Abstract
The integration of pathology with molecular biology is vital if we are to enhance the translational value of cancer research. Pathology represents a bridge between medicine and basic biology, it remains the gold standard for cancer diagnosis, and it plays an important role in discovery studies. In the past, pathology and cancer research were closely associated; however, the molecular biology revolution has shifted the focus of investigators toward the molecular alterations of tumors. The reductionist approach taken in molecular studies is producing great insight into the inner workings of neoplasia, but it can also minimize the importance of histopathology and of understanding the disease as a whole. In turn, pathologists can underestimate the role of molecular studies in developing new ancillary techniques for clinical diagnosis. A multidisciplinary approach that integrates pathology and molecular biology within a translational research system is needed. This process will require overcoming cultural barriers and can be achieved through education, a more effective incorporation of pathology into biological research, and conversely an integration of biological research into the pathology laboratory.
Keywords: cancer research, molecular biology, pathology, systems biology, translational research
Introduction
The field of cancer research is in an exciting historical period as spectacular advances in molecular and cellular biology are unveiling many of the fundamental mechanisms of carcinogenesis [1, 2]. For example, novel technologies for high throughput molecular profiling such as microarrays and next generation DNA sequencing are identifying unique molecular profiles and mutation patterns in cancer cells [3, 4]. Entire new mechanisms of gene expression regulation including epigenetics, i.e. DNA methylation, the histone code, and microRNAs, have been identified as key components in the process of cell differentiation and tumorigenesis [5, 6]. A new bioinformatics discipline has evolved and is now essential for the analysis and integration of large-scale molecular data sets [7, 8]. Moreover, the online availability of biomedical databases, such as Medline, allows a near real-time evaluation of research being conducted across the world, accelerating scientific inquiry [9]. Together, these advances are generating a comprehensive understanding of the complex mechanisms involved in cancer and represent an ongoing scientific revolution [3, 10, 11]. According to Thomas Kuhn [12], progress is based on periodic phases in which a paradigm shift opens a new dimension of knowledge that offers novel insights into phenomena that could not be explained with the previous worldview, even permeating beyond science into the cultural framework. In this regard, molecular and cellular biology are offering novel insights into previously unexplained phenomena, such as the mechanisms of Darwinian evolution, and these are percolating into modern culture and presenting new realities and ethical challenges [13, 14].
Naturally, cancer research involves the study of the neoplastic cell and many of today’s cancer research efforts are aimed at understanding the fundamental mechanisms that underlie carcinogenesis. Indeed, an important body of knowledge has been obtained via the study of cells retrieved from patients with cancer and grown in the laboratory, i.e. cell lines [15]. However, analyses of the tumor microenvironment have revealed that cancer cells also have critically important interactions with non-cancer, normal host cell populations, such as fibroblasts and inflammatory cells [16–18]. As a consequence, the study of tumors in their natural environment – the tissue – is of vital importance in cancer research and is a valuable complement to studying cancer cell lines and other in vitro model systems (Fig. 1) [19–22].
Figure 1.
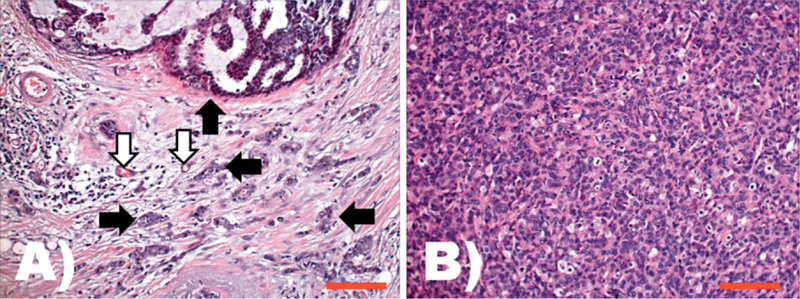
Comparison of phenotypic features of cancer cells in their natural environment versus a xenograph implanted into a mouse. Both A and B are images taken from H&E stained slides at the same original magnification (200 ×); the scale bars represents 200 µm. A: Typical aspect of a ductal carcinoma, the most common histological type of human breast cancer. Tumor epithelial cells are arranged in diverse architectural patterns (e.g. see upper side of this field and black arrows). The tumor epithelial cells are in intimate relation with the stroma that is composed of multiple non-tumor cell types including blood vessels (white arrows), fibroblasts, and inflammatory cells. The functional unit of tumor and cancer associated mesenchymal cells can be considered as the cancer histion. B: Morphological phenotype of human breast ductal carcinoma isolated and cultured as a cell line, and subsequently implanted as a xenograft into a mouse. The morphology of this model system is distinctly different from that observed in patients; here the ductal carcinoma cells grow as solid sheets, showing higher atypia, increased mitotic activity, and a different shape and size than in their natural tissue environment. The functional structure of the tumor microenvironment – the cancer histion – is lost in this model.
During the first half of the last century, cancer research and pathology were fully integrated disciplines in which pathology represented a natural bridge between biology and medicine. The systematic histopathological study of tumors allowed classification and grading of neoplasms, the prediction of tumor behavior, and the determination of appropriate therapies. However, in the second half of the 20th century, the focus of researchers shifted more to the molecular mechanisms of the cancer cell and less on an integrated understanding of the complex nature of cancer as a biomedical entity with multiple levels of organization, including: molecular, subcellular, cellular, microenvironment, whole organ, and patient [23, 24]. To many, it is increasingly evident that pathology is now an underutilized bridge between biology and medicine, and that a multidisciplinary effort integrating pathology with molecular research is necessary in order to improve cancer research efforts and ultimately benefit patients.
What is pathology?
