Abstract
Parkinson’s disease (PD) is the second-most common progressive neurodegenerative disorder. Although the clinical diagnosis of PD is still based on its cardinal motor dysfunctions, several non-motor symptoms (NMS) have been established as integral part of the disease. Unlike motor disorders, development of therapies against NMS are still challenging and remain a critical unmet clinical need. During the last decade, several studies have characterised the molecular, physiological and behavioural alterations of the circadian system in PD patients. As a consequence, and given the ubiquitous nature of circadian rhythms in the entire organism, the biological clock has emerged as a potential therapeutic target to ease suffering from both motor and NMS in PD patients. Here we discuss the emerging field of using bright light, physical exercise and melatonin as chronotherapeutic tools to alleviate motor disorders, sleep/wake alterations, anxiety and depression in PD patients. We also highlight the potential of these readily available therapies to improve the general quality of life and wellbeing of PD patients. Finally, we provide specific data- and mechanisms-driven recommendations that might help improve the therapeutic benefit of light and physical exercise in PD patients.
Keywords: Light therapy, Chronobiology, Non-motor symptoms, Exercise, Sleep, Circadian system
1. Introduction
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disease and affects between 2 and 3% worldwide of the population over the age of 65 (Poewe et al., 2017; Schapira et al., 2017). Clinically, PD is a multisystem disease characterised by motor and non-motor disabilities (Poewe et al., 2017). The main motor symptoms consist of bradykinesia, resting tremor, rigidity and postural instability while the non-motor symptoms span a wide range of dysfunctions including mood and other neuropsychiatric alterations, cardiovascular and respiratory dysfunctions, impaired olfaction, gastrointestinal problems, circadian and sleep/wake cycle alterations (Poewe et al., 2017; Schapira et al., 2017). Several of these non-motor symptoms are manifest during the prodromal stage of the disease and will inevitably worsen over disease progression to ultimately dominate the clinical picture at advanced stages.
PD remains an incurable neurodegenerative disease (Poewe et al., 2017). Since its introduction 50 years ago, L-DOPA remains the most effective therapeutic option to alleviate motor symptoms of the illness (Poewe et al., 2017). However, the beneficial effect of L-DOPA wanes over time and, on the average, 5 years after initiation of L-DOPA intake, motor fluctuations and adventitious involuntary movements re-emerge. Moreover, additional non-motor adverse effects such as excessive daytime sleepiness and dopamine dysregulation syndrome have been associated with dopaminergic (DA) medications (Arnulf and Leu-Semenescu, 2009). To overcome these side effects of DA medications, the development of surgical deep brain stimulation (DBS) has led to the alleviation of both the intrinsic motor abnormalities and the side effects related to long term DA intake (Fasano et al., 2012). This remarkable achievement was tempered by the discovery of behavioral problems related to DBS such as abulia, depression, reduced verbal fluency, weight gain and difficulty with social adjustment (Krack et al., 2003). Rare fatalities such as intracerebral haemorrhages and suicide have also been reported (Voon et al., 2008). Additionally, both DA replacement medication and DBS do not alleviate the wide spectrum of non-motor symptoms including sleep and circadian dysfunctions that may dominate the clinical picture of the disease in advanced stages (Chaudhuri et al., 2006; Chaudhuri and Shapira, 2009; Fasano et al., 2012). Rather DA medications contribute negatively to the exacerbation of these non-motor disorders particularly in advanced stages (Chaudhuri and Shapira, 2009). Only few pharmacological agents are available for the management of the NMS of PD. Currently, therapeutics trategies that would target NMS along with neuroprotective or disease-modifying therapies remain a critical unmet clinical need (Meissner et al., 2011). In view of the diverse and common occurrence of NMS, patients with PD frequently take numerous medications, which itself is a well-established risk factor for adverse drug reactions and serious side effects in elderly (Lai et al., 2012; Chang et al., 2012).
Emerging evidence points to utility of non-pharmacological treatment approaches in PD. Among these, light therapy and physical exercise are well-tolerated and widely available treatment choices. As an alternative to polypharmacy, light therapy was introduced as an adjuvant to L-DOPA medication in PD. The aim of this article is to discuss the rational, safety and efficiency of using bright light and physical exercise as adjuvant therapies in PD. Caveats and consequently, recommendations to improve these emerging therapies will be highlighted.
2. The Rationale of light therapy use in Parkinson’s Disease
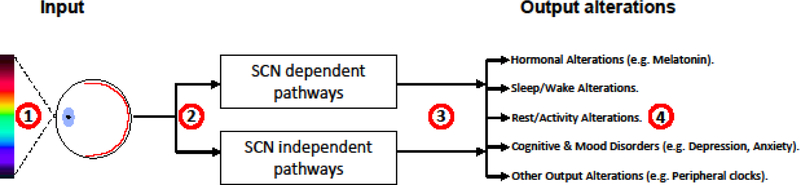
Alterations of the circadian system are increasingly being recognized as non-motor manifestations of PD. Though still a new emerging field of research, alterations of the biological clock at the behavioural, physiological and molecular levels have been recently reported in PD (Box 1, Videnovic and Golombek, 2013; Videnovic et al., 2014; Videnovic and Willis, 2016; Mattis and Sehgal, 2016; Musiek and Holtzman, 2016; La Morgia et al., 2017; Li et al., 2017; Videnovic and Golombek, 2017; De Pablo-Fernández et al., 2017; Fifel, 2017). The circadian system is essentially a genetically encoded anticipatory mechanism that governs physiological homeostasis (Lowrey and Takahashi, 2004; Bell-Pedersen et al., 2005; Takahashi et al., 2008). In mammals, the central clock in the suprachiasmatic nucleus (SCN) plays a central role in the synchronisation of multiple central and peripheral clocks in a coherent timing system (Bell-Pedersen et al., 2005; Takahashi et al., 2008). To achieve this, the SCN integrates photic information received from the specialized photoreceptor cells expressing the pigment melanopsin in the retina and then uses both neural and humoral signals in order to orchestrate the temporal organisation of circadian rhythmicity at the molecular, physiological and organismal levels (Bell-Pedersen et al., 2005; Takahashi et al., 2008). In addition to light, which is the main circadian synchronizer, the circadian system receives and integrates several non-photic stimuli such as alternating cycles of feeding/fasting and exercise (Dibner et al., 2010; Sulli et al., 2018). Although still mechanistically not fully understood, a proper interplay of all these photic and non-photic cues both in time and space is essential for physiological homeostasis and good health (Bass and Lazar, 2016).
Box 1: Typology of the Circadian system in Parkinson’s Disease.
Although the pace of acquiring scientific knowledge about the circadian system in PD has been slow and lagging long behind the motor aspects of the disease, a growing number of studies have described several aspects of health alterations in PD patients directly linked with the circadian timing system (Videnovic and Golombek, 2013; Videnovic et al., 2014; Videnovic and Willis, 2016; Mattis and Sehgal, 2016; Musiek and Holtzman, 2016 La Morgia et al., 2017; Li et al., 2017; Videnovic and Golombek, 2017; De Pablo-Fernández et al., 2017; Fifel, 2017). The scope of these alterations encompasses several behavioural, physiological and molecular circadian overt-rhythms. The most evident behavioural alteration of the circadian system in PD consists of the progressive deterioration of the quality of sleep/wake and rest/activity cycles (Arnulf and Leu-Semenescu, 2009; Schapira et al., 2017). Fragmentation of sleep/wake behaviour as well as intrusions of abnormal motor actions into the resting phase of the light/dark cycle are common complaints of PD patients. Worsening of these symptoms over disease progression can even lead to a dramatic drop in the amplitude of rest/activity cycle (Niwa et al., 2011; Fifel, 2017; Madrid-Navarro et al., 2018). In severe advanced stages, this might manifest as a quasi-arrhythmia (Niwa et al., 2011). At the physiological level, alteration of several biological rhythms has been described in PD. These include; reduced amplitude of 24h corticol (Hartmann et al., 1997) and melatonin release rhythms (Videnovic et al., 2014; Breen et al., 2014), phase advance of melatonin rhythm (Bordet et al., 2003; Bolitho et al., 2014), Reversal or complete loss of both the daily rhythm of blood pressure (Ejaz et al., 2006; Vetrano et al., 2015) and heart rate variability (Stuebner et al., 2013), dampening of the daily rhythm of urine excretion (Hineno et al., 1994) and the alterations of rhythmic modulation of visual contrast sensitivity (Struck et al., 1990). In de novo medication-free PD patients, no alterations in the 24h circadian profile of growth hormone, thyrotropin and prolactin were observed (Aziz et al., 2011a). Similar results were found in another study of newly diagnosed PD patients for several metabolic markers including; adipokines, leptin, adiponectin and resistin (Aziz et al., 2011b). Molecular studies investigating clock genes in PD patients are sparse (Cai et al., 2010; Ding et al., 2011; Breen et al., 2014). Nevertheless, available data extracted from clock genes expression in blood mononuclear cells showed either a dampening (Cai et al., 2010) or a complete loss of the 24h rhythmic expression of BMAL1 (Breen et al., 2014). Other genes (Per2 and REV-ERBs) showed also altered rhythmic expressions (Ding et al., 2011; Breen et al., 2014). Collectively, all these studies strongly reveal alteration of several aspects of the circadian system in PD patients.
