Introduction
Peripheral neuropathy incidence is more than 70 per 100,000 person-years, and rises with age and diabetes.[11; 20; 21] The prevalence of pain in those with diabetic peripheral neuropathy is approximately 35%.[7] Limited disease modifying treatments exist, thus management typically focuses on treatment of neuropathic pain.[6] Systematic reviews from the American Academy of Neurology (AAN), the European Federation of Neurological Societies (EFNS), the Agency for Healthcare Research and Quality (AHRQ), and others report a consensus that the medications with the best evidence to reduce pain in diabetic peripheral neuropathy are serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and gabapentinoids.[3; 4; 10; 14; 22] While opioid medications have also demonstrated short term benefit in the treatment of this disabling condition, the risks include overdose, dependency, addiction, and death.[13] These risks have led the AAN and the Centers for Disease Control and Prevention (CDC) to recommend against the treatment of chronic non-cancer pain, such as painful peripheral neuropathy, with opioid medications.[9; 13] Thus, peripheral neuropathy is unique amongst prevalent painful conditions in that multiple non-opioid evidence-based medications exist, and recommendations explicitly prioritize these medications above opioids.
A recent regional study demonstrated that 69.4% of peripheral neuropathy patients receive opioid medications with 18.8% receiving chronic opioid therapy, although other chronic pain conditions were not excluded.[16] Furthermore, opioid medications were associated with increased depression, overdose, and dependence without improved in functional status.[16] On the other hand, previous studies demonstrate that painful peripheral neuropathy is likely undertreated.[7] Patients do not always discuss their neuropathic pain with their physicians, and even when they do, their physicians often do not prescribe guideline-recommended medications.[7] To optimize treatment of painful peripheral neuropathy, information on the current prescription practice patterns of neuropathic pain medications is needed.
We aimed to investigate the longitudinal pattern of neuropathic pain medication utilization in patients with peripheral neuropathy with a focus on opioids and guideline-recommended medications. We also determined the patient related factors associated with neuropathic pain medication use and pain specialist visits.
Methods
Population
We used the de-identified Clinformatics™ Datamart (OptumInsight, Eden Prairie, MN) database, which consists of detailed medical claims data, including pharmaceutical claims, on more than 59.7 million unique individuals insured by United Healthcare from 2001–2014. United Healthcare is the largest private insurer in the country and the population demographics closely match those of the under 65 and privately insured population within the United States. We identified individuals with peripheral neuropathy using a validated ICD-9 code claims definition (356.x, 357.1–8, 357.82, 357.89 and 357.9),[15] but we excluded ICD-9 autonomic neuropathy diagnoses (337.0 and 337.1). Patients were required to be ≥18 years old and have 4 years of continuous insurance enrollment with at least one year before and 3 years after the incident peripheral neuropathy diagnosis. Importantly, claims data are unable to distinguish if patients had neuropathic pain. Therefore, to focus on peripheral neuropathy as the main indication for pain treatment, we excluded patients that had other diagnoses that result in chronic pain (e.g. low back pain, osteoarthritis, fibromyalgia, lumbar and cervical radiculopathy, rheumatoid arthritis, irritable bowel syndrome) at or prior to their peripheral neuropathy diagnosis as previously described.[8]
Exposures (patient characteristics)
The database contains patient demographic information including age, gender, race, education, and household income. Using ICD-9 codes from visits before the initial neuropathy diagnosis, we defined diabetes as (249.xx, 250.xx, 362.01–362.03, 357.2), depression as (311.00, 296.30–296.36, 296.2x), and anxiety as (300.0x). Charlson comorbidity index was used to estimate patient comorbidities as previously described with removal of the diabetes components to avoid double counting this comorbidity.[19]
Main outcomes and measures
Opioid medications were defined using the American Hospital Formulary Service (AHFS) class (Opiate antagonists: 281000, Opiate partial agonists: 280812, and Opiate agonists: 280808). Chronic opioid use was defined by having 90 continuous days of opioid prescription, allowing for less than 10 days between prescriptions. This is the same definition previously employed by Hoffman et al,[16] which is based on the treatment duration of chronic pain as defined by the International Association for the study of pain (greater than or equal to 90 continuous days).[1] Guideline-recommended medications included tricyclic anti-depressants (TCAs, e.g. amitriptyline and nortriptyline), serotonin norepinephrine reuptake inhibitors (SNRIs, e.g. venlafaxine and duloxetine), and gabapentinoids (gabapentin and pregabalin).[3; 4; 10; 14; 22] Visits to pain specialists were considered office visits (CPT codes 99201–99205, 99211–99215, and 99241–99245) with provider category codes for neurologists, physiatrists, and anesthesiologists on or after the index diagnosis.