A brief historical background
Pathology is a medical specialty with the main goal of understanding the mechanisms involved in disease and the use of this information to enhance clinical diagnosis and patient care. Until the 19th century, pathology studies were limited to the gross examination of morphological alterations of organs of the human body inspected at autopsy, which was often performed by the same physician who treated the patient before death [25]. An important contribution to the field was the work of Giovanni Battista Morgagni, an Italian physician, who in the 18th century published a comprehensive study of more than 600 cases in which the clinical findings were correlated with the alterations found at the anatomical level [25]. Morgagni’s work influenced medicine for the next century and established anatomic pathology as a distinct medical specialty [25]. A second break- through occurred in the 19th century, secondary to advances in optical microscopy [25]. The German pathologist Rudolf Virchow systematically correlated the microscopic alterations at the cellular and tissue levels with the gross findings at autopsy and with the clinical background of patients [25, 26]. His work included the seminal microscopic descriptions of tumors that were the bedrock for the classification of cancer, establishing the basis of modern histopathology [25, 26]. Virchow’s findings led him to propose the concept of cellular pathology: that the cell is both the basic unit of life and also the basic unit of disease [25, 26], and he focused on understanding the mechanisms of illnesses through cellular alterations. During the 20th century, American surgeons and pathologists such as James Ewing and Arthur Purdy Stout applied histopathology as a practical clinical tool, using small samples of tissue or cells retrieved from patients for an expedited and functional diagnosis that guided therapeutic decisions [27–29]. This resulted in the development of the primary subdiscipline of surgical pathology. Today, surgical pathology is the gold standard for the diagnosis of cancer and is complemented with ancillary techniques such as immunohistochemistry (IHC), and more recently, molecular testing and profiling [30].
Molecular pathology
The discovery of biomarkers that are clinically relevant for diagnostics, therapy, and prognosis, together with the development of consistent and reliable molecular techniques, led to the incorporation of molecular testing as an ancillary technique for surgical pathology [30]. A novel subspecialty called molecular pathology evolved as a clinical practice and many universities and medical institutions offer fellowships in this discipline for the training of young pathologists (Box 1) [31]. As a specific example, detection of genetic alterations that are characteristically present in tumors is a test that is performed in a molecular pathology laboratory [32]. In parallel, these pathologists often conduct research for the identification of novel biomarkers, an area of research which is at the intersection of histology and molecular/cellular biology; however, in general their role is typically focused on clinical applications for diagnostic pathology as opposed to molecular research per se. Also, since these are clinical tests that affect patient care, an important aspect of the subspecialty is quality control testing and monitoring.
Box 1. Pathology, surgical pathologists, and cytogeneticists
Pathology means the study of disease; thus, this term can be widely applied in biology, for example, plant pathology. However, in this article we use the terms “pathology” and “anatomic pathology” to refer to the human medical field with a diagnostic emphasis, encompassing morphological analysis at the anatomical and histological levels, as well as related molecular alterations. “Histopathology” specifically refers to the histological, microscopic alterations related to specific pathologies. “Surgical pathology” is focused on the clinical diagnosis of biopsies or surgical specimens removed from patients. Due to the complexity of human disease, anatomic pathology has evolved into several subspecialists.
A pathologist is a physician who specializes in anatomic pathology and provides clinical diagnoses from biopsies, cytological, and surgical specimens from live patients, and also performs the medical autopsies.
A surgical pathologist is a pathologist specially focused on the clinical diagnosis of biopsies and surgical specimens removed from patients. In general, a surgical pathologist receives specialty training through a clinical fellowship after completion of an anatomic pathology residency and usually works as an active member of a clinical team. Surgical pathology can be further subspecialized into specific groups of diseases such as hematopathology or dermatopathology. Because of the important clinical role, the major training programs in pathology today are focused on surgical pathology.
A cytogeneticist is a highly specialized biologist, often with a doctoral degree (PhD), who focuses on both research and cytogenetic tests of clinical samples, for example, the detection of specific translocations using the fluorescence in situ hybridization (FISH) technique for the diagnosis of a particular neoplasm.
In summary, a pathologist is a physician who integrates clinical information from patients with morphologic and IHC findings, including the data provided by molecular or cytogenetic tests. A board certified pathologist is qualified to sign the final clinical diagnosis in a pathology report, thus undertaking the medico-legal responsibility regarding subsequent therapeutic decisions for the patient based on the report.
Why is pathology important in cancer research?
Cancerous tissues are complex, involving interactions between different tissue types
There is ample evidence that cancer cells actively interact with non-tumor cells in the surrounding tumor microenvironment (Fig. 1) [16]; thus, tissue specimens are an important resource for both primary research efforts and for validation of biological findings that are made in the laboratory [19–22]. However, working with tissues presents four major challenges to investigators. The first is sample acquisition, including the ethical and legal rules that protect patient privacy and confidentiality. Tissue samples obtained through biopsies and surgical resections from patients, or even at autopsy, have a diagnostic priority. Clinical specimens can be used for research only when: (1) the patient consents after being carefully informed about the research study, (2) the planned studies have been officially approved by an Institutional Review Board (IRB), and (3) the clinical analyses of the specimen have been fully completed. As a practical matter, the acquisition of tissue samples is best facilitated in centers where biological researchers and pathologists interact as a multidisciplinary team and can closely coordinate their efforts.
A second challenge is the sampling of the tissue for histology and research [33, 34]. After the removal of a tissue specimen from a patient, a gross inspection first needs to be performed. The pathologist ensures that appropriate processing and sampling of the specimen is completed for histological examination and potential molecular testing [33–35]. Since tissues and organs that contain tumors are highly heterogeneous with distinct areas of cancer, necrosis, inflammation and normal tissue, gross inspection, and precise sampling are essential for accurate clinical diagnosis [33–35].
