bias for each included study and documented their judgement with supporting evidence (see: Characteristics of included studies).
Exploring the influence of attrition and follow‐up interval on effect size.
We explored the possible influence of attrition and follow‐up duration on effect size. We plotted attrition (in percent of participants lost at follow‐up from participants randomized) versus effect size (log odds ratio) for the major groups of studies investigating regional anaesthesia for the prevention of persistent postoperative pain, where we had most studies with repeated measurements. We connected repeated sequential effect measures at consecutive follow‐up visits within one study. We wanted to test the hypothesis that increasing attrition and outcome reporting at later follow‐ups leads to bias in the effect size estimation. If we found evidence to refute our null hypothesis of no association, then pooling studies reporting outcomes at different follow‐up intervals or with differential attrition might lead to biased pooled estimates and we would avoid this mode of analysis.
Measures of treatment effect
As the summary statistic for our dichotomous primary outcome, we chose the odds ratio (OR) (Bland 2000). We reported the OR with 95% confidence intervals (CI). We calculated the number needed to treat for an additional beneficial outcome (NNTB) for the surgical subgroups, for example, for thoracotomy and breast cancer surgery, but not for the overall effect across all types of surgery (Cook 1995). We used the open source statistical software package R (R 2015), to compute the NNTB and its 95% CI according to the Cochrane Handbook for Systematic Reviews of Interventions chapter 12.5.4.3 Computing absolute risk reduction or NNTB from an OR (Schünemann 2011a), as documented in Appendix 5.
Risk ratios (RR) and ORs are equally accepted measures of treatment effect (Deeks 2011). The planned integration of dichotomous outcomes with continuous outcomes implied the use of ORs (see: Data synthesis). After this integration turned out to be of marginal importance for our analysis, we decided to stick to our protocol to eliminate any reasonable doubt about a postanalysis decision that might inappropriately influence our results (Andreae 2008).
For the continuous pain scales we calculated the mean difference between groups when all studies in a given subgroup used the same scale, and standardized mean differences (SMD) between groups when studies being compared used different scales.
Unit of analysis issues
Some studies have the surgical site (e.g. left or right hernia) as unit of analysis as opposed to the study participant (Bell 2001; Kurmann 2015), which could, in theory, confound results as absorbed lidocaine from the treated site could exert effects on the non‐treated site if they were randomized to discordant interventions (Strichartz 2008).
For our inclusive evidence synthesis (Analysis 1.1; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11), we pooled studies reporting outcomes at variable follow‐up intervals. When one study reported the results at several subsequent follow‐up intervals, we used only the latest outcome reported, because the most sustained effect would be most interesting clinically.
1.1. Analysis.
Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 1 PPP three to 18 months after thoracotomy.
1.3. Analysis.
Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 3 PPP three to twelve months after breast cancer surgery.
1.4. Analysis.
Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 4 PPP three to eight months after caesarean section.
1.5. Analysis.
Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 5 Pain score three to six months after caesarean section.
1.6. Analysis.
Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 6 PPP three to 55 months after Iliac crest bone graft.
1.7. Analysis.
Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 7 PPP six to 12 months after amputation.
1.8. Analysis.
Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 8 PPP six to 12 months after laparotomy.
1.9. Analysis.
Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 9 PPP three to 12 months after hernia repair.
1.10. Analysis.
Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 10 Pain score three months after prostatectomy.
1.11. Analysis.
Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 11 SF‐36 bodily pain score at three to six months after hysterectomy.
Dealing with missing data
We checked with the study authors for any missing information and reported data inconsistencies in the Characteristics of included studies. We specified in the tables if we were unable to obtain data.
Assessment of heterogeneity
We grouped studies in subgroups based on surgical interventions. Depending on the surgery, PPP has a different natural history (MacRae 2008). We feel these differences argue against pooling or comparing studies across surgical disciplines (Deeks 2011). We investigated study heterogeneity at the subgroup level using a Chi2 test and calculation of the I2 statistic (Higgins 2002). We followed the thresholds suggested in the Cochrane Handbook for Systematic Reviews of Interventions for the interpretation of I2 statistic (Deeks 2011).
Assessment of reporting biases
We contacted study authors to request missing data. We countered time lag bias by repeating our search just prior to submission of our work.
We considered an examination of publication bias using graphical and statistical tests (e.g. funnel plot, Egger's test (Sterne 2011)).
Data synthesis
In anticipation of diversity in reporting (Andreae 2012), in this update including additional studies with earlier and later follow‐up intervals at three months and beyond 12 months, we planned to pool studies reporting outcomes at different intervals after surgery and to build one coherent hierarchical Bayesian model (Andreae 2017a; Carter 2015), described in detail elsewhere (Andreae 2015). We thereby followed Ioannidis 2008, who explicitly proposed Bayesian methods to synthesize heterogeneous studies to overcome disparity in study design and reporting. In addition we performed a classical (frequentist) stratified evidence synthesis by surgical subgroup and follow‐up interval as in our initial publication (Andreae 2012). Frequentist inference, throughout this review, refers to the classical statistical approaches of significance and hypothesis testing proposed by Fisher and Neyman–Pearson, respectively, in contrast to the Bayesian statistical paradigm of updating a prior probability with new data (Andreae 2015c; Andreae 2018; Gelman 2014).
Inclusive model
For the inclusive evidence synthesis, we did not pool the data across different surgical disciplines. Instead, we grouped studies in broad surgical categories (e.g. thoracotomy, limb amputation, breast cancer surgery, etc.) based on the different natural history of PPP after each surgery. Where we had sufficient studies for a surgical procedure, that is, the inclusive analysis in breast surgery (Analysis 1.3), we organized the studies according to the regional anaesthesia intervention employed.
Pooling across different follow‐up intervals
We pooled studies reporting results at different follow‐up intervals to get a single stable estimate of the effect in a given surgical subgroup. Stratifying both by follow‐up and surgical subgroup would have led to very few studies at each follow‐up for each subgroup and hence unstable and variable pooled effect estimates. We counted each study only once, using the last follow‐up, if results were reported at more than one, and ordered them in the forest plots according to the duration of follow‐up. For example, in Analysis 1.3 synthesizing the dichotomous outcome persistent postoperative pain after breast cancer surgery, we pooled studies reporting this outcome at three, six and 12 months.
The underlying assumption is that follow‐up duration and attrition do not alter the effect estimate and we tested this hypothesis as described under Assessment of risk of bias in included studies and Incomplete outcome data (attrition bias), (Levene 2015). We describe how we dealt with unit of analysis issues in studies reporting outcomes at several follow‐up intervals under (Unit of analysis issues), and for the Bayesian model below.
Stratified analysis
We compared the results of our inclusive model with a classical (frequentist) stratified analysis where we only pooled studies with similar follow‐ups in each surgical subgroup. This predictably would lead to smaller bins and hence to more variability in the estimate, including possibly contradicting results when pooling the same studies, but repeatedly at subsequent intervals. If follow‐up varied only by weeks to one month, we considered follow‐up intervals to be the same, for example data at 24 weeks or at five months with data at six months.
For both stratified and inclusive analysis, we used the inverse‐variance approach, adjusting study weights based on the extent of variation, or heterogeneity, among the varying intervention effects (Deeks 2011). Confidence intervals for the average intervention effect would be wider with the more conservative random‐effects model; this would account for any potential between‐study heterogeneity and result in a more cautious estimate of any treatment effect (DerSimonian 1986).
We pooled treatment effects following the random‐effects meta‐analysis using the Cochrane statistical software RevMan 2014, as detailed in Chapter 8.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Following the process of GRADE assessment (GRADE Working Group 2004), we generated 'Summary of findings' tables as detailed in Chapter 11.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011b) using the computer software GRADEpro GDT 2015.
Bayesian model
Anticipating that some studies would report only dichotomous outcomes while other studies would report only continuous outcomes (Andreae 2012), we had planned to pool the results in one comprehensive Bayesian hierarchical model (Andreae 2017a; Ioannidis 2008).
We started with a Bayesian hierarchical model for the surgical subgroup of iliac crest bone graft harvesting (ICBG). Where dichotomous aggregate data were not available, we estimated the dichotomous data from the continuous data presented for Blumenthal 2005 (Andreae 2013b). We then pooled the data in a Bayesian model (Andreae 2013b), implemented in the statistical software OpenBugs (Lunn 2009), with the model code presented in Appendix 6.
Bayesian statistics and our all‐inclusive Bayesian hierarchical model are described elsewhere in greater detail (Andreae 2015; Andreae 2017b; Carter 2015; Gelman 2014), but essentially we first obtained study‐level estimates for studies reporting outcomes at several subsequent follow‐up intervals by pooling these in a random‐effects model. Then we pooled these study‐level pooled effect estimates with the study‐level data of studies reporting only at one specific follow‐up interval by subgroups according to surgical intervention, as described above for the classical (frequentist) model. Finally we pooled the group‐level effect estimates to obtain an overall effect estimate. We used weak priors for effect estimates. We pooled the estimates of the within‐study variance between subsequent follow‐ups across all studies, assuming that the variability of effect estimates within a study would not depend on the surgical intervention but rather on the outcome measurement, which would be similar across all studies. We pooled the within‐group variance across studies and as a sensitivity analysis estimated between‐study variance for each group. We chose our prior for the variance of the overall effect estimate, the between‐group variability to force it to represent our prior belief that effects in one group will be almost independent of effects in another surgical group, reflecting the identical approach executed in both the classical and the inclusive analysis, described above. We used one study, identified during the initial search and selection, but subsequently excluded as non‐randomized (Brull 1992), to inform our Bayesian priors for the hierarchical Bayesian model of the subgroup of ICBG. We compared results based on this informative prior with results based on a weak uninformative prior (Andreae 2013b; Andreae 2015; Gelman 2014). In this we considered the argument by Shrier, that observational studies did not differ in their effects of interventions (Shrier 2007).
Model estimation, implementation and convergence testing
We used Marcov Chain Monte Carlo (MCMC) methods implemented in OpenBugs (Carter 2015; Lunn 2009) for our ICBG Bayesian model and the Hamiltonian Monte Carlo (HMC) algorithm implemented in the probabilistic programming language Stan (RStan 2.5), to fit our all‐inclusive model. We assessed convergence looking at trace plots of our simulations. We explored the multidimensional autocorrelation of parameters using shinyStan, our purpose‐built software, to visualize objects created in the Stan language (ShinyStan 1.0). We investigated tree depth and other HMC‐specific convergence parameters (Gelman 2014). We used the Gelman‐Rubin statistic Ř to assess convergence of all parameters (Gelman 2014). Even though convergence was satisfactory, we ran the final model with four chains, and 100,000 iterations in OpenBugs (Lunn 2009), and 5000 iterations, (including 2500 warm‐up iterations) in (RStan 2.5).
Pooling groups with different timing of regional anaesthesia interventions or varying use of adjuvants in regards to the surgical intervention
For studies with several groups using local or regional anaesthesia, albeit with varying use of adjuvants or different timing of the intervention with regards to the surgical procedure, or both, we pooled all groups employing local or regional anaesthesia and compared them against the comparator. If the first group received a regional anaesthesia intervention before incision (preoperative or pre‐emptive) and the second group received it after incision (postoperative or preventive), we pooled the (first and second) groups employing local anaesthetics against the (third) control groups not employing any local anaesthetics (that is using only conventional pain control instead). Similarly, if there were multiple study groups using (different) regional anaesthesia, one with and one without an adjuvant analgesic, we pooled the results from both groups and compared them to the control group using conventional analgesic methods.
Subgroup analysis and investigation of heterogeneity
Where there were enough studies in one group, we calculated the I2 statistic (Higgins 2002). We followed the thresholds suggested in the Cochrane Handbook for Systematic Reviews of Interventions for the interpretation of the I2 statistic (Deeks 2011).
We investigated studies employing adjuvant therapy, using different regional anaesthesia modalities, and studies providing continuous postoperative regional anaesthesia as a subgroup.
Sensitivity analysis
We tested the sensitivity of our results to our model assumptions and calculated the effect estimates for our pooled subgroups (e.g. breast cancer surgery and thoracotomy) for the random‐effects model versus the fixed‐effect model. For the Bayesian model, we tested the influence of different priors on the pooled estimate (Gelman 2014), comparing the use of a non‐RCT (Brull 1992), to inform our Bayesian priors versus the use of a weak, non‐informative prior for our Bayesian hierarchical model, for the subgroup of Illiac crest bone graft harvesting only, as reported in greater detail elsewhere (Andreae 2013b).
'Summary of findings' table and GRADE
We used the GRADE approach to assess the quality of the evidence (Langendam 2013). We imported import data from RevMan 2014, using GRADEpro GDT 2015, to create 'Summary of findings' tables (Table 1; Table 2; Table 3; Table 4; Table 5). These tables summarize the magnitude of the effects of the interventions examined, the total sum of all available data and their consistency, weighing them against the internal and external validity of the studies, or lack thereof. We assessed the overall quality of evidence for each outcome. We downgraded the evidence from 'high quality' by one level for serious (or by two levels for very serious) study limitations (risk of bias, e.g. performance bias, shortcomings in allocation concealment, considerable attrition and incomplete outcome data) serious inconsistency, heterogeneity or imprecision of effect estimates. We reported the effect of local or regional anaesthesia on the prevention of PPP at three months or beyond by surgical subgroups after thoracotomy (Table 1), breast cancer surgery (Table 2; Table 5), caesarean section (Table 3), and ICBG (Table 4).
Summary of findings for the main comparison. Thoracic epidural anaesthesia versus conventional pain control to prevent persistent pain after open thoracotomy.
| Should thoracic epidural anaesthesia or conventional pain control be used to prevent persistent pain after open thoracotomy | ||||||
| Patient or population: people undergoing open thoracotomy Settings: university and teaching hospitals in China, Turkey and Canada Intervention: thoracic epidural anaesthesia Comparison: conventional pain control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional pain control | Thoracic epidural anaesthesia | |||||
|
Persistent pain 3 to 18 months after thoracotomy (We defined persistent postsurgical pain as new pain that did not exist before the operation, measured using differences in scores based on validated pain scales; patient interview between 3 to 18 months after surgery.) |
Study population | OR 0.52 (0.32 to 0.84) | 499 (7 studies) | ⊕⊕⊕⊝ moderate1,2,3 | All studies investigated persistent pain after open thoracotomy. The results cannot be extended to video‐assisted thoracotomy or other (minimally invasive) surgeries of the chest. The five of the seven included studies using thoracic epidural anaesthesia showed the strongest effect. The results cannot be extended to other interventions like paravertebral blocks. Conventional pain control with opioids and NSAID was the comparator. Event rates of persistent pain after thoracotomy were reported between 25% to 65% Regional anaesthesia may prevent persistent (chronic) pain after open thoracotomy in one out of seven people treated, thoracic epidural anaesthesia in one out of five people treated. |
|
| 525 per 1000 | 332 per 1000 (230 to 453) | |||||
| Low | ||||||
| 250 per 1000 | 130 per 1000 (83 to 200) | |||||
| Moderate | ||||||
| 500 per 1000 | 310 per 1000 (213 to 429) | |||||
| Adverse effects of epidural anaesthesia ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Adverse effects of epidural anaesthesia were not systematically reported and due to their low frequency are better investigated in patient registries. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NSAID: nonsteroidal anti‐inflammatory drugs; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1While outcome observers' blinding was described, study participants were not blinded; this is acceptable because participant and provider blinding is difficult in regional anaesthesia. 2We downgraded quality of evidence by one level because none of the studies performed an intention‐to‐treat analysis. Considerable attrition might have led to bias. 3There was no evidence of statistical heterogeneity. Studies that failed to improve immediate postoperative pain control had lower effect estimates beyond three months (null bias).
Summary of findings 2. Regional anaesthesia compared to conventional pain control for breast cancer surgery.
| Should regional anaesthesia or conventional pain control be used to prevent persistent pain following breast cancer surgery | ||||||
| Patient or population: women with breast cancer undergoing elective surgery Settings: cancer, community and university hospitals in Europe, China and North America Intervention: various regional anaesthesia techniques including paravertebral block, nerve blocks or local infiltration Comparison: conventional pain control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional pain control | Paravertebral block | |||||
|
Persistent pain 3 to 12 months after breast cancer surgery (We defined persistent postsurgical pain as new pain that did not exist before the operation, measured using differences in scores based on validated pain scales; patient interview between 3 to 12 months after surgery.) |
Study population | OR 0.43 (0.28 to 0.68) | 1297 (18 studies) | ⊕⊕⊝⊝ low1,2 | Conventional pain control with opioids and NSAID was the comparator. Event rates of persistent pain after breast cancer were reported around 30%. Pooling all studies, regional anaesthesia may prevent persistent pain after breast surgery in one out of every seven women. Limiting the analysis to paravertebral block, the number of women needed to treat for one person to benefit was 11. |
|
| 427 per 1000 | 239 per 1000 (162 to 340) | |||||
| Low | ||||||
| 200 per 1000 | 95 per 1000 (61 to 147) | |||||
| High | ||||||
| 600 per 1000 | 387 per 1000 (281 to 509) | |||||
| Adverse effects of paravertebral block for breast cancer surgery | See comment | See comment | Not estimable | ‐ | See comment | Adverse effects of regional anaesthesia after breast surgery were not systematically reported and due to their low frequency are better investigated in registries. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NSAID: nonsteroidal anti‐inflammatory drugs; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1We downgraded quality of evidence by one level because conclusions may be considerably weakened by performance bias, shortcomings in allocation concealment, considerable attrition and incomplete outcome data. 2We downgraded quality of evidence by one level because there was evidence of heterogeneity. The effect estimates were contingent on the type of surgery and the anaesthesia intervention.
Summary of findings 3. Local or regional anaesthesia for the prevention of chronic pain after caesarean section.
| Should local or regional anaesthesia be used for the prevention of chronic pain after caesarean section | ||||||
|
Patient or population: women after caesarean section
Settings: maternity and university hospitals in South and North America, Egypt and Europe
Intervention: local or regional anaesthesia Comparison: conventional pain control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Local or regional anaesthesia | |||||
|
Persistent pain 3 to 8 months after caesarean section (We defined persistent postsurgical pain as new pain that did not exist before the operation, measured using differences in scores based on validated pain scales; patient interview between 3 to 8 months after surgery.) |
Study population | OR 0.46 (0.28 to 0.78) | 551 participants (4 studies1) | ⊕⊕⊕⊝ moderate2,3 | Event rates of persistent pain after caesarean section are reported around 10%. The number of women needed to be treated for one woman to benefit from regional anaesthesia after caesarean section was 19. |
|
| 179 per 1000 | 91 per 1000 (58 to 145) | |||||
| Low | ||||||
| 50 per 1000 | 24 per 1000 (15 to 39) | |||||
| Moderate | ||||||
| 100 per 1000 | 49 per 1000 (30 to 80) | |||||
| Adverse effects of local or regional anaesthesia ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Adverse effects of local or regional anaesthesia after caesarean section were not systematically reported and due to their low frequency are better investigated in registries. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1The results are based on only four, mostly smaller studies. Meta‐analysis results based on small numbers tend to overestimate the effects. 2The methodological quality of the larger trial was good, but only intermediate for the remaining studies. 3We downgraded quality of evidence by one level, because of the above noted two concerns, and because the pooled effect estimate is mainly driven by one larger study (Shahin 2010).
Summary of findings 4. Continous donor site local anaesthetic infusion for the prevention of persistent postoperative pain after iliac crest bone graft harvesting.
| Should continuous donor site local anaesthetic infusion or conventional pain control be used for the prevention of persistent postoperative pain after iliac crest bone graft harvesting | ||||||
|
Patient or population: people after iliac crest bone graft harvesting
Settings: university hospitals in Europe and North America
Intervention: continuous donor site local anaesthetic infusion Comparison: conventional pain control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Continous donor site local anaesthetic infusion | |||||
|
Persistent pain 3 to 55 months after iliac crest bone graft harvesting (We defined persistent postsurgical pain as new pain that did not exist before the operation, measured using differences in scores based on validated pain scales; patient interview between 3 to 55 months after surgery) |
Low | OR 0.20 (0.04 to 1.09) | 123 (3 studies1) | ⊕⊕⊝⊝ low1 | We accepted study author classification of the presence of persistent postoperative pain. Some assessed only pain vs no pain, others pain and dysaesthesia vs none. Event rates of persistent pain after iliac crest bone graft harvesting were reported between 20% to 40% and was assumed to be around 30%. |
|
| 200 per 1000 | 48 per 1000 (10 to 214) | |||||
| Moderate | ||||||
| 400 per 1000 | 118 per 1000 (26 to 421) | |||||
| High | ||||||
| 600 per 1000 | 231 per 1000 (57 to 620) | |||||
| Adverse effects of continuous local anaesthetic infusion ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Adverse effects of regional anaesthesia after iliac crest bone graft harvesting were not systematically reported and due to their low frequency are better investigated in registries. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1The results are based on only three small studies. Meta‐analysis results based on small numbers tend to overestimate the effects. Including an additional RCT with continuous outcomes in a Bayesian evidence synthesis further strengthens the evidence favouring the intervention (Blumenthal 2005).
Summary of findings 5. Continous intravenous local anaesthetic infusion for the prevention of persistent pain after breast cancer surgery.
| Should continuous intravenous local anaesthetic infusion or conventional pain control be used for the prevention of persistent pain after breast cancer surgery | ||||||
|
Patient or population: women with breast cancer undergoing elective surgery
Settings: university hospitals in Ireland and the USA
Intervention: continuous intravenous local anaesthetic infusion Comparison: conventional pain control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Continous intravenous local anaesthetic infusion | |||||
|
Persistent pain 3 to 6 months after breast cancer surgery (We defined persistent postsurgical pain as new pain that did not exist before the operation, measured using differences in scores based on validated pain scales; patient interview between 3 to 6 months after surgery.) |
Study population | OR 0.24 (0.08 to 0.69) | 97 (2 studies)1 | ⊕⊕⊕⊝ moderate1 | Event rates of persistent pain after breast cancer surgery ranged in this population between 20% to 40%. One in three women benefited on average from continuous intravenous infusion of local anaesthetics after breast cancer surgery. |
|
| 370 per 1000 | 123 per 1000 (45 to 288) | |||||
| Low | ||||||
| 200 per 1000 | 57 per 1000 (20 to 147) | |||||
| High | ||||||
| 600 per 1000 | 265 per 1000 (107 to 509) | |||||
| Adverse effects of continuous local anaesthetic infusion ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Adverse effects of intravenous infusion of local anaesthetics after breast cancer surgery were not systematically reported and due to their low frequency are better investigated in registries. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1We downgraded quality of evidence by one level because conclusions may be considerably weakened by the small number of studies included. These two studies are however consistent and of high methodological quality. Still, meta‐analysis results based on small numbers tend to overestimate the effects.
Results
Description of studies
Results of the search
The searches for this updated review were undertaken in September 2014 to January 2015, again in April 2015, and for a final time in December 2016. We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 12), Ovid MEDLINE (1946 to April 2016), and Ovid Embase (1980 to April 2016).
For the original review, the searches were undertaken in February and March 2008 and rerun between February and August 2010 and again between April and May 2012 (Andreae 2012).
The search and selection process is illustrated in a flow diagram (Figure 1).
Electronic search
The electronic search yielded a total of 4717 references matching the predefined search parameters: 773 in CENTRAL, 1765 in MEDLINE, 2179 in Embase; among them were 1371 duplicates. The review authors (EJW, JLL, MSC, JC, MHA and DAA) screened these and excluded 2787 references as irrelevant or not RCTs. We added 11 study reports from an updated search in December 2017 to Studies awaiting classification.
Handsearch
We did not repeat the handsearch for this update. For the first version of this review (Andreae 2012), in our handsearch of the conference proceedings, we looked at 2101 references. We found 372 references in the reference lists of included studies or review articles, or by following links in PubMed and Google to other relevant studies. This resulted in a total of 2473 references; 175 were duplicates and 2293 were excluded as irrelevant or not RCTs.
Unpublished data
We identified one unpublished study, which was included in the meta‐analysis (Katz 1996).
Selection process
Three review authors (EJW, JLL, MHA) obtained full‐text copies of 564 articles for further assessment (see: Figure 1). Six review authors (EJW, JLL, MSC, JC, MHA and DAA) selected 63 studies for inclusion in this review (see: Characteristics of included studies). We found seven ongoing studies for assessment upon completion (ISRCTN46621916; Liew 2011; Michael 2014; NCT00418457; NCT01626755; NCT02002663; Theodoraki 2016) (see Characteristics of ongoing studies).
Data extraction
Seven study reports were only available as a conference abstracts. For three of these, we could not identify any follow‐up report and obtained no additional data (Katsuly‐Liapis1996; Okur 2016; Smaldone 2010). We were able to resolve all disagreements with regard to data extraction, study inclusion and quality assessment by informal discussion. Data extraction and quality assessment for the remaining four studies was resolved with help from the respective study authors (Besic 2014; Choi 2016; Micha 2012; Tecirli 2014).
Incomplete and raw data
In spite of contacting study authors, we were unable to obtain appropriate or adequate data for five studies (Burney 2004; Chiu 2008; Di‐Gennaro 2013; McKeen 2014; Pinzur 1996).
Included studies
We identified 63 RCTs studying regional anaesthesia or local anaesthetics for the prevention of PPP in this updated review (see: Characteristics of included studies), 40 of these were newly included in this update. For ease of orientation, Appendix 7 summarizes the surgical operations, type of anaesthesia, timing of intervention, adjuvant therapy and outcomes of the pooled studies. Four included studies reported their results in several published manuscripts (Kairaluoma 2006; Katz 1996; Katz 2004; Singh 2007). When two manuscripts were published by the same authors and reported the same participant numbers, we judged them to be reporting on just one and the same study; we used this data set only once (Kairaluoma 2006; Katz 1996; Katz 2004; Singh 2007). We reviewed studies reported in English and several other languages, including Danish (Bach 1988), French (Baudry 2008; Mounir 2010), German (Weihrauch 2005), Japanese (Hirakawa 1996), Mandarin (Lu 2008), and Spanish (Ibarra 2011).
Descriptive characteristics of participants
We pooled the data of 3027 study participants in our inclusive analysis (Appendix 8), with 499 participants after thoracotomy, 1297 participants after breast cancer surgery, 661 participants after caesarean section, 123 participants after ICBG, 150 participants after prostatectomy, 297 participants after hysterectomy, with outcomes ranging from 3 to 48 months after surgery.
We pooled the data organized by surgery type with outcomes at 3, 6, 12, 20, or 48 months. A breakdown of the number of participants by surgery and time point is provided in Appendix 8. One study on participants undergoing pectus excavatum repair took place in children and adolescents older than 10 years, but was the only study of its surgery type and we did not, therefore, include it in the meta‐analysis (Weber 2007). Only adults (> 18 years) could be included in the meta‐analysis; the youngest population had a mean age in the experimental group of 25 years plus or minus a standard deviation of five years (Blumenthal 2005).
Patient characteristics
Reflecting the diversity of surgical interventions, the participants' age, sex and comorbidities varied widely and were sparsely reported. Breast surgery and caesarean section studies included only female participants. Studies on limb amputation included predominantly male participants.
Types of surgery
We listed the surgical interventions investigated in the pooled studies (thoracotomy, breast cancer surgery, hysterectomy, ICBG, caesarean section, prostatectomy) in Appendix 7. We grouped studies in broad categories (thoracotomy, cardiac surgery, breast surgery, caesarean section, laparotomy, and prostatectomy) with similar characteristics. We reported breast surgery (Albi‐Feldzer 2013; Baudry 2008; Besic 2014; Fassoulaki 2000; Fassoulaki 2001; Fassoulaki 2005; Grigoras 2012; Ibarra 2011; Kairaluoma 2006; Karmakar 2014; Lee 2013; Micha 2012; Strazisar 2012; Strazisar 2014; Tecirli 2014; Terkawi 2015b) including cosmetic breast surgery (Bell 2001), in the same subgroup, but performed a sensitivity analysis excluding plastic surgery.
Characteristics of regional anaesthesia interventions
Regional anaesthesia modalities and timing of perioperative blockade
We summarized the use of regional techniques in (Appendix 7).
Epidural anaesthesia was used in majority of the thoracotomy studies (Can 2013; Comez 2015; Ju 2008; Lu 2008; Senturk 2002). Exceptions included one study using intercostal nerve block (Katz 1996), and one employing wound irrigation (Liu 2015). Wound irrigation and instillation were used in three of the studies on ICBG (Blumenthal 2005; Gundes 2000; Singh 2007), while local infiltration techniques were used in the others (Barkhuysen 2010; O'Neill 2014). For laparotomy surgery, both studies employed epidural anaesthesia (Katz 2004; Lavand'homme 2005), whereas in hysterectomy both studies employed spinal anaesthesia (Sprung 2006; Wodlin 2011). Within the other surgical subgroups, studies investigated different regional anaesthetic techniques: for breast surgery, mostly paravertebral block (Gacio 2016; Ibarra 2011; Kairaluoma 2006; Karmakar 2014; Lam 2015; Lee 2013), with and without some local infiltration (Albi‐Feldzer 2013), some used intravenous local anaesthesia (Grigoras 2012; Terkawi 2015b), others used only local infiltration (Baudry 2008; Besic 2014; Strazisar 2012; Strazisar 2014); for caesarean section, mostly transverse abdominal plain block (Bollag 2012; Loane 2012; McKeen 2014; Singh 2013), and peritoneal instillation (Shahin 2010); for hernia repair, mainly local/wound infiltration.
The experimental arms in two studies on breast cancer surgery used intravenous lidocaine (Grigoras 2012; Terkawi 2015b). Dermal patches, Bier block, ultra long‐acting or slow‐release local anaesthetic compounds were not studied.
In thoracotomy, all studies used continuous regional anaesthesia in the perioperative period. In the breast cancer surgery subgroup, only those with topical (Fassoulaki 2000; Fassoulaki 2005), or intravenous administration (Grigoras 2012; Terkawi 2015b), of local anaesthesia used continuous perioperative regional anaesthesia. Caesarean section studies employed mostly single‐shot interventions with the exception of two studies that used continuous wound irrigation perioperatively (Lavand'homme 2007; O'Neill 2012). In ICBG, three of the studies used continuous postoperative wound irrigation (Blumenthal 2005; O'Neill 2014; Singh 2007). In the remaining surgical subgroups, there were only a handful of studies utilizing continuous application of regional anaesthetics (Brown 2004; Chiu 2008; Gupta 2006; Lavand'homme 2005; Pinzur 1996; Vrooman 2015).
Two studies tested the hypothesis that blocking ischaemic limb pain prior to amputation prevents the central sensitization that might otherwise lead to persistent pain afterwards (Karanikolas 2006; Katsuly‐Liapis1996). The latter comparison was not planned in our protocol and hence these data were not presented.
Primary outcomes
As a prerequisite for inclusion, studies had to employ an instrument to subjectively measure patient discomfort (Appendix 7). The study authors primarily used a dichotomous outcome, that is presence or absence of (phantom) pain. They also used several continuous pain scales (verbal rating scale (VRS), visual analogue scale (VAS), numeric rating scale (NRS), bodily pain sub‐component of the Short Form Health Survey (SF‐36)). Nine studies did not record pain as a dichotomous outcome but only used continuous pain scales (Blumenthal 2005; Chiu 2008; Gupta 2006; McKeen 2014; O'Neill 2014; Singh 2013; Sprung 2006; Vrooman 2015; Wodlin 2011). One did record pain as a dichotomous outcome but did not report it in the manuscript, and provided the review authors with the data via email (Kurmann 2015). Nine studies (Brown 2004; Burney 2004; Gupta 2006; Karanikolas 2006; Karmakar 2014; Katz 2004; ; McKeen 2014; Sprung 2006; Wodlin 2011), reported continuous complex outcome instruments, like the McGill questionnaire (Dworkin 2009b), or the Short Form Health Survey (SF‐36) (Ware 1992), which are recommended in consensus statements for the assessment of chronic pain (Gewandter 2015; Turk 2006).
Duration of follow‐up
A minimum of three months' follow‐up was required for inclusion. Most studies focused on, and most patient data were collected at three or six months' follow‐up (Appendix 7).
Secondary outcomes
Allodynia and hyperalgesia and other outcome measures
Nine studies investigated allodynia and hyperalgesia (Bell 2001; Blumenthal 2005; Bollag 2012; Grigoras 2012; Gundes 2000; Ju 2008; Kurmann 2015; Lavand'homme 2005; Lavand'homme 2007). The heterogeneity of surgical interventions precluded any evidence synthesis. Fifteen studies used other (additional) outcome measures, like McGill questionnaire (Dworkin 2009b), Short Form Health Survey (SF‐36) (Ware 1992), Mental Health Inventory 18 (Beusterien 1996), Pain Disability Index (Tait 1990), or "interference with life" (Bollag 2012; Brown 2004; Burney 2004; Gupta 2006; Karanikolas 2006; Katz 2004; Lavand'homme 2005; McKeen 2014; Pinzur 1996; Sprung 2006; Wodlin 2011).
Reporting of adverse effects
Most reporting on long‐term adverse effects was sparse, sporadic and anecdotal, rather than prospective and systematic. Two RCTs investigated the risk of women in labour developing backache after epidural anaesthesia during labour as primary outcome (Howell 2001; Loughnan 2002), but did not meet the inclusion criteria of the main analysis.
Risk factors and pre‐existing pain
The included studies did not elicit or compare the known risk factors for the development of PPP between the experimental and control groups. We are therefore unable to comment on to what degree a difference between the groups may have introduced bias (Fassoulaki 2008). As people who present for thoracotomy and breast cancer are usually pain free, pre‐existing pain is unlikely to be a confounder for these pooled subgroups (Gottschalk 2006). This may be very different for people undergoing limb amputation; they may have suffered from prolonged and excruciating ischaemic pain prior to surgery.
Excluded studies
We excluded 79 studies, a summary of which can be found in the Characteristics of excluded studies table. No study was excluded exclusively for lack of observer blinding. We excluded three studies for pseudo‐randomization (Bach 1988; da Costa 2011; Nikolajsen 1997). One study (da Costa 2011), also failed other inclusion criteria.
Studies awaiting classification
As reported on 22 January 2009, SS Reuben was accused of publishing fraudulent data. Up to 22 papers have been, or will be, retracted by the journals in which they have been published, as detailed in the retraction notice in Anesthesia and Analgesia, 20 February 2009 (Shafer 2009). It appears that Reuben 2006 is not among the list of retracted manuscripts, however we have placed it in the classification pending section on the advice of Cochrane Anaesthesia, Critical and Emergency Care.
Further, 11 studies from an updated search in December 2017 are currently awaiting classification (see Characteristics of studies awaiting classification).
Ongoing studies
There are seven ongoing studies (ISRCTN46621916; Liew 2011; Michael 2014; NCT00418457; NCT01626755; NCT02002663; Theodoraki 2016). These seven studies will be assessed when they have been completed. A summary of the studies can be found in the Characteristics of ongoing studies table.
Risk of bias in included studies
The risk of bias is detailed in the risk of bias tables (Characteristics of included studies), the risk of bias graph (Figure 2), and is summarized in the methodological quality summary (Figure 3).
2.
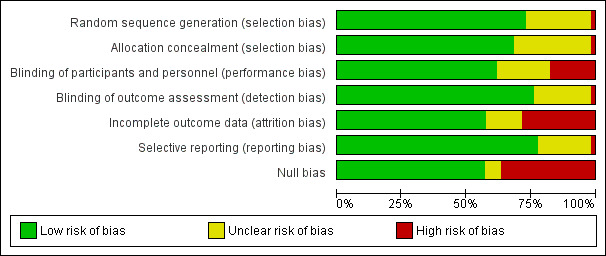
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
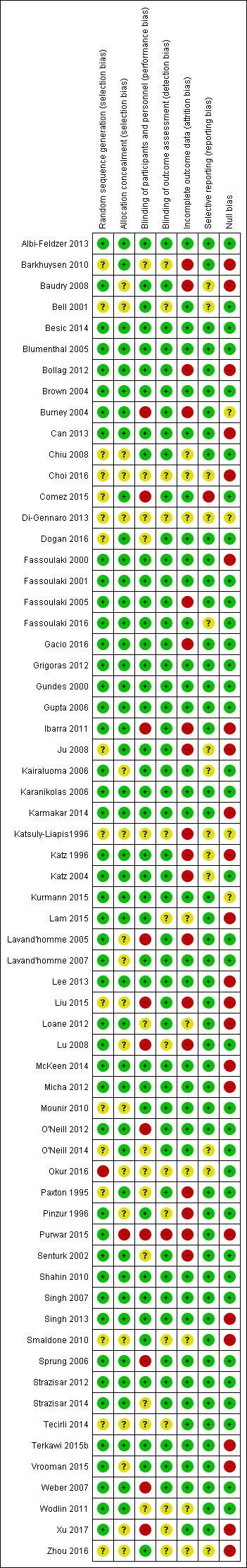
Methodological quality summary: review authors' judgements about each methodological quality item for each included study
Allocation
Sequence generation
Twelve studies did not detail the process of sequence generation (Bell 2001; Chiu 2008; Choi 2016; Comez 2015; Dogan 2016; Gacio 2016; Ju 2008; Katsuly‐Liapis1996; Liu 2015; Mounir 2010; Paxton 1995; Zhou 2016). Study authors' responses provided additional unpublished information for some studies (Can 2013; Fassoulaki 2000; Fassoulaki 2001; Gacio 2016; Gundes 2000; Ibarra 2011; Lavand'homme 2007; Purwar 2015; Senturk 2002). We excluded three studies for pseudo‐randomization (Bach 1988; da Costa 2011; Nikolajsen 1997) (Appendix 9). A general finding was that the most recently published articles overall provided much more detail on this process in their study manuscripts.
Concealment of allocation
The majority of studies utilized adequate concealment of allocation, using sealed, opaque envelopes opened just prior to the regional anaesthesia intervention. Allocation concealment was not detailed in 16 studies (Baudry 2008; Bell 2001; Chiu 2008; Choi 2016; Kairaluoma 2006; Katsuly‐Liapis1996; Lavand'homme 2005; Lavand'homme 2007; Liu 2015; Lu 2008; Mounir 2010; Okur 2016; Pinzur 1996; Vrooman 2015; Xu 2017; Zhou 2016).
Blinding
We did not exclude any studies for detection bias, and only outcome assessment blinding was a prerequisite for inclusion. Some study authors reported difficulties in keeping the participants and providers blinded due to the need to adjust dosing or preoperative pain control prior to limb amputation (Nikolajsen 1997), or the obvious immediate clinical effects of regional anaesthesia, that is numbness of the affected body part (Lavand'homme 2005; Senturk 2002). Most participants will note the obvious effects of regional anaesthesia, like motor weakness and sensory loss, and guess their allocation. This made effective blinding of participants and practitioners almost impossible. In other cases, different methods of anaesthesia between the groups led to awareness of group allocation by participants and physicians conducting the study, such as one group with spinal anaesthesia versus another with spinal‐epidural anaesthesia (O'Neill 2012), or thoracic epidural anaesthesia in the intervention arm versus patient‐controlled analgesia (PCA) in the control arm (Weber 2007).
Many study authors detailed (in the publication or via further communications) efforts to blind study participants, physicians and caregivers well as outcome assessors (Albi‐Feldzer 2013; Baudry 2008; Blumenthal 2005; Bollag 2012; Brown 2004; Can 2013; Chiu 2008; Fassoulaki 2000; Fassoulaki 2001; Fassoulaki 2005; Fassoulaki 2016; Gacio 2016; Grigoras 2012; Gundes 2000; Gupta 2006; Ju 2008; Kairaluoma 2006; Karanikolas 2006; Karmakar 2014; Katz 1996; Katz 2004; Kurmann 2015; Lavand'homme 2007; McKeen 2014; Mounir 2010; Shahin 2010; Singh 2007; Terkawi 2015b; Vrooman 2015). Some reported double blinding but did not provide details (Bell 2001; Comez 2015; Paxton 1995; Pinzur 1996). Six studies described outcome assessor blinding, without detail on personnel or participant blinding (Burney 2004; Dogan 2016; Ibarra 2011; Lam 2015; Lavand'homme 2005; O'Neill 2012), but nine other studies neither described nor confirmed it (Bell 2001; Choi 2016; Katsuly‐Liapis1996; Liu 2015; Lu 2008; Okur 2016; Wodlin 2011; Xu 2017; Zhou 2016).
Obviously, performance bias may weaken the conclusions of our review. The placebo effect may be particularly strong for pain outcomes and remains unknown for long‐term outcomes. Our conclusions are considerably weakened by shortcomings in allocation concealment, considerable attrition and incomplete outcome data. Six studies employed adjuvants (Bollag 2012; Brown 2004; Fassoulaki 2005; Gacio 2016; Lavand'homme 2005; Sprung 2006), only in the experimental group, potentially introducing bias, but this did not affect the results for the breast cancer surgery subgroup and was not pertinent for the thoracotomy subgroup.
Incomplete outcome data
There was a trend toward more adequate addressing of incomplete outcome data in more recent studies (Albi‐Feldzer 2013; Bell 2001; Blumenthal 2005; Brown 2004; Can 2013; Comez 2015;Dogan 2016; Fassoulaki 2000; Fassoulaki 2001; Fassoulaki 2016; Gacio 2016; Grigoras 2012; Gundes 2000; Gupta 2006; Kairaluoma 2006; Karanikolas 2006; Karmakar 2014; Kurmann 2015; Lavand'homme 2007; McKeen 2014; Mounir 2010; O'Neill 2012; Okur 2016; Purwar 2015; Shahin 2010; Singh 2007; Sprung 2006; Terkawi 2015b; Vrooman 2015; Weber 2007; Xu 2017). compared to those that are older (Katsuly‐Liapis1996; Katz 1996; Katz 2004; Lavand'homme 2005; Paxton 1995; Senturk 2002). Study authors reported high attrition rates, due to loss to follow‐up as well as the high mortality of the participant groups studied. This potentially introduces bias. One study excluded randomized participants that the surgeon deemed inoperable but did not consider an intention‐to‐treat analysis (Senturk 2002). Only seven studies performed a formal intention‐to‐treat analysis (Albi‐Feldzer 2013; Kairaluoma 2006; Karmakar 2014; Kurmann 2015; Singh 2007; Sprung 2006; Terkawi 2015b). In four studies, there was no attrition at all (Comez 2015; Grigoras 2012; ; ; Weber 2007; Xu 2017).
In our graphical exploration of the influence of attrition and follow‐up interval on effect size shown in an attrition effect size graph (Figure 4), we did not find any association. In other words, we found no evidence to reject the null hypothesis that attrition and follow‐up intervals have no influence on effect size estimation.
4.
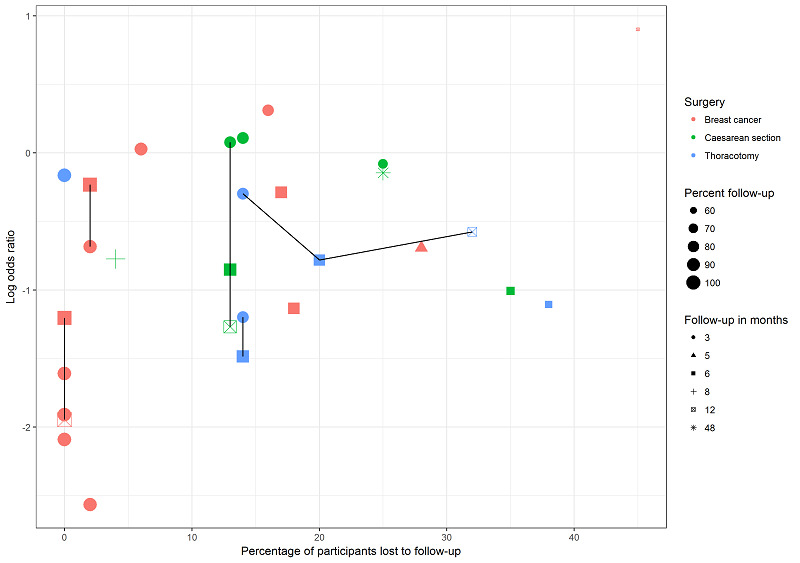
This graph plots attrition versus effect size (log odds ratio) for studies investigating regional anaesthesia for the prevention of persistent pain after thoracotomy (blue), breast surgery (pink) and caesarean section (green). Symbol size decreases with attrition. Repeated follow‐ups within one study are linked with a black line. We are unable to discern any association between attrition, follow‐up time and effect measure; this lends support to our decision to pool studies reporting outcomes at different follow‐up intervals and with different attrition.
Selective reporting
We contacted the authors of 37 included studies during this update, and 23 in the original systematic review for clarification of study methodology or to obtain further unpublished data. We found no contact information for the authors of three studies (Choi 2016; Katsuly‐Liapis1996; Zhou 2016).
Selective reporting was a concern regarding adverse effects. Several studies reported adverse effects as 'none', but did not detail, if patients were asked about any side effects and if so which. This may reflect reporting bias (Albi‐Feldzer 2013; Karmakar 2014; Pinzur 1996). Where reported, information on adverse effects in the included studies was mostly anecdotal and not reported separately by group (Can 2013; Kairaluoma 2006; Katz 2004; Lavand'homme 2007; Paxton 1995; Singh 2007; Weber 2007). The studies made very general statements about the side effects, such as, "no clinical signs or symptoms of local anaesthetic toxicity were noted in any patient" (Gundes 2000), and "Only one patient (in the placebo group) developed lymphedema, while no post‐surgery infection or other complications were reported" (Terkawi 2015b).
Undue sponsor influence (conflict of interest)
The source of funding and conflict of interest statements for many studies were either addressed in the manuscript or clarified in correspondence with study authors, with the exception of eleven studies for which no information was available (Baudry 2008; Brown 2004; Chiu 2008; Choi 2016; Gupta 2006; Ibarra 2011; Kairaluoma 2006; Katsuly‐Liapis1996; Lam 2015; Lu 2008; Paxton 1995). The studies were mostly supported by funds from the department or the institution. For those studies that described support by outside funding, we did not find any undue influence by the sponsors.
Null bias
The occurrence of ‘null bias’ is due to interventions being insufficiently well delivered (Higgins 2011a; Woods 1995). A number of included studies report insufficient pain control in the immediate postoperative period, as evidenced by inconsequential differences in pain scores between groups perioperatively, or similar requirements of rescue analgesic medications between groups in the immediate postoperative period (Barkhuysen 2010; Baudry 2008; Bollag 2012; Can 2013; Choi 2016; Fassoulaki 2000; Ibarra 2011; Ju 2008; Karmakar 2014; Katz 1996; Lam 2015; Lee 2013; Liu 2015; Loane 2012; McKeen 2014; Micha 2012; Purwar 2015; Singh 2013; Smaldone 2010; Terkawi 2015b; Vrooman 2015; Xu 2017; Zhou 2016). These studies are at high risk of null bias as the intervention was possibly not applied correctly or at high enough dosages for a true treatment effect in the immediate postoperative period. This likely blunted the treatment effect at three or more months postoperatively, because poor pain control in the postoperative period is probably an important driver of persistent pain after surgery (Lewis 2015; Gottschalk 2006).
Other potential sources of bias
Reporting bias
The small numbers of studies found in each subgroup precluded a formal study of publication bias by graphical analysis or the test proposed by Egger 1997 in most subgroups. At least 10 studies should be included in the meta‐analysis to make a funnel plot or an Egger test useful because with fewer studies the power of the tests is insufficient to distinguish chance from real asymmetry (Sterne 2011). We present a funnel plot for the breast surgery subgroup (Figure 5), which is inconclusive, especially considering that it is based on only 11 studies and includes several repeated observations for some among them. We acknowledge some degree of publication bias. Some studies, which failed to demonstrate substantial benefit beyond three months, could not be included because published aggregate data were insufficient for inclusion. In some studies we could not get the individual participant data (Blumenthal 2005; Burney 2004; Chiu 2008; McKeen 2014; Pinzur 1996), even though this did not affect any inferences we made.
5.
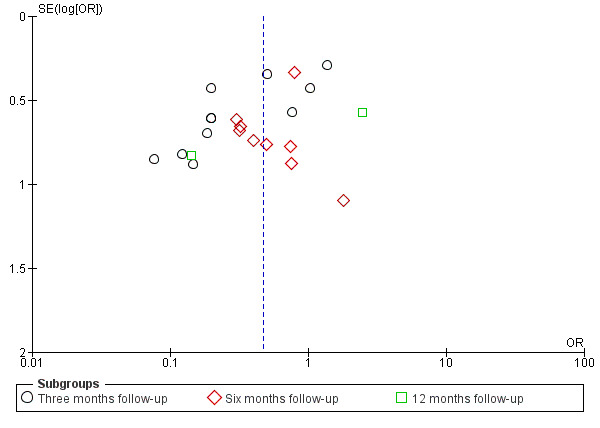
The funnel plot for breast surgery including all outcomes at any follow‐up interval for all breast surgery studies is inconclusive for publication bias.
In spite of considerable efforts outcome data were not available for some studies, as detailed also in the table Characteristics of included studies, this potentially introduced bias in our review and may reflect underlying publication bias.
Assessment of pre‐existing pain and risk factors for persistent postsurgical pain
There are risk factors for the development of PPP (Kehlet 2006). The severe ischaemic pain prior to limb amputation may be a predictor for PPP after amputation (Karanikolas 2006). Most studies did not assess risk factors or baseline pain. An exception to this were studies reporting continuous outcomes via the Short Form Health Survey (SF‐36), in which some studies report baseline values for comparison (Brown 2004; Gupta 2006; Karmakar 2014; Sprung 2006; Wodlin 2011).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Regional anaesthesia for the prevention of persistent postoperative pain three or more months after surgery
We report an inclusive evidence synthesis (Data synthesis/inclusive model), whereby we synthesize outcomes observed at different follow‐up intervals (Analysis 1.1; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11; Figure 6). We used only the latest available follow‐up time point for each study included in the analysis (Data synthesis/inclusive model). We compared our results with the classical (frequentist) evidence synthesis stratified by follow‐up interval as in the previous versions of this review (Andreae 2012) (Analysis 2.1; Analysis 2.3; Analysis 2.4;Analysis 2.5Analysis 2.6; Analysis 2.7; Analysis 2.8). A census of included participants grouped according to surgery is in Appendix 8. We presented the data in 'Summary of findings' tables (Table 1; Table 2; Table 3; Table 4; Table 5), for persistent pain after thoracotomy, breast cancer surgery, caesarean section subgroups, intravenous local anaesthetic infusion and for local infiltration to reduce the risk of persistent pain at the donor site after iliac crest bone graft harvesting.
6.
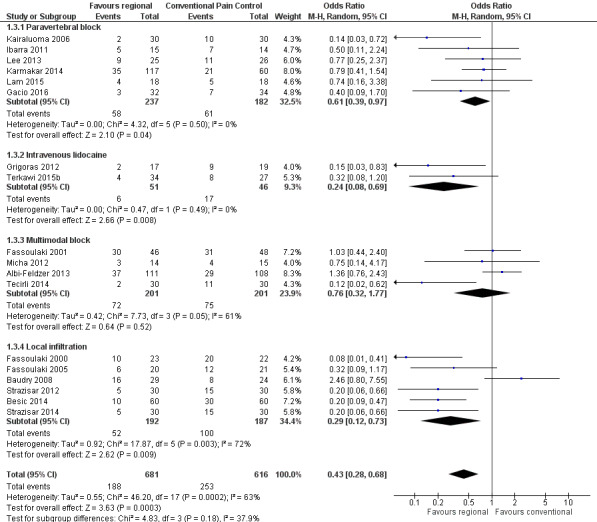
Forest plot of comparison 1. Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), outcome 1.3, PPP three to 12 months after breast cancer surgery
2.1. Analysis.
Comparison 2 Local or regional anaesthesia for the prevention of persistent postoperative pain (classical analysis), Outcome 1 PPP after thoracotomy.
2.3. Analysis.
Comparison 2 Local or regional anaesthesia for the prevention of persistent postoperative pain (classical analysis), Outcome 3 PPP after breast cancer surgery.
2.4. Analysis.
Comparison 2 Local or regional anaesthesia for the prevention of persistent postoperative pain (classical analysis), Outcome 4 PPP after caesarean section.
2.5. Analysis.
Comparison 2 Local or regional anaesthesia for the prevention of persistent postoperative pain (classical analysis), Outcome 5 PPP after amputation.
2.6. Analysis.
Comparison 2 Local or regional anaesthesia for the prevention of persistent postoperative pain (classical analysis), Outcome 6 PPP after laparotomy.
2.7. Analysis.
Comparison 2 Local or regional anaesthesia for the prevention of persistent postoperative pain (classical analysis), Outcome 7 PPP after hernia repair.
2.8. Analysis.
Comparison 2 Local or regional anaesthesia for the prevention of persistent postoperative pain (classical analysis), Outcome 8 PPP after hysterectomy.
1. Thoracotomy
In an inclusive analysis summarized in (Table 1), including the latest possible time point for each study for an overall estimate of effect, we found an overall benefit to regional anaesthesia for preventing persistent post‐thoracotomy pain (Analysis 1.1). This analysis included a total 499 participants from seven studies (Can 2013; Comez 2015; Ju 2008; Katz 1996; Liu 2015; Lu 2008; Senturk 2002) and found an overall effect clearly favouring regional anaesthesia, with an OR of 0.52, (95% CI 0.32 to 0.84, P = 0.008). The I² statistic, (measuring between‐study heterogeneity), was 14%, indicating little statistical heterogeneity between the studies pooled. Limiting the analysis only to those five studies (Can 2013; Comez 2015; Ju 2008; Lu 2008; Senturk 2002) that had employed epidural anaesthesia favoured regional anaesthesia even more (OR 0.41, 95% CI 0.25 to 0.67), without changing the inferences.
Including 499 participants in seven studies, the NNTB for the subgroup thoracotomy is 7 with a 95% CI 4 to 23, for an assumed corresponding risk of 0.5. High risk of bias from missing data across a number of included studies reduced our confidence in the findings. However, the risk of detection bias was low in the included studies on PPP after thoracotomy. Cryotherapy can arguably cause neuropathy (Ju 2008; Mustola 2011), and is clinically different from conventional pain therapy. Liu 2015, used continuous wound infiltration instead of the epidural analgesia employed in all the other included studies. To perform a sensitivity analysis, we excluded Ju 2008 or Liu 2015, or both; while this reduced I2, the statistical heterogeneity observed, the exclusions did not alter the inferences. In other words, the resulting change in confidence intervals are not clinically relevant.