Another emerging feature from these studies is the high heterogeneity in the nature and the extent of circadian alterations in both central and peripheral organs. Recently, Roenneberg and Merrow proposed a comprehensive taxonomy of circadian alterations in which they describe potential states of circadian systems in the SCN and peripheral circadian clocks both in health and disease (Roenneberg and Merrow, 2016). Below, we apply this taxonomy in order to summarise circadian alterations in PD patients based on currently available data. This taxonomy is composed of four possible states for circadian systems (Fig. A); green icons (state 1) represent optimal entrainment in the whole body, blue icons (state 2) refer to rhythms that show abnormal phase relationship to their respective zeitgebers (light, food, endogenous signals). Gray icons (state 3) represent free running rhythms that failed to entrain to their zeitgebers. Such state has not been described in PD patients so far. The last state (state 4) is shown by red icons and refer to a complete loss of rhythmicity.
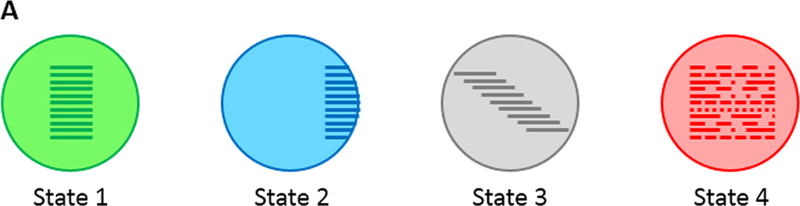
The state of any circadian system can be described as a mixture of these potential states (Roenneberg and Merrow, 2016). In the figures below, the circadian state of the SCN is represented by the larger symbol on the left while the state of peripheral clock is displayed as the small symbol(s) to the right of the larger symbol.
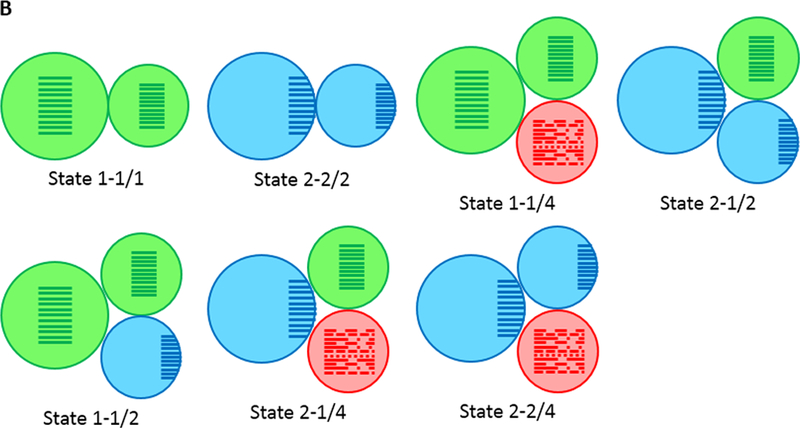
If all peripheral tissues and organs show the same state, a single icon is indicated. If different tissues or functions adopt different relative entrained phases, then two icons are indicated. Years before developing PD, subjects would show optimal entrainment (state 1–1/1 or 2–2/2 for extreme chronotypes). Before, and up to diagnosis, during which PD patients show normal entrainment of several physiological functions (for a review, see Fifel, 2017), sleep/wake behaviour might show a fragmented pattern (state 1–1/4; although the degree of fragmentation does not reach behavioural arrhythmia). When PD patients start to take dopamine medications, a phase advance in melatonin rhythm has been reported in some studies (Bordet et al., 2003; Bolitho et al., 2014). The pathophysiology of this circadian alteration is currently unknown (Fifel, 2017). This could either reflect an abnormal expression of clock genes in the SCN (state 2–1/2) or an abnormal entrainment of peripheral clocks in output structures responsible for the generation of melatonin rhythm (state 1–1/2). During disease progression, several motor and non-motor symptoms deteriorate (Maetzler et al., 2009). These impairments have been shown to negatively impact the general quality of life in PD patients (Videnovic et al., 2014; Fifel, 2017) and exacerbate the alterations of both the amplitude and entrainment of several circadian rhythms (states 1–2/4, 2–1/4, 2–2/4).
As mentioned before, a high degree of variability in the alterations of the circadian system is evident among PD patients (Fifel and Deboer, 2014; Fifel, 2017), it is therefore more likely to regard these different states as a plastic continuum that might change as a function of the influence of internal (i.e. biology) and external factors (i.e. environment and medication) behind the development of PD. Fortunately, such plasticity constitute also as asset allowing the application of chronotherapies for PD.
The ubiquitous nature of circadian rhythmicity at every level of organisation and throughout the body and their intertwined functional connections imply that any internal mismatch between these circadian cycles might precipitate health disorders (Bass and Lazar, 2016; Sulli et al., 2018). Indeed, a plethora of diseases have been associated with circadian disruption as a result of the chronic challenge to the circadian system imposed by our modern life style (light pollution, Jet travel, social jet-lag, light emitting devices and 24/7 food availability, (Bass and Lazar, 2016; Sulli et al., 2018). Because multiple external stimuli can independently modulate the circadian system, several circadian-based strategies have been developed to restore proper circadian functioning in several diseases (Schroeder and Colwell, 2013; Sulli et al., 2018). The same scientific rationale underlies the use of light as a therapeutic tool to treat or at least improve motor and non-motor symptoms of PD. While light may exhibit its effects on physiology and behaviour via circadian mechanisms, it is important to also point to the Potential alerting effects of light via mechanisms that are not well defined to date (Terman and Terman, 2005; LeGates et al., 2014).
Because of the debilitating effects of motor and NMS, PD patients generally adopt a sedentary lifestyle which, through vicious cycles (Fifel, 2017), aggravates the quality of their life (Speelman et al., 2011; Fifel, 2017). As a consequence of this sedentary lifestyle, PD patients spend most of their time indoors and are therefore likely to be exposed to lower intensities of light. Additionally, optic neuropathy is well documented in PD patients (Archibald et al., 2009). This neuropathology encompasses retinal degeneration of both dopaminergic amacrine cells as well as retinal ganglion cells (Archibald et al., 2009). Furthermore, α-synuclein deposition, which is a hallmark of PD neuropathology, have been found in the retina of PD patients (Beach et al., 2014; Ortuño-Lizarán et al., 2018a). Recently, by examining retinas from advanced PD patients, Ortuño-Lizarán et al., 2018 reported a significant 33.3 % decrease in the density of ipRGCs in PD patients relative to age-matched controls. This study also reported significant morphological impairments in the remaining ipRGCs in retinas from PD patients. These alterations manifested as a significant decrease in the number of intersections, branches and terminals in PD melanopsin ganglion cells (Ortuño-Lizarán et al., 2018b). Degeneration and impairment of ipRGCs in PD might therefore contribute to decrease sensitivity to light and consequently dysfunctional melanopsin-mediated responses. Collectively, all these factors strongly point to the possibility that PD patients might be amenable to and benefit from light therapy.
3. Light therapy in Parkinson’s disease: State of the art
Light is the most potent environmental cue regulating majority of behavioural and physiological functions (LeGates et al., 2014). Accordingly, light therapy has been widely applied to improve several disorders including circadian misalignment, depression, sleep disorders and cognitive dysfunction (Schroeder and Colwell, 2013; LeGates et al., 2014). All these disturbances may co-exist with PD syndrome (Chaudhuri et al., 2006; Chaudhuri and Shapira, 2009) and are therefore expected to benefit from appropriate light regimes. Potential insights from the use of light therapy in animal models of PD are discussed in Box 2. In the PD population, six studies so far have assessed the safety and therapeutic efficacy of light therapy (Willis and Turner, 2007; Paus et al., 2007; Willis et al., 2012; Rios Romenets et al., 2013; Videnovic et al., 2017; Martino et al., 2018). In 2007, two studies were published (Willis and Turner, 2007; Paus et al., 2007). Willis et al. exposed 12 PD patients to 1–1.5h of bright light pulses of 1.000–1.500 Lux in intensity 1h before bedtime during 1 to 5 weeks (Willis and Turner, 2007). The investigators reported significant improvement of motor function including bradykinesia and rigidity. Tremor was not improved by light therapy. Bright light therapy had an anti-depressant effect that lasted several weeks following treatment and was exteriorised by increased socialisation. Subjective self-assessment of sleep habits showed also significant improvement (Willis and Turner, 2007).