Statistical Analysis
Descriptive statistics were used to characterize the peripheral neuropathy population in terms of patient socio-demographic (age, gender, race, education, household income, insurance type geographic location) and clinical characteristics (Charlson Comorbidity Index, presence of diabetes, depression, and/or anxiety, visits to anesthesiologists, neurologists and physiatrists).
Current practice patterns
To describe longitudinal patterns of neuropathic pain medication use, we examined patient’s prescription states over time. First we defined the following eight discrete prescription states: no neuropathic pain medication use, any chronic opioid use (consuming state) and six different states for the number of guideline-recommended medications prescribed (#1–6). At the time of any new prescription, a patient would change states and we tabulated the probabilities of each state transition.
Trends in utilization of neuropathic pain medications
To evaluate the change in the frequency of neuropathic pain prescriptions over time, we plotted the number of prescriptions adjusted for the number of patient-days (number of patients with peripheral neuropathy multiplied by the amount of time continuously enrolled in insurance plan for that year) for opioid medications and for each guideline-recommended medication for each year (2002–2014).
Association of patient level factors with neuropathic pain medication use and pain specialists
Separate multivariable logistic regression models were used to evaluate the association between patient level variables and the dependent variables of opioid medication use, guideline-recommended medication use, and pain specialist visits (neurologists, physiatrists, or anesthesiologists) after the incident peripheral neuropathy diagnosis. For opioid medication use we used two different logistic regression models to predict factors associated with any opioid medication use and to predict chronic opioid medication use amongst those patients who received any opioid medications. Given that little is known about the patient level variables that are associated with these outcomes in patients with peripheral neuropathy, we included all of the patient socio-demographic and clinical characteristics (see above) in our models. As a sensitivity analysis, we excluded tramadol from the models evaluating opioid medication use.
All analysis was performed with SAS, version 9.4 (SAS Institute Inc.). Figure 2 was created using the ggplot2 package in R v3.4.2.
Figure 2: Frequency of opioid and guideline-recommended medication prescriptions for painful peripheral neuropathy.
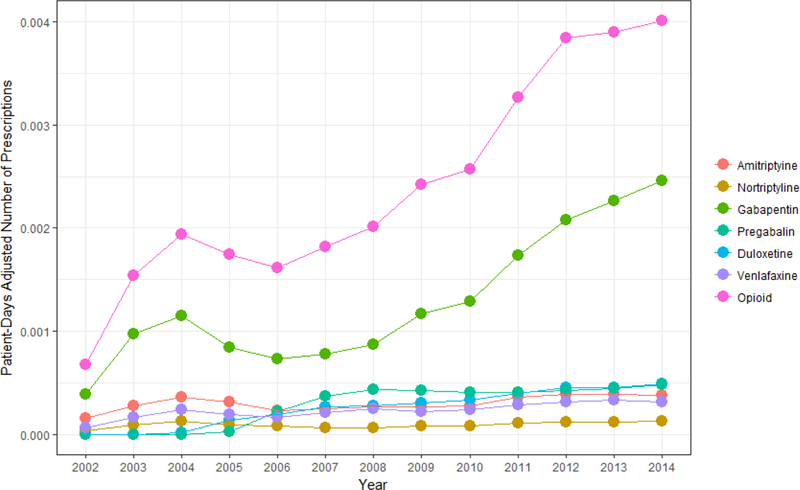
The number of opioid and specific guideline-recommended medication prescription adjusted for patient days for neuropathic pain per year from 2002–2014.
Standard Protocol Approvals, Registrations, and Patient Consents
The University of Michigan IRB determined that this study was exempt.
Results
Patients were followed for a mean (SD) of 3.1 (1.7) years before and 4.5 (1.4) years after the incident peripheral neuropathy diagnosis. Mean (SD) age was 43.1 years (2.8) and 52.4% were men (Table 1). Of the 14,426 patients with peripheral neuropathy, 11.5% were African-American, 10.4% Hispanic, 2.5% Asian, and 75.6% non-Hispanic white. In this population, 46.5% had diabetes, 7.8% anxiety, and 4.2% depression.