A third challenge is the selection of the proper preservation technique for the tissue specimen [35, 36]. Importantly, preservation is an “irreversible step” that will determine the usefulness of the specimen for histopathological diagnosis and subsequent molecular testing and research [36, 37]. The proper preservation of the tissue morphology and molecular profile actually begins before the removal of the tissue sample. A prolonged time of ischemia – when the tissue is devoid of blood supply – can profoundly affect the quality of the specimen. Thus, good coordination between the surgeon and the pathologist can help to reduce the time of cold ischemia, i.e. after the tissue sample is removed and before fixation, resulting in better molecular preservation [35, 36]. The two primary preservation techniques are chemical fixation, usually with a formalin solution followed by paraffin wax embedding, and snap freezing [36, 37]. The microscopic examination of formalin-fixed, paraffin-embedded tissue samples thin-sectioned onto glass slides and then stained with hematoxylin and eosin (H&E) is the gold standard diagnostic technique for surgical pathology [30]. Unfortunately, formalin induces significant chemical alterations that lead to loss of integrity of the biomolecules in the specimens and compromises many downstream molecular tests [36, 37]. Alternatively, snap-freezing tissue samples better preserves the integrity of tissue biomolecules; however, frozen tissues require special conditions for storage and handling, and also result in lower histological quality, thus the pathological evaluation of frozen sections can be difficult for many diseases [38].
Finally, a fourth challenge that investigators face when studying tissues is that specimens are a complex mixture of normal and pathological cell types and thus require a careful histopathological analysis as part of the research project to ensure that cells are accurately identified [34, 35, 39]. Specifically, histopathological evaluation helps to identify the presence and amount of tumor cells, including type, patterns of invasion, and grade, as well as viability (vital vs. necrotic), and the presence and amount of other cell populations such as inflammatory cells and fibroblasts [35, 39]. Furthermore, histopathological evaluation may also include the analysis of IHC staining patterns and levels of target protein expression.
The lack of attention to pathological evaluation affects the quality of clinical diagnosis and biological research
Indubitably, a lack of attention to pathological variables when studying tissue specimens can have detrimental effects for the patient in the clinical setting as well as interpretation of the molecular results in the laboratory [33, 34, 40, 41]. For example, there are cases in which removed tissue was not sent for histopathological examination. One such case was a healthy young patient with a small subcutaneous lump in the upper gluteus region that arose after a subcutaneous vaccination. The physician assumed the lump was a local inflammatory reaction and removed it for aesthetical reasons without sending the tissue to pathology. A few years later, the lump recurred at the same site; a second physician performed a biopsy with subsequent pathological examination that demonstrated the lump was actually a dermatofibrosarcoma protuberans, a locally aggressive low-grade soft tissue tumor. This time, the patient received the proper therapy, including a resection with wide margins, local radiation therapy, and follow up examinations. In this instance, even though the clinical history strongly suggested a benign process, proper pathological evaluation when the tissue was removed for the first time could have avoided recurrence of the tumor.
Although the case selection for a study can be correctly based on the pathological diagnosis, the tissue block that will be used for molecular extraction needs to be carefully examined. For example, in a large-scale laboratory-based epigenetic study of lymph node specimens with follicular lymphoma and follicular hyperplasia, two cases showed molecular data that did not fit well in the global epigenetic classification, thus the investigators performed a more extensive pathological analysis that showed the two tissue samples used for the epigenetic profiling were not representative of the pathological condition being analyzed and, therefore, were excluded from the study [42].
Another problem identified in research studies using animal specimens is what Cardiff et al. have called the “do it yourself” (DIY) pathology attitude [34, 40, 41]. These authors pointed out that many investigators perform their own pathological analysis, from the animal necropsy sampling to the histopathological evaluation, often done by students or investigators lacking proper training in pathology, with the risk of an inadequate pathological study that can seriously affect the research results [34, 40, 41]. Concrete examples cited by Couto and Cardiff [34] include incorrect sampling methods, such as transgenic mice in a mouse model for thyroid cancer in which a consulting pathologist received only the submandibular lymph nodes with no thyroid tissue and consequently, the transgenic mice were not utilized properly. Another example is suboptimal tissue preservation, such as in cases of mouse uteri with uninterpretable histology due to poor fixation causing serious and irreversible damage to the sample that necessitates that the experiments are repeated [34]. Finally, DIY pathology can lead to diagnostic errors including the incorporation of tissues with benign processes, such as inflammation, instead of actual tumor; examples include inflammation secondary to bacterial infection mistakenly diagnosed as multiple myeloma, or ovarian cyst diagnosed as lymphoma [34]. The development of DIY-pathology has increased recently due to the shortage of pathologists, particularly those trained in assessing genetically engineered mouse models and in comparative pathology, a worrisome trend for future cancer research efforts [22, 33, 34, 40, 41].
Lastly, our own experience in developing and implementing laser capture microdissection (LCM) emphasizes the importance of pathological variables when working with tissue specimens for molecular cancer research [35, 39, 43]. LCM has become an important tool for analyzing tissue samples by facilitating a microdissection-based isolation or enrichment of specific cell types, such as cancer cells from complex specimens. Histopathological evaluation is required since the molecular data depends on the correct identification of the cells for dissection. Moreover, there is increasing scientific evidence demonstrating that molecular profiling data obtained from whole tissue specimens, which contains multiple cell types, is different from the profiling data obtained from microdissected cells of interest from these samples [44–48]. Therefore, the use of upfront enrichment techniques such as LCM or immuno-guided LCM is often necessary in order to obtain accurate molecular data, and this approach depends on a proper histopathological tissue evaluation [47–49]. Table 1 shows some of the most common issues related to inadequate pathological examination that we have observed in our LCM Core lab.