Stratified analysis
We compared this with a classical (frequentist) analysis and pooled five studies on regional anaesthesia for the prevention of PPP after thoracotomy in 428 participants with dichotomous outcomes at three months after thoracotomy (Analysis 2.1). This resulted in an OR 0.70 (95% CI 0.40 to 1.20) favouring regional anaesthesia, but the results are imprecise leaving doubts as to their clinical relevance (Can 2013; Comez 2015; Ju 2008; Liu 2015; Lu 2008). Excluding Liu 2015, the only study employing wound infiltration instead of epidural analgesia, resulted in similar inferences (OR 0.60, 95% CI 0.35 to 1.02, P = 0.06). We pooled these same four studies (Can 2013; Comez 2015; Ju 2008; Lu 2008) plus one more (Senturk 2002), with dichotomous pain outcomes at six months after thoracotomy including data from 370 participants (Analysis 2.1). This resulted in OR 0.39 (95% CI 0.24 to 0.63), strongly favouring regional anaesthesia (P = 0.0001). Only one study, Ju 2008, an insufficient number for meta‐analysis, reported outcomes at 12 months in 77 participants, but results were inconclusive with an OR of 0.56 (95% CI 0.23 to 1.39). Similarly, only one small study (Katz 1996) reported outcomes at 20 months in 23 participants, showing no benefit for the intervention with an OR 1.17 (95% CI 0.22 to 6.08).
2. Cardiac surgery
We did not conduct any meta‐analysis of the three studies in cardiac surgery (Chiu 2008; Dogan 2016; Vrooman 2015), due to very high statistical heterogeneity (I2 = 83%), possibly due to different regional anaesthesia modalities employed. Chiu 2008 employed a continuous wound infusion, parasternal blocks were utilized in Dogan 2016, while Vrooman 2015 used lidocaine patches.
3. Breast cancer surgery
In our inclusive analysis of overall effect (Analysis 1.3; Table 2; Figure 6), we included 18 studies (Albi‐Feldzer 2013; Baudry 2008; Besic 2014; Fassoulaki 2000; Fassoulaki 2001; Fassoulaki 2005; Gacio 2016; Grigoras 2012; Ibarra 2011; Kairaluoma 2006; Karmakar 2014; Lam 2015; Lee 2013; Micha 2012; Strazisar 2012; Strazisar 2014; Tecirli 2014; Terkawi 2015b) and 1297 participants, which resulted in an overall treatment effect (OR 0.43, 95% CI 0.28 to 0.68) suggesting a clear benefit of regional anaesthesia (P = 0.0003). The inferences were not affected whether or not we included the study on plastic surgery of the breast (Bell 2001), or the study investigating intravenous infusions of local anaesthetics (Terkawi 2015b). (As an aside, Bell 2001 randomized participants to receive local anaesthetic infiltration of one breast, while the other side was infiltrated with placebo. Absorbed systemic lidocaine might have attenuated the development of PPP on the untreated side, leading to a diminished signal). We observed substantial heterogeneity among included studies (I2 = 63%). Limiting the studies to those six studies (participants = 419) that investigated paravertebral block as the intervention (Gacio 2016; Ibarra 2011; Kairaluoma 2006; Karmakar 2014; Lam 2015; Lee 2013), still favoured regional anaesthesia (OR 0.61, 95% CI 0.39 to 0.97; NNTB 11), and reduced the statistical heterogeneity to zero (I2 = 0%). Including 1297 participants in 18 studies, the NNTB for the subgroup breast cancer surgery is 7 with a 95% CI 6 to 13, for an assumed corresponding risk of 0.3.
This review was not planned as a comparison of different regional anaesthesia modalities and it is problematic to make inference by a crude subgroup stratification as in (Analysis 1.3). We will plan an a priori‐designed network analysis and meta‐regression for our next review update (Andreae 2015c; Andreae 2018; Thompson 2002).
Stratifed analysis
We compared this inclusive analysis with the stratified classical (frequentist) analyses by follow‐up interval; we pooled 11 studies on regional anaesthesia for breast surgery with dichotomous pain outcomes at three months postoperatively (Albi‐Feldzer 2013; Besic 2014; Fassoulaki 2000; Fassoulaki 2001; Fassoulaki 2005; Grigoras 2012; Karmakar 2014; Lee 2013; Strazisar 2012; Strazisar 2014; Tecirli 2014), including a total of 966 participants (Analysis 2.3). Their evidence synthesis (OR 0.34, 95% CI 0.19 to 0.61) favoured regional anaesthesia (P = 0.0003). However, an I² statistic of 72% suggested considerable statistical heterogeneity.
Similarly, we pooled nine studies on regional anaesthesia for breast surgery with dichotomous pain outcomes at six months postoperatively (Bell 2001; Fassoulaki 2005; Gacio 2016; Ibarra 2011; Kairaluoma 2006; Karmakar 2014; Lam 2015; Micha 2012; Terkawi 2015b), including a total of 515 participants (Analysis 2.3). The result strongly favoured regional anaesthesia (OR 0.56, 95% CI 0.37 to 0.84; P = 0.005; I2 = 0%). For a more conservative estimate, we had included the only one of the seven studies that investigated plastic surgery of the breast (Bell 2001), which has a different pathologic mechanism of persistent pain after breast cancer surgery, and the study investigating intravenous infusion of local anaesthetics (Terkawi 2015b); however, the inferences were the same with or without inclusion of these studies (OR 0.56, 95% CI 0.37 to 0.87). The results at six months are much less heterogeneous (I² statistic = 0%).
Finally, we present the pooled results of two studies on regional anaesthesia with dichotomous pain outcomes at 12 months after breast cancer surgery (Baudry 2008; Kairaluoma 2006), including 113 participants in total (Analysis 2.3), but caution that these studies are highly heterogeneous, both statistically (I² statistic = 88%), and clinically, as one utilized local infiltration (Baudry 2008), and the other paravertebral block (Kairaluoma 2006). In Baudry 2008, the experimental treatment failed to reduce the severity of immediate postoperative pain and the results at 12 months did not favour regional anaesthesia, with an OR of 2.46, and wide confidence interval which crosses the midline (95% CI 0.80 to 7.55). Kairaluoma 2006, with improved immediate pain control in the experimental group, however, did strongly favour the experimental intervention, with an OR of 0.14 (95% CI 0.03 to 0.72).
4. Caesarean section
In an inclusive analysis (Analysis 1.4), evaluating the overall effect across all time points, we included four studies (Bollag 2012; Lavand'homme 2007; Loane 2012; Shahin 2010), totaling 551 participants, but excluding O'Neill 2012, which had zero events in both arms (Deeks 2011). The results strongly favoured the use of regional anaesthesia for the prevention of PPP after caesarean section, with an OR of 0.46 (95% CI 0.28 to 0.78, P = 0.004) and little heterogeneity at both the study and subgroup level (I2 = 0% for both); the NNTB for caesarean section is 19 with a 95% CI (14 to 49) for an assumed corresponding risk of 0.1 (Table 3).
We performed an inclusive analysis (Analysis 1.5) evaluating two studies reporting continuous outcomes on 110 participants but it was inconclusive (pooled SMD 0.14 (95% CI ‐0.34 to 0.61) (McKeen 2014; Singh 2013). Neither study demonstrated a clear improvement in immediate postoperative pain control or a reduction of the risk of persistent postoperative pain.
Stratified analysis
We again compared the results of our inclusive analysis with a conservative stratified analysis, where we pooled two studies after caesarean section (Pfannenstiel incision), including 137 participants with dichotomous pain outcomes at three months postoperatively (Bollag 2012; Loane 2012) but excluding O'Neill 2012, which had zero events in both arms (Deeks 2011) (Analysis 2.4). Evidence synthesis resulted in an OR of 1.09 (95% CI 0.39 to 3.07), suggesting no benefit of regional anaesthesia. Both of these studies (Bollag 2012; Loane 2012), used transversus abdominis plane blocks, with single‐shot interventions suggesting relative clinical homogeneity, which is complemented by the lack of statistical heterogeneity (I² statistic = 0%) in this analysis. We did not pool one study in this analysis (O'Neill 2012), as there were no events in either arm (making the OR undeterminable). The Cochrane Handbook for Systematic Reviews of Interventions suggests the standard practice in this instance is to exclude these studies from a meta‐analysis (Deeks 2011).
We pooled three studies after caesarean section (Pfannenstiel incision), including 492 participants (Bollag 2012; Lavand'homme 2007; Shahin 2010), with dichotomous pain outcomes at six months postoperatively (Analysis 2.4). Their analysis resulted in an OR of 0.44 (95% CI 0.26 to 0.74), clearly favouring regional anaesthesia at this follow‐up interval. Bollag 2012 administered transversus abdominus plane block, Lavand'homme 2007 used continuous postoperative wound irrigation, and Shahin 2010, peritoneal instillation, both as single‐shot interventions. The interventions were clinically heterogeneous, and one must be cautious when interpreting this evidence synthesis. However, all three studies individually favoured regional anaesthesia.
We decided not to include two studies in our analysis above (Bamigboye 2013; Kindberg 2009), because they studied chronic pelvic pain (Bamigboye 2013) and dyspareunia (Kindberg 2009) as their outcomes after postpartum surgical repair. These conditions are materially different from persistent postoperative pain, our primary outcome. The pre‐existing pain in Bamigboye 2013 and the nonelective traumatic nature of the surgical intervention in Kindberg 2009 led the authors ultimately to exclude the studies from the review. However, a sensitivity analysis including those two studies did not alter the inferences.
5. Iliac crest bone graft
We performed the inclusive analysis (Analysis 1.6; Table 4) to synthesize the effect of local anaesthesia on PPP after iliac crest bone grafting across all available time points, and included three studies with a total of 123 participants (Barkhuysen 2010; Gundes 2000; Singh 2007). This analysis could not include Blumenthal 2005, which reported only continuous outcomes. The overall OR for the effect was 0.20 (95% CI 0.04 to 1.09, P = 0.06), with an I2 statistic demonstrating moderate heterogeneity, but was inconclusive.
We were able to include one additional study (Blumenthal 2005), in a Bayesian analysis (Appendix 6). We could not include one study reporting no pain outcome (O'Neill 2014). We described the approach separately (Andreae 2013b). We pooled four RCTs with 159 participants with continuous (Blumenthal 2005), or dichotomous (Barkhuysen 2010; Gundes 2000; Singh 2007), pain outcomes at 3, 6 and 12 months after iliac crest bone graft harvesting in our Bayesian evidence synthesis. Results favoured continuous infusion of the donor site with local anaesthetic after iliac crest bone graft harvesting with an OR 0.1, (95% Bayesian credible intervals (BCI 95%) 0.01 to 0.59); NNTB 3 (BCI 95% 2 to 10). Clinical inferences were unaffected by the minor changes in effect estimates (OR 0.12, BCI 95% 0.02 to 0.63; NNTB 3, BCI 95% 2 to 10), whether we included a fifth non‐randomized observational study (Brull 1992), as proposed in Andreae 2013b, or not.
Stratifed analysis
No classical (frequentist) analysis was possible for the effects of local anaesthesia on PPP following iliac crest bone graft, as there were only three studies that met our inclusion criteria, one with available data at three months (Gundes 2000), one with data at 12 months (Barkhuysen 2010), and one other study with available data at 55 months postoperatively (Singh 2007). Two additional studies in the iliac crest bone graft surgical subcategory met the inclusion criteria, but reported only continuous pain data (Blumenthal 2005) or no pain outcome (O'Neill 2014). The study at three months (Gundes 2000), included a total of 45 participants and found that perioperative wound instillation of bupivacaine decreased postoperative pain, with an OR of 0.14 (95% CI 0.02 to 0.86). At almost four years postoperatively, one study with 20 participants (Singh 2007) also found that wound irrigation with local anaesthetic reduced chronic pain after iliac crest bone graft, with an OR 0.03 (95% CI 0.00 to 0.68). However, local infiltration of bupivacaine showed no clear reduction in persistent postoperative pain in another study at 12 months (Barkhuysen 2010).
6. Limb amputation
We did not pool two studies investigating the effect of epidural anaesthesia on chronic pain (phantom limb pain) after limb amputation at six months (Karanikolas 2006; Katsuly‐Liapis1996). PPP may be different from phantom limb pain and timing of nociception may be much more important for the latter (Karanikolas 2006). Pooling groups of participants receiving epidural analgesia during different pre‐, intra‐ and postoperative intervals may be seen as arbitrary and controversial. We did not pool these studies in Analysis 1.7 and Analysis 2.5 for these reasons. We excluded two studies on pre‐amputation epidural analgesia (Bach 1988; Nikolajsen 1997) for pseudo‐randomization, as discussed in Appendix 9.
7. Laparotomy
We did not pool data from two studies with data at six months on 189 laparotomy participants (Analysis 1.8; Analysis 2.6), because the I² statistical estimate of 82% and 90%, respectively suggested excessive statistical heterogeneity.
The study on epidural anaesthesia for laparotomy for major gynaecological surgery (Katz 2004), provided insufficient evidence to reject the null hypothesis of no effect with an OR of 0.81 (95% CI 0.35 to 1.88) at six months, while the study on thoracic epidural anaesthesia for colonic resection (xiphopubic incision) (Lavand'homme 2005), favoured regional anaesthesia with an OR of 0.04 (95% CI 0.01 to 0.22) at six months and OR of 0.08 (95% CI 0.01 to 0.45) at 12 months. We can only hypothesize that the more effective pain control in Lavand'homme 2005, compared to the no‐improved‐pain‐control in the immediate postoperative period in the experimental group in Katz 2004 might explain the heterogeneity. Alternatively, differences in surgical specialties may explain this heterogeneity.
8. Hernia repair
We did not pool data for our inclusive analysis (Analysis 1.9), including only the six‐month time point for Mounir 2010 and the 12‐month time point for Kurmann 2015, not synthesizing the data on hernia repairs, because statistical heterogeneity at both the study and subgroup level was deemed excessive with an I2 statistic of 93%.
Stratified analysis
We did not pool two studies after inguinal hernia repair, including 389 hernias (Kurmann 2015; Mounir 2010), with outcome data at three months postoperatively (Analysis 2.7). An I2 statistic of 93% suggested marked heterogeneity; one study used participants while the other used hernias as unit of analysis. Both studies employed infiltration locally or into the wound, with a single shot post‐incision. However, Mounir 2010 used spinal anaesthesia, whereas Kurmann 2015 employed either spinal or general anaesthesia, at the request of the participant. The OR for Mounir 2010, using spinal anaesthesia with wound infiltration was 0.01 (95% CI 0.00 to 0.15) at three months and 0.01 (95% CI 0.00 to 0.09) at six months. In contrast, the OR of 2.61 (95% CI 0.80 to 8.48) at three months for (Kurmann 2015), favoured conventional postoperative analgesia over local infiltration. Notably, Kurmann 2015 could not show a clear and precise improvement in pain in the immediate postoperative period, while pain was improved immediately postoperatively in Mounir 2010.
9. Prostatectomy
We pooled two studies after prostatectomy that utilized regional anaesthesia with pain outcomes at three months postoperatively (Brown 2004; Gupta 2006), including a total of 150 participants. While only one of the two studies on prostatectomy collected dichotomous outcomes (Brown 2004), both reported continuous outcome data, in the form of the Short Form Health Survey (SF‐36) (Analysis 1.10). The pooled standard mean difference was inconclusive with a SMD of 0.06 (95% CI ‐0.26 to 0.38) not suggesting any benefit of regional anaesthesia (P = 0.71). Both studies reported outcomes at the same time point, (three months after surgery), thus approach and results are the same using the inclusive or the classical analysis.
10. Hysterectomy
We performed an inclusive analysis on the effect of the intervention on PPP in hysterectomy, pooling 297 participants from three studies (Purwar 2015; Sprung 2006; Wodlin 2011) performed across the above named time points (Analysis 1.11). Each study recorded the pain data as the bodily pain subcomponent of the Short Form Health Survey (SF‐36) questionnaire, which is a continuous outcome, and thus we used the mean difference as the outcome measure. The results remained inconclusive, with an overall mean difference of 1.70 (95% CI ‐1.06 to 4.46), with little heterogeneity (I2 statistic across both study and subgroup level = 0%). We performed classical analysis (Analysis 2.8) for the effects of regional or local anaesthesia on PPP after hysterectomy at three months (Purwar 2015; Sprung 2006). There were 135 participants included in the analysis, which yielded a mean difference of 1.90 (95% CI ‐1.23 to 5.02), which is inconclusive.
11. Additional comparisons
We performed an additional analysis of the effect of intravenous local anaesthesia on persistent pain after breast surgery (Table 5); breast cancer surgery was the only surgical subgroup which has been studied thus far (Analysis 1.3.2). Two studies, one with outcomes at three months (Grigoras 2012), and one with outcomes at six months (Terkawi 2015b), and a total of 97 participants, were included in this evidence synthesis, demonstrating a meaningful benefit of the use of intravenous local anaesthetics in preventing persistent postsurgical pain in breast surgery (OR 0.24, 95% CI 0.08 to 0.69, P = 0.008).
One study on the use of regional anaesthesia for the prevention of pain after repair of pectus excavatum in children and young adults met the inclusion criteria for our review, but we were unable to include it in the primary analysis as it was the only study of its surgical subgroup (Weber 2007). Due to the rare incidence of pain in this study, the effect of epidural anaesthesia on PPP was inconclusive at both three months (OR 0.32, 95% CI 0.01 to 8.26) and six months (inestimable due to 0 events) postoperatively. We also report on a single study (Paxton 1995), that favoured local injection of bupivacaine to the vas deferens for pain after vasectomy, with an OR 0.02 (95% CI 0.00 to 0.33). Finally, we report on one study performed on plastic surgery of the breast (Bell 2001), excluded from the rest of the breast surgery subgroup as the nature of plastic surgery and the population studied are likely quite different. The results of this small study (Bell 2001), did not show a benefit to local infiltration of the wound in this subgroup at six months, with an OR 1.80 (95% CI 0.21 to 15.41).
12. Extended perioperative nociception
When we excluded single‐shot interventions to test if continuous prolonged antinociception was more effective in reducing the risk of persistent pain after surgery, the results were unchanged because either the same or too few studies were left for meta‐analysis in each surgical subgroup.
13. Anaesthesia modality
While we explored the influence of anaesthesia modality on risk reduction afforded by regional anaesthesia in sensitivity analysis, the small number of studies precluded a formal subgroup analysis of anaesthesia technique. Only epidural anaesthesia was used for thoracotomy, limb amputation and laparotomy. For other surgical interventions, studies investigated a variety of regional anaesthesia techniques (Appendix 7), with the marked diversity especially in breast surgery possibly explaining the observed heterogeneity of effect.
14. Adjuvant therapy
We examined studies employing adjuvant therapy. Because they investigated surgeries of different body parts (Fassoulaki 2005; Lavand'homme 2005), we did not pool the data (Data synthesis). A separate Cochrane Review on pharmacological interventions to prevent PPP has recently been completed (Chaparro 2013).
15. Adverse effects and long‐term sequelae after regional anaesthesia
Reporting of adverse effects and long‐term sequelae after regional anaesthesia (e.g. permanent nerve damage) was mostly anecdotal; they were not our primary or secondary outcomes and we report them only for completeness. Three studies systematically compared adverse effects between the experimental and the control groups, but these studies and the collected data sets were too heterogeneous for meta‐analysis. Details are listed in Appendix 10.
Sensitvity analysis of model assumptions
We had decided a priori to use the random‐effects model for evidence synthesis regardless of the observed I² statistic, because we anticipated clinically relevant heterogeneity and felt that the absence of observed proof for heterogeneity would be no proof for homogeneity.
Discussion
Summary of main results
Of the 63 studies identified, we pooled the data from 39 studies, enrolling a total of 3027 participants in our inclusive analysis. Follow‐up was for 1293 participants at three months, 1365 participants at six months, 326 participants at 12 months, and 43 participants at 20 or more months after surgery (Appendix 8), favouring regional anaesthesia for the prevention of persistent pain after surgery after thoracotomy, breast cancer surgery, caesarean section and iliac crest bone graft harvesting as detailed below.
Inclusive analysis
Our inclusive evidence synthesis (Data synthesis/inclusive analysis), is presented in five summary of findings tables (Table 1; Table 2; Table 3; Table 4; Table 5).
Thoracotomy
Including 499 participants in seven studies (Can 2013; Comez 2015; Ju 2008; Katz 1996; Liu 2015; Lu 2008; Senturk 2002), with outcomes between 3 and 18 months, results favoured regional anaesthesia for thoracotomy with an OR of OR of 0.52 (0.32 to 0.84), leading to a NNTB of 7, 95% CI (4 to 23) (Analysis 1.1) (Table 1).
Cardiac surgery
We did not conduct any meta‐analysis of the three studies in cardiac surgery (Chiu 2008; Dogan 2016; Vrooman 2015), due to a very high statistical heterogeneity (I2 = 83%), possibly due to different regional anaesthesia modalities employed (Chiu 2008 employed a continuous wound infusion, parasternal blocks were utilized in Dogan 2016, while Vrooman 2015 used lidocaine patches).
Breast cancer surgery
For breast cancer surgery, based on 1297 participants in 18 studies (Albi‐Feldzer 2013; Baudry 2008; Besic 2014; Fassoulaki 2000; Fassoulaki 2001; Fassoulaki 2005; Gacio 2016; Grigoras 2012; Ibarra 2011; Kairaluoma 2006; Karmakar 2014; Lam 2015; Lee 2013; Micha 2012; Strazisar 2012; Strazisar 2014; Tecirli 2014; Terkawi 2015b), we estimated the NNTB for breast cancer surgery as 7 with a 95% CI of 6 to 13 (Table 2), calculated from (OR 0.43, 95% CI 0.28 to 0.68) (Analysis 1.3; Figure 6).
Caesarean section
For caesarean section (Analysis 1.4), evaluating the overall effect across all time points, we included four studies (Bollag 2012; Lavand'homme 2007; Loane 2012; Shahin 2010), totaling 551 participants. The results strongly favoured the use of regional anaesthesia for the prevention of PPP after caesarean section with an OR of 0.46 (95% CI 0.28 to 0.78). The NNTB for caesarean section is 19 with a 95% CI (14 to 49) with an assumed corresponding risk of 0.1 (Table 3).
Illiac crest bone graft harvesting
Bayesian evidence synthesis (Data synthesis/Bayesian Evidence Synthesis), of data from 159 participants enrolled in four RCTs (Barkhuysen 2010; Blumenthal 2005; Gundes 2000; Singh 2007), favoured continuous infusion of the donor site with local anaesthetic for the reduction of PPP risk after iliac crest bone graft harvesting with an OR 0.1 (BCI 95% 0.01 to 0.59); NNTB 3 (BCI 95% 2 to 10) (Andreae 2013b), but our frequentist analysis (Analysis 1.6), (unable to include the study Blumenthal 2005, reporting only continuous outcomes) was inconclusive, pooling data from three studies (Barkhuysen 2010; Gundes 2000; Singh 2007), including a total of 123 participants (OR 0.20, 95% CI 0.04 to 1.09) (Table 4).
Other surgical subgroups, interventions, continuous pain outcomes and results in children
We did not pool the studies investigating local or regional anaesthesia after limb amputation (Karanikolas 2006; Katsuly‐Liapis1996), laparotomy (Katz 2004; Lavand'homme 2005), or hernia repair (Kurmann 2015; Mounir 2010), as the sparse study data were clinically and statistically too heterogeneous (Analysis 1.7; Analysis 1.8; Analysis 1.9).
The inclusive analysis of two studies (Brown 2004; Gupta 2006), reporting continuous outcomes for prostatectomy were inconclusive, with a SMD of 0.06 (95% CI ‐0.26 to 0.38) (Analysis 1.10), as were those pooling three studies (Purwar 2015; Sprung 2006; Wodlin 2011), for hysterectomy, with a MD of 1.70, 95% CI (‐1.06 to 4.46) (Analysis 1.11). A subgroup comparison pooling two studies (Grigoras 2012; Terkawi 2015b), with 97 participants showed a statistically meaningful benefit of intravenous local anaesthetics in reducing the risk of persistent postsurgical pain after breast surgery with an OR of 0.24 (95% CI 0.08 to 0.69) (Analysis 1.3.2), and a NNTB 4 95% CI (3 to 11) (Table 5).
We included only one RCT in children and adolescents undergoing pectus excavatum repair; this study was inconclusive (Weber 2007). A single study favoured local injection of bupivacaine to the vas deferens for pain after vasectomy, with an OR 0.02 (95% CI 0.00 to 0.33) (Paxton 1995). The results of one small study on local infiltration of the breast for plastic surgery did not show a benefit to local infiltration of the wound in this subgroup at six months, with an OR 1.80 (95% CI 0.21 to 15.41) (Bell 2001).
Classical stratified analysis
Classical (frequentist) evidence synthesis pooling studies separately at different follow‐up intervals within the same surgical subgroup led to sometimes disparate, contradictory results. For thoracotomy, evidence synthesis at three months of data from five studies (Can 2013; Comez 2015; Ju 2008; Liu 2015; Lu 2008), with a total of 428 participants favoured epidural anaesthesia with an OR 0.70 but failed to reach statistical significance with a 95% CI from 0.40 to 1.20 (Analysis 2.1); in contrast at six months, data from five studies (Can 2013; Comez 2015; Ju 2008; Lu 2008; Senturk 2002), (including four studies with outcomes at three months), with 370 participants favoured epidural anaesthesia for the prevention of persistent postoperative pain at six months (OR 0.39, 95% CI 0.24 to 0.63) (Analysis 2.1). At all time points, the four studies completed at different institutions and in several countries (China and Turkey) were remarkably homogeneous in their estimates of effect measure (I2 statistic = 0% and 19%).
Likewise, in the breast cancer surgery subgroup, statistical and clinical heterogeneity was notable for the outcomes observed at three months after surgery, but much less for outcomes observed six months after surgery, with both comparisons clearly favouring regional anaesthesia. In the most conservative analysis limiting our analysis only to the two studies (Ibarra 2011; Kairaluoma 2006), using paravertebral block for breast cancer surgery at six months, evidence synthesis favoured the intervention (OR 0.37, 95% CI 0.14 to 0.94; NNTB 5; analysis shown in the previous version of this review (Andreae 2012)). Also, for example after caesarean section (Analysis 2.4), pooled effect estimates including data from 492 participants in three studies (Bollag 2012; Lavand'homme 2007; Shahin 2010), with outcomes at six months showed a strong and statistically meaningful effect (OR 0.44, 95% CI 0.26 to 0.74). However, pooling data from three studies reporting outcomes at three months after caesarean section did not favour regional anaesthesia (OR 1.09, 95% CI 0.39 to 3.07). The same studies showed different results at different follow‐up intervals (Bollag 2012; Loane 2012; O'Neill 2012).
This emphasizes the utility and need for an inclusive analysis (Data synthesis/inclusive analysis) and for more advanced (Bayesian) modelling in evidence synthesis (Andreae 2013b), reflecting the hierarchical, nested structure of interventions and outcome reporting:
at the very least, results in some subgroups can inform estimates of between‐study heterogeneity in other subgroups and
taking into account the correlation of effects observed at subsequent follow‐up intervals can lead to better estimation of the credible intervals of the pooled effect estimates.
Clinical and statistical heterogeneity of effects
While there is consistent evidence favouring regional anaesthesia for the prevention of persistent pain after surgery across different but not all surgical subgroups, regardless of which approach we chose for the analysis, we observed important statistical heterogeneity, possibly explained by 'null bias', clinical heterogeneity or differences in follow‐up intervals or attrition (Incomplete outcome data (attrition bias) between studies and diversity in the follow‐up intervals used for many of the other subgroups (Appendix 7). We failed to completely explain the observed disparity in the effect estimates for outcomes reported at different follow‐up intervals: for example after caesarean section (Analysis 2.4). The same was true to a lesser extent after thoracotomy (Analysis 2.1), where the pooled effect estimate confidence interval touched the midline at three months but not at six months in our classical (frequentist) analysis.
As in our first review (Andreae 2012), we noted a pattern at the study level, in that if pain control was not improved in the immediate postoperative period, persistent postoperative pain was less likely to be improved at three, six or twelve months (e.g. Baudry 2008; Can 2013; Ju 2008; Karmakar 2014; Kurmann 2015; Loane 2012). This may be an example of ‘null bias’ due to interventions being insufficiently well delivered (Higgins 2011a; Woods 1995). On the other hand, 'null bias' may simply reflect the clinical reality that providers with different training and skill levels provide regional anaesthesia of variable quality.
On one hand, especially in the breast surgery subgroup, (as illustrated by Figure 6, ordered by regional anaesthesia modality), local infiltration consistently failed to reduce the risk of persistent postoperative pain (Baudry 2008; Bell 2001), and as mentioned often failed to have an effect in the immediate postoperative period. At first sight, this seems to contradict the finding that intravenous administration of lidocaine did reduce the risk of persistent postoperative pain in two studies (Grigoras 2012; Terkawi 2015b), and evidence synthesis of their data favoured intravenous lidocaine over control (OR 0.24, 95% CI 0.08 to 0.69; participants = 97; I2 = 0%). We had planned to include studies that administered local anaesthetics systemically in our initial protocol (Andreae 2008), because we felt there is a physiological rationale for effect several months later (Strichartz 2008). We hypothesize that the lack of effect observed in the infiltration study (Bell 2001), is the result of systemic absorption of local anaesthetics, which would attenuate the effect in the untreated breast and diminish the effect difference observed between the breast infiltrated versus non‐infiltrated.
Surgical and anaesthetic complications were too sparsely and inconsistently reported for any conclusions to be drawn from the data included in this review. It is probable that large observational studies would be more suited to accurately estimating these risks, particularly the rare but serious risk of persistent long‐term neurological injuries after regional anaesthesia (Brull 2007; Schnabel 2010).
Overall completeness and applicability of evidence
Participants
Most included studies were performed in university settings. Other than this limitation, the inclusion and exclusion criteria did not limit the applicability of the results to people in the community. We deplore the dearth of paediatric studies (Weber 2007). On a cautionary note, there is still insufficient evidence to extrapolate the effect of one regional anaesthesia technique to another. For example, with our data on epidural anaesthesia for thoracotomy and on paravertebral block for breast cancer surgery, we cannot conclude that paravertebral blocks prevent PPP after thoracotomy.
Interventions
When we limited our evidence synthesis to almost identical regional techniques for very similar surgical interventions (epidural anaesthesia for thoracotomy or paravertebral blocks for breast cancer surgery) (data shown in the previous version of this review (Andreae 2012)), heterogeneity of effect measures was clearly reduced (Figure 6). Some may take the stance that pooling studies using different techniques, different adjuvants, even different local anaesthetic agents is never appropriate. A sceptical reader may consider different regional anaesthesia techniques or different surgical interventions clinically too diverse to justify pooling in a meta‐analysis (Deeks 2011). Others may argue that such evidence synthesis is warranted (and this type of clinical heterogeneity is immaterial) and that effective pain control in the immediate postoperative period would be a better criterion to include or exclude studies. We were not comfortable to base our decision to pool or not solely on the observed statistical heterogeneity, not least because lack of evidence for heterogeneity obviously constitutes no proof for homogeneity. Results of our evidence synthesis were indifferent to choosing a classical or more inclusive approach and suggested that regional anaesthesia reduces persistent postoperative pain after breast surgery, thoracotomy, caesarean section and iliac crest bone graft harvesting.
Comparator
Our review compared local and regional anaesthesia to conventional pain control (Appendix 1). Only one study (Lavand'homme 2005) compared the effects of the localized (for example wound infiltration) versus the systemic (for example intravenous) administration of local anaesthetics on PPP (Strichartz 2008). There is insufficient evidence to support or refute the notion that systemically administered local anaesthetics are equally effective in reducing the risk of persistent pain after surgery (Lavand'homme 2005; Strichartz 2008; Vigneault 2011), but there is evidence that intravenous local anaesthetics are also effective in reducing the risk of persistent pain after (breast cancer) surgery (Analysis 1.3).
Outcomes and follow‐up intervals
Outcomes were reported at three, six and 12 months, and beyond. We compared our inclusive analysis (which pooled studies reporting outcomes at different intervals) with a classical approach (only pooling outcomes reported at similar follow‐up intervals) (Data synthesis); we also built a novel Bayesian hierarchical model, which first pooled outcomes observed at subsequent intervals in the same study to a study‐level pooled estimated, which we then used to inform the group‐level estimate. The inclusive analysis and the Bayesian approach gave more consistent and coherent results than the classical stratified evidence synthesis, (grouping studies strictly by time to follow‐up), reminding us that meta‐analysis results are contingent on modelling choices in any approach (Deeks 2011).
Dichotomous outcomes were reported by most studies. While neither optimal nor comprehensive, dichotomous outcomes are meaningful and easy to understand for people, physicians, payers, politicians and the public alike; in other words, the media, congress aides and insurance administrators will find it easier to comprehend the benefit of regional anaesthesia when outcomes are expressed simply as a 'pain versus no pain' alternative. Many continuous outcome measures of chronic pain represent not just similar scales measuring the same outcome, but rather, different dimensions of the human pain experience that hence cannot be pooled easily by meta‐analysis. We acknowledge that the dichotomous outcomes used in our review fall short of a comprehensive assessment of the full impact of PPP on peoples' quality of life (Turk 2006).
The summary statistics extracted from the included studies did not provide the detail required to differentiate between mild and severe disabling PPP six months after surgery (Gewandter 2015). Mild versus severely disabling PPP may make an important difference (Kehlet 2006) for the individual. However, persistent pain after thoracotomy can decrease function even at low levels of pain (Gottschalk 2006). Considering the impact of even minor pain on quality of life (Gottschalk 2006; MacRae 2008), we feel that the prevention of minor PPP after thoracotomy or breast cancer surgery is clinically meaningful; this is even more so after minor or benign elective interventions like caesarean section, vasectomy, lumpectomy or iliac bone graft harvesting. Similar to responder analysis, the state of the art for the evaluation of interventions for chronic pain (Dworkin 2009a), our dichotomous effect measure is also appropriate to investigate if regional anaesthesia reduces the risk of PPP.
To judge the clinical meaningfulness of regional anaesthesia we must weigh its risks and costs against short‐term benefits, such as enhanced recovery and improved immediate pain control (Dworkin 2009a; Gottschalk 2006), plus the reduced risk for persistent postsurgical pain suggested by our evidence synthesis. Long‐term sequelae secondary to regional anaesthesia are better studied in registries, then in RCTS and meta‐analysis (Jeng 2010). The risk of regional anaesthesia is deemed very low (Brown 1995; Jeng 2010; Neal 2008; Schnabel 2010). An overall assessment of the clinical usefulness of regional anaesthesia should probably be reserved for a Cochrane Review overview.
Quality of the evidence
The 'Risk of bias' graph gives an overview of risk of bias in the included studies (Figure 2), detailed in the methodological quality summary (Figure 3). We noted several important limitations in the quality of the evidence. The nature of the interventions made participant blinding effectively impossible. Hence, performance bias may weaken the conclusions of our review. The placebo effect may be particularly strong for pain outcomes and remains unknown for long‐term outcomes. Several studies employed adjuvants only in the experimental group, potentially introducing bias, although this did not affect the pooled results for the breast cancer surgery subgroup and was not pertinent for the thoracotomy subgroup. Our conclusions are considerably weakened by high risk of bias due to incomplete outcome data, high risk of selection bias due to lack of allocation concealment and high risk of performance bias due to incomplete participant blinding across a number of the included studies (Hewitt 2005).
Influence of attrition and follow‐up interval on effect size
The included studies investigating long‐term outcomes after regional anaesthesia tended to vary in the follow‐up intervals at which they collected and reported outcomes (Appendix 7). By pooling studies with disparate outcome reporting, we greatly increased our power, because more studies and more data are available for inferences. However, this could lead to bias, if the (estimation of the) effect of the intervention were associated with the duration of follow‐up or with attrition; the attrition is likely to increase with the duration of the follow‐up period. Several reasons for a biased estimate are conceivable.
PPP might slowly subside with time; this would lead to lower estimates of the prevalence of PPP at later follow‐up visits, which would bias the estimates of the effects of regional anaesthesia on PPP towards the null, because both the treatment and the control group prevalence would be diminished.
Attrition might have a similar effect of biasing the effect estimates towards the null, simply by decreasing the sample size of available observed outcomes.
Attrition might however bias the effect estimates in unforeseeable ways, if loss to follow‐up were associated with the outcomes, the intervention, or other predictors of effect or risk factors for poor outcome (PPP). Indeed, it is very well conceivable that people with persistent pain are more likely to be retained in a study; people with chronic painful symptoms are more likely to continue to follow‐up and see their physician than those who have no complaints and hence no reason to attend subsequent visits. This increased probability to keep people with pain in the study (and to loose people who no longer have persistent pain), could lead to a (spurious) increase in the observed prevalence of persistent pain in the control or the treatment group and hence to false estimates of effect, even when the intervention is not (as) effective.
To refute this concern, we explored the association of attrition and follow‐up duration with effect size estimation graphically in an attrition effect size plot; we are unaware of any description of a similar graphical test, especially in the context of meta‐analysis. The resultant graph (Figure 4; Levene 2015), does not suggest any correlation of effect size estimation with follow‐up or attrition to our best judgement. Effect sizes at later follow‐up visits sometimes lead to lower and sometimes to higher estimates of effect. Loss to follow‐up leads to higher effect size estimates in some and to lower estimates in other studies, without any apparent trend. This absence of evidence to reject this null hypothesis (no association between attrition and effect), while there is no proof of lack of association, reassures us regarding our decision to pool studies with disparate follow‐up intervals or attrition (Data synthesis/inclusive analysis; Effects of interventions/inclusive analysis).
We compared our inclusive analysis with the approach taken in the previous version of this review, a classical meta‐analysis stratified by follow‐up interval (Andreae 2012); the classical approach produced contradictory results with strong evidence at one follow‐up interval and inconclusive results at another; sometimes the same studies showed conflicting results at subsequent follow‐up intervals. We feel that this is likely the result of the generally small study size leading to variability in effect estimates and therefore a priori planned to pool the studies across follow‐up periods to obtain more robust and consistent results (Data synthesis/inclusive analysis). As discussed above, we found no evidence in our graphical exploration that attrition and follow‐up duration bias effect estimates (Figure 4; Levene 2015). We also compared our analysis with a Bayesian hierarchical model and described the results elsewhere in more detail (Andreae 2015). While we obtained similar inferences in our Bayesian model, we found the estimates of the credible intervals for the OR to change substantially with our modelling choices. Classical meta‐analysis may underestimate the between‐study variability for small numbers of studies, making estimates for the confidence intervals less reliable when they rely only on a small number of studies (Cornell 2014; Song 2012).
The statistical and clinical heterogeneity in some subgroups, the dependence of the estimates of effects on model choices or the duration of follow‐up, high risk of bias from incomplete outcome data and lack of participant blinding across a number of included studies may lead sceptical readers to question the strength of the evidence favouring regional anaesthesia for the prevention of persistent pain after surgery; the variability of results is in part explained by the small size of the included studies, which some consider a risk of bias in its own right (Moore 2013).
Potential biases in the review process
Reporting and selection bias
Not all outcome data were available for inclusion (Figure 1; Results of the search; Assessment of reporting biases; Appendix 11). This potentially introduced bias in our review and may reflect publication bias. A formal analysis of publication bias by using a funnel plot or the test proposed by Egger 1997 was precluded by the small numbers of studies found in most subgroups and their similar sizes. Even though we feel that the funnel plot for breast surgery (Figure 5) is inconclusive for publication bias, we acknowledge the possibility of underlying publication bias, as we were clearly unable to include data of all identified studies as detailed in Other potential sources of bias.
Predefining subgroups based on surgical interventions, pooling studies across subsequent follow‐up intervals and identification of studies with high risk of null bias effectively reduced unexplained effect size variability, but failed to explain all statistical heterogeneity. Our results were robust in different models used in the analysis, but are undeniably contingent on model assumptions. For several subgroups study design and reporting disparity were deemed clinically too heterogeneous for classical evidence synthesis.
Additionally, though we attempted to conduct a comprehensive search, the 12 studies currently awaiting classification may be a source of potential bias.
Agreements and disagreements with other studies or reviews
Two previous narrative reviews were rather sceptical as to the potential of regional anaesthesia for the prevention of PPP (Kehlet 2006; MacRae 2008), but did not quote all the evidence analysed in this review. We are only aware of one new attempt to synthesize the evidence on regional anaesthesia for the prevention of chronic pain after surgery (Terkawi 2015a). He investigated the prevention of persistent postoperative pain after breast cancer surgery but only pooled studies employing paravertebral block. The three available RCTs reported outcomes at disparate endpoints. His evidence synthesis was inconclusive, favouring the intervention at some but not all follow‐up intervals studied (Terkawi 2015a). Several (major) studies are underway on regional anaesthesia for PPP (ISRCTN46621916; Liew 2011; Michael 2014; NCT01626755), plus one study where this is likely to be an important, albeit not the primary outcome (NCT00418457).
The effects of intravenous lidocaine several months after surgery are remarkable and match findings from another excluded study in spine surgery (Farag 2013). Another Cochrane Review on pharmacotherapy to prevent PPP in adults was published before the second included study (Terkawi 2015b) became available and hence did attempt an evidence synthesis for this outcome (Analysis 1.3.2) (Chaparro 2013).
Authors' conclusions
Implications for practice.
Epidural anaesthesia should be considered for people undergoing open thoracotomy, and paravertebral block should be considered for women undergoing breast cancer surgery to reduce their risk of persistent postoperative pain (PPP) beyond three months after surgery. Women in labour may benefit from regional anaesthesia (e.g. continuous wound infiltration with local anaesthetics) to reduce the risk of PPP beyond three months, (number needed to treat for an additional beneficial outcome (NNTB) 19, 95% CI (14 to 49), moderate‐quality evidence). Using epidural anaesthesia may reduce the risk of experiencing persistent pain several months after thoracotomy in one patient out of every six treated (NNTB 7, 95% CI 4 to 23, moderate‐quality evidence) (Table 1); the NNTB for paravertebral block for breast cancer surgery is seven people (95% CI 6 to 13), low‐quality evidence (Table 2). The NNTB after caesarean section is 19, (95% CI 14 to 49), moderate‐quality evidence, (Table 3). Continous infusion of local anaesthetics after iliac crest bone graft harvesting may reduce the risk of PPP beyond three months. However, while classical evidence synthesis was inconclusive (OR 0.20, 95% CI 0.04 to 1.09; participants = 123, low‐quality evidence), Bayesian evidence synthesis, including additional study data, suggested a NNTB of three people, low‐quality evidence (Table 4). Continuous intravenous local anaesthetic infusion (Table 5), may reduce the risk of PPP after breast cancer surgery in about one out of every three people treated (NNTB 4, 95% CI (3 to 11), moderate‐quality evidence). Our findings were robust to sensitivity analysis and independent of model assumptions. However, our conclusions may be considerably weakened by performance bias, shortcomings in allocation concealment, considerable attrition and incomplete outcome data. We caution that except for breast surgery, our evidence synthesis is based on only a few small studies. There are seven ongoing studies (Characteristics of ongoing studies), and 12 studies awaiting classification (Characteristics of studies awaiting classification), which may change the conclusions of our review. On a cautionary note, we cannot extend our conclusions to other surgical interventions or regional anaesthesia techniques, for example we cannot conclude that paravertebral block reduces the risk of PPP after thoracotomy.
Implications for research.
Future clinical studies
Participants
We urgently need RCTs on the effects of regional anaesthesia on PPP in children.
Interventions
We need to study the effects of adjuvant medications and more diverse regional anaesthesia interventions, for example paravertebral blocks for thoracotomy.
Control groups
Studies should compare the experimental regional anaesthesia intervention to a conventional pain control comparator and to an intravenous local anaesthetic control group. The latter would confirm or refute the hypothesis that intravenous local anaesthetics are equally effective, while being much easier to administer (Grigoras 2012; Lavand'homme 2005; Strichartz 2008; Terkawi 2015b; Vigneault 2011).
Outcomes in clinical studies
Outcomes should include dichotomous pain data, eliciting analgesic consumption and employing complex psychosocial instruments (Turk 2006). Studies should assess the baseline pain prior to surgery, in particular when pain before surgery warrants regional anaesthesia, as for limb amputation (Bach 1988). Risk factors should be elicited and reported separately for each group (Kehlet 2006).
Research on adverse effects
Studies should include adverse effects, separated by group, as primary outcomes.
Study design
Randomizing participants to receive the intervention on one side of the body with the contralateral site untreated as control, may not improve signal strength, see discussion on Bell 2001 in Effects of interventions. Absorbed systemic lidocaine might attenuate the development of PPP on the untreated side, leading to a diminished signal. Future studies should employ more rigorous methodology, to, for example, address patient attrition, such as intention‐to‐treat analysis.
Future evidence synthesis
The increasingly large number of RCTs investigating various modalities of regional anaesthesia for breast surgery may allow a network analysis and/or meta‐regression, to control for baseline risk or investigate which modality is most effective in preventing persistent pain after breast surgery (Andreae 2015c; Andreae 2018; Thompson 2002). These analyses should be planned with a detailed a priori protocol (Thompson 2002).
What's new
| Date | Event | Description |
|---|---|---|
| 25 February 2019 | Amended | The Cochrane review was co‐published in the Journal of Clinical Anesthesia (Levene 2019) |
History
Protocol first published: Issue 2, 2008 Review first published: Issue 10, 2012
| Date | Event | Description |
|---|---|---|
| 4 October 2018 | Amended | Acknowledgement section amended to include Co‐ordinating Editor |
| 13 June 2018 | New citation required but conclusions have not changed | We corrected the number of pooled studies and participants in the following sections: abstract (main results); results of the search (descriptive characteristics of participants); PRISMA figure; and Appendix 8 (removal of cardiac studies). |
| 13 June 2018 | Amended | We corrected the number of participants, and studies which were pooled in the inclusive analysis throughout the text and captions. Previously the text referred to 41 pooled studies (3143 participants). We corrected the text to read 39 studies, enrolling a total of 3027 participants. We removed the cardiac studies from Appendix 8 'Table of included participants', where previously the cardiac studies had erroneously been listed; even though due to heterogeneity, no data synthesis was performed for this surgical subgroup. Figure 1 and Appendix 8, and the text now consistently refer to the studies we included and pooled in our inclusive analysis. |
| 8 December 2016 | New search has been performed | We updated the review. We ran the search to December 2016. We identified 40 new RCTs and seven ongoing studies that met our inclusion criteria. We reran the search in December 2017 and added 11 studies to Studies awaiting classification. |
| 8 December 2016 | New citation required and conclusions have changed | Several authors have joined the team (Weinstein EJ, Levene JL, Cohen MS, Chao JY, Johnson M, Hall CB). The conclusions are changed by the inclusion of new studies, leading to stronger inferences in some subgroups and new inferences in others. We have updated the methods by including any outcomes after three months, with the inclusion of Bayesian hierarchical modelling and the inclusive analysis of studies by subgroup. In particular, we added additional analysis to estimate study level effects from outcomes observed at subsequent follow‐up visits in a study for a more coherent and stable effect estimate for the surgical groups. |
| 2 July 2013 | Amended | Journal version of review (Andreae 2013a) cited in ‘Other published versions of this review’ |
Acknowledgements
We would like to thank Mike Bennett (content editor), Cathal Walsh (statistical editor), Alka Kaushal, Chi Wai Cheung, Russel J Roberts, Jean‐Pierre C Estebe, Argyro Fassoulaki (peer reviewers), and Andrew Smith (Co‐ordinating Editor), for their help and editorial advice during the preparation of the updated systematic review. In particular we would like to thank Janet Wale (consumer editor) for her valuable help in writing the Plain language summary.
We would like to thank Associate Professor Michael Bennet (content editor), Prof Nathan Pace (statistical editor), Dr Ewan McNicol and Dr Patricia Lavand’homme (peer reviewers), and Durhane Wong‐RIeger (consumer reviewer) for their help and editorial advice during the preparation of the first version of this systematic review (Andreae 2012).
We would like to thank Dr Jane Ballantyne (content editor), Dr W Scott Beattie and Professor Martin Tramèr (peer reviewers), and Janet Wale (consumer) for their help and editorial advice during the preparation of the protocol for this systematic review (Andreae 2008). We would like to thank Dr Hung‐Mo Lin for her help in extracting methodological information from the article in Chinese (Lu 2008). We would like to thank Dr Jing Song for her help in reviewing an abstract in Chinese (Wang 1992).
We acknowledge the responses of the authors of 19 studies to our request for additional information (Besic 2014; Burney 2004; Can 2013; Fassoulaki 2000; Fassoulaki 2001; Gacio 2016; Gundes 2000; Ibarra 2011; Kairaluoma 2006; Katz 1996; Kurmann 2015; Lavand'homme 2005; Lavand'homme 2007; Micha 2012; Obata 1999; Ochroch 2006; Purwar 2015; Senturk 2002; Tecirli 2014), in addition to one author regretting that the data were no longer accessible (Pinzur 1996); and the author of one study not meeting the inclusion criteria (Ilfeld 2004).
Appendices
Appendix 1. Lay explanation of intervention and comparator: regional anaesthesia versus conventional analgesia
Local anaesthetics and regional anaesthesia
Local anaesthetics are drugs used to block pain conduction. If local anaesthetics are applied locally at the site of surgery this is called local anaesthesia. If local aesthetics are applied close to nerves, but at a distance from the surgical site, this is called regional anaesthesia. Local anaesthetics block nerve conduction, if applied close to nerves. Sometimes, local aesthetics are also applied intravenously.We included studies that applied local anaesthetics close to peripheral nerves (nerve block), close to a nerve plexus (plexus block) or in the spinal canal (spinal or epidural anaesthesia). We also included studies that irrigated the operative field with local anaesthetics or infused local anaesthetics in the wound, or confined local anaesthetics to the operated limb and extremity by using a tourniquet (Bier Block). We included the intravenous delivery of local anaesthetics (IVRA), as local anaesthetics might also have beneficial anti‐hyperalgesic (Strichartz 2008) and anti‐inflammatory properties (Herroeder 2007), even if administered systemically.
We included studies where local anaesthetics were given as a single shot or as a continuous infusion through catheters or controlled‐release preparations, dermal patches etc.
Adjuvants like ketamine may enhance the effect of local anaesthetics. They act through different receptors on the nerves. We included studies regardless of whether they also employed adjuvants or opioids, either locally or systemically in the experimental and/or in the control groups. We included studies that employed local or regional analgesia for any length of time during the perioperative period, for example only for the 24 hours preceding the operation or only for postoperative pain control.
We compared whether local anaesthetics work better than conventional pain control in reducing the event rate of persistent pain after surgery. Hence, we excluded studies that only compared different regional anaesthesia techniques or varying dose regimens of local anaesthetics during the same perioperative time span and studies using local anaesthetics for other than anaesthetic or analgesic purposes (for example as anti‐arrhythmics).
Conventional analgesia
Drugs used to treat pain are called analgesics or painkillers. They act on receptors of the peripheral and central nervous systems. Painkillers are mainly divided into opioids and non‐opioids. Non‐opioids include paracetamol (acetaminophen in the USA) and the non‐steroidal anti‐inflammatory drugs (NSAIDs), a well‐known example being aspirin. Opioids include weaker opioids like codeine and stronger ones like morphine and fentanyl.
A disadvantage is that painkillers work systemically, in other words in the entire body not just locally where the pain is felt. Painkillers have adverse and side effects. Typical side effects of NSAIDs range from mild stomach upset to severe gastrointestinal bleeding. Ketorolac, the only intravenous NSAID approved in the USA, is used with caution as it can potentially cause kidney damage. In higher doses all NSAIDs can damage the kidneys. Newer (COX‐2 antagonists) and older NSAIDs except aspirin, may increase the risk of myocardial infarction and stroke. Opioids often cause nausea and vomiting, drowsiness and constipation. In the elderly in particular they can cause delirium and hallucinations. At higher doses opioids can cause potentially dangerous respiratory depression, in other words causing patients to stop breathing. People often describe that opioids take the edge off the pain and make it bearable, but do not completely suppress the pain.
The WHO pain ladder is often used to titrate the painkillers to effect: mild pain is treated ideally with just NSAIDs. Stronger pain is treated with a combination of NSAID and mild or stronger opioids as needed. After surgery, patients sometimes cannot eat right away; hence medication cannot be administered orally, but has to be given intravenously. Opioids are sometimes administered by patient‐controlled analgesia (PCA). A PCA machine administers intravenous opioids when the patient presses a button. This allows the patient to titrate the medication to better meet his or her individual needs. The PCA machine is programmed such that the patient cannot overdose by pressing the PCA button too often. In spite of the ubiquitous availability and the relatively low price for conventional painkillers in the industrialized world, many patients find their pain under‐treated.
Appendix 2. CENTRAL (Ovid SP) search strategy
1. analgesia, epidural/ or interpleural analgesia/ or anesthesia, conduction/ or anesthesia, epidural/ or anesthesia, caudal/ or anesthesia, spinal/ or nerve block/
2. ((an?esthesia adj3 (conduction or regional or epidural)) or (block$ adj3 (epidural or spinal or plexus or bier)) or (Ropivacain$ or Lidocain$ or Bupivacain$ or Tetracain$ or Mepivacain$ or Prilocain$ or levobupivacain$)).ti,ab,tw.
3. anesthetics, local/ or anesthesia, local/
4. Anesthetics, Local.mp.
5. limit 4 to pharmacologic actions
6. 1 or 2 or 3 or 5
7. (phantom limb or mastectomy or thoracotomy).sh,tw.
8. postsurgical.af.
9. pain.sh,tw.
10. visual analog scale.sh. or (visual analog scale or numeric rating scale or SF‐36 or McGill pain questionnaire or McGill pain score).tw.
11. (7 or 8) and (9 or 10)
12. (hyperalgesia or allodynia).sh,tw.
13. Pain, Postoperative.sh. or postoperative pain.tw.
14. Phantom Limb/pc or Pain, Postoperative/pc
15. (preventive analgesia or (preventive analgesia or preventive analgesic) or (pre emptive analgesia or pre emptive analgesic or pre emptive analgesics) or (preemptive analgesia or preemptive analgesic or preemptive analgesics)).af.
16. 11 or 12 or 13 or 14 or 15
17. (chronic or weeks or months or persistent).af.
