Abstract
OBJECTIVE:
Higher triglyceride (TG) is a risk factor for incident type 2 diabetes mellitus (T2DM), but paradoxically, genetic susceptibility for higher TG has been associated with lower T2DM risk. There is also evidence that the genetic association may be modified by baseline TG. Whether such associations can be replicated and the interaction is selective for certain TG-rich lipoprotein particles (TRLP) remain to be explored.
APPROACH AND RESULTS:
Cox regression involving TG, TRLPs, and genetic determinants of TG was performed among 15,813 participants with baseline fasting status in the Women’s Genome Health Study (WGHS), including 1,453 T2DM incident cases over a mean 18.6 (SD=5.3) years of follow-up. A weighted, 40-SNP TG genetic risk score (TG-wGRS40) was inversely associated with incident T2DM (HR [95%CI] =0.66 [0.58, 0.75]/10 TG risk alleles, P-value=<0.0001) with adjustment for baseline BMI, HDL-C and TG. TG-associated risk was higher among individuals in the low compared with the highest TG-wGRS40 tertile (HR [95%CI] =1.98 [1.83, 2.14] vs. 1.68 [1.58, 1.80] per mmol/L, Pinteraction=0.0007). In TG-adjusted analysis, large and medium but not small TRLPs were associated with higher T2DM incidence for successively lower TG-wGRS40 tertiles, Pinteraction=0.013, 0.012 and 0.620 across tertiles, respectively.
CONCLUSIONS:
Our results confirm the previous observations of the paradoxical associations of TG with T2D while focusing attention on the larger TRLP subfractions, suggesting their importance in clinical profiling of T2DM risk.
Keywords: Epidemiology, Biomarkers, Type 2 Diabetes, Risk Factors, Lipids and Cholesterol, Genetic Association Studies, Clinical Studies, Pathophysiology, Genetics
INTRODUCTION
Elevated serum triglyceride (TG) level is an independent risk factor for T2DM incidence, even after controlling for body mass index (BMI) and all other conventional risk factors 1, 2. From a clinical perspective, elevated TG levels may be observed as much as 10 years before diagnosis of T2DM 3, 4. In conventional analysis, TG is substantially confined to the chylomicron and very low density lipoprotein (VLDL) fractions, collectively termed as triglyceride rich lipoproteins particles (TRLP). However, TRLPs are highly heterogeneous, and technological developments using nuclear magnetic resonance (NMR) allow detection of five discrete TRLP subfractions including very large, large, medium, small, and very small as defined by lipoprotein particle diameter 5, each of which may have distinct clinical properties. Therefore, further delineating associations between TG with T2DM according to TRLP subfractions may provide clinical insight.
Since 2007, through genome-wide association studies (GWAS), about 40 independent genetic loci have been discovered for association with TG levels 6, 7. When combined as a genetic risk score (GRS), the TG-raising alleles at these loci provide a quantitative and continuous measure of genetic susceptibility to elevated TG levels. Recently, Klimentidis et al., 8 reported a significant interaction between a TG-specific weighted GRS (TG-wGRS) and baseline TG for association with T2DM risk. The authors observed that TG was more strongly associated with T2DM incidence in low compared to high TG-wGRS individuals. Klimentidis et al., 8 and Li et al., 9 also reported that many of the TG-associated genetic variants at or near CILP2, GCKR, NAT2, PLA2G6, CETP and APOA1 genes were positively associated with TG but negatively associated with glycemic traits and T2DM. Meanwhile, formal Mendelian randomization analysis using TG-specific genetic instruments found no evidence of causal association between plasma TG and T2DM, and instead suggested a protective effect of genetically raised TG levels on T2DM 10, 11.
In the current study, we seek to confirm and further explore these paradoxical findings by using the large, prospective population based sample of middle-aged women from the Women’s Genome Health Study (WGHS) to evaluate whether established TG-associated loci modify the association of baseline TG with the incidence of T2DM over a mean 18.6 years of follow-up. We also aimed to investigate whether the relationship between TG with T2DM risk can be refined to specific TRLP subfractions, potentially informing which biological aspects of lipid metabolism influence development of T2DM.
MATERIALS AND METHODS
Study Population
Individual level data for the WGHS is not currently publicly available but scripts for performing the analysis described in this report will be provided upon request. The study participants of the WGHS are derived from the Women’s Health Study (WHS) that was designed as a placebo-controlled randomized trial evaluating the impact of low-dose of aspirin and vitamin E for the primary prevention of CVD 12, 13 among initially healthy middle age (45 years or older) health-care professionals. Subsequent to the conclusion of the trial, the study population has been followed in observational mode. The WGHS consists of the approximately 72% of WHS participants with a baseline blood sample. For the current analysis, we have 23,294 participants with available genotype data and self-reported European ancestry that was also confirmed through genetics 12. Klimentidis et al., 8 performed analyses in the fasting sample, and considering this, for the current primary analyses we have 15,813 of 21,848 participants without diabetes at baseline who also had more than 8 hours of fasting status at baseline. Secondary analyses were performed among all participants (fasting and non-fasting). Analysis in the WGHS was approved by institutional review board of Brigham and Women’s Hospital, Boston.
At baseline, clinical characteristics were collected through self-reported questionnaire regarding age and body mass index (BMI), as described previously 14. All lipid levels including TG and HDL cholesterol (HDL-C) were enzymatically measured through Hitachi 917 analyzer (Roche Diagnostics) using baseline plasma. TGs were measured enzymatically with correction for endogenous glycerol.
Incident T2DM ascertainment
Incident T2DM cases were initially identified by self-report using annual questionnaires that asked if the participant had been diagnosed with diabetes since baseline 15. Self-reported incident T2DM cases were confirmed through physician-administered telephone interviews or the questionnaires that inquired about clinical manifestations of diabetes including diabetes medications, diagnostic diabetes testing and disease symptoms 16.
Measurement of Triglyceride-rich Lipoprotein (TRLP) Subfractions
NMR-based measures were performed for quantifying TRLPs, as has been reported previously 17, 18. Briefly, H-NMR spectra (400 MHz) of plasma were deconvoluted with the LipoProfile IV assay (LipoScience) to resolve concentrations (nmol/L) of distinct TRLP subfractions, differentiated according to diameter, i.e. particle size. For the current analysis, we considered very large (90–240 nm), large (50–89 nm), medium (37–49 nm), small (30–36 nm) and very small (24–29 nm) TLRPs. Mean particles size (TRLZ) ranged from 30–100 nm. The TG and cholesterol content of the combined TRLPs, designated as TRLTG and TRLTC respectively, were also determined by NMR.