Box 2: Light therapy in animal models of PD.
In the field of neurodegenerative diseases, animal models are valuable tools enabling progress in both our mechanistic understanding of pathology and the development of efficient therapies (Dawson et al., 2018; Fifel et al., 2016). Currently, the mainstay symptomatic therapies used for PD patients (i.e. L-dopa and DBS) have been developed and refined using rodent and non-human primate models of PD (Duty & Jenner, 2011). However, all attempts to develop disease-modifying therapies using current models have failed, not only for PD, but also for other neurodegenerative disorders (Dawson et al., 2018; Fifel et al., 2016). As we discuss in the main text, the available clinical trials in PD patients suggest the potential use of bright light as both a symptomatic and a disease-modifying therapy. It is surprising and unfortunate to discover therefore that, to date, no single study has assessed the impact of bright light therapy on both motor and non-motor aspects of PD using current animal models. By comparison, two recent reports have shown that chronic administration of bright light significantly improves motor performances, sleep/wake cycle and survival in mouse models of Huntington’s disease (Wang et al., 2017; Ouk et al., 2017). In PD, the only reports available relate to the intracranial administration of Near-infrared Light (NiL) in MPTP- and 6OHDA-treated animal models (Johnstone et al., 2016). Despite its promising potential, the low energy of NiL necessitates DBS-like invasive probes to effectively reach the midbrain. Additionally, the restriction of NiL therapy to midbrain DA structures limits the potential of this type of light therapy to interfere with the widespread neuropathology in several extra-DA areas. Given these technical limitations, it is necessary to conduct experiments in which the therapeutic potential of NiL therapy is compared with the safer application of bright light therapy. Performing such preclinical studies using animal model of PD will reveal added values of NiL therapy relative to bright light therapy (if any) and help justify any translation to human trials. In the meantime, preclinical light therapy studies with animal models of PD should capitalize on results obtained from published clinical trials in order to fine-tune our current protocols (Fifel and Videnovic, 2018). Given the sensitivity of several components of the dopaminergic neurotransmission to ambient light (see section 4.3), we do expect clinically important insights from testing light therapy in preclinical animal models of PD.
Another study by Paus et al. was a randomized, placebo-controlled and double-blind pilot clinical trial of light therapy (Paus et al., 2007). The experimental group consisting of 18 PD patients received 30 min of bright light of 7.500 lux and was compared to 18 PD patients receiving placebo light pulses of 950 lux. Light was administered 1h after awakening daily for 2 weeks. Patients receiving bright light showed improvement in depression. With the exception of a slight attenuation of tremor, no improvements were reported in motor symptoms (i.e. bradykinesia and rigidity). Regarding the sleep-wake cycles, the function assessed was excessive daytime sleepiness that improved with light therapy in both the active and placebo group with no significant in between-group difference. Although showing promising results, the main limitation of these two studies was the short-term duration of light administration which may explain the modest treatment effects and preclude any conclusion regarding the safety and efficacy of the desirable long-term administration of light therapy to PD patients in routine clinical settings (Willis and Turner, 2007; Paus et al., 2007).
A larger retrospective study was conducted and published by Willis et al. in 2012 (Willis et al., 2012). 129 participants with PD received light therapy pulses at a dose of 4.000–6.000 lux daily, one hour prior to bedtime. A crucial finding of this study was the significant and persistent improvement in both motor and non-motor symptoms as long as the patients remained adherent to light therapy program. Beneficial effects were most significant among patients that fully adhered to the intervention compared to both semi-adherent patients and PD patients that withdrew prematurely from the program. The motor symptoms that showed improvement were; bradykinesia, tremor, rigidity, nocturnal movement, dyskinesia, and balance. The improved non-motor symptoms included; insomnia, anxiety and depression. As expected, in patients that withdrew from the program, all these manifestations deteriorated over the study period. Whether the benefits observed in this study resulted fully or in part from interference with the neurodegeneration processes or were fully symptomatic remains to be determined. The recovery rate depended on the symptoms; for example, anxiety and insomnia improved first (within one month), while the improvement of the motor symptoms lagged but, once present, persisted over the course of several months to years. The main limitation of this study was its un-blinded study design (Willis et al., 2012).
More recently, the same group published results of another large and long-term retrospective study involving 140 PD patients (Martino et al., 2018). In this study, the authors focused mainly on the impact of a daily exposure to a 1h light pulse of intensity ranging from 3.000 to 4.000 Lux on insomnia and REM Behaviour Disorder (RBD). Light pulses were administered 1 to 4 hours before habitual sleep onset. Similar to the previous study (Willis et al., 2012), the authors described a progressive and persistent improvement of insomnia. This positive response was induced in as little as one month after light therapy initiation and persisted for long durations as long as the patients adhered to light therapy protocol (up to 6 years). Comparable dynamic was also observed for the reduction of the RBD symptoms. Although not systematically analysed, this study also reported a less fragmented sleep/wake behaviour after exposure to light pulses. In addition of its design being also open-label, the main limitation of this study is the use of questionnaire to subjectively assess sleep quality. No EEG polysomnography was used. Nevertheless, this study is the first to suggest that the most debilitating sleep disorder found in some PD patients (i.e. RBD) might benefit from light therapy.
Rios Romenets et al. compared the efficiency of a combined cognitive behaviour therapy and bright light therapy with the effects of a pharmacological treatment using doxepin (Rios Romenets et al., 2013). Although the authors assessed multiple sleep and behavioural outcomes, the main focus of the study was to evaluate the effects of two study interventions on insomnia in PD patients. With the combination of cognitive behavioural therapy and sleep hygiene recommendations, pulses of bright light of 10.000 lux during 30 min were administered either in the morning (for patients suffering from sleep onset insomnia) or in the afternoon (for patients complaining from sleep maintenance insomnia) for 6 weeks. The results revealed a significant improvement in the insomnia severity index by bright light therapy, which was comparable to the improvement induced by doxepin. No improvements were observed in daytime sleepiness, fatigue, depression and sleep quality. Although actigraphy measurements showed no significant changes, the motor part (Part III) of the Unified Parkinson’s Disease Rating Scale (UPDRS) scale revealed a worsening of the motor functions over the treatment period which was in contrast with the stability of the motor function in the patients receiving doxepin (Rios Romenets et al., 2013). Given the intrinsic variability in PD-related phenotypes as well as their underlying pathophysiology (Thenganatt, and Jankovic, 2014), the main limitation of this study is the very small study cohort (n=6 per group; Rios Romenets et al., 2013). Additionally, the choice of the time of light administration was not based on any objective physiological marker of the endogenous circadian clock (Rios Romenets et al., 2013). Furthermore, the combination of light therapy with cognitive behavioural therapy in this study makes it impossible to decipher the relative contribution of light therapy to the behavioural component of the intervention. Combined, these limitations restrict the interpretation of the results and preclude strong conclusions regarding the efficacy of bright light therapy in PD.