18. 6 and 16
19. limit 18 to abstracts
20. 18 not 19
21. 17 and 19
22. 20 or 21
Appendix 3. MEDLINE (Ovid SP) search strategy
1 analgesia, epidural/ or interpleural analgesia/ or anesthesia, conduction/ or anesthesia, epidural/ or anesthesia, caudal/ or anesthesia, spinal/ or nerve block/ 2 ((an?esthesia adj3 (conduction or regional or epidural)) or (block$ adj3 (epidural or spinal or plexus or bier)) or (Ropivacain$ or Lidocain$ or Bupivacain$ or Tetracain$ or Mepivacain$ or Prilocain$ or levobupivacain$)).ti,ab,tw. 3 anesthetics, local/ or anesthesia, local/ 4 Anesthetics, Local.mp. 5 limit 4 to pharmacologic actions 6 1 or 2 or 3 or 5 7 (phantom limb or mastectomy or thoracotomy or hernia repair).sh,tw. 8 (post?surgical or postoperative).af. 9 pain.sh,tw. 10 visual analog scale.sh. or (visual analog scale or numeric rating scale or SF‐36 or Short‐Form Health Survey or McGill pain questionnaire or McGill pain score).tw. 11 (7 or 8) and (9 or 10) 12 (hyperalgesia or allodynia).sh,tw. 13 Pain, Postoperative.sh. or postoperative pain.tw. 14 Phantom Limb/pc or Pain, Postoperative/pc 15 (preventive analgesia or (preventive analgesia or preventive analgesic) or (pre emptive analgesia or pre emptive analgesic or pre emptive analgesics) or (preemptive analgesia or preemptive analgesic or preemptive analgesics)).af. 16 11 or 12 or 13 or 14 or 15 17 (chronic or weeks or month$ or persistent).af 18 6 and 16 19 limit 18 to abstracts 20 18 not 19 21 17 and 19 22 20 or 21 23 (randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab. 24 exp animals/ not humans.sh. 25 23 not 24 26 22 and 25
Appendix 4. Embase (Ovid SP) search strategy
1 analgesia, epidural/ or interpleural analgesia/ or anesthesia, conduction/ or anesthesia, epidural/ or anesthesia, caudal/ or anesthesia, spinal/ or nerve block/ 2 ((an?esthesia adj3 (conduction or regional or epidural)) or (block$ adj3 (epidural or spinal or plexus or bier)) or (Ropivacain$ or Lidocain$ or Bupivacain$ or Tetracain$ or Mepivacain$ or Prilocain$ or levobupivacain$)).ti,ab,tw. 3 anesthetics, local/ or anesthesia, local/ 4 Anesthetics, Local.mp. 5 limit 4 to pharmacologic actions [Limit not valid in Embase; records were retained] 6 1 or 2 or 3 or 5 7 (phantom limb or mastectomy or thoracotomy or hernia repair).sh,tw. 8 (post?surgical or postoperative).af. 9 pain.sh,tw. 10 visual analog scale.sh. or (visual analog scale or numeric rating scale or SF‐36 or Short‐Form Health Survey or McGill pain questionnaire or McGill pain score).tw. 11 (7 or 8) and (9 or 10) 12 (hyperalgesia or allodynia).sh,tw. 13 Pain, Postoperative.sh. or postoperative pain.tw. 14 Phantom Limb/pc or Pain, Postoperative/pc 15 (preventive analgesia or (preventive analgesia or preventive analgesic) or (pre emptive analgesia or pre emptive analgesic or pre emptive analgesics) or (preemptive analgesia or preemptive analgesic or preemptive analgesics)).af. 16 11 or 12 or 13 or 14 or 15 17 (chronic or weeks or month$ or persistent).af. 18 6 and 16 19 limit 18 to abstracts 20 18 not 19 21 17 and 19 22 20 or 21 23 (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐ clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or factorial* or placebo* or volunteer* or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*))).ti,ab.) not (animals not (humans and animals)).sh. 24 22 and 23
Appendix 5. Calculations for number needed to treat for an additional beneficial outcome (NNTB)
Function implemented in the statistical software package R (R 2015) to calculate the NNTB:
function(OR,lower, upper, ACR){ #function returns NNTB from OR and ACR with 95% CI # OR := odds ratio # lower := lower bound of OR 95% confidence interval # upper := upper bound of OR 95% confidence interval # ACR := assumed control risk # NNTB =: Number needed to treat # Cochrane handbook chapter 12.5.4.3 Computing absolute risk reduction or NNTB from an odds ratio
## calculate effect on risk per 1000 and NNT:
Effect_per_1000 <‐ 1000* ( ACR ‐ (OR*ACR)/(1‐ACR + OR*ACR) ) NNTB <‐ 1000/Effect_per_1000 ## calculate lower bound effect on risk per 1000 and for NNT:
Effect_per_1000_lower <‐ 1000* ( ACR ‐ (lower*ACR)/(1‐ACR + lower*ACR) ) NNT_lower <‐ 1000/Effect_per_1000_lower ## calculate effect on risk per 1000:
Effect_per_1000_upper <‐ 1000* ( ACR ‐ (upper*ACR)/(1‐ACR + upper*ACR) ) NNT_upper <‐ 1000/Effect_per_1000_upper result <‐ list(NNT, NNT_lower, NNT_upper) return(result)
Appendix 6. OpenBugs Model code
model{
############################## # Blumenthal # ############################## for(i in 0:3){ logL[1,i+1] <‐ i*log(p[1,1]) +(18‐i)*log(1‐p[1,1]) ‐ logfact(i) ‐ logfact(18‐i) + logfact(18) L[1,i+1] <‐ exp(logL[1,i+1]) p1[i+1] <‐ L[1,i+1]/sum(L[1,1:4]) } for(i in 16:18){ logL[2,i‐15] <‐ i*log(p[1,2]) + (18‐i)*log(1‐p[1,2]) ‐ logfact(i) ‐ logfact(18‐i)+logfact(18) L[2,i‐15] <‐ exp(logL[2,i‐15]) p2[i‐15] <‐ L[2,i‐15]/sum(L[2,1:3]) } for(i in 1:2){ d[i] <‐ 1 d[i] ˜ dbern(LogLike[i]) LogLike[i] <‐ mean(L[i,1:(r[i])]) } ###################################### ################################## # Other Studies # ################################## for(i in 1:3){ for(j in 1:2){ x[i,j] ˜ dbin(p[i+1,j],N[i,j]) } } #################################### #################################### # Priors # #################################### for(i in 1:4){ for(j in 1:2){ logit(p[i,j]) <‐ gamma[i,j] } gamma[i,1:2] ˜ dmnorm(gamma[5,1:2],Tau[1:2,1:2]) #gamma[i,1] ˜ dnorm(gamma[5,1], or[i] <‐ exp(gamma[i,1]‐gamma[i,2]) } gamma[5,1] ˜ dnorm(0,0.001) gamma[5,2] ˜ dnorm(0,0.001) or[5] <‐ exp(gamma[5,1]‐gamma[5,2]) logit(p[5,1]) <‐ gamma[5,1] logit(p[5,2]) <‐ gamma[5,2] nnt <‐ 1/(p[5,2] ‐ p[5,1]) Sigma[1] ˜ dt(0,3,1)T(0,) Sigma[2] ˜ dt(0,3,1)T(0,) rho ˜ dunif(‐1,1) Sigma[3] <‐ rho*sqrt(Sigma[1]*Sigma[2]) det <‐ Sigma[1]*Sigma[2] ‐ Sigma[3] * Sigma[3] Tau[1,1] <‐ Sigma[2]/det Tau[2,2] <‐ Sigma[1]/det Tau[1,2] <‐ ‐Sigma[3]/det Tau[2,1] <‐ Tau[1,2] }
Appendix 7. Table of surgeries, interventions, timing and outcomes by subgroup of pooled studies
| Study ID | Regional technique | Timing of intervention | Adjuvants | Outcomes | Continuous | Follow‐up(month) |
| Breast cancer surgery | ||||||
| Albi‐Feldzer 2013 | Wound instillation and intervertebral block | Postincision, single shot vs placebo | None | Pain/no pain | Brief Pain Index | 3, 6 and 12 months |
| Baudry 2008 | Local Infiltration | Single shot, postincision vs control | None | Pain/no pain | McGill results not reported | 18 months |
| Besic 2014 | Local Infiltration | Postincision, continuous post‐op vs control | None | Pain/no pain | None | 3 months |
| Fassoulaki 2000 | Topical application | Preincision, continuous post‐op vs placebo | Propoxyphene | Pain/no pain | Verbal Intensity Scale | 3 months |
| Fassoulaki 2001 | Brachial plexus block | Postincision, single shot vs placebo | Mexiletine, propoxyphene | Pain/no pain | VAS | 3 months |
| Fassoulaki 2005 | Topical application | Postincision, continuous postop vs control | Gabapentin | Pain/no pain | Analgesic consumption | 6 months |
| Gacio 2016 | Paravertebral block | Single shot, preincision vs control | Parecoxib, fentanyl, morphine, and adrenaline | Pain/no pain | None | 6 months |
| Grigoras 2012 | IV lidocaine | Preincision, continuous intra‐op vs placebo | None | Pain/no pain | Short‐form McGill Pain Questionnaire | 3 months |
| Ibarra 2011 | Single shot, paravertebral block | Single shot, preincision vs control | None | Myofascial, phantom or neuropathic pain | None | 3 and 5 months |
| Kairaluoma 2006 | Single shot, paravertebral block | Single shot, preincision vs control | None | NRS > 3 | Analgesic consumption | 12 months |
| Karmakar 2014 | Thoracic paravertebral block | Single shot, preincision vs pre incision, continuous vs control | Epinephrine | Pain/no pain | VRS | 3 and 6 months |
| Lam 2015 | Paravertebral block | Not specified | None | Pain/no pain | None | 6 months |
| Lee 2013 | Paravertebral block | Preincision, continuous intra‐op and post‐op vs control | Pregabalin | Pain/no pain | Short‐form McGill Pain Questionnaire | 3 months |
| Micha 2012 | Local infiltration with brachial plexus and interscalene block | Postincision, single shot vs placebo | None | DN4 | None | 6 months |
| Strazisar 2012 | Local infiltration | Postincision, continuous post‐op vs control | None | Pain/no pain | None | 3 months |
| Strazisar 2014 | Local infiltration | Postincision, continuous post‐op vs control | None | Pain/no pain | None | 3 months |
| Tecirli 2014 | Intercostal nerve block | Postincision, single shot vs control | None | DN4 | VAS | 3 months |
| Terkawi 2015b | IV lidocaine | Preincision, continuous intra‐op and post‐op vs placebo | None | Pain/no pain | VAS | 6 months |
| Caesarean section | ||||||
| Bollag 2012 | Transversus abdominis plane block | Single shot, post‐op vs placebo | Clonidine | None | Short form McGill Pain Questionnaire | 3, 6 and 12 months |
| Lavand'homme 2007 | Wound irrigation | Preincision, continuous post‐op vs control | None | Pain/no pain | Analgesic consumption | 6 months |
| Loane 2012 | Transversus abdominis plane block | Postincision, single shot vs placebo | None | Pain/no pain | None | 3 months |
| McKeen 2014 | Transversus abdominis plane block | Postincision, single shot vs placebo | None | None | SF‐36 | 6 months |
| Shahin 2010 | Peritoneal instillation | Postincision, single shot vs placebo | None | Pain/no pain | NRS | 8 months |
| Singh 2013 | Transversus abdominis plane block | Postincision, single shot vs placebo | None | None | NRS | 3 months |
| Iliac crest bone graft | ||||||
| Barkhuysen 2010 | Local infiltration | Postincision, single shot vs control | Epinephrine | Pain/no pain | None | 1 Year |
| Gundes 2000 | Wound instillation | Postincision, single shot vs placebo | None | Pain and dysaesthesia vs none | None | 3 months |
| Singh 2007 | Wound irrigation | Postincision, continuous post‐op vs control | None | Pain/no pain | VAS, pain frequency, functional activity score, overall satisfaction | 4.7 years |
| Prostatectomy | ||||||
| Brown 2004 | Spinal | Preincision, continuous intra‐op vs placebo | Clonidine | Pain/no pain | Numerical Pain Scale, SF‐36 | 3 months |
| Gupta 2006 | Epidural | Continuos, post‐op vs placebo | Adrenaline | None | SF‐36 | 3 months |
| Thoracatomy | ||||||
| Can 2013 | Epidural | Single shot, preincision vs preincision, continuous vs control | None | Pain/no pain | VAS, patient satisfaction | 6 months |
| Comez 2015 | Epidural | Preincision, continuous intra‐op vs control | Dexketoprofen, morphine, and fentanyl | Pain/no pain | VAS | 3 and 6 months |
| Ju 2008 | Epidural | Preincision and post‐op vs control | None | Pain/no pain | Allodynia | 12 months |
| Katz 1996 | Intercostal nerve block | Single shot, postincision vs control | None | Pain/no pain | VRS, analgesic consumption | 18 months |
| Liu 2015 | Wound irrigation | Postincision, continuous post‐op vs control | Fentanyl | Pain/no pain | None | 3 months |
| Lu 2008 | Epidural | Preincision vs post‐op vs control | None | Pain/no pain | None | 6 months |
| Senturk 2002 | Epidural | Preincision vs post‐op vs control | None | Pain/no pain | NRS, pain affecting daily living | 6 months |
| Vaginal hysterectomy | ||||||
| Purwar 2015 | Spinal | Single shot, preincision vs control | Fentanyl | None | VAS, SF‐36 | 3 months |
| Sprung 2006 | Spinal | Single shot, preincision vs control | Clonidine | None | NRS, SF‐36 | 3 months |
| Abdominal hysterectomy | ||||||
| Wodlin 2011 | Spinal | Single shot, preincision vs control | None | None | SF‐36 | 6 months |
| DN4: Douleur Neuropathique 4, a pain questionnaire; NRS: numerical rating scale; SF‐36: Short Form Health Survey; VAS: visual analogue scale; VRS: verbal rating scale | ||||||
Appendix 8. Table of included participants
| Participants included | Inclusive analysis | 3 months | 6 months | 12 months | 20 months | 48 months |
| Thoracotomy | 499 (7 studies) | 120 | 279 | 77 | 23 | 0 |
| Breast cancer surgery | 1297 (18 studies) | 745 | 439 | 113 | 0 | 0 |
| Caesarean section (dichotomous) | 551 (4 studies) | 59 | 414 | 78 | 0 | 0 |
| Caesarean section (continuous) | 110 (2 studies) | 39 | 71 | |||
| Iliac crest bone graft | 123 (3 studies) | 45 | 0 | 58 | 0 | 20 |
| Prostatectomy | 150 (2 studies) | 150 | 0 | 0 | 0 | 0 |
| Hysterectomy | 297 (3 studies) | 135 | 162 | 0 | 0 | 0 |
| Sum | 3027 (39 studies) | 1293 | 1365 | 326 | 23 | 20 |
| The table of included participants provides a detailed census of the 3027 participants in 39 studies pooled in our inclusive analysis (Data synthesis/inclusive analysis). We provide a breakdown of the number of participants that contributed data at different follow‐up intervals. The first column lists the total number of participants pooled for each surgical subgroup; subsequent columns break the participants down by follow‐up interval. The last row sums participants at different follow‐ups. Most of the study data were observed at three and six months after surgery. If a study reported outcomes at more than one follow‐up, we counted the study data only once, at the last follow‐up reported for that study (Unit of analysis issues). | ||||||
Appendix 9. Pseudo‐randomization
We excluded one study, Nikolajsen 1997, for pseudo‐randomization, even though the exclusion did not alter our results. This was a double‐blinded (participants and outcome assessors) pseudo‐randomized controlled clinical trial on preoperative epidural analgesia for limb amputation with a follow‐up of 12 months including 60 adults in a university setting in Aarhus, Denmark.
We detail our risk of bias assessment below:
Randomization: high risk of bias
“We stratified patients into two groups according to the intensity of their preamputation pain.” "Patients were assigned to a group 'by the toss of a coin',..." “The next patient ... was assigned to the opposite treatment.” “We randomized women and men separately.”
Many authors would include this as an acceptable method of randomization. The review authors feel that the "toss of a coin" is not an adequate method of sequence generation, because it is open to tampering and prone to errors. If in doubt, the adequacy of sequence generation should be questioned (Higgins 2011a).
Allocation concealment: high risk of bias
“The first patient who entered the study with a preamputation pain intensity of less than 30 mm on a VAS was assigned to the blockade or control group by the toss of a coin. The next patient with a VAS score of less than 30 mm was assigned to the opposite treatment. We followed this procedure for patients with a preamputation pain intensity of 30 mm or greater on VAS. If the first patient with a VAS of 30 mm or more was assigned to the blockade group by the coin method, the next patient would automatically be assigned to the control group. We randomized women and men separately.
Attempts to conceal allocation were not reported. “The next patient ... was assigned to the opposite treatment." This made allocation predictable. The review authors take the view that this is pseudo‐randomisation because the allocation for every second patient is ‘pre‐ordained’ (Higgins 2011a).
Blinding of participants and personnel (performance bias): high risk of bias
“SI was responsible for pain treatment before and during the amputation” but also did the randomization. Also the interoperative provider had to know allocation to adjust doses “to epidural pain treatment (blockade group) or not (control group).” Postop, patients could not identify the group they had been allocated to, when "To assess masked conditions among patients, SI asked patients at the 6‐month interview what treatment they received before amputation (epidural blockade or oral/intramuscular morphine).”
Blinding of outcome assessment (detection bias): low risk of bias
“LN was informed about stratification by preamputation pain intensity, but was otherwise unaware of treatment assignment. Staff (apart from the attending nurse anaesthetist who was informed for safety reasons) and patients were not informed about treatment assignment."
Incomplete outcome data (attrition bias): low risk of bias
"Patients who underwent amputation during follow‐up were excluded from further analysis." Attrition was reported in detail also with respect to group assignments, but no intention‐to‐treat analysis was considered.
Appendix 10. Adverse effects
Adverse effects
Reporting of adverse effects was mostly anecdotal. Three studies reported no adverse effects (Albi‐Feldzer 2013; Karmakar 2014; Pinzur 1996). Several studies reported anecdotal adverse effects. Adverse effects included cardiac arrhythmias (Brown 2004; ), hypotension (Sprung 2006), cutaneous allergy to topical study drug (Fassoulaki 2000), transient leg paralysis (Kurmann 2015 ) chronic backache after epidural analgesia (Lavand'homme 2005), wound or regional anaesthesia catheter infection (Can 2013; Lavand'homme 2007; Paxton 1995; Singh 2007), including one subcutaneous infection and a case of meningitis attributed to the regional anaesthesia catheter (Nikolajsen 1997). Cases of severe intraoperative chest rigidity and severe nausea were reported (Katz 2004). One patient convulsed during regional anaesthesia (Kairaluoma 2006).
Systematic between‐group comparisons of adverse effects
Eleven included studies (Blumenthal 2005; Fassoulaki 2000; Fassoulaki 2005; Grigoras 2012; Ju 2008; Kurmann 2015; Lavand'homme 2005; Lavand'homme 2007; O'Neill 2012; Sprung 2006; Weber 2007) compared adverse effects between the experimental and the control group, but the studies and the collected data sets were too heterogeneous for meta‐analysis. Blumenthal 2005 found no meaningful difference in the incidence of nausea and vomiting, pruritis, or neurologic damage of the lateral cutaneous, ilioinguinal or superior cluneal nerves between the two groups, and no patient experienced signs of inflammation or infection at the site of the catheter. Fassoulaki 2000 only reported adverse events pertaining to a cutaneous allergy to eutectic mixture of local anaesthetics (EMLA), used in the intervention group, who was then excluded. Fassoulaki 2005 reported higher event rates of adverse effects (depression, local inflammation and thrombosis) in the control groups, but deemed them unrelated to the anaesthesia intervention. Grigoras 2012 reports sedation score and the presence of nausea and/or vomiting by group, which was minimal in both groups with immaterial differences. Ju 2008 compared side effects of opioid neuraxial treatment between groups and found a similar event rate of nausea, vomiting and sedation similar between groups, but pruritus was more frequent in the regional anaesthesia arm. One participant in the intervention group in Kurmann 2015 experienced a transient leg paralysis lasting 24 hours, which was reportedly due to deviation from injection protocol. Lavand'homme 2005 compared adverse effects between groups prospectively and found that orthostatic hypotension was less frequent in participants in the control arm, receiving intravenous analgesics. Lavand'homme 2005 reported no adverse psychomimetic effects of adjuvant low‐dose, intravenous ketamine in the same study. Lavand'homme 2007 reported no statistically meaningful differences between groups, with respect to blood drainage, time to return of bowel function, first oral intake, and scar infections or delayed wound healing. O'Neill 2012 found a difference in incidence of adverse events between groups: in the continuous wound infusion group, participants experienced less pruritis, nausea/vomiting and urinary retention compared to the epidural morphine group, while there was no statistically meaningful difference in the number of participants who re‐established bowel function by 48 hours after surgery. Sprung 2006 found that participants in the spinal group received more doses of vasopressors intraoperatively when compared to the general anaesthesia group. Weber 2007 reported that there was no meaningful difference between groups with respect to sedation, nausea and pruritis.
Two prospective randomized trials on long‐term adverse effects after labour epidural analgesia did not fulfil the inclusion criteria of this review (Howell 2001; Loughnan 2002).
Appendix 11. Study data not pooled in meta‐analysis
| Surgery | Study ID | Reason for non‐inclusion |
| Cardiac surgery | Dogan 2016 | Data N/A |
| Chiu 2008 | Too heterogeneous | |
| Vrooman 2015 | Too heterogeneous | |
| Breast cancer surgery | Di‐Gennaro 2013 | Data N/A |
| Plastic surgery of the breast | Bell 2001 | Different type of intervention |
| Iliac crest bone graft | Blumenthal 2005 | Data N/A |
| O'Neill 2014 | Data N/A | |
| Laparotomy | Katz 2004 | Too heterogeneous |
| Lavand'homme 2005 | Too heterogeneous | |
| Caesarean section | O'Neill 2012 | No events |
| Hernia repair | Burney 2004 | Data N/A |
| Kurmann 2015 | Too heterogeneous | |
| Mounir 2010 | Too heterogeneous | |
| Okur 2016 | Data N/A | |
| Prostatectomy | Smaldone 2010 | Data N/A |
| Vasectomy | Paxton 1995 | Single study |
| Limb amputation | Kairaluoma 2006 | Inconsistent regional application |
| Katsuly‐Liapis1996 | ||
| Pinzur 1996 | Data N/A | |
| Pectus excavatum | Weber 2007 | Single study |
| Cholecystectomy | Fassoulaki 2016 | Single study |
| Spinal surgery | Xu 2017 | Single study |
| Thyroidectomy | Choi 2016 | Single study |
| Craniotomy | Zhou 2016 | Single study |
Data and analyses
Comparison 1. Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PPP three to 18 months after thoracotomy | 7 | 499 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.32, 0.84] |
| 2 PPP three to six months after cardiac surgery | 2 | 116 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.73, 0.21] |
| 3 PPP three to twelve months after breast cancer surgery | 18 | 1297 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.28, 0.68] |
| 3.1 Paravertebral block | 6 | 419 | Odds Ratio (M‐H, Random, 95% CI) | 0.61 [0.39, 0.97] |
| 3.2 Intravenous lidocaine | 2 | 97 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.08, 0.69] |
| 3.3 Multimodal block | 4 | 402 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.32, 1.77] |
| 3.4 Local infiltration | 6 | 379 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.73] |
| 4 PPP three to eight months after caesarean section | 4 | 551 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.28, 0.78] |
| 5 Pain score three to six months after caesarean section | 2 | 110 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.34, 0.61] |
| 6 PPP three to 55 months after Iliac crest bone graft | 3 | 123 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.04, 1.09] |
| 7 PPP six to 12 months after amputation | 2 | 108 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.21, 1.33] |
| 8 PPP six to 12 months after laparotomy | 2 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 PPP three to 12 months after hernia repair | 2 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10 Pain score three months after prostatectomy | 2 | 150 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.26, 0.38] |
| 11 SF‐36 bodily pain score at three to six months after hysterectomy | 3 | 297 | Mean Difference (IV, Random, 95% CI) | 1.70 [‐1.06, 4.46] |
1.2. Analysis.
Comparison 1 Local or regional anaesthesia for the prevention of persistent postoperative pain (inclusive analysis), Outcome 2 PPP three to six months after cardiac surgery.
Comparison 2. Local or regional anaesthesia for the prevention of persistent postoperative pain (classical analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PPP after thoracotomy | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Three months follow‐up | 5 | 428 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.40, 1.20] |
| 1.2 Six months follow‐up | 5 | 370 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.63] |
| 2 PPP after cardiac surgery | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Three months follow‐up | 2 | 116 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.74, 0.20] |
| 3 PPP after breast cancer surgery | 19 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Three months follow‐up | 11 | 966 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.19, 0.61] |
| 3.2 Six months follow‐up | 9 | 515 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.37, 0.84] |
| 3.3 12 months follow‐up | 2 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.04, 10.47] |
| 4 PPP after caesarean section | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Three months follow‐up | 2 | 137 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.39, 3.07] |
| 4.2 Six months follow‐up | 3 | 492 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.26, 0.74] |
| 5 PPP after amputation | 2 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 PPP after laparotomy | 2 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 PPP after hernia repair | 2 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 PPP after hysterectomy | 2 | 135 | Mean Difference (IV, Random, 95% CI) | 1.90 [‐1.23, 5.02] |
| 8.1 Three months follow‐up | 2 | 135 | Mean Difference (IV, Random, 95% CI) | 1.90 [‐1.23, 5.02] |
2.2. Analysis.
Comparison 2 Local or regional anaesthesia for the prevention of persistent postoperative pain (classical analysis), Outcome 2 PPP after cardiac surgery.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Albi‐Feldzer 2013.
| Methods | Triple‐blinded (participant, provider, outcome assessor) clinical RCT Assignments were computer‐generated Follow‐up: 1 year |
|
| Participants | Participants: 260 women aged 18‐85 from 4 cancer hospitals in France Operation: breast cancer surgery (both breast‐conserving and mastectomy with or without axillary or sentinel node dissection) 2 groups, size: 117/119 Age (± SD): 56 (± 12), 57 (± 13) Men/women: 0/117, 0/119 Patient co‐morbidities: breast‐conserving surgery with axillary lymph node dissection, group 1, 2 (± SD) 53 (± 45.3), 62 (± 52.1), mastectomy with axillary lymph node dissection or sentinel lymph node dissection, group 1, 2 (± SD): 53 (± 45.3), 48 (± 40.3), mastectomy without axillary lymph node dissection or sentinel lymph node dissection, group 1, 2 (± SD): 11 (± 9.4), 9 (± 7.6) |
|
| Interventions |
Group 1 (ropivacaine): at end of surgery before suturing, 3 mL‐4 mL infiltration of 0.375% ropivacaine along each site of SC and deep layers of breast and axillary incisions, 2nd and 3rd intercostal space, humeral insertion of major pectoralis (received 3 mg/kg of 0.375% ropivacaine) Group 2 (saline): at end of surgery before suturing, 3 mL‐4 mL infiltration of saline along each site of SC and deep layers of breast and axillary incisions, 2nd and 3rd intercostal space, humeral insertion of major pectoralis (receive 0.8 mL/kg saline Both groups: premedicated with oral hydroxyzine (2 mg/kg) 1 h before surgery. GA induction with propofol, sufentanil, maintenance with nitrous oxide in O2, sevoflurane or desflurane, sufentanil bolus as required. Post‐op pain control with oral paracetamol and ketoprofen and rescue with morphine PCA for 24 h (bolus dose 1 mg on demand, lockout 5 min). Ondanestron 4 mg for nausea/vomiting +/‐ droperidol 1.25 mg every 8 h Adjuvants: none Immdiate post‐op pain control: significantly improved |
|
| Outcomes | Dichotomous: pain/no pain at 3 months only Continuous: BPI score at 3, 6, 12 months Other reported: neuropathic pain score, hospital anxiety and depression score at 3, 6, 12 months |
|
| Notes | For dichotomous pain, BPI score of ≥ 3 was used as cut off Funding sources: support was from institutional/departmental sources. The study author responded to our request that "Astra Zeneca only paid the insurance for the study and Astra Zeneca had no role in conceiving the study, designing the protocol, executing the trial and or analysing and interpreting the results" Conflicts of interest: there were no other conflicts of interest to report. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a balanced block stratified randomization scheme was used for patient allocation. Stratification was performed on the basis of hospital and type of surgery (conservative or not). Patients were randomized in randomly permuted blocks of four or six patients in each striatum. Assignments were computer generated" |
| Allocation concealment (selection bias) | Low risk | Quote: [Assignments were] "maintained in sequentially numbered, opaque, sealed envelopes...the envelope was opened in an isolated room on the day of surgery, and patients were assigned to either the placebo group or the ropivacaine group" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "before induction of anaesthesia, an operating room nurse read the results of randomization to prepare the solution of normal saline or ropivacaine in identical syringes... The solution was prepared in an isolated room and the nurse did not have any further contact with the patient. No other physician or nursing staff member was aware of the contents" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "pain was evaluated by a nurse who was blinded to the treatment group". Patients filled out questionnaires at inclusion and 3 months, 6 months and 1 year after surgery to evaluate chronic pain |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24 participants were excluded after randomization because of withdrawal of consent or failure to meet inclusion criteria. The groups to which these belonged was not reported, but there were fairly equal numbers in those that were included and received treatment (117 vs 119). At 3 months, there were 6 participants who were lost to follow‐up or had missing outcome data in the ropivacaine group, and 11 participants lost to follow‐up or with missing BPI data in the placebo group. these are low numbers when compared to the total studied population, and fairly balanced and reasons are listed for each group. No report on the exact number of participants with missing data at 6 or 12 months' follow‐up, only states "The maximum percentage of missing data for each point (0, 3, 6, and 12 months) in both arms was less than 5% (range: 0%‐5%). ITT was performed |
| Selective reporting (reporting bias) | Low risk | The primary and secondary outcomes listed in the protocol were all reported. |
| Null bias | Low risk | Quote: "measurement of pain on the VAS showed lower scores at rest and during mobilization in the first 90 min after the end of surgery in the ropivacaine group than in the control group (P < 0.001)... Ropivacaine wound infiltration decreased immediate postoperative pain in the PACU and increased the percentage of pain‐free patients (VAS = 0) for the first 48h" |
Barkhuysen 2010.
| Methods | Double‐blinded, clinical RCT Randomization scheme not described Follow‐up: 1 year |
|
| Participants | Participants: 200 adults in a hospital setting in Nijmegen, Netherlands Operation: ICBG for cranio‐maxillofacial surgery 2 groups, size: 100/100 Age (range): 56 (21‐74), 57 (21‐80) Men/women: 25/31, 14/28 |
|
| Interventions |
Group 1 (bupivacaine): intraop: after wound closure, participants received a single dose of bupivacaine (10 cc of 2.5 mg/mL bupivacaine with 1:80.000 epinephrine) Group 2 (control): no intervention given Adjuvants: epinephrine Immediate post‐op pain control: no difference between VAS and post‐op NSAID use between groups |
|
| Outcomes | Dichotomous: pain/no pain questionnaire at 1 year Continuous: none Other reported: use of paracetamol (Acetaminophen) and ibuprofen after surgery, duration of surgery, blood loss, and length of incision Adverse events: perforation of the lateral cortex of the iliac crest, haematoma |
|
| Notes | Financial support statement: "none." Conflict of interest statement: "none declared" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization scheme was not described |
| Allocation concealment (selection bias) | Low risk | Quote: "for each patient an envelope was drawn" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel were not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of the outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "79 questionnaires were sent out . After exclusion of the incorrectly filled and nonreturned questionnaires, 58 remained for evaluation (59%)." |
| Selective reporting (reporting bias) | Low risk | No protocol available but all specified outcomes were reported on |
| Null bias | High risk | Quote: "No statistically significant differences in outcome were detected between these groups...". |
Baudry 2008.
| Methods | Quadruple‐blinded (participant, provider, surgeon, outcome assessor), randomized, placebo‐controlled clinical trial Sequence generation by random number tables Follow‐up: 1 year (effectively, in treatment group: 17 months, control group 15 months) |
|
| Participants | Participants: 96 women included (78 analysed), from 1 university hospital, Besancon, France Operation: breast cancer surgery (mastectomy and lumpectomy with sentinel node biopsy) 2 groups, size: 40/38 Age (groups 1, 2): 52.4 years (SD ± 11.2), 57.7 (SD ± 12.6) Only women |
|
| Interventions |
Group 1 (postsurgical breast infiltration): GA (sufentanil 0.3 µg/kg), at wound closure single‐shot local infiltration with ropivacaine (0.475%, 40 mL), post‐op: paracetamol (1 g, intravenously, every 6 h), ketoprofen (100 mg, intravenously, every 12 h) rescue analgesic (if VAS > 30/100) nalbuphine 0.2 mg/kg Group 2 (placebo postsurgical breast infiltration): GA (sufentanil 0.3 µg/kg), at wound closure single‐shot placebo infiltration with normal saline (40 mL), post‐op: paracetamol (1 g, intravenously, every 6 h), ketoprofen (100 mg, intravenously, every 12 h) rescue analgesic (if VAS > 30/100) nalbuphine 0.2 mg/kg Adjuvants: none reported Immediate post‐op pain control: analgesic rescue medication and VAS were not different between groups |
|
| Outcomes | Dichotomous: pain/no pain at 1 year (effectively at 17 months in the experimental and at 15 months in the control group) Continuous: McGill Questionnaire described, but results not reported Effective regional anaesthesia not reported, and treatment did not reduce the severity of immediate postoperative pain or the consumption of rescue pain medication |
|
| Notes | Article in French, extracted by authors Funding sources: none reported Conflicts of interest: no conflict of interest statement was provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized with the use of a “randomization table” |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomized “after inclusion”. Unclear how the allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “the anaesthetist in charge, the surgeon, the investigator were blinded”. "The anaesthetic was administered with the patients anaesthetized". “The solution was prepared by personnel not taking care of the patient”. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the investigator was blinded". “The solution was prepared by personnel not taking care of the patient". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Significant attrition due to post hoc exclusion/lost participants and lost data that were reported but not analysed with ITT. Unclear how many participants were initially randomized to which group, hence attrition cannot even be assessed. Participants initially excluded for missing data were later included for the 1‐year analysis. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes fully reported on |
| Null bias | High risk | Quote: "au cours des 24 premières heures postopératoire, l'EVA a varié significativement au cours du temps...sans différence significative entre les deux groupes... Le nombre de patientes ayant eu recours au traitement antalgiue de secours et la dose de nalbuphine consummée n'était pas statistiquement différente entre les deux groupes". Analogical visual scale pain score, antalgic consumption were similar between groups |
Bell 2001.
| Methods | Double‐blinded (participants, outcome assessors), placebo‐controlled, clinical RCT Sequence generation randomized but not described Follow‐up: 6 months |
|
| Participants | Participants: 8 adults in a university setting in Bergen, Norway Operation: bilateral reduction mammoplasty 2 groups, size: 8/8 Age: 28.5 years (range 18‐34) Men/women: 0/8 Remarks: body sides, not participants randomized |
|
| Interventions |
Breast group 1 (preop infiltration): GA (fentanyl), preincision: infiltration with lidocaine (0.5%, 100 mL with epinephrine 5 µg/mL), post‐op as needed ketobemidone (oral, 5 mg) and paracetamol (1000 mg 3 x daily) Breast group 2 (placebo): GA (fentanyl), preincision: infiltration with normal saline (100 mL with epinephrine 5 µg/mL), post‐op as needed ketobemidone (orally, 5 mg) and paracetamol (1000 mg 3 x daily) Adjuvants: none Immediate post‐op pain control: significantly improved in treated breasts |
|
| Outcomes | Dichotomous: pain at 6 months Continuous: none reported Secondary: thermal thresholds were reported as tables, touch allodynia, or hyperalgesia |
|
| Notes | Some details, reported as graphs, are difficult to compare and extract. We acknowledge the study author's response regarding sources of funding and conflict of interest statement. Funding sources: the author informed us that this was an investigator‐initiated study, supported by an unrestricted grant from Astra Zeneca initially to study the effects of ropivacaine. When the study authors could not obtain approval to study this drug, the company maintained their support. The study author wrote that "the results were analysed with the help of a statistician at Astra Zeneca... we were allowed to keep the equipment... and that Astra financed my travel to a conference..." Conflicts of interest: the author had "no conflict of interest... and did not receive any [other] salary or economic compensation from Astra Zeneca." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients’ breasts were randomized to test and control groups”, but the method was not described in detail. |
| Allocation concealment (selection bias) | Unclear risk | Efforts to conceal allocation were not described. Bias is rather unlikely, because body sides, not participants were randomized. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the procedure was performed double blind", however blinding of participants and personnel not explicitly described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "the procedure was performed double blind", however outcome assessor blinding not explicitly described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and attrition reported as none, except one participant excluded for drug spillage. With only one withdrawal, body parts randomized not participants, even though no ITT analysis was performed, bias seems unlikely. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "some details, reported as graphs, are difficult to compare and extract" |
| Null bias | Low risk | Quote: "the sum of VAS scores for pain intensity was significantly lower in the lidocaine group than in the placebo group for the entire registration period of 10 h after wound closure" |
Besic 2014.
| Methods | Double‐blinded (patient/outcome assessor), RCT Sequence generation by a computer‐based, random numbers generator Follow‐up: 3 months |
|
| Participants | Participants: 120 women in a hospital setting in Ljubljana, Slovenia Operation: axillary lymphadenectomy and breast reconstruction Groups, size: 60/60 Age (lymphadenectomy, reconstruction): 60, 48 All female participants Comorbidities: none |
|
| Interventions |
Group 1 (levobupivacaine): intraop: before wound closure, a fenestrated wound catheter was placed under the pectoralis major muscle and upon the entire length over the upper side of the wound. The wound catheter was fenestrated along 15 cm in the distal part. A bolus of 15 mL of 0.25% levobupivacaine was injected into the wound through the catheter immediately after wound closure. Surgical drains and the fenestrated catheter were clamped for 5 min to enable bolus absorption. Elastomeric pump was connected containing 100 mL of 0.25% levobupivacaine. Infusion at 2 mL/h was continuous for 50 h. Group 2 (piritramide): intraop: continuous intravenous infusion with piritramide (30 mg), metoclopramide (20 mg) and metamizole (2.5 g) in 100 mL of 0.9% sodium chloride (3 mL/h‐6 mL/h) until 24 h postoperatively Adjuvants: none Immediate post‐op pain control: significantly improved, significantly reduced analgesic consumption |
|
| Outcomes | Continuous: none Dichotomus: overall pain/no pain at 3 months No adverse events reported |
|
| Notes | Study characteristics and data combined with Strazisar 2014. Axillary lymphadenectomy and breast reconstruction performed on 60 participants per procedure. Results from both procedures were combined to best represent pain outcomes. Funding sources: financial support was not described. Conflicts of interest: no conflict of interest statement was provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the research nurse performed randomization using random numbers generated by a computer..." |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization and numbers were placed in sealed opaque envelopes to ensure concealment of allocation at enrollment" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "participants were randomly grouped" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "clinicians who recorded data about chronic pain were blinded about randomisation group of patients." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the follow‐up evaluation. |
| Selective reporting (reporting bias) | Low risk | No subgroup analysis or selective reporting was noted. |
| Null bias | Low risk | Quote: "a smaller portion of patients treated with local anesthetics had chronic pain in comparison to the control group." "Chronic pain three months after operation is less frequent in the test group." |
Blumenthal 2005.
| Methods | Triple‐blinded (participant, provider, outcome assessor) randomized placebo‐controlled clinical trial Sequence generation via randomized list Follow‐up: 3 months |
|
| Participants | Participants: 36 adult participants at a university clinic in Zurich, Switzerland Operation: Bakart repair for shoulder instability using autogenous bone graft, harvested from iliac crest 2 groups, size: 18/18 Age (± SD), group 1, 2: 25 (± 5), 26 (± 4) Men/women, group 1, 2: 14/4, 13/5 Comorbidities: none reported Remarks: autogenous bone harvested through lateral oblique incision just cephalic to anterior iliac crest using classical surgical technique |
|
| Interventions |
Group 1 (ropivacaine): at end of surgery, bolus of 30 mL ropivacaine 0.5% via iliac crest catheter and in PACU, continuous infusion 0.2% ropivacaine at 5 mL/h started, continued for total of 48 h. Group 2 (placebo): at end of surgery, bolus of 30 mL saline via iliac crest catheter, in PACU, continuous infusion saline 5 mL/h started, continued for total of 48 h. Both groups: premedicated with midazolam 1 h before arrival to induction room, and interscalene brachial plexus block performed. GA with propofol, rocuronium and fentanyl. Autogenous bone harvested through lateral oblique incision cephalad to anterior iliac crest using classical surgical technique. Catheter placed in direct contact with self‐resorbing foam pad dressing touching bone, tunnelled and secured to skin using sutures and adhesive dressing. In PACU, all participants also received continuous interscalene analgesia with 0.2% ropivacaine at 10 mL/h 6 h after initial block. Both groups got IV PCA containing 1 mg/mL morphine, 2 mg dose lockout interval 15, no baseline, or 4 h limit, with 2 mg IV morphine top up by nurse for VAS > 30. After discharge, 25 mg oral rofecoxib/d and 2 mg oral paracetamol as needed during 3 weeks post‐op Adjuvants: none Immediate post‐op pain control: pain significantly lower at the iliac crest donor site at rest (except at t40 h) and during motion (except at t48 h) in the ropivacaine group with significantly decreased morphine consumption at 24 h and 48 h. |
|
| Outcomes | Dichotomous: none Continuous: VAS at rest and on motion at iliac crest at 3 months Other reported: post‐op pain at shoulder and presence of numbness/paraesthesias/neurologic damage at 3 months Adverse events: post‐op nausea/vomiting, pruritis, inflammation at catheter site |
|
| Notes | Interscalene block performed in both groups. Comparison of interest is ropivacaine vs placebo continuous infusion at iliac crest donor site. Funding sources: "support was provided solely from institutional and/or departmental sources." Conflicts of interest: no conflict of interest statement was provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were given a number between 1 and 36...according to a randomization list" |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were given a number between 1 and 36 by choosing a sealed envelope containing a number.. Each patient’s number was passed on to a pharmacist, who prepared the anaesthetic set (bolus and maintenance package) of either ropivacaine or placebo, according to a randomization list" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind study". Participants, block performers/anaesthesiologists, post‐op providers all blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all the patients were observed independently by a surgeon and an anaesthesiologist 3 months after surgery to assess the pain (anaesthesiologist) at rest and during motion at the operated IC and operated shoulder". Only pharmacy was aware of contents of anaesthetic set based on randomization list. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "all patients completed the study. All interscalene catheters were successfully placed, and no disconnection or other technical problems were encountered during the course of the study" |
| Selective reporting (reporting bias) | Low risk | Primary outcomes fully reported on |
| Null bias | Low risk | Quote: "pain was significantly lower at the donor site at rest (except at t40hrs) and during motion (except at t48hrs) in the ropivacaine group" |
Bollag 2012.
| Methods | Triple‐blinded (participant, provider, outcome assessor) RCT Sequence generation with computer‐generated list of random numbers Follow‐up: 12 months |
|
| Participants | Participants: 90 healthy non‐labouring pregnant women from Maternity Hospital in Sao Paulo, Brazil Operation: caesarean delivery, scheduled (under SA with Pfannenstiel incision) Three groups, size: 30/25/26 Age (± SD), group 1, 2, 3: 30.5 (± 6.7), 31.8 (± 4.5), 29.5 (± 6.7) Only female participants Comorbidities: previous caesarean delivery (%), group 1, 2, 3: 46/48/35. Gestational age in weeks, mean (± SD), group 1, 2, 3: 38 (± 1), 38 (± 1), 38 (± 1.5) |
|
| Interventions |
Group 1 (placebo/control): TAP block with 20.5 mL 0.9% NaCL per side. Group 2 (bupivacaine TAP): TAP block with 20 mL bupivacaine 0.375% + 0.5 mL NaCl 0.9% per side. Group 3 (bupivacaine + clonidine group): TAP block with 20 mL bupivacaine 0.375% + 75 µg (0.5 mL) clonidine per side All TAP blocks were performed in PACU within 1 h post‐op All groups: spinal anaesthetic with 12 mg hyperbaric bupivacaine, 25 µg fentanyl, 100 µg morphine. IV ketoralac at skin closure. Post‐op analgesia: in PACU, IV morphine as needed; in postpartum unit paracetamol (1 g every 6 h standing) and diclofenac (75 mg every 8 h standing), with tramadol 50 mg as needed Adjuvants: clonidine (group 3 only) Immediate post‐op pain control: significantly reduced morphine use in TAP groups compared to placebo in PACU but no change in resting pain scores. Effective regional anaesthesia: reported. "Block success and dermatomal extent of the sensory analgesia were assessed bilaterally by pinprick after recovery from the spinal anaesthetic". |
|
| Outcomes | Dichotomous: pain/no pain at 3, 6, 12 months Continuous: short‐form McGill Pain questionnaire at 3, 6 and 12 months |
|
| Notes | We contacted the study author who provided dichotomous pain data for 3, 6, and 12 months' follow‐up. Funding sources: no financial support was received for the study. Conflicts of interest: "the authors declare no conflict of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer‐generated list of random numbers was used (www.randomizer.org) for group allocation of the participants". |
| Allocation concealment (selection bias) | Low risk | Quote: "each woman was assigned a study number upon enrolment and received a TAP block with the corresponding numbered syringe. The allocation sequence was concealed from investigators and patients". While it does not state method with which allocation was concealed, it states it was concealed thus little risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "an investigator with no clinical involvement in the trial prepared the solutions following exact preparation guidelines. All syringes were labelled with the amount and concentrations of all possible contents, as well as a study number. Both operator [who performed TAP block] and patient were blinded to the study group." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "hyperalgesia was evaluated by the same research investigator (who was not involved in placement or evaluation of the TAP blocks in the PACU)". "At 3, 6, and 12 months, telephone interviews were performed to assess development of chronic postoperative pain using the Short‐Form McGill Pain Questionnaire 2 (SF‐MPQ‐2)". While it does not explicitly state chronic pain assessment was performed by a blinded investigator, based on the other descriptions of how participants were assigned to groups and blinding was maintained, it seems very unlikely the telephone interviewers knew which group they were assigned to. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "five women from [group 2] and 4 women from [group 3] were excluded from the study because of block failure (absence of sensory block on the abdomen assessed by pinprick after recovery from the spinal anesthetic)". No ITT analysis was performed, only per‐protocol. Flow diagram depicts loss of follow‐up for each group at 3‐, 6‐, 12‐month periods, with 2 participants in the control, 6 participants in [group 2] and 5 participants in [group 3] lost at 12 months, and fewer in each group at 3 and 6 months. SF‐36 survey reports "return rate" at each time point in terms of percent but does not provide raw numbers. Discordance between flow diagram and numbers included in analysis in neuropathic pain descriptors (table 4) |
| Selective reporting (reporting bias) | Low risk | Protocol reviewed and primary outcomes fully reported on |
| Null bias | High risk | Quote: "the incidence of wound hyperalgesia and the WHI were similar among groups at 24 hours (Fig. 2). At 48 hours, the incidence of wound hyperalgesia was not different among groups". |
Brown 2004.
| Methods | Triple‐blinded (participant, provider, outcome assessor) clinical RCT Sequence via computer‐generated list Follow‐up: 3 months |
|
| Participants | Participants: 100 men at university hospital in Minnesota, USA Operation: elective radical retropubic prostatectomy 2 groups, size: 50/49 (completed) Age ± SD (group 1, 2): 61.0 (± 7.5), 61.6 (± 7.0) All male participants Exclusion criteria: age < 35 or > 85 |
|
| Interventions |
Group 1 (control): after sedation, lumbar region injected with 1% lidocaine SC in one of lumbar interspaces between 2nd‐5th vertebral bodies. SC injection of sterile saline instead of intrathecal injection into subarachnoid space. Received IV fentanyl citrate bolus (4 µg/kg) immediately after induction, followed by continuous infusion (2 µg/kg/h) until fascial closure.