Genotyping and Genetic Risk Score
In the WGHS, genotyping was performed with the HumanHap300 Duo array using the Infinium II assay 19, 20. Imputation for the SNPs that were not present in the genotyping array was performed using MACH (v. 1.0.16) using the 1000 genomes phase I v. 3 (March 2012) cosmopolitan reference panel. For the current analyses, we used 40-TG associated genetic variants that have been previously reported in association with TG 6, 7, as our primary genetic predictor regarding analyses because 40-TG associated variants are similar to the 31-TG associated genetic variants in the publication by Klimentidis et al8. In addition, we performed secondary analyses using recently published 127-TG associated genetic variants21. Most of the SNPs were directly genotyped. The SNPs that were imputed had high imputation quality score (MACH r2≥0.85). For the 40 TG-associated SNPs, effect allele frequencies, chromosomal position and gene names have been described previously22 and for the 127-TG associated SNPs21, this information is shown in Supplemental Table I. Weighted genetic risk scores (TG-wGRSs) were created using published effect estimates from the 40-TG (TG-wGRS40) and 127-TG (TG-wGRS127) associated genetic variants separately and scaled to such that each unit reflects 10 alleles, as described previously22.
Stastical Analysis
Cox proportional regression models were used to assess the association of TG, TRLPs, SNPs, and the TG-wGRSs with the incident T2DM. Interaction analyses were performed by additionally introducing a term of TG-associated SNPs or the TG-wGRSs × TG in the Cox models. For interaction analyses, the TG-wGRSs were used as continuous variables or as a tertiles (coded as 0, 1 and 2). TG was used as a continuous variable. The basic models were adjusted with principle components (PCs) (to control for European population substructure), age, and randomization to treatment in the WHS parent cohort (vitamin E and aspirin). The fully adjusted models included covariates from the basic model plus measures of HDL-C and BMI, similar to Klimentidis et al.,8. All clinical covariates were determined at baseline. For the single SNP analysis, we considered a significant threshold p≤0.00125 (=0.05/40) after Bonferroni correction for the 40 single SNPs tests performed. Very large and large TRLP variables were not normally distributed as the distribution of these variables were left skewed. To assess the significance of associations with these variables, we used permutation procedures to derive empirical association P-values. We repeated the Cox regression over 10,000 iterations, each time resampling the TG-wGRS40 at random without replacement to generate a null distribution of beta coefficients. The empirical p-values were derived as the proportion of beta coefficients from the empirical null distribution with absolute value greater than the absolute value of the beta coefficient from the unpermuted model. Also, because many of the study participants have lower even undetectable concentrations of very large TRLP particles, we created variable combining the very large and large TRLP particles and tested that combined variable in interaction with TG-wGRS40 in predicting T2DM incidence. In sensitivity analyses, we generated trait residuals by regressing TG (and other TRLPs) against the TG-wGRS40 to eliminate collinearity, and used the residuals as an interactor variable with the TG-wGRS40 to assess association with T2DM. To investigate whether interactions with the TG-wGRS40 were consistent with the implicit assumption that the ratio of SNP effects between strata of TG was similar across all SNPs, we regressed SNP effects for incident T2DM (as beta coefficients) in the group with TG below or equal to median TG against the group with TG above median. SNPs with disproportionate influence on the regression relationship were identified using the R function “influence.measures” that flags extreme points for any of a series of regression diagnostics, including Cook’s D, leave-one-out analysis, covariance ratio, and leverage.
RESULTS
Clinical and general characteristics of the study participants are shown in Table 1. For the current analyses, there were N=15,813 with fasting status (and N=21,848 in total) female participants from the WGHS among whom 1,453 experienced incident cases for T2DM during follow-up (N=1,944 incident T2DM cases in total sample). The mean follow-up duration for T2DM incident was 18.6 (SD=5.3) years.
Table 1.
Baseline Characteristics of the total and with fasting Participants a in the WGHS Cohort
| Fasting sample | Total sample | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Number of participants, N | 15813 | 21848 | ||
| Age (years) | 54.91 | 7.2 | 54.62 | 7.1 |
| Triglycerides (mmol/L) | 1.49 | 0.8 | 1.55 | 0.9 |
| BMI, kg/m2 | 25.75 | 4.9 | 25.76 | 4.9 |
| HDL, mmol/L | 1.41 | 0.4 | 1.40 | 0.4 |
| TRLP (nmol/L) | 173.42 | 62.2 | 172.25 | 60.8 |
| Very large TRLP (nmol/L) | 0.23 | 0.5 | 0.25 | 0.5 |
| Large TRLP (nmol/L) | 2.55 | 3.0 | 2.70 | 3.0 |
| Medium TRLP (nmol/L) | 18.03 | 13.3 | 18.45 | 13.3 |
| Small TRLP (nmol/L) | 62.14 | 38.1 | 61.76 | 37.7 |
| Very small TRLP (nmol/L) | 90.47 | 42.9 | 89.08 | 42.2 |
| TRLZ (nm) | 43.72 | 7.3 | 44.08 | 7.5 |
| TRLTG (mmol/L) | 2.38 | 1.3 | 2.33 | 1.3 |
| TRLTC (mmol/L) | 0.77 | 0.3 | 0.77 | 0.3 |
| Genetic-predisposition score | 40.09 | 4.2 | 40.11 | 4.2 |
BMI: body mass index; HDL: high density lipoproteins; TRLP: triglycerides rich lipoprotein particles;
TRLZ: mean triglyceride-rich particles size; TRLTG: TG rich TRLP particles;
TRLTC: cholesterol rich TRLP particles.
Associations of TG, TG-wGRS and incident T2DM
Although 40 SNPs have been identified through GWAS for TG, Klimentidis et al. 8 performed analysis using a TG-wGRS based on 31 TG-associated SNPs as they observed that this TG-wGRS was more strongly associated with TG. However, by reviewing the literature, we found that three SNPs, COBL1 rs10195252, APOB rs1042034, and APOE rs439401 were not primarily or secondarily associated with TG in the most recent GWAS 6. We therefore constructed a TG-wGRS and performed all of our primary analyses using 40-TG associated SNPs after excluding these three inconsistent SNPs but including 12 additional SNPs (PIGV-NR0B2 rs12748152, LRPAP1 rs6831256, VEGFA rs998584, RSPO3 rs719726, MIR148A rs4719841, MET rs38855, AKR1C4 rs1832007, PDXDC1 rs3198697, FTO rs1121980, MPP3 rs8077889, INSR rs7248104 and PEPD rs731839). All of the included genetic variants were primarily or secondarily associated with TG at genome-wide significance 6.