Table 1.
Problems associated with pathological evaluation of specimens for laser microdissection or histopathological analysis for research studies observed at the laser capture microdissection (LCM) core laboratory
| Problem | Description or examples |
|---|---|
| Lack of H&E slides from the tissue specimens | Pathology review from each tissue block is a necessary step before any experiment involving tissue samples; this step also helps with proper planning of the experiments |
| Lack of knowledge of the histopathology of the specimens | Learning the histopathology of the type of tissues and pathologies used in a specific project helps with the correct LCM of the target cells and also helps with the correct analysis and interpretation of the data, such as IHC results |
| Lack of validation of animal cancer models with human cancer | Using animal models representative of specific cancers without comparing the histopathology of the animal model with the human counterpart |
| Inappropriate preservation methods | For example, using formalin fixed tissue specimens may present limitations in molecule integrity for certain large-scale platforms like gene expression arrays |
| Inappropriate storing or shipping methods | For example, forcing fresh big tissue samples into small tubes and freezing them. The integrity of the sample can be irremediably affected in this way |
| Inappropriate freezing methods | Certain tissues like brain require special care, i.e. very fast freezing and cryopreservation techniques, otherwise water crystals secondary to a slow freezing process will irreversibly affect the tissue integrity |
| Inappropriate tissue sampling | Certain studies may require a specific type of sampling; for example, regional inflammatory colon diseases may require systematic samples from proximal, medium, and distal colon for a proper histopathological evaluation |
| Lack of controls on IHC | IHC should be routinely performed with positive and negative controls |
| Equivocal interpretation of IHC results | IHC must be carefully interpreted after satisfactory evaluation of positive and negative controls to rule out unspecific background staining. Knowledge of the expected subcellular location of the targeted protein usually helps in the interpretation of IHC |
Pathology studies often precede molecular characterization of cancers
The clinical diagnostic process in pathology involves the analysis of morphological alterations in tissues and the correlation with the clinical background of the patients, including medical history, radiological studies, and relevant laboratory tests. Therefore, pathologists are in a unique and privileged position to enable translational cancer research. For instance, pathology plays a continuing role in the identification and characterization of new types of cancer as well as the clinical classification of tumors [29, 30, 50, 51]. As a specific example, the discovery and development of targeted therapy of gastrointestinal stromal tumors (GIST) began when pathologists recognized a morphologically different type of spindle cell tumor in the gastrointestinal tract [30]. Biomedical researchers then discovered a mutation in the c-kit receptor present in these particular tumors. Today, pathologists utilize immunostaining to characterize c-kit expression in cases of GIST that helps in predicting clinical outcome and assists oncologists in employing targeted therapy [30]. As a second example, the new World Health Organization (WHO) classification of lymphoid neoplasms represents a successful multiparametric approach to developing a clinically useful schema for lymphomas [50]. Many of the tumor types contained therein are characterized by distinct, although not unique, molecular alterations such as translocations involving the c-MYC gene and the immunoglobulin genes in Burkitt’s lymphoma. Notably, the identification of the molecular alteration in virtually all instances came after the pathological identification of the tumor type, and not the reverse [50]. One might suppose that discovery of new molecular aberrations would lead to the identification of new tumor types, but the discoveries generally occur in the opposite sequence. Once the molecular finger print of a tumor type is discovered, however, it becomes a valuable diagnostic tool. Additionally, knowledge of the molecular phenotype leads to improved delineation of the pathological entity and a better understanding of its clinical behavior and response to treatment [50].
Additionally, the integration of pathology with cancer research has led to several seminal biomedical discoveries with important clinical value. Examples include the (t11;22)(p13:q12) translocation resulting in a EWS/WT1 transcript present in the desmoplastic small round cell tumor, the discovery, and characterization of TMPRSS2-ERG gene fusions in prostate carcinoma, the molecular characterization of solid carcinomas such as lung adenocarcinoma, and the molecular genetics and pathological characterization of the multiple endocrine neoplasia type 1 (MEN1) syndrome [11, 30, 52–54]. Pathologists have also actively collaborated in the discovery of neoplasia associated with pathogens. Examples include Epstein-Barr virus, Burkitt’s lymphoma, Hodgkin’s lymphoma and nasopharyngeal carcinoma, and Human Papilloma Virus and cervical cancer, among others [55, 56].
Creating synergies between paradigms in pathology and cancer research
Cancer affects several biological levels of organization
One way to understand the complexity of biological systems is based on the concept of levels of organization [57, 58], starting with the cell as the basic unit of life and disease, followed by tissues as a functional group of cells, organ systems, and ultimately the whole organism [57]. The historical evolution of anatomic pathology and cancer research echoes the levels of organization of biology (Fig. 2). For instance, diseases were first characterized as clinical entities at the organism level, and then correlated with alterations of anatomy and organs, and finally Virchow introduced the study of disease at the tissue and cellular level. Today the revolution of cellular biology has opened another new window: a molecular-level mechanistic understanding of pathological processes.
Figure 2.