Table 1:
Demographic and clinical variables in patients with incident neuropathy diagnosis
| Demographics and clinical variables (N, %) unless otherwise specified |
|
|---|---|
| Age (mean, SD) | 43.1 (2.8) |
| Male | 7558 (52.4%) |
| Race Asian African-American Hispanic Non-Hispanic White |
334 (2.5%) 1550 (11.5%) 1401 (10.4%) 10227 (75.6%) |
| Census level East North Central East South Central Middle Atlantic Mountain New England Pacific South Atlantic West North Central West South Central |
1730 (12.1%) 594 (4.1%) 786 (5.5%) 1358 (9.5%) 448 (3.1%) 1926 (13.4%) 3401 (23.7%) 1826 (12.7%) 2276 (15.9%) |
| Education Less than 12th Grade High School Diploma Less than Bachelor Degree Bachelor Degree Plus |
94 (0.7%) 4043 (28.8%) 7668 (54.7%) 2214 (15.8%) |
| Household income <$40K $40K-$49K $50K-$59K $60K-$74K $75K-$99K $100K+ |
3061 (26.0%) 1057 (9.0%) 982 (8.3%) 1323 (11.2%) 1799 (15.3%) 3551 (30.2%) |
| Diabetes | 6701 (46.5%) |
| Anxiety | 1120 (7.8%) |
| Depression | 603 (4.2%) |
| Insurance type EPO HMO IND Other POS PPO |
1105 (7.7%) 4832 (33.5%) 1492 (10.3%) 335 (2.3%) 4898 (34.1%) 1764 (12.2%) |
| Charlson score (mean, SD) | 0.7 (1.0) |
| Diagnosis by provider type Anesthesiologist Neurologist Primary Care Physician Physiatrist Other |
39 (0.3%) 1413 (9.8%) 6241 (43.3%) 210 (1.5%) 6523 (45.2%) |
| Seen by pain specialist at or after neuropathy diagnosis Any Neurologist Physiatrist Anesthesiologist |
5175 (35.9%) 4013 (27.8%) 1251 (8.7%) 919 (6.4%) |
| Opioid Use Any >/= 90 continuous days |
9512 (65.9%) 1266 (8.8%) |
| Guideline-recommended medications for neuropathic pain Any TCAs Amitriptyline Nortriptyline Other TCA SNRIs Duloxetine Venlafaxine Other SNRI Gabapentinoids Gabapentin Pregabalin |
5143 (35.7%) 972 (6.7%) 326 (2.3%) 193 (1.3%) 817 (5.7%) 510 (3.5%) 80 (0.01%) 3562 (24.7%) 1195 (8.3%) |
| Number of guideline-recommended medications for neuropathic pain 0 1 2 3 4 5 6 or more |
9283 (64.4%) 3352 (23.2%) 1241 (8.6%) 408 (2.8%) 113 (0.8%) 25 (0.2%) 4 (0.02%) |
| Number of years prior to neuropathy diagnosis (mean, SD) | 3.1 (1.7) |
| Number of years after neuropathy diagnosis (mean, SD) | 4.5 (1.4) |
EPO= Exclusive Provider Organization, HMO= Health Maintenance Organization, IND= Indemnity, POS=Point of Service, PPO= Preferred Provider Organization, TCA= tricyclic antidepressant, SNRI=serotonin reuptake inhibitor
Opioid medications were prescribed to 65.9% of patients and 8.8% received chronic opioid therapy. Excluding tramadol, opioid medications were prescribed to 62.4% of patients and 6.9% received chronic opioid therapy. At least one guideline-recommended medication was prescribed to 35.7% of the population, 12.4% received two or more, 3.8% received three or more, and 1.0% received four or more of these medications. In those with diabetes, opioid medications were prescribed to 68.5% of patients, 10.2% received chronic opioid therapy, and 39.2% received at least one guideline-recommended medication. The most common guideline-recommended medications were gabapentin (24.7%), pregabalin (8.3%), amitriptyline (6.7%), and duloxetine (5.7%). Pain specialists were involved in the care of 35.9% of these patients, of which, 27.8% saw a neurologist, 8.7% a physiatrist, and 6.4% an anesthesiologist.
Current practice patterns
Of the 14,426 peripheral neuropathy patients, 8,953 (62.1%) never received a guideline-recommended medication and never received chronic opioid therapy (Figure 1). Prior to receiving any guideline-recommended medications, 6.5% received chronic opioid therapy. Of those receiving chronic opioid therapy (n=1266), 26.4% received at least one guideline-recommended medication prior to chronic opioid status, 6.4% at least two medications, 1.6% at least three medications, and 0.2% at least four different medications.Of the 5,473 patients who received neuropathic pain medications, 83% received a guideline-recommended medication while 17% received chronic opioid therapy before receiving any guideline-recommended medications. Of those receiving at least one guideline-recommended medication and receiving more neuropathic pain medications, 84% received a second guideline medication and 16% received chronic opioid therapy before receiving any additional guideline-recommended medications. Similar proportions were seen for those receiving at least two and three guideline-recommended medication and receiving more neuropathic pain medications.