The TG-wGRS40 was positively associated with TG (β[SE]=0.38[0.01] mmol/L per 10-TG associated risk alleles, P-value≤0.0001) when the regression model was adjusted with age, BMI, HDL-C and PCs. TG was positively associated with T2DM incidence (HR [95% CI] =1.70[1.63, 1.77] per mmol/L, P-value≤0.0001) when the regression analysis was adjusted with age and PCs. The association of TG with T2DM was attenuated (HR [95% CI] = 1.37[1.30, 1.43] per 1mmol/L, P-value≤0.0001) when the regression analysis was additionally adjusted with BMI and HDL-C (Table 2). In a Cox model adjusted with age and PCs, we observed that the TG-wGRS40 was associated with a non-significant reduction in T2DM incidence (HR [95% CI] =0.94 [0.83, 1.06] per 10-TG associated risk alleles, P-value=0.30). However, when the model was additionally adjusted for baseline age, TG, BMI and HDL-C, the TG-wGRS40 was significantly associated with reduced T2DM incidence (HR [95% CI] =0.66 [0.58, 0.75] per 10-TG associated risk alleles, P-value ≤0.0001). Similarly, we observed that the TG-wGRS127 was negatively but non-significantly (HR [95% CI] =0.98 [0.93, 1.03] per mmol/L, P-value= 0.39) associated with the incidence of T2DM when the analyses were adjusted with basic model covariates. In the fully adjusted regression models, TG-wGRS127 was significantly negatively associated with T2DM incidence (HR [95% CI] =0.87 [0.83, 0.92] per mmol/L, P-value≤0.0001).
Table 2.
Association of TG and TG-rich lipoproteins with the incidence of T2DM and TG-wGRS x biomarkers interactions in the incidence of T2DM in the WGHS (fasting sample)
| Model | HR | HR (95% CIs) | P-value | ¥Pinteraction | £Pinteraction | ||
|---|---|---|---|---|---|---|---|
| Triglycerides (mmol/L) | Basic | 1.70 | 1.63 | 1.77 | <0.0001 | 0.002 | 0,008 |
| Full | 1.37 | 1.30 | 1.43 | <0.0001 | 0.030 | 0,043 | |
| TRLP (nmol/L) | Basic | 1.01 | 1.01 | 1.01 | <0.0001 | 0.182 | 0,129 |
| Full | 1.00 | 1.00 | 1.00 | <0.0001 | 0.824 | 0,294 | |
| Full + TG | 1.00 | 1.00 | 1.00 | 0.0163 | 0.429 | 0,089 | |
| Very large TRLP | Basic | 1.56 | 1.49 | 1.64 | <0.0001 | 0.598 | 0,111 |
| Full | 1.31 | 1.24 | 1.39 | <0.0001 | 0.171 | 0,195 | |
| Full + TG | 1.08 | 1.01 | 1.17 | 0.034 | 0.107 | 0,120 | |
| Large TRLP | Basic | 1.15 | 1.14 | 1.17 | <0.0001 | 0.002* | 0,700 |
| Full | 1.07 | 1.06 | 1.09 | <0.0001 | 0.043 | 0,385 | |
| Full + TG | 0.99 | 0.96 | 1.01 | 0.298 | 0.016 | 0,076 | |
| Medium TRLP (nmol/L) | Basic | 1.02 | 1.02 | 1.03 | <0.0001 | 0.014 | 0,035 |
| Full | 1.00 | 1.00 | 1.01 | 0.0446 | 0.141 | 0,159 | |
| Full + TG | 0.98 | 0.97 | 0.98 | <0.0001 | 0.047 | 0,039 | |
| Small TRLP (nmol/L) | Basic | 1.00 | 1.00 | 1.00 | 0.1178 | 0.041 | 0,009 |
| Full | 1.00 | 1.00 | 1.00 | 0.0008 | 0.518 | 0,083 | |
| Full + TG | 1.00 | 1.00 | 1.00 | 0.0091 | 0.891 | 0,231 | |
| Very small TRLP (nmol/L) | Basic | 1.01 | 1.01 | 1.01 | <0.0001 | 0.514 | 0,864 |
| Full | 1.00 | 1.00 | 1.01 | <0.0001 | 0.325 | 0,688 | |
| Full + TG | 1.00 | 1.00 | 1.00 | 0.0923 | 0.828 | 0,696 | |
| TRLZ (nm) | Basic | 1.06 | 1.06 | 1.07 | <0.0001 | 0.746 | 0,682 |
| Full | 1.04 | 1.03 | 1.04 | <0.0001 | 0.396 | 0,630 | |
| Full + TG | 1.02 | 1.01 | 1.03 | <0.0001 | 0.132 | 0,258 | |
| TRLTG (mmol/L) | Basic | 2.02 | 1.89 | 2.15 | <0.0001 | 0.012 | 0,022 |
| Full | 1.39 | 1.28 | 1.51 | <0.0001 | 0.181 | 0,127 | |
| Full + TG | 0.69 | 0.59 | 0.80 | <0.0001 | 0.045 | 0,013 | |
| TRLTC (mmol/L) | Basic | 3.26 | 2.79 | 3.82 | <0.0001 | 0.11 | 0,074 |
| Full | 1.47 | 1.22 | 1.77 | <0.0001 | 0.621 | 0,192 | |
| Full + TG | 0.60 | 0.48 | 0.76 | <0.0001 | 0.211 | 0,036 |
Basic model was adjusted with age, PCs, Vitamin E and Aspirin
Fully adjusted model was adjusted with basic model + BMI, HDL
Full + TG: Fully adjusted model + TG; *Empirical Pinteraction = 0.0024 after 10,000 permutations (Methods).
We observed a significant interaction of baseline TG levels and the TG-wGRS40 (Pinteraction= 0.002) for incident T2DM when the Cox model was adjusted for age and PCs. This interaction was also observed when the regression model was additionally adjusted for baseline BMI and HDL-C (Pinteraction=0.030) (Table 2). Through residual trait analysis, we observed that the TG-wGRS40 explained 4.99% trait variance in TG, similar to previously reported estimates 22. When we replaced the TG with GRS-residualized TG in the analysis, the TG-wGRS40 × trait-residual interactions for incident T2DM remained significant (Pinteraction= 0.005 for the basic model and Pinteraction= 0.05 for the fully adjusted model). In the Cox model adjusted for age and PCs, we observed that TG was associated with higher incidence of T2DM in the low TG-wGRS40 tertile (HR [95% CI] =1.98 [1.83, 2.14] per mmol/L) compared to the high TG-wGRS40 tertile (HR [95% CI] =1.68 [1.58, 1.80] per mmol/L), with a statistically significant interaction (Pinteraction=0.0007) (Figure S1). Similar interactions were observed when the regression models were additionally adjusted with BMI and HDL-C (Table 3). Similar to the finding with the TG-wGRS40, we observed significant interactions of TG-wGRS127 and TG levels in the risk of T2DM in the basic model (Pinteraction=0.0157) and in the fully adjusted model (Pinteraction=0.0432) (Table 2)
Table 3.