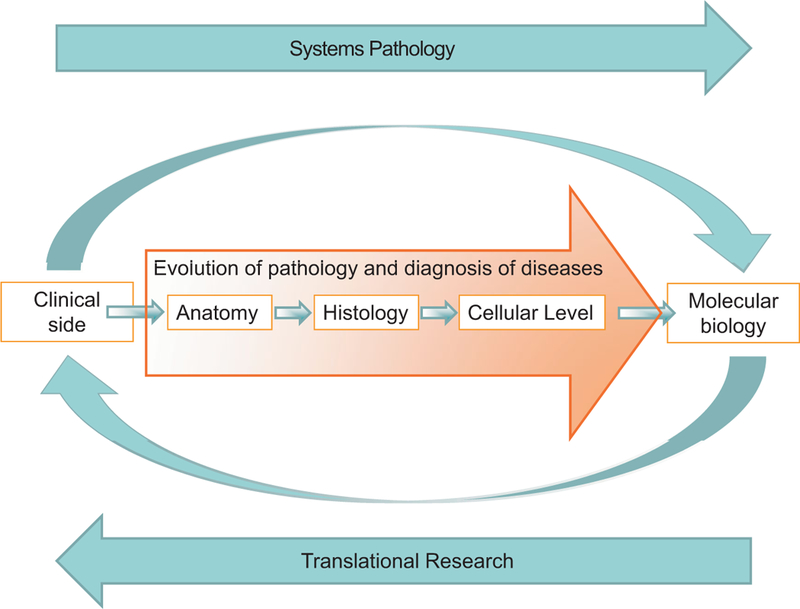
Schematic diagram showing the evolution of pathology from the anatomical level to the molecular level, in which clinical information is integrated with morphological changes at the anatomical and histological levels and with the alterations at the molecular level. Translational research allows novel biomarkers, discovered or validated in pathological tissues using molecular studies, to be used in the clinic as potential markers for diagnosis, prognosis, or therapy. Finally, a new systems pathology approach is needed to enhance integration of molecular biology and pathology.
During the process of carcinogenesis, molecular alterations influence the machinery of cancer cells as well as non-cancerous cells in the tumor microenvironment [16, 59–61]. Because of the complex structure of cancer and its functional interaction with multiple non-cancer cell types, tumors can be considered as organs [16]. From a histopathological point of view, one can consider cancer a disease of the histion, which is a general pathology concept defined as the basic functional unit of the tissue that composes a particular organ, for example, the nephron in the kidney [58, 62–64]. The histion is comprised of parenchymal cells, for example, the epithelium, and the associated stromal cells, for example, fibroblasts, blood vessels, macrophages, etc. In the same way, the cancerous tissue can be considered as a cancer histion; a basic functional unit composed of the cancer cells and the cancer-associated stroma in which complex cancer biology processes such as angiogenesis, epithelial-to-mesenchymal transition, and invasion take place (Fig. 1).
Understanding the emergent effects of cancer requires a holistic approach
Taking into account biological complexity, there are at least two ways to study cancer: a reductionistic approach and a holistic or systems biology approach [23]. Reductionism considers that a complex system can be understood in terms of its simpler parts and focuses on analysis of individual components [23, 24, 58]. However, since many emergent properties of a system cannot be explained strictly by reductionism, a systems approach is also needed to understand the complexity of cancer [23]. For example, the characterization and description of the types and varieties of p53 gene mutations in tumor cells enables a mechanistic understanding of the function of the gene. However, the clinical consequences and effects on the patient require a holistic approach that combines information on tumor type, location, grading, stage, clinical history, and available therapeutic options.
A new paradigm: A multiparameter model for the classification of cancer
Since cancer affects several levels of organization requiring a holistic approach, new models of cancer classification integrating clinical, pathological, and molecular data are required that are more efficient in terms of both biomedical research and clinical application. An example of the success of a multiparameter model is the new WHO classification of lymphomas mentioned previously, in which the integration of immunology, molecular biology, pathology, and clinical parameters resulted in a more accurate classification of lymphoid neoplasms that enhances clinical interpretation and translational studies [50].
Ideally, a systems pathology approach should be applied to cancer diagnosis in the future. The synthesis of clinical information, morphology, and molecular biology will increase the accuracy of cancer diagnosis. As an example, the diagnosis of sarcomas, malignant soft tissue tumors, benefits from a multiparameter strategy and has been remarkably enhanced with the incorporation of IHC and molecular markers, such as the detection of particular translocations. However, these parameters alone are not sufficient for an accurate tumor diagnosis and the correlation between clinical information (i.e. age, gender, and location of the primary tumor), morphology (H&E), immuno-markers, and cytogenetics must be incorporated in the diagnostic process [30].
Pathology and translational cancer research as an integrative approach
Translational research can be defined as the effort to “transform scientific discoveries arising from laboratory, clinical, or population studies into clinical applications to reduce cancer incidence, morbidity, and mortality” (www.cancer.gov/researchandfunding/trwg/TRWG-definition-and-TR-continuum, National Cancer Institute, last accessed January 10th, 2011). In a general sense, it is a biomedical effort to apply new knowledge generated from research studies into clinical practice, often referred to as “from bench to bedside”. As a multidisciplinary approach, translational research can efficiently close the gap between basic science and medical practice as it aims to explain both the complex mechanisms of cancer as a biological entity, and the clinical applications that benefit the patient from the therapeutic standpoint (Fig. 2).
Progress has been made in recent years, yet an intensified integration of both disciplines is still needed and will require overthrowing cultural barriers that sometimes are obstacles (see Box 2). Today, the challenge for pathology is similar to when, a century ago, Virchow incorporated cell-based studies into pathology. In the same way, pathologists today are called to include the new molecular dimension of disease within an established histopathological framework of knowledge and a clinical perspective. Stenzinger et al. [26] have suggested that today Virchow would probably reinvent morphological-based- pathology at a complex functional level: systems pathology. Indeed, a systems biology approach would be the ultimate future endpoint of pathology as a clinical practice [65–67].
Box 2. Cultural barriers that hinder the integration of pathology with molecular cancer research
Better integration between pathologists and basic investigators will require overcoming cultural barriers:
The clinical priority of modern surgical pathology. In the past, pathology was focused on the causes of diseases and was closely associated with cancer research; however, during the 20th century surgical pathology became a medical speciality with a clinical diagnostic purpose that shifted the focus of pathologists toward a pragmatic application of morphologic and molecular tools, and distancing surgical pathologists from the basic research environment.