Figure 1: The sequence of neuropathic pain medications in the peripheral neuropathy population.
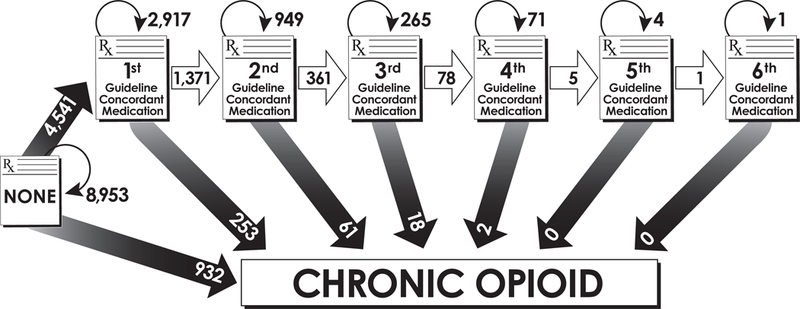
The sequence of neuropathic pain medications, specifically guideline-recommended medications and the start of chronic opioid use, in patients with incident peripheral neuropathy
Trends in utilization of neuropathic pain medications
From 2002–2014, the frequency of opioid prescriptions increased faster than all other guideline-recommended medications (Figure 2). Amongst guideline-recommended medications, gabapentin had the largest increase.
Association of patient level factors with neuropathic pain medication use
No patient level factors were associated with both high opioid utilization (initiation and chronic use) and low guideline-recommended medication utilization. Older patients and Asians had significantly less initiation of opioid therapy and less guideline-recommended medication (Table 2). Men and Hispanics had significantly less initiation of opioids, less transition to chronic opioid therapy, and less guideline-recommended medications. Diabetes, depression, Charlson score, and pain specialists were all significantly associated with more initiation of opioid therapy, more transition to chronic opioid therapy, and more guideline-recommended medication. Anxiety was significantly associated with more transition to chronic opioid therapy and more guideline-recommended medication. Higher household income was significantly associated with more transition to chronic opioid therapy. Excluding tramadol had minimal effects on the odds ratios for our models investigating initiation of opioids and transition to chronic opioids.
Table 2:
Demographic and clinical factors associated with guideline-recommended medications for neuropathic pain and opioid medications in the peripheral neuropathy population**
| Any opioid use OR (95% CI) |
>/= 90 days of opioid use OR (95% CI) |
Guideline- recommended medications OR (95% CI) |
|
|---|---|---|---|
| Male (ref=Female) | 0.89 (0.82,0.97)* | 0.77 (0.67,0.90)* | 0.68 (0.62,0.74)* |
| Age | 0.87 (0.86,0.89)* | 0.97 (0.94,1.01) | 0.95 (0.93,0.96)* |
| Race (ref=White) | |||
| Asian | 0.57 (0.45,0.73)* | 0.54 (0.27,1.08) | 0.61 (0.46,0.82)* |
| Black | 1.02 (0.89,1.16) | 0.84 (0.67,1.06) | 0.90 (0.79,1.03) |
| Hispanic | 0.87 (0.75,1.00)* | 0.64 (0.48,0.84)* | 0.76 (0.66,0.89)* |
| Education (ref= Less than 12th Grade) | |||
| High School Diploma | 1.08 (0.63,1.83) | 3.76 (0.87,16.24) | 0.78 (0.46,1.32) |
| Less than Bachelor Degree | 1.00 (0.59,1.70) | 2.85 (0.66,12.33) | 0.62 (0.36,1.04) |
| Bachelor Degree Plus | 0.74 (0.43,1.28) | 1.74 (0.39,7.17) | 0.50 (0.29,0.87)* |
| Household income (Ref= <$40K) |
|||
| $40K-$49K |
1.05 (0.89,1.23) | 1.74 (1.37,2.20)* | 1.00 (0.85,1.17) |
| $50K-$59K |
1.03 (0.87,1.21) | 1.44 (1.08,1.92)* | 1.01 (0.86,1.19) |
| $60K-$74K |
0.95 (0.82,1.10) | 1.34 (0.99,1.80) | 1.07 (0.93,1.24) |
| $75K-$99K |
1.21 (1.05,1.39)* | 1.51 (1.16,1.98)* | 0.99 (0.87,1.14) |
| $100K+ | 1.02 (0.89,1.16) | 1.05 (0.81,1.36) | 0.86 (0.76,0.99)* |
| Diabetes | 1.38 (1.27,1.50)* | 1.32 (1.13,1.53)* | 1.52 (1.39,1.65)* |
| Anxiety | 1.14 (0.97,1.33) | 1.43 (1.13,1.82)* | 1.54 (1.33,1.79)* |
| Depression | 1.25 (1.01,1.55)* | 1.48 (1.09,2.03)* | 1.65 (1.35,2.02)* |
| Charlson score | 1.15 (1.10,1.20)* | 1.24 (1.17,1.32)* | 1.16 (1.11,1.21)* |
| Pain specialist Neurologist Physiatrist Anesthesiologist |
1.23 (1.12,1.36)* 1.97 (1.67,2.34)* 3.90 (3.07,4.95)* |
1.16 (0.99,1.36) 1.77 (1.44,2.16)* 4.32 (3.57,5.23)* |
1.92 (1.75,2.10)* 1.55 (1.35,1.79)* 3.07 (2.60,3.61)* |
| Insurance type (Ref=PPO) EPO HMO IND Other POS |
1.03 (0.85,1.25) 0.94 (0.81,1.09) 1.06 (0.88,1.27) 0.68 (0.51,0.90)* 1.11 (0.96,1.29) |
0.80 (0.56,1.14) 1.10 (0.85,1.43) 0.95 (0.71,1.28) 1.94 (1.20,3.12)* 1.07 (0.83,1.38) |