PD patients suffer from several sleep abnormalities (Chahine et al., 2017). These include excessive daytime sleepiness, insomnia, sleep fragmentation, REM sleep behaviour disorder and restless legs syndrome (Chahine et al., 2017). A recent meta-analysis of studies investigating therapeutic effects of light therapy on sleep problems has shown the effectiveness of bright light in improving sleep quality (Van Maanen et al., 2016). All the PD-related studies discussed so far have assessed the efficacy of light on either excessive daytime sleepiness (EDS) (Paus et al., 2007) or insomnia (Willis et al., 2012). Willis and Turner interviewed patients about the general quality of their sleep without emphasis on specific aspects of sleep alterations (Willis and Turner, 2007). More recently, in a randomized clinical trial, Videnovic et al. assessed the safety and efficacy of light therapy for impaired sleep-wake cycles in PD (Videnovic et al., 2017). EDS was assessed using Epworth Sleepiness Scale (ESS), and sleep quality with the Pittsburgh Sleep Quality Index (PSQI) and Parkinson’s disease Sleep Scale (PDSS). Self-reported study assessments included sleep diaries and visual analog scales for daytime sleepiness. In addition, actigraphy was used to estimate characteristics of sleep/wake cycle and as a more objective assessment of the general activity of the patients. Bright light pulses of 1-hour duration and intensity of 10.000 lux were administered to 16 PD patients twice daily over 14 days. As a control condition, a dim-red light of an intensity of less than 300 lux was used in a separate group of PD patients (n=15). Participants treated with bright light therapy had significant improvements in EDS and insomnia. Sleep became more consolidated as shown by the significant decrease in the number of overnight awakenings. Additionally, light therapy was associated with increased daily physical activity as revealed by actigraphy. This improvement in motor activity was corroborated by significant improvement in several motor items of the UPDRS. Remarkably, these improvements in sleep-wake cycle, especially in excessive daytime sleepiness persisted at a follow-up examination, two weeks after discontinuation of light therapy (Videnovic et al., 2017).
Rutten et al. published recently a study protocol of a double-blind randomized controlled trial to assess the effect of bright light therapy on depression, sleep/wake behaviour and the biological circadian system (Rutten et al., 2016). This study has addressed several limitations of earlier studies and controlled for several parameters. The result of this ongoing study will therefore be more informative about the potential of light therapy in PD patients. A large cohort (n=84) of PD patients were exposed twice a day to 30 min of 10.000 light pulses during 3 months. The administration of the morning light pulse was determined based on the individual chronotype of PD patients. The blind study design was achieved by installing neutral density filters on the control light device so that it appears identical to the experimental device when switched-off. When switched on, and at a distance of 30 cm, the experimental device emits light with the intensity of 10.000 lux while at a distance of 40 cm, the control device emits light at an intensity of 200 lux. Despite these technical measures, and because the reduced intensity of light could point out that the subjects in the control condition were not receiving bright light therapy, the authors are planning to control for this potential bias by rating the expectances of PD subjects before the start of the treatment. The primary outcome measure that will be assessed in this study is the change in depressive symptoms relative to baseline. Additionally, the acute and long-term effects of light on the motor symptoms, the subjective (questionnaire-based) and objective (actigraphy-based) quality of sleep as well as on the robustness of circadian of circadian rhythms. Given its long post-treatment follow-up period (6 months), this study will also reveal how long the beneficial effect of bright light on motor and NMS of PD lasts, which might inform about the optimal frequency of light administration in future applications.
Light therapy has been used for over 30 years as adjuvant therapy for several medical conditions including depression, circadian sleep phase disorders, cognitive alterations and many more (Terman and Terman, 2005; Terman, 2007; Van Maanen et al., 2016). One of the many advantages of light therapy over medication is the transient and benign nature of the side-effects (Terman and Terman, 2005). A consistent finding in all studies that assessed the safety of light therapy in PD is the excellent toleration of the therapy. Like is other patients (Terman and Terman, 2005), headache, hypomania, agitation, sleepiness and fine tremor were reported in PD patients shortly after the initiation of light treatment (Willis et al., 2012; Videnovic et al., 2017). However, these side effects were short lived and dissipated within days even in the PD population studies by Willis et al. for up to 8 years (Willis et al., 2012; Videnovic et al., 2017). These results corroborate the findings from other studies in which bright light was administered chronically in both middle-age and elderly patients for several years (Gallin et al., 1995; Riemersma-van der Lek et al., 2008) suggesting therefore the safety of chronic application of light therapy in PD patients.
4. Light therapy in Parkinson’s disease: Unresolved questions and recommendations
Although the results of the studies discussed so far point to a potential therapeutic benefit of bright light therapy for both motor and non-motor symptoms in patients with PD, the field is still in its infancy and several critical questions remain. One of the major unresolved question related to light therapy in PD is related to its dose properties that must be defined and optimized similar to any other pharmacological and non-pharmacological treatment approach. This encompasses duration, timing, frequency and spectral properties and intensity of light therapy. Below we discuss recommendations and areas requiring further research in order to improve the efficacy of light therapy in PD (Fig. 1).
Figure 1. Potential mechanisms of the therapeutic effect of light therapy and physical exercise on motor and NMS in PD.
Exposure to bright light can improve motor and multiple NMS in PD patients, however, the optimal dose of light for PD patients in terms of intensity, time and duration of exposure and spectral proprieties still need improvements (1). Additionally, the mechanisms of light therapy in PD are still poorly understood (2). While PD patients suffer from several NMS, the impact of light was investigated so far only on motor disorders, sleep alterations, anxiety and depression. Future studies should also study the effect of light therapy on other NMS (3). Preclinical as well as clinical studies suggest potential benefit of timed physical exercise on several health parameters (Mrosovsky and Salmon, 1987; Van Reeth and Turek, 1989; Van Someren et al., 1997; Hillman et al., 2008; Reid et al., 2010; Speelman et al., 2011; Hughes and Piggins, 2012; Speelman et al., 2014). The translation of these findings into PD patients might prove beneficial (4). All these limitations are still halting the optimal benefit from these chronotherapies. Ongoing progress in this field is expected to optimize these therapies. The implementation of improved protocols in the clinic holds the promise of significantly improving the quality of life in PD patients (see text for details).
4.1. Defining the optimal dose of light therapy in PD
Although the therapeutic mechanism of light is unlikely to be restricted to its impact on the circadian system (see below), the therapeutic outcomes of light exposure depend on the relative time of administration within the 24h day (Terman and Terman, 2005). This is related to the fact that the magnitude and direction of phase-resetting is dependent on the circadian phase at which the light stimulus occurs. The circadian system is most sensitive to light during the biological night, during which most individuals are sleeping in darkness. Therefore, bright light exposure is most effective in normalizing circadian misalignment if administered during twilight time either shortly after awakening, or shortly before bedtime. In humans, and most diurnal animals, early morning light therapy will advance the endogenous clock while light exposure in the evening is more likely to delay circadian rhythms (Terman and Terman, 2005). Therefore, depending on the individual chronotype of PD patients, the time of light exposure should be selected accordingly. Light exposure to PD patients 1h after awakening improved depression, quality of life and tremor without affecting bradykinesia and insomnia (Paus et al., 2007) while the application of bright light 1h before bedtime improved, in addition of mood, insomnia, bradykinesia and rigidity in PD patients (Willis and Turner, 2007; Willis et al., 2012). These results suggest that exposure of light at different times of the day yields different clinical outcomes. However, these results could also suggest a degree of heterogeneity among PD patients in the nature and the degree of alterations of the circadian system. Indeed, recent studies investigating circadian alterations in PD patients revealed variability in the degree of alterations (different degrees of amplitude reduction) as well as in the nature of the circadian desynchronisation of the circadian system in PD patients (unaffected, delayed or advanced rhythms) (reviewed in Videnovic and Golombek, 2013; Videnovic et al., 2014; Videnovic and Willis, 2016; Mattis and Sehgal, 2016; La Morgia et al., 2017; Li et al., 2017; Videnovic and Golombek, 2017; De Pablo-Fernández et al., 2017; Fifel, 2017; Musiek and Holtzman, 2016). Like in depressed patients (Terman and Terman, 2005; Terman, 2007), this heterogeneity would make PD patients benefit the most from light exposure at different times of the day. Practical solution to this issue would require personalized monitoring of the chronotype of PD patients using for example reliable physiological markers such as melatonin onset, which correlates with a high degree of accuracy with the internal clock. Alternatively, the Morning-Evening Questionnaire (MEQ) might be used. However, given the severe alterations of circadian rhythms (Videnovic and Golombek, 2013; Videnovic et al., 2014; Videnovic and Willis, 2016; Mattis and Sehgal, 2016; La Morgia et al., 2017; Li et al., 2017; Videnovic and Golombek, 2017; De Pablo-Fernández et al., 2017; Fifel, 2017; Musiek and Holtzman, 2016) and sleep/wake cycle in PD patients (Arnulf and Leu-Semenescu, 2009; Poewe et al., 2017; Schapira et al., 2017), a validation of the MEQ against more reliable markers (i.e. melatonin onset or temperature) is necessary before its usage in order to estimate the chronotype of PD patients. These easily-administered tools have not been implemented in the six studies conducted so far with PD. An alternative approach to overcome these costly measures would be to expose patients to regular and robust 12h/12h Light/dark cycles or to two bright pulses per day (1 in the morning and 1 in the evening) instead of single pulse at different times of the day. Such protocols would reinforce temporal patterning of SCN oscillation, strengthen entrainment to the external world, and better orchestrate temporal organisation of the internal physiology. Indeed, evidence for such beneficial reinforcement of the circadian system by robust LD cycles are substantial from both animal (Schroeder and Colwell, 2013; Lucassen et al., 2016) and human studies including elderly patients (Riemersma-van der Lek et al., 2008; Schroeder and Colwell, 2013).