Association of TG with the incidence of T2DM across tertiles of GRS in the WGHS (fasting sample)
| 1st GRS tertile | 2nd GRS tertile | 3rd GRS tertile | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | HR | HR (95% CIs) | HR | HR (95% CIs) | HR | HR (95% CIs) | Pinteraction | ||||
| Triglycerides (mmol/L) | Basic | 1.98 | 1.83 | 2.14 | 1.72 | 1.60 | 1.85 | 1.68 | 1.58 | 1.80 | 0.0007 |
| Full | 1.55 | 1.41 | 1.71 | 1.35 | 1.24 | 1.48 | 1.40 | 1.30 | 1.51 | 0.032 | |
| TRLP (nmol/L) | Basic | 1.01 | 1.01 | 1.01 | 1.01 | 1.00 | 1.01 | 1.01 | 1.01 | 1.01 | 0.161 |
| Full | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.650 | |
| Full + TG | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.336 | |
| Very large TRLP | Basic | 1.67 | 1.54 | 1.82 | 1.45 | 1.32 | 1.58 | 1.64 | 1.52 | 1.76 | 0.824 |
| Full | 1.42 | 1.30 | 1.56 | 1.20 | 1.08 | 1.35 | 1.38 | 1.26 | 1.50 | 0.504 | |
| Full + TG | 1.11 | 0.98 | 1.26 | 0.95 | 0.82 | 1.10 | 1.14 | 1.01 | 1.28 | 0.319 | |
| Large TRLP | Basic | 1.21 | 1.18 | 1.24 | 1.15 | 1.13 | 1.18 | 1.14 | 1.12 | 1.17 | 0.0006 |
| Full | 1.11 | 1.08 | 1.15 | 1.07 | 1.04 | 1.09 | 1.07 | 1.04 | 1.10 | 0.025 | |
| Full + TG | 1.02 | 0.97 | 1.06 | 0.99 | 0.94 | 1.03 | 0.97 | 0.93 | 1.01 | 0.013 | |
| Medium TRLP (nmol/L) | Basic | 1.03 | 1.02 | 1.04 | 1.03 | 1.02 | 1.03 | 1.02 | 1.01 | 1.03 | 0.007 |
| Full | 1.01 | 1.00 | 1.02 | 1.01 | 1.00 | 1.01 | 1.00 | 1.00 | 1.01 | 0.039 | |
| Full + TG | 0.98 | 0.97 | 0.99 | 0.98 | 0.98 | 0.99 | 0.97 | 0.97 | 0.98 | 0.012 | |
| Small TRLP (nmol/L) | Basic | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.99 | 1.00 | 0.018 |
| Full | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.99 | 1.00 | 0.317 | |
| Full + TG | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.620 | |
| Very small TRLP (nmol/L) | Basic | 1.01 | 1.01 | 1.01 | 1.01 | 1.01 | 1.01 | 1.01 | 1.01 | 1.01 | 0.298 |
| Full | 1.01 | 1.00 | 1.01 | 1.00 | 1.00 | 1.00 | 1.01 | 1.01 | 1.01 | 0.253 | |
| Full + TG | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.01 | 0.648 | |
| TRLZ (nm) | Basic | 1.07 | 1.06 | 1.08 | 1.06 | 1.05 | 1.07 | 1.07 | 1.06 | 1.08 | 0.675 |
| Full | 1.05 | 1.04 | 1.06 | 1.03 | 1.02 | 1.04 | 1.04 | 1.03 | 1.05 | 0.429 | |
| Full + TG | 1.03 | 1.01 | 1.04 | 1.01 | 0.99 | 1.02 | 1.02 | 1.01 | 1.04 | 0.171 | |
| TRLTG (mmol/L) | Basic | 2.64 | 2.29 | 3.04 | 1.97 | 1.77 | 2.20 | 2.03 | 1.83 | 2.27 | 0.004 |
| Full | 1.65 | 1.39 | 1.95 | 1.37 | 1.19 | 1.58 | 1.47 | 1.28 | 1.68 | 0.111 | |
| Full + TG | 0.73 | 0.56 | 0.95 | 0.69 | 0.51 | 0.91 | 0.72 | 0.56 | 0.93 | 0.038 | |
| TRLTC (mmol/L) | Basic | 4.60 | 3.38 | 6.27 | 3.10 | 2.37 | 4.06 | 3.46 | 2.63 | 4.55 | 0.078 |
| Full | 1.81 | 1.28 | 2.56 | 1.44 | 1.05 | 1.98 | 1.74 | 1.26 | 2.39 | 0.414 | |
| Full + TG | 0.60 | 0.39 | 0.92 | 0.61 | 0.40 | 0.92 | 0.72 | 0.48 | 1.06 | 0.132 | |
Basic model was adjusted with age, PCs, VE and ASA
Fully adjusted model was adjusted with basic model + BMI, HDL
Full + TG: fully adjusted model + TG
TG-rich lipoproteins, TG-wGRS and T2DM risk
In Cox models adjusted for basic covariates, total TRLP, very large, large, medium, very small TRLPs, TRLZ, TRLTG and TRLTC (except small TRLP) were significantly associated with T2DM (all P-values ≤0.0001), as has been published previously in a similar WHS sample 16. Regarding the TG-wGRS40, when the regression models were fully adjusted for BMI and HDL-C along-with total TG at baseline, we observed significant interactions between TG-wGRS40 and large TRLP (Pinteraction=0.016) and medium TRLP (Pinteraction=0.047) with T2DM (Table 2). No significant interactions were observed for very large (Pinteraction= 0.107), very small (Pinteraction=0.891) or small (Pinteraction=0.828) TRLPs. Similarly, a variable combining large and very large TRLP particles showed significant interaction with TG-wGRS40 in the incident T2DM (Pinteraction =0.0035). For both the large TRLPs and the combination of large and very large TRPLs, in models adjusted with basic covariates, we also observed significant interaction using a resampling approach to derive an empirical procedure to address the skew in the TRLP distribution (Pinteraction, empirical = 0.0024 and 0.0057, respectively). Similarly, we observed concordant interactions for the different TG-rich lipoprotein subfractions (as shown in Table 2).
Accordingly, we observed a stronger association of large TRLPs with incident of T2DM among individuals in the lower TG-wGRS40 tertile (HR [95% CI] =1.02 [0.97–1.06]) compared to the individuals in the higher TG-wGRS40 tertile (HR [95% CI] = 0.97 [0.93–1.01]) with a significant interaction (Pinteraction=0.013). Similar interactions were observed regarding medium TRLPs (Pinteraction= 0.012) (Table 3). However, the interactions with tertiles of TG-wGRS40 were non-significant for small and very small TRLPs (Pinteraction= 0.620) and (Pinteraction= 0.648), respectively. We observed materially similar results when the analyses were performed in the total WGHS sample, including both fasting and non-fasting participants (Supplemental Table I).