0.85 (0.70,1.03) 0.92 (0.79,1.06) 1.02 (0.86,1.21) 1.06 (0.79,1.41) 0.94 (0.81,1.09) |
*p<0.05
**Models adjusted for census level division
EPO= Exclusive Provider Organization, HMO= Health Maintenance Organization, IND= Indemnity, POS=Point of Service, PPO= Preferred Provider Organization
Association of patient level factors with pain specialists
Older patients and Hispanics had less visits to pain specialists (Table 3). Asians also had less visits to pain specialists, but this was only significant for anesthesiologists. Seeing other pain specialists is significantly associated with more involvement of the other pain specialists. Higher household income and higher Charlson scores were significantly associated with more involvement of neurologists and physiatrists. Diabetes was significantly associated with less involvement of neurologists and physiatrists. Depression and anxiety were significantly associated with more involvement of neurologists. Higher educational level was associated with less involvement of anesthesiologists.
Table 3:
Demographic and clinical factors associated with seeing a pain specialist within the peripheral neuropathy population**
| Neurologist OR (95% CI) |
PMR OR (95% CI) |
Anesthesiologist OR (95% CI) |
|
|---|---|---|---|
| Male (ref=Female) | 0.99 (0.91,1.08) | 0.82 (0.72,0.94)* | 0.94 (0.80,1.10) |
| Age | 0.92 (0.90,0.93)* | 0.93 (0.90,0.95)* | 0.97 (0.94,1.00) |
| Race (ref=White) | |||
| Asian | 0.89 (0.67,1.18) | 1.04 (0.67,1.60) | 0.43 (0.20,0.92)* |
| Black | 0.99 (0.86,1.14) | 0.99 (0.79,1.24) | 0.81 (0.62,1.05) |
| Hispanic | 0.76 (0.64,0.90)* | 0.72 (0.54,0.95)* | 0.69 (0.51,0.94)* |
| Education (ref= Less than 12th Grade) | |||
| High School Diploma | 0.99 (0.49,1.99) | 3.22 (0.44,23.80) | 0.39 (0.17,0.90)* |
| Less than Bachelor Degree | 1.12 (0.55,2.25) | 3.62 (0.49,26.71) | 0.42 (0.18,0.97)* |
| Bachelor Degree Plus | 1.34 (0.66,2.73) | 3.93 (0.53,29.23) | 0.32 (0.13,0.75)* |
| Household income (Ref= <$40K) |
|||
| $40K-$49K |
1.16 (0.98,1.38) | 1.29 (0.98,1.70) | 0.97 (0.73,1.29) |
| $50K-$59K |
1.03 (0.87,1.24) | 1.75 (1.35,2.27)* | 0.82 (0.60,1.12) |
| $60K-$74K |
1.20 (1.02,1.41)* | 1.25 (0.96,1.61) | 0.91 (0.69,1.20) |
| $75K-$99K |
1.32 (1.14,1.53)* | 1.45 (1.15,1.83)* | 0.80 (0.61,1.03) |
| $100K+ | 1.33 (1.15,1.53)* | 1.48 (1.18,1.85)* | 0.81 (0.63,1.04) |
| Diabetes | 0.59 (0.54,0.65)* | 0.79 (0.69,0.91)* | 1.04 (0.89,1.22) |
| Anxiety | 1.31 (1.12,1.53)* | 1.07 (0.84,1.36) | 1.08 (0.82,1.42) |
| Depression | 1.27 (1.03,1.58)* | 1.04 (0.74,1.47) | 1.16 (0.80,1.68) |
| Charlson score | 1.07 (1.03,1.12)* | 1.12 (1.05,1.19)* | 1.01 (0.94,1.09) |
| Pain specialist Neurologist Physiatrist Anesthesiologist |
1.57 (1.37,1.80)* 1.72 (1.46,2.02)* |
1.59 (1.38,1.83)* 2.53 (2.07,3.10)* |
1.71 (1.46,2.01)* 2.53 (2.07,3.10)* |
| Insurance type (Ref=PPO) EPO HMO IND Other POS |
1.01 (0.84,1.23) 0.87 (0.75,1.02) 1.05 (0.88,1.26) 1.04 (0.75,1.43) 1.00 (0.86,1.16) |
1.07 (0.80,1.43) 0.85 (0.67,1.09) 1.39 (1.07,1.81)* 1.35 (0.84,2.17) 0.95 (0.76,1.20) |
1.09 (0.77,1.54) 0.86 (0.65,1.14) 1.03 (0.75,1.42) 0.76 (0.43,1.36) 0.99 (0.75,1.30) |
*p<0.05
**Models adjusted for census level division
EPO= Exclusive Provider Organization, HMO= Health Maintenance Organization, IND= Indemnity, POS=Point of Service, PPO= Preferred Provider Organization
Discussion