Independent of its synchronization effects on circadian rhythms, exposure to bright light is associated with an acute increase in subjective, as well as objective measures of alertness and arousal (LeGates et al., 2014, Rahman et al., 2017). Recent studies indicate that bright light enhances alertness, improves reaction times in behavioural tasks and induces changes in the waking electroencephalogram (EEG) that are consistent with increased arousal. These studies suggest a wake promoting effect of bright light exposure that is independent of its circadian-mediating effects (Rahman et al., 2017). Based on this arousal effect of bright light exposure, we would expect insomnia that PD patients suffer from to be exacerbated. Paradoxically however, studies that examined this (Willis et al., 2007; Willis et al., 2012; Martino et al., 2018) have shown that bright light exposure before bedtime rather mitigated insomnia in PD patients. thorough mechanistic investigation of these findings might contribute to fine-tuning protocols of light therapy in PD. Such knowledge is expected to also shed some light on the potential contribution of reduced sensitivity or exposure to environmental light to excessive daytime sleepiness by PD patients.
Efficacy of light therapy is also modulated by the intensity, duration and spectral proprieties of the administered light (Terman and Terman, 2005; Terman, 2007; LeGates et al., 2014). The intensity of the light pulses used so far ranges from 1.000 to 10.000 with a duration of either 30 min (Paus et al., 2007; Rios Romenets et al., 2013), 1h (Willis and Turner, 2007; Willis et al., 2012; Videnovic et al., 2017; Martino et al., 2018) or 1.5h (Willis and Turner, 2007). The intensities used is probably based on field studies showing that exposure to bright artificial light (typically 2.500–10.000 lux) improves sleep-wake quality and mood (Terman and Terman, 2005; Terman, 2007). However, several clinical studies have shown that exposure to bright monochromatic blue light (460nm) is more effective that other wavelengths (like green light; 555nm) at phase-resetting the circadian clock and suppressing the release of melatonin during night-time from the pineal gland (Rahman et al., 2017). Given the mediation of light therapy by blue light-sensitive photopigment melanopsin (Güler et al., 2008), whether PD patients will benefit most from blue-enriched light despite suffering from several retinopathologies (Archibald et al., 2009; Beach et al., 2014) is still unknown. Given the limited number of studies as well as their limitations, it is still premature to draw reliable conclusion regarding the optimal parameters of light administration in PD patients. Large clinical trials with multiple treatment arms will be required to this end. None of the studies have introduced variations in the wavelength spectrum in order to assess the optimal wavelengths with the best therapeutic benefits (Fig. 1).
4.2. Alterations of the circadian system in PD patients and its relevance for circadian-based treatments in PD
Recently, several studies have documented diverse molecular, physiological and behavioural disruptions of the circadian system in PD patients (Box 1, Fifel, 2017). detailed description of these circadian alterations is beyond the scope of this article, and interested readers are referred to recent excellent reviews on the topic (Videnovic and Golombek, 2013; Videnovic et al., 2014; Videnovic and Willis, 2016; Mattis and Sehgal, 2016; Musiek and Holtzman, 2016; La Morgia et al., 2017; Li et al., 2017; Videnovic and Golombek, 2017; De Pablo-Fernández et al., 2017; Fifel, 2017). The main conclusions of these studies are; first, the nature and the degree of circadian disruptions are heterogeneous among PD patients and second; the neuroanatomical sites underlying these alterations are unknown. Both of these limitations have direct implications on the efficiency of the implementation of bright light therapy in PD patients. As mentioned before, well designed (and if necessary personalised) protocols of light administration might practically remedy the phenotypic and neuropathological variability in circadian alterations observed in patients with PD (Thenganatt and Jankovic, 2014). Furthermore, there is a need of additional detailed and multicenter investigations of different aspects of the circadian system in PD with emphasis on the underlying neurophysiology. Such knowledge is also pivotal for the optimization of light therapy protocols. For example, and as mentioned before, significant degeneration and morphological impairments of retinal photosensitive melanopsin ganglion cells, which constitute the neuronal pathway mediating photic entrainment (Güler et al., 2008), have just been recently reported in PD (Ortuño-Lizarán et al., 2018b). In line with these anatomical findings, ipRGC-mediated post-illumination pupil response to blue light exposure was found to be impaired in PD (Joyce et al., 2018). More precisely, a 14.73% reduction in the sustained constriction of pupil diameter was found in PD patients relative to controls (Joyce et al., 2018). Theoretically, with this knowledge it is possible to estimate the magnitude and spectral proprieties of light sensitivity in PD patients and adjust the parameters of light therapy accordingly. Similarly, the involvement of the central clock in the SCN vs downstream central and peripheral structures over PD progression is still not fully understood. Answering these questions might inform us about the optimal wavelength spectrum of light with potentially the maximum therapeutic benefit.
4.3. Mechanisms of bright light therapy with specific relevance to PD
The mechanism behind the therapeutic effect of bright light is not fully understood. Light, by acting directly on the circadian system, consolidates and strengthens the internal synchrony and coordination of cellular processes, physiological functions and behaviours with the external light/dark cycle (Takahashi et al., 2008; Dibner et al., 2010; Schroeder and Colwell, 2013; LeGates et al., 2014; Bass and Lazar, 2016). However, none of the six studies conducted so far have specifically assessed the impact of light therapy on molecular and/or physiological overt rhythms in order to verify whether light exerts its effects by correcting the internal desynchrony of physiological rhythms in PD. If it is the case, one needs, furthermore, to show that the degree of correction of circadian alterations correlates with the magnitude of improvement. In patients suffering from winter depression, in which such studies and analysis have been conducted, it has been shown that the impact of light on the circadian timing system accounts for only 14% of the variance suggesting the involvement of other circadian-independent mechanisms of light therapy (Terman et al., 2001). The results of the ongoing study by Rutten et al. (2016) are expected to shed some light on the relative contribution of circadian-based vs circadian-independent processes to the therapeutic effect of light in PD patients.
Another potential mechanism of bright light worth further investigation is the possible enhancement of the dopaminergic neurotransmission. This mechanism is supported by multiple lines of evidence. First, electrophysiological studies in rodents showed an acute activatory response of dopamine-containing regions following brief exposure to light pulses (Nieoullon et al., 1977; Dommett et al., 2005; Schultz et al., 2009). These neural responses were short-lived, declining few milliseconds after light offset, which implies that light directly influences these regions through short-distant relays (Comoli et al., 2003). Second, light therapy has been shown to remarkably increase L-DOPA efficacy in patients with PD (Willis and Turner, 2007; Willis et al., 2012). Patients under bright light therapy significantly and stably reduced the amount of L-DOPA required to 50% while maintaining therapeutic efficacy compared to a 17% increase observed in unexposed patients (Willis and Turner, 2007; Willis et al., 2012). Further evidence for this light enhancing effect of DA signalling comes from studies showing a seasonal modulation of DA neurotransmission with an up-regulation during summer compared to winter (Eisenberg et al., 2010; Tsai et al., 2011). Recently, bright light therapy was shown to normalize the abnormal rest/activity rhythms induced by winter-like short photoperiod in a diurnal rodent (i.e. Arvicanthis ansorgei). Interestingly, these impacts were paralleled with the strengthening and normalisation of the circadian modulation of DA signalling in the striatum (Itzhacki et al., 2018). onversely, chronic light deprivation for 6 weeks in rats induces neurodegeneration of monoaminergic neuronal systems (including DA neurons) which was associated with depressive behavioural phenotype (Gonzalez et al., 2008). Collectively, all these studies indicate that ambient lighting conditions affect the DAergic system. Additional investigation of how light enhances DA neuro-signalling will help to further improve the efficacy of light therapy in PD patients and likely in many other neurodegenerative disorders in which DA signalling is impaired.
Finally, and in specific relation to PD, light therapy might nurture positive feedbacks from improved motor and non-motor symptoms of the disease following light treatment. The progressive impairment of motor and several non-motor symptoms over disease progression in PD contributes, through negative feedbacks, to the alteration of circadian rhythms (Fifel, 2017). The potential improvement of motor functions, depression, anxiety and sleep/wake behavior by light therapy in PD patients in expected to feedback positively on health in general by improving and normalizing the circadian organization of physiological functions. The extend of this mechanism in improving the quality of life in PD patients has, however, not yet been investigated.