Justification of using a genetic risk score for testing interactions with TG
One assumption in using a genetic risk score (GRS) for gene-environment interaction studies, is that the ratio of individual SNP effects across environmental strata should be approximately similar for all SNPs. We tested this assumption by comparing SNP-T2DM association between two strata of TG (dichotomous variable was created based upon the median value: low ≤ 1.29 mmol/L vs high > 1.29 mmol/L). In a linear model of SNP effects in the two strata, we observed that four of the SNPs (KLHL8 rs442177, APOA1 rs964184, CAPN3 rs2412710 and CILP2 rs10401969) had disproportionate influence on the regression fit (Supplemental Table II and Figure S2). We performed sensitivity analysis of TG-wGRS40 × TG interactions in T2DM after removing the most influential SNPs (KLHL8 rs442177, APOA1 rs964184, CAPN3 rs2412710 and CILP2 rs10401969) from the TG-wGRS40 and observed significant interactions with TG (Pinteraction=0.016) for the basic model but not for the fully adjusted model (Pinteraction=0.27).
Pleiotropic effects
In order to reduce the potential influence of known pleiotropic effects on our conclusions, we removed genetic variants from the TG-wGRS40 that associate at genome-wide significance with T2DM related traits (GCKR rs1260326 [T2DM, fasting glucose, beta-cell function, fasting insulin], IRS1 rs2972146 [fasting glucose, T2DM, homeostatic model assessment of β-cell function, homeostatic model assessment of insulin resistance], FADS1–2-3 rs174546 [fasting glucose, T2DM, homeostatic model assessment of β-cell function], FTO rs1121980 [T2DM], CILP2 rs10401969 [T2DM] and PEPD rs731839 [T2DM])23. As was found for the primary 40 SNP TG-wGRS, this 34 SNP TG-wGRS was associated with a non-significant reduction in T2DM incidence (HR [95% CI] =0.96 [0.84, 1.10] per 10-TG associated risk alleles, P-value=0.57). However, in models additionally adjusted for baseline TG, HDL-C and BMI, the 34 SNP TG-wGRS was negatively associated with T2DM (HR [95% CI] =0.67 [0.58, 0.77] per 10-TG associated risk alleles, P-value ≤0.0001). Similar to the 40 SNP TG-wGRS, we observed significant interactions of the 34 SNP TG-wGRS with very large TRLPs (Pinteraction=0.016) and large TRLPs (Pinteraction= 0.001) for association with T2DM outcomes (Supplemental Table III). In order to remove any of the potential pleiotropic effects from the TG-wGRS127, we excluded 15 genetic variants that associate with T2DM related traits. As with the TG-wGRS127, the 112 SNPs TG-wGRS was associated with non-significant decrease in T2DM (HR [95% CI] =0.97 [0.91, 1.02] per 10-TG associated risk alleles, P-value=0.26) but a significant decrease in the fully adjusted model (HR [95% CI] =0.86 [0.82, 0.91] per 10-TG associated risk alleles, P-value ≤0.0001). As was found for the TG-wGRS127, we observed significant interactions between 112 SNPs TG-wGRS and TG for the T2DM outcome in the basic model (Pinteraction=0.021) or and the fully adjusted model (Pinteraction=0.047).
Individual SNPs, TG and T2DM incidence
In single SNP analyses, we observed that 5 (out of 40) SNPs including FADS1–2-3 rs174546, APOA1 rs964184, CETP rs3764261, CILP2 rs10401969 and PLA2G6 rs5756931 showed significantly negative association (P-value≤0.05) with T2DM incidence but after correction for multiple testing, only APOA1 rs964184 remained statistically significant (Table 4). Moreover, 18 of the 40 SNPs were directionally negatively associated with T2DM in models adjusted for age, PCs, BMI and HDL. Regarding single SNPs × TG interactions on T2DM incidence, we observed that a significant excess (P≤0.001) of 9 (out of 40) variants showed nominally significant interactions (P≤0.05) but after correction for multiple testing none of the variants remained statistically significant (Table 4).
Table 4.
List of 40-triglyceride associated regarding their genomic position, effect allele, TG effect size (weights used for GRS), association with T2DM incidence and interaction with TG in WGHS (fasting sample)
| Main Effect on T2DM incidence | Interaction | ||||||
|---|---|---|---|---|---|---|---|
| SNP | Locus | Effect Allele | HR | HR (95% CIs) | P-value | Pinteraction | |
| rs12748152 | PIGV-NR0B2 | T | 1.12 | 0.98 | 1.28 | 0.091 | 0.553 |
| rs2131925 | ANGPTL3 | T | 0.95 | 0.88 | 1.03 | 0.211 | 0.406 |
| rs4846914 | GALNT2 | G | 0.95 | 0.88 | 1.03 | 0.184 | 0.781 |
| rs1260326 | GCKR | T | 1.02 | 0.95 | 1.10 | 0.602 | 0.140 |
| rs2972146 | IRS1 | T | 1.00 | 0.92 | 1.08 | 0.934 | 0.460 |
| rs645040 | MSL2L1 | T | 1.04 | 0.95 | 1.14 | 0.380 | 0.243 |
| rs6831256 | LRPAP1 | G | 1.02 | 0.94 | 1.09 | 0.703 | 0.370 |
| rs442177 | KLHL8 | T | 1.01 | 0.93 | 1.08 | 0.901 | 0.001 |
| rs9686661 | MAP3K1 | T | 0.96 | 0.87 | 1.05 | 0.369 | 0.467 |
| rs6882076 | TIMD4 | C | 1.05 | 0.98 | 1.14 | 0.186 | 0.027 |
| rs998584 | VEGFA | A | 1.05 | 0.95 | 1.16 | 0.321 | 0.200 |
| rs719726 | RSPO3 | T | 1.01 | 0.93 | 1.09 | 0.908 | 0.012 |
| rs4719841 | MIR148A | G | 1.00 | 0.93 | 1.08 | 0.941 | 0.346 |
| rs13238203 | TYW1B | C | 0.06 | 0.00 | 2.16 | 0.125 | 0.337 |
| rs17145738 | MLXIPL | C | 0.96 | 0.86 | 1.08 | 0.522 | 0.045 |
| rs38855 | MET | A | 0.98 | 0.91 | 1.06 | 0.589 | 0.002 |