In a large, national, privately-insured population, we found that almost two thirds of peripheral neuropathy patients receive at least one opioid prescription with nearly 9% eventually receiving chronic opioid therapy even after excluding patients with other diagnoses that result in chronic pain. In contrast, approximately one third of patients receive at least one guideline-recommended medication for peripheral neuropathy. Importantly, a substantial proportion of patients receive chronic opioid therapy before trying even one guideline-recommended medications for peripheral neuropathy. Very few patients receive more than one guideline-recommended medication with a substantial proportion receiving chronic opioid therapy instead of trying additional evidence-based medications. Furthermore, opioid prescriptions are increasing faster in this population than any of the guideline-recommended medications.
These results illustrate three main problems with the current practice of treating painful peripheral neuropathy. One, most patients with peripheral neuropathy are exposed to opioid therapy at some point after diagnosis, which is consistent with two previous studies in patients with peripheral neuropathy.[16; 18] Given the magnitude of this issue, efforts to curb initiation of opioids in this population would likely help reduce the large numbers of patients that eventually transition to chronic opioid therapy. In fact, the peripheral neuropathy population may be an ideal group to study opioid reducing interventions because of the high prevalence of both initiation and transition to chronic opioid therapy. Two, chronic opioid therapy is common even though AAN and CDC recommendations advise against their use for chronic non-cancer pain.[9; 12] As successful interventions are developed to taper patients off chronic opioid therapy, the enriched peripheral neuropathy population should be targeted. Three, patients with painful peripheral neuropathy often transition to chronic opioid therapy without trying other medications that are known to be effective. No previous studies have focused on the sequence of neuropathic pain medications given to peripheral neuropathy patients. Given that the best evidence supports the use of non-opioids, optimal treatment algorithms may require that a patient fail at least three guideline recommended medications, one in each of the three effective classes (SNRIs, TCAs, and gabapentinoids) before trying alternative therapies.[2; 4; 10; 14; 22] Perhaps even better would be failure of at least two medications in each class, including their combinations, prior to consideration of opioid therapy. However, our data shows that only 2.8% of the patients tried three guideline recommended medications and only 0.02% tries six or more medications. Improving guideline concordant care has the potential to not only improve pain control in this population, but also simultaneously decrease both opioid initiation and chronic opioid therapy and should be the focus of implementation efforts.
Besides decreasing opioid medications and increasing guideline recommended medications for neuropathic pain, improvement of neuropathic pain treatment would also be facilitated with better data on how to treat painful peripheral neuropathy. We currently do not know which guideline recommended medications are most efficacious because comparative effectiveness data is lacking.[4] We also do not know the likelihood of response to additional medications if an initial guideline recommended medication fails, which would inform how many medications to try. We have little information on the benefits of trying a second medication in the same therapeutic class if the first one fails although pregabalin treatment after gabapentin failure is a common prescription practice pattern. We also do not know what interventions to try if the guideline recommended medications all fail. Similarly, where non-pharmacologic approaches to pain treatment fit into the algorithm is unclear. Finally, whether there is any role for opioid therapy in this population is unknown. Future studies should answer these questions so that clinicians are armed with the necessary data to optimally treat neuropathic pain.