4.4. Should Bright Light Therapy focus on non-motor symptoms, motor symptoms, or both?
As stated before, PD is characterised by a plethora of non-motor symptoms (Chaudhuri et al., 2006; Chaudhuri and Schapira, 2009). So far, the impact of bright light has been assessed only on a few non-motor symptoms namely; sleep/wake behaviour, anxiety and depression (Willis and Turner, 2007; Paus et al., 2007; Willis et al., 2012; Rios Romenets et al., 2013; Videnovic et al., 2017; Martino et al., 2018). Although the effects of bright light on sleep have been evaluated using valid questionnaires, there is a need to objectively assess how bright light improves sleep alterations in PD using EEG polysomnography. Large scale and controlled trial in demented elderly have demonstrated the efficiency of bright light therapy to significantly attenuate cognitive decline and positively influence mood (Riemersma-van der Lek et al., 2008). It is therefore reasonable to expect similar benefits for cognitive dysfunctions seen in patients with PD. Similarly, given the already shown beneficial effect of bright light on L-DOPA efficiency and dosage, it might be expected that bright light therapy would at least attenuate the highly debilitating side effect of dopamine therapy in PD such as involuntary movements, hallucinations and psychosis (Chaudhuri et al., 2006; Chaudhuri and Schapira, 2009; Poewe et al., 2017). Therefore, it is necessary to investigate in future studies the impact of bright light, not only on motor dysfunctions, but also on the wide range of non-motor symptoms affecting PD patients.
5. Chronotherapeutic potential of Exercise in Parkinson’s disease
A second complementary approach that has shown efficiency in improving the quality of life in patients with PD is physical activity and exercise. A recent review listed 10 potential reasons why an active lifestyle might benefit patients with PD (Speelman et al., 2011). Interestingly, many symptoms that responded positively to exercise overlap with the symptoms sensitive to light therapy. These include cognitive function, mood, sleep, motor performance, dopaminergic signalling and L -DOPA efficacy. Additionally, osteoporosis, cardiovascular function, constipation and fatigue improved significantly after physical training (Speelman et al., 2011). Adaptive neuroplasticity defined as the dynamic capacity of the brain to adjust through neuronal re-organisation, is thought to be the main mechanism through which aerobic physical training exerts its effects (Hillman et al., 2008). Another, yet not systematically investigated approach to further improve the benefit of physical activity in patients with PD is the use of timed physical activity as non-photic stimuli to boost physiological resonance of the circadian system. Several studies both in rodents and humans suggest indeed the presence of a specific time window where the benefits of exercise are maximal (reviewed in Hughes and Piggins, 2012; Schroeder and Colwell, 2013). Based on our current knowledge, it is difficult to propose standard schedules for physical exercise in PD patients. This difficulty stems from at least 3 facts. First, like light therapy, the heterogenous nature of circadian insults in PD will make patients benefit the most from exercise at different times of the day (Fifel and Deboer, 2014; Fifel, 2017). Second, although it is established that nocturnal exercise can phase delay the clock, reports of exercise-induced phase advances of circadian rhythms are rarer (Atkinson et al., 2007). Finally, the best timing of exercise therapy will depend also on the symptoms being targeted. For example, although both exercise in the morning and late afternoon improved cognitive abilities in aged individuals, late-afternoon exercise improved more measures of cognitive abilities (Benloucif et al., 2004). Similarly, in older adults with insomnia, sleep quality also improved when exercise was performed during the afternoon or early evening (Reid et al., 2010). However, long-term fitness exercise in the middle of the day improved the consolidation and robustness of sleep/wake cycle in older men (Van Someren et al., 1997). Given the many insults on circadian neurocircuitry in PD (Fifel, 2017), it is unlikely that exercise will have similar impacts on PD patients as in healthy elderly. It will be therefore more efficient to personalize the timing of physical exercise after careful and thorough examination of medical needs of PD patients. Such approaches are needed to reveal the importance of physical exercise as a major non-photic synchronizing agent in the PD population. A major barrier towards the implementation of physical training as a long-lasting lifestyle change in patients with PD is not only the motor disability, but also the diverse non-motor problems (Speelman et al., 2011). In particular, anxiety and fear of falling should be taken into account. These hurdles can, however, be overcome through specific coaching and counselling as has been shown recently by the positive outcomes of the ParkFit study (Speelman et al., 2014).
Interestingly, behavioral studies on the interactions between light and physical activity on one hand and sleep, cognition, the circadian system and physiology on the other showed that these interactions likely operate multi-directionally and dynamically influence each other in complex ways (see Box 3 for bidirectional interactions between the circadian system and sleep/wake behaviour). For example, in rodents the latency to fully re-entrain to the 8h phase advance of the LD cycle (which simulate the jetlag encountered by humans following trans-meridian air travels) can be dramatically reduced from 8 to 2–3 days by 3 h of locomotor activity at the beginning of the dark phase on the first day in the new LD cycle (Mrosovsky and Salmon, 1987; Van Reeth and Turek, 1989). In aged demented patients, chronic supplementation of bright light and melatonin yielded better outcomes on sleep, cognitive abilities and quality of life than did bright light or melatonin alone (Riemersma-van der Lek et al., 2008). Conversely, improving the quality of sleep can also improve health. Evidence for such positive feedbacks comes from animal and, more recently, human studies (reviewed in Schroeder and Colwell, 2013). In a mouse model of Huntington’s disease, both the circadian system and the sleep/wake behavior deteriorate as the disease progresses (Pallier et al., 2007; Pallier and Morton, 2009). Pharmacological improvement of sleep/wake cycles in these animals improved significantly health span, cognition and motor abilities (Pallier et al., 2007; Pallier and Morton, 2009). In humans, better sleep consolidation attenuated the risk of Alzheimer’s disease and reduced age-related cognitive decline and the development of neurofibrillary tangle pathology in individuals carrying the apolipoprotein E ɛ4 allele and are therefore at higher risk for incident Alzheimer’s disease (Lim et al., 2013). Similar follow-up studies have not yet been conducted with PD patients. Taken together however, these tight multidirectional feedbacks and modulations between different systems provide a promising opportunity to improve health in general using bright light and physical training in patients with PD.
Box 3: Sleep regulation and its dysfunction in PD.
Models of sleep regulation have emphasized two distinct processes for the regulation of the timing of sleep: a homeostatically regulated sleep process and a circadian oscillatory process (Achermann and Borbély, 2011). The circadian oscillator is responsible for the tendency to sleep during specific windows of the 24-hour cycle and by opposing homeostatic pressure gates consolidated bouts of sleep and wakefulness. The sleep homeostat in contrast is responsible for monitoring and adjusting the duration and intensity of sleep depending on prior amounts of sleep and wakefulness.
In contrast to our advanced molecular and mechanistic understanding of the circadian system and the potential neural pathways by which the central clock regulates the timing of sleep-wake behaviour (Mistlberger, 2005; Takahashi et al., 2008), the brain structures and mechanisms that regulate the homeostatic sleep drive remain unclear. However, there is increasing evidence that a build-up of a substantial number of sleep-initiating metabolic by-products (e.g. adenosine and GABA) in specific brain regions (especially the basal forebrain) provides the molecular basis for sleep propensity during wakefulness (Zeitzer et al., 2006; Wigren et al., 2007; Landolt, 2008; Krueger et al., 2008). The production of these sleep-inducing endogenous metabolites during wakefulness is proportional to the duration and intensity of wakefulness. These metabolites, upon reaching a critical level, act by suppressing activity in wake-promoting structures leading to the initiation of sleep (Datta and Maclean, 2007).