Group 2 (active intrathecal block): after sedation, lumbar region injected with 1% lidocaine SC in one of lumbar interspaces between 2nd‐5th vertebral bodies. Mixture of bupivacaine (15 mg isobaric, 0.75%), clonidine (75 µg), morphine (0.2 mg) injected into subarachnoid space. No intraoperative fentanyl in this group, rather equal volume of saline as a bolus and infusion. Both groups had sedation with IV fentanyl and midazolam. Standardized GA with sodium thiopental, succinylcholine, cisatracurium, isoflurane and nitrous oxide in O2. When study drug infusion discontinued, IV ketoralac 30 mg to both groups. Phenylephrine and ephedrine were used as needed to maintain an adequate blood pressure. In PACU, both groups treated with morphine (1 mg to 2 mg IV every 10 min as needed), droperidol for nausea, then naloxone if persisted diphenhydramine for pruritus initially then naloxone infusion if persisted. Once on the floor, postoperative pain management with scheduled Ketoralac (15 mg IV every 6 h x 6 doses), PCA morphine (1 mg bolus, 10‐min lockout, no basal infusion) for 24 h then oral paracetamol/codeine (650/30 mg) every 6 h as needed. Adjuvants: clonidine Immediate post‐op pain control: significantly improved, significantly reduced analgesic consumption |
|
| Outcomes | Dichotomous: pain/no pain at 3 months Continuous: numerical pain scale, SF‐36 at 3 months Other reported: none |
|
| Notes | Funding sources: not reported Conflicts of interest: no conflict of interest statement was provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by a "computer‐generated list that made assignments based on enrolment number" |
| Allocation concealment (selection bias) | Low risk | Quote: "assigned to a treatment group using a sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "patients and providers were masked to treatment assignments...To maximize masking of the study, a consulting anaesthesiologist familiar with the study but not responsible for the intraoperative care of the patient performed the regional procedure. During this time, the anaesthesiologist for the clinical conduct of anaesthesia left the operating room...the anaesthesia team was blinded to the identity of the bolus and infusion" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "patients and providers were masked to treatment groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant assigned to active block group had severe bradycardia after induction and surgery was cancelled. 3 participants in control group, 2 in active block group could not be reached at 12 weeks. Balanced numbers, low attrition rate, low risk of bias |
| Selective reporting (reporting bias) | Low risk | Primary outcomes fully reported on |
| Null bias | Low risk | Quote: "iIntrathecal analgesia improved current, least, and worst pain scores on the day of surgery and current and worst pain scores at 06:00 h the next day." |
Burney 2004.
| Methods | Single‐blinded (outcome assessor), clinical RCT Sequence generation by random number tables Follow‐up: 6 months |
|
| Participants | Participants: 34 adults in a university setting in Ann Arbor, Michigan, USA Operation: unilateral inguinal hernia repair 2 groups, size: 15/18 Age: not reported Men/women: not reported Remarks: recurrent hernias or bilateral hernias were excluded |
|
| Interventions |
Group 1 (spinal): spinal with lidocaine (5% with 7.5% dextrose, volume not reported), postincision: illio‐inguinal block with bupivacaine (0.5%, 8 mL to 10 mL), post‐op regimen not reported Group 2 (control): GA (fentanyl), postincision: illio‐inguinal block with bupivacaine (0.5%, 8‐10 mL), post‐op regimen not reported. Adjuvants: none Immediate post‐op pain control: significantly improved |
|
| Outcomes | Dichotomous: none reported Continuous: health status measured by SF‐36 at 6 months, but without randomization list |
|
| Notes | We contacted the study author for missing information on SF‐36 outcome. He provided original data and comments, but regretted that the randomization list was no longer available. Therefore the data could not be included. Funding sources: this study was supported by a grant from the Aetna Foundation, Hartford, Conn, USA. Conflicts of interest: no conflict of interest statement was provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was carried out using a blocked and balanced random number table." |
| Allocation concealment (selection bias) | Low risk | Quote: "a sealed opaque envelope with the randomization assignment was opened only after the patient had given informed consent for the study." The well‐described method makes bias unlikely. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and caregivers were not blinded, but this is acceptable. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinding was not reported, but participants filled out the questionnaire alone. Study author responded: "research assistants collecting the data were blinded as to experimental groups during initial data collection. All data collection was by questionnaire. Research assistants were present for early data collection, but at 6 months I think it was only by mail." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up reported, but not assigned to groups or outcomes. Initially 34 participants were recruited, but only 23 questionnaires were collected at 6 months. Participants erroneously assigned to the wrong group were analysed with ITT. Bias is likely due to the unclear group allocation of participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes fully reported on |
| Null bias | Unclear risk | Quote: "twelve (80%) of 15 patients in group 1 and 17 (94%) of 18 in group 2 received pain medication in the PACU (P = .3). In group 1, 10 (67%) of 15 patients received narcotic medication, and 6 (40%) of 15 patients received non‐ narcotic medication. In the group 2, 17 (94%) of 18 received narcotic medication, and 7 (39%) of 18 received nonnarcotic medication (P = .07 for narcotic medication; P > 0.99 for nonnarcotic medication)." No significantly decreased analgesic consumption in the PACU, however pain scores not reported. |
Can 2013.
| Methods | Double‐blind, clinical RCT Randomization using "the envelope method" but no report on sequence generation technique. Follow‐up: 6 months |
|
| Participants | Participants: 60 adult participants from university‐affiliated hospital in Turkey Operation: thoracotomy, elective 3 groups, size: 20/20/20 Age (± SD), group 1, 2, 3: 52.20 (± 17.05), 45.00 (± 17.46), 50.9 (± 16.12) Men/women, group 1, 2, 3: 15/5, 15/5, 15/5 Comorbidities: no concomitant disease |
|
| Interventions |
Group 1 (control): preoperative and intraoperative analgesia with 0.25 µg/kg/h to 0.60 µg/kg/h remifentanil infusion. No epidural analgesic medication before or during operation through epidural catheter Group 2 (incision‐sensitized): preoperative analgesia with 0.25 µg/kg/h to 0.60 µg/kg/h remifentanil infusion. 10 min after surgical incision, epidural admin 10 mL to 15 mL 0.1% levobupivacaine and remifentanil infusion then remifentanil continued for 20 more min for a total of 30 min then 10 mL 0.1% levobupivacaine epidural every 45 min Group 3 (pre‐emptive analgesia group): preop analgesia: 0.1% levobupivacaine 10 mL to 15 mL at 2nd dermatome superior and inferior to incision dermatome (between T4 to T10) through epidural catheter prior to induction. Intraop analgesia: 10 mL 0.1% levobupivacaine epidural injection every 45 min. In all groups epidural catheters were placed preoperatively at 6th‐7th or 7th‐8th thoracic intervals. All received the same GA regimen. Postoperatively all received morphine (3 mg) + fentanyl (50 µg) in 15 mL isotonic solution via epidural route at skin closure and every 12 h for 48 h Adjuvants: none Immediate post‐op pain control: not significantly improved |
|
| Outcomes | Dichotomous: pain/no pain at 3 and 6 months Continuous: VAS score 3 and 6 months Other reported: participant satisfaction levels at discharge and at month 6 |
|
| Notes | Presence of chronic pain defined as VAS score > 3. Epidural catheters were placed in all participants, and after placement a 3 mL test dose of 2% lidocaine with 1/200,000 adrenalin was injected. Thus, all participants did receive small amount of lidocaine via epidural catheter. We acknowledge the study author's response on allocation concealment, blinding, source of funding and whether there was any conflict of interest. Funding sources: response from study author, "the authors declare... [their] university... funded this study" Conflicts of interest: "the authors declare... that they have no conflict of interest to the publication of this article..." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized envelopes drawn "when patient come to operation room a staff get an envelope and open it", from study author |
| Allocation concealment (selection bias) | Low risk | On questioning, study author responded "Envelopes are opaque and include equal groups symbols. When patient come to operation room a staff get an envelope and open it." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind" study. When questioned, study author responded "The personal collecting the pain data was not involved in the previous study phases" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the outcome assessor collecting pain levels postoperatively and at 1, 3, 6 months was blinded" says the study author |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "2 patients from control group and 1 patient from preemptive analgesia group died and 1 patient from preemptive analgesia and other one patient from incision sensitized group wound infection were excluded" stated author. "New participants that were compliant with the inclusion criteria were enrolled." |
| Selective reporting (reporting bias) | Low risk | No protocol available but all specified outcomes were reported on. |
| Null bias | High risk | Table 3 demonstrates no significant difference in VAS scores between the 3 groups at hours 1, 4, 24 or 48 after surgery |
Chiu 2008.
| Methods | Triple‐blind (participant, provider, outcome assessor) placebo‐controlled, clinical RCT Sequence generation method not described Follow‐up: 3 months |
|
| Participants | Participants: 40 adults at a teaching hospital in New Taipei City, Taiwan Operation: minimally invasive cardiac surgery (coronary artery bypass performed through left thoracotomy via 4th or 5th intercostal space without cardiopulmonary bypass, valvular surgery through a right lateral thoracotomy via 4th intercostal space with cardiopulmonary bypass) 2 groups, size: 19/19 (actually completed) Age (± SD), group 1, 2: 57.4 (± 15.2), 59.7 (± 13.8) Men/women (group 1, 2): 12/7, 13/6 Remarks: 40 participants were randomized, but 2 were excluded, 1 per group, because of protocol violation Surgery type: coronary artery bypass/valve surgery (group 1, 2): 5/14, 6/13 |
|
| Interventions |
Group 1 (placebo): 10 mL saline infused via catheter at end of operation, continuous infusion saline 2 mL/h x 48 h Group 2 (thoracotomy wound infusion): 10 mL 0.15% bupivacaine infused at end of operation then continuous infusion 2 mL/h x 48 h Both groups had same GA regimen with etomidate, fentanyl, rocuronium and sevoflurane and multi‐orifice catheter placed at a SC layer during wound closure. Post‐op breakthrough analgesia for both groups with IV PCA (morphine 0.5 mg/mL, fentanyl 5 µg/mL, tenoxicam 0.8 mg/mL) basal infusion rate 0.1 mL/h, bolus 1 mL, lockout 15 min. After 72 h, oral or parenteral NSAIDs or opioids were used. Adjuvants: none Immediate post‐op pain control: significantly improved, significantly reduced analgesic consumption |
|
| Outcomes | Dichotomous: none Continuous: VAS Other reported: IV PCA consumption in first 72 h post‐op |
|
| Notes | Funding sources: source of funding not reported. Conflicts of interest: no conflict of interest statement given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned" but no description of method of randomization or at what time point it was done. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the nurse connecting the infusion bag to the catheter, the surgeons, the patient...were all blinded to the nature of the infusion". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The nurse evaluating the pain score was blinded to the nature of the infusion. Does not explicitly say, but likely the individual evaluating pain score at 90 days after was also blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant in each group was excluded as a result of "protocol violation (limited consciousness)". No ITT analysis was done. Did not report on the number of individuals assessed at 3‐month follow‐up time point (or if any lost to follow‐up) |
| Selective reporting (reporting bias) | Low risk | No protocol available but primary outcomes specified in paper were fully reported on |
| Null bias | Low risk | Quote: "not only did the bupivacaine wound infusion reduce pain during the first 48‐hour infusion period, but it also provided reduced pain at 24 hours after cessation of the infusion" |
Choi 2016.
| Methods | Placebo‐controlled, RCT Sequence generation not described Follow‐up for 3 months |
|
| Participants | Participants: 84 adults in a university setting in Korea Operation: robot‐assisted thyroidectomy 2 groups, size: 41/43 Age (± SD), group 1, 2: not described Men/women, group 1, 2: not described Exclusion criteria: not described |
|
| Interventions |
Group 1 (lidocaine): after induction of anaesthesia, participants received a bolus of 2 mg/kg of lidocaine intravenously followed by continuous infusion at a rate of 3 mg/kg/h during surgery. Further details of anaesthetic regimen were not provided. Group 2 (control): same as above except 0.9% saline was substituted for lidocaine. Adjuvants: none Immediate post‐op pain control: no improvement |
|
| Outcomes | Dichotomous: pain vs no pain Continuous: none Other reported: quality of recovery and pain scores during 24 h and 48 h postoperatively |
|
| Notes | Study published only as an abstract. We were unable to obtain additional information about methods, randomization or blinding methods from the study author. Funding sources: funding of study not described Conflicts of interest: conflicts of interest statement not provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were "randomly allocated" but no further description of sequence generation was included |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Degree of attrition not described |
| Selective reporting (reporting bias) | Unclear risk | Use of subgroup analysis not described |
| Null bias | High risk | Quote: "pain scores for 2 days after surgery were not different between the two groups." |
Comez 2015.
| Methods | Double‐blinded (participant, outcome assessor), RCT Sequence generation not described Follow‐up for 3 and 6 months |
|
| Participants | Participants: 60 adults in a university setting in Turkey Operation: thoracotomy 3 groups, size: 20/20/20 Age (± SD), group 1, 2, 3: 45.95 (18.248), 51.05 (19.324), 44.35 (19.712) Men/women, group 1, 2, 3: 10/10, 15/5, 11/9 Exclusion criteria: no concomitant systemic disease with functional limitations, ASA III‐IV |
|
| Interventions |
Group 1 (control): an epidural catheter was inserted using an 18 Ga. Tuohy needle with the help of the negative pressure hanging drop method from the levels of thoracic 6‐7 or thoracic 7‐8 in the preoperative period. Following the determination of epidural catheter, 2 mL 2% lidocaine was applied to cases as a test dose. No IV dexketoprofen and pre‐emptive epidural analgesic medication was applied to cases. Intraoperative analgesia was provided with 50‐100 mcg/h fentanyl citrate and O2/N2O 40% to 60% Pre‐oxygenation was provided for all cases with 6 L/min‐8 L/min 100% O2 (3‐5 min) Following 2 mg/kg propofol induction and the sufficient muscle relaxation that was provided with 0.6 mg/kg‐1 mg/kg rocuronium bromide, the cases were intubated using a double‐lumen endobronchial tube. The area of the endobronchial tube was confirmed with fibreoptic bronchoscopy. The maintenance of the anaesthesia was provided with 6%‐8% desflurane within 45% O2, between MAC 1 to1.5. During one‐lung ventilation (OLV), the amount of oxygen was increased according to the saturation of the case. 50 mcg/h fentanyl and O2 + 50%‐60% N2O were given for the analgesia in the intraoperative period. Dosage of the fentanyl was increased to 100 mcg/h during the OLV. At the end of the operation, 1.5 mg neostigmine and 0.5 mg atropine were applied for the antagonism of the muscle relaxant. Postoperative analgesia was provided with 3 mg morphine + 50 mcg fentanyl within 15 mL 0.9% NaCl through epidural catheter shortly before the operation while stitching the skin sutures. Analgesia of the cases was followed for 48 h and postoperative epidural analgesic fluid was applied at intervals of 12 h. When the VAS score became ≥ 3, an additional dose of postoperative epidural analgesic fluid was applied. Group 2 (pre‐emptive epidural): same GA technique used as above. 10 mL to 15 mL 0.125% levobupivacaine was given to cases in 5 mL with intervals of 5 min pre‐emptively through epidural catheter before the anaesthesia induction to provide the analgesia at two dermatome levels below and above the surgical incision dermatome (T4 to T10). Sufficiency of the analgesia was determined by performing hot‐cold test and the anaesthesia induction was then started. Intraoperative analgesia was provided with 10 mL 0.125% levobupivacaine injection, which was repeated every 60 min through epidural catheter. Group 3 (pre‐emptive epidural and dexketoprofen): same GA technique as described for previous 2 groups. Levobupivacaine applied as described in group 2. In addition, 50 mg dexketoprofen trometamol was given within 100 mL 0.9% NaCl with IV infusion in 15 min, and it was finished 15 min before the surgical incision. Adjuvants: dexketoprofen, morphine, and fentanyl Immediate post‐op pain control: significantly improved |
|
| Outcomes | Dichotomous: pain vs no pain Continuous: VAS Secondary: participant satisfaction scores at 1, 3, 6 months, surgery duration, and VAS scores and frequency of pain at 1 h, 4 h, 24 h, 48 h, discharge, and 1 month |
|
| Notes | We were unable to obtain additional information about randomization and blinding methods from the study author. Funding sources: funding of study not described Conflicts of interest: study authors had no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation for randomization not described |
| Allocation concealment (selection bias) | Low risk | Quote: "the cases, who were not informed about which study group they were included in, were divided into 3 groups ... with the random envelope method" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Sham block was used, however the control group did not receive LA or sham saline loading |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assesors were masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no attrition |
| Selective reporting (reporting bias) | High risk | Epidurals that were not effective were excluded from the analysis. |
| Null bias | Low risk | Quote: "A statistically significant decrease was determined in the VAS score in Group PED ... compared to the other groups." |
Di‐Gennaro 2013.
| Methods | Data not available | |
| Participants | Participants: 80 women, ASA II, aged 30‐55, in Italy Operation: central quadrantectomy and reconstruction with Grisotti's inferior dermo‐glandular flap for retroareolar breast cancer 2 groups, size: 40/40 |
|
| Interventions |
Group 1 (tramadol): participants of group 1 were administered tramadol 100 mg/20 mL Group 2 (levobupivacaine): participants of group 2 were administered levobupivacaine 2.5% 20 mL Both groups: perioperative pain management was treated with paracetamol 1000 mg/100 mL postoperatively (3 times/d for 48 h) Adjuvants: none Immediate post‐op pain control: data not available |
|
| Outcomes | NRS data not available | |
| Notes | Multiple attempts to contact study author were not successful and thus we were unable to obtain results from study Funding sources: funding source not described Conflicts of interest: conflict of interest statement not given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Concelament of allocation was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel was not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data collection and outcomes not described |
| Selective reporting (reporting bias) | Unclear risk | Selective reporting not described |
| Null bias | Unclear risk | No results reported |
Dogan 2016.
| Methods | Double‐blinded (participant, outcome assessor), clinical RCT Sequence generation not described Follow‐up for 6 months |
|
| Participants | Participants: 81 adults in a university setting in Turkey Operation: coronary artery bypass graft 2 groups, size: 40/41 Age (± SD), group 1, 2: 64.18 (10.46), 60.22 (13.27) Men/women, group 1, 2: 31/9, 32/9 Exclusion criteria: allergy to any of the study medications, severe renal, pulmonary, liver, or endocrine systemic disease, a history of alcohol or drug abuse, a history of chronic pain, psychiatric problems, or difficulty in communication. During the postoperative period, participants who needed postoperative revision for haemostasis, who had haemodynamic instability or infections, or severe bleeding, or who died were also excluded |
|
| Interventions |
Group 1 (parasternal block): anaesthesia was induced by etomidate 0.2‐0.5 mg/kg and fentanyl 3 µg/kg in addition to rocuronium 0.9 mg/kg for tracheal intubation. For maintenance of anaesthesia, desflurane 1 MAC, remifentanyl infusion (0.25 µg/kg/min) and rocuronium (0.1 mg/kg/h) following induction was used in both groups. The participants were ventilated with a tidal volume of 6‐8 mL/kg, fraction of inspired oxygen (FiO2 ) of 50% in air, the respiratory rate was modulated to keep the end‐tidal carbon dioxide at normal values of 35‐45 mm Hg and adjusted to arterial PCO2 values, and a positive end‐expiratory pressure of 5 cm H2O was applied. Coronary artery bypass graft surgery was initiated with a sternotomy incision. The participants were anticoagulated with 300 U/kg of heparin to provide an activated clotting time (ACT) > 400 s. Cardiopulmonary bypass (CPB) was started following the cannulation of the aorta and the right atrium. Membrane oxygenators (Terumo Corporation, Tokyo, Japan) were primed with 1000‐1500 mL of Ringer’s lactate to maintain a hematocrit level of 26% ± 2%. A nonpulsatile pump flow was set at 2.2 to 2.4 L/min/m2 to maintain mean arterial pressure between 50 and 70 mmHg. CPB was performed at mild hypothermia with a core temperature of 33°C. Intermittent antegrade cardioplegia was used for myocardial protection. The participants were rewarmed to a temperature of 37°C. When the heart was paced in the atrioventricular sequential mode at a rate of 90 beats/min, the participants were weaned from CPB. Protamine sulfate was used to antagonize the heparin. Before sternal wire placement, sternotomy and mediastinal tube sites were infiltrated with 50 mL of study solution (levobupivacaine 25 mL (chirocaine, 50 mg/10 mL, Abbott Lab) + fentanyl 100 µg + 23 mL saline) by the surgeon. This mixture was infiltrated as follows: bilateral 5 costa levels (underside of them) and every level 2 mL on both sides of the sternum, over sternal periosteum 20 mL and the entrance of chest tubes deep infiltration 10 mL. At the end of the surgery, 1 g paracetamol and 1 mg/kg tramadol were given to all participants. At the end of the surgery, all anaesthetics were discontinued and participants were transferred to the intensive care unit (ICU) where they were mechanically ventilated. The participants were extubated if they met the following criteria: participant awake and responsive to commands, fully warmed with core temperature > 36°C, haemodynamically stable without significant dysrhythmias, well‐perfused with adequate urine output ( > 1.0 mL/kg/h), no active bleeding, respiratory rate 10‐30/min, SpO2 > 95 when 50% oxygen + air. Patients were to receive tramadol infusion with an intravenous PCA device for postoperative analgesia when they came to the ICU. The PCA device was set to deliver a 10 mg/h continuous dose and a 20 mg/h demand dose with a lock‐out interval of 30 min and with a maximum 4‐h limit of 200 mg for every participant. All participants were given additional IV NSAID. Group 2 (control): same anaesthetic regimen as described above except no LA was applied before sternal wire placement. Adjuvants: fentanyl Immediate post‐op pain control: significantly improved |
|
| Outcomes | Dichotomous: pain vs no pain Continuous: VAS Other reported: presence of allodynia, thermal pain, or dysesthesia, tramadol consumption, cross clamp time, duration of operation, left internal mammary artery harvested or not, duration of mechanical ventilation, haemodynamic parameters, VAS at 1, 2, 3, 4, 8, 24, and 48 h postoperatively. |
|
| Notes | We were unable to obtain additional information regarding continuous pain outcomes or about randomization and blinding methods from the study author. Pain on a dichotomous scale was defined as Leeds Assessment of Neuropathic Symptoms and Signs > 12 Funding sources: "no financial support was received for this study." Conflicts of interest: "the author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomly allocated by opening an envelope ... before the entry in the operating room." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of personnel not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "six months after surgery, an investigator who was blinded to acute pain treatment examined the patients' chronic pain." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost to follow‐up and ITT analysis was performed |
| Selective reporting (reporting bias) | Low risk | No subgroup analysis was performed |
| Null bias | Low risk | Quote: "parasternal block had a beneficial effect on the management of postoperative acute pain." |
Fassoulaki 2000.
| Methods | Triple‐blinded (participants, providers, outcome assessors) randomized placebo‐controlled clinical trial Sequence generation was randomized but not described Follow‐up: 3 months |
|
| Participants | Participants: 46 female participants at a university hospital in Athens, Greece Operation: modified radical mastectomy or lumpectomy and axillary lymph node dissection 2 groups, size: 23/22 (completed) Age ± SD (group 1, 2): 49 ± 6, 49 ± 8 All female participants Exclusion criteria: age > 60 years Remarks: participants undergoing modified radical mastectomy with axillary node dissection/lumpectomy (group 1, 2): 10/13, 7/15. Participants undergoing chemotherapy post‐op (group 1/2): 16/16. Participants undergoing radiotherapy post‐op (group 1/2): 13/8 |
|
| Interventions |
Group 1 (EMLA): 5 g EMLA to sternal area 5 min before induction. Immediately after extubation 5 g EMLA on supraclavicular area, 10 g around axilla (away from site of incision), then covered with Tegaderm. Same total dose of cream (20 g) applied daily on the 4 days after surgery. Group 2 (control/placebo): exactly the same as above, only placebo cream was used. Both groups received premedication with droperidol and metoclopramide and the same GA technique with thiopental and propofol, sevoflurane and nitrous oxide in O2 with rocuronium. No analgesics were given to either group during surgery. Post‐op analgesia in all participants: 75 mg propoxyphene and 600 mg paracetamol IM as needed x 24 h, then paracetamol oral or paracetamol/codeine oral ± hydroxyzine Adjuvants: propoxyphene Immediate post‐op pain control: no significant improvement in post‐op pain or analgesic consumption. Time to first analgesic requirement was significantly longer in EMLA group |
|
| Outcomes | Dichotomus: pain/no pain at 3 months (also broken down by site, including chest wall, arm, axilla) Continous: verbal intensity scale of 0 = no pain to 3 = severe pain at 3 months Other reported: absent/decreased sensation, home analgesic use at 3 months |
|
| Notes | We acknowledge the response by the study author providing details on allocation concealment, blinding, and sources of support and conflict of interest statement. Funding sources: study author replied, "the study was funded from Departmental sources only." Conflicts of interest: study author replied, "none of the authors has conflict of interest relevant to the study," |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized before induction of anesthesia using sealed opaque envelopes containing code A or B" |
| Allocation concealment (selection bias) | Low risk | Quote: study author responded "sealed opaque envelopes containing code A or B" were used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the EMLA or the placebo cream was applied by an anaesthesiologist who was not involved in patients' anaesthesia or data collection. All other anaesthesiologists, anaesthetic or ward nurses, as well as the patient, were not aware of the group of assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "an independent observer who was not involved in patient randomization or anaesthesia administration was assessing and recording pain scores" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant in the EMLA group with cutaneous allergy was excluded and not replaced. Otherwise no other participants lost. No ITT analysis was done, only per‐protocol. |
| Selective reporting (reporting bias) | Low risk | No protocol available for review but pre‐specified outcomes within manuscript were reported on. |
| Null bias | High risk | Quote: "The VAS scores at rest and after movement recorded 0, 3, 6, 9, and 24 h, as well as 2, 3, 4, 5, and 6 days postoperatively did not differ significantly between the 2 groups" |
Fassoulaki 2001.
| Methods | Double‐blinded, placebo‐controlled randomized clinical trial Sequence generation via "coded envelopes", but not explicitly described Follow‐up: 3 months |
|
| Participants | Participants: 100 adult women at a university hospital in Athens, Greece Operation: breast cancer surgery (modified radical mastectomy or lumpectomy + axillary node dissection) 4 groups, size: 23/24/25/24 (completed) Age, group 1, 2, 3,4 (SD not reported): 46, 46, 44, 44 All female participants Exclusion criteria: women over 59 years of age or those who received radiotherapy or chemotherapy preoperatively Number of participants who underwent modified radical mastectomy (group 1, 2, 3, 4): 8, 10, 11, 7. Number of participants who underwent radiotherapy post‐op (group 1, 2, 3, 4): 9, 9, 4, 12. Number of participants who underwent chemotherapy post‐op (group 1, 2, 3, 4): 18, 15, 23, 18 |
|
| Interventions |
Group 1 (ropivacaine and mexiletine): mexiletine 200 mg by mouth evening before surgery and 200 mg twice daily for first 6 post‐op days, brachial plexus infiltrated 12 mL ropivacaine 10 mg/mL and 6 mL 3rd‐5th intercostal spaces after axillary dissection. Group 2 (ropivacaine and placebo): placebo tablet oral evening before surgery and twice daily for first 6 post‐op days, brachial plexus infiltrated 12 mL ropivacaine 10 mg/mL and 6 mL 3rd‐5th intercostal spaces after axillary dissection. Group 3 (placebo and mexiletine): mexiletine 200 mg by mouth evening before surgery and 200 mg twice daily for first 6 post‐op days, brachial plexus infiltrated 12 mL saline and 6 mL 3rd‐5th intercostal spaces after axillary dissection. Group 4 (placebo and placebo): placebo tablet oral evening before surgery and twice daily for first 6 post‐op days, brachial plexus infiltrated 12 mL saline and 6 mL 3rd‐5th intercostal spaces after axillary dissection. All groups received IV metoclopramide and droperidol 5 min before induction. Standardized GA regimen with thiopental, propofol, recouronium, sevoflurane, nitrous oxide in O2. All groups received same post‐op analgesia regimen of 75 mg propoxyphene + 600 mg paracetamol IM every 5 h as needed x first 24 h then post‐op day 2, oral tablet of 10 mg codeine + 400 mg paracetamol every 5 h as needed. Adjuvants: mexiletine (2/4 groups), propoxyphene (4/4 groups) Immediate post‐op pain control: significantly improved, significantly reduced analgesic consumption in group 2 compared with all other groups |
|
| Outcomes | Dichotomous: pain/no pain at 3 months (also reported by site, including chest, axilla) Continuous: VAS at 3 months Other: absent/decreased sensation, analgesic use at 3 months |
|
| Notes | We acknowledge the response by the study author providing details on randomization, allocation concealment, blinding of participants, personnel and outcome assessors as well as sources of support and conflicts of interest. Funding sources: study author responded, "The study was funded from Departmental sources only." Conflicts of interest: study author responded, "None of the authors has conflict of interest relevant to the study," |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study author stated, "twenty five opaque envelopes were prepared for each group, each containing a note with [a] code...The night before surgery the anaesthesiologist pulled out one envelop from the bag containing the 100 envelops and according to the code inside administered to the patient the capsule from the jar with the same code" |
| Allocation concealment (selection bias) | Low risk | The study author stated: "twenty five opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study author stated: "patients surgeons and anaesthesiologists ALL were blinded except for an anaesthesiologist not participating in the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study author responded that the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "four patients failed to complete the protocol and were not replaced. Data are unavailable for chronic follow up of two others". Does not state which group specifically the participants belonged to, but can see the numbers of attrition in each group. Overall low numbers and fairly balanced. |
| Selective reporting (reporting bias) | Low risk | No available protocol but primary outcome specified in manuscript completely reported on |
| Null bias | Low risk | Quote: "regional block reduced the number of intramuscular (IM) injections required the first 24 hours (P = 05), the R +PL group requiring less injections versus the PL + M group (P = .037). Three hours postoperatively, the R +PL group had less pain at rest when compared with all other groups" |
Fassoulaki 2005.
| Methods | Double‐blind (participant, outcome assessor), placebo‐controlled, randomized clinical trial Sequence generation by computer‐generated random number tables Follow‐up: 6 months |
|
| Participants | Participants: 50 adults in a university setting in Athens, Greece Operation: breast surgery (modified radical mastectomy and lumpectomy plus axillary dissection) for breast cancer 2 groups, size: 25/25 Age (group 1, 2): 49 years (SD ± 8.4), 48 (SD ± 8.1) Men/women: 0/50 |
|
| Interventions |
Group 1 (multimodal): GA, brachial plexus irrigation with ropivacaine (0.75%, 10 mL), intercostal ropivacaine (0.75%, 3 mL) at intercostal spaces 3‐5, post‐op for 3 d topical (wound, sternum, axilla) EMLA cream (20 g, 2.5% lidocaine/prilocaine), codeine, paracetamol Group 2 (control): GA, brachial plexus irrigation with normal saline, sham intercostal block at intercostal spaces 3‐5, post‐op for 3 d topical (wound and axilla) placebo cream, codeine, paracetamol Adjuvants: Group 1: gabapentin (400 mg, orally every 6 h starting the night before surgery) for 8 d, Group 2: placebo as above Immediate post‐op pain control: significantly improved |
|
| Outcomes | Dichotomous: pain, analgesic consumption at 6 months Continuous: none reported Adverse effects, withdrawal and attrition were reported with group allocation. |
|
| Notes | We contacted the study author and we acknowledge the response, providing details on source of funding and conflict of interest. Funding sources: study author responded "the study was funded from Departmental sources only." Conflicts of interest: the study author responded "none of the authors has conflict of interest relevant to the study." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "fifty envelopes, 25 containing odd and 25 containing even numbers, obtained from a computer‐generated table, were prepared and sealed...," this is an adequate description of an acceptable randomization technique. Bias is unlikely. |
| Allocation concealment (selection bias) | Low risk | Quote: "an independent anesthesiologist, who did not participate in the study or data collection, read the number contained in the envelope and made group assignments." Bias is unlikely. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "except for the independent anesthesiologist, [not involved in the study] no other physician or nursing staff member was aware of the interventions administered to each patient." "Regarding EMLA cream and possible interference with blinding, EMLA or placebo was applied in the morning after pain assessment"... "pain was assessed by an anesthesiologist blinded to group assignment." "Placebo capsules were identical in appearance with the gabapentin capsules. The same number of capsules was packaged in group‐specific bottles and coded as bottle A and bottle B for the control and treatment groups, respectively. A white odourless cream was the control treatment corresponding to the EMLA cream. Similarly, cream for each group was kept in boxes labelled as A and B for the control and treatment groups, respectively." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "except for the independent anesthesiologist, (not involved in the study) no other physician or nursing staff member was aware of the interventions administered to each patient." "Pain was assessed by an anesthesiologist blinded to group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study authors provide a good account of attrition, including group allocation, but considered no ITT analysis: dropouts, participants lost to follow‐up, failures, etc were all excluded. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes fully reported on |
| Null bias | Low risk | Quote: "the treatment group consumed less paracetamol in the PACU... and fewer Lonalgal® tablets... than the controls, exhibited lower visual analog scale scores at rest in the PACU... and on postoperative Days 1, 3, and 5" |
Fassoulaki 2016.
| Methods | Triple‐blind (participant, provider, and outcome assessor), placebo controlled, randomized clinical trial Sequence generation by computer‐generated random number tables Follow‐up: 3 months |
|
| Participants | Participants 110 adults in a university setting in Greece Operation: laparoscopic cholecystectomy 2 groups, size: 55/55 Age (± SD), group 1, 2: 51 years (11.2), 48 (SD ± 12.5) Men/women, group 1, 2: 17/38, 14/41 Exclusion criteria: central nervous system, kidney, or liver disease, chronic pain, or consumption of analgesics and/or calcium channel blockers during the last month |
|
| Interventions |
Group 1 (ropivacaine): premedication was omitted in all cases. In the operating room an 18‐G catheter was inserted in a peripheral vein on the dorsum of the left hand and metoclopramide 10 mg, ranitidine 50 mg, and droperidol 0.75 mg were injected IV before induction of anaesthesia. Pulse oximetry, electrocardiogram, noninvasive blood pressure, inspired and end tidal oxygen concentration, capnography, inspired and end tidal sevoflurane concentration, and neuromuscular block were monitored (Datex Ohmeda S/5TM, Anesthesia Monitor, Helsinki, Finland) (Multistim VARIO, Pajunk, Geisingen, Germany). Participants were preoxygenated for 3 min. Thiopental (5‐6 mg/kg) and fentanyl (2 mg/kg) were administered to induce anaesthesia, followed by rocuronium (0.6 mg/kg) to facilitate tracheal intubation. Anaesthesia was maintained with sevoflurane 2%‐3% inspired concentration in an oxygen nitrous oxide mixture of 1:1 L/min. Diclophenac (75 mg IV) was infused slowly within 30 min before pneumoperitoneum. After induction of anaesthesia and before beginning the operation the surgeon inserted SC a “PAINfusor” multihole catheter 75 mm long (PLAN 1 Health, Baxter, Amaro‐UD, Italy) below and parallel to the subcostal area under aseptic conditions. The catheter was connected to a 130 mL elastomeric pump (Baxter Health‐Care Corporation, Deerfield, IL) delivering fluid at 2 mL/h. The pump was filled with 48 mL of 0.75% ropivacaine under sterile conditions by an anaesthetic nurse not participating in the study and having access to the randomization sets. The infusion was maintained for the first 24 h. Laparoscopic cholecystectomy using the 4‐port technique was performed by the same surgeon in all participants. During the pneumoperitoneum the intra‐abdominal pressure ranged between 12 and 14 mmHg. The total amount of CO2 used was recorded. At the end of the procedure each of the 4 holes was infiltrated with 2 mL of ropivacaine 0.75%. After skin closure residual neuromuscular block was reversed with sugammadex (2 mg/kg), and the participant was extubated and transferred to the PACU. In the PACU, the participants were asked to score their pain using the VAS and received paracetamol IV 1 g if VAS was > 40 mm or if the participant asked for analgesia. If paracetamol was not effective then tramadol (100 mg IV) was administered. Participants who experienced vomiting were given ondansetron 4 mg IV. During the first 48 h postoperatively participants were given paracetamol (400 mg) and codeine (10 mg) (Lonarid tablets) on demand or when the VAS scores exceeded the 40 mm in the VAS 100 mm scale. If the participant experienced nausea/vomiting, then ondansetron (4 mg IV) was given. Group 2 (control): the same intervention as above was used except 0.9% saline was substituted for ropivacaine Adjuvants: none Immediate post‐op pain control: no difference |
|
| Outcomes | Dichotomous: pain vs no pain Continuous: VAS scores Other reported: pain at rest and pain during cough recorded 2, 4, 8, 24, and 48 h postoperatively, paracetamol and tramadol consumption in the PACU and cumulative Lonarid tablets consumption during the first postoperative 48 h, incidence of shoulder pain |
|
| Notes | Funding sources: source of funding not stated Conflicts of interest: "the authors declare no conflicts of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was carried out by means of a computer‐generated table with 1 set of 55 numbers for the range 1‐110. In a second set the remaining 55 numbers were included corresponding to the control group. |
| Allocation concealment (selection bias) | Low risk | Each number for the ropivacaine and the control group remained unique. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The pump was filled with 48 mL of 0.75% ropivacaine or equal volume of saline 0.9% under sterile conditions by an anesthetic nurse not participating in the study and having access to the randomization sets." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Sham block was used to maintain blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were low and ITT analysis was performed. |
| Selective reporting (reporting bias) | Unclear risk | Not discussed |
| Null bias | Low risk | Quote: "Subcutaneous ropivacaine ...was associated with less pain in the PACU and 4 hours after surgery." |
Gacio 2016.
| Methods | Triple‐blind (participant, provider, outcome assessor), clinical RCT Sequence generation was randomized but not described Follow‐up: 6 months |
|
| Participants | Participants: 80 participants at a university hospital in Portugal Operation: lumpectomy with axillary dissection, modified radical mastectomy (MRM), and mastectomy with or without axillary dissection 2 groups, size: 40/40 Age (± SD), group 1, 2: 55.10 (9.8), 52.68 (8.9) All women Exclusion criteria: allergy to NSAIDs, LAs, propofol, opioids, paracetamol, or antiemetics, participants on chronic treatment with antibiotics, obesity (BMI > 30), bilateral or multiple surgical procedures, contraindication to PVB (including coagulation disorders/anatomical changes), severe respiratory disease, pregnancy, inability to understand the VAS |
|
| Interventions |
Group 1 (ropivacaine PVB): before the induction of anaesthesia, peripheral route catheterization was performed, and participants were monitored according to ASA standards and bispectral index (BIS) anaesthetic depth. PVB was performed with single‐injection, according to the classic technique at the T4 level with Tuohy needle 18 G, with 0.5% ropivacaine + adrenaline 3 g/mL, with a volume of 0.3 mL/kg (maximum total volume of 30 mL). Subsequently, anaesthesia was induced with propofol (1.5 mg kg−1 h−1) and fentanyl (2 g kg−1) and LMA was inserted. Anaesthesia was induced with propofol (1.5 mg kg−1 h−1) and fentanyl (2g kg−1) and LMA was inserted. The maintenance of anaesthesia was performed in both groups with desflurane to maintain BIS values at 45‐60 with a mixture of O2/air. Both groups received parecoxib 40 mg IV before the start of surgery. During maintenance, fentanyl (1.5 g kg−1) was administered if there was an increase of 20% from baseline values of mean arterial pressure (MAP) and heart rate (HR). For maintenance of haemodynamic stability, ephedrine or atropine was administered, at the anaesthesiologist’s discretion, if verified a decreased in MAP > 20% or HR < 50 beats/min of baseline values. The institutional protocol for the prevention of nausea and vomiting was administered, according to the predictive model by Apfel and colleagues, with three antiemetic intervention lines. At the end of surgery, PCA with morphine was initiated, programmed with bolus of 2 mg on demand and 5 min lock‐out and a maximum dose of 6 mg h−1 during the first 24 h postoperatively. Group 2 (general anaesthesia): same anaesthetic technique as above but no PVB was administered Adjuvants: parecoxib, fentanyl, morphine, and adrenaline Immediate post‐op pain control: significantly improved |
|
| Outcomes | Dichotomous: pain vs no pain Continuous: none Other reported: anxiety was assessed using the Hospital Anxiety and Depression scale (HADS), pain at rest according to the VAS score (0‐10), as well as pain with mobilization of the ipsilateral arm interpreted as 90° arm abduction 0 h, 1 h, 6 h, and 24 h after surgery, postoperative nausea and vomiting at 24 hours after surgery |
|
| Notes | Pain defined as DN4 score > 4 We acknowledge the study author's response regarding blinding and randomization technique. Funding sources: funding for the study was not described. Conflicts of interest: "the authors declare no conflicts of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study author responded, quote: "a stratified randomization was performed using Excel software for that purpose." |
| Allocation concealment (selection bias) | Low risk | The study author responded, quote: " in this study the anesthesiologist who proceeded to the technique became aware of the randomization sequence (in groups of 4 patients) the same day of the procedure." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study author responded, quote: " the surgical team did not know the group to which the patient belongs." However, "In the first part of the study (assessment of acute pain in the peri‐operative and up to the first 24 hours) the anesthesiologist who proceeded to the technique knew in which group the patient was." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study author responded, quote: "the investigator who interviewed the patients and carried out the records in the peri‐operative period.did not know the group to which the patient belongs." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14 participants were not included in the final analysis |
| Selective reporting (reporting bias) | Low risk | No subgroup analysis was performed |
| Null bias | Low risk | "The Visual Analog Scale (VAS) values of paravertebral group at rest were lower throughout the 24 h of study" |
Grigoras 2012.
| Methods | Triple‐blind (participants, providers, outcome assessors) randomized controlled study Sequence generation by computer‐generated codes Follow‐up: 3 months |
|
| Participants | 36 participants at Cork University Hospital in Cork, Ireland Operation: mastectomy or wide local excision + axillary node dissection, including sentinel node 2 groups, size: 17/19, all women Age (± SD): 55.9 (± 10.4), 56.8 (± 14.4) |
|
| Interventions |
Group 1 (lidocaine group): immediately after intubation, IV bolus lidocaine (1.5 mg/kg in 10 min) followed by continuous IV infusion (1.5 mg/kg/h), stopped 60 min after skin closure. Group 2 (control group): immediately after intubation, IV bolus saline followed by continuous IV infusion of saline, stopped 60 min after skin closure. Neither group received preanaesthetic medication. Both groups had the same GA protocol, including propofol and fentanyl for induction, sevoflurane and nitrous oxide in O2 for maintenance. The remaining analgesic regimen was identical between groups, including intraoperative paracetamol 1 g and diclofenac 75 mg IV with morphine as needed and postoperative morphine PCA (1 mg max every 5 min), diclofenac (50 mg oral/rectal every 12 h as needed), paracetamol (1 g oral/rectal every 6 h as needed), tramadol (100 mg IM/oral as needed as rescue) Adjuvants: none Immediate post‐op pain control: improved |
|
| Outcomes | Dichotomous: pain/no pain at 3 months Continuous: short form McGill Pain Questionnaire (SF‐MPQ) at 3 months Other reported outcomes: measurement of area of peri‐incisional hyperalgesia, pain catastrophizing scale at 3 months post‐op (broken down by question), Hosptial Anxiety and Depression scale at 3 months post‐op |
|
| Notes | Funding Sources: source of funding not stated Conflicts of interest: "the authors declare no conflict of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly allocated to 1 of 2 groups based on computer generated codes" |
| Allocation concealment (selection bias) | Low risk | Codes were, quote: "maintained in sequentially numbered opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "on the morning of surgery an anaesthetist who was not involved in the patient’s evaluation opened the envelope and prepared either 1% lidocaine or normal saline in coded 50mL syringes. None of the investigators involved in patient management or data collection were aware of the group assignment...The anaesthetist, surgeon, and nursing staff were all blinded to the group allocations" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"a dedicated investigator, unaware of the patients’ group assignment" performed the outcome assessments. "None of the investigators involved in patient management or data collection were aware of the group assignment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts; all participants randomized were included in the final analysis at 3 months. |
| Selective reporting (reporting bias) | Low risk | A post‐hoc analysis of preoperative factors comparing participants who did and those who did not develop persistent postsurgical pain was done, but this was specified. The rest of listed outcomes were all reported. |
| Null bias | Low risk | Quote: "VAS pain scores at rest, 4 hours postoperatively were less in lidocaine group compared with control group" |
Gundes 2000.
| Methods | Triple‐blind (participant, provider, outcome assessor) clinical RCT Sequence generation was randomized but not described Follow‐up: 3 months |
|
| Participants | Participants: 45 participants (no age requirement) at a university hospital in Kocaeli, Turkey Operation: iliac crest bone harvesting (surgical procedures included vertebral fusion, fracture grafting and grafting for tumour resection) 3 groups, size: 15/15/15 Age (range), group 1, 2, 3: 46 (16‐70), 48 (18‐71), 51 (19‐73) Men/women, group 1, 2, 3: 5/10, 6/9, 6/9 Comorbidities: vertebral fusion (n), group 1, 2, 3: 6, 5, 6. Fracture grafting (n), group 1, 2, 3: 6, 7, 7. Tumour grafting (n), group 1, 2, 3: 3, 3, 2. |
|
| Interventions |
Group 1 (control): 20 mL of 0.9% sodium chloride solution via iliac crest catheter within 10 min after surgery Group 2 (bupivacaine only): 20 mL of 0.9% NaCl with 50 mg bupivacaine via iliac crest catheter within 10 min after surgery Group 3 (morphine‐bupivacaine group): 20 mL of 0.9% NaCl solution with 5 mg morphine and 50 mg bupivacaine via iliac crest catheter within 10 min after surgery. All groups: standardized general anaesthesia with thiopental, vecuronium, N2 in O2 and isoflurane. Regional infusions via fine bore epidural catheter at iliac crest donor site, tip between muscle and bone at lateral surface of ilium, started 10 min after surgery. Post‐op pain control: participants requested reinjection of LA at iliac crest when donor site became painful (5 mL 0.9% NaCl with 12.5 mg bupivacaine), morphine PCA 1 mg bolus, 5 min lockout, 4‐h limit 20 mg Adjuvants: none Immediate post‐op pain control: significantly improved, significantly reduced analgesic consumption |
|
| Outcomes | Dichotomous: pain and dysaesthesia vs none at 3 months post‐op Continuous: none Other reported: none |
|
| Notes | Postoperatively, all participants in all groups received reinjection of LA (5 mL NaCl and 12.5 mg bupivacaine) into iliac crest when donor site became painful. Thus, control group did receive some bupivacaine in post‐op period. Average number of injections received reported by group. We acknowledge the response provided by the study author regarding blinding, randomization, allocation concealment and source of funding and conflict of interest statement. Funding sources: the study author reports the study was "not funded by any kind of resource." Conflicts of interest: "the authors have no conflict of interests of any kind (financial, commercial or otherwise)." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study author responded that he "did a simple randomization; as every second patient was included in group two; every third patient was included in group three, then reversing it as every fourth patient in group three, every fifth patient in group two, every sixth patient in group one; and so on". He did not mention this to his collaborators and he did not perform or attend any surgeries in the study. He did not mention his randomization technique to the other collaborators |
| Allocation concealment (selection bias) | Low risk | Study author responded, quote: "all the medications had been prepared by senior anesthesiology resident, according to me or my chief residents' instructions. All were prepared in 50 cc identical syringes without any label" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study author responded they, quote: "blinded both the patients and anaesthesiologists" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study author states "Dr L.K (anaesthesiologist) did the postoperative (24 hour) evaluation of the patient including VAS score without knowing the group of the patient. He also evaluated patients 12 weeks after the surgery, also without knowing the group of the patient. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes |
| Null bias | Low risk | Quote: "the VAS score, analgesic consumption and request for reinjection of local anaesthetic into the donor site in the early postoperative period (24th hour) were significantly higher in the control group than in the other two study groups" |
Gupta 2006.
| Methods | Triple‐blinded (participants, providers, outcome assessors) randomized placebo‐controlled trial Sequene generation by computer‐generated randomized numbers Follow‐up: 3 months |
|
| Participants | Participants: 60 men from a university hospital in Orebro, Sweden Operation: radical retropubic prostatectomy (for prostatic cancer) 2 groups, size: 28/28 (completed) Age (± SD), group 1, 2: 64.5 (± 4.9), 61.1 (± 4.3) All male participants Exclusion criteria: age > 70 Remarks: Gleason score, median (range), group 1, 2: 6 (5‐9), 6 (5‐9) |
|
| Interventions |
Group 1 (epidural group): on arrival to PACU, ropivacaine‐fentanyl‐adrenaline epidurally at 10 mL/h, IV PCA with 0.9% saline (bolus dose 1 mL, lockout 6 min, used NRS > 3). Group 2 (placebo group): on arrival to PACU, 0.9% saline via epidural at 10 mL/h, IV PCA with 1 mg/mL morphine (bolus dose 1 mg, lockout 6 min, used NRS > 3). In both groups, preoperative anxiolysis with 10 mg diazepam oral 1 h before scheduled surgery and 1 mg‐2 mg midazolam as needed during catheter placement. Standardized placement of epidural at T10 to 12 interspace, tested using 3 mL mepivacaine 2% with adrenaline then bolus dose of 3 mL to 4 mL mepivacaine 2% with adrenaline. Sensory blockade at T8 level. Standardized GA with propofol (participants 1‐55) or thiopentone (participants 56‐60), fentanyl, rocuronium, nitrous oxide in O2, sevoflurane. Intraoperative analgesia with 2% mepivacaine with 2 mL/h‐5 mL/h adrenaline by epidural infusion in all participants. Immediately before transfer to PACU epidural infusion was turned off. In PACU, nurse allowed to administer 1 mg‐2mg morphine bolus as needed if NRS > 5. 1 g paracetamol oral before surgery and every 6 h post‐op during hospitalization. Adjuvants: adrenaline Immediate post‐op pain control: significantly improved |
|
| Outcomes | Dichotomous: none Continuous: SF‐36 at 3 months Adverse effects: postoperative nausea, vomiting, sedation and bleeding were reported |
|
| Notes | We contacted study author for clarification on attrition, source of funding and conflict of interest but received no response. Funding sources: source of funding not reported. Conflicts of interest: conflict of interest statement not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomized numbers", randomized "after successful insertion of the epidural catheter" |
| Allocation concealment (selection bias) | Low risk | Quote: "every precaution was taken to achieve double blinding...hospital pharmacy sent two double‐blinded bags" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the patients and surgeons, anaesthesiologists and nurses involved in patient treatment were unaware of method of analgesia and every precaution was taken to achieve double blinding" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the SF‐36 was given before and 1 and 3 months after the operation to each patient". Participants, as well as providers, were blinded and the participants filled out the questionnaire themselves |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 60 participants were randomized, 4 participants were excluded after randomization with reasons and group assignments listed and balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes fully reported |
| Null bias | Low risk | Quote: "median pain at rest at the incision site was low (< 4) and significantly lower in group E compared with group P at 4–24 h after the operation" |
Ibarra 2011.
| Methods | Blinded (PACU nurses, outcome assessor), controlled, randomized clinical trial Computer‐generated randomization in blocks of 2 using sealed, opaque envelopes Follow‐up: 5 months |
|
| Participants | Participants: 40 adults in a university hospital setting in Albacete, Spain Operation: radical mastectomy and conservative breast surgery for breast cancer 2 groups, size: 20/20 Age: not reported Men/women: 0/40 |
|
| Interventions |
Group 1 (preoperative PVB): single shot PVB at T4 with ropivacaine (0.5% without epinephrine, 25 mL to 30 mL, doses maximum 150 mg; using nerve stimulations according to Naja but only one single injection), GA (LMA using sevoflurane and remifentanil 0.05 to 0.1 mcg/kg/min only in the first 20‐30 min), post‐op: intravenous morphine (0.1 mg/kg), dexketoprofen 50 mg IV plus 25 mg every 8 h as needed for pain and paracetamol (1 g every 6 h) Group 2 (no block): no block, GA (LMA using sevoflurane and remifentanil 0.05 mcg/kg/min to 0. 02 mcg/kg/min), post‐op: IV morphine (0.1 mg/kg), dexketoprofen 50 mg IV plus 25 mg every 8 h as needed for pain and paracetamol (1 g every 6 h) Adjuvants: none Immediate post‐op pain control: not significantly improved |
|
| Outcomes | Dichotomous: number of participants with pain (including detailed number per group on myofascial pain, breast phantom pain or neuropathic pain) at 3 and 5 months per group Continuous: not reported Effective regional anaesthesia: one participant had an unsuccessful block but was NOT excluded, yet PVBs did not reduced the severity of postoperative pain. |
|
| Notes | We acknowledge the study author's response regarding randomization, allocation concealment and blinding, dosing and attrition Funding sources: source of funding not stated Conflicts of interest: conflict of interest not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “computer generated list”, “randomization in blocks of two”. Low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: “patients were assigned as they arrived in the preoperative clinic”, “The anaesthesiologist [enrolling the participant] did not know in which group the patient was going to be enrolled”. “The anaesthesiologist [in the OR] did not know the group allocation, until the patient reached the operating room.” “The randomization number was included in the chart in a sealed opaque envelope.” Low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “the recovery room nurses did not know the anaesthetic technique used in each case.” “The surgeon knew” if a block was performed. Participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “the outcome observer conducting the interview did not know the group allocation.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The numbers excluded in each group for radiotherapy and lost to follow‐up, respectively are unclear. Significant attrition with unclear group allocation may have caused bias, but no ITT analysis considered. |
| Selective reporting (reporting bias) | Low risk | Expected primary outcomes fully reported on |
| Null bias | High risk | Quote: "no significant differences in acute pain were observed" |
Ju 2008.
| Methods | Double‐blind (participants and outcome assessor), sham epidural‐controlled, clinical RCT Sequence generation was randomized, but not described Follow‐up: 12 months |
|
| Participants | Participants: 114 adults in a university setting in Beijing, China Operation: posterolateral thoracotomy for lung and oesophageal disease 2 groups, size: 57/57 Age (group 1, 2): 61.80 years (SD ± 13.78), 61.41 (SD ± 11.78) Men/women (group 1, 2): 41/13, 38/15 (completed the protocol) Remarks: pulmonary/oesophageal operation (group 1, 2): 28/26, 25/28 7 participants with dislodged catheters were excluded. |
|
| Interventions |
Group 1 (preincision epidural): epidural at T6/7/8, preincision epidural ropivacaine (0.5%, bolus 5 mL to 10 mL), GA (fentanyl), post‐op for 72 h PCEA (0.125% bupivacaine + 0.05 mg/mL morphine + 0.02 mg/mL droperidol, basal 3 mL/h, demand 3 mL, lock out 15 min). Group 2 (control/cryotherapy): sham epidural at T6/7/8, GA (fentanyl), cryoalgesia, post‐op for 72 h PCA through sham epidural (SC, 1 mg/mL morphine, demand 2 mL, lock‐out in 30 min, no basal) Adjuvants: none Immediate post‐op pain control: not significant |
|
| Outcomes | Dichotomous: pain at 6 and 12 months Continuous: not reported Secondary: allodynia at 6 and 12 months |
|
| Notes | Funding sources: study supported by grants from Research and Development Foundation of Peking University People's Hospital Conflicts of interest: no conflict of interest statement given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were stratified by disease sites (lung or oesophagus), and blinded randomized to receive either epidural analgesia (Epidural Group, Group E) or intercostal nerve cryoanalgesia (Cryo Group, Group C), in order to ensure that both groups had comparable operation methods." Randomization method not detailed, but otherwise well documented. |
| Allocation concealment (selection bias) | Low risk | Participants unaware of allocation, concealment of allocation for providers described: "After obtaining ... written informed consent from the prospective patient cases, 114 physical status I or II patients scheduled for posterolateral thoracotomy for lung or oesophagus diseases were enrolled in the study." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intraoperative anaesthesia providers were not blinded. An effort was made to blind study participants. Quote: "in order to make the patients blinded to the analgesic method, SC infusion catheters were inserted at upper back (T7‐8 level) in Group C." This is acceptable, bias is unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor "who was blinded to the postoperative pain management, interviewed patients by telephone, using a standard questionnaire." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was reported, but no ITT analysis was considered. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available, but pre‐specified outcomes within manuscript were all reported on. |
| Null bias | High risk | Quote: "no statistically significant differences were found between the two groups with respect to NRS pain scores at rest or on motion within three days following surgery" |
Kairaluoma 2006.
| Methods | Triple‐blinded (participant, providers, outcome assessor), sham‐ and placebo‐controlled, randomized clinical trial Sequence generation was not described Follow‐up: 12 months |
|
| Participants | Participants: 60 adults in a university setting in Helsinki, Finland Operation: conservative breast surgery with sentinel lymph node biopsy for cancer 2 groups, size: 30/30 Age: not reported Men/women: 0/60 |
|
| Interventions |
Group 1 (preincision PVB): single shot PVB at T3 with bupivacaine (0.5%, 1.5 mL/kg), GA, post‐op: oral ibuprofen (10 mg/kg) and paracetamol (1 g, 3 x daily ) rescue analgesia: paracetamol (500 mg with codeine 30 mg) or tramadol (50‐100 mg) Group 2 (sham PVB): sham PVB at T3 with normal saline, GA, post‐op: oral ibuprofen (10 mg/kg) and paracetamol (1 g, 3 x daily) rescue analgesia: paracetamol (500 mg with codeine 30 mg) or tramadol (50‐100 mg) Adjuvants: none Immediate post‐op pain control: significantly improved |
|
| Outcomes | Dichotomous: NRS larger 3 at 6 and at 12 months, use of pain medication at 6 and 12 months Continuous: pain at rest and in motion reported as NRS, number of pain descriptors, all at 6 and 12 months Effective regional anaesthesia not reported, but treatment reduced the severity of postoperative pain and oxycodone consumption, postoperatively |
|
| Notes | We acknowledge the study author's response regarding randomization and allocation concealment Funding sources: source of funding not reported Conflicts of interest: conflict of interest statement not provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants "were randomly assigned." Sequence generation was "randomized", "performed in a randomized fashion", but the exact method of randomization was not explained. The study author responded "The randomization was done using the opaque sealed envelope method." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described in the original report |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the patients and the study anaesthesiologists who performed the analysis remained blinded to the use of PVB with bupivacaine or a sham block throughout the entire study period." "Procedure behind a drape curtain" The study author responded, also that "the patient, the anaesthesiologist providing anaesthesia and the staff taking care of the patient were blinded to the study group. The curtains and drapes were hung so that the block was performed behind the curtains on the back side of the patient while the patient's head and front side and her nurse were on the other side of the curtains. The anaesthesiologist and nursing staff giving general anaesthesia were blinded to the study group..." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the patients and the study anaesthesiologists who performed the analysis remained blinded to the use of PVB with bupivacaine or a sham block throughout the entire study period.", "telephone interviews by a blinded interviewer." "A group‐blinded study assistant conducted all telephone interviews." The study author responded also that "A non‐medical study assistant blinded to the study group performed the follow‐up telephone interviews at predestined time points up to 12 months postoperatively". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition explained in detail, ITT analysis performed |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes fully reported |
| Null bias | Low risk | Quote: "the patients given PVB with bupivacaine had less postoperative pain, as indicated by longer times to first analgesic dose, lower VAS scores, and 40% smaller oxycodone consumption in the PACU... On the first postoperative day, the number of patients who experienced continuous aching pain and pain at rest was significantly smaller in the PVB group" |
Karanikolas 2006.
| Methods | Double‐blind (participants, outcome assessor) placebo‐controlled, randomized clinical trial Sequence generation was randomized Follow‐up: 6 months |
|
| Participants | Participants: 65 adults in a university setting in Patras, Greece Operation: lower limb amputation with pain score > 60/100 VAS 48 h prior to amputation 5 groups, group size: 13 Age: group means ranging 69.2 to 74.3 with largest SD 13 Men/women: 35/53 |
|
| Interventions |
Group 1 (Epi/Epi/Epi): preop: lumbar epidural analgesia bupivacaine (0.2%, fentanyl 2 µg/mL at 4 mL/h to 8 mL/h) for 48 h, GA preincision: epidural bupivacaine (0.5% 10 mL to 15 mL, fentanyl 100 µg), post‐op epidural bupivacaine (0.2% fentanyl 2 µg/mL at 4 mL/h to 8 mL/h) Group 2 (PCA/Epi/Epi): preop: PCA fentanyl (IV, demand 25 µg, lockout 20 min), preincision: epidural bupivacaine (0.5% 10 mL to 15 mL, fentanyl 100 µg), post‐op epidural bupivacaine (0.2%, fentanyl 2 µg/mL at 4 mL/h to 8 mL/h) Group 3 (PCA/Epi/PCA): preop: PCA fentanyl (IV, demand 25 µg, lockout 20 min), preincision: epidural bupivacaine (0.5% 10 mL to 15 mL, fentanyl 100 µg), post‐op PCA fentanyl (IV, demand 25 µg, lockout 20 min) Group 4 (PCA/GA/PCA): preop: PCA fentanyl (IV, demand 25 µg, lockout 20 min), general anaesthesia with LMA, sevoflurane and remifentanil infusion, post‐op PCA fentanyl (IV, demand 25 µg, lockout 20 min) Group 5 (control/GA/control): preop: meperidine (50 mg 4‐6 x/d IM) paracetamol/codeine 30/500 mg orally plus as‐needed IV paracetamol 650 mg 3 x/d and parecoxib 40 mg 2 x/d, GA with LMA, sevoflurane and remifentanil infusion, post‐op: meperidine (IM) paracetamol/codeine 30/500 mg orally plus as‐needed IV paracetamol 650 mg 3 x/d and parecoxib 40 mg 2 x/d Immediate pain control: significantly improved preop and post‐op |
|
| Outcomes | Dichotomous: phantom limb pain at 6 months Continuous: VAS and McGill pain questionnaire and phantom limb pain frequency scores for phantom and stump pain at 6 months Effective regional anaesthesia not reported, but interventions reduced the severity of pain pre‐ and postoperatively. |
|
| Notes | There are minor discrepancies regarding the dosing described between the preliminary report of the ongoing registered trial (Karanikolas 2006) and the final report. We reported the treatment according to the latest publication. We contacted the study author for confirmation and additional information, but received no response. Hence, we could only use the data extracted from the publications and the information provided on clinicaltrials.gov/ct2/show/NCT00443404. Funding sources: "support was provided solely from institutional and/or departmental sources." Conflicts of interest: no conflict of interest statement was provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as "prospective, randomized, clinical trial", with “computer generated blocks with five treatment groups and 13 patients per group.” |
| Allocation concealment (selection bias) | Low risk | Quote: “sequentially numbered sealed envelope... concealed until after consent was obtain.” Recruitment, outcome assessment and protocol management clearly separated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial is described as "double‐blind" in the title. Detailed description of blinding procedures. Quote: “control group patients had an epidural catheter placed subcutaneously.” D.A. i.e. the person “responsible for adjusting the epidural...” may not have been blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Detailed description of blinding procedures. Quote: “a second blinded investigator interviewed all participants.” “A third blinded investigator conducted all interviews during the analgesic protocol.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only minor attrition is reported, and attributed to groups. Seemingly, attrition affected mainly the control groups. ITT analysis is reported. Per protocol or ITT analysis did not change results. |
| Selective reporting (reporting bias) | Low risk | Protocol review and primary outcomes fully reported on |
| Null bias | Low risk | Quote: "all patients had severe ischemic pain before analgesia started, but pain scores improved markedly and were significantly lower in all intervention groups compared with control at all times while the protocol was in effect" |
Karmakar 2014.