The advancing specialization of the medical and scientific fields. The increasing amount of medical and scientific knowledge is opening new fields of specialization and subspecialization in both science and medicine. For example, pathology has evolved to surgical pathology and then into subspecialized pathology experts. In the same way, biological investigators have specialized in particular fields and study specific molecular aspects involved in cancer, such as particular pathways involved in carcinogenesis or the role of a specific family of proteins in cellular metabolism. The amount of scientific knowledge has grown to such magnitude that today it is not possible for one person to be an expert across all fields. The high specialization in sciences and medicine forces investigators and physicians to choose a career focus, for example, pathology practice versus basic cancer research. This is a dilemma that has been recognized in the career of young pathologists [68], which can slow down progress by loss of a big picture view.
A multidisciplinary integration among diverse specialists is the best strategy in moving translational research forward, providing both physicians and basic investigators with the opportunity to exchange ideas, understanding, and visions, leading ultimately to the eradication of disease in cancer patients.
As a practical matter, the integration of pathology into translational research can be accomplished in multiple ways, including: educational meetings between pathologists and cancer researchers; reinforcement of the value of comparative pathology that analyzes the similarities and differences between human samples and tissue from model systems; and, incorporation of novel analysis techniques for tumors that enable molecular measurements while preserving histopathological information. On one side, education is a powerful tool for teaching biologists the basic histopathological concepts needed to improve their research and to compare and validate the experimental data with the disease process as they occur in the human patient [22, 33, 34]. On the other side, a comprehensive education in the molecular and cellular biology of cancer, basic bioinformatics tools, and systems biology will be an important complement to the curriculum of anatomic pathology trainees. Because of the shortage of pathologists available for biological research [33], career stimulus for investigative pathology and comparative pathology will be of strategic importance for translational research as a distinct scientific discipline in the future.
Conclusions
We are in the midst of a scientific revolution in molecular biology that is providing novel mechanistic explanations for biological phenomena ranging from Darwinian evolution to medicine, including cancer research. However, the advance of a scientific discipline into a new dimension of knowledge should not necessarily nullify previous principles if they have been properly validated. Pathology represents a vast body of knowledge that has been clinically validated over decades, therefore, molecular cancer research efforts should seek to build upon, advance, and integrate with validated pathological principles and observations. As a matter of fact, cancer is a highly complex and heterogeneous group of diseases, thus cancer research requires a holistic and multidisciplinary approach in order to understand its multidimensional characteristics.
Neglecting pathology in cancer research involves the risk of generating a growing pool of molecular profiling information for cancers that includes poorly validated data. This can slow down progress in translational medicine and further increase the gap between biological discoveries and their efficient clinical application in patients with cancer. Modern pathology continues to play an active role in the discovery and characterization of diseases. Consequently, pathology will continue to represent a natural bridge between medicine and biology that can inform and enable investigators’ research efforts in the laboratory, and in turn assist clinicians in translating new scientific knowledge into medical practice for the benefit of the patient.
Acknowledgments
We thank Kamran Ghoreschi for his assistance with Fig. 2, and Jodie M. Fleming for providing the histological slide of a xenograft from human breast ductal carcinoma (Fig. 1B). This work was supported in part by the intramural program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health (NIH). M. R. E.-B. is an inventor on all NIH patents covering laser-capture microdissection technology and receives royalty payments through the NIH technology transfer program.
Abbreviations:
- DIY
Do it yourself
- GIST
gastrointestinal stromal tumor
- H&E
hematoxylin and eosin
- IHC
immunohistochemistry
- LCM
laser capture microdissection
- MEN1
syndrome of multiple endocrine neoplasia type 1
- WHO
World Health Organization
References
- 1.Hanahan D, Weinberg RA 2000. The hallmarks of cancer. Cell 100: 57–70. [DOI] [PubMed] [Google Scholar]
- 2.Hahn WC, Weinberg RA 2002. Rules for making human tumor cells. N Engl J Med 347: 1593–603. [DOI] [PubMed] [Google Scholar]
- 3.Perou CM, Sorlie T, Eisen MB, van de Rijn M, et al. 2000. Molecular portraits of human breast tumours. Nature 406: 747–52. [DOI] [PubMed] [Google Scholar]
- 4.Pfeifer GP, Hainaut P. 2010. Next-generation sequencing: emerging lessons on the origins of human cancer. Curr Opin Oncol 23: 62–8. [DOI] [PubMed] [Google Scholar]
- 5.Iacobuzio-Donahue CA 2009. Epigenetic changes in cancer. Annu Rev Pathol 4: 229–49. [DOI] [PubMed] [Google Scholar]