Importantly, no patient level factors were associated with high opioid use and low guideline-recommended medication use as all variables studied had similar associations with both types of medications. Therefore, the specific population that should be the focus of interventions to improve neuropathic pain treatment practices is unclear. However, patient level factors associated with both low guideline-recommended medications and low opioid use may require interventions to increase guideline-recommended medications without the unintended consequence of increasing opioid medications as well. Conversely, patient level factors associated with both high guideline-recommended medications and high opioid use may require interventions to lower opioid use without the unintended consequence of decreasing guideline-recommended medications as well.
We found that older age, male sex, Hispanics, and Asians were associated with less guideline-recommended medications and either less initiation of opioids or transition to chronic opioid therapy. According to a 2012 National Health Interview Survey, Asians and men self-report less severe pain in general compared to other races and women.[17] Whether this is true of peripheral neuropathy patients is unknown, and we also do not know if this is the sole reason for differences in utilization. However, Hispanics report the same proportion of severe pain and older patients report more severe pain compared to other races and younger patients.[17] Physicians may be concerned about the side effects of pain medications in older patients, but both of these observations require further study. Diabetes, anxiety, depression, higher charlson scores, and seeing pain specialists were all associated with higher utilization of opioid and guideline-recommended medications. Patients with more medical problems and comorbid psychiatric diagnoses would be expected to have more pain and higher pain medication utilization.[17] Interestingly, high household income was associated with a higher transition to chronic opioid therapy. The implication of this result is that the prescription opioid epidemic in peripheral neuropathy patients does not preferentially affect the poor.
Pain specialists were not associated with less opioid therapy; although this is likely at least partly explained by differences in unmeasured patient characteristics.[5] However, the magnitude of the associations makes interventions to increase pain specialist referrals unlikely to succeed in reducing opioid prescriptions. Older age, Hispanic ethnicity, and Asian race were all associated with lower utilization of pain specialists. For older patients and Hispanics this is likely not secondary to less severe pain as previously stated. However, given that pain specialists are associated with both more guideline-recommended and opioid medications, these differences are unlikely to lead to meaningful changes in long term pain management given the downsides of chronic opioid therapy. Targeting interventions to pain specialists may be promising given the potential for their practice patterns to influence other physicians.
Limitations include the use of claims data to diagnose peripheral neuropathy, which may lead to misclassification bias. However, a previous study revealed that 93.4% of patients meeting this definition had peripheral neuropathy after medical record review and only 5.1% of controls not meeting this definition had peripheral neuropathy.[15] Moreover, we are unable to link the medications specifically as treatments for peripheral neuropathy, but we were able to exclude patients with other indications for chronic pain. Claims data does not have detailed clinical information to allow judgment of appropriate clinical decision making in individual patients. However, these data can provide important information on the typical prescribing practice patterns in peripheral neuropathy patients to identify areas of potential improvement. Furthermore, the guideline-recommended medications have other indications such as epilepsy and anxiety/depression, but exclusion of treatment for these conditions would only have accentuated the finding of opioid medication use early and often for peripheral neuropathy prior to these medications. The findings pertaining to pain specialists need to be interpreted with caution given that patients that see pain specialists are likely different than those that do not based on unmeasured variables. For our analyses pertaining to opioid therapy, we could not exclude patients with acute or chronic post-traumatic pain, but we were able to exclude many chronic pain conditions.[8] Current analyses focused on logistic regression models. Future studies could take advantage of the longitudinal data available.
Peripheral neuropathy patients receive opioids early and often in the treatment of their pain. Few patients try multiple guideline-recommended medications despite the large proportion that end up on chronic opioid therapy. Decreasing opioid prescriptions and increasing guideline-recommended medications should be the focus of future intervention efforts. Without interventions, opioid prescriptions in the peripheral neuropathy population are likely to continue to rise.
Acknowledgements:
None
Study Funding:
Dr. Callaghan is supported by a NIH K23 grant (NS079417) and a VA CSRD Merit (CX001504). Dr. Kerber is supported by NIH/NCRR K23 RR024009, AHRQ R18 HS017690, NIH/NIDCD R01DC012760, and AHRQ R18HS022258. Dr. Skolarus is supported by NIH/NIMHD R01MD00887, NIH/NIMHD R01MD011516, NIH/NMHD U01MD010579. Dr. Burke is supported by NINDS K08 NS082597 and NIH/NIMHD R01MD00887.