Until recently, circadian and homeostatic processes were thought to act independently of each other in the regulation of sleep-wake behaviour (Yasenkov and Deboer, 2012). Several studies however have provided compelling evidence that sleep feeds back to the clock. Scheduling of sleep affects circadian phase in blind patients (Klerman et al., 1998) and night shift workers (Santhi et al., 2005). Sleep deprivation under dim light conditions modulates the phase of the melatonin rhythm (Cajochen et al., 2003). Similarly, sleep deprivation without exercise in the Syrian hamster induces a phase shift in their rest/activity cycle and suppresses expression of the immediate early gene c-fos in the SCN (Antle and Mistlberger, 2000). Gradual shifts of sleep timing lead to changes in the phase of body temperature and melatonin rhythms (Danilenko et al., 2003). The most direct evidence that the quality of sleep modulates the proprieties of the central clock was obtained in rats by simultaneous recording of EEG and SCN electrical neuronal activity (Deboer et al., 2003). Sleep states were found to affect electrical activity in SCN neurons. Neuronal firing rates were high during wakefulness and REM sleep and low during NREM sleep. During selective NREM sleep deprivation, SCN activity remained high and failed to show the normal decrease in firing rate, whereas during REM sleep deprivation, SCN neuronal firing remained low and failed to display increases in firing rates (Deboer et al., 2003). Similar functional feedbacks of sleep and wakefulness states on the activity of the SCN area have also been shown in humans (Schmidt et al., 2009). Through these modulatory effects on the excitability of SCN neurons, sleep may influence directly the genetic clockwork of the SCN (Colwell, 2011). Altogether then, it has become clear that sleep and the circadian system are highly intertwined and that the interactions between the two are continuously bidirectional.
PD patients complain from several alterations of their sleep/wake behaviour (Arnulf and Leu-Semenescu, 2009; Schapira et al., 2017). These alterations include; insomnia, excessive daytime sleepiness, restless legs syndrome and RBD (Arnulf and Leu-Semenescu, 2009). Although the pathophysiology of sleep/wake disturbances is still poorly understood, available evidence points to a multifactorial origin implicating dysfunctional central sleep regulatory areas (Arnulf and Leu-Semenescu, 2009), adverse effects of anti-parkinsonian drugs and secondary negative feedbacks of motor and several non-motor symptoms of the disease (Arnulf and Leu-Semenescu, 2009).
The alterations of sleep in PD patients in both quality and quantity might also reflect dysfunctions in homeostatic and circadian processes of sleep regulation. A growing number of studies have shown the extent of circadian alterations in PD patients (Box 1). Several components of the neuronal circuitry governing circadian rhythms have been shown to be dysfunctional or even displaying signs of neurodegeneration (Arnulf and Leu-Semenescu, 2009). Less in known about the homeostatic drive of sleep/wake cycle in PD patients. Although adenosine signalling is known to be alerted in the striatum of PD patients (Nazario et al., 2017), whether it is affected in key sleep centers such as basal forebrain is unknown. Additionally, the time course of purinergic signalling alteration over disease progression is currently unknown. The elucidation of the contribution of dysfunctional homeostatic processes to sleep alterations in PD relative to other factors (i.e. circadian alterations and/or neurodegeneration of sleep centers) will be crucial in the development of successful therapies.
6. Therapeutic effects of melatonin intake in Parkinson’s Disease
The powerful antioxidant property of melatonin (Pandi-Perumal et al., 2013) as well as its role in the internal synchronisation of circadian and sleep/wake rhythms (Lewy et al., 2006; Hardeland, 2012) have made it a therapeutic option as both a neuroprotective and a chronotherapeutic drug. Accordingly, melatonin has been successfully used to treat insomnia and alleviate, at least partially, circadian alterations in older adults (Lewy et al., 2006). In patients with PD, only two studies have assessed the efficacy of melatonin to alleviate sleep/wake cycle alterations (Dowling et al., 2005; edeiros et al., 2007). Both study protocols were based on the soporific action of melatonin, rather on its effects as a modulator of the circadian system. In the first study, melatonin at doses ranging from 5 to 50 mg/day was administered 30 min before bedtime for a period of 2 weeks (Dowling et al., 2005). The second study used 3 mg of melatonin, 1h before bedtime over 4 weeks (Medeiros et al., 2007). Both studies reported significant improvement in the subjective quality of sleep (Dowling et al., 2005; Medeiros et al., 2007). Although statistically significant, clinical improvement manifested as increases in total sleep time for 10 minutes in one of these studies diminishes the enthusiasm for this medication (Dowling et al., 2005). Furthermore, when sleep was assessed objectively using polysomnography, no beneficial effects on sleep were observed (Medeiros et al., 2007). Motor symptoms were unchanged with administration of melatonin (Medeiros et al., 2007). Although both studies reported that melatonin, even at high dosage, was tolerated, the short duration of the treatment (maximum of 4 weeks) precludes conclusions about long-term tolerability. Indeed, when melatonin was taken alone for longer period by elderly people, it was shown to adversely affect mood (Riemersma-van der Lek et al., 2008). These negative effects were counteracted by the combination of melatonin intake with bright light therapy (Riemersma-van der Lek et al., 2008). These findings suggest that the combination of light and melatonin therapies might be more successful than light administration or melatonin intake alone. Based on current evidence, it is not clear whether melatonin has beneficial effects on sleep-wake cycles in PD patients. Further trials using the newly developed melatonin agonists might yield better outcomes (Schroeder and Colwell, 2013).
7. Concluding remarks and future perspectives
Parkinson’s disease is a multisystem disorder characterised clinically by motor and non-motor symptoms. The significant improvement in symptomatic therapy of motor difficulties have been tempered recently by the recognition of the debilitating aspect of several non-motor symptoms particularly in advanced stages of the disease (Chaudhuri et al., 2006; Chaudhuri and Schapira, 2009; Fasano et al., 2012; Poewe et al., 2017). Specific management of these non-motor features is still very poor, and the efficiency of available treatments is still limited by their negative side effects (Chaudhuri et al., 2006; Chaudhuri and Schapira, 2009; Fasano et al., 2012; Poewe et al., 2017). As a remedy to this situation, and taking advantage of the accumulated data on its application over 30 years is psychiatric and other clinical settings (Terman and Terman, 2005; Terman, 2007; Van Maanen et al., 2016), bright light therapy has been introduced as an adjuvant to pharmacological therapies in PD. The inexpensive and non-invasive nature of light therapy and exercise makes them attractive supplemental therapies to potentially postpone the onset of parkinsonism or even prevent the manifestations of multiple non-motor symptoms. This possibility is all the more pertinent given the emerging strategies used to identify people who are at high risk of developing PD (Stephenson et al., 2009; Sherer, 2011; Chen-Plotkin, 2014). Implementation of bright light and exercise therapies in such individuals as a pre-emptive strategy could potentially confer beneficial results. Because of its non-invasive nature, this strategy does not face the ethical issues related to the long-term side effects associated with chemical-based neuroprotective therapies (Obeso et al., 2010). However, no study has examined the chronotherapeutic potential of exercise in PD and regarding light therapy, over the last decade, only six studies have been published (Willis and Turner, 2007; Paus et al., 2007; Willis et al., 2012; Rios Romenets et al., 2013; Videnovic et al., 2017; Martino et al., 2018) and one double-blind randomized controlled trial is currently underway (Rutten et al., 2016). Yet these studies provide the ‘proof of concept’ that light therapy could be a powerful tool to ease suffering from motor and multiple non-motor symptoms of PD. Future studies should focus on optimizing the protocol as well as the parameters of light exposure before the expected broad application of bright light therapy yield better outcomes in improving symptoms and quality of life or even slow down disease progression in patients with PD (Fifel and Videnovic, 2018).
Acknowledgments
K.F. gratefully acknowledges support during the writing of this article from the European Community’s Marie Sklodowska-Curie IEF Programme under contract 655135, “The Role of Dopamine in the Regulation of Sleep and Circadian Rhythms (CIRCADOPAMINE)” and from the Japanese Society for the Promotion of Science (JSPS) under contract P18073. A.V. received research grant support from the National Institutes of Health (R01NS099055).
Glossary
- PD
Parkinson’s disease
- NMS
Non Motor Symptoms
- DBS
Deep Brain Stimulation
- SCN
suprachiasmatic nucleus
- UPDRS
Unified Parkinson’s Disease Rating Scale
- REM
Rapid Eye Movement
- EDS
Excessive Daytime Sleepiness
- ESS
Epworth Sleepiness Scale
- PSQI
Pittsburgh Sleep Quality Index
- MEQ
Morning-Evening Questionnaire
- EEG
Electroencephalogram
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests
The authors declare no conflict of interests.