| Methods | Blinded (outcome assessor), RCT Sequence generation by computer‐generated allocation number Follow‐up: 6 months |
|
| Participants | Participants: 180 adult women in University Hospital in Hong Kong, China Operation: modified radical mastectomy (including axillary lymph node clearance) 3 groups, size: 60, 57, 60 Age (± SD), group 1, 2, 3: 51 (± 9), 54 (± 9), 53 (± 8) All female participants |
|
| Interventions |
Group 1 (GA group): standardized GA as described below Group 2 (GA + single shot PVB + placebo infusion): pre‐op thoracic paravertebral catheter placed opposite third thoracic spine, ipsilateral to side of surgery, ropivacaine (2 mg/kg) + epinephrine (5 µg/mL) in total volume of 20 mL with normal saline injected slowly then epidural catheter inserted into thoracic paravertebral space. Intraoperatively, continuous infusion of 0.9% saline started at 0.10 mL/kg/h via catheter and maintained constant until 72 h post‐op. Group 3 (GA+ PVB): pre‐op thoracic paravertebral catheter placed opposite third thoracic spine, ipsilateral to side of surgery, ropivacaine (2 mg/kg) + epinephrine (5 µg/mL) in total volume of 20 mL with normal saline injected slowly then epidural catheter inserted into thoracic paravertebral space. Intraoperatively, continuous infusion of ropivacaine 0.25% started at 0.10 mL/kg/h via catheter, maintained constant until 72 h post‐op. All participants had standardized GA, which included IV fentanyl, propofol and rocuronium. Intraoperative morphine (0.1 mg/kg) IV to every participant, then morphine (1 mg IV) as needed, ondansetron 4 mg IV 30 min before end of surgery. In the PACU, all participants had nurse‐administered IV morphine for rescue analgesia as needed. On post‐op ward, analgesia was with diclofenac (75 mg) oral 2 x 72 h, IM morphine (0.1 mg/kg, as needed every 3 h) or Dologesic (paracetamol 325 mg and dextropropoxyphene 32.5 mg, 2 tablets as needed every 6 h) as rescue. Adjuvants: none Immediate pain control: not significantly improved |
|
| Outcomes | Dichotomous: incidence of chronic pain at all sites (operated site, axilla, arm) and over operated site at 3 and 6 months Continuous: chronic pain scores at rest and on movement at all sites (operated site, axilla, arm) and over operated site at 3 and 6 months Other reported outcomes: HRQOL (Chinese‐HK version of SF‐36) at 3 and 6 months, Chronic pain symptom and sign score at 3 and 6 months, physical health summary score, mental health summary score (of SF‐36) at 3 and 6 months |
|
| Notes | Funding sources: this research work was fully funded by a grant from the Research Grants Council of the Hong Kong Special Administrative Region, China (RGC reference no. CUHK4406/05, project code 2140452). Conflicts of interest: the study authors declare no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized to 1 of 3 study groups... with a computer‐generated allocation number" |
| Allocation concealment (selection bias) | Low risk | Quote: "sequentially numbered, coded, sealed opaque envelopes...The sealed envelopes were prepared by a third party (research assistant) who took no further part in the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "patients in group1, who had received standardized GA with no paravertebral intervention, could not be blinded for obvious reasons..For the other 2 study groups that had a thoracic paravertebral catheter placed, we adopted a double‐blind methodology... The principal investigator performed all the thoracic paravertebral catheter placements, collected procedural data, injected the ropivacaine bolus for the TPVB [thoracic paravertebral block], conducted the GA, and took no further part in data collection.. The paravertebral infusion (ropivacaine 0.25% or 0.9% saline) was prepared.. by a postanaesthetic care unit (PACU) nurse not involved in the study ... A single surgeon, who was also blinded to the group allocation, performed or supervised all the surgical procedures" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "a research nurse blinded to the group allocation recorded data preoperatively, in the PACU, and at regular intervals in the postoperative ward...The telephone interview at 3 and 6 months after surgery was also conducted by the same research nurse (blind to group allocation)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "the primary analyses were performed on a modified intention‐to‐ treat basis (i.e., patients were analysed according to their randomized allocated groups but were excluded from the analysis if they did not adhere to the protocol after randomization)". 1 participant lost to follow‐up in group 2 and reason given (returned overseas after surgery). 2 excluded from the analysis in group 2 because of protocol violation/diagnosed contralateral breast cancer. Very small numbers of attrition, with reasons reported for each exclusion and modified ITT protocol used. |
| Selective reporting (reporting bias) | Low risk | All primary outcomes in protocol were fully reported on |
| Null bias | High risk | Quote: "there was no significant difference in acute pain scores at rest (Fig. 2) or on movement (Fig. 3) between the study groups (both P = 0.22) during the 72 hours after surgery". |
Katsuly‐Liapis1996.
| Methods | clinical RCT Sequence generation randomized, but not described Follow‐up: one year |
|
| Participants | Participants: 45 adults in a university setting in Athens, Greece Operation: lower limb amputation 3 groups, size: 15/12/18 Age: not reported Men/women: not reported |
|
| Interventions |
Group 1 (preoperative epidural): for 72 h preop: bupivacaine (0.25% and morphine) via epidural catheter (level not specified), (intraop anaesthesia not specified), post‐op for 72 h epidural bupivacaine infusion (not specified) Group 2 (post‐op epidural): for 72 h preop: opioids and NSAIDs (not specified), (intraop anaesthesia not specified), post‐op for 72 h epidural bupivacaine infusion (not specified) Group 3 (control): for 72 h preop: opioids and NSAID (not specified), (intraop anaesthesia not specified), post‐op opioids and NSAIDs (not specified) Adjuvants: none Immediate post‐op pain control: not reported, phantom pain risk not significantly reduced for the first three days |
|
| Outcomes | Dichotomous: phantom limb pain at 6 and 12 months Continuous: none reported |
|
| Notes | We were unable to find the contact information for any of the authors using Google and PubMed or the institution and therefore no additional information beyond the abstract could be obtained or extracted. Funding sources: no source of funding reported. Conflicts of interest: no conflict of interest statement given. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were "randomly allocated", but the exact method was not explained. |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not reported in the abstract. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding was not reported in the abstract. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition is not reported. ITT analysis is not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available for review and only abstract available |
| Null bias | Unclear risk | Immediate post‐op pain control not reported, however phantom pain risk not significantly reduced for the first three days. |
Katz 1996.
| Methods | Triple‐blind (participants, providers, outcome assessors), sham/placebo‐controlled, randomized clinical trial Sequence generation was by random number tables Follow‐up: 18 months |
|
| Participants | Participants: 30 adults in a university setting in Toronto, Ontario, Canada Operation: lateral thoracotomy for pulmonary or oesophageal disease 2 groups, size: 15/15 Age (group 1, 2): 54.6 years (range 19‐75), 58.9 (range 46‐72) Men/women (group 1, 2): 5/10, 8/7 |
|
| Interventions |
Group 1 (preincision intercostal block): placebo rectal suppository, intramuscular midazolam (0.05 per kg), GA (fentanyl 1 µg/kg), preincision intercostal nerve block with bupivacaine (0.5% with epinephrine (1:200.000), 3 mL/interspace) 2 spaces above and below planned incision, post‐op for 72 h PCA morphine (demand 1.5 mg‐2 mg, lockout 6 min, max dose 30 mg/4 h) Group 2 (sham/placebo block): IM morphine (0.15 mg/kg) and perphenazine (0.03 mg/kg), indomethacin (100 mg, rectal suppository), GA (fentanyl 1 µg/kg), preincision sham intercostal nerve block with normal saline (3 mL/level) 2 spaces above and below planned incision, post‐op for 72 h PCA morphine (demand 1.5 mg‐2 mg, lockout 6 min, max dose 30 mg/4 h) Adjuvants: none Immediate post‐op pain control: initial analgesic consumption reduced |
|
| Outcomes | Dichotomous: pain and analgesic consumption at 18 months Continuous: verbal rating scale at 18 months Secondary: allodynia at 6 and 12 months |
|
| Notes | We contacted the study author for missing information. He provided a data table with unpublished data from the follow‐up study to Kavanagh 1994, the second manuscript reporting on (Katz 1996). Funding sources: "this study was supported by a research scholarship from the Medical Research Council of Canada (MRC) and by MRC grant MT‐12052 to Dr Katz." Conflicts of interest: a conflict of interest statement was not given. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a table of random numbers was used to allocate patients." |
| Allocation concealment (selection bias) | Low risk | Quote: "..investigator (who had no further involvement with that patient) who administered the medications in accordance with the instructions in the envelope...". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the patients and all other personnel involved in subsequent patient management and assessment were completely blinded as to group allocation, ...thus maintain the blind and (patients) also received a placebo rectal suppository." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "other personnel involved in subsequent patient management and assessment were completely blinded as to group allocation...,thus maintain the blind..." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was described with regards to group allocation. Per‐participant analysis was performed, with no ITT analysis considered. Bias is unlikely, as an ITT analysis would not alter the lack of the statistical significance. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes fully reported on |
| Null bias | High risk | Quote: "in the original study, use of preemptive multimodal analgesia during surgery was not found to be more effective than the placebo in reducing the intensity of acute postoperative pain" |
Katz 2004.
| Methods | Double‐blinded, placebo/sham‐controlled, randomized clinical trial Sequence generation by computer‐generated random numbers Follow‐up: 6 months |
|
| Participants | Participants: 152 adults in a university setting in Toronto, Canada Operation: laparotomy for major gynaecological surgery 3 groups, size: 49/56/47 Age: 44 years (SD ± 8.9), 47 (SD ± 10.6), 44 (SD ± 9.6) Men/women: women only |
|
| Interventions |
Group 1 (preincisional epidural): epidural catheter at L2/3/4 tested, GA, preincision: lidocaine (2% with epinephrine (1:200,000), 12 mL plus 0.8 mL for each 2.5cm (1 inch) of height above 152cm (60 inch), plus 4 µg/kg fentanyl), 40 min after incision epidural normal saline (12 mL), post‐op morphine PCA (loading dose 4 mg, then bolus 1.0‐1.5 mg, lockout time 5 min, max 40 mg in 4 h, no basal rate) Group 2 (postincision epidural): epidural catheter at L2/3/4 tested, GA, preincision: epidural normal saline (12 mL), 40 min after incision: lidocaine (2% with epinephrine (1:200,000), 12 mL plus 0.8 mL for each inch of height above 60 inch, plus 4 µg/kg fentanyl), post‐op morphine PCA (loading dose 4 mg, then bolus 1.0‐1.5 mg, lockout time 5 min, max 40 mg in 4 h, no basal rate) Group 3 (sham epidural): sham epidural catheter at L2/3/4 tested, GA (fentanyl 1 µg/kg), preincision: epidural normal saline (12 mL), 40 min after incision epidural normal saline (12 mL), post‐op morphine PCA (loading dose 4 mg, then bolus 1.0‐1.5 mg, lockout time 5 min, max 40 mg in 4 h, no basal rate) Adjuvants: none Immediate post‐op pain control: not significant |
|
| Outcomes | Dichotomous: pain at 6 months, analgesic consumption at 6 months Continuous: Pain Disability Index, Mental Health Inventory‐18 and McGill Pain Questionnaire at 6 months Secondary: allodynia/hyperalgesia |
|
| Notes | Funding sources: supported by grants from the National Institutes of Health and the Canadian Institutes of Health. Conflicts of interest: conflicts of interest were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “a randomization schedule was computer generated by a biostatistician.” |
| Allocation concealment (selection bias) | Low risk | Quote: "an opaque envelope containing the patient number and group assignment was prepared, sealed, and numbered for each patient by the hospital pharmacist, not involved in the study otherwise...All patients and personnel involved in patient management and data collection were unaware of the group to which the patient had been allocated. The anesthesiologist in charge of the case was aware of group allocation for control group patients and was not involved in postoperative management or data collection." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "all patients and personnel involved in patient management and data collection were unaware of the group to which the patient had been allocated. The anaesthesiologist in charge of the case was aware of group allocation for control group patients and was not involved in postoperative management or data collection." but the anaesthesiologist in charge of the case was aware of group allocation for control group participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "neither the person conducting the interview nor the patient was aware of the group to which the patient had been assigned," "personnel involved in ... data collection were unaware of the group to which the patient had been allocated." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "both an intention to treat analysis and a protocol‐compliant analysis were performed." "There was no appreciable difference in the results of the intention‐to‐treat analyses and the protocol compliant analyses. Data and results of significance tests reported below are therefore based on the intention to treat analyses." But ITT was only done for early outcomes, not for questionnaire data at 6 months, when significant attrition occurred. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes fully reported on |
| Null bias | Low risk | Quote: "preincisional administration of epidural lidocaine and fentanyl was associated with a significantly lower rate of morphine use, lower cumulative morphine consumption, and reduced hyperalgesia compared with a sham epidural condition" |
Kurmann 2015.
| Methods | Triple‐blinded (participants, providers and outcome assessors) placebo‐controlled, group sequential clinical trial Sequence generation with computer‐generated block sequences Follow‐up: 12 months |
|
| Participants | Participants: 357 adult participants underwent 403 hernia operations at a teaching hospital in Lucerne, Switzerland Operation: single‐ or double‐sided primary or recurrent inguinal hernia repair 2 groups, participant population size: 162/174 Age (± SD), group 1, 2: 50 (± 16), 51 (± 15) Men/women, group 1, 2: 145/8, 161/8 Comorbidities: unilateral/bilateral hernia (n), group 1, 2: 148/14, 162/12 Primary/recurrent hernia (n), group 1, 2: 167/14, 186/12 Remarks: the unit of analysis published was the hernia not the participant |
|
| Interventions |
Group 1 (placebo): "operative procedures were performed under general or SA at the request of the patient". After closure of the incision, infiltration of 20 mL saline 0.9% in specified region Group 2 (intervention): "operative procedures were performed under general or SA at the request of the patient". After closure of the incision, infiltration of 20 mL bupivacaine 0.25% in specified region Both groups: infiltration started with the laterocranial puncture 1 finger below and 1 finger medial to the anterior superior iliac spine at the lateral end of the incision; 10 mL of study drug was injected in a fan‐shaped manner lateral to and 4 mL medial to the laterocranial puncture. The mediocaudal puncture was located directly above the pubic tubercle; 4 mL of study drug were injected in a fan‐shaped manner lateral to and 2 mL medial to the mediocaudal puncture. Adjuvants: none Immediate post‐op pain control: not reported |
|
| Outcomes | Dichotomous: pain/no pain at 3 (and at 12 months, but not published) Continuous: VAS at rest, with various types of movements at 3 and 12 months Other: quality of life at 1 year, neuralgia at 3 and 12 months |
|
| Notes | Unit of analysis was the hernia in the original publication. The study authors provided additional information on methodological quality. Absorbed lidocaine from 1 hernia may have mitigated the chronic pain for the other hernia in those with discordant randomization, i.e. participants undergoing bilateral hernia repair in whom one side was treated while the other was not. Funding sources: funding provided by NIH grant NCT00484731 Conflicts of interest: Drs Anita Kurmann, Henning Fischer, Salome Dell‐Kuster, Rachel Rosenthal, Laurent Audigé, Guido Schüpfer, Jürg Metzger, and Philipp Honigmann have no conflicts of interest or financial ties to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the randomization, based on computer‐generated block randomization sequences, was performed in a 1:1 ratio between investigational and control arms" |
| Allocation concealment (selection bias) | Low risk | Quote: "the hospital pharmacy provided similar‐looking syringes containing either bupivacaine 0.25% or saline 0.9% solution according to the randomization sequence". In the protocol states the syringes are numbered according to "randomization sequence that is kept confidential" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the patient, surgeon, and the physician performing the examinations during follow‐up visits were blinded to the treatment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the patient, surgeon, and the physician performing the examinations during follow‐up visits were blinded to the treatment. Unblinding was performed after completion of the analysis as described in the study protocol". Sham techniques would make it difficult for the practitioner to know which group he or she was working with. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was 16% in intervention group and 11.2% in the placebo group at 3 months post‐op for primary endpoint. One participant was excluded from placebo group because syringe became unsterile. Participants were excluded retrospectively because did not meet inclusion criteria. Numbers lost to follow‐up at each stage clearly delineated. ITT analysis was done, with exception of 1 participant excluded from placebo group described above. |
| Selective reporting (reporting bias) | Low risk | Protocol available and reviewed. Primary outcome of pain at 3 months measured by VAS was fully reported on |
| Null bias | Unclear risk | No data on immediate postoperative pain control. |
Lam 2015.
| Methods | Placebo‐controlled, randomized clinical trial Sequence generation by computer‐generated random numbers Follow‐up for 6 months |
|
| Participants | Participants: 36 adults in a university setting in Alberta, Canada Operation: unilateral total breast mastectomy +/‐ axillary lymph node dissection 2 groups, size: 18/18 Age (± SD), group 1, 2, 4: 63.9 years (16.7), 60.2 (13.1) All women Exclusion criteria: not specified |
|
| Interventions |
Group 1 (PVB): participants received an ultrasound‐guided PVB (regional anaesthetic not specified) or combined with a multimodal regimen consisting of propofol‐based total intravenous anaesthesia with ketorolac, gabapentin, ranitidine, paracetamol, and ondansetron. Group 2 (control): same intervention as above except sham block was substituted for local anaesthesia. Adjuvants: none Immediate post‐op pain control: no improvement |
|
| Outcomes | Dichotomous: pain vs no pain Continuous: none Other reported: propofol and fentanyl consumption, postoperative morphine equivalent consumption, frequency of postoperative nausea and vomiting |
|
| Notes | We were unable to obtain additional information about randomization and blinding methods from the study author. Funding sources: funding for the study not reported Conflicts of interest: there was no statement on conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "following patient allocation with a computer‐generated sequence..." |
| Allocation concealment (selection bias) | Low risk | Quote: "consenting patients were randomized to either the treatment group or the control group via sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Sham block was used and participants were well blinded. No comment on personnel blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Degree of attrition not described |
| Selective reporting (reporting bias) | Low risk | No subgroup analysis noted |
| Null bias | High risk | Quote: "pain scores were similar at all time points within the first 24 hours" |
Lavand'homme 2005.
| Methods | Double‐blinded (participant, outcome assessor), placebo/sham‐controlled, randomized clinical trial Sequence generation by computer‐generated random numbers Follow‐up for 12 months |
|
| Participants | Participants: 85 adults in a university setting in Brussels, Belgium Operation: colonic resection (xiphopubic incision) of rectal adenocarcinoma 4 groups, size: 20/20/20/20 Age (group 1, 2, 3, 4): 53 years (SD ± 8), 54 (SD ± 8), 55 (SD ± 8), 53 (SD ± 10) Men/women (total: group 1, 2, 3, 4): 49/31: 12/8, 13/7, 12/8, 12/8 Remarks: intraoperative discovery of an extended tumour resulted in participants' exclusion from the study. |
|
| Interventions |
Group 1 (IV/IV): epidural catheter at T8, GA (sufentanil 2.5 µg) IV (lidocaine 2 mg/kg + 0.5 mg/kg/h, clonidine 4 µg/kg + 1 µg/kg/h, sufentanil 0.1 µg/kg + 0.07 µg/kg/h) post‐op IV PCA (lidocaine bolus per request 7.5 mg, clonidine bolus per request 15 µg, morphine bolus per request 1.3 mg) (0.75 mL solution per demand, lockout time 7 min, max 15 mL per 4 h) Group 2 (IV/epidural): epidural catheter at T8, GA (sufentanil 2.5 µg); IV (lidocaine 2 mg/kg + 0.5 mg/kg/h, clonidine 4 µg/kg + 1 µg/kg/h, sufentanil 0.1 µg/kg + 0.07 µg/kg/h), before recovery (epidural bolus 7 mL bupivacaine 0.5%, clonidine 1 µg/kg, sufentanil 0.03 µg/kg) post‐op epidural PCEA (bupivacaine 5 mL 0.0675% + 5 mL/h 0.0675%, clonidine 3.5 µg + 3.5 µg/kg/h, sufentanil 0.05 µg + 0.05 µg/h) (continuous infusion of 5 mL and bolus of 5 mL on request, 40 min lockout time) Group 3 (epidural/epidural): epidural catheter at T8, GA (sufentanil 2.5 µg), preincision epidural (bupivacaine 7 mL 0.5% + 5 mL/h 0.125%, clonidine 1 µg/kg + 0.5 µg/kg/h, sufentanil 0.03 µg/kg + sufentanil 0.015 g/kg/h) post‐op epidural PCEA (bupivacaine 5 mL 0.0675% + 5 mL/h 0.0675%, clonidine 3.5 µg + 3.5 µg/kg/h, sufentanil 0.05 µg + 0.05 µg/h) (continuous infusion of 5 mL and bolus of 5 mL on request, 40 min lockout time) Group 4 (epidural/IV): epidural catheter at T8, GA (sufentanil 2.5 µg), preincision epidural (bupivacaine 7 mL 0.5% + 5 mL/h 0.125%, clonidine 1 µg/kg + 0.5 µg/kg/h, sufentanil 0.03 µg/kg + sufentanil 0.015 g/kg/h), post‐op IV PCA (lidocaine bolus per request 7.5 mg, clonidine bolus per request 15 µg, morphine bolus per request 1.3 mg) (0.75 mL solution per demand, lockout time 7 min, max 15 mL per 4 h) Adjuvants: ketamine from skin incision to the end of surgery (0.5 mg/kg bolus followed by continuous infusion at 0.25 mg/kg/h), clonidine as detailed above Immediate post‐op pain control: significantly improved |
|
| Outcomes | Dichotomous: pain at 6 and 12 months Continuous: Pain Disability Index at 6 months, Mental Health Inventory‐18 at 6 months Secondary: punctuate wound hyperalgesia was reported for the first 72 h |
|
| Notes | We contacted the study authors for missing data and they responded, but with some data inconsistencies that could not be verified or corrected. The study authors reported an unusually high success rate of epidural analgesia with only 2 failures in 60 participants. Funding sources: "support was provided solely from institutional and/or departmental sources." Conflicts of interest: no conflict of interest statement provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "according to a computer‐generated table of random number assignments, each patient was assigned to one of four double‐blinded groups." Bias is unlikely. |
| Allocation concealment (selection bias) | Unclear risk | The timing of allocation and concealment not detailed. Risk of bias is unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "all of the analgesic solutions were prepared by an anesthesiologist who was not involved in the patients' care." Testing the epidural in the PACU "prevented a true double blinding in the postoperative period." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | However, (quote:) "postoperative parameters were recorded by an anesthesiologist who was not aware of the intraoperative treatment administered to the patient", "mobilization assessed by a blinded observer", telephone interviews were "performed by the research nurse." The study author responded: " the research nurse (outcome assessor) was blinded to the group allocation ..." as there was no random code on questionnaire. Bias is unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adverse effects and attrition were reported with group allocation. “Absence of thermoanalgesia level as well as intraoperative discovery of an extended tumor resulted in the patient’s exclusion from the study.” ”One was excluded during surgery after discovery of widespread neoplastic disease, and two other patients were excluded for postoperative early dislocation of epidural catheter (before 72‐h follow‐up).” “... one who died of a cardiac arrest at home 2 months" before completion. Results reported on a per‐participant basis, with no ITT analysis considered. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes fully reported on |
| Null bias | Low risk | Quote: "patients in group 1 (intravenous–intravenous) experienced significantly more severe pain than patients in the three other groups. Cumulative number of satisfied analgesic requirements was significantly higher in group 1 (intravenous–intravenous) than in the other groups " |
Lavand'homme 2007.
| Methods | Triple‐blinded (participants, provider, outcome assessor), placebo/sham‐controlled, randomized clinical trial Sequence generation by computer‐generated random numbers Follow‐up: 6 months |
|
| Participants | Participants: 92 adults in a university setting in Brussels, Belgium Operation: elective caesarean section (Pfannenstiel incision) 3 groups, size: 30/30/30 Age (group 1, 2, 3): 33 years (SD ± 5), 31 (SD ± 5), 31 (SD ± 6) Men/women: 0/92 Remarks: no previous caesarean delivery |
|
| Interventions |
Group 1 (ropivacaine): spinal bupivacaine (1.8‐2 mL hyperbaric 0.5%, sufentanil 1 µg/kg), post‐op for 48 h continuous wound irrigation (ropivacaine (0.2%, 5 mL/h), every 12 h diclofenac (75 mg in 50 mL/20 min)), PCA (morphine, no basal rate, demand 1 mg, lockout 5 min, max 25 mg/4 h), as needed paracetamol (1 g/6 h) Group 2 (diclofenac): spinal bupivacaine (1.8 mL‐2 mL hyperbaric 0.5%, sufentanil 1 µg/kg), post‐op for 48 h continuous wound irrigation (diclofenac (300 mg in 240 mL, 5 mL/h) IV saline 50 mL/20 min every 12 h), PCA (morphine, no basal rate, demand 1 mg, lockout 5 min, max 25 mg/4 h), as needed paracetamol (1 g/6 h) Group 3 (saline): spinal bupivacaine (1.8 mL to 2 mL hyperbaric 0.5%, sufentanil 1 µg/kg), post‐op for 48 h continuous wound irrigation (saline (5 mL/h), every 12 h diclofenac (75 mg in 50 mL/20 min)), PCA (morphine, no basal rate, demand 1 mg, lockout 5 min, max 25 mg/4 h), as needed paracetamol (1 g/6 h) Adjuvants: none Immediate post‐op pain control: pain and analgesic consumption significantly improved |
|
| Outcomes | Dichotomous: "chronic postsurgical pain" and scar/wound pain at 6 months Continuous: none reported Secondary: punctuate wound hyperalgesia for the first 48 h. Analgesic consumption at 6 months. Wound healing and complications such as hypotension, nausea or vomiting |
|
| Notes | The study author responded to our request for clarification, but with information differing from the published data. Funding sources: "support was provided solely from institutional and/or departmental sources." Conflicts of interest: no conflict of interest statement was given. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...according to a randomized, prospective, blinded protocol...The parturients were randomly assigned using computer‐generated random numbers..." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not explicitly described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the patient, the person in charge of perioperative management,... were not aware of the patient group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the staff involved in data collection were not aware of the patient group assignment." The study author responded to our inquiry that "the research nurse was blinded to the group allocation‐ there was no code on the questionnaire, she used." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A per‐participant analysis was performed, with no attrition reported. But the study author responded: "patients were excluded from the data analysis (intraoperative failure of intrathecal anaesthesia and intra‐wound catheter out, which did not allow a 48h postoperative follow up). We continued the inclusion of patients following the randomisation and at the end of the random list, we add 1 patient in ropivacaine group and 1 patient in diclofenac group (in the same order than those patients were excluded from the study).” Even though no formal ITT analysis was performed, only 2/90 participants were excluded, reducing the likelihood of bias. |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but published report includes all the expected outcomes. |
| Null bias | Low risk | Quote: "for the first 12 h after surgery, patients receiving a subcutaneous infusion of ropivacaine reported lower VAS pain scores at rest and during movement than those receiving local saline infusion... Wound infiltration with ropivacaine was also more effective than saline to relieve visceral pain at 12 h after surgery." |
Lee 2013.
| Methods | Single‐blinded (outcome assessor) clinical RCT Sequence generation using random numbers table Follow‐up: 3 months |
|
| Participants | Participants: 51 adults in a university setting in Cork, Ireland Operation: breast surgery (mastectomy or breast tumour resection) with axillary node clearance 2 groups, size: 26/25 Age, years (± SD), group 1, 2 : 57.8 (± 14.5), 54.3 (± 11.5) Men/women: all women Comorbidities: wide local excision/mastectomy/mastectomy and reconstruction, n (group 1, 2): 16/9/1, 13/11/1. Chemotherapy, n (group 1, 2): 13, 18. Further surgery, n: None/wide local excision/mastectomy/wide local excision and mastectomy (group 1, 2): 18/4/1/3, 18/3/2/2. Remarks: exclusion criteria included pre‐existing pain conditions other than those due to breast lump biopsy |
|
| Interventions |
Group 1 (Group C, control): as needed morphine IV intro. Post‐op morphine 2 mg IV as needed in PACU until morphine PCA x 48 h post‐op (2 mg bolus, 5 min lockout, no background, max dose 30 mg 4 h), diclofenac 50 mg oral/PR every 8 h as needed, paracetamol 1 g oral/PR/IV every 6 h as needed Group 2 (Group P, paracetamol and paravertebral): paravertebral catheter inserted prior to induction, 10 mL bupivacaine 0.25% injected with repeat aspiration tests then catheter inserted. 10 mL bupivacaine 0.25% 4 h post‐op then every 12 h x 48 h Both groups: GA induction with propofol 2‐2.5 mg/kg, maintenance with sevoflurane in O2/N2O mixture, vecuronium with 75 mg IV diclofenac sodium and 1 g IV paracetamol intraoperatively. All participants received 100 mg tramadol oral as rescue if required. Adjuvants: pregabalin Immediate post‐op pain control: not significantly improved, but with significantly decreased analgesic consumption |
|
| Outcomes | Dichotomous: pain/no pain at 3 months Continuous: Short‐form McGill Pain questionnaire at 3 months Secondary: Hospital Anxiety and Depression score, Spielberger Tate‐Trait Anxiety Inventory at 3 months, allodynia/hyperalgesia |
|
| Notes | Funding sources: "PL received a research grant from the South of Ireland Association of Anaesthetists." Conflicts of interest: "nothing to declare" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a random numbers table, patients were randomly allocated to one of two groups" |
| Allocation concealment (selection bias) | Low risk | Upon contacting study author: quote: "these pieces of paper were then placed in opaque sealed numbered envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Upon contacting study author: quote: "the envelopes were not opened until all study information was gathered and data analysis had begun" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "patients were interviewed three months postoperatively...by an investigator blinded to their group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost to follow‐up. ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported on. |
| Null bias | High risk | Quote: "patients in the two groups were similar in terms of reported pain intensity in the early postoperative period," |
Liu 2015.
| Methods | Assessor‐blinded, randomized clinical trial Sequence generation not described Follow‐up for 3 months |
|
| Participants | Participants: 120 adults in a university setting in China Operation: open thoracotomy 2 groups, size: 60/60 Age (± SD), group 1, 2: 61 (10), 58 (10) Men/women, group 1, 2: 33/27, 36/24 Exclusion criteria: paralysis, known allergy to LAs, active bacterial infection, clinically severe liver or kidney diseases, neurologic dysfunction, chronic use of systemic lidocaine, NSAIDs or opioids, insulin‐dependent diabetes mellitus and para‐aminobenzoic acid |
|
| Interventions |
Group 1 (ropivacaine wound infusion): the moment participants entered the operating room, standard monitoring was performed by 5‐lead electrocardiography, pulse oximetry, and non‐invasive arterial pressure measurement. GA was induced with midazolam at 0.05 mg/kg, propofol at 1.5 mg/kg to 2.5 mg/kg and fentanyl at 3 µg/kg. When loss of consciousness was confirmed, a bolus of 0.8 mg/kg rocuronium was intravenously injected for tracheal intubation. Anaesthesia was maintained with continuous infusion of propofol and a bolus of fentanyl at 1 µg/kg/h to 2 µg/kg/h in order to keep the bispectral index monitor (BIS, Aspect 1000, Aspect Medical System Inc., Natick, MA, USA) between 40 and 60. Neuromuscular blockade was conducted by continuous infusion of cis‐atracurium at 0.06‐0.07 mg/kg/h. Participants in both groups were accessible to rescue analgesia via pethidine, if needed, during the postoperative period. The catheter was positioned in the SC tissues above the fascia along the inferior edge of the rib along the incision. The catheter consisted of a multi‐orifice tube that was connected to an elastomeric infusion pump (Beijing tech‐bio‐med medical equipment Corporation, China) for postoperative continuous SC infusion with an anaesthetic at the end of surgery. After skin closure, the infusion pump containing 0.5% ropivacaine (Naropin®‐produced by AstraZeneca) was connected, and the wound was infused at 2 mL/h. Group 2 (control): same intervention induction procedure as above. No catheter was inserted. Sufentanil was injected intravenously via an analgesia pump after surgery, followed by intravenous PCA with sufentanil at 2 mL/h Adjuvants: fentanyl Immediate post‐op pain control: no difference |
|
| Outcomes | Dichotomous: pain vs no pain Continuous: none Secondary: the level of sedation, severity of pain at rest and movement, the amount of opioid analgesics administered, and participants’ satisfaction with their postoperative pain management |
|
| Notes | We were unable to obtain additional information about randomization and blinding methods from the study author. Funding sources: "this work was supported by Natural Science Foundation of Jinling Hospital." Conflicts of interest: the study authors have no conflicts of interest to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization technique not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "postoperative evaluations were performed by an observer blind to this study." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was a substantial degree of attrition. |
| Selective reporting (reporting bias) | Low risk | ITT principle was used and no subgroup analysis was performed |
| Null bias | High risk | Quote "There were no statistical differences in the VAS scores... between the two groups" |
Loane 2012.
| Methods | Double‐blind (participant, outcome assessor) randomized clinical trial Sequence generation by computer‐generated table Follow‐up: 3 months |
|
| Participants | Participants: 69 adult women at university hospital in Vancouver, British Columbia, Canada Operation: elective caesarean delivery with low transverse incision (under SA) 2 groups, size: 33/33 (completed) Age (± SD), group 1, 2: 35 (± 3), 34 (± 5) All female participants Comorbidities: number of multiparous women (group 1, 2): 25/21 |
|
| Interventions |
Group 1 (intrathecal morphine): 100 µg intrathecal morphine at time of spinal insertion. At end of surgery, sham TAP block with capped needle pushing against skin Group 2 (TAP block): no intrathecal morphine was given. At the end of surgery, TAP block 5 mL increments of ropivacaine into transversus abdominis plane on each side (0.5% ropivacaine, 1.5 mg/kg on each side to max of 100 mg (20 mL)) Both groups received standardized SA with 0.75% hyperbaric bupivacaine 11.25 mg + fentanyl 10 µg and at the end of surgery, rectal naproxen 500 mg + paracetamol 975 mg. Both had same post‐op analgesia regimen with 500 mg naproxen every 12 h standing, oral hydromorphone 2 mg‐4 mg every 4 h as needed with IV PCA (bolus 1.5 mg, lockout 7 min, max 10 mg/h) if needed Adjuvants: none Immediate post‐op pain control: pain scores were higher in participants receiving a TAP block at all time points but this was only significant at 10 h; statistically significant increase in morphine consumption 24 h post‐op in TAP group, but not at earlier time point |
|
| Outcomes | Dichotomous: pain/no pain "in the operative area" at 3 months Continuous: none Adverse events: incidence of wound infection, nausea/vomiting, pruritus, sedation |
|
| Notes | We contacted the study author for clarification on participant flow details, but received no response. Funding sources: "the authors received no external funding for this project." Conflicts of interest: "Dr Joanne Douglas is an Editor of the International Journal of Obstetric Anesthesia. She had no involvement with the editorial process or decision to accept this article." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned using "computer‐generated table" after consent and enrolment |
| Allocation concealment (selection bias) | Low risk | Quote: "group allocation was concealed in an opaque envelope until the woman was consented and enrolled" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "women, postoperative care providers..were blinded to treatment group...The anaesthesiologist caring for the woman, as well as the anaesthesiologist performing the TAP block, were not blinded". Bias during operation by non‐blinded providers possible, e.g. by administering additional morphine, but not very likely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "women, postoperative care providers and research staff collecting postoperative data were blinded to treatment group" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 69 women were randomized, but 1 in intrathecal morphine group and 2 in TAP group were excluded because of protocol violation. 3‐month follow‐up was obtained from 31 (of 33) in group 1 and 28 (of 33) in group 2. Numbers of attrition provided per group, fairly balanced. However, numbers presented in text do not match the numbers presented in the flow chart (reversed groups) |
| Selective reporting (reporting bias) | Low risk | Primary outcome in protocol fully reported on. Investigator left the study and this led to premature termination of the study before the intended time. |
| Null bias | High risk | Quote: "pain scores on rest and movement were higher in the TAP block group at all times although this only reached statistical significance at 10 h (P = 0.001)" |
Lu 2008.
| Methods | Placebo‐controlled, randomized clinical trial Sequence generation was randomized Follow‐up: 6 months |
|
| Participants | Participants: 105 adults in a university setting in Guangdong, China Operation: thoracotomy for tumour resection 3 groups, size randomized (completed): 36 (32)/36 (30)/33 (28) Age (median group 1, 2, 3): 57, 55, 59 years Men/women (group 1, 2, 3): 24/8, 18/12, 20/8 Remarks: 2 participants excluded intraop, 13 participants excluded post‐op with group allocation not specified |
|
| Interventions |
Group 1 (preincision epidural): epidural at T7/8, 3 mL 1% lidocaine (test dose), preincision 10 mL ropivacaine (0.25%, with morphine 0.2 mg/mL) epidurally, GA, post‐op 2 mL/h (0.15% ropivacaine and 1.5 µg/kg/mL morphine) epidurally for 48 h, additional analgesics and rescue medication not described Group 2 (post‐op epidural): epidural at T7/8, 3 mL 1% lidocaine (test dose), GA, post‐op 2 mL/h (0.15% ropivacaine and 1.5 µg/kg/mL morphine) epidurally for 48 h, additional analgesics and rescue medication not described Group 3 (control): GA (0.1 mg fentanyl), post‐op IV fentanyl (0.25 µg/kg/mL at basal 2 mL/h + 0.05 mg/mL demand) for 48 h, additional analgesics and rescue medication not described Adjuvants: none Immediate post‐op pain control: significantly improved |
|
| Outcomes | Dichotomous: pain at 3 and 6 months Continuous: not reported |
|
| Notes | Article published in Mandarin. Data extracted from the abstract and tables, methodological information extracted with the help of a Mandarin‐speaking statistician. Funding sources: source of funding not reported Conflicts of interest: conflict of interest statement not given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation was by "random numbers generation". Bias is unlikely. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. Bias is possible, but unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the attending physician called the patient". No detail provided neither in the English abstract nor the Mandarin methods section. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "the attending physician called the patient". No detail provided neither in the English abstract nor the Mandarin methods section. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was described with reasons, but it is unclear what the reasons for the attrition were in each group. Attrition was larger in control group. No ITT analysis described. Bias is likely. |
| Selective reporting (reporting bias) | Low risk | No protocol available, primary outcomes specified in text fully reported on |
| Null bias | Low risk | Quote: "VAS scores in the first 48h after operation were significantly lower in group PE and group E than in the group IV (P < 0.05)" |
McKeen 2014.
| Methods | Double‐blinded (participant, outcome assessor) randomized placebo‐controlled clinical trial Sequence generation by computer‐generated random numbers Follow‐up: 6 months |
|
| Participants | Participants: 74 pregnant women from university hospital in Halifax, Canada Operation: scheduled caesarean delivery (planned SA) 2 groups, size: 35/39 (completed) Age (± SD), group 1, 2: 32.1 (± 5.3), 31.4 (± 5.8) All female participants Comorbidities: gravidity (n) 1/2/3/4/5, group 1, 2: 1/1/11/16/5, 2/1/12/15/9; parity (n) 0/1/2/3, group 1, 2: 7/21/7/0, 10/18/10/1 |
|
| Interventions |
Group 1 (ropivacaine): at conclusion of surgery, 20 mL 0.25% ropivacaine injected deep to tissue fascial plane between interior oblique and transversus abdominis Group 2 (placebo): at conclusion of surgery, 20 mL 0.9% saline injected deep to tissue fascial plane between interior oblique and transversus abdominis. All participants received antacid prophylaxis. Standardized spinal anaesthetic technique hyperbaric bupivacaine, fentanyl, morphine. At conclusion of procedure, ketorolac, ondansetron, paracetamol and bilateral TAP blocks under ultrasound. Post‐op pain control with naproxen 250 mg every 8 h, paracetamol 1 g every 6 h, and oxycodone 2.5 mg‐5mg every 6 h as needed. Adjuvants: none Immediate post‐op pain control: no significant decrease in pain or morphine consumption |
|
| Outcomes | Dichotomous: none Continuous: SF‐36 Other: adverse effects reported on include nausea, vomiting, pruritus, urine retention |
|
| Notes | We acknowledge the study author's response that no dichotomous pain data were collected at 6 months, only SF‐36 Funding sources: "Dr McKeen acknowledges the support of the Canadian Anesthesiologists’ Society (CAS) GE Healthcare Canada Research Award in Perioperative Imaging Operating Grant. Dr George held an IWK Recruitment & Establishment Grant and acknowledges the support of a CAS Career Scientist Award. Dr Allen held a Canadian Institutes of Health Research New Investigator Award and a Dalhousie University Clinical Research Scholar Award. Dr Pink acknowledges Dalhousie University Medical Research Foundation Summer Research Studentship Funding." Conflicts of interest: "none declared" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated block randomized table. Blocks were permuted at ten patients per block with equal allocation of patients between the two groups" |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed opaque envelopes" labelled with a study number based on order of recruitment with randomization to 1 of two groups (A or B) inside envelope. The pharmacy supplied sterile blinded study drug syringes labelled TAP Block Study Drug ‘‘A’’ or ‘‘B’’ |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The pharmacy supplied sterile blinded study drug syringes labelled TAP Block Study Drug ‘‘A’’ or ‘‘B’’. Quote: "prior to each patient’s discharge from the PACU (once spinal motor block had regressed), one of the investigators (D.M. or R.G.) assessed the adequacy of the TAP." This was only known after the participant had left the PACU and was receiving the same ward orders no matter what group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "research personnel unaware of the patients’ randomization or adequacy of block assessment collected data until the patients left the PACU (minimum two hours), then 24 h and 48 h postoperatively via a ward visit... research personnel contacted patients via telephone at 30 days and six months to complete a five minute Short Form‐36 Health Survey (SF‐36)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Balanced, low rates of attrition between groups. Reasons for exclusion/missing data are listed for each group. |
| Selective reporting (reporting bias) | Low risk | Quote: "trial registration was not congruent with the final study protocol and did not include cumulative opioid consumption at 24 h postoperatively as a primary outcome". However, this value was not statistically significant and did not add effect to their results, thus low risk of reporting bias. |
| Null bias | High risk | Quote: "pain scores at 24 hr were slightly higher in the TAP 0.25% ropivacaine group. These differences were not statistically significant" |
Micha 2012.
| Methods | Double‐blinded (participant/outcome assessor), placebo‐controlled, randomized clinical trial Sequence generation by computer‐generated random numbers Follow‐up: 6 months |
|
| Participants | Participants: 35 adults in a hospital setting, Athens, Greece Operation: modified radical mastectomy with axillary dissection Groups, size: 17/18 Age: not specified All female participants, 13/7 Comorbidities: none included |
|
| Interventions |
Group 1 (ropivacaine): intra‐op: infiltration before wound closure with 10 mL ropivacaine 7.5 mg/mL. 3 mL of the solution was infiltrated around the route sheath of brachial plexus and the rest of it in the 1st‐7th intercostal spaces. Group 2 (saline): intra‐op: same method as above with infiltration of saline Adjuvants: none Immediate post‐op pain control: no difference |
|
| Outcomes | Dichotomus: pain questionnaire at 6 months Continuous: none Other reported: none Adverse events: none reported |
|
| Notes | Funding sources: no explanation of financial support Conflicts of interest: conflict of interest statement was not provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Response from study author: "Randomisation was done by means of a computer generated table" |
| Allocation concealment (selection bias) | Low risk | Response from study author: "Mrs Vassi was the only person throughout the study period that was aware of the allocation group. She didn’t participate in any other part of the study pre‐ or postoperatively nor did she have any contact with the patients at any time." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Response from study author: "The anaesthesiologists in the operating room were unaware of the allocation group and so was the surgeon." The participants were also unaware of their group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Response from study author: "...the record of the pain medicines administered and the telephone contact 6 months postoperatively were performed by me, who I was unaware of the study group throughout the study period." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "thirty‐five patients were enrolled in the study and six of them were excluded (failure to be contacted by phone)." |
| Selective reporting (reporting bias) | Low risk | No subgroup analysis or selective reporting was noted |
| Null bias | High risk | Quote: "no difference was documented...in chronic neuropathic pain." "Ropivacaine infiltration does not seem to attenuate chronic neuropathic pain...after modified radical mastectomy." |
Mounir 2010.
| Methods | Double‐blinded (participant/outcome assessor), placebo‐controlled, randomized clinical trial Sequence generation unclear Follow‐up: 6 months |
|
| Participants | Participants: men in a military teaching hospital in Rabat, Morocco Operation: inguinal hernia repair groups, size: 20/22 Age: years (range ): 46 ± 5; 40 ± 4 Men/women (group 1, 2): 20/0; 22/0 Comorbidities (group 1, 2, 3): none reported Remarks: only ASA I and II |
|
| Interventions |
Group 1 (bupivacaine wound infiltration): spinal (12.5 mg hyperbaric bupivacaine + 25 µg fentanyl, intrathecally), postincision SC infiltration of the skin with bupivacaine (0.5%, 20 mL), post‐op 1 g paracetamol, ketoprofen (100 mg), morphine 3 mg as needed for breakthrough pain Group 2 (saline/placebo wound infiltration): spinal (12.5 mg hyperbaric bupivacaine + 25 µg fentanyl, intrathecally), postincision SC infiltration of the skin with saline (0.9%, 20 mL), post‐op 1 g paracetamol, ketoprofen (100 mg), morphine 3 mg as needed for breakthrough pain Adjuvants: none Immediate post‐op pain control: significantly improved |
|
| Outcomes | Dichotomous: pain/no pain at 3 and 6 months, (pain differentiated in mild, moderate and severe) Continuous: none Secondary: |
|
| Notes | The report leaves it unclear if postoperative analgesics were given intravenously or orally. We contacted the study author for clarification of randomisation, allocation and blinding methods, but did not get a response. Funding sources: no funding sources specified Conflicts of interest: no conflict of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “etude prospective randomisee”, (prospective randomized trial) “La randomisation etait realise au cours de la visite preanesethesique par envelopes cachetees et numerotees...” (the randomization was realized during the preoperative visit with numbered and sealed envelopes) Even so the study is reportedly "randomized", the randomization method is not explained, hence bias is possible. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “la randomisation etait realise au cours de la visite preanesethesique par envelopes cachetees et numerotees...” It is unclear if and how and how long the allocation was concealed to the person enrolling the participants or to the anaesthesia provider. Bias is therefore possible. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “l’anesthesiste remettait au chirurgien une seringue”, “le chirurgien, qui ignorait la solution de infiltration”, (The anesthesiologist passed a syringe to the surgeon, ... the surgeon did not know the solutions to be infiltrated.) Possibly no blinding of the anaesthesia providers, but participant and surgeon were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:” a six mois" "evaluee grace a un questionnaire rempli par tous les patients lors de leur consultation de chirurgie de controle?”. (at six months ... evaluated by a questionnaire filled out by all participants during their surgical follow‐up visit) The outcome observer (surgeon) was blinded and the outcome was reported with the use of a questionnaire. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The uneven numbers of 22 and 20 in both groups leaves open the possibility of an error in the allocation process, cross over, attrition or incorrect randomisation and this is not addressed in the report. Bias seems still unlikely, due to the low attrition. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes fully reported on |
| Null bias | Low risk | Quote: "there was a significant reduction of postoperative pain in the bupivacaine group at rest as well as with coughing" |
O'Neill 2012.
| Methods | Single‐blind (outcome assessor), RCT Sequence generation by computer‐generated random numbers Follow‐up: 3 months |
|
| Participants | Participants: 67 women aged 18‐50 years, gestational age 37‐42 at hospital setting in Lisbon, Portugal Operation: elective caesarean section delivery (with Pfannenstiel incision) Groups, size: 29/29 Age (years ± SD; group 1, group 2): 33 ± 5, 33 ± 5 Men/women (group 1, 2): 0/29, 0/29 Primary caesarean delivery (n, group 1/2): 25/24 |
|
| Interventions |
Group 1 (continuous wound infusion group): anaesthesia was performed through SAB with hyperbaric bupivacaine and sufentanil with single‐shot SA. Intra‐op: catheter placed in wound below fascia after peritoneum closed, 10 mL ropivacaine 10 mg/mL injected during wound closure, then continuous infusion ropivacaine 2 mg/mL at 5 mL/h for 48 h Group 2 (epidural morphine): anaesthesia initiated with combined spinal‐epidural technique to site epidural catheter, single‐shot SA. Intra‐op: upon partial recovery from motor blockade (Bromage score 2), initiated 2 mg/10 mL bolus epidural morphine every 12 h (x 4 times). Neither group received any preanaesthetic medication. Both received standardized post‐op analgesia with paracetamol 1 g every 6 h x 48 h, breakthrough pain (VAS > 3) with IM diclofenac 75 mg every 6 h as needed, ondansetron 4 mg IV for nausea or vomiting as needed Adjuvants: none Immediate post‐op pain control: significantly improved |
|
| Outcomes | Continuous: presence or absence of "residual pain related to the scar or pain that the patient related to caesarean delivery" at 3 months Dichotomus: none Other reported: neurologic sequelae (paraesthesia, tactile hyperaesthesia), surgical wound healing impairment, surgical wound infection, impact on care provided to newborn/relationship, satisfaction score all at 3 months Adverse events: nausea, vomiting and anti‐emetic therapy requirements, incidence of pruritus, urinary retention, sedation, incidence of neurologic alterations (paraesthesia, tactile hyperaesthesia, headache) |
|
| Notes | Because no events were detected in either arm, we could not include the study in the meta‐analysis. Funding sources: "Dr Patricia O'Neill received speaker fees from Baxter Healthscore in 2010. B. Brain and Baxter were contacted simultaneously by authors to provide devices to perform the study. B Braun declined and Baxter showed interest and provided the devices for the study. Dr O'Neill helped design the study, conduct the study, analyse the data and write the manuscript and was paid by the company providing the devices for the study, to speak, after the study was finished being conducted but the results were not yet published. All four other authors reported no conflict of interest." Conflicts of interest: "we do not see a conflict of interest for the authors and no risk of bias of undue sponsor influence." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated random number list" |
| Allocation concealment (selection bias) | Low risk | Quote: " list concealed in an opaque envelope". Randomization was done after consent and prior to initiation of anaesthesia. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The intraoperative and postoperative anaesthesia managers were not blinded, nor were the surgeons, This is acceptable for inclusion. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Three months after discharge, patients were interviewed by telephone by an investigator blinded to group assignment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Per protocol analysis done, no ITT analysis. Number of participants in each group who were excluded is given, as well as the reasons for exclusion (e.g. accidental removal of catheter, did not receive allocated intervention, etc). Low overall attrition, fairly balanced numbers between groups. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes listed in manuscript completely reported on. No protocol available for review. |
| Null bias | Low risk | Pain scores (quote:) "at rest at 2, 6, and 48 hours were lower in the continuous wound infusion group than in the epidural morphine group... (pain scores) evaluated at mobilization were higher in the epidural morphine group at 2 and 6 hours" |
O'Neill 2014.
| Methods | Double‐blinded (participant/outcome assessor), placebo‐controlled, randomized clinical trial Sequence generation unclear Follow‐up: 4‐6 months |
|
| Participants | Participants: 40 adults in a university setting, Nashville, TN, USA Operation: ICBG for spinal fusion Groups, size: 20/20 Age (± SD), group 1, 2: 66 (± 12), 62 (± 8) Men/women (group 1, 2): 13/7, 13/7 Comorbidities: tobacco use, group 1, 2 (18, 16); alcohol use, group 1, 2 (7, 6) |
|
| Interventions |
Group 1 (bupivacaine): intra‐op: rectangular window of approximately 4 × 1 cm was created in the cortex of the posterior superior iliac spine using osteotomes and was then hinged open to allow access to cancellous bone. After graft harvest, a gel foam soaked in 10 mL 0.25% bupivacaine was packed into the wound. The cortical bone window was replaced and the wound closed. Group 2 (saline): intra‐op: same method of gel‐foam packing into cortex of posterior superior iliac spine. Gel was soaked in 10 mL 0.9% saline. Adjuvants: none Immediate post‐op pain control: not reported |
|
| Outcomes | Continuous: VAS at 4‐6 months Dichotomus: none Other reported: surgical data included the type of surgery, surgical indication, number of levels fused, the use of instrumentation, and the operative time. Health outcomes were back and neck pain, satisfaction with surgical results, and mental/physical states as determined by the Short Form‐12. Adverse events: 1 participant in the saline group had infection |
|
| Notes | The reported continuous data were insufficient for inclusion in the additional Bayesian inclusive analysis. Funding sources: "the authors have no relevant financial relationships to disclose." Conflicts of interest: conflicts of interest statement not provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a block randomization scheme was used," but the method of randomization was not described. |
| Allocation concealment (selection bias) | Low risk | Quote: "a sealed envelope containing the group assignment was opened and the appropriate intervention was performed" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and surgeons were blinded, but knowledge of anaesthesia team not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all forms were administered and collected by a research nurse without knowledge of the assigned group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19/20 in the treatment group and 17/20 in the control group completed the final evaluation. Quote: "this met the goal of 17 patients per group as determined from the sample size calculation." |
| Selective reporting (reporting bias) | Unclear risk | The protocol defined the VAS at 3 months as the primary outcome, but it remained unclear from the manuscript if the pain was recorded at rest or at movement and if the current or the average pain was the initial primary outcome. |
| Null bias | Low risk | Experimental treatment was effective in improving immediate postoperative pain control for some outcome measures at least. |
Okur 2016.