- 6.Ma L, Weinberg RA 2008. MicroRNAs in malignant progression. Cell Cycle 7: 570–2. [DOI] [PubMed] [Google Scholar]
- 7.Schramm G, Kannabiran N, Konig R 2010. Regulation patterns in signaling networks of cancer. BMC Syst Biol 4: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raghavan K, Ruskin HJ, Perrin D, Goasmat F, et al. 2010. Computational micromodel for epigenetic mechanisms. PLoS One 5: e14031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aalai E, Gleghorn C, Webb A, Glover SW 2009. Accessing public health information: a preliminary comparison of CABI’s GLOBAL HEALTH database and MEDLINE. Health Info Libr J 26: 56–62. [DOI] [PubMed] [Google Scholar]
- 10.Lander ES, Linton LM, Birren B, Nusbaum C, et al. 2001. Initial sequencing and analysis of the human genome. Nature 409: 860–921. [DOI] [PubMed] [Google Scholar]
- 11.Weir BA, Woo MS, Getz G, Perner S, et al. 2007. Characterizing the cancer genome in lung adenocarcinoma. Nature 450: 893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn TS. 1996. The Structure of Scientific Revolutions 3rd edition. Chicago, IL, USA: University of Chicago Press. [Google Scholar]
- 13.Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG 2009. Educational and social-ethical issues in the pursuit of molecular medicine. Mol Med 15: 60–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waltz E 2009. GM crops: battlefield. Nature 461: 27–32. [DOI] [PubMed] [Google Scholar]
- 15.Borrell B 2010. How accurate are cancer cell lines? Nature 463: 858. [DOI] [PubMed] [Google Scholar]
- 16.Egeblad M, Nakasone ES, Werb Z 2010. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell 18: 884–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson AM, Woodson K, Wang Y, Rodriguez-Canales J, et al. 2007. Global expression analysis of prostate cancer- associated stroma and epithelia. Diagn Mol Pathol 16: 189–97. [DOI] [PubMed] [Google Scholar]
- 18.Steidl C, Lee T, Shah SP, Farinha P, et al. 2010. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med 362: 875–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Compton C 2007. Getting to personalized cancer medicine: taking out the garbage. Cancer 110: 1641–3. [DOI] [PubMed] [Google Scholar]
- 20.Gillespie JW, Ahram M, Best CJ, Swalwell JI, et al. 2001. The role of tissue microdissection in cancer research. Cancer J 7: 32–9. [PubMed] [Google Scholar]
- 21.Emmert-Buck MR, Strausberg RL, Krizman DB, Bonaldo MF, et al. 2000. Molecular profiling of clinical tissues specimens: feasibility and applications. J Mol Diagn 2: 60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardiff RD, Rosner A, Hogarth MA, Galvez JJ, et al. 2004. Validation: the new challenge for pathology. Toxicol Pathol 32: 31–9. [DOI] [PubMed] [Google Scholar]
- 23.Ahn AC, Tewari M, Poon CS, Phillips RS. 2006. The limits of reductionism in medicine: Could systems biology offer an alternative? PLoS Med 3: e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heng HH 2008. The conflict between complex systems and reductionism. JAMA 300: 1580–1. [DOI] [PubMed] [Google Scholar]
- 25.Long ER 1965. A History of Pathology New York: Dover Publications, Inc. [Google Scholar]
- 26.Stenzinger A, Klauschen F, Wittschieber D, Weichert W, et al. 2010. Would Virchow be a systems biologist? A discourse on the philosophy of science with implications for pathological research. Virchows Arch 456: 599–607. [DOI] [PubMed] [Google Scholar]
- 27.Azar HA. 1984. Arthur Purdy Stout (1885– 1967). The man and the surgical pathologist. Am J Surg Pathol 8: 301–7. [PubMed] [Google Scholar]
- 28.Rosai J 2004. Rosai and Ackerman’s Surgical Pathology, Chapter 1: Historical Perspective Philadelphia, PA: Mosby: (Elsevier; ). [Google Scholar]
- 29.Huvos AG 1998. James Ewing: cancer man. Ann Diagn Pathol 2: 146–8. [DOI] [PubMed] [Google Scholar]
- 30.Rosai J 2007. Why microscopy will remain a cornerstone of surgical pathology. Lab Invest 87: 403–8. [DOI] [PubMed] [Google Scholar]
- 31.Talbert ML, Dunn ST, Hunt J, Hillyard DR, et al. 2009. Competency-based education for the molecular genetic pathology fellow: a report of the association for molecular pathology training and education committee. J Mol Diagn 11: 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Igbokwe A, Lopez-Terrada DH 2011. Molecular testing of solid tumors. Arch Pathol Lab Med 135: 67–82. [DOI] [PubMed] [Google Scholar]
- 33.Barthold SW, Borowsky AD, Brayton C, Bronson R, et al. 2007. From whence will they come? – a perspective on the acute shortage of pathologists in biomedical research. J Vet Diagn Invest 19: 455–6. [DOI] [PubMed] [Google Scholar]
- 34.Couto SS, Cardiff RD 2008. The genomic revolution and endocrine pathology. Endocr Pathol 19: 139–47. [DOI] [PubMed] [Google Scholar]
- 35.Bova GS, Eltoum IA, Kiernan JA, Siegal GP, et al. 2005. Optimal molecular profiling of tissue and tissue components: defining the best processing and microdissection methods for biomedical applications. Mol Biotechnol 29: 119–52. [DOI] [PubMed] [Google Scholar]
- 36.Leiva IM, Emmert-Buck MR, Gillespie JW 2003. Handling of clinical tissue specimens for molecular profiling studies. Curr Issues Mol Biol 5: 27–35. [PubMed] [Google Scholar]
- 37.Perlmutter MA, Best CJ, Gillespie JW, Gathright Y, et al. 2004. Comparison of snap freezing versus ethanol fixation for gene expression profiling of tissue specimens. J Mol Diagn 6: 371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Francz M, Egervari K, Szollosi Z. 2011. Intraoperative evaluation of sentinel lymph nodes in breast cancer: comparison of frozen sections, imprint cytology and immunocyto- chemistry. Cytopathology 22: 36–42. [DOI] [PubMed] [Google Scholar]