| rs11776767 | PINX1 | C | 0.96 | 0.88 | 1.04 | 0.282 | 0.622 |
| rs1495741 | NAT2 | G | 1.05 | 0.96 | 1.15 | 0.252 | 0.883 |
| rs12678919 | LPL | A | 0.96 | 0.85 | 1.09 | 0.554 | 0.029 |
| rs2954029 | TRIB1 | A | 1.03 | 0.95 | 1.11 | 0.496 | 0.827 |
| rs1832007 | AKR1C4 | A | 1.06 | 0.96 | 1.18 | 0.245 | 0.089 |
| rs10761731 | JMJD1C | A | 0.95 | 0.88 | 1.03 | 0.195 | 0.492 |
| rs2068888 | CYP26A1 | G | 0.98 | 0.91 | 1.06 | 0.619 | 0.954 |
| rs174546 | FADS1–2-3 | T | 0.90 | 0.83 | 0.97 | 0.007 | 0.716 |
| rs964184 | APOA1 | G | 0.77 | 0.69 | 0.87 | <.0001 | 0.010 |
| rs11613352 | LRP1 | C | 0.96 | 0.88 | 1.04 | 0.318 | 0.284 |
| rs4765127 | ZNF664 | G | 1.04 | 0.96 | 1.12 | 0.332 | 0.602 |
| rs2412710 | CAPN3 | A | 1.23 | 0.96 | 1.59 | 0.105 | 0.845 |
| rs2929282 | FRMD5 | T | 1.12 | 0.94 | 1.33 | 0.224 | 0.963 |
| rs1532085 | LIPC | A | 1.00 | 0.93 | 1.08 | 0.910 | 0.124 |
| rs3198697 | PDXDC1 | C | 1.01 | 0.91 | 1.11 | 0.932 | 0.485 |
| rs11649653 | CTF1 | C | 1.03 | 0.95 | 1.12 | 0.494 | 0.630 |
| rs1121980 | FTO | A | 1.01 | 0.94 | 1.09 | 0.790 | 0.047 |
| rs3764261 | CETP | C | 0.91 | 0.83 | 1.00 | 0.040 | 0.578 |
| rs8077889 | MPP3 | C | 0.96 | 0.87 | 1.05 | 0.315 | 0.800 |
| rs7248104 | INSR | G | 1.03 | 0.95 | 1.11 | 0.500 | 0.690 |
| rs10401969 | CILP2 | T | 0.83 | 0.73 | 0.95 | 0.007 | 0.487 |
| rs731839 | PEPD | G | 1.06 | 0.98 | 1.15 | 0.135 | 0.566 |
| rs6065906 | PLTP | C | 0.98 | 0.89 | 1.07 | 0.618 | 0.047 |
| rs5756931 | PLA2G6 | T | 0.90 | 0.83 | 0.97 | 0.007 | 0.469 |
Models were adjusted with age, PCs, BMI and HDL
DISCUSSION
By using data from a well-powered prospective sample of initially healthy US females with homogeneous European ancestry, our study provides a compelling evidence that genetic risk for higher TG derived from a 40-SNP TG-wGRS is associated with lower T2DM risk, contrary to the positive observational associations with TG levels. Further, there was a significant interaction between the TG-wGRS40 and TG levels for predicting incident T2DM such that individuals with lower genetic predisposition to elevated TG had relatively stronger TG-based risk of T2DM than individuals with high genetic predisposition to elevated TG. We observed similar findings when the analyses were performed using a TG-wGRS based upon 127 TG-associated genetic variants. These results confirm findings from Klimentidis et al., 8 who studied a TG-wGRS based on 31 SNPs and found both reduced risk of T2DM with increased genetic risk of TG and an interaction between the TG-wGRS and TG levels for T2DM. The 40 SNPs in our TG-wGRS excluded three SNPs from the TG-wGRS of Klimentidis that only had weak evidence of TG association while including 12 additional SNPs with primary association for TG.
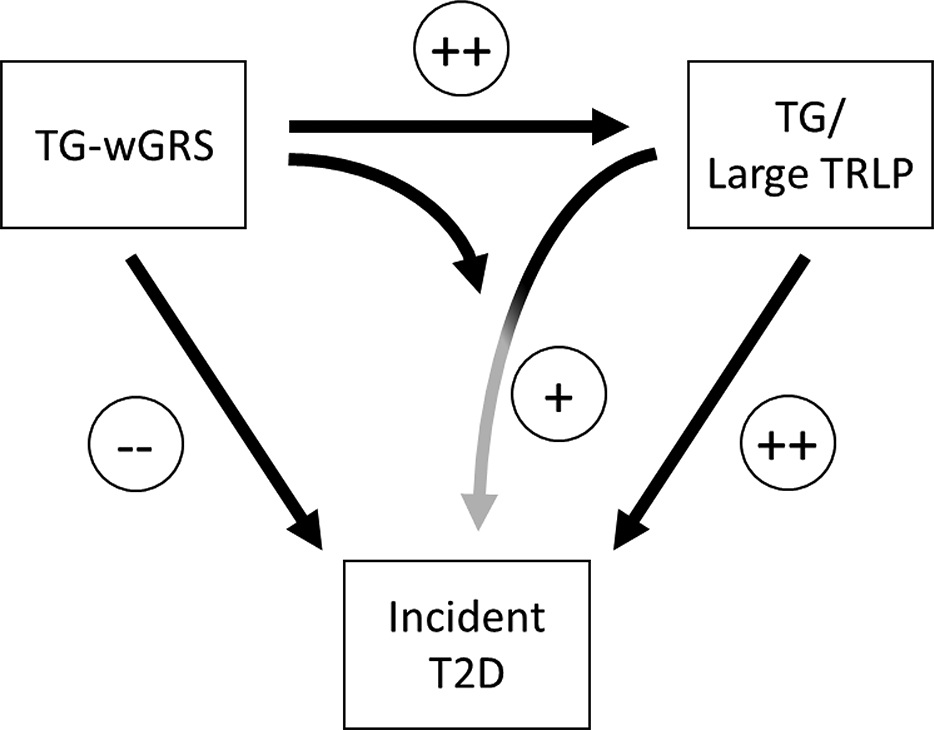
The current study also extends the previously reported discordant effects of a TG-wGRS on TG and T2DM incidence and the interaction of TG with the TG-wGRS in Klimentidis et al., 8. Our investigation focuses on the interaction effects with TG to the large, and medium but not small or very small TRLPs, even after adjustment with TG. Given the significant interaction of medium and large TRLPs, it may have been surprising that there was no significant interaction with the very large TRLPs (findings are summarized in Figure 1). However, approximately 20 % of the sample had no detectable very large TRLPs leading to lower variability in very large TRLPs compared to the others. The combined variable of large and very large TRLP particles showed significant interaction with TG-wGRS40 for incident T2DM and the significance was affirmed by the empirically p-values.
Figure 1.
Central figure describes the TG-wGRS association with TG, TRLPs and the risk of T2DM.
Previously, Mora et al., 16 using the same sample, reported that large TRLPs were associated with higher risk of T2DM compared to the smaller TRLPs, which was further confirmed in two independent cohorts of multiethnic men and women 24, 25. Similarly, Garvey et al., 26 reported that higher insulin resistance was associated with large but not small TRLPs. Festa et al., 27 reported that larger but not smaller TRLPs were associated with increased risk of T2DM among 800 individuals of the Insulin Resistance Atherosclerosis Study who were followed-up for 5-years. Although the interactions persisted in TG adjusted analysis, the biochemical mechanism of our observed interactions may nevertheless be related to the larger amount of TG that is carried by larger compared with smaller TRLPs. Comparing individuals having insulin sensitivity to those with insulin resistant phenotypes, hepatic overproduction of larger TRLPs is a key feature of dyslipoproteinemia of insulin resistance and T2DM 28. It has been reported through in vivo lipoprotein kinetic experiments that increased larger TRLPs can change the composition of HDL particles through the action of cholesteryl ester transfer protein (CETP) and hepatic lipase, which targets TG, and this process leads to formation of small dense HDL particles and also increased catabolism of these particles 29. In turn, catabolism of HDL particles is associated with the formation of small density LDL (sdLDL) particles, which have been associated with insulin resistance and T2DM related phenotypes 30.