Footnotes
Disclosures:
Dr. Callaghan receives research support from Impeto Medical Inc. He performs medical consultations for Advance Medical, consults for a PCORI grant, consults for the immune tolerance network, and performs medical legal consultations. Dr. Skolarus has received compensation from Bracket Global. Dr. Burke has received compensation from Astra Zeneca for his role on the adjudication committee of the SOCRATES trial. Drs. Banerjee, Kerber, and Mr. Reynolds report no disclosures.
References
- [1].Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Supplement 1986;3:S1–226. [PubMed] [Google Scholar]
- [2].Attal N, Cruccu G, Baron R, Haanpaa M, Hansson P, Jensen TS, Nurmikko T. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol 2010;17(9):1113–e1188. [DOI] [PubMed] [Google Scholar]
- [3].Attal N, Cruccu G, Baron R, Haanpaa M, Hansson P, Jensen TS, Nurmikko T, European Federation of Neurological S. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. European journal of neurology 2010;17(9):1113–e1188. [DOI] [PubMed] [Google Scholar]
- [4].Bril V, England J, Franklin GM, Backonja M, Cohen J, Del Toro D, Feldman E, Iverson DJ, Perkins B, Russell JW, Zochodne D. Evidence-based guideline: Treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2011;76(20):1758–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Callaghan BC, Burke JF, Kerber KA, Skolarus LE, Ney JP, Magliocco B, Esper GJ. The association of neurologists with headache health care utilization and costs. Neurology 2018;90(6):e525–e533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Callaghan BC, Price RS, Feldman EL. Distal Symmetric Polyneuropathy: A Review. Jama 2015;314(20):2172–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ. Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabet Med 2004;21(9):976–982. [DOI] [PubMed] [Google Scholar]
- [8].Davis JA, Robinson RL, Le TK, Xie J. Incidence and impact of pain conditions and comorbid illnesses. J Pain Res 2011;4:331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. Jama 2016;315(15):1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. The Lancet Neurology 2015;14(2):162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Forrest KY, Maser RE, Pambianco G, Becker DJ, Orchard TJ. Hypertension as a risk factor for diabetic neuropathy: a prospective study. Diabetes 1997;46(4):665–670. [DOI] [PubMed] [Google Scholar]
- [12].Franklin GM. Opioids for chronic noncancer pain: a position paper of the American Academy of Neurology. Neurology 2014;83(14):1277–1284. [DOI] [PubMed] [Google Scholar]
- [13].Franklin GM, American Academy of N. Opioids for chronic noncancer pain: a position paper of the American Academy of Neurology. Neurology 2014;83(14):1277–1284. [DOI] [PubMed] [Google Scholar]
- [14].Griebeler ML, Morey-Vargas OL, Brito JP, Tsapas A, Wang Z, Carranza Leon BG, Phung OJ, Montori VM, Murad MH. Pharmacologic interventions for painful diabetic neuropathy: An umbrella systematic review and comparative effectiveness network meta-analysis. Annals of internal medicine 2014;161(9):639–649. [DOI] [PubMed] [Google Scholar]
- [15].Hoffman EM, Staff NP, Robb JM, St Sauver JL, Dyck PJ, Klein CJ. Impairments and comorbidities of polyneuropathy revealed by population-based analyses. Neurology 2015;84(16):1644–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hoffman EM, Watson JC, St Sauver J, Staff NP, Klein CJ. Association of Long-term Opioid Therapy With Functional Status, Adverse Outcomes, and Mortality Among Patients With Polyneuropathy. JAMA neurology 2017;74(7):773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. The journal of pain : official journal of the American Pain Society 2015;16(8):769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Patil PR, Wolfe J, Said Q, Thomas J, Martin BC. Opioid use in the management of diabetic peripheral neuropathy (DPN) in a large commercially insured population. The Clinical journal of pain 2015;31(5):414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical care 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- [20].Sands ML, Shetterly SM, Franklin GM, Hamman RF. Incidence of distal symmetric (sensory) neuropathy in NIDDM. The San Luis Valley Diabetes Study. Diabetes Care 1997;20(3):322–329. [DOI] [PubMed] [Google Scholar]
- [21].Visser NA, Notermans NC, Linssen RS, van den Berg LH, Vrancken AF. Incidence of polyneuropathy in Utrecht, the Netherlands. Neurology 2015;84(3):259–264. [DOI] [PubMed] [Google Scholar]
- [22].Waldfogel JM, Nesbit SA, Dy SM, Sharma R, Zhang A, Wilson LM, Bennett WL, Yeh HC, Chelladurai Y, Feldman D, Robinson KA. Pharmacotherapy for diabetic peripheral neuropathy pain and quality of life: A systematic review. Neurology 2017;88(20):1958–1967. [DOI] [PubMed] [Google Scholar]