- 39.Erickson HS, Albert PS, Gillespie JW, Rodriguez-Canales J, et al. 2009. Quantitative RT-PCR gene expression analysis of laser microdissected tissue samples. Nat Protoc 4: 902–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardiff RD, Ward JM, Barthold SW 2008. ‘One medicine – one pathology’: Are veterinary and human pathology prepared? Lab Invest 88: 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ince TA, Ward JM, Valli VE, Sgroi D, et al. 2008. Do-it-yourself (DIY) pathology. Nat Biotechnol 26: 978–9. [DOI] [PubMed] [Google Scholar]
- 42.Killian JK, Bilke S, Davis S, Walker RL, et al. 2009. Large-scale profiling of archival lymph nodes reveals pervasive remodeling of the follicular lymphoma methylome. Cancer Res 69: 758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emmert-Buck MR, Bonner RF, Smith PD, Chuaqui RF, et al. 1996. Laser capture micro- dissection. Science 274: 998–1001. [DOI] [PubMed] [Google Scholar]
- 44.Harrell JC, Dye WW, Harvell DM, Sartorius CA, et al. 2008. Contaminating cells alter gene signatures in whole organ versus laser capture microdissected tumors: a comparison of experimental breast cancers and their lymph node metastases. Clin Exp Metastasis 25: 81–8. [DOI] [PubMed] [Google Scholar]
- 45.El-Serag HB, Nurgalieva ZZ, Mistretta TA, Finegold MJ, et al. 2009. Gene expression in Barrett’s esophagus: laser capture versus whole tissue. Scand J Gastroenterol 44: 787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klee EW, Erdogan S, Tillmans L, Kosari F, et al. 2009. Impact of sample acquisition and linear amplification on gene expression profiling of lung adenocarcinoma: laser capture micro-dissection cell-sampling versus bulk tissue-sampling. BMC Med Genomics 2: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silvestri A, Colombatti A, Calvert VS, Deng J, et al. 2010. Protein pathway biomarker analysis of human cancer reveals requirement for upfront cellular-enrichment processing. Lab Invest 90: 787–96. [DOI] [PubMed] [Google Scholar]
- 48.Eberle FC, Rodriguez-Canales J, Wei L, Hanson JC, et al. 2011. Large scale DNA methylation analysis can distinguish between gray zone lymphoma, classical Hodgkin’s lymphoma and primary mediastinal large B- cell lymphoma. Haematologica 96: 558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eberle FC, Hanson JC, Killian JK, Wei L, et al. 2010. Immunoguided laser assisted microdissection techniques for DNA methylation analysis of archival tissue specimens. J Mol Diagn 12: 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaffe ES, Harris NL, Stein H, Isaacson PG 2008. Classification of lymphoid neoplasms: the microscope as a tool for disease discovery. Blood 112: 4384–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eberle FC, Mani H, Jaffe ES. 2009. Histopathology of Hodgkin’s lymphoma. Cancer J 15: 129–37. [DOI] [PubMed] [Google Scholar]
- 52.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, et al. 2005. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310: 644–8. [DOI] [PubMed] [Google Scholar]
- 53.Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, et al. 1997. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 276: 404–7. [DOI] [PubMed] [Google Scholar]
- 54.Emmert-Buck MR, Lubensky IA, Dong Q, Manickam P, et al. 1997. Localization of the multiple endocrine neoplasia type I (MEN1) gene based on tumor loss of heterozygosity analysis. Cancer Res 57: 1855–8. [PubMed] [Google Scholar]
- 55.Moore PS, Chang Y 2010. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer 10: 878–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snow AN, Laudadio J 2010. Human papillomavirus detection in head and neck squamous cell carcinomas. Adv Anat Pathol 17: 394–403. [DOI] [PubMed] [Google Scholar]
- 57.Pave A 2010. in Pumain D (ed), Hierarchy in Natural and Social Sciences (Methodos Series); Hierarchical Organization of Biological and Ecological Systems pp. 39–70. [Google Scholar]
- 58.Chuaqui B 1995. On the reductionism of sciences. Rev Med Chil 123: 1306–12. [PubMed] [Google Scholar]
- 59.Orimo A, Weinberg RA 2006. Stromal fibro- blasts in cancer: a novel tumor-promoting cell type. Cell Cycle 5: 1597–601. [DOI] [PubMed] [Google Scholar]
- 60.Baird L, Terskikh A 2010. The tumor microenvironment. Adv Exp Med Biol 671: 67–73. [DOI] [PubMed] [Google Scholar]
- 61.Mbeunkui F, Johann DJ Jr. 2009. Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol 63: 571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Letterer E 1959. Allgemeine Pathologie, Grundlagen und Probleme: ein Lehrbuch Stuttgart: Georg Thieme Verlag. [Google Scholar]
- 63.Kitamura H 1964. The fine structure of lung alveoli and its reactions. (Histopathological studies by electron microscope). Acta Pathol Jpn 14: 147–66. [DOI] [PubMed] [Google Scholar]
- 64.Gossner W 2003. Target cells in internal dosimetry. Radiat Prot Dosimetry 105: 39–42. [DOI] [PubMed] [Google Scholar]
- 65.Costa J 2008. Is clinical systems pathology the future of pathology? Arch Pathol Lab Med 132: 774–6. [DOI] [PubMed] [Google Scholar]
- 66.Donovan MJ, Costa J, Cordon-Cardo C 2009. Systems pathology: a paradigm shift in the practice of diagnostic and predictive pathology. Cancer 115: 3078–84. [DOI] [PubMed] [Google Scholar]
- 67.Donovan MJ, Kotsianti A, Bayer-Zubek V, Verbel D, et al. 2009. A systems pathology model for predicting overall survival in patients with refractory, advanced non- small-cell lung cancer treated with gefitinib. Eur J Cancer 45: 1518–26. [DOI] [PubMed] [Google Scholar]
- 68.Armstrong S 2008. A Matter of Life and Death, Conversations with Pathologists p. 107. [PubMed]