| Methods | Randomized clinical trial Sequence generation by "simple random sampling" Follow‐up for 6 months |
|
| Participants | Participants: 90 adults in a university setting in Turkey Operation: inguinal herniorrhaphy 3 groups, size: 30/30/30 Age (± SD), group 1, 2, 3: not described Men/women, group 1, 2, 3: not described Exclusion criteria: not described |
|
| Interventions |
Group 1 (spinal): SAB was administered. Further detail about anaesthetic regimen and timing of intervention was not provided. Group 2 (TAP): in addition to SAB, TAP block was performed. No additional detail about anaesthetic regimen or timing of intervention provided. Group 3 (IINB): in addition to SAB, ilioinguinal/iliohypogastric nerve block was performed. No additional detail about anaesthetic regimen or timing of intervention provided. Adjuvants: none Immediate post‐op pain control: significantly improved |
|
| Outcomes | Dichotomous: none Continuous: NRS score Other reported: NRS score and amount of analgesia given in perioperative period |
|
| Notes | Published only as abstract. We were unable to obtain data on pain outcomes or additional information about randomization and blinding methods from the study author. Funding sources: funding of study not described Conflicts of interest: the study authors have no conflicts of interest to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generation by, quote: "simple random sampling" |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Rate of attrition not described |
| Selective reporting (reporting bias) | Unclear risk | Unclear if subgroup analysis performed |
| Null bias | Low risk | Quote: "NRS scores ... in TAP block were significantly smaller in all measurements..."." |
Paxton 1995.
| Methods | Double‐blind, placebo‐controlled, randomized clinical trial Sequence generation "at random", but not described Follow‐up: 12 months |
|
| Participants | Participants: 70 adults from a university setting in Belfast, Northern Ireland Operation: vasectomy for contraception 2 groups, size: 70 total, (group size not given) Age: years (range ): 35 years (range 26‐45), 34 years (28‐45) Men/women: 70/0 Remarks: in the intervention group, body sides were randomized to receive treatment or placebo. |
|
| Interventions |
Group 1a (intervention, body side treated): GA, intraop: bupivacaine (0.5% 1 mL) injected into the lumen of the vas deferens, post‐op NSAID Group 1b (intervention, placebo body side): GA, intraop: normal saline injected into the lumen of the vas deferens, post‐op NSAID Group 2 (control, both sides): GA, intraop: no injection, post‐op NSAID Adjuvants: none Immediate post‐op pain control: significantly improved |
|
| Outcomes | Dichotomous: testicular discomfort at 12 months Continuous: duration of testicular discomfort Secondary: none |
|
| Notes | No available contact info to email study author to inquire about study sponsorship Funding sources: source of funding not reported Conflicts of interest: no conflict of interest statement given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly....at random..," but exact method of sequence generation not reported. Still, with excellent description of allocation concealment and blinding, we judge that bias is unlikely. |
| Allocation concealment (selection bias) | Low risk | Allocation was done after education and enrolment, (it remains unclear when the vas deferens side was randomized, but this is unlikely to cause bias.) Bias is unlikely. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Bias during operation by non‐blinded providers possible, e.g. by administering additional fentanyl, but not very likely. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all the replies were analysed by one of the authors who was unaware of the treatment". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "the questionnaire was valid for 61 (91%) patients only." Six participants did not respond and "...three were excluded because of development of wound infection and scrotal haematoma." A per‐participant analysis was performed, withdrawals and attrition were reported, but allocation to groups or subgroup was not reported. Bias is likely, but unlikely to change the result of the study. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all specified outcomes were reported on. |
| Null bias | Low risk | Quote: "the VAS scores for pain on days 1..were significantly lower on the side of the bupivacaine infiltration in the treatment group compared with the saline side of this group and the control group" |
Pinzur 1996.
| Methods | Double‐, possibly triple‐blind (participant, provider and possibly outcome assessor), placebo/sham‐controlled randomized clinical trial Sequence generation "with use of a table of random numbers" Follow‐up: 6 months |
|
| Participants | Participants: 21 adults, at a university setting, Chicago, Illinois, USA Operation: lower limb amputation because of ischaemic necrosis secondary to peripheral vascular disease 2 groups, size: 11/10 Age: 68.3 years (SD ± 12.96) Men/women: 10/11 Comorbidities: diabetes mellitus in 9 participants |
|
| Interventions |
Group 1 (treatment): GA or spinal, post‐op nerve sheath irrigation (bupivacaine 0.5%, 1 mL/h) and PCA (morphine, no basal rate, demand 2 mg, lockout 15 min, max 30 mg/4 h) for 72 h Group 2 (placebo): GA or spinal, post‐op nerve sheath irrigation (normal saline, 1 mL/h) and PCA (morphine, no basal rate, demand 2 mg, lockout 15 min, max 30 mg/4 h) for 72 h Adjuvants: none Immediate post‐op pain control: significantly improved analgesic consumption |
|
| Outcomes | Dichotomous: pain at 6 months Continuous: McGill Pain Questionnaire at 6 months Secondary: none |
|
| Notes | Reported data not allocated to groups. No graphics that reported data. We contacted the study author for missing information and outcome data. He responded that the data were not accessible. Hence, outcome data could not be included. Funding sources: "no benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. No funds were received in support of this study." Conflicts of interest: no conflicts of interest statement given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were 'divided into two groups with use of a table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the patients and the staff were blinded to the contents of the bag, which were known only to the research pharmacist." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinding was not described, but (quote:) "the patients and the staff were blinded to the contents of the bag, which were known only to the research pharmacist." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The study authors report on attrition, (2 participants died, 5 did not participate in the questionnaire), but patients lost to follow up were neither allocated to groups nor considered for an ITT analysis. The authors found no statistically meaningful difference in phantom pain, but it remains unclear which participant numbers were taken as the basis for their analysis. An ITT analysis would likely only have confirmed the lack of significance, however. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes appropriately reported on |
| Null bias | Low risk | Quote: "the patients in Group A used significantly less morphine during the first and second days after the operation than did those in Group B" |
Purwar 2015.
| Methods | Randomized clinical trial Sequence generation by computer‐generated random numbers Follow‐up: 3 months |
|
| Participants | Participants: 60 adults in a university setting in the UK Operation: vaginal surgery for pelvic floor disorders (tape, repair, or hysterectomy) 2 groups, size: 29/31 Age (± SD), group 1, 2: 65.1 (12.5), 60.6 (11.5) All women Exclusion criteria: American Society of Anesthesiologists (ASA) grade 3, contraindication to Spinal Anesthesia (SA), a lack of capacity to provide consent, and an inability to read and write in English. |
|
| Interventions |
Group 1 (GA): anaesthesia was induced with propofol (3 mg/kg) and maintained with isoflurane in oxygen‐enriched air to achieve an inspired oxygen fraction (FiO2) of 33%. Ondansetron 4 mg IV was given as prophylaxis against postoperative nausea and vomiting. The operating surgeon was a urogynaecology consultant (JC) or specialist trainee signed off as competent for independent practice for the type of surgery performed. Anaesthesia was provided by 1 of two anaesthetic specialists (NT or AF). Anaesthesia was augmented by surgical infiltration with LA solution comprising 30 mL of 0.5% levobupivacaine, 27 mL of normal saline and 3 mL of adrenaline 1:10,000. Hypotension (systolic blood pressure < 85 mmHg) was treated with metaraminol in aliquots of 0.5 mg and bradycardia (heart rate < 60 beats per min) was treated with glycopyrrolate in aliquots of 200 µg. Women were prescribed ibuprofen 400 mg every 4 h orally with food when required and either co‐codamol (30/500) two tablets every 4 h or paracetamol 1 g IV or orally every 4 h. If pain was not controlled with the above regimen, morphine was prescribed. Postoperative nausea and vomiting were initially treated with prochlorperazine 12.5 mg IM every 6 h with ondansetron 4 mg to8 mg IV if required. Group 2 (SA): a 25‐G Whitacre needle was inserted at the L3‐L4 interspace following skin infiltration with 1% lidocaine, under aseptic conditions, the participant in the sitting position. Initially, the SA regimen consisted of 1 mL of 0.5% hyperbaric bupivacaine with 10 µg of fentanyl diluted to a volume of 3.0 mL using normal saline. Participants remained in the sitting position for 5 min following the introduction of SA. However, owing to suboptimal pain control in the first few participants, the protocol was revised and the spinal anaesthetic mixture was amended to 2.0 mL 0.5% heavy bupivacaine with 10 µg fentanyl, diluted to 3 mL, with the participant’s position immediately changed to semi‐recumbent following spinal injection. Participants’ complaints of pain were treated with IV fentanyl in aliquots of 50 µg. Additional intraoperative sedation was achieved by IV midazolam as required. Levobupivacaine was used to augment anaesthesia as described above. Hypotension was treated as described above. Adjuvants: fentanyl Immediate post‐op pain control: no improvement |
|
| Outcomes | Dichotomous: none Continuous: VAS score, SF‐36 Other reported: VAS in the perioperative period 2 h, 24 h, 2 weeks, Incontinence Modular Questionnaire on Vaginal Symptoms (ICIQ‐VS), data regarding the time taken from the induction of anaesthesia to commencing surgery, operating time, duration of stay in the postoperative recovery room in min, use of analgesia postoperatively, and length of hospital stay |
|
| Notes | We acknowledge the response provided by the study author regarding blinding, randomization, allocation concealment and source of funding and conflict of interest statement. Funding sources: "this study was funded by a Research Award from the North Staffordshire Medical Institute, UK." Conflicts of interest: the study authors have no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "an internet‐based sequence allocation randomisation was carried out by the Nottingham (UK) Clinical Trials Support Unit with random permuted blocks of randomly varying size." |
| Allocation concealment (selection bias) | High risk | Quote: "The anaesthetist was informed of the random allocation allocated by the computer." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study author responded, quote: "Owing to the nature of the interventions, it was not possible to blind either patients or the assessing team to the intervention given." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Significant attrition |
| Selective reporting (reporting bias) | Low risk | No subgroup analysis was performed |
| Null bias | High risk | Quote: "no statistically significant differences were noted between the groups with regard to pain..." |
Senturk 2002.
| Methods | Single‐blind (outcome assessor), clinical RCT Sequence generation was random, but not described Follow‐up: 6 months |
|
| Participants | Participants: 112 adults at a university setting in Istanbul, Turkey Operation: open thoracotomy for a mix of lung resections 3 groups, size: 28/29/28 Age (group 1, 2, 3): 49 (SD 9), 52 (SD 11), 50 (SD 11) years Men/women: 56/13 (reported at end of study) Comorbidities: not reported |
|
| Interventions |
Group 1 (preincision): epidural at T7‐8, preincision bupivacaine bolus 10 mL, 7 mL/h infusion (0.1% + 0.1 mg/mL morphine), GA, post‐op 48 h PCEA (0.1% bupivacaine + 0.05 mg/mL morphine, basal rate 5 mL/h, demand 3 mL, lockout 30 min) Group 2 (postsurgery): epidural at T7‐8, GA (fentanyl), postsurgical bupivacaine bolus 10 mL (0.1% + 0.1 mg/mL morphine), post‐op 48 h PCEA (0.1% bupivacaine + 0.05 mg/mL morphine, basal rate 5 mL/h, demand 3 mL, lock time 30 min) Group 3 (control): GA (fentanyl), PCA (morphine, bolus 5 mg, no basal rate, demand 2 mg, lockout 15 min) Adjuvants: none Immediate post‐op pain control: significantly improved |
|
| Outcomes | Dichotomous: pain at 6 months, pain affecting daily life at 6 months Continuous: NRS at 6 months Secondary: none |
|
| Notes | Regional anaesthesia catheter placement was verified under fluoroscopy. The study author responded and provided additional information regarding randomization allocation concealment, sources of funding and conflicts of interest. Funding sources: "the study was not funded" Conflicts of interest: the authors "have no conflict of interest" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were "randomly divided into three groups", "using sealed envelopes technique." |
| Allocation concealment (selection bias) | Low risk | Quote: “randomization was performed at the first presentation of the patient to our department, i.e. 5‐7 days before the operation (just before the anaesthetic evaluation). The result of the randomization was "hidden" by the secretary of the department until the operation date.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "patients were not blinded to group, anaesthesia providers aware of allocation at least during treatment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors "were blinded to the analgesic method." Blinding of only outcome assessors is acceptable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Allocation of excluded participants is not reported, no ITT analysis was considered. Considerable attrition prior to, during and after intervention make bias likely. Adverse effects were not, but attrition was described albeit without group allocation 27 participants were excluded preoperatively, 6 intraoperatively, and 10 postoperatively, without specification of their group allocation. Comorbidities were the preoperative, inoperability the intraoperative and recurrence of pain due to metastasis & reoperation were the postoperative exclusion criteria. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes included |
| Null bias | Low risk | Quote: "during movement and cough, Group Pre‐TEA had significantly less pain compared with the other two groups during the entire period. At rest, patients in Group Pre‐TEA reported having significantly lower pain scores during the first 12 h compared with those in Group Post‐TEA and during the first 48 h compared with those in Group IV‐PCA. There were statistically significant differences between Group Post‐TEA and Group IV‐PCA during rest from 8 h after surgery until the end of 48 h, but no difference during cough or movement was recorded" |
Shahin 2010.
| Methods | Double‐blinded (participant/outcome assessor), placebo/sham‐controlled, randomized clinical trial Sequence generation by computer‐generated random numbers Follow‐up: 8 months |
|
| Participants | Participants: parturients in a university setting in Assiut, Egypt Operation: caesarean section for delivery groups, size: 185/185 Age: 25 years (SD ± 1.5 ) Men/women (group 1, 2): 0/185, 0/185 Comorbidities (group 1/2/3): none reported Remarks: |
|
| Interventions |
Group 1 (intraperitoneal lidocaine instillation): spinal (details not reported), postincision, preperitoneal closure single‐shot instillation of peritoneal lidocaine (2%, 10 mL) into the pelvis, post‐op paracetamol 1 g intravenously every 6 h for 36 h, rectal suppository of 10 mg followed by oral 400 mg ibuprofen for 72 h, plus intravenous morphine 2 mg for breakthrough pain Group 2 (intraperitoneal placebo/saline instillation): spinal (details not reported), postincision, preperitoneal closure single‐shot instillation of peritoneal saline (0.9%, 10 mL) into the pelvis, post‐op paracetamol 1 g intravenously every 6 h for 36 h, rectal suppository of 10 mg followed by oral 400 mg ibuprofen for 72 h, plus intravenous morphine 2 mg for breakthrough pain Adjuvants: none Immediate post‐op pain control: significantly improved |
|
| Outcomes | Dichotomous: overall pain/no pain at 8 months, differentiated also in wound, global abdominal and epigastric pain Continuous: at 8 months: NRS |
|
| Notes | Funding sources: "No ... funding acknowledgement was declared by either of the authors." Conflicts of interest: the study authors have no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based random allocation |
| Allocation concealment (selection bias) | Low risk | Placed in sealed, opaque, consecutively numbered envelopes... just after providing consent the women were given the next number on the random list..., (allocation) was concealed from the residents and caregivers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the surgeon involved complied with the instruction but was not further involved" data "collection sheets with corresponding codes,.. a number of syringes equal in size;" "preparation and administration of the medication was carried out by a nurse not involved in the management of the patient", "access to randomization code was only available to the secretary of the statistics department", "randomization code was not broken until the completion of the study". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "access to randomization code was only available to the secretary of the statistics department", "randomization code was not broken until the completion of the study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was per protocol, not ITT, but the low number of participants lost to follow‐up with almost equal attrition in both groups and the similar demographics in both groups make bias unlikely. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all outcomes specified in the article were reported on. |
| Null bias | Low risk | Quote: "control group patients received significantly more morphine injections in the first 24 hours than lidocaine patients". Significantly more participants in the control group reported pain in all sites in the first 24 h than in the lidocaine group. |
Singh 2007.
| Methods | Triple‐blind (participant/provider/outcome assessor), placebo‐controlled, clinical RCT Sequence generation by a computer‐based, random numbers generator Follow‐up: mean of 4.7 years (range 4.5–5.4 years) |
|
| Participants | Participants : 26 adults in a university setting, Houston, Texas, USA Operation: ICBG for spinal arthrodesis 2 groups, size: 11/14 Age (all, 1, 2): 64 (range 34‐84), 66, 63 years Sex: not reported Comorbidities: not reported Remarks: 11 anterior ICBG included in the initial stage were later excluded |
|
| Interventions |
Group 1 (treatment): GA, at closure continuous wound irrigation (bupivacaine hydrochloride and epinephrine (Marcaine) 0.5% 2 mL/h) for 48 h post‐op + PCA (hydromorphone hydrochloride (Dilaudid)) (basal, bolus and lock‐out time not specified) Group 2 (control): GA, at closure continuous wound irrigation (normal saline, 2 mL/h) for 48 h post‐op + PCA (Dilaudid) (basal, bolus and lock‐out time not specified) Adjuvants: none Immediate post‐op pain control: significantly improved |
|
| Outcomes | Dichotomous: graft site pain at around 55 months Continuous: VAS at around 55 months Secondary: pain frequency in days, functional activity score, overall satisfaction with the surgical procedure at around 55 months |
|
| Notes | Funding sources: "no funds were received in support of this work" Conflicts of interest: "no benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the method used to generate the randomization consisted of a computer‐based number generator. Moreover, to account for the size of the sample groups, randomization attempted to balance baseline characteristics by stratification, such as age." |
| Allocation concealment (selection bias) | Low risk | Quote: "the participants were randomized and allocated by a different individual than the one who enrolled the patient." "Randomization and allocation to group type was concealed and not made public to the individual enrolling the patients, the treating physician, or to the nursing staff." "Patients were assigned to receive either one or the other (treatment) solutions at the time of surgery based on a coded sequence enclosed within an envelope." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "blinded and identical in appearance, solutions of saline and Marcaine were prepared." "Physicians, patients, nursing staff, and research personnel conducting the statistical analyses were blinded to the infusion solution until the end of the study to minimize potential for performance and detection bias." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the physician conducting the telephone interview as well as recording the data were blinded to the treatment group." "Research personnel conducting the statistical analyses were blinded to the infusion solution until the end of the study to minimize potential for performance and detection bias." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors report details of attrition with reference to the groups participants were randomized to. "An intent‐to‐treat analysis was considered to preserve randomization and to offer the best representation of the clinical population." "Even if we assume that any treatment patient that was lost to follow‐up (n = 6 patients) was considered to be a failure (chronic dysesthesias, an ICBG VAS score of 8, 15 days of narcotic usage/mo, functional activity score of 4, and an overall dissatisfaction with the procedure), a statistical difference was still noted in the 2 groups (p = 0.05)." |
| Selective reporting (reporting bias) | Low risk | Primary outcomes fully reported on |
| Null bias | Low risk | Quote: "narcotic dosage, demand frequency, and mean VAS pain score were significantly less in the treatment (Marcaine) group at 24 and 48 hours" |
Singh 2013.
| Methods | Double‐blinded (participant/outcome assessor), randomized clinical trial Sequence generation by a computer‐based, random numbers generator Follow‐up: 3 months |
|
| Participants | Participants: 60 women at a university hospital in Ontario, Canada Operation: caesarean section Groups, size: 20/20/20 Age (± SD), group 1, 2, 3: 33 (± 3), 32 (± 7), 33 (± 4) All female participants Comorbidities: previous caesarean delivery, groups 1, 2, 3 (16, 14, 15) Remarks: ASA I, II, and III |
|
| Interventions | All participants received SA with 0.75% bupivacaine 10 mg‐12 mg, fentanyl 10 µg and morphine 150 µg Group 1 (high‐ropivacaine): post‐op: a 22‐G, 50 mm or 80mm Pajunk Uniplex nanoline needle was introduced into the fascia between the internal oblique and transversus abdominis muscles. After confirmation of needle placement, the study solution was injected in 5 mL increments after negative aspiration. Study solution for high‐ropivacaine group consisted of 0.5% ropivacaine 3 mg/kg (up to a maximum of 300 mg) plus saline to total 60 mL of fluid. TAP blocks were performed bilaterally. Group 2 (low‐ropivacaine): post‐op: same method as group 1, but study solution consisted of 0.25% ropivacaine 1.5 mg/kg (up to a maximum of 150 mg) plus saline to total 60 mL.TAP blocks were performed bilaterally. Group 3 (placebo): post‐op: TAP blocks consisting of 60 mL of saline were administered bilaterally using same method as groups 1 and 2. Adjuvants: none Immediate post‐op pain control: no difference |
|
| Outcomes | Dichotomus: none Continuous: NRS at 3 months Other reported: the time to first request for additional analgesia, the total consumption of opioids, antiemetics and anti‐pruritics 72 h postoperatively Adverse events: none reported |
|
| Notes | Funding sources: "this study was supported in part by a grant from the Lawson Health Research Institute." Conflicts of interest: "the authors have no conflicts of interest to declare." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned using a computer generated table of random numbers to one of three groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "group allocations were concealed in sealed opaque envelopes that were opened only after patient consent was obtained.." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the patients, anesthesiologists, and nursing staff involved in direct patient care were unaware of the study group allocations." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "patients were interviewed at regular intervals by an investigator unaware of group allocation..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 60 participants enrolled, 59 completed the study. |
| Selective reporting (reporting bias) | Low risk | No subgroup analysis or selective reporting was noted. |
| Null bias | High risk | Quote: "neither high‐ or low‐dose TAP blocks as part of a multimodal analgesia regimen including intrathecal morphine improved pain scores." |
Smaldone 2010.
| Methods | Double‐blinded (participant/outcome assessor), randomized clinical trial Sequence generation not specified Follow‐up: 3, 6 months |
|
| Participants | Participants: 60 men in a hospital setting in Philadelphia, PA Operation: open radical retropubic prostatectomy Groups, size: 29/31 Age: not specified All male participants |
|
| Interventions |
Group 1 (multimodal analgesia): pre‐op: PVB with 5 mL of 0.5% ropivacaine per level (T10‐T12) and oral celecoxib (400 mg preoperatively and 200 mg twice daily for 7 days postoperatively). Intra‐op: IV ketamine (10 mg) following induction. Post‐op: all participants had access to morphine (PCA) Group 2 (PCA): pre‐op: participants received placebo equivalents as treatment group ‐ sham tablets and sham saline injections. Post‐op: all participants had access to morphine (PCA) Adjuvants: none Immediate post‐op pain control: significantly improved, significantly reduced analgesic consumption |
|
| Outcomes | Continuous: SF‐36 at 3, 6 months Dichotomus: none Other reported: VAS at 24 hours, morphine consumption postoperatively Adverse events: none reported |
|
| Notes | We were unable to obtain additional information regarding pain outcomes or about randomization and blinding methods from the study author. Funding sources: none received Conflicts of interest: conflict of interest not discussed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "all patients, staff and physicians were blinded to treatment group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Amount of follow‐up and attrition not specified |
| Selective reporting (reporting bias) | Low risk | No subgroup analysis or selective reporting was noted |
| Null bias | High risk | Quote: "there were no significant differences detected in SF‐36 scores at 2, 12, and 24 weeks." |
Sprung 2006.
| Methods | Single‐blinded (outcome assessor), randomized clinical trial Sequence generation via computer‐generated list Follow‐up: 3 months |
|
| Participants | Participants: 89 women from a university hospital in Minnesota, USA Operation: elective vaginal hysterectomy (with or without repair of cystocoele and rectocoele) 2 groups, size: 45/44 Age (± SD), group 1, 2: 52.2 (± 11.9), 51.8 (± 12.8) All female participants Comorbidities: postmenopausal, group 1, 2: 21/17. Procedure, group 1, 2: hysterectomy only 27/27, hysterectomy + cystocoele 1/1, hysterectomy + rectocoele 4/4, hysterectomy + cystocoele + rectocoele 13/7 |
|
| Interventions |
Group 1 (regional): sedation with IV midazolam and propofol. SAB performed in lumbar region between 3rd and 5th vertebral bodies. After cerebrospinal fluid free flow, 0.75% hyperbaric bupivacaine (15 mg), preservative‐free clonidine (1 µg/kg), morphine (2 µg/kg, max 200 µg) injected to subarachnoid space. Intraoperative sedation with IV midazolam and propofol as needed. No intraoperative IV opioids. 30 mg ketorolac IV at end of surgery. On floor IV PCA 1.0 mg every 10 min with 4‐h lock out max of 15 mg in regional group (lower than general group, to decrease likelihood of delayed respiratory depression). Additional IV morphine per attending physician as needed Group 2 (general): 2 µg/kg fentanyl after pre‐oxygenation GA with sodium thiopental, succinylcholine, vecuronium bromide, isoflurane and 50% inspired nitrous oxide. A morphine sulphate 0.1 mg/kg IV in divided doses, no additional morphine was allowed. All participants received 30 mg IV ketoralac at end of surgery. On floor IV PCA 1.0 mg every 10 min, 4‐h lockout max of 30 mg Both groups: in PACU 2 mg IV morphine every 5‐10 min as needed for NRS > 3. On floor, morphine PCA, with differences in maximum noted above. Scheduled ketorolac 30 mg IM every 8 h until oral D3. After 24 h, IV PCA stopped and oral paracetamol and codeine (650 mg/30 mg) every 6 h as needed. In both groups, pruritis managed with diphenhydramine then naloxone if needed. Nausea/vomiting managed with droperidol, if later stages ondansetron, then naloxone if persisted. Adjuvants: clonidine (into subarachnoid space) Immediate post‐op pain control: significantly improved, significantly reduced analgesic consumption |
|
| Outcomes | Dichotomous: none Continuous: NRS at 3 months, SF‐36 pain subcomponent at 3 months Secondary: none Effective regional anaesthesia: reported. "Confirmation of an adequate dermatomal level of blockade" Adverse events reported on included use of intraoperative pressors, nausea/vomiting, pruritis |
|
| Notes | We acknowledge the study author's clarification on blinding methods. Funding sources: "intramural grant from the Mayo Foundation." Conflicts of interest: "none declared." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated list" |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were randomized...using a sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The anaesthesiologist, participants and providers were not blinded. This is acceptable for our purposes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | SF‐36 was filled out by participant and mailed in at 12 weeks. Study author contacted, stated the research co‐ordinator performing telephone follow‐up "was blinded regarding the study group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote. "in three patients in the SAB group, the block failed and the patient received general anesthesia. For all analyses presented in this report these patients are included in the SAB group (intention‐to‐treat)". Fairly balanced, low rate of participants lost to follow‐up at 12‐week follow‐up. |
| Selective reporting (reporting bias) | Low risk | All primary outcomes fully reported on. |
| Null bias | Low risk | Quote: "the patients in the general anesthesia group received more morphine in the PACU... compared to patients receiving SAB" and this continued into the 12 hours after PACU discharge. Numerical pain score values tended to be lower in participants receiving SAB compared to the general anesthesia group through 14:00 hr on postoperative day two (the day after surgery), with significant differences noted at the time of floor arrival and at 14:00 hr on postoperative day two" |
Strazisar 2012.
| Methods | Double‐blinded (participant/outcome assessor), randomized clinical trial Sequence generation by a computer‐based, random numbers generator Follow‐up: 3 months |
|
| Participants | Participants: 60 women in a hospital setting in Ljubljana, Slovenia Operation: breast cancer surgery with axillary lymphadenectomy Groups, size: 30/30 Age (all, 1, 2): 60 (30‐84), 57.4, 62.9 All female participants Comorbidities: diabetes, groups 1, 2 (4, 8); depression, groups 1, 2 (1, 4) Remarks: ASA I, II, and III |
|
| Interventions |
Group 1 (levobupivacaine): intra‐op: before wound closure, a fenestrated wound catheter was placed near the axillary vein and upon the whole length over the upper side of the wound. The wound catheter was fenestrated along 15 cm in the distal part. A bolus of 15 mL of 0.25% levobupivacaine was injected into the wound through the catheter immediately after wound closure. Surgical drains and the fenestrated catheter were clamped for 5 min to enable bolus absorption. Elastomeric pump was connected containing 100 mL of 0.25% levobupivacaine. Infusion at 2 mL/h was continuous for 50 h Group 2 (piritramide): intra‐op: continuous IV infusion with piritramide (30 mg), metoclopramide (20 mg) and metamizole (2.5 g) in 100 mL of 0.9% sodium chloride (3 mL/h to 6 mL/h) until 24 h postoperatively Adjuvants: none Immediate post‐op pain control: significantly improved, significantly reduced analgesic consumption |
|
| Outcomes | Continuous: none Dichotomus: overall pain/no pain at 3 months Other reported: nausea, opioid consumption, and length of hospital stay and were measured Adverse events: 3 participants (2, 1) underwent additional surgical procedures due to haematoma and 9 participants (5, 4) experienced inflammation postoperatively |
|
| Notes | Funding sources: no funding source given Conflicts of interest: "no potential conflicts of interest were disclosed." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed using random numbers generated by a computer |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization and numbers were placed in sealed opaque envelopes to ensure concealment of allocation at enrollment." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "participants were randomly grouped." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "clinicians who recorded data about chronic pain were blinded about randomisation group of patients." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the follow‐up evaluation. |
| Selective reporting (reporting bias) | Low risk | No subgroup analysis or selective reporting was noted. |
| Null bias | Low risk | Quote: "pain (at 3 months) was reported by 17% and 50% of patients." Continuous infusion of local anesthetic reduced pain compared to control. |
Strazisar 2014.
| Methods | Doubl‐ blinded (participant/outcome assessor), randomized clinical trial Sequence generation by a computer‐based, random numbers generator Follow‐up: 3 months |
|
| Participants | Participants: 60 women in a hospital setting in Ljubljana, Slovenia Operation: radical mastectomy and breast reconstruction Groups, size: 30/30 Age (range, 1, 2): 25‐64, 47.6, 48.0 All female participants Comorbidities: smoking, groups 1, 2 (9, 10); depression, groups 1, 2 (3, 1) Remarks: ASA I, II, and III |
|
| Interventions |
Group 1 (levobupivacaine): intra‐op: before wound closure, a fenestrated wound catheter was placed under the pectoralis major muscle and upon the entire length over the upper side of the wound. The wound catheter was fenestrated along 15 cm in the distal part. A bolus of 15 mL of 0.25% levobupivacaine was injected into the wound through the catheter immediately after wound closure. Surgical drains and the fenestrated catheter were clamped for 5 min to enable bolus absorption. Elastomeric pump was connected containing 100 mL of 0.25% levobupivacaine. Infusion at 2 mL/h was continuous for 50 h. Group 2 (piritramide): intra‐op: continuous IV infusion with piritramide (30 mg), metoclopramide (20 mg) and metamizole (2.5 g) in 100 mL of 0.9% sodium chloride (3 mL/h to 6 mL/h) until 24 h postoperatively. Adjuvants: none Immediate post‐op pain control: significantly improved, significantly reduced analgesic consumption |
|
| Outcomes | Continuous: none Dichotomus: overall pain/no pain at 3 months Other reported: nausea, opioid consumption, and length of hospital stay were measured. Adverse events: 2 participants (1, 1) underwent additional surgical procedures due to haematoma, 4 participants (1, 3) experienced inflammation postoperatively, and unilateral lymphoedema of the arm was present in 2 participants (1, 1) |
|
| Notes | Funding sources: "study was entirely financed by the Institute of Oncology as a part of public service." Conflicts of interest: "the authors declare that they have no competing interests." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was made by using random numbers generated by a computer." |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization and numbers were placed in sealed opaque envelopes to ensure concealment of allocation at enrollment." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were blinded, but no description of medical staff's knowledge other than, quote: "after randomization... the principal investigator was informed about the treatment allocation of the patient." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "data about pain were collected by nursing staff, that is, by an independent observer." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the follow‐up evaluation. |
| Selective reporting (reporting bias) | Low risk | No subgroup analysis or selective reporting was noted. |
| Null bias | Low risk | Quote: "in the test and the control groups of patients, pain was reported in 16.7% (5/30) and 50% (15/30), respectively." "We observed that patients treated with a LA experienced a lower frequency of chronic pain compared to patients treated with standard analgesic." |
Tecirli 2014.
| Methods | Double‐blinded (participant/outcome assessor), randomized clinical trial Sequence generation not described Follow‐up: 3 months |
|
| Participants | Participants: 60 women in university hospital in Ankara, Turkey Operation: radical mastectomy (with axillary lymph node dissection) Groups, size: 30/30 Age: not listed All female participants Comorbidities: not listed |
|
| Interventions |
Group 1 (bupivacaine): intra‐op: intercostobrachial nerve was blocked with 10 cc 0.5% bupivacaine before being sectioned Group 2 (control): intra‐op: intercostobrachial nerve sectioned without blockage Adjuvants: none Immediate post‐op pain control: no difference |
|
| Outcomes | Continuous: VAS at 3 months Dichotomus: pain questionnaire at 3 months Other reported: analgesic consumption Adverse events: reported as none |
|
| Notes | Pain score ≥ 4 was accepted as pain Funding sources: no explanation of financial support Conflicts of interest: no conflict of interest statement given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not explained |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not explained |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of medical personnel not explained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Knowledge of outcome assessors not indicated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the follow‐up evaluation |
| Selective reporting (reporting bias) | Low risk | No subgroup analysis or selective reporting was noted |
| Null bias | Low risk | Quote: "this study shows that intercostobrachial nerve block is an effective method to reduce the chronic neuropathic pain development after a breast cancer surgery." |
Terkawi 2015b.
| Methods | Triple‐blind (participant/provider/outcome assessor), placebo‐controlled, randomized clinical trial Sequence generation using website random number generator Follow‐up: 6 months |
|
| Participants | Participants: 61 adult patients at a university hospital in Virginia, USA Operation: mastectomy (including simple and modified radical, with or without axillary dissection) for breast cancer surgery 2 groups, size: 27/34 Age (± SD), group 1, 2: 55.2 (± 10.9), 55.0 (± 13.7) All female participants Exclusion criteria: Age > 80 Comorbidities: simple mastectomy (n), group 1, 2: 19/20. Modified radical (n), group 1, 2: 8/14. Axillary direction (n), group 1, 2: 3/13. Breast implant (n), group 1, 2: 5/8. Chemotherapy, (n), group 1, 2: 11/18. Radiotherapy (n), group 1, 2: 9/14. Hormone therapy (n), group 1, 2: 10/7 Remarks: the demographic data above are for participants who were available for follow‐up at 6 months and included in the analysis. |
|
| Interventions |
Group 1 (placebo): 0.9% NaCl IV infusion beginning before induction, at equal volume to lidocaine group, until 2 h after arrive to PACU or at discharge from PACU (whichever earlier) Group 2 (lidocaine): 2 mg/kg/h IV lidocaine infusion beginning before induction (max 200 mg/h) until 2 h after arrive to PACU or at discharge from PACU (whichever earlier) Both groups: lidocaine bolus before induction, up to 1.5 mg/kg, max 150 mg. Premedication, induction drug, muscle relaxant for GA chosen by anaesthesiologist. Maintenance sevoflurane. Post‐op analgesia fentanyl 50 µg every 10 min as needed or morphine 4 mg every 20 min as needed, with morphine PCA if needed. Nausea treated with ondansetron 4 mg IV as needed then promethazine 6.25 mg IV every 20 min as needed. Adjuvants: none Immediate post‐op pain control: no significant improvement |
|
| Outcomes | Dichotomous: pain/no pain at 6 months Continuous: VAS collected but not reported Other: logistic regression model (Best model) to assess efficacy of lidocaine Adverse events: incidence of lymphoedema, evidence of lidocaine toxicity, post‐surgery infection or complications |
|
| Notes | Funding sources: "the study was funded by the Department of Anesthesiology, University of Virginia, Charlottesville, VA." Conflicts of interest: "the authors declare no conflict of interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a website random number generator was used (www.randomization.com)...and the patient was asked to select one envelope on the morning of surgery." |
| Allocation concealment (selection bias) | Low risk | Quote: "numbers were concealed in opaque sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "both the patients and research team remained blinded until after all data were analysed." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "a research associate, who was blinded to treatment group and management, conducted a telephone interview with the patients 6 months after surgery." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "seven patients in the placebo group and 3 in the lidocaine group could not be reached for follow‐up, despite multiple phone call attempts (14% dropout). Therefore, we analysed 61 patients, 27 in the placebo group and 34 in the lidocaine group". Slightly higher loss in the placebo group but overall low numbers of attrition. |
| Selective reporting (reporting bias) | Low risk | The study maintained a defined protocol, which they did not deviate from. |
| Null bias | High risk | Quote: "the mean postoperative pain scores at rest (Fig. 2A) were 3.88 ± 2.92 at 2 hours, 2.66 ± 2.66 at 24 hours, and 3.09 ± 2.80 at 48 hours in the placebo group, whereas they were 2.94 ± 2.74 at 2 hours, 2.91 ± 2.21 at 24 hours, and 2.72 ± 2.25 at 48 hours in the lidocaine group. Overall pain scores in both groups were similar with no statistical difference by repeated‐measures ANOVA ". No significant difference in pain scores on movement or perioperative morphine consumption either. |
Vrooman 2015.
| Methods | Triple‐blinded (participant, provider, outcome assessor), placebo‐controlled, randomized clinical trial Sequence generation by computer‐generated random numbers Follow‐up for 3 and 6 months |
|
| Participants | Participants: 78 adults in a university setting in USA Operation: robotic cardiac surgery 2 groups, size: 39/39 Age (± SD), group 1, 2: 56 (11), 58 (10) Men/women, group 1, 2: 31/8, 29/10 Exclusion criteria: history of severe psychiatric issues (e.g. depression, somatoform conversion disorder, and borderline personality disorder); addiction to alcohol, opioids, or illegal substances; known history of sensitivity to amide LAs; severe hepatic disease; or pregnant |
|
| Interventions |
Group 1 (lidocaine): anaesthetic technique not described. The 5% lidocaine transdermal patches contained 700 mg of lidocaine. Each self‐adhesive patch was 10 cm x 14 cm. Up to 3 patches were applied to maximize analgesia while reducing the risk of systemic toxicity. Patches were applied for 12 h, removed for the subsequent 12 h, and then new patches were applied. This process was continued for 6 months or until participants no longer required analgesia. Additional postoperative analgesia was provided by participant‐controlled fentanyl (20 mg bolus, 6‐min lockout, no hourly limit). Morphine or hydromorphone was substituted in participants reporting sensitivity to fentanyl. PCA was continued for up to 3 days, with the exception of a single participant who was treated for 5 days, until participants could tolerate oral opioid medications such as oxycodone 5 mg to 10 mg every 4‐6 hours as needed. Participants who required more than 40 mg of oxycodone, or equivalent, per day were supplemented with fentanyl 25 mg/h transdermal patches Group 2 (control): same intervention as above except sham patches were used. Adjuvants: none Immediate post‐op pain control: no improvement |
|
| Outcomes | Dichotomous: none Continuous: VAS/VRS Secondary: VAS at POD 3; VRS at 1 week and 1 month, the Depression Anxiety Stress Score recorded the day before surgery, GPE‐a measure of participant satisfaction, recorded after 1 week, 1 month, 3 months, and 6 months. PDI at 3 and 6 months |
|
| Notes | Funding sources: funding for the study was provided by Endo Pharmaceuticals. Conflicts of interest: "none of the authors has a personal financial interest in this research." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed by our Research Pharmacy and was based on computer‐generated codes" |
| Allocation concealment (selection bias) | Unclear risk | Allocation of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "all investigators and clinicians were fully blinded to treatment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "incisional pain was evaluated over 6 months with data collected by an independent study coordinator who was blinded to treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no attrition and ITT analysis was performed |
| Selective reporting (reporting bias) | Low risk | No subgroup analysis was performed |
| Null bias | High risk | Quote: "lidocaine 5% patches did not influence any measure of acute or persistent incisional pain" |
Weber 2007.
| Methods | Single‐blinded (outcome observer) clinical RCT Sequence generation via computer‐generated randomization list Follow‐up: 6 months |
|
| Participants | Participants children and adolescents ≥ 10 years at a university hospital in Vienna, Austria Operation: pectus excavatum repair (minimally invasive using a thorascope for creation of retrosternal tunnel) 2 groups, size: 20/20 Age (± SD), group 1, 2: 16.7 (± 5.2), 14.8 (± 4.2) Men/women, group 1, 2: 17/3, 15/5 Comorbidities: except for 1 participant in TEA group, all procedures were primary operations. Vertebral index (vertebral diameter x 100/sagittal diameter + vertebral diameter), group 1, 2 (± SD) = 32.05 (± 36.2), 31.85 (± 4.15) |
|
| Interventions |
Group 1 (PCA): post‐op IV PCA 0.02 mg/kg morphine bolus, lockout 6 min, max 6 bolus/h, no continuous rate. Postoperatively, both groups 1 mg/kg diclofenac IV every 8 h scheduled until POD 4, rescue pain medication with IV paracetamol 15 mg/kg, followed by 1.5 mg piritramide IV bolus as needed Group 2 (TEA): catheter placed once in operating room by median approach at T6/7 or T7/8 corresponding with likely insertion site of steel bar. After induction, bolus of 0.2 mg/kg ropivacaine 0.2% with 2 µg/mL fentanyl, then continuous rate of 0.2 mL/h same mixture throughout surgery, continued until POD 4 (96 h). Post‐op scheduled 1 mg/kg diclofenac IV every 8 h until POD 4 rescue pain medication with IV paracetamol 15 mg/kg, followed by epidural bolus of 0.1 mL/kg ropivacaine 0.2% with 2 µg/mL fentanyl as needed. Both groups received standardized GA with propofol, fentanyl, rocuronium. 15 min before end, IV paracetamol bolus Adjuvants: none Immediate post‐op pain control: significantly improved |
|
| Outcomes | Dichotomous: pain/no pain at 3 and 6 months Continuous: VAS pain score 3 and 6 months Secondary: satisfaction with type of anaesthesia at 3 and 6 months Adverse events reported: sedation, nausea, pruritis |
|
| Notes | Presence of pain defined by VAS ≥ 3. We acknowledge the study author for providing response regarding VAS cutoff for presence of pain, allocation concealment, blinding and source of funding. Funding sources: "AstraZeneca, Bristol Myers‐Squibb, and Smiths Medical Austria supported the study with an unrestricted grant". We contacted the study author on their specific involvement, who responded, "Funding by the three companies included just paying for the insurance (approximately one third by each company). None of the companies were involved in conducting the study or writing the manuscript." Conflicts of interest: no direct conflicts of interest statement given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated randomization list" |
| Allocation concealment (selection bias) | Low risk | Study author specified "Group allocation was concealed in an opaque envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, surgeons and providers were not blinded. The study author clarified that "the PCA pump and the TEA continuous infusion (depending on the study group) were hidden from the persons assessing the VAS scores". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study author stated "For postoperative data collection, the PCA pump and the TEA continuous infusion (depending on the study group) were hidden from the persons assessing the VAS scores. The persons who made the follow up questioning [at 3 and 6 months] were unaware to which group the patients were assigned". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study author specified "All 40 patients were available at three and 6 months for follow‐up". |
| Selective reporting (reporting bias) | Low risk | Primary outcomes fully reported on |
| Null bias | Low risk | Quote: "Patients treated with a thoracic epidural catheter after pectus excavatum repair reported lower postoperative pain scores... than did patients treated with intravenous PCA containing morphine. Postoperative pain scores in the intravenous PCA group were higher despite higher intraoperative fentanyl use in the intravenous PCA group" |
Wodlin 2011.
| Methods | Single‐blinded (outcome assessor), clinical RCT Sequence generation using computer‐generated block randomization table Follow‐up: 6 months |
|
| Participants | Participants: 162 women aged 18‐60 from five hospitals in Sweden Operation: abdominal subtotal or total hysterectomy (for benign gynaecological disorders) 2 groups, size: 80/82 Age (range), groups 1, 2: 45 (33‐58), 46 (35‐58) All female participants Exclusion criteria: former or concomitant bilateral oophorectomy, postmenopausal without hormone therapy, gynaecological malignancy (cervical dysplasia not included) Comorbidities: indication of hysterectomy, group 1, 2: bleeding disturbances: 46, 46, mechanical symptoms: 27, 29, cervical dysplasia or endometrial hyperplasia: 4, 5, endometriosis or dysmenorrhoea: 3, 2. Total abdominal hysterectomy, group 1, 2: 55/51. Subtotal abdominal hysterectomy, group 1, 2: 25, 31. Mode of skin incision, group 1, 2: midline: 6, 7, low transverse 74, 75 |
|
| Interventions |
Group 1 (GA): GA with propofol, fentanyl, rocuronium. 5 mg IV morphine administered 20 min before surgery complete Group 2 (SA): at L3/4 or L2/3 intervertebral space, 20 mg hyperbaric bupivacaine (5 mg/mL) and 0.2 mg morphine (0.4 mg/mL) administered. 15 min later, confirmed neural blockade with cold test. Sedation throughout operation with continuous IV propofol Both groups, 2 g oral paracetamol 1 h preoperatively. Surgeon injected 40 mL bupivacaine (2.5 mg/mL) SC and pre‐fascially in abdominal wall before end of surgery. Postoperatively, oral paracetamol and diclofenac scheduled 3 x day during hospitalization. Oral or IV opioids given if necessary. Rescue antiemetic with droperidol, then 5‐HT3 receptor antagonist if still necessary. Pruritus treated with clementine and if necessary, naloxone Adjuvants: none Immediate post‐op pain control: significantly reduced analgesic consumption |
|
| Outcomes | Dichotomous: none Continuous: SF‐36 at 6 months Other reported: list of major and minor complications |
|
| Notes | Funding sources: "the Medical Research Council of South East Sweden, Linköping University and the County Council of Östergötland supported the trial financially." Conflicts of interest: "the authors have stated explicitly that there are no conflicts of interest in connection with this article." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer generated the randomisation sequences into blocks of ten, with an equal number of the two modes of anaesthesia for each of the five participating centres" |
| Allocation concealment (selection bias) | Low risk | Quote: "the allocated mode of anaesthesia, written on a label, was sealed in opaque consecutively numbered envelopes. At each centre the envelopes were opened in consecutive number order of patient inclusion in the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "blinding and/or placebo control was not possible in this study. The temporary paralysis of the lower extremities after SA would, for obvious reasons, be observed immediately by the patient, as well as by the staff. The lack of blinding may pose a risk of bias. In order to reduce such potential bias the women were informed and monitored in a standardised fashion, and the mode of incision and type of abdominal hysterectomy were decided prior to randomisation" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported on whether outcome assessor was blinded or not |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "in the SF‐36, a missing cell was substituted by the truncated mean value of the other items in the specific subscale for the individual. If all cells in a subscale were missing, the cells were substituted by the truncated mean value of each cell in the group. If a questionnaire was missing completely on one occasion, each cell was substituted by the truncated mean value of the cell for the group on that occasion. Missing cells for the SF‐36 on all three occasions made up 0.44%, and a complete SF‐36 was missing in 2.26% (11 of 486 cases). " |
| Selective reporting (reporting bias) | Low risk | Primary outcomes fully reported |
| Null bias | Low risk | Quote: "spinal anaesthesia was associated with a significantly lower use of opioids" compared to general anaesthesia |
Xu 2017.
| Methods | Clinical RCT Sequence generation by computer‐generated random numbers Follow‐up for 3 months |
|
| Participants | Subjects: 71 adults in a military hospital in China Operation: thoracolumbar spinal surgery 2 groups, size: 35/36 Age (± SD), group 1, 2: 51.91 (11.44), 49.06 (11.20) Men/women, group 1, 2: 19/16, 19/17 Exclusion criteria: a history of cardiopulmonary disease, coagulation and merging with multiple injuries |
|
| Interventions |
Group 1 (ropivacaine): continuous wound infusion with ropivacaine was used as primary analgesia. This group received an initial wound infiltration with 6 mL 1% ropivacaine (100 mg; AstraZeneca AB, Sweden) and followed by continuous infusion with 0.33% ropivacaine via a double lumen catheter system at a rate of 5 mL/h (disposable postoperative local analgesia system, Beijing Heng Yuan Tongji Medical Technology Corporation, China) for 48 h. Participants in this group did not receive postoperative IV continuous constant‐dose analgesia (ICCA) for pain control. Participants were premedicated with phenobarbital 100 mg and atropine 0.5 mg, 30 min before the induction of anesthesia. After baseline measurements of heart rate, noninvasive blood pressure, respiratory rate and oxygen saturation, each participant was preoxygenated for 3 min before induction. All participants received the target‐controlled infusion with propofol 2‐ 3 μg/mL using the Marsh pharmacokinetic model and remifentanil at 3 ng/mL to 4 ng/mL using the Minto pharmacokinetic model for induction. Following the induction of anaesthesia, cisatracurium 0.15 mg/kg was given as an IV injection. After tracheal intubation, mechanical ventilation was initiated with 100% oxygen and adjusted to maintain the end tidal carbon dioxide tension between 35 mmHg and 45 mmHg. Intermittent bolus injection of cisatracurium was used to maintain full muscle relaxation. At the end of surgery, residual neuromuscular block was reversed, if needed, with a mixture of atropine and neostigmine. Participants were given pentazocine 60 mg when surgery was completed prior to extubation. All participants expanded on the use of the supplementary analgesic (flurbiprofen 50 mg IV injection) if necessary (VAS > 4) Group 2 (control): exactly the same as described above except there was no wound infiltration with ropivacaine. Additionally, this group relied on ICCA for postoperative pain control involving flurbiprofen axetil 150 mg, pentazocine 240 mg and palonosetron 0.5 mg in 100 mL normal saline, at a rate of 2 mL/h. All participants expanded on the use of the supplementary analgesic (flurbiprofen 50 mg IV injection) if necessary (VAS > 4) Adjuvants: none Immediate post‐op pain control: no improvement |
|
| Outcomes | Dichotomous: pain vs no pain Continuous: none Other reported: demographic and operation data including disease, date of birth, gender, operating time, preoperative VAS, perioperative remifentanil and propofol doses, and length of surgical incision, pain score at rest during first 48 h postoperative using VAS, and Ramsay scores, times of rescue analgesia requests, incidence of postoperative nausea and vomiting, antiemetic therapy requirements and incidence of pruritus (participants were asked about the desire to scratch) at 2, 4, 6, 12, 24, 36 and 48 h postoperatively |
|
| Notes | We were unable to obtain additional information about randomization and blinding methods from the study author. Funding sources: funding for the study was provided by Guangzhou General Hospital of Guangzhou Military Command. Conflicts of interest: "all the authors declare they have no competing of interests." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "All participants were randomly assigned using a computer‐generated random number table." |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No sham was employed and blinding of participants/personnel not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All enrolled patients successfully completed the study and were included in the main analysis." |
| Selective reporting (reporting bias) | Low risk | No subgroup analysis was performed |
| Null bias | High risk | "There were no significant differences in the pain level between the two groups" |
Zhou 2016.