It is common in GRS interaction effects analysis to draw SNP weights in constructing GRS from single SNP marginal association effects. This approach assumes that the magnitude of main and interaction effects across SNPs are highly correlated, and indeed, genetic variants that show main effect association with a trait are generally good candidates for interaction effects 31. In a previous interaction analysis with adiposity, we have shown that the assumption was largely valid for the TG-wGRS40 in relation to TG as the outcome across normal compared to overweight/obese strata 22. By adopting that same approach, we demonstrate that the results here were largely valid also for interaction with TG in predicting T2DM, although the assumption of proportionality was challenged for four of the genetic variants (KLHL8 rs442177, APOA1 rs964184, CAPN3 rs2412710 and CILP2 rs10401969). Excluding these SNPs, the interaction of the GRS and TG-wGRS40 for T2DM remained significant.
The biological and pathophysiological mechanisms through which higher TG-wGRSs were protective for the risk of T2DM, after adjusting with baseline TG and HDL, are not clear. The effects of the TG-associated genetic variants on T2D may reflect their role in hepatic de-novo lipogenesis 32 through which they might regulate glucose uptake and glycolysis and may subsequently increase plasma TG levels 33. A potential explanation of the discordant effects of TG and T2DM is that higher glucose utilization and glycolytic flux leads to downregulation of glucose-6-phosphatase and upregulation of glucokinase (GCK), phosphofructokinase and fatty acid synthases. These changes lead to higher glycogen synthesis, increased concentration of malonyl CoA concentration and production of larger TRLP subfractions 34. Our observation that TG-wGRSs positively associate with TG but with lower risk to T2DM is consistent with this hypothesis.
Our study has many strengths, including a large number of incident T2DM cases, the homogenous study sample, a comprehensive collection of TG-associated genetic variants, longitudinal study design, and complete profiles of TRLP subfractions and standard lipids. The long follow-up duration for incident T2D also reduces the likelihood of reverse causation. Our study has several limitations including that our sample was based upon European ancestry women participants only; the generalizability of the current findings still needs to be established by testing among ancestrally diverse populations. TG, HDL and LDL are highly correlated lipids and it is difficult to distinguish the specific effects of one lipid fraction versus another. To limit such potential pleiotropic effects, TG-wGRS and T2DM incidence analyses were additionally adjusted with HDL and LDL and the results were materially similar. However, the findings from Klimentidis et al., 8 were based upon both male and female participants and were similar to our findings suggesting that they are independent of sex.
In summary, our findings provide novel information understanding the complex relationship between TG and T2DM by focusing this relationship on the large TRLP component of TG. At the clinical level, the gene-based relationship between TG and T2DM related traits was opposite to the observational relationship, suggesting a complex gene regulation and cardiometabolic interplay between TG and T2DM related traits. One expects that the mechanisms underlying our associations most likely occur in tissues where lipids and T2DM related biomolecules are synthesized and stored. Our findings thus suggests future studies employing systematic biological approaches using liver, muscle and adipose tissues are warranted to help further understand the causal links between lipids, glycemic traits, and their consequences to T2DM risk.
Supplementary Material
Highlights.
Previous results had shown that while higher plasma triglycerides (TG) is a risk for type 2 diabetes (T2DM), genetic predisposition to higher TG was paradoxically protective for T2DM.
Moreover, a significant interaction between a genetic risk score of TG-associated variants (TG-wGRS) and baseline TG for association with T2DM risk has been observed. In the interaction, plasma TG was more strongly associated with T2DM incidence among individuals with lower compared to higher genetic predisposition to elevated TG.
By using TG-wGRSs based on 40-triglyceride and 127-triglyceride associated genetic variants, we tested whether such associations can be replicated and also whether the interaction is selective for certain triglyceride-rich lipoprotein particles (TRLP).
In our cohort with 18.6 years of follow-up observation, our results confirm the paradoxical associations of TG with incident T2DM as well as the genetic interactions with TG. Moreover, the results add novelty by demonstrating the role of larger but not smaller TRLP subfractions in the interaction
The results focus attention on the influence of large TRLPs on incident T2DM and may ultimately contribute to identifying population groups with greater susceptibility to T2DM through a combination of genetic risk and plasma lipid profile.
Acknowledgments
Sources of Funding
The WGHS is supported by the National Cancer Institute (CA047988 and UM1CA182913) and the National Heart, Lung, and Blood Institute (HL043851 and HL080467) with collaborative scientific support and funding for genotyping. Dr. Shafqat Ahmad was supported through a fellowship from Nutricia Research Foundation (2016-T1), Swedish Heart-Lung Foundation (20150711) and Henning och Johan Throne-Holst stiftelse Fellowship Sweden. Dr. Mora was supported by the research grants from American Heart Association (0670007N), the Sandra A. Daugherty Foundation and the NHLBI (K08 HL094375) regarding NMR measurements, as well as from the National Heart, Lung, and Blood Institute (R01HL134811 and K24 HL136852), and the National Institute of Diabetes and Digestive and Kidney Diseases (DK112940). Also, without additional costs, LabCorp provided the LipoProfile IV results. Daniel Chasman is supported by the NINDS (R21NS092963, R21NS104398) and the NEI (R01EY021900).
Nonstandard Abbreviations and Acronyms
- TRLP
Triglyceride-rich lipoprotein subfraction particles
- TG-wGRS
Triglyceride-associated weighted genetic risk score
- WGHS
Women’s Genome Health Study
- TRLZ
Triglyceride rich lipoprotein particles size
- TRLTG
TG rich TRLP particles
- TRLTC
Cholesterol rich TRLP particles
Footnotes
Disclosures:
Samia Mora received research grant support from Atherotech Diagnostics for research outside the current work, served as a consultant to Quest Diagnostics, and has a patent application regarding the use of an NMR biomarker (not related to lipoproteins) in relation to colorectal cancer risk. All other coauthors have no conflict of interests.
REFERENCES
- 1.Hjellvik V, Sakshaug S, Strom H. Body mass index, triglycerides, glucose, and blood pressure as predictors of type 2 diabetes in a middle-aged norwegian cohort of men and women. Clin Epidemiol. 2012;4:213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dotevall A, Johansson S, Wilhelmsen L, Rosengren A. Increased levels of triglycerides, bmi and blood pressure and low physical activity increase the risk of diabetes in swedish women. A prospective 18-year follow-up of the beda study. Diabet Med. 2004;21:615–622 [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139:802–809 [DOI] [PubMed] [Google Scholar]
- 4.Perry IJ, Wannamethee SG, Walker MK, Thomson AG, Whincup PH, Shaper AG. Prospective study of risk factors for development of non-insulin dependent diabetes in middle aged british men. BMJ. 1995;310:560–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870 [DOI] [PubMed] [Google Scholar]
- 6.Global Lipids Genetics C, Willer CJ, Schmidt EM, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klimentidis YC, Chougule A, Arora A, Frazier-Wood AC, Hsu CH. Triglyceride-increasing alleles associated with protection against type-2 diabetes. PLoS Genet. 2015;11:e1005204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N, van der Sijde MR, LifeLines Cohort Study G, Bakker SJ, Dullaart RP, van der Harst P, Gansevoort RT, Elbers CC, Wijmenga C, Snieder H, Hofker MH, Fu J. Pleiotropic effects of lipid genes on plasma glucose, hba1c, and homa-ir levels. Diabetes. 2014;63:3149–3158 [DOI] [PubMed] [Google Scholar]
- 10.De Silva NM, Freathy RM, Palmer TM, et al. Mendelian randomization studies do not support a role for raised circulating triglyceride levels influencing type 2 diabetes, glucose levels, or insulin resistance. Diabetes. 2011;60:1008–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White J, Swerdlow DI, Preiss D, Fairhurst-Hunter Z, Keating BJ, Asselbergs FW, Sattar N, Humphries SE, Hingorani AD, Holmes MV. Association of lipid fractions with risks for coronary artery disease and diabetes. JAMA Cardiol. 2016;1:692–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridker PM, Chasman DI, Zee RY, Parker A, Rose L, Cook NR, Buring JE, Women’s Genome Health Study Working G. Rationale, design, and methodology of the women’s genome health study: A genome-wide association study of more than 25,000 initially healthy american women. Clin Chem. 2008;54:249–255 [DOI] [PubMed] [Google Scholar]
- 13.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin e in the primary prevention of cardiovascular disease and cancer: The women’s health study: A randomized controlled trial. JAMA. 2005;294:56–65 [DOI] [PubMed] [Google Scholar]
- 14.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the women’s health study. J Womens Health Gend Based Med. 2000;9:19–27 [DOI] [PubMed] [Google Scholar]
- 15.Pradhan AD, Cook NR, Manson JE, Ridker PM, Buring JE. A randomized trial of low-dose aspirin in the prevention of clinical type 2 diabetes in women. Diabetes Care. 2009;32:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mora S, Otvos JD, Rosenson RS, Pradhan A, Buring JE, Ridker PM. Lipoprotein particle size and concentration by nuclear magnetic resonance and incident type 2 diabetes in women. Diabetes. 2010;59:1153–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawler PR, Akinkuolie AO, Ridker PM, Sniderman AD, Buring JE, Glynn RJ, Chasman DI, Mora S. Discordance between circulating atherogenic cholesterol mass and lipoprotein particle concentration in relation to future coronary events in women. Clin Chem. 2017;63:870–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otvos JD, Shalaurova I, Wolak-Dinsmore J, Connelly MA, Mackey RH, Stein JH, Tracy RP. Glyca: A composite nuclear magnetic resonance biomarker of systemic inflammation. Clin Chem. 2015;61:714–723 [DOI] [PubMed] [Google Scholar]
- 19.Gunderson KL, Kuhn KM, Steemers FJ, Ng P, Murray SS, Shen R. Whole-genome genotyping of haplotype tag single nucleotide polymorphisms. Pharmacogenomics. 2006;7:641–648 [DOI] [PubMed] [Google Scholar]
- 20.Gunderson KL, Steemers FJ, Ren H, et al. Whole-genome genotyping. Methods Enzymol. 2006;410:359–376 [DOI] [PubMed] [Google Scholar]
- 21.Liu DJ, Peloso GM, Yu H, et al. Exome-wide association study of plasma lipids in >300,000 individuals. Nat Genet. 2017;49:1758–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad S, Mora S, Franks PW, Orho-Melander M, Ridker PM, Hu FB, Chasman DI. Adiposity and genetic factors in relation to triglycerides and triglyceride-rich lipoproteins in the women’s genome health study. Clin Chem. 2018;64:231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prasad RB, Groop L. Genetics of type 2 diabetes-pitfalls and possibilities. Genes (Basel). 2015;6:87–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dugani SB, Akinkuolie AO, Paynter N, Glynn RJ, Ridker PM, Mora S. Association of lipoproteins, insulin resistance, and rosuvastatin with incident type 2 diabetes mellitus : Secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:136–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackey RH, Mora S, Bertoni AG, Wassel CL, Carnethon MR, Sibley CT, Goff DC Jr. Lipoprotein particles and incident type 2 diabetes in the multi-ethnic study of atherosclerosis. Diabetes Care. 2015;38:628–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, Pugh K, Jenkins AJ, Klein RL, Liao Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52:453–462 [DOI] [PubMed] [Google Scholar]
- 27.Festa A, Williams K, Hanley AJ, Otvos JD, Goff DC, Wagenknecht LE, Haffner SM. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the insulin resistance atherosclerosis study. Circulation. 2005;111:3465–3472 [DOI] [PubMed] [Google Scholar]
- 28.Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:1225–1236 [DOI] [PubMed] [Google Scholar]
- 29.Rashid S, Watanabe T, Sakaue T, Lewis GF. Mechanisms of hdl lowering in insulin resistant, hypertriglyceridemic states: The combined effect of hdl triglyceride enrichment and elevated hepatic lipase activity. Clin Biochem. 2003;36:421–429 [DOI] [PubMed] [Google Scholar]
- 30.Feingold KR, Grunfeld C, Pang M, Doerrler W, Krauss RM. Ldl subclass phenotypes and triglyceride metabolism in non-insulin-dependent diabetes. Arterioscler Thromb. 1992;12:1496–1502 [DOI] [PubMed] [Google Scholar]
- 31.Aschard H A perspective on interaction effects in genetic association studies. Genet Epidemiol. 2016;40:678–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanders FW, Griffin JL. De novo lipogenesis in the liver in health and disease: More than just a shunting yard for glucose. Biol Rev Camb Philos Soc. 2016;91:452–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beer NL, Tribble ND, McCulloch LJ, Roos C, Johnson PR, Orho-Melander M, Gloyn AL. The p446l variant in gckr associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum Mol Genet. 2009;18:4081–4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orho-Melander M, Melander O, Guiducci C, et al. Common missense variant in the glucokinase regulatory protein gene is associated with increased plasma triglyceride and c-reactive protein but lower fasting glucose concentrations. Diabetes. 2008;57:3112–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.