| Methods | Double‐blinded, placebo controlled, randomized clinical trial Sequence generation not described Follow‐up for 3 months |
|
| Participants | Subjects: 106 adults in a university setting in China Operation: craniotomy 2 groups, size: 53/53 Age (± SD), group 1, 2: not described Men/women, group 1, 2: not described Exclusion criteria: not described |
|
| Interventions |
Group 1 (ropivacaine): after the anesthesia induction, skin along the incision was infiltrated with 0.5% ropivacaine. Morphine was used as rescue analgesic postoperatively. Anaesthetic regimen not further described. Group 2 (control): exactly the same as above except 0.9% saline was substituted for ropivacaine. Adjuvants: none Immediate post‐op pain control: significantly improved |
|
| Outcomes | Dichotomous: pain vs no pain Continuous: VAS Other reported: morphine consumption, heart rate and mean arterial pressure were recorded before anesthesia induction, after anesthesia induction, after scalp infiltration, during skull drilling, mater cutting, and skin closure |
|
| Notes | We were unable to obtain additional information about randomization and blinding methods from the study author. Funding sources: funding of study not described Conflicts of interest: study authors declare no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Sham block was used. Blinding of personnel not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Rate of attrition not described |
| Selective reporting (reporting bias) | Unclear risk | Unclear if subgroup analysis was performed |
| Null bias | High risk | Quote: "the incidence of pain... showed no difference between groups." |
5‐HT3: 5‐hydroxytryptamine; ANOVA: analysis of variance; ASA: American Society of Anesthesiology perioperative risk classification; BPI: brief pain inventory; EMLA: eutectic mixture of local anaesthetics; Epi: epinephrine; GA: general anaesthesia; h: hour; HRQOL: health‐related quality of life; ICBG: iliac crest bone graft harvesting; IM: intramuscular; ITM: intrathecal morphine; ITT: intention‐to‐treat; IV: intravenous; Kg: kilogram; L2: lumbar segment number 2; LA: local anaesthetic; LMA: laryngeal mask airway; MAC: minimum alveolar concentration; mg: milligram; mL: millilitre; NIH: National Institute of Health; NSAID: nonsteroidal anti‐inflammatory drugs; NRS: numerical rating scale; paracetamol: acetaminophen; PACU: postanaesthesia care unit; PCA: participant controlled analgesia; PCEA: patient controlled epidural analgesia; POD: postoperative day; PVB: paravertebral block; RCT: randomized controlled trial; SA: spinal anaesthesia; SAB: subarachnoid block; SC: subcutaneous; SD: standard deviation; SF‐36: Short Form (36) Health Survey; SF‐MPQ‐2: Short Form MacGill Pain Questionaire; T4: thoracic segment 4; TAP: transabdominal plane block; TEA: thoracic epidural analgesia; µg: microgram; VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdel‐Salam 1975 | Study comparing different epidural LA mixtures for analgesic effect, 2 days after surgery. No long‐term outcomes recorded |
| Aveline 2011 | Participants undergoing day‐case open inguinal hernia repair with mesh given TAP block or ilioinguinal/iliohypogastric nerve block. No control group. VAS scores at 3 and 6 months |
| Bach 1988 | Pseudo‐clinical RCT (sequence generation by means of patients' year of birth) investigating epidural analgesia before limb amputation for chronic phantom pain with a follow‐up of 12 months |
| Bamigboye 2013 | Outcome was attenuation of (pre‐existing) chronic pelvic pain. The primary outcome of interest for this review, (new onset wound pain persisting for > 3 months after surgery) was not measured |
| Baral 2010 | Study assessing effectiveness of preoperative IV lidocaine infusion on post‐op pain, however, no chronic pain outcomes assessed |
| Batoz 2009 | Follow‐up only 2 months in this RCT of scalp infiltration for craniotomy |
| Blumenthal 2011 | Comparing regional technique against combination of regional techniques |
| Borgeat 2001 | Outcome: regional anaesthesia complications associated with interscalene block |
| Borghi 2010 | Non‐randomized prospective trial of perineural catheter for phantom limb pain |
| Brull 1992 | Non‐randomized observational study of continuous infusion through an iliac crest catheter for postoperative analgesia after ICBG harvesting |
| Cerfolio 2003 | Preincision epidural anaesthesia vs none for thoracotomy, but no control (as both groups had post‐op epidural anaesthesia) |
| Chelly 2011 | All participants received local wound infiltration and there was no control group without application of local or regional anaesthesia |
| Corsini 2013 | Article in French. Single‐dose intraincisional infiltration of levobupivacaine or placebo into wound after scheduled C‐section. Longest pain outcome at 2 months |
| da Costa 2011 | Excluded for pseudo‐randomization, this prospective trial investigated different anaesthetic techniques for the prevention of regional pain syndrome after carpal tunnel release |
| De Kock 2001 | Comparing IV ketamine to epidural ketamine to control as adjuvant therapy; all patients receiving LAs via epidural catheter |
| Duale 2009 | Comparison of ketamine or placebo in people undergoing thoracotomy. All participants received local ropivacaine administration at the edges of the thoracotomy and chest drainage orifices and in the inter pleural space postoperatively (thus no control group) |
| Eisenach 2010 | RCT comparing intrathecal bupivacaine with ketoralac vs saline for prevention of postoperative pain. All participants received intrathecal bupivacaine thus no control group |
| El‐Morsy 2012 | Randomized, blinded study comparing outcome of paravertebral block vs thoracic epidural block for post‐thoracotomy incision pain in paediatric patients. The primary objective was evaluation of immediate postoperative analgesia. Secondary objectives included hormonal responses, side effects, failure rate, and pulmonary function. No long‐term outcomes were measured |
| Elman 1989 | Comparing different doses of bupivacaine intrapleurally, no long‐term pain outcomes were measured |
| Farag 2013 | Patient on chronic opioids preoperatively |
| Gottschalk 1998 | Follow‐up only 9.5 weeks, in a double‐blind clinical RCT of 100 people undergoing elective radical retropubic prostatectomy for the treatment of prostate cancer. Epidural bupivacaine, epidural fentanyl, or no epidural drug was administered prior to induction of anaesthesia and throughout the entire operation resulting in more pain‐free participants at 9.5 weeks |
| Haythornthwaite 1998 | Study on prostatectomy with 3 groups: epidural anaesthesia only, combined epidural and general anaesthesia and general anaesthesia only. Total of 6‐month follow‐up. However, excluded because epidural PCA was provided with bupivacaine and fentanyl for all participants in the postoperative period, thus no control group |
| Hirakawa 1996 | Not randomized |
| Hivelin 2011 | Not a randomized trial but only a prospective blinded study of TAP block in breast reconstruction |
| Howell 2001 | Study designed to investigate differences in backache as complication/adverse effect of labour epidural |
| Ilfeld 2004 | Not a clinical RCT, but only case reports on 3 paediatric patients with continuous regional anaesthesia catheters, 2 patients with pain outcomes at 3 months |
| Ilfeld 2015 | Comparison of continuous vs single shot (regional vs regional) anesthesia |
| Jahangiri 1994 | Prospective, but not randomized study of preoperative epidural anaesthesia for phantom pain after limb amputation |
| Jirarattanaphochai 2007 | Excluded because chronic pain present at baseline and is reason for surgery |
| Joseph 2012 | RCT in which all participants received epidural catheter with participant‐controlled ropivacaine administration, comparing IV ketamine vs no ketamine in people undergoing thoracotomy. Follow‐up of 3 months post‐op |
| Kairaluoma 2010 | Comparing paravertebral block against local infiltration for hernia repair under SA |
| Kindberg 2009 | RCT comparing use of ear acupuncture vs LA in primiparous women with a vaginal delivery at term undergoing surgical repair of lacerations to the labia or the vagina, perineal lacerations of first or second degree or mediolateral episiotomies. Excluded because of traumatic reason for 'surgical' intervention (suturing), not an elective procedure |
| Kumar 1989 | Non‐randomized pilot study of 20 patients to examine post‐cholecystectomy pain relief of paravertebral block with bupivacaine, with or without adrenaline added. Alternating participants received adrenaline or did not |
| Kumar 2009 | Men undergoing totally extra‐peritoneal repair of groin hernia were randomized to pre‐peritoneal bupivacaine vs saline after mesh placement. All prospective trocar sites were infiltrated by bupivacaine in all cases, thus no control group without regional analgesia |
| Lambert 2001 | Comparing regional against regional technique: clinical RCT comparing preoperative epidural vs postoperative perineural catheter for risk reduction of phantom pain after limb amputation |
| Lebreux 2007 | Not comparing regional vs nonregional anaesthesia. 20 healthy parturients undergoing elective caesarean section under SA were randomized to receive spinal clonidine. Outcome was pain up to 6 months and hyperalgesia |
| Lee 2012 | RCT of patients undergoing video‐assisted thoracic surgery, with all participants receiving epidural ropivacaine and fentanyl, with or without magnesium sulphate |
| Loughnan 2002 | Controlled clinical trial designed to detect difference in backache as complication/adverse effect of labour epidural |
| Mendola 2012 | RCT evaluating use of S(+)‐ketamine for prevention of post thoracotomy pain syndrome at 6 months. Patients undergoing thoracotomy under general anaesthesia, with thoracic epidural catheter placed +/‐ IV infusion of ketamine vs IV placebo with 6 months post‐op follow‐up. All participants received epidural catheter with levobupivacaine, thus no control group |
| Milligan 2002 | Comparison of LA vs LA |
| Muthukumar 2012 | Prospective‐double blind RCT investigating haemodynamic effects, quality of surgical field and postoperative analgesia following surgical field infiltration with different concentrations of adrenaline with and without lignocaine in children undergoing cleft lip repair. Only immediate postop pain was recorded, no long‐term outcomes measured |
| Nabhan 2011 | Patients undergoing endoscopic carpal tunnel release under LA (prilocaine) vs IV regional anaesthesia (prilocaine) |
| Nikolajsen 1997 | Study excluded for pseudo‐randomization as discussed in (Appendix 9). Double‐blinded (patients and outcome assessors), pseudo‐randomized (sequence generation was by "the toss of a coin") controlled clinical trial on preoperative epidural analgesia for limb amputation with a follow‐up of 12 months including 60 adults in a university setting in Aarhus, Denmark |
| Obata 1999 | Comparing preincisional vs postincisional epidural anaesthesia for thoracotomy |
| Ochroch 2006 | Comparing preincisional vs postincisional epidural anaesthesia for thoracotomy |
| Ouaki 2009 | Prospective study examining continuous infusion of ropivacaine at iliac crest donor site in paediatric patients undergoing ICBG. However, non‐randomized with only 1 study group, all with same treatment (no control group) |
| Panos 1990 | RCT comparing IV vs epidural fentanyl, not LA vs control |
| Perniola 2009 | RCT of intra‐abdominal LA for abdominal hysterectomy. Follow‐up 3 months. Excluded because all 3 groups used LA infusions |
| Pompeo 2007 | Comparison of awake video‐assisted thoracoscopic bullectomy with pleural abrasion using thoracic epidural anaesthesia vs general anaesthesia (control) in treatment of spontaneous pneumothorax. No long‐term pain outcomes measured; follow‐up at 12 months was to elicit recurrences of pneumothorax |
| Rosen 2009 | Patients undergoing laparoscopic ventral hernia repair randomized to receive elastomeric pain pump with continuous LA vs saline. Each trocar site injected with LA in either group thus both groups received LAs. Total follow‐up 3 months |
| Royse 2007 | Measured outcome was a depression score, no chronic postsurgical pain measured |
| Ryu 2011 | Comparison of pre‐emptive thoracic epidural analgesia with or without ketamine in people undergoing operations using classic posterolateral thoracotomy incisions. Thus, no control group. Total follow‐up of 3 months post‐op |
| Saber 2009 | Follow‐up only 2 months |
| Salengros 2010 | RCT investigating pre‐ vs postoperative epidural anaesthesia after thoracotomy |
| Schaan 2004 | Pain outcomes measured < 3 months |
| Schley 2007 | Study on effect of adjuvants for LAs to prevent chronic postsurgical pain. All 19 participants received a continuous brachial plexus block for 1 week after the amputation of an upper extremity. In addition they were treated with the NMDA antagonist memantine or placebo for 4 weeks |
| Sen 2009 | RCT of 60 men aged 20‐40 years undergoing inguinal herniorrhaphy, comparing preoperative oral gabapentin to placebo and the effects on acute and long‐term pain. All participants received intrathecal bupivacaine. Follow‐up total of 6 moths post‐op |
| Shikano 1994 | RCT looking at the effect of wound infiltration with bupivacaine before insertion of trocars on post‐op pain and respiratory impairment in people undergoing laparoscopic cholecystectomy. No long‐term pain outcomes measured |
| Sim 2012 | Randomized trial investigating pre‐ vs postincisional pre‐emptive thoracic epidural analgesia for thoracotomy with outcomes at 6 months, but with no control group without regional anaesthesia |
| Suvikapakornkul 2009 | Pain outcomes measured only until 24 h post‐op; 3‐month follow‐up was only for recurrence and complications |
| Suzuki 2006 | Studying the adjuvant effect of IV ketamine vs placebo in 49 thoracotomy patients, all participants receiving ropivacaine with morphine via epidural analgesia for 2 days |
| Verma 2006 | Patients with chronic cholecystitis divided into 4 groups, to receive either saline or different combinations of bupivacaine at gallbladder bed and trocar sites. No long‐term pain outcome measures |
| Vigneau 2011 | Pain outcomes measured only up to 2‐month follow‐up in this RCT on would infiltration after breast surgery |
| Wang 1992 | Article in Mandarin. No comparison group without regional anaesthesia |
| Weihrauch 2005 | Comparing block vs block with no pain outcome measured |
| Wilson 2008 | RCT on patients undergoing lower limb amputation received combined intrathecal/epidural anaesthetic for surgery followed by epidural infusion with bupivacaine with ketamine vs bupivacaine with placebo (saline). No control group as both received LA |
| Yang 2012 | We acknowledge the study author's response to our inquiry; pain data only measured until 2 months post‐op |
ICBG: iliac crest bone graft; IV: intravenous; NMDA : N‐methyl‐D‐aspartate receptor; PCA: patient controlled analgesia; RCT: randomized controlled trial; SA: spinal anaesthetic; TAP: transabdominal plane block; VAS: visual analogue scale
Characteristics of studies awaiting assessment [ordered by study ID]
Capdevila 2017.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Found during top‐up search December 2017 |
Choi 2017.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Found during top‐up search December 2017 |
Elkaradawy 2012.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Found during top‐up search December 2017 |
Fiorelli 2016.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Found during top‐up search December 2017 |
Iohom 2006.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Found during top‐up search December 2017 |
Jendoubi 2017.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Found during top‐up search December 2017 |
Kendall 2018.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Found during top‐up search December 2017 |
Kim 2017.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Found during top‐up search December 2017 |
Oh 2017.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Found during top‐up search December 2017 |
Okur 2017.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Found during top‐up search December 2017 |
Reuben 2006.
| Methods | Double‐blinded (patient and outcome assessor), placebo‐controlled, RCT Sequence generation randomized follow‐up: 12 months |
| Participants | Participants : 80 adults, at a teaching hospital, Springfield, MA, USA Operation: lower limb amputation because of ischaemic necrosis, secondary to peripheral vascular disease 2 groups, size: 40/40 Age (group 1, 2): 68 years (SD ± 12 ), 65 years (SD ± 17) Men/women (group 1, 2): 23/17, 25/15 Comorbidities (group 1, 2): BKA:AKA ratio 29:11, 26:14 |
| Interventions |
Group 1 (treatment): GA (fentanyl), intra‐op perineural injection of bupivacaine 10 mL 0.25% and clonidine 100 µg, post‐op morphine IV and paracetamol (acetaminophen)/oxycodone orally Group 2 (placebo): GA (fentanyl), intra‐op perineural injection of placebo, post‐op morphine IV and paracetamol/oxycodone orally Adjuvants: clonidine perineurally Immediate post‐op pain control: statistically meaningful reduction in analgesic consumption |
| Outcomes | Dichotomous: phantom limb pain and stump pain at 12 months Continuous: not reported Secondary: not reported |
| Notes | The sciatic nerve was infiltrated for AKA or the posterior tibial nerve for BKA. We could not make sense of some numbers reported on attrition. As reported 22 January 2009, SS Reuben was accused of publishing fraudulent data. Up to 22 papers have been or will be retracted by the journals in which they have been published (Retraction notice Anesthesia and Analgesia 20 February 2009 (Shafer 2009)). This article appears not to be among the retracted manuscripts. We placed it in the classification pending section on the advice of Cochrane Anaesthesia, Critical and Emergency Care. |
Zwaans 2017.
| Methods | Not yet assessed |
| Participants | Not yet assessed |
| Interventions | Not yet assessed |
| Outcomes | Not yet assessed |
| Notes | Found during top‐up search December 2017 |
AKA: above‐the‐knee amputation; BKA: below‐the‐knee amputation; GA: general anaesthesia
Characteristics of ongoing studies [ordered by study ID]
ISRCTN46621916.
| Trial name or title | Study protocol for a double blind, randomised, placebo‐controlled trial of continuous subpectoral local anaesthetic infusion for pain and shoulder function following mastectomy: SUB‐pectoral Local anaesthetic Infusion following MastEctomy (SUBLIME) study |
| Methods | Single‐blinded (outcome observer) clinical RCT Sequence generation via computer‐generated randomization list follow‐up: 6 months |
| Participants | Participants: all women presenting for unilateral mastectomy surgery at the Royal Cornwall Hospitals NHS Trust and Royal Devon and Exeter NHS Foundation Trust, aged ≥ 18 years Operation: mastectomy with or without axillary involvement 2 groups, size: N/A Age (range), groups 1, 2: N/A All female participants Exclusion criteria: inability to give informed consent; primary reconstructive surgery; hypotension, hypovolaemia or any form of shock; known allergy or sensitivity to LA agents, morphine, paracetamol or ondansetron; pregnancy; daily opioid analgesic use; inability to understand or use a PCA device; inability to understand or complete the visual analogue assessment tools; concurrent participation in another interventional study that might conflict with this study |
| Interventions |
Group 1 (saline, control arm): 0.9% sodium chloride, is sourced from standard NHS supplies at the participating sites, delivered by means of an infusion catheter and device, supplied as a sterile prepacked kit and licensed for the delivery of LA. At the end of the surgical procedure the surgeon inserts the infusion catheter percutaneously into the subpectoral plane under direct vision within the surgical field. After skin closure, a 20 mL bolus of comparator treatment is given via the catheter, which is then connected to the infusion device to provide an infusion of study treatment at a continuous rate of 5 mL/h for 24 h. Group 2 (levobupivacaine): 0.25% levobupivacaine (chirocaine), an established LA infusion agent, prepared as a 2.5 mg/mL solution and packaged by the manufacturer (Abbott) delivered by means of an infusion catheter and device, supplied as a sterile prepacked kit and licensed for the delivery of LA. At the end of the surgical procedure the surgeon inserts the infusion catheter percutaneously into the subpectoral plane under direct vision within the surgical field. After skin closure, a 20 mL bolus of active or comparator treatment is given via the catheter, which is then connected to the infusion device to provide an infusion of study treatment at a continuous rate of 5 mL/h for 24 h. In the active treatment arm this equates to a 50 mg bolus of levobupivacaine followed by an infusion of 12.5 mg/h. Both groups: paracetamol 1 g IV, ondansetron 4 mg IV, and dexamethasone 3.3 mg (+/‐ 0.1 mg) IV unless clinically contraindicated. Intubation and ventilation at anaesthetist’s discretion ‐ with muscle relaxant of anaesthetist’s choice. Sevoflurane in air: depth of anaesthesia at anaesthetist’s discretion. Fentanyl: 3 µg/kg to 6 µg/kg IV during surgery. Fluids: at anaesthetist’s discretion. All other nonopiate and nonantiemetic drugs: at anaesthetist’s discretion. IV rescue morphine in recovery unit, 2 mg increments IV morphine PCA, 1 mg bolus, 5 min lockout. Paracetamol 1 g 6‐hourly orally. Ibuprofen 400 mg 8‐hourly orally unless contraindicated as needed: ondansetron 4 mg (IV) 8‐hourly and cyclizine 50 mg (IV) 8‐hourly Adjuvants: none Immediate postop pain control: data not available |
| Outcomes | Dichotomous: none Continuous: VAS pain scores at rest at 24 h, 14 days and 6 months after surgery; BPI at 6 months. Secondary: total morphine consumption (mg) in the first 24 h (defined as the 24 h following start of the subpectoral infusion), including all morphine given in the recovery unit and cumulative PCA use as recorded by the PCA device and (2) total pain over the first 24 h, as defined by measurement of the area‐under‐the‐curve of each participant’s self‐reported pain scores at rest, measured using a VAS. VAS pain scores are recorded in the recovery unit and then at 4‐hourly intervals for the first 24 h. Secondary outcome measures include the number of PCA attempts in the first 24 h following start of infusion. Incidence of postoperative nausea and/or vomiting and use of supplemental analgesics and postoperative antiemetics in the first 24 h; self‐reported analgesia use at 14 days and 6 months; duration of hospital stay; shoulder movement assessed by goniometry at 24 h, 14 days and 6 months following surgery; shoulder function (as measured by the validated 31) at 6 months. Following the participant’s discharge, the length of stay in hospital is recorded by the research nurse. Adverse events reported: data not available |
| Starting date | 15 October 2012 |
| Contact information | Dr Roger Langford, roger.langford@rcht.cornwall. nhs.uk |
| Notes |
Liew 2011.
| Trial name or title | Postoperative pain relief after laparoscopic gynaecological surgery: a pilot study of pre‐emptive superior hypogastric plexus block versus placebo using ropivacaine. The LAP‐HYPOPLEX study |
| Methods | Quote: a "prospective double‐blind randomised controlled trial" with parallel assignment; this is an efficacy study, single centre |
| Participants | Women undergoing (quote:) "gynaecological diseases for complex laparoscopic surgery" |
| Interventions | The superior hypogastric plexus is identified with the laparoscope during surgery, the women receive pre‐emptive infiltration of 20 mL of 0.75% ropivacaine or placebo. |
| Outcomes | Participants are contacted 6 months after surgery with a postal questionnaire and telephone interview to assess chronic pain syndrome. |
| Starting date | Unclear, before 2012 |
| Contact information | Liew A: Anaesthetics, Sydney Women's Endosurgery Centre, St George Private Hospital, Sydney, NSW, Australia |
| Notes | www.aaic.net.au/document/?D=20110649 |
Michael 2014.
| Trial name or title | Continuous transgluteal sciatic nerve block to prevent phantom limb pain after trans‐femoral amputation |
| Methods | Prospective, randomized double‐blind trial Single centre |
| Participants | Ages eligible for study: not specified Genders eligible for study: both Estimated enrolment: 40 People undergoing trans‐femoral lower limb amputation |
| Interventions | Quote. "a pre‐operative transgluteal sciatic perineural catheter is placed for 5‐days continuous infusion of L‐Bupivacaine vs saline." |
| Outcomes | Quote: "pain assessment via Mc Gill score and OBAS (Overall Benefits of Analgesia Score) test on at 3, 6, and 12 months." |
| Starting date | December 2013 |
| Contact information | Michael Michael, MD e‐mail: medici.anestesia@ospedale.varese.it |
| Notes | We were unable to contact the study author to request more information |
NCT00418457.
| Trial name or title | Regional anaesthesia and breast cancer recurrence: prospective, randomized, double‐blinded, multicenter clinical trial to compare postoperative analgesia and cancer outcome after combined paravertebral versus thoracic epidural versus general anaesthesia for breast cancer surgery |
| Methods | Prevention, randomized, open‐label, active‐control, parallel‐assignment, efficacy study |
| Participants | Ages eligible for study: 18‐85 years Genders eligible for study: women only Estimated enrolment: 1600 Women undergoing mastectomies or isolated lumpectomy with axillary node dissection |
| Interventions | Combined paravertebral vs thoracic epidural vs general anaesthesia |
| Outcomes | Primary outcome is cancer recurrence with a follow‐up of 5 years. Secondary outcomes include chronic pain, among others, with a follow‐up of 6 and 12 months |
| Starting date | January 2007 |
| Contact information | Nancy Graham, RN Tel: +1216‐445‐7530 e‐mail: grahamn@ccf.org |
| Notes |
NCT01626755.
| Trial name or title | Prevention of phantom limb pain after transtibial amputation (PLATA) |
| Methods | Randomized, double‐blind (participant, caregiver, outcomes assessor), parallel‐assignment, efficacy study, multi‐centred |
| Participants | Ages eligible for study: ≥ 18 years Genders eligible: both Estimated enrolment: 400 |
| Interventions | Quote. "all patients will receive standard optimised intravenous anaesthesia and analgesia (opiate patient‐controlled analgesia (PCA), intravenous ketamine). People in the intervention group will receive additional infusion of local anaesthetic via a sciatic nerve catheter placed under ultrasound guidance." |
| Outcomes | Point prevalence of chronic phantom limb pain (time frame: 12 months after amputation) |
| Starting date | August 2013 |
| Contact information | Philipp Lirk, MD Tel: +31(20)566 ext 4032 Email: p.lirk@amc.uva.nl |
| Notes | ClinicalTrials.gov Identifier: NCT01626755 |
NCT02002663.
| Trial name or title | Continuous wound infusion of local anaesthetic and steroid after major abdominal surgery: study protocol for a randomized controlled trial |
| Methods | Double‐blinded (participant and outcome assessor) clinical RCT Sequence via computer‐generated list follow‐up: 3 months |
| Participants | Participants: 120 men and women at university hospital in Italy Operation: major abdominal surgery by laparotomy 2 groups, size: 60/60 Age: 18‐85 years old Men/women: not reported Exclusion criteria: regular use of opioid analgesics, history of drugs or alcohol abuse (or both), postoperative hospitalisation in intensive care with sedation or mechanical ventilation (or both), neurological disorders, any heart conduction disease, any cognitive or mental disorder hindering a participant from providing informed consent, BMI > 30, diabetes (type I or II), allergy to study drugs, and use of epidural analgesia |
| Interventions |
Group 1 (ropivacaine infusion): GA is given using propofol and midazolam (as deemed appropriate by the anaesthesiologist), opioids (fentanyl 0.2 μg/kg or remifentanil 0.1‐0.25 mg/kg/min or both), and muscle relaxants (cisatracurium/rocuronium) and maintained with sevoflurane. A morphine bolus of 0.15 mg/kg is given 30‐45 min before the end of surgery. An infusion catheter is placed by the surgeon in the fascial plane between peritoneum and fascia transversalis, and a 10 mL bolus of 0.2% ropivacaine is administered immediately after muscular plane closure; the catheter is then connected to an electronic pump to give a continuous infusion of pain medications. During the first 24 h, all participants receive ropivacaine 0.2% + methylprednisolone 1 mg/kg, 10 mL/h (total volume of 240 mL in 24 h) continuous wound infusion; additionally, either paracetamol (acetaminophen) 1000 mg or ketorolac 30 mg every 8 h is prescribed. Rescue analgesia in the first 48 h is provided by PCA pump with morphine (0.5 mg/mL, bolus 1 mg, lock‐out 5 min, 20 mg limit every 4 h) Group 2 (control): exactly the same as above, except after 24 h, 10 mL/h continuous infusion of saline 0.9% given to control group Adjuvants: methylprednisolone Immediate post‐op pain control: not reported |
| Outcomes | Dichotomous: none Continuous: NRS Other reported: acute postoperative pain, use of morphine equivalents, analgesic consumption, side effects (postoperative nausea and vomiting, sedation, and any signs of LA or steroid systemic toxicity), and differences in terms of wound healing or wound infections. |
| Starting date | October 2013 |
| Contact information | Dario Bugada, M.D. Email: dariobugada@gmail.com |
| Notes | ClinicalTrials.gov Identifier: NCT02002663 |
Theodoraki 2016.
| Trial name or title | The effect of transversus abdominis plane block on acute and chronic pain after inguinal hernia repair |
| Methods | Double‐blinded (participant, outcome assessor), placebo‐controlled, randomized clinical trial Sequence generation not described Follow‐up for 6 months |
| Participants | Participants: 35 adults in a university setting in Athens, Greece Operation: inguinal hernia repair 2 groups, size: not specified Age (± SD), group 1, 2: not specified Men/women, group 1, 2: not specified Exclusion criteria: inability to consent to the study; BMI > 40 kg/m2; skin infection at the puncture site; contraindication to monoamide LAs, paracetamol, NSAID's (parecoxib); preoperative use of opioids or NSAIDs for chronic pain conditions |
| Interventions |
Group 1 (ropivacaine): during the operation participants all received remifentanil infusion titrated as to maintain heart rate and systolic arterial pressure within 20% of baseline. In the PACU, participants received morphine boluses, until the NRS score was ≤ 3. They also had access to PCA device administering 1 mg doses of morphine as rescue analgesia. TAP block was applied intraoperatively using 20 mL of 0.75% ropivacaine Group 2 (control): same intervention as above except saline was substituted for ropivacaine for TAP block Adjuvants: none Immediate post‐op pain control: meaningful improvement |
| Outcomes | Dichotomous: none Continuous: NRS Secondary: intraoperative dose of remifentanil, mg of IV morphine used in the PACU, and total dose of morphine administered via the PCA device |
| Starting date | January 2014 |
| Contact information | Anne Theodoraki, M.D. Email: ktheodoraki@hotmail.com |
| Notes | ClinicalTrials.gov Identifier: NCT02030223 |
BMI: body mass index; BPI: Brief Pain Inventory; g: gram; GA: general anaesthesia; h: hours; IV: intravenous; mg: milligram; LA: local anaesthetic; N/A: not applicable; NHS: National Health Service; NRS: numerical rating scale; OBAS: overall benefits of analgesia score; PACU: postanaesthesia care unit; PCA: patient controlled analgesia; TAP: transversus abdominis plane; TEA: thoracic epidural anaesthesia; VAS: visual analogue scale; µg: microgram
Differences between protocol and review
We made the following changes to the published protocol (Andreae 2008), and the first version of this review (Andreae 2012).
Updating the title
We updated the title to "Local anaesthetics and regional anaesthesia for preventing persistent postoperative pain in adults and children", to be consistent with the new scientific nomenclature and usage, describing the condition as persistent postoperative pain and to be in full compliance with Cochrane's guidance regarding the inclusion of the population in the title.
Searching major databases only
In the first version of this review (Andreae 2012), we found the yield of our electronic search low in CINHAL, a small electronic database of nursing and allied health literature; where we did find relevant studies, they were duplicates already identified in Pubmed, CENTRAL or Embase. Hence, we decided not to update our search with this database. Equally, we found the yield of our handsearch for the first version of this review so low that we did not repeat the handsearch for this update, just two years later.
Criteria for considering studies for this review
We attempted to extract and pool data on adverse events, which we had not explicitly specified in the original protocol, but incomplete reporting precluded this additional evidence synthesis.
Exploring the effect of attrition and bull bias on effect size.
We explored the effect of attrition and length of follow‐up on effect size with graphical tools. We extracted evidence for null bias in included studies, but we did not perform a planned subgroup analysis on improved pain control defined at the participant level and not at the study level, because of the risk of time‐dependent bias.
Timing of local or regional anaesthesia
We focused exclusively on the prevention of the risk of persistent pain by local anaesthetics regardless of the timing of the intervention to improve clarity and prevent confusion about pre‐emptive versus preventive analgesia.
Data synthesis
We fit a Bayesian analysis and pooled studies reporting outcomes at different follow‐up intervals in our inclusive analysis, both planned a priori. We did not pool the dichotomous data with the continuous data by calculating odds ratios based on the standardized mean differences (a secondary analysis detailed in the protocol).
Sensitivity analysis
We had not planned to test the sensitivity of our results to the model assumptions (Sensitivity analysis).
Change in authors
Erica Weinstein, Marc Cohen, Jerry Chao and Jake Levene joined the review team in 2014 for the update. Dr Hall joined in 2013 as statistician and Dr Johnson in 2013 for the Bayesian meta‐analysis of the ICBG data.
Contributions of authors
Erica J Weinstein (EJW), Jacob L Levene (JLL), Marc S Cohen (MSC), Doerthe A Andreae (DAA), Jerry Y Chao (JYC), Matthew Johnson (MJ), Charles B Hall (CBH), Michael H Andreae (MHA)
All the review authors read and approved the manuscript before submission. Review authors' contribution to the previous version of this review are detailed in Andreae 2012; below we attribute the contributions of review authors for the update only.
Conceiving the review: MHA Co‐ordinating the review: MHA Undertaking manual searches: Screening search results: EJW, MSC, JLL, DAA, JYC, MHA Organizing retrieval of papers: EJW, JLL, MHA Screening retrieved papers against inclusion criteria: EJW, MSC, JLL, JYC, DAA, MHA Appraising quality of papers: EJW, JLL, MSC, JYC, DAA, MHA Abstracting data from papers: EJW, JLL, MSC, JYC, DAA, MHA Writing to authors of papers for additional information: EJW, JLL, MHA Providing additional data about papers: Obtaining and screening data on unpublished studies: MHA, EJW, JLL. Data management for the review: MHA, EJW, JLL Entering data into Review Manager 5 (RevMan 5) (RevMan 2014): EJW, JLL, MSC, DAA, MHA RevMan 5 statistical data: updated: EJW, JLL, MSC, MHA Other statistical analysis not using RevMan 5: MHA, MJ (Bayesian models for iliac crest bone graft harvesting), CBH Double entry of data: updated: EJW, JLL, MSC, DAA, MHA Interpretation of data: MHA, EJW, DAA, JYC, CBH, MJ Statistical inferences: MHA, MJ (Bayesian models for iliac crest bone graft harvesting), CBH Writing the review: MHA, EJW, MSC, CBH, JLL Securing funding for the review: MHA Performing previous work that was the foundation of the present study: MHA, DAA Guarantor for the review: MHA Responsible for reading and checking review before submission: MHA, EJW, JLL, DAA, CBH, MSC
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institutes of Health (NIH), USA.
This publication was supported in part by grants (5KL2TR001071‐03; UL1 TR001073) from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCATS or the NIH.
Declarations of interest
Erica J Weinstein: none known Jacob L Levene: none known Marc S Cohen: none known Doerthe A Andreae: none known Jerry Chao: none known Matthew Johnson: none known Charles B Hall: none known Michael Andreae: none known
Edited (no change to conclusions)
References
References to studies included in this review
Albi‐Feldzer 2013 {published data only}
- Albi‐Feldzer A, Mouret‐Fourme EE, Hamouda S, Motamed C, Dubois PY, Jouanneau L, et al. A double‐blind randomized trial of wound and intercostal space infiltration with ropivacaine during breast cancer surgery: effects on chronic postoperative pain. Anesthesiology 2013;118(2):318‐26. [PUBMED: 23340351] [DOI] [PubMed] [Google Scholar]
Barkhuysen 2010 {published data only}
- Barkhuysen R, Meijer GJ, Soehardi A, Merkx MA, Borstlap WA, Berge SJ, et al. The effect of a single dose of bupivacaine on donor site pain after anterior iliac crest bone harvesting. International Journal of Oral and Maxillofacial Surgery 2010;39(3):260‐5. [PUBMED: 19959335] [DOI] [PubMed] [Google Scholar]
Baudry 2008 {published data only}
- Baudry G, Steghens A, Laplaza D, Koeberle P, Bachour K, Bettinger G, et al. Ropivacaine infiltration during breast cancer surgery: postoperative acute and chronic pain effect [Infiltration de ropivacaıne en chirurgie carcinologique du sein: effet sur la douleur postoperatoire aigue et chronique]. Annales Françaises d'Anesthésie et de Réanimation 2008;27(1769‐6623 (Electronic), 12):979‐86. [PUBMED: 19013751] [DOI] [PubMed] [Google Scholar]
Bell 2001 {published data only}
- Bell RF, Sivertsen A, Mowinkel P, Vindenes H. A bilateral clinical model for the study of acute and chronic pain after breast‐reduction surgery. Acta Anaesthesiologica Scandinavica 2001;45(5):576‐82. [PUBMED: 11309007] [DOI] [PubMed] [Google Scholar]
Besic 2014 {published data only}
- Besic N, Strazisar B. Incidence of chronic pain after continuous local anesthetic in comparison to standard systemic pain treatment after axillary lymphadenectomy or primary reconstruction with a tissue expander in breast carcinoma patients: a prospective randomized study. Annals of Surgical Oncology 2014;21(1):S47‐8. [Google Scholar]
- Strazisar B, Besic N. Continuous infusion of local anesthetic into surgical wound after breast cancer operations efficiently reduces pain. Regional Anesthesia and Pain Medicine 2014;39(5):e219. [Google Scholar]
Blumenthal 2005 {published data only}
- Blumenthal S, Dullenkopf A, Rentsch K, Borgeat A. Continuous infusion of ropivacaine for pain relief after iliac crest bone grafting for shoulder surgery. Anesthesiology 2005;102(2):392‐7. [PUBMED: 15681956] [DOI] [PubMed] [Google Scholar]
Bollag 2012 {published and unpublished data}
- Bollag L, Richebe P, Siaulys M, Ortner CM, Gofeld M, Landau R. Effect of transversus abdominis plane block with and without clonidine on post‐cesarean delivery wound hyperalgesia and pain. Regional Anesthesia and Pain Medicine 2012;37(5):508‐14. [PUBMED: 22683707] [DOI] [PubMed] [Google Scholar]
Brown 2004 {published data only}
- Brown DR, Hofer RE, Patterson DE, Fronapfel PJ, Maxson PM, Narr BJ, et al. Intrathecal anesthesia and recovery from radical prostatectomy: a prospective, randomized, controlled trial. Anesthesiology 2004;100(4):926‐34. [PUBMED: 15087629] [DOI] [PubMed] [Google Scholar]
Burney 2004 {published data only}
- Burney RE, Prabhu MA, Greenfield ML, Shanks A, O'Reilly M. Comparison of spinal vs general anesthesia via laryngeal mask airway in inguinal hernia repair. Archives of Surgery 2004;139(2):183‐7. [PUBMED: 14769578] [DOI] [PubMed] [Google Scholar]
Can 2013 {published and unpublished data}
- Can A, Erdem AF, Aydin Y, Ahiskalioglu A, Kursad H. The effect of preemptive thoracic epidural analgesia on long‐term wound pain following major thoracotomy. Turkish Journal of Medical Sciences 2013;43(4):515‐20. [CENTRAL: CN‐00919217; EMBASE: 2013483422] [Google Scholar]
Chiu 2008 {published data only}
- Chiu KM, Wu CC, Wang MJ, Lu CW, Shieh JS, Lin TY, et al. Local infusion of bupivacaine combined with intravenous patient‐controlled analgesia provides better pain relief than intravenous patient‐controlled analgesia alone in patients undergoing minimally invasive cardiac surgery. The Journal of Thoracic and Cardiovascular Surgery 2008;135(6):1348‐52. [PUBMED: 18544384] [DOI] [PubMed] [Google Scholar]
Choi 2016 {published data only}
- Choi KW, Nam KH, Lee JR, Chung WY, Kang SW, Joe YE, et al. The effects of intravenous lidocaine infusions on the quality of recovery and chronic pain after robotic thyroidectomy: a randomized, double‐blinded, controlled study. World Journal of Surgery 2016;1:e138. [PUBMED: 27896411] [DOI] [PubMed] [Google Scholar]
Comez 2015 {published data only}
- Comez M, Celik M, Dostbil A, Aksoy M, Ahiskalioglu A, Erdem AF, et al. The effect of pre‐emptive intravenous dexketoprofen + thoracal epidural analgesia on the chronic post‐thoracotomy pain. International Journal of Clinical and Experimental Medicine 2015;8(5):8101‐7. [PUBMED: 26221376] [PMC free article] [PubMed] [Google Scholar]
Di‐Gennaro 2013 {published data only}
- Gennaro T, Passavanti M, Pace M, Coletta F, Sansone P, Pota V, et al. Chronic postsurgical pain prevention: surgical site infiltration of tramadol vs levobupivacaine. Regional Anesthesia and Pain Medicine 2013;38(5 Suppl 1):E226‐7. [PUBMED: 25215629]25215629 [Google Scholar]
Dogan 2016 {published data only}
- Dogan Baki E, Kavrut Ozturk N, Ayoglu RU, Emmiler M, Karsli B, Uzel H. Effects of parasternal block on acute and chronic pain in patients undergoing coronary artery surgery. Seminars in Cardiothoracic and Vascular Anesthesia 2016;20(3):205‐12. [PUBMED: 25900900] [DOI] [PubMed] [Google Scholar]
Fassoulaki 2000 {published data only}
- Fassoulaki A, Sarantopoulos C, Melemeni A, Hogan Q. EMLA reduces acute and chronic pain after breast surgery for cancer. Regional Anesthesia and Pain Medicine 2000;25(4):350‐5. [PUBMED: 10925929] [DOI] [PubMed] [Google Scholar]
Fassoulaki 2001 {published data only}
- Fassoulaki A, Sarantopoulos C, Melemeni A, Hogan Q. Regional block and mexiletine: the effect on pain after cancer breast surgery. Regional Anesthesia and Pain Medicine 2001;26(3):223‐8. [PUBMED: 11359221] [DOI] [PubMed] [Google Scholar]
Fassoulaki 2005 {published data only}
- Fassoulaki A, Triga A, Melemeni A, Sarantopoulos C. Multimodal analgesia with gabapentin and local anesthetics prevents acute and chronic pain after breast surgery for cancer. Anesthesia and Analgesia 2005;101(5):1427‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fassoulaki 2016 {published data only}
- Fassoulaki A, Vassi E, Korkolis D, Zotou M. Perioperative continuous ropivacaine wound infusion in laparoscopic cholecystectomy: a randomized controlled double‐blind trial. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2016;26(1):25‐30. [PUBMED: 26679680] [DOI] [PubMed] [Google Scholar]
Gacio 2016 {published data only}
- Gacio MF, Lousame AM, Pereira S, Castro Cl, Santos J. Paravertebral block for management of acute postoperative pain and intercostobrachial neuralgia in major breast surgery. Brazilian Journal of Anesthesiology 2016;66(5):475‐84. [DOI] [PubMed] [Google Scholar]
Grigoras 2012 {published data only}
- Grigoras A, Lee P, Sattar F, Shorten G. Perioperative intravenous lidocaine decreases the incidence of persistent pain after breast surgery. The Clinical Journal of Pain 2012;28(7):567‐72. [PUBMED: 22699129] [DOI] [PubMed] [Google Scholar]
Gundes 2000 {published data only}
- Gundes H, Kilickan L, Gurkan Y, Sarlak A, Toker K. Short‐ and long‐term effects of regional application of morphine and bupivacaine on the iliac crest donor site. Acta Orthopaedica Belgica 2000;66(4):341‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Gupta 2006 {published data only}
- Gupta A, Fant F, Axelsson K, Sandblom D, Rykowski J, Johansson JE, et al. Postoperative analgesia after radical retropubic prostatectomy: a double‐blind comparison between low thoracic epidural and patient‐controlled intravenous analgesia. Anesthesiology 2006;105(4):784‐93. [PUBMED: 17006078] [DOI] [PubMed] [Google Scholar]
Ibarra 2011 {published data only}
- Ibarra MM, S‐Carralero GC, Vicente GU, Cuartero del Pozo A, Lopez Rincon R, Fajardo del Castillo MJ. Chronic postoperative pain after general anesthesia with or without a single‐dose preincisional paravertebral nerve block in radical breast cancer surgery [Comparacion entre anestesia general con o sin bloqueo paravertebral preincisional con dosis unica y dolor cronico postquirurgico, en cirugia radical de cancer de mama]. Revista Espanola de Anestesiologia y Reanimacion 2011;58(5):290‐4. [PUBMED: 21692253] [DOI] [PubMed] [Google Scholar]
Ju 2008 {published data only}
- Ju H, Feng Y, Yang BX, Wang J. Comparison of epidural analgesia and intercostal nerve cryoanalgesia for post‐thoracotomy pain control. European Journal of Pain 2008;12(3):378‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kairaluoma 2006 {published data only}
- Kairaluoma PM, Bachmann MS, Korpinen AK, Rosenberg PH, Pere PJ. Single‐injection paravertebral block before general anesthesia enhances analgesia after breast cancer surgery with and without associated lymph node biopsy. Anesthesia and Analgesia 2004;99(6):1837‐43. [PUBMED: 15562083] [DOI] [PubMed] [Google Scholar]
- Kairaluoma PM, Bachmann MS, Rosenberg PH, Pere PJ. Preincisional paravertebral block reduces the prevalence of chronic pain after breast surgery. Anesthesia and Analgesia 2006;103(3):703‐8. [PUBMED: 16931684] [DOI] [PubMed] [Google Scholar]
Karanikolas 2006 {published data only (unpublished sought but not used)}
- Karanikolas M, Aretha D, Monantera G, TsolakisI, Swarm RA, Filos KS. Rigorous perioperative analgesia decreases phantom pain frequency and intensity after lower limb amputation. A prospective, randomized, double‐blind clinical trial. XXV Annual Congress of the European Society of Regional Anaesthesia, Monte Carlo, Monaco 2006.
- Karanikolas M, Aretha D, Tsolakis I, Monantera G, Kiekkas P, Papadoulas S, et al. Optimized perioperative analgesia reduces chronic phantom limb pain intensity, prevalence, and frequency: a prospective, randomized, clinical trial. Anesthesiology 2011;114(5):1144‐54. [PUBMED: 21368651] [DOI] [PubMed] [Google Scholar]
Karmakar 2014 {published data only}
- Karmakar MK, Samy W, Li JW, Lee A, Chan WC, Chen PP, et al. Thoracic paravertebral block and its effects on chronic pain and health‐related quality of life after modified radical mastectomy. Regional Anesthesia and Pain Medicine 2014;39(4):289‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Katsuly‐Liapis1996 {published data only}
- Katsuly‐Liapis I, Georgakis P, Tierry C. Preemptive extradural analgesia reduces the incidence of phantom pain in lower limb amputees. British Journal of Anaesthesia 1996;76 Suppl 2:125: A410. [not found in PubMed] [Google Scholar]
Katz 1996 {published and unpublished data}
- Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long‐term post‐thoracotomy pain. The Clinical Journal of Pain 1996;12(1):50‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kavanagh BP, Katz J, Sandler AN, Nierenberg H, Roger S, Boylan JF, et al. Multimodal analgesia before thoracic surgery does not reduce postoperative pain. British Journal of Anaesthesia 1994;73(2):184‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Katz 2004 {published data only}
- Katz J, Cohen L. Preventive analgesia is associated with reduced pain disability 3 weeks but not 6 months after major gynaecological surgery by laparotomy. Anesthesiology 2004;101:169‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Katz J, Cohen L, Schmid R, Chan VW, Wowk A. Postoperative morphine use and hyperalgesia are reduced by preoperative but not intraoperative epidural analgesia: implications for preemptive analgesia and the prevention of central sensitization. Anesthesiology 2003;98(6):1449‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kurmann 2015 {published and unpublished data}
- Honigmann P, Fischer H, Kurmann A, Audige L, Schupfer G, Metzger J. Investigating the effect of intra‐operative infiltration with local anaesthesia on the development of chronic postoperative pain after inguinal hernia repair. A randomized placebo controlled triple blinded and group sequential study design. BMC surgery 2007;7:22. [NCT00484731; PUBMED: 17986324] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurmann A, Fischer H, Dell‐Kuster S, Rosenthal R, Audige L, Schupfer G, et al. Effect of intraoperative infiltration with local anesthesia on the development of chronic pain after inguinal hernia repair: a randomized, triple‐blinded, placebo‐controlled trial. Surgery 2015;157(1):144‐54. [PUBMED: 25482469] [DOI] [PubMed] [Google Scholar]
Lam 2015 {published data only}
- Lam D, Green J, Henschke S, Cameron J, Hamilton S, Wiingaarden‐Stephens M, et al. Abstract 378: paravertebral block vs. sham in the setting of a multimodal analgesia regimen and total intravenous anesthesia for mastectomy: a prospective, randomized, controlled trial. 40th Annual Regional Anesthesiology and Acute Pain Medicine Meeting. 2015.
Lavand'homme 2005 {published data only}
- Lavand'homme P, Kock M, Waterloos H. Intraoperative epidural analgesia combined with ketamine provides effective preventive analgesia in patients undergoing major digestive surgery. Anesthesiology 2005;103:813‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lavand'homme 2007 {published data only}
- Lavand'homme PM, Roelants F, Waterloos H, Kock MF. Postoperative analgesic effects of continuous wound infiltration with diclofenac after elective cesarean delivery. Anesthesiology 2007;106(6):1220‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Lee P, McAuliffe N, Dunlop C, Palanisamy M, Shorten G. A comparison of the effects of two analgesic regimens on the development of persistent post‐surgical pain (PPSP) after breast surgery. Jurnalul Roman de Anestezie Terapie Intensiva/Romanian Journal of Anaesthesia and Intensive Care October 2013;20(2):83‐93. [EMBASE: 2013701143] [Google Scholar]
Liu 2015 {published data only}
- Liu FF, Liu XM, Liu XY, Tang J, Jin L, Li WY, et al. Postoperative continuous wound infusion of ropivacaine has comparable analgesic effects and fewer complications as compared to traditional patient‐controlled analgesia with sufentanil in patients undergoing non‐cardiac thoracotomy. International Journal of Clinical and Experimental Medicine 2015;8(4):5438‐45. [PUBMED: 26131121] [PMC free article] [PubMed] [Google Scholar]
Loane 2012 {published data only}
- Loane H, Preston R, Douglas MJ, Massey S, Papsdorf M, Tyler J. A randomized controlled trial comparing intrathecal morphine with transversus abdominis plane block for post‐cesarean delivery analgesia. International Journal of Obstetric Anesthesia 2012;21(2):112‐8. [PUBMED: 22410586] [DOI] [PubMed] [Google Scholar]
Lu 2008 {published data only}
- Lu YL, Wang XD, Lai RC, Huang W, Xu M. Correlation of acute pain treatment to occurrence of chronic pain in tumor patients after thoracotomy. Aizheng 2008;27(2):206‐9. [MEDLINE: ] [PubMed] [Google Scholar]
McKeen 2014 {published data only}
- McKeen DM, George RB, Boyd JC, Allen VM, Pink A. Transversus abdominis plane block does not improve early or late pain outcomes after Cesarean delivery: a randomized controlled trial. Canadian Journal of Anesthesia/Journal Canadien d'Anesthésie 2014;61(7):631‐40. [PUBMED: 24764186] [DOI] [PubMed] [Google Scholar]
Micha 2012 {published data only}
- Micha G, Vassi A, Balta M, Panagiotidou O, Saleh M, Chondreli S. The effect of local infiltration of ropivacaine on the incidence of chronic neuropathic pain after modified radical mastectomy. European Journal of Anaesthesiology 2012;29:199. [Google Scholar]
Mounir 2010 {published data only}
- Mounir K, Bensghir M, Elmoqaddem A, Massou S, Belyamani L, Atmani M, et al. Efficiency of bupivacaine wound subfascial infiltration in reduction of postoperative pain after inguinal hernia surgery [Efficacité de l'infiltration cicatricielle subfasciale par la bupivacaine dans la réduction de la douleur postopératoire des hernies inguinales]. Annales Françaises d'Anésthesie et de Réanimation 2010;29(4):274‐8. [PUBMED: 20117910] [DOI] [PubMed] [Google Scholar]
O'Neill 2012 {published data only}
- O'Neill P, Duarte F, Ribeiro I, Centeno MJ, Moreira J. Ropivacaine continuous wound infusion versus epidural morphine for postoperative analgesia after cesarean delivery: a randomized controlled trial. Anesthesia and Analgesia 2012;114(1):179‐85. [PUBMED: 22025490] [DOI] [PubMed] [Google Scholar]
O'Neill 2014 {published data only}
- O'Neill KR. Bupivacaine for pain reduction after iliac crest bone harvest. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000‐ [cited 2015 Jun 15] Available from: clinicaltrials.gov/ct2/show/NCT01087931?term=o%27neill&rank=1 NLM Identifier: NCT01087931.
- O'Neill KR, Lockney DT, Bible JE, Crosby CG, Devin CJ. Bupivacaine for pain reduction after iliac crest bone graft harvest. Orthopedics 2014;37(5):e428‐34. [PUBMED: 24810818] [DOI] [PubMed] [Google Scholar]
Okur 2016 {published data only}
- Okur O, Tekgul ZT, Erkan N. Abstract PR506: a prospective randomised controlled open‐labelled study: comparison of efficacy of transversus abdominis plane block and ilioinguinal nerve block for postoperative pain management in patients undergoing inguinal herniorrhaphy with spinal anaesthesia. Anesthesia and Analgesia. [DOI] [PubMed]
Paxton 1995 {published data only}
- Paxton LD, Huss BK, Loughlin V, Mirakhur RK. Intra‐vas deferens bupivacaine for prevention of acute pain and chronic discomfort after vasectomy. British Journal of Anaesthesia 1995;74(5):612‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pinzur 1996 {published data only}
- Pinzur MS, Garla PG, Pluth T, Vrbos L. Continuous postoperative infusion of a regional anesthetic after an amputation of the lower extremity. A randomized clinical trial. Journal of Bone and Joint Surgery. American volume 1996;78(10):1501‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Purwar 2015 {published data only}
- Purwar B, Ismail KM, Turner N, Farrell A, Verzune M, Annappa M, et al. General or spinal anaesthetic for vaginal surgery in pelvic floor disorders (GOSSIP): a feasibility randomised controlled trial. International Urogynecology Journal 2015;26(8):1171‐8. [PUBMED: 25792351] [DOI] [PubMed] [Google Scholar]
Senturk 2002 {published data only}
- Senturk M, Ozcan PE, Talu GK, Kiyan E, Camci E, Ozyalcin S, et al. The effects of three different analgesia techniques on long‐term postthoracotomy pain. Anesthesia and Analgesia 2002;94(1):11‐5, table of contents. [PUBMED: 11772793] [DOI] [PubMed] [Google Scholar]
Shahin 2010 {published data only}
- Shahin AY, Osman AM. Intraperitoneal lidocaine instillation and postcesarean pain after parietal peritoneal closure: a randomized double blind placebo‐controlled trial. Clinical Journal of Pain 2010;26(2):121‐7. [PUBMED: 20090438] [DOI] [PubMed] [Google Scholar]
Singh 2007 {published data only}
- Singh K, Phillips FM, Kuo E, Campbell M. A prospective, randomized, double‐blind study of the efficacy of postoperative continuous local anesthetic infusion at the iliac crest bone graft site after posterior spinal arthrodesis: a minimum of 4‐year follow‐up. Spine 2007;32(25):2790‐6. [PUBMED: 18245999] [DOI] [PubMed] [Google Scholar]
- Singh K, Samartzis D, Strom J, Manning D, Campbell‐Hupp M, Wetzel FT, et al. A prospective, randomized, double‐blind study evaluating the efficacy of postoperative continuous local anesthetic infusion at the iliac crest bone graft site after spinal arthrodesis. Spine 2005;30(22):2477‐83. [PUBMED: 16284583] [DOI] [PubMed] [Google Scholar]
Singh 2013 {published data only}
- Singh S, Dhir S, Marmai K, Rehou S, Silva M, Bradbury C. Efficacy of ultrasound‐guided transversus abdominis plane blocks for post‐cesarean delivery analgesia: a double‐blind, dose‐comparison, placebo‐controlled randomized trial. International Journal of Obstetric Anesthesia 2013;22(3):188‐93. [DOI] [PubMed] [Google Scholar]
Smaldone 2010 {published data only}
- Smaldone M, Chelly J, Nelson J. A prospective, randomized, double‐blind, placebo controlled trial of multimodal anesthesia compared to patient controlled opioid anesthesia in patients undergoing radical prostatectomy. Journal of Urology 2010;183(4):e605. [Google Scholar]
Sprung 2006 {published data only}
- Sprung J, Sanders MS, Warner ME, Gebhart JB, Stanhope CR, Jankowski CJ, et al. Pain relief and functional status after vaginal hysterectomy: intrathecal versus general anesthesia. Canadian Journal of Anesthesia 2006;53(7):690‐700. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Strazisar 2012 {published data only}
- Strazisar B, Besic N. Comparison of continuous local anesthetic and systemic pain treatment after axillary lymphadenectomy in breast carcinoma patients ‐ a prospective randomized study ‐ final results. Regional Anesthesia and Pain Medicine 2012;37(5):E218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strazisar 2014 {published data only}
- Strazisar B, Besic N, Ahcan U. Does a continuous local anaesthetic pain treatment after immediate tissue expander reconstruction in breast carcinoma patients more efficiently reduce acute postoperative pain‐‐a prospective randomised study. World Journal of Surgical Oncology 2014;12:16. [PUBMED: 24433317] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tecirli 2014 {published data only}
- Tecirli AT, Inan N, Inan G, Kurukahveci O, Kuruoz S. The effects of intercostobrachial nerve block on acute and chronic pain after unilateral mastectomy and axillary lymph node dissection surgery. Pain Practice 2014;14:63. [Google Scholar]
Terkawi 2015b {published data only}
- Terkawi AS, Durieux ME, Gottschalk A, Brenin D, Tiouririne M. Effect of intravenous lidocaine on postoperative recovery of patients undergoing mastectomy: a double‐blind, placebo‐controlled randomized trial. Regional Anesthesia and Pain Medicine 2014;39(6):472‐7. [PUBMED: 25275577] [DOI] [PubMed] [Google Scholar]
- Terkawi AS, Sharma S, Durieux ME, Thammishetti S, Brenin D, Tiouririne M. Perioperative lidocaine infusion reduces the incidence of post‐mastectomy chronic pain: a double‐blind, placebo‐controlled randomized trial. Pain Physician 2015;18(2):E139‐46. [PUBMED: 25794212] [PubMed] [Google Scholar]
Vrooman 2015 {published data only}
- Vrooman B, Kapural L, Sarwar S, Mascha EJ, Mihaljevic T, Gillinov M, et al. Lidocaine 5% patch for treatment of acute pain after robotic cardiac surgery and prevention of persistent incisional pain: a randomized, placebo‐controlled, double‐blind trial. Pain Medicine (Malden, Mass.) 2015;16(8):1610‐21. [PUBMED: 26176878] [DOI] [PubMed] [Google Scholar]
Weber 2007 {published data only}
- Weber T, Matzl J, Rokitansky A, Klimscha W, Neumann K, Deusch E. Superior postoperative pain relief with thoracic epidural analgesia versus intravenous patient‐controlled analgesia after minimally invasive pectus excavatum repair. The Journal of Thoracic and Cardiovascular Surgery 2007;134(4):865‐70. [PUBMED: 17903498] [DOI] [PubMed] [Google Scholar]
Wodlin 2011 {published data only}
- Wodlin NB, Nilsson L, Arestedt K, Kjolhede P. Mode of anesthesia and postoperative symptoms following abdominal hysterectomy in a fast‐track setting. Acta Obstetricia et Gynecologica Scandinavica 2011;90(4):369‐79. [PUBMED: 21332679] [DOI] [PubMed] [Google Scholar]
- Wodlin NB, Nilsson L, Kjolhede P. Health‐related quality of life and postoperative recovery in fast‐track hysterectomy. Acta Obstetricia et Gynecologica Scandinavica 2011;90(4):362‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Xu 2017 {published data only}
- Xu B, Ren L, Tu W, Wu Z, Ai F, Zhou D, et al. Continuous wound infusion of ropivacaine for the control of pain after thoracolumbar spinal surgery: a randomized clinical trial. European Spine Journal 2017;26(3):825‐31. [PUBMED: 25935145] [DOI] [PubMed] [Google Scholar]
Zhou 2016 {published data only}
- Zhou H, Ou M, Yang Y, Ruan Q, Pan Y, Li Y. Effect of skin infiltration with ropivacaine on postoperative pain in patients undergoing craniotomy. SpringerPlus 2016;5(1):1180. [PUBMED: 27512639] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdel‐Salam 1975 {published data only}
- Abdel‐Salam A, Scott B. Bupivacaine and etidocaine in epidural block for post‐operative relief of pain. Acta Anaesthesiologica Scandinavica. Supplementum 1975;60:80‐2. [PUBMED: 1101617] [PubMed] [Google Scholar]
Aveline 2011 {published data only}
- Aveline C, Hetet H, Roux A, Vautier P, Cognet F, Vinet E, et al. Comparison between ultrasound‐guided transversus abdominis plane and conventional ilioinguinal/iliohypogastric nerve blocks for day‐case open inguinal hernia repair. British Journal of Anaesthesia 2011;106(3):380‐6. [PUBMED: 21177284] [DOI] [PubMed] [Google Scholar]
Bach 1988 {published data only}
- Bach S, Noreng MF, Tjéllden NU. Phantom limb pain in amputees during the first 12 months following limb amputation, after preoperative lumbar epidural blockade. Pain 1988;33:297‐301. [PUBMED: 3419837] [DOI] [PubMed] [Google Scholar]
- Noreng MF, Tjellden NU, Bach S. Preoperative epidural blockade and phantom pain after below‐knee amputation [Praeoperativ epidural blokade og fantomsmerter efter crusamputation]. Ugeskr Laeger 1988;150(50):3111‐3. [PUBMED: 3206720] [PubMed] [Google Scholar]
Bamigboye 2013 {published data only}
- Bamigboye AA, Hofmeyr J, Labeodan M. Caesarean section wound infiltration with ropivacaine versus placebo: survey of chronic pelvic pain after 4 years' follow‐up. South African Journal of Obstetrics and Gynaecology 2013;19(3):75‐6. [CENTRAL: CN‐00916001; EMBASE: 2013571611] [Google Scholar]
- Bamigboye AA, Justus HG. Ropivacaine abdominal wound infiltration and peritoneal spraying at cesarean delivery for preemptive analgesia. International Journal of Gynaecology and Obstetrics 2008;102(2):160‐4. [PUBMED: 18538773] [DOI] [PubMed] [Google Scholar]
Baral 2010 {published data only}
- Baral BK, Bhattarai BK, Rahman TR, Singh SN, Regmi R. Perioperative intravenous lidocaine infusion on postoperative pain relief in patients undergoing upper abdominal surgery. Nepal Medical College journal : NMCJ 2010;12(4):215‐20. [PUBMED: 21744761] [PubMed] [Google Scholar]
Batoz 2009 {published data only}
- Batoz H, Verdonck O, Pellerin C, Roux G, Maurette P. The analgesic properties of scalp infiltrations with ropivacaine after intracranial tumoral resection. Anesthesia and Analgesia 2009;109(1):240‐4. [PUBMED: 19535716] [DOI] [PubMed] [Google Scholar]
Blumenthal 2011 {published data only}
- Blumenthal S, Borgeat A, Neudorfer C, Bertolini R, Espinosa N, Aguirre J. Additional femoral catheter in combination with popliteal catheter for analgesia after major ankle surgery. British Journal of Anaesthesia 2011;106(3):387‐93. [PUBMED: 21169609] [DOI] [PubMed] [Google Scholar]
Borgeat 2001 {published data only}
- Borgeat A, Ekatodramis G, Kalberer F, Benz C. Acute and nonacute complications associated with interscalene block and shoulder surgery: a prospective study. Anesthesiology 2001;95(4):875‐80. [PUBMED: 11605927] [DOI] [PubMed] [Google Scholar]
Borghi 2010 {published data only}
- Borghi B, D'Addabbo M, White PF, Gallerani P, Toccaceli L, Raffaeli W, et al. The use of prolonged peripheral neural blockade after lower extremity amputation: the effect on symptoms associated with phantom limb syndrome. Anesthesia and Analgesia 2010;111(5):1308‐15. [PUBMED: 20881281] [DOI] [PubMed] [Google Scholar]
Brull 1992 {published data only}
- Brull SJ, Lieponis JV, Murphy MJ, Garcia R, Silverman DG. Acute and long‐term benefits of iliac crest donor site perfusion with local anesthetics. Anesthesia and analgesia 1992;74(1):145‐7. [PUBMED: 1734778] [PubMed] [Google Scholar]
Cerfolio 2003 {published data only}
- Cerfolio RJ, Bryant AS, Bass CS, Bartolucci AA. A prospective, double‐blinded, randomized trial evaluating the use of preemptive analgesia of the skin before thoracotomy. The Annals of Thoracic Surgery 2003;76(4):1055‐8. [PUBMED: 14529984] [DOI] [PubMed] [Google Scholar]
Chelly 2011 {published data only}
- Chelly JE, Ploskanych T, Dai F, Nelson JB. Multimodal analgesic approach incorporating paravertebral blocks for open radical retropubic prostatectomy: a randomized double‐blind placebo‐controlled study. Canadian Journal of Anesthesia/Journal Canadien d'Anésthesie 2011;58(4):371‐8. [PUBMED: 21174182] [DOI] [PubMed] [Google Scholar]
- Smaldone M, Chelly J, Nelson J. A prospective, randomized, double‐blind, placebo controlled trial of multimodal anesthesia compared to patient controlled opioid anesthesia in patients undergoing radical prostatectomy. Journal of Urology 2010;1:e605. [EMBASE: 70145238] [Google Scholar]
Corsini 2013 {published data only}
- Corsini T, Cuvillon P, Forgeot A, Chapelle C, Seffert P, Chauleur C. Single‐dose intraincisional levobupivacaine infiltration in caesarean postoperative analgesia: a placebo‐controlled double‐blind randomized trial [Infiltration peropératoire de lévobupivacaine après césariennes: étude randomisée en double insu contre placebo]. Annales francaises d'anesthésie et de réanimation 2013;32(1):25‐30. [PUBMED: 23260628] [DOI] [PubMed] [Google Scholar]
da Costa 2011 {published data only}
- Costa VV, Oliveira SB, Fernandes Mdo C, Saraiva RA. Incidence of regional pain syndrome after carpal tunnel release. Is there a correlation with the anesthetic technique?. Revista Brasileira de Anestesiologia 2011;61(4):425‐33. [PUBMED: 21724005] [DOI] [PubMed] [Google Scholar]
De Kock 2001 {published data only}
- Kock M, Lavand'homme P, Waterloos H. 'Balanced analgesia' in the perioperative period: is there a place for ketamine?. Pain 2001;92(3):373‐80. [PUBMED: 11376910] [DOI] [PubMed] [Google Scholar]
Duale 2009 {published data only}
- Duale C, Sibaud F, Guastella V, Vallet L, Gimbert YA, Taheri H, et al. Perioperative ketamine does not prevent chronic pain after thoracotomy. European Journal of Pain (London, England) 2009;13(5):497‐505. [PUBMED: 18783971] [DOI] [PubMed] [Google Scholar]
Eisenach 2010 {published data only}
- Eisenach JC, Curry R, Rauck R, Pan P, Yaksh TL. Role of spinal cyclooxygenase in human postoperative and chronic pain. Anesthesiology 2010;112(5):1225‐33. [PUBMED: 20395820] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elman 1989 {published data only}
- Elman A, Debaene B, Magny‐Metrot C, Orhant E, Jolis P. Intrapleural analgesia with bupivacaine after thoracotomy is ineffective. Controlled study and pharmacokinetics [L'analgésie intrapleurale à la bupivacaine après thoracotomie est inefficace. Etude controlée et pharmacocinétique]. Annales Françaises d'Anesthésie et de Réanimation 1989;8 Suppl:R181. [PUBMED: 2604141] [PubMed] [Google Scholar]
El‐Morsy 2012 {published data only}
- El‐Morsy GZ, El‐Deeb A, El‐Desouky T, Elsharkawy AA, Elgamal MA. Can thoracic paravertebral block replace thoracic epidural block in pediatric cardiac surgery? A randomized blinded study. Annals of Cardiac Anaesthesia 2012;15(4):259‐63. [PUBMED: 23041682] [DOI] [PubMed] [Google Scholar]
Farag 2013 {published data only}
- Farag E, Ghobrial M, Sessler DI, Dalton JE, Liu J, Lee JH, et al. Effect of perioperative intravenous lidocaine administration on pain, opioid consumption, and quality of life after complex spine surgery. Anesthesiology 2013;119(4):932‐40. [PUBMED: 23681143] [DOI] [PubMed] [Google Scholar]
Gottschalk 1998 {published data only}
- Gottschalk A, Smith DS, Jobes DR, Kennedy SK, Lally SE, Noble VE, et al. Preemptive epidural analgesia and recovery from radical prostatectomy: a randomized controlled trial. JAMA 1998;279(14):1076‐82. [PUBMED: 9546566] [DOI] [PubMed] [Google Scholar]
Haythornthwaite 1998 {published data only}
- Haythornthwaite JA, Raja SN, Fisher B, Frank SM, Brendler CB, Shir Y. Pain and quality of life following radical retropubic prostatectomy. The Journal of Urology 1998;160(5):1761‐4. [PUBMED: 9783947] [PubMed] [Google Scholar]
- Shir Y, Frank SM, Brendler CB, Raja SN. Postoperative morbidity is similar in patients anesthetized with epidural and general anesthesia for radical prostatectomy. Urology 1994;44(2):232‐6. [PUBMED: 8048199] [DOI] [PubMed] [Google Scholar]
- Shir Y, Raja SN, Frank SM. The effect of epidural versus general anesthesia on postoperative pain and analgesic requirements in patients undergoing radical prostatectomy. Anesthesiology 1994;80(1):49‐56. [PUBMED: 8291729] [DOI] [PubMed] [Google Scholar]
Hirakawa 1996 {published data only}
- Hirakawa N, Fukui M, Takasaki M, Harano K, Totoki T. The effect of preemptive analgesia on the persistent pain following thoracotomy. Masui To Sosei. Hiroshima Journal of Anesthesia 1996;32(3):263‐6. [Google Scholar]
Hivelin 2011 {published data only}
- Hivelin M, Wyniecki A, Plaud B, Marty J, Lantieri L. Ultrasound‐guided bilateral transversus abdominis plane block for postoperative analgesia after breast reconstruction by DIEP flap. Plastic and Reconstructive Surgery 2011;128(1):44‐55. [PUBMED: 21701318] [DOI] [PubMed] [Google Scholar]
Howell 2001 {published data only}
- Howell CJ, Dean T, Lucking L, Dziedzic K, Jones PW, Johanson RB. Randomised study of long term outcome after epidural versus non‐epidural analgesia during labour. BMJ 2002;325(7360):357. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell CJ, Kidd C, Roberts W, Upton P, Lucking L, Jones PW, et al. A randomised controlled trial of epidural compared with non‐epidural analgesia in labour. BJOG 2001;108(1):27‐33. [PUBMED: 11213000] [DOI] [PubMed] [Google Scholar]
Ilfeld 2004 {published data only}
- Ilfeld BM, Smith DW, Enneking FK. Continuous regional analgesia following ambulatory pediatric orthopedic surgery. The American Journal of Orthopedics 2004;33(8):405‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Ilfeld 2015 {published data only}
- Ilfeld BM, Madison SJ, Suresh PJ, Sandhu NS, Kormylo NJ, Malhotra N, et al. Persistent postmastectomy pain and pain‐related physical and emotional functioning with and without a continuous paravertebral nerve block: a prospective 1‐year follow‐up assessment of a randomized, triple‐masked, placebo‐controlled study. Annals of Surgical Oncology 2015;22(6):2017‐25. [PUBMED: 25413267] [DOI] [PubMed] [Google Scholar]
Jahangiri 1994 {published data only}
- Jahangiri M, Jayatunga AP, Bradley JW, Dark CH. Prevention of phantom pain after major lower limb amputation by epidural infusion of diamorphine, clonidine and bupivacaine. Annals of the Royal College of Surgeons of England 1994;76(5):324‐6. [PUBMED: 7979074] [PMC free article] [PubMed] [Google Scholar]
Jirarattanaphochai 2007 {published data only}
- Jirarattanaphochai K, Jung S, Thienthong S, Krisanaprakornkit W, Sumananont C. Peridural methylprednisolone and wound infiltration with bupivacaine for postoperative pain control after posterior lumbar spine surgery: a randomized double‐blinded placebo‐controlled trial. Spine 2007;32(6):609‐16; discussion 617. [PUBMED: 17413463] [DOI] [PubMed] [Google Scholar]
Joseph 2012 {published data only}
- Joseph C, Gaillat F, Duponq R, Lieven R, Baumstarck K, Thomas P, et al. Is there any benefit to adding intravenous ketamine to patient‐controlled epidural analgesia after thoracic surgery? A randomized double‐blind study. European Journal of Cardio‐thoracic Surgery 2012;42(4):e58‐65. [PUBMED: 22790008] [DOI] [PubMed] [Google Scholar]
Kairaluoma 2010 {published data only}
- Kairaluoma P, Bachmann M, Alatalo S, Rosenberg P, Pere P. Paravertebral block vs. local anaesthetic wound infiltration for analgesia after open inguinal hernia repair performed under spinal anaesthesia. Regional Anesthesia and Pain Medicine 2010;35(5):E117. [DOI: ; EMBASE: 70287448] [Google Scholar]
Kindberg 2009 {published data only}
- Kindberg S, Klunder L, Strom J, Henriksen TB. Ear acupuncture or local anaesthetics as pain relief during postpartum surgical repair: a randomised controlled trial. BJOG 2009;116(4):569‐76. [PUBMED: 19120322] [DOI] [PubMed] [Google Scholar]
Kumar 1989 {published data only}
Kumar 2009 {published data only}
- Kumar S, Joshi M, Chaudhary S. 'Dissectalgia' following TEP, a new entity: its recognition and treatment. Results of a prospective randomized controlled trial. Hernia 2009;13(6):591‐6. [PUBMED: 19644647] [DOI] [PubMed] [Google Scholar]
Lambert 2001 {published data only}
- Lambert AW, Dashfield AK, Cosgrove C, Wilkins DC, Walker AJ, Ashley S. Randomized prospective study comparing preoperative epidural and intraoperative perineural analgesia for the prevention of postoperative stump and phantom limb pain following major amputation. Regional Anesthesia and Pain Medicine 2001;26(4):316‐21. [PUBMED: 11464349] [DOI] [PubMed] [Google Scholar]
Lebreux 2007 {published data only}
- Lavand'homme P, Roelants F, Fuzier‐Mercier V, Waterloos H. Postoperative analgesic and antihyperalgesic effects of spinal clonidine for cesarean section. Anesthesiology 2006;105:A997. [Google Scholar]
- Lebreux L, Roelants F, Waterloos H, Lavand'homme P. Postoperative analgesic and antihyperalgesic effect of spinal clonidine used during elective cesarean section. Acta Anaesthesiologica Belgica 2007;58(1):71. [EMBASE: 2007190608] [Google Scholar]
Lee 2012 {published data only}
- Lee JH, Yang WD, Han SY, Noh JI, Cho SH, Kim SH, et al. Effect of epidural magnesium on the incidence of chronic postoperative pain after video‐assisted thoracic surgery. Journal of Cardiothoracic and Vascular Anesthesia 2012;26(6):1055‐9. [PUBMED: 22883445] [DOI] [PubMed] [Google Scholar]
Loughnan 2002 {published data only}
- Loughnan BA, Carli F, Romney M, Dore CJ, Gordon H. Epidural analgesia and backache: a randomized controlled comparison with intramuscular meperidine for analgesia during labour. British Journal of Anaesthesia 2002;89(3):466‐72. [PUBMED: 12402727] [PubMed] [Google Scholar]
Mendola 2012 {published data only}
- Mendola C, Cammarota G, Netto R, Cecci G, Pisterna A, Ferrante D, et al. S+ ‐ketamine for control of perioperative pain and prevention of post thoracotomy pain syndrome: a randomized, double‐blind study. Minerva Anestesiologica 2012;78(7):757‐66. [PUBMED: 22441361] [PubMed] [Google Scholar]
Milligan 2002 {published data only}
- Milligan MP, Etokowo G, Kanumuru S, Mannifold N. Microwave endometrial ablation: patients' experiences in the first 3 months following treatment. Journal of Obstetrics and Gynaecology 2002;22(2):201‐4. [PUBMED: 12521709] [DOI] [PubMed] [Google Scholar]
Muthukumar 2012 {published data only}
- Muthukumar M, Arya VK, Mathew PJ, Sharma RK. Comparison of haemodynamic responses following different concentrations of adrenaline with and without lignocaine for surgical field infiltration during cleft lip and cleft palate surgery in children. Anaesthesia and Intensive Care 2012;40(1):114‐9. [PUBMED: 22313070] [DOI] [PubMed] [Google Scholar]
Nabhan 2011 {published data only}
- Nabhan A, Steudel WI, Dedeman L, Al‐Khayat J, Ishak B. Subcutaneous local anesthesia versus intravenous regional anesthesia for endoscopic carpal tunnel release: a randomized controlled trial. Journal of Neurosurgery 2011;114(1):240‐4. [PUBMED: 20415525] [DOI] [PubMed] [Google Scholar]
Nikolajsen 1997 {published data only}
- Nikolajsen L, Ilkjaer S, Christensen JH, Kroner K, Jensen TS. Randomised trial of epidural bupivacaine and morphine in prevention of stump and phantom pain in lower‐limb amputation. Lancet 1997;350(9088):1353‐7. [PUBMED: 9365449] [DOI] [PubMed] [Google Scholar]
- Nikolajsen L, Ilkjaer S, Jensen TS. Effect of preoperative extradural bupivacaine and morphine on stump sensation in lower limb amputees. British Journal of Anaesthesia 1998;81(3):348‐54. [PUBMED: 9861117] [DOI] [PubMed] [Google Scholar]
Obata 1999 {published data only}
- Obata H, Saito S, Fujita N, Fuse Y, Ishizaki K, Goto F. Epidural block with mepivacaine before surgery reduces long term postthoracotomy pain. Canadian Journal of Anasthesia/Journal Canadien d'Anesthésie 1999;46:1127‐32. [PUBMED: 10608205] [DOI] [PubMed] [Google Scholar]
Ochroch 2006 {published data only}
- Gottschalk A, Ochroch EA. Clinical and demographic characteristics of patients with chronic pain after major thoracotomy. The Clinical Journal of Pain 2008;24(8):708‐16. [PUBMED: 18806536] [DOI] [PubMed] [Google Scholar]
- Ochroch EA, Gottschalk A, Augostides J, Carson KA, Kent L, Malayaman N, et al. Long term pain and activity during recovery from major thoracotomy using thoracic epidural anesthesia. Anesthesiology 2002;97:1234‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ochroch EA, Gottschalk A, Troxel AB, Farrar JT. Women suffer more short and long‐term pain than men after major thoracotomy. The Clinical Journal of Pain 2006;22(5):491‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ouaki 2009 {published data only}
- Ouaki J, Dadure C, Bringuier S, Raux O, Rochette A, Captier G, et al. Continuous infusion of ropivacaine: an optimal postoperative analgesia regimen for iliac crest bone graft in children. Paediatric Anaesthesia 2009;19(9):887‐91. [PUBMED: 19691695] [DOI] [PubMed] [Google Scholar]
Panos 1990 {published data only}
- Panos L, Sandler AN, Stringer DG, Badner N, Lawson S, Koren G. Continuous infusions of lumbar epidural fentanyl and intravenous fentanyl for post‐thoracotomy pain relief. I: analgesic and pharmacokinetic effects. Canadian Journal of Anesthesia 1990;37(4 Pt 2):S66. [PUBMED: 2193761] [PubMed] [Google Scholar]
Perniola 2009 {published data only}
- Perniola A, Gupta A, Crafoord K, Darvish B, Magnuson A, Axelsson K. Intraabdominal local anaesthetics for postoperative pain relief following abdominal hysterectomy: a randomized, double‐blind, dose‐finding study. European Journal of Anaesthesiology 2009;26(5):421‐9. [PUBMED: 19521298] [DOI] [PubMed] [Google Scholar]
Pompeo 2007 {published data only}
- Pompeo E, Tacconi F, Mineo D, Mineo TC. The role of awake video‐assisted thoracoscopic surgery in spontaneous pneumothorax. Journal of Thoracic and Cardiovascular Surgery 2007;133(3):786‐90. [PUBMED: 17320585] [DOI] [PubMed] [Google Scholar]
Rosen 2009 {published data only}
- Rosen MJ, Duperier T, Marks J, Onders R, Hardacre J, Ponsky J, et al. Prospective randomized double‐blind placebo‐controlled trial of postoperative elastomeric pain pump devices used after laparoscopic ventral hernia repair. Surgical Endoscopy 2009;23(12):2637‐43. [PUBMED: 19357918] [DOI] [PubMed] [Google Scholar]
Royse 2007 {published data only}
- Royse C, Remedios C, Royse A. High thoracic epidural analgesia reduces the risk of long‐term depression in patients undergoing coronary artery bypass surgery. Annals of Thoracic and Cardiovascular Surgery 2007;13(1):32‐5. [PUBMED: 17392668] [PubMed] [Google Scholar]
Ryu 2011 {published data only}
- Ryu HG, Lee CJ, Kim YT, Bahk JH. Preemptive low‐dose epidural ketamine for preventing chronic postthoracotomy pain: a prospective, double‐blinded, randomized, clinical trial. The Clinical Journal of Pain 2011;27(4):304‐8. [PUBMED: 21178605] [DOI] [PubMed] [Google Scholar]
Saber 2009 {published data only}
- Saber AA, Elgamal MH, Rao AJ, Itawi EA, Martinez RL. Early experience with lidocaine patch for postoperative pain control after laparoscopic ventral hernia repair. International Journal of Surgery (London, England) 2009;7(1):36‐8. [PUBMED: 18951860] [DOI] [PubMed] [Google Scholar]
Salengros 2010 {published data only}
- Salengros JC, Huybrechts I, Ducart A, Faraoni D, Marsala C, Barvais L, et al. Different anesthetic techniques associated with different incidences of chronic post‐thoracotomy pain: low‐dose remifentanil plus presurgical epidural analgesia is preferable to high‐dose remifentanil with postsurgical epidural analgesia. Journal of Cardiothoracic and Vascular Anesthesia 2010;24(4):608‐16. [PUBMED: 20005744] [DOI] [PubMed] [Google Scholar]
Schaan 2004 {published data only}
- Schaan M, Schmitt N, Boszczyk B, Jaksche H. Reduction in late postoperative pain after iliac crest bonegraft harvesting for cervical fusion: a controlled double‐blinded study of 100 patients. Acta Neurochirurgica 2004;146(9):961‐5. [PUBMED: 15340805] [DOI] [PubMed] [Google Scholar]
Schley 2007 {published data only}
- Schley M, Topfner S, Wiech K, Schaller HE, Konrad CJ, Schmelz M, et al. Continuous brachial plexus blockade in combination with the NMDA receptor antagonist memantine prevents phantom pain in acute traumatic upper limb amputees. European Journal of Pain 2007;11(3):299‐308. [PUBMED: 16716615] [DOI] [PubMed] [Google Scholar]
Sen 2009 {published data only}
- Sen H, Sizlan A, Yanarates O, Senol MG, Inangil G, Sucullu I, et al. The effects of gabapentin on acute and chronic pain after inguinal herniorrhaphy. European Journal of Anaesthesiology 2009;26(9):772‐6. [PUBMED: 19424073] [DOI] [PubMed] [Google Scholar]
Shikano 1994 {published data only}
- Shikano S Yamashita H, Kawahara M, Shimizu N, Nomura S, Nobusawa S, et al. Effect of wound infiltration with bupivacaine for postoperative pain after laparoscopic cholecystectomy. Surgical Laparoscopy and Endoscopy 1994;4(6):500. [Google Scholar]
Sim 2012 {published data only}
- Sim WS, Lee SH, Roe HJ. Does preemptive thoracic epidural analgesia enhance post‐thoracotomy pain control and pulmonary function?. Pain Practice 2012;12:137. [EMBASE: 70654960] [Google Scholar]
Suvikapakornkul 2009 {published data only}
- Suvikapakornkul R, Valaivarangkul P, Noiwan P, Phansukphon T. A randomized controlled trial of preperitoneal bupivacaine instillation for reducing pain following laparoscopic inguinal herniorrhaphy. Surgical Innovation 2009;16(2):117‐23. [PUBMED: 19468036] [DOI] [PubMed] [Google Scholar]
Suzuki 2006 {published data only}
- Suzuki M, Haraguti S, Sugimoto K, Kikutani T, Shimada Y, Sakamoto A. Low‐dose intravenous ketamine potentiates epidural analgesia after thoracotomy. Anesthesiology 2006;105(1):111‐9. [PUBMED: 16810002] [DOI] [PubMed] [Google Scholar]
Verma 2006 {published data only}
- Verma GR, Lyngdoh TS, Kaman L, Bala I. Placement of 0.5% bupivacaine‐soaked Surgicel in the gallbladder bed is effective for pain after laparoscopic cholecystectomy. Surgical Endoscopy 2006;20(10):1560‐4. [PUBMED: 16897291] [DOI] [PubMed] [Google Scholar]
Vigneau 2011 {published data only}
- Vigneau A, Salengro A, Berger J, Rouzier R, Barranger E, Marret E, et al. A double blind randomized trial of wound infiltration with ropivacaine after breast cancer surgery with axillary nodes dissection. BMC Anesthesiology 2011;11:23. [PUBMED: 22114900] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 1992 {published data only}
- Wang DF, Liu XM, Xue CX. A comparative study of intra‐pleural administration of bupivacaine in different volume for pain relief after thoracotomy. Chinese Journal of Anesthesiology 1992; Vol. 12, issue 6:370‐2. [0254‐1416]
Weihrauch 2005 {published data only}
- Weihrauch JO, Jehmlich M, Leischik M, Hopf HB. Are peripheral nerve blocks of the leg (femoralis in combination with anterior sciatic blockade) as sole anaesthetic technique an alternative to epidural anaesthesia? [Ist die periphere nervenblockade des beines (femoralis‐ in kombination mit anteriorer ischiadikusblockade) als alleinige anasthesietechnik eine alternative zur periduralanasthesie fur arthroskopische eingriffe am kniegelenk?]. Anasthesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie: AINS 2005;40(1):18‐24. [PUBMED: 15645383] [DOI] [PubMed] [Google Scholar]
Wilson 2008 {published data only}
- Wilson JA, Nimmo AF, Fleetwood‐Walker SM, Colvin LA. A randomised double blind trial of the effect of pre‐emptive epidural ketamine on persistent pain after lower limb amputation. Pain 2008;135(1‐2):108‐18. [PUBMED: 17583431] [DOI] [PubMed] [Google Scholar]
Yang 2012 {published and unpublished data}
- Yang HC, Lee J, Song I, Lee J, Choi W, Cho S, et al. Pain control of thoracoscopic major pulmonary resection: is pre‐emptive local bupivacaine injection able to replace intravenous patient‐controlled analgesia?. Interactive Cardiovascular and Thoracic Surgery 2012;15(Suppl. 2):S104‐S105. [EMBASE: 70930928] [Google Scholar]
References to studies awaiting assessment
Capdevila 2017 {published data only}
- Capdevila X, Moulard S, Plasse C, Peshaud JL, Molinari N, Dadure C, et al. Effectiveness of epidural analgesia, continuous surgical site analgesia, and patient‐controlled analgesic morphine for postoperative pain management and hyperalgesia, rehabilitation, and health‐related quality of life after open nephrectomy: a prospective, randomized, controlled study. Anesthesia and Analgesia 2017;124(1):336‐45. [PUBMED: 27918333] [DOI] [PubMed] [Google Scholar]
Choi 2017 {published data only}
- Choi KW, Nam KH, Lee JR, Chung WY, Kang SW, Joe YE, et al. The effects of intravenous lidocaine infusions on the quality of recovery and chronic pain after robotic thyroidectomy: a randomized, double‐blinded, controlled study. World Journal of Surgery 2017;41(5):1305‐12. [PUBMED: 27896411] [DOI] [PubMed] [Google Scholar]
Elkaradawy 2012 {published data only}
- Elkaradawy S, Nasr M, Elkerm Y, Deeb M, Yassine O. The effect of multimodal balanced anaesthesia and long term gabapentin on neuropathic pain, nitric oxide and interleukin‐1β following breast surgery. Egyptian Journal of Anaesthesia 2012;28(1):67‐78. [Google Scholar]
Fiorelli 2016 {published data only}
- Fiorelli A, Natale D, Rimessi A, Sansone P, Pace C, Passavanti B, et al. Preventive application of lidocaine patch in adjunction to intravenous morphine analgesia for management of post‐thoracotomy pain: results of a randomized, double blind, placebo controlled study. Interactive Cardiovascular and Thoracic Surgery 2016;23(suppl 1):i2. [Google Scholar]
Iohom 2006 {published data only}
- Iohom G, Abdalla H, O'Brien J, Szarvas S, Larney V, Buckley E, et al. The associations between severity of early postoperative pain, chronic postsurgical pain and plasma concentration of stable nitric oxide products after breast surgery. Anesthesia and Analgesia 2006;103(4):995‐1000. [PUBMED: 17000819] [DOI] [PubMed] [Google Scholar]
Jendoubi 2017 {published data only}
- Jendoubi A, Naceur IB, Bouzouita A, Trifa M, Ghedira S, Chebil M, et al. A comparison between intravenous lidocaine and ketamine on acute and chronic pain after open nephrectomy: a prospective, double‐blind, randomized, placebo‐controlled study. Saudi Journal of Anaesthesia 2017;11(2):177‐84. [PUBMED: 28442956] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kendall 2018 {published data only}
- Kendall MC, McCarthy RJ, Panaro S, Goodwin E, Bialek JM, Nader A, et al. The effect of intraoperative systemic lidocaine on postoperative persistent pain using Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials criteria assessment following breast cancer surgery: a randomized, double‐blind, placebo‐controlled trial. Pain Practice 2018;18(3):350‐9. [DOI: 10.1111/papr.12611; PUBMED: 28691269] [DOI] [PubMed] [Google Scholar]
Kim 2017 {published data only}
- Kim MH, Lee KY, Park S, Kim SI, Park HS, Yoo YC. Effects of systemic lidocaine versus magnesium administration on postoperative functional recovery and chronic pain in patients undergoing breast cancer surgery: a prospective, randomized, double‐blind, comparative clinical trial. PloS one 2017;12(3):e0173026. [PUBMED: 28253307] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oh 2017 {published data only}
- Oh J, Page MG, Zhong T, McCluskey S, Srinivas C, O'Neill AC, et al. Chronic postsurgical pain outcomes in breast reconstruction patients receiving perioperative transversus abdominis plane catheters at the donor site: a prospective cohort follow‐up study. Pain Practice 2017;17(8):999‐1007. [PUBMED: 27996199] [DOI] [PubMed] [Google Scholar]
Okur 2017 {published data only}
- Okur O, Tekgul ZT, Erkan N. Comparison of efficacy of transversus abdominis plane block and iliohypogastric/ilioinguinal nerve block for postoperative pain management in patients undergoing inguinal herniorrhaphy with spinal anesthesia: a prospective randomized controlled open‐label study. Journal of Anesthesia 2017;31(5):678‐85. [PUBMED: 28616651] [DOI] [PubMed] [Google Scholar]
Reuben 2006 {published data only}
- Reuben SS, Raghunathan K, Roissing S. Evaluating the analgesic effect of the perioperative perineural infiltration of bupivacaine and clonidine at the site of injury following lower extremity amputation. Acute Pain 2006;8(13):117‐23. [Google Scholar]
Zwaans 2017 {published data only}
- Zwaans WA, Mair LH, Scheltinga MR, Roumen RM. Spinal versus general anaesthesia in surgery for inguinodynia (SPINASIA trial): study protocol for a randomised controlled trial. Trials 2017;18(1):23. [PUBMED: 28088218] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN46621916 {published data only}
- ISRCTN46621916. SUBpectoral Local anaesthetic Infusion following MastEctomy ‐ version 1 (SUBLIME). doi.org/10.1186/ISRCTN46621916 first received 5 December 2012.
- Langford R, Brown I, Vickery J, Mitchell K, Pritchard C, Creanor S. Study protocol for a double blind, randomised, placebo‐controlled trial of continuous subpectoral local anaesthetic infusion for pain and shoulder function following mastectomy: SUB‐pectoral Local anaesthetic Infusion following MastEctomy (SUBLIME) study. BMJ Open 2014;4(9):e006318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liew 2011 {published data only}
- Liew A, Reftymann L, Chou D, Aust T, Rosen D, Cario G. Postoperative pain relief after laparoscopic gynaecological surgery: a pilot study of pre‐emptive superior hypogastric plexus block versus placebo using ropivacaine. The LAP‐HYPOPLEX study. Anaesthesia and Intensive Care 2011;39(5):964. [Google Scholar]
Michael 2014 {published data only}
- Michael M, Tozzi M, Blesi L, Torchio S, Binda S, Tarallo A, et al. Continuous transgluteal sciatic nerve block to prevent phantom limb pain after trans‐femoral amputation in patient with copa. Regional Anesthesia and Pain Medicine 2014;39(5):e319. [Google Scholar]
NCT00418457 {published data only}
- NCT00418457. Regional anesthesia and breast cancer recurrence. clinicaltrials.gov/ct2/show/NCT00418457 first received 4 January 2007.
NCT01626755 {published data only}
- Lirk P, Stadlbauer KH, Hollmann MW. ESA Clinical Trials Network 2012: PLATA‐‐Prevention of Phantom Limb Pain After Transtibial Amputation: randomised, double‐blind, controlled, multicentre trial comparing optimised intravenous pain control versus optimised intravenous pain control plus regional anaesthesia. European Journal of Anaesthesiology 2013;30(5):202‐4. [DOI] [PubMed] [Google Scholar]
- NCT01626755. Prevention of Phantom Limb Pain After Transtibial Amputation (PLATA). clinicaltrials.gov/ct2/show/NCT01626755 first received 25 June 2012.
NCT02002663 {published data only}
- Bugada D, Gregori M, Compagnone C, Muscoli C, Raimondi F, Bettinelli S, et al. Continuous wound infusion of local anesthetic and steroid after major abdominal surgery: study protocol for a randomized controlled trial. Trials 2015;16:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02002663. Continuous local anesthetic and steroid infusion in abdominal surgery (GR‐CWI). clinicaltrials.gov/ct2/show/NCT02002663 first received 6 December 2013.
Theodoraki 2016 {published data only}
- Theodoraki K, Argyra E, Papacharalampous P. The effect of transversus abdominis plane block on acute and chronic pain after inguinal hernia repair. Regional Anesthesia and Pain Medicine 2016;41(5 (suppl 1)):ESRA6‐0147. [DOI] [PubMed] [Google Scholar]
Additional references
Andreae 2013b
- Andreae MH, Johnson M, Sacks H. Bayesian responder meta‐analysis of regional anaesthesia to prevent chronic pain after iliac crest bone graft harvesting. Regional Anesthesia and Pain Medicine 2013;38(1):A1. [Google Scholar]
Andreae 2015
- Andreae MH. Local and regional anesthesia to prevent persistent pain after surgery. A systematic review and bayesian meta‐analysis for the Cochrane Collaboration [Masters thesis]. New York, NY: Columbia University, 2015. [Google Scholar]
Andreae 2015c
- Andreae MH, Carter GM, Shaparin N, Suslov K, Ellis RJ, Ware MA, et al. Inhaled cannabis for chronic neuropathic pain: a meta‐analysis of individual patient data. Journal of Pain 2015;16(12):1221‐32. [PUBMED: 26362106] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreae 2017a
- Andreae MH, Gabry JS, Goodrich B, White RS, Hall C. Antiemetic prophylaxis as a marker of health care disparities in the national anesthesia clinical outcomes registry. Anesthesia and Analgesia 2017;126(2):588‐99. [DOI: 10.1213/ANE.0000000000002582; PUBMED: 29116968] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreae 2017b
- Andreae MH, Nair S, Gabry JS, Goodrich B, Hall C, Shaparin N. A pragmatic trial to improve adherence with scheduled appointments in an inner‐city pain clinic by human phone calls in the patient's preferred language. Journal of Clinical Anesthesia 2017;42:77‐83. [DOI: 10.1016/j.jclinane.2017.08.014.; PUBMED: 28841451] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreae 2018
- Andreae MH, Pace, NL. A novel approach to synthesize the evidence on analgesic adjuvants for postoperative pain. Anesthesia and Analgesia 2018;126(2):377‐81. [DOI: 10.1213/ANE.0000000000002589; PUBMED: 29346200] [DOI] [PubMed] [Google Scholar]
Atchabahian 2015a
- Atchabahian A, Schwartz G, Hall CB, Lajam CM, Andreae MH. Regional analgesia for improvement of long‐term functional outcome after elective large joint replacement. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD010278.pub2; PUBMED: 26269416] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atchabahian 2015b
- Atchabahian A, Andreae M. Long‐term functional outcomes after regional anesthesia: a summary of the published evidence and a recent Cochrane Review. Refresher Courses in Anesthesiology 2015;43(1):15‐26. [PUBMED: 26456997] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayman 2014
- Bayman EO, Brennan TJ. Incidence and severity of chronic pain at 3 and 6 months after thoracotomy: meta‐analysis. Journal of Pain 2014;15(9):887‐97. [PUBMED: 24968967] [DOI] [PubMed] [Google Scholar]
Beusterien 1996
- Beusterien KM, Steinwald B, Ware JE Jr. Usefulness of the SF‐36 Health Survey in measuring health outcomes in the depressed elderly. Journal of Geriatric Psychiatry and Neurology 1996;9(1):13‐21. [PUBMED: 8679058] [DOI] [PubMed] [Google Scholar]
Bland 2000
- Bland JM, Altman DG. Statistics notes. The odds ratio. BMJ (Clinical Research Ed.) 2000;320(7247):1468. [PUBMED: 10827061] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bong 2005
- Bong CL, Samuel M, Ng JM, Ip‐Yam C. Effects of preemptive epidural analgesia on post‐thoracotomy pain. Journal of Cardiothoracic and Vascular Anesthesia 2005;19(6):786‐93. [PUBMED: 16326309] [DOI] [PubMed] [Google Scholar]
Booth 2012
- Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M, et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Systematic Reviews 2012;1:2. [PUBMED: 22587842] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 1995
- Brown DL, Ransom DM, Hall JA, Leicht CH, Schroeder DR, Offord KP. Regional anesthesia and local anesthetic‐induced systemic toxicity: seizure frequency and accompanying cardiovascular changes. Anesthesia and Analgesia 1995;81(2):321‐8. [PUBMED: 7618723] [DOI] [PubMed] [Google Scholar]
Brull 2007
- Brull R, McCartney CJ, Chan VW, El‐Beheiry H. Neurological complications after regional anesthesia: contemporary estimates of risk. Anesthesia and Analgesia 2007;104(4):965‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carter 2015
- Carter GM, Indyk D, Johnson M, Andreae M, Suslov K, Busani S, et al. Micronutrients in HIV: a Bayesian meta‐analysis. PloS one 2015;10(4):e0120113. [DOI: ; PUBMED: 25830916] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaparro 2013
- Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD008307.pub2; PUBMED: 23881791] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ (Clinical Research Ed.) 1995;310(6977):452‐4. [PUBMED: 7873954] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cornell 2014
- Cornell JE, Mulrow CD, Localio R, Stack CB, Meibohm AR, Guallar E, et al. Random‐effects meta‐analysis of inconsistent effects: a time for change. Annals of Internal Medicine 2014;160(4):267‐70. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [PUBMED: 3802833] [DOI] [PubMed] [Google Scholar]
DistillerSR [Computer program]
- Evidence Partners. DistillerSR. Ottawa, Canada: Evidence Partners, 2014.
Duarte 2005
- Duarte AM, Pospisilova E, Reilly E, Mujenda F, Hamaya Y, Strichartz GR. Reduction of postincisional allodynia by subcutaneous bupivacaine: findings with a new model in the hairy skin of the rat. Anesthesiology 2005;103(1):113‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dworkin 2009a
- Dworkin RH, Turk DC, McDermott MP, Peirce‐Sandner S, Burke LB, Cowan P, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 2009;146(3):238‐44. [PUBMED: 19836888] [DOI] [PubMed] [Google Scholar]
Dworkin 2009b
- Dworkin RH, Turk DC, Revicki DA, Harding G, Coyne KS, Peirce‐Sandner S, et al. Development and initial validation of an expanded and revised version of the Short‐Form McGill Pain Questionnaire (SF‐MPQ‐2). Pain 2009;144(1‐2):35‐42. [PUBMED: 19356853] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fassoulaki 2008
- Fassoulaki A, Melemeni A, Staikou C, Triga A, Sarantopoulos C. Acute postoperative pain predicts chronic pain and long‐term analgesic requirements after breast surgery for cancer. Acta Anaesthesiologica Belgica 2008;59(4):241‐8. [PUBMED: 19235522] [PubMed] [Google Scholar]
Gelman 2014
- Gelman A Carlin JB, Stern HS, Rubin DB. Bayesian Data Analysis. 2. Abdindon, OXON, UK: Taylor & Francis, 2014. [Google Scholar]
Gewandter 2015
- Gewandter JS, Dworkin RH, Turk DC, Farrar JT, Fillingim RB, Gilron I, et al. Research design considerations for chronic pain prevention clinical trials: IMMPACT recommendations. Pain 2015;7:1184‐97. [PUBMED: 25887465] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association : JMLA 2006;94(2):130‐6. [PUBMED: 16636704] [PMC free article] [PubMed] [Google Scholar]
Gottschalk 2006
- Gottschalk A, Cohen SP, Yang S, Ochroch EA. Preventing and treating pain after thoracic surgery. Anesthesiology 2006;104:594‐600. [PUBMED: 16508407] [DOI] [PubMed] [Google Scholar]
GRADE Working Group 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 1 June 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Herroeder 2007
- Herroeder S, Pecher S, Schonherr ME, Kaulitz G, Hahnenkamp K, Friess H, et al. Systemic lidocaine shortens length of hospital stay after colorectal surgery: a double‐blinded, randomized, placebo‐controlled trial. Annals of Surgery 2007;246(2):192‐200. [PUBMED: 17667496] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hewitt 2005
- Hewitt C, Hahn S, Torgerson DJ, Watson J, Bland JM. Adequacy and reporting of allocation concealment: review of recent trials published in four general medical journals. BMJ (Clinical Research Ed.) 2005;330(7499):1057‐8. [PUBMED: 15760970] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;15(21):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ioannidis 2008
- Ioannidis JP, Patsopoulos NA, Rothstein HR. Reasons or excuses for avoiding meta‐analysis in forest plots. BMJ (Clinical Research Ed.) 2008;336(7658):1413‐5. [PUBMED: 18566080] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeng 2010
- Jeng CL, Torrillo TM, Rosenblatt MA. Complications of peripheral nerve blocks. British Journal of Anaesthesia 2010;105 Suppl 1:i97‐107. [PUBMED: 21148659] [DOI] [PubMed] [Google Scholar]
Jung 2003
- Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain 2003;104(1‐2):1‐13. [PUBMED: 12855309] [DOI] [PubMed] [Google Scholar]
Kehlet 2006
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367(9522):1618‐25. [PUBMED: 16698416] [DOI] [PubMed] [Google Scholar]
Kissin 1996
- Kissin I. Preemptive analgesia: why its effect is not always obvious. Anesthesiology 1996;84:1015‐9. [PUBMED: 8623993] [DOI] [PubMed] [Google Scholar]
Kissin 2000
- Kissin I. Preemptive analgesia. Anesthesiology 2000;93(4):1138‐43. [PUBMED: 11020772] [DOI] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schunemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;2:81. [PUBMED: 24059250] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lavand'homme 2011
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Levene 2015
- Levene J, Weinstein E, Cohen M, Hall C, Johnson M, Andreae M. The impact of attrition on effect size in meta‐analysis: a graphical test. www.asaabstracts.com/ (last accessed November 2015):A2092.
Lewis 2015
- Lewis GN, Rice DA, McNair PJ, Kluger M. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta‐analysis. British Journal of Anaesthesia 2015;114(4):551‐61. [PUBMED: 25542191] [DOI] [PubMed] [Google Scholar]
Lunn 2009
- Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: evolution, critique and future directions. Statistics in Medicine 2009;28(25):3049. [DOI] [PubMed] [Google Scholar]
MacRae 2001
- MacRae WA. Chronic pain after surgery. British Journal of Anaesthesia 2001;87:88‐98. [PUBMED: 11460816] [DOI] [PubMed] [Google Scholar]
MacRae 2008
- MacRae WA. Chronic post‐surgical pain: 10 years on. British Journal of Anaesthesia 2008;101(1):77‐86. [PUBMED: 18434337] [DOI] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of metaanalyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Metaanalyses. Lancet 1999;354:1896‐900. [PUBMED: 10584742] [DOI] [PubMed] [Google Scholar]
Moher 2010
Montes 2015
- Montes A, Roca G, Sabate S, Lao Jose I, Navarro Ai, Cantillo J, et al. Genetic and clinical factors associated with chronic postsurgical pain after hernia repair, hysterectomy, and thoracotomy. A two‐year multicenter cohort study. Anesthesiology 2015;122(5):1123‐41. [DOI] [PubMed] [Google Scholar]
Moore 2013
- Moore RA, Derry S, Wiffen PJ. Challenges in design and interpretation of chronic pain trials. British Journal of Anaesthesia 2013;111(1):38‐45. [PUBMED: 23794643] [DOI] [PubMed] [Google Scholar]
Movassaghian 2013
- Movassaghian S, Afzalifar R, Alaeddini M. Clinical anesthetic effectiveness of intraoral mucoadhesive tablets of amitriptyline in healthy volunteers. Journal of Oral and Maxillofacial Surgery 2013;71(1):23‐8. [PUBMED: 23089653] [DOI] [PubMed] [Google Scholar]
Mustola 2011
- Mustola ST, Lempinen J, Saimanen E, Vilkko P. Efficacy of thoracic epidural analgesia with or without intercostal nerve cryoanalgesia for postthoracotomy pain. The Annals of Thoracic Surgery 2011;91(3):869‐73. [PUBMED: 21353017] [DOI] [PubMed] [Google Scholar]
Møiniche 2002
- Møiniche S, Kehlet H, Dahl JB. A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: the role of timing of analgesia. Anesthesiology 2002;96:725‐41. [PUBMED: 11873051] [DOI] [PubMed] [Google Scholar]
Neal 2008
- Neal JM, Bernards CM, Hadzic A, Hebl JR, Hogan QH, Horlocker TT, et al. ASRA practice advisory on neurologic complications in regional anesthesia and pain medicine. Regional Anesthesia and Pain Medicine 2008;33(5):404‐15. [PUBMED: 18774509] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ong 2005
- Ong CK, Lirk P, Seymour RA, Jenkins BJ. The efficacy of preemptive analgesia for acute postoperative pain management: a metaanalysis. Anesthesia and Analgesia 2005;100:757‐73. [PUBMED: 15728066] [DOI] [PubMed] [Google Scholar]
Perkins 2000
- Perkins FM, Kehlet H. Chronic pain after surgery: a review of predictive factors. Anesthesiology 2000;93:1123‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pubmed Central
- National Center for Biotechnology Information. PubMed Central. www.ncbi.nlm.nih.gov/pubmed/ (last accessed August 2010).
R 2015 [Computer program]
- R core Team. R: a language and environment for statistical computing. Vienna Austria: R foundation for statistical computing, 2015.
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodriguez‐Navarro 2011
- Rodriguez‐Navarro AJ, Berde CB, Wiedmaier G, Mercado A, Garcia C, Iglesias V, et al. Comparison of neosaxitoxin versus bupivacaine via port infiltration for postoperative analgesia following laparoscopic cholecystectomy: a randomized, double‐blind trial. Regional Anesthesia and Pain Medicine 2011;36(2):103‐9. [PUBMED: 21425506] [DOI] [PubMed] [Google Scholar]
RStan 2.5 [Computer program]
- Stan Development Team. RStan: the R interface to Stan, Version 2.5. New York: Stan Development Team, 2014.
Schnabel 2010
- Schnabel A, Reichl SU, Kranke P, Pogatzki‐Zahn EM, Zahn PK. Efficacy and safety of paravertebral blocks in breast surgery: a meta‐analysis of randomized controlled trials. British Journal of Anaesthesia 2010;105(6):842‐52. [PUBMED: 20947592] [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shafer 2009
ShinyStan 1.0 [Computer program]
- ShinyStanTeam. ShinyStan: R package for the interactive exploration of Marcov Chain Monte Carlo Output Version 1.0. New York: ShinyStanTeam, 2014.
Shrier 2007
- Shrier I, Boivin JF, Steele RJ, Platt RW, Furlan A, Kakuma R, et al. Should meta‐analyses of interventions include observational studies in addition to randomized controlled trials? A critical examination of underlying principles. American Journal of Epidemiology 2007;166(10):1203‐9. [PUBMED: 17712019] [DOI] [PubMed] [Google Scholar]
Sng 2009
- Sng BL, Sia AT, Quek K, Woo D, Lim Y. Incidence and risk factors for chronic pain after caesarean section under spinal anaesthesia. Anaesthesia and Intensive Care 2009;37(5):748‐52. [PUBMED: 19775038] [DOI] [PubMed] [Google Scholar]
Song 2012
- Song F, Clark A, Bachmann MO, Maas J. Simulation evaluation of statistical properties of methods for indirect and mixed treatment comparisons. BMC Medical Research Methodology 2012;12:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Strichartz 2008
- Strichartz GR. Novel ideas of local anaesthetic actions on various ion channels to ameliorate postoperative pain. British Journal of Anaesthesia 2008;101(1):45‐7. [PUBMED: 18487246] [DOI] [PubMed] [Google Scholar]
Tait 1990
- Tait RC, Chibnall JT, Krause S. The Pain Disability Index: psychometric properties. Pain 1990;40(2):171‐82. [PUBMED: 2308763] [DOI] [PubMed] [Google Scholar]
Terkawi 2015a
- Terkawi AS, Tsang S, Sessler DI, Terkawi RS, Nunemaker MS, Durieux ME, et al. Improving analgesic efficacy and safety of thoracic paravertebral block for breast surgery: a mixed‐effects meta‐analysis. Pain Physician 2015;18(5):E757‐80. [PUBMED: 26431130] [PubMed] [Google Scholar]
Thompson 2002
- Thompson SG, Higgins JPT. How should meta‐regression analyses be undertaken and interpreted?. Statistics in Medicine 2002;21(11):1559‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Turk 2006
- Turk DC, Dworkin RH, Burke LB, Gershon R, Rothman M, Scott J, et al. Developing patient‐reported outcome measures for pain clinical trials: IMMPACT recommendations. Pain 2006;125:208‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
University of Alberta Library Guide 2014
- University of Alberta Library Guides. Systematic reviews‐searching the literature. guides.library.ualberta.ca/systematicreviews (Accessed 20 September 2014).
Vigneault 2011
- Vigneault L, Turgeon AF, Cote D, Lauzier F, Zarychanski R, Moore L, et al. Perioperative intravenous lidocaine infusion for postoperative pain control: a meta‐analysis of randomized controlled trials. Canadian Journal of Anesthesia/Journal Canadien d'Anesthésie 2011;58(1):22‐37. [PUBMED: 21061107] [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical care 1992;30(6):473‐83. [PUBMED: 1593914] [PubMed] [Google Scholar]
Wilder‐Smith 2006
- Wilder‐Smith OH, Arendt‐Nielsen L. Postoperative hyperalgesia, its clinical importance and relevance. Anesthesiology 2006;104:601‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Woods 1995
- Woods KL. Mega‐trials and management of acute myocardial infarction. Lancet 1995;346(8975):611‐4. [PUBMED: 7651008] [DOI] [PubMed] [Google Scholar]
Woolf 1993
- Woolf CJ, Chong MS. Preemptive analgesia: treating postoperative pain by preventing the establishment of central sensitisation. Anesthesia and Analgesia 1993;77:362‐79. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Woolf 2000
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science 2000;288:1765‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Andreae 2008
- Andreae MH, Andreae DA, Motschall E, Rücker G, Timmer A. Local anaesthetics and regional anaesthesia for preventing chronic pain after surgery. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD007105] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreae 2012
- Andreae MH, Andreae DA. Local anaesthetics and regional anaesthesia for preventing chronic pain after surgery. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD007105.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreae 2013a
- Andreae MH, Andreae DA. Regional anaesthesia to prevent chronic pain after surgery: a Cochrane systematic review and meta‐analysis. British Journal of Anaesthesia 2013;epublication:1‐10. [DOI: 10.1093/bja/aet213] [DOI] [PMC free article] [PubMed] [Google Scholar]
Levene 2019
- Levene JL, Weinstein EJ, Cohen MS, Andreae DA, Chao JY, Johnson M, et al. Local anesthetics and regional anesthesia versus conventional analgesia for preventing persistent postoperative pain in adults and children: a Cochrane systematic review and meta‐analysis update. Journal of Clinical Anesthesia 2019;55:116‐27. [ 10.1016/j.jclinane.2018.12.043; PUBMED: 30640059] [DOI] [PMC free article] [PubMed] [Google Scholar]