References
- Achermann P, Borbély AA, 2011. Sleep homeostasis and models of sleep regulation. In: Principles and practice of sleep medicine, Ed 5 (Kryger MH, Roth T, Dement WC, eds), pp 431–444. St. Louis: Saunders. [Google Scholar]
- Antle MC, Mistlberger RE, 2000. Circadian clock resetting by sleep deprivation without exercise in the Syrian hamster. J. Neurosci 20, 9326–9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald NK, Clarke MP, Mosimann UP, Burn DJ, 2009. The retina in Parkinson’s disease. Brain 132, 1128–1145. [DOI] [PubMed] [Google Scholar]
- Arnulf I, Leu-Semenescu S, 2009. Sleepiness in Parkinson’s disease. Parkinsonism Relat. Disord 15, S101–104. [DOI] [PubMed] [Google Scholar]
- Atkinson G, Edwards B, Reilly T, Waterhouse J, 2007. Exercise as a synchroniser of human circadian rhythms: an update and discussion of the methodological problems. Eur. J. Appl. Physiol 99, 331–341. [DOI] [PubMed] [Google Scholar]
- Aziz NA, Pijl H, Frolich M, Roelfsema F, Roos RA 2011b. Leptin, adiponectin, and resistin secretion and diurnal rhythmicity are unaltered in Parkinson’s disease. Mov. Disord 26, 760–761. [DOI] [PubMed] [Google Scholar]
- Aziz NA, Pijl H, Frolich M, Roelfsema F, Roos RA, 2011a. Diurnal secretion profiles of growth hormone, thyrotrophin and prolactin in Parkinson’s disease. J. Neuroendocrinol 23, 519–524. [DOI] [PubMed] [Google Scholar]
- Bass J, Lazar MA, 2016. Circadian time signatures of fitness and disease. Science 354, 994–999. [DOI] [PubMed] [Google Scholar]
- Beach TG, Carew J, Serrano G, Adler H, Shill H, Sue LI, Sabbagh MN, Akiyama H, Cuenca N, Arizona Parkinson’s Disease Consortium., 2014. Phosphorylated α-synuclein-immunoreactive retinal neuronal elements in Parkinson’s disease subjects. Neurosci. Lett 13, 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ, 2005. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nature Rev. Genet 6, 544–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benloucif S, Orbeta L, Ortiz R, Janssen I, Finkel SI, Bleiberg J, Zee PC, 2004. Morning or evening activity improves neuropsychological performance and subjective sleep quality in older adults. Sleep 271542–1551. [DOI] [PubMed]
- Bolitho SJ, Naismith SL, Rajaratnam SM, Grunstein RR, Hodges JR, Terpening Z, Rogers N, Lewis SJ 2014. Disturbances in melatonin secretion and circadian sleep-wake regulation in Parkinson disease. Sleep Med 15, 342–347. [DOI] [PubMed] [Google Scholar]
- Bordet R, Devos D, Brique S, Touitou Y, Guieu JD, Libersa C, Destée A, 2003. Study of circadian melatonin secretion pattern at different stages of Parkinson’s disease. Clin. Neuropharmacol 26, 65–72. [DOI] [PubMed] [Google Scholar]
- Breen P, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB, Barker RA, 2014. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol 71, 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Liu S, Sothern RB, Xu S, Chan P, 2010. Expression of clock genes Per1 and Bmal1 in total leukocytes in health and Parkinson’s disease. Eur. J. Neurol 17, 550–554. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Jewett ME, Dijk DJ, 2003. Human circadian melatonin rhythm phase delay during a fixed sleep-wake schedule interspersed with nights of sleep deprivation. J. Pineal. Res 35, 149–157. [DOI] [PubMed] [Google Scholar]
- Chahine LM, Amara AW, Videnovic A, 2017. A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Med. Rev 35, 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YP, Huang SK, Tao P, Chien CW, 2012. A population-based study on the association between acute renal failure (ARF) and the duration of polypharmacy. BMC Nephrol 30, 13:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri KR, Healy DG, Schapira AH, National Institute for Clinical Excellence. 2006. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 5, 235–245. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Schapira AH, 2009. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 8, 464–474. [DOI] [PubMed] [Google Scholar]
- Chen-Plotkin AS, 2014. Unbiased approaches to biomarker discovery in neurodegenerative diseases. Neuron 84: 594–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS 2011. Linking neural activity and molecular oscillations in the SCN. Nat. Rev. Neurosci 12, 553–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoli E, Coizet V, Boyes J, Bolam JP, Canteras NS, Quirk RH, Overton PG, Redgrave P, 2003. A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nat. Neurosci 6, 974–980. [DOI] [PubMed] [Google Scholar]
- Danilenko KV, Cajochen C, Wirz-Justice A, 2003. Is sleep per se a zeitgeber in humans? J. Biol. Rhythms 18, 170–178. [DOI] [PubMed] [Google Scholar]
- Datta S, Maclean RR 2007. Neurobiological mechanisms for the regulation of mammalian sleep-wake behavior: reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neurosci. Biobehav. Rev 31, 775–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Golde TE, Lagier-Tourenne C, 2018. Animal models of neurodegenerative diseases. Nat. Neurosci 21, 1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pablo-Fernández E, Breen DP, Bouloux PM, Barker RA, Foltynie T, Warner TT,. 2017. Neuroendocrine abnormalities in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 88, 176–185. [DOI] [PubMed] [Google Scholar]
- Deboer T, Vansteensel MJ, Détári L, Meijer JH, 2003. Sleep states alter activity of suprachiasmatic nucleus neurons. Nat. Neurosci 6, 1086–1090. [DOI] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U, 2010. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol 72, 517–549. [DOI] [PubMed] [Google Scholar]
- Ding H, Liu S, Yuan Y, Lin Q, Chan P, Cai Y, 2011. Decreased expression of Bmal2 in patients with Parkinson’s disease. Neurosci. Lett 499, 186–188. [DOI] [PubMed] [Google Scholar]
- Dommett E, Coizet V, Blaha CD, Martindale J, Lefebvre V, Walton N, Mayhew JE, Overton PG, Redgrave P, 2005. How visual stimuli activate dopaminergic neurons at short latency. Science 307, 1476–1479. [DOI] [PubMed] [Google Scholar]
- Dowling GA, Mastick J, Colling E, Carter JH, Singer CM, Aminoff MJ, 2005. Melatonin for sleep disturbances in Parkinson’s disease. Sleep Med 6, 459–466. [DOI] [PubMed] [Google Scholar]
- Duty S, Jenner P, 2011. Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol 164, 1357–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DP, Kohn PD, Baller EB, Bronstein JA, Masdeu JC, Berman KF, 2010. Seasonal effects on human striatal presynaptic dopamine synthesis. J. Neurosci 30, 14691–14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejaz AA, Sekhon IS, Munjal S, 2006. Characteristic findings on 24-h ambulatory blood pressure monitoring in a series of patients with Parkinson’s disease. Eur. J. Intern. Med 17, 417–420. [DOI] [PubMed] [Google Scholar]
- Fasano A, Daniele A, Albanese A, 2012. Treatment of motor and non-motor features of Parkinson’s disease with deep brain stimulation. Lancet Neurol 11, 429–442. [DOI] [PubMed] [Google Scholar]
- Fifel K, 2017. Alterations of the circadian system in Parkinson’s disease patients. Mov. Disord 32, 682–692. [DOI] [PubMed] [Google Scholar]
- Fifel K, DeBoer T, 2014. The central clock in patients with Parkinson disease. JAMA Neurol 71, 1455–1456. [DOI] [PubMed] [Google Scholar]
- Fifel K, Piggins H, Deboer T, 2016. Modeling sleep alterations in Parkinson’s disease: How close are we to valid translational animal models? Sleep Med. Rev 25, 95–111. [DOI] [PubMed] [Google Scholar]
- Fifel K, Videnovic A, 2018. Light Therapy in Parkinson’s Disease: Towards Mechanism-Based Protocols. Trends Neurosci 41, 252–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin PF, Terman M, Remé CE, Rafferty B, Terman JS, Burde RM, 1995. “Ophthalmologic examination of patients with seasonal affective disorder, before and after bright light therapy.” American Journal of Ophthalmology 119, 202–210. [DOI] [PubMed] [Google Scholar]
- Gonzalez MM, Aston-Jones G, 2008. Light deprivation damages monoamine neurons and produces a depressive behavioral phenotype in rats. Proc. Natl. Acad. Sci. USA 105, 4898–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güler AD, Ecker JL, Lall GS, Haq S, Altimus M, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S, 2008. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature 453, 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeland R, 2012. Melatonin in aging and disease -multiple consequences of reduced secretion, options and limits of treatment. Aging Dis 3, 194–225. [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Veldhuis JD, Deuschle M, Standhardt H, Heuser I, 1997. Twenty-Four hour cortisol release profiles in patients with Alzheimer’s and Parkinson’s Disease compared to normal controls: ultradian secretory pulsatility and diurnal variation. Neurobiol. Aging 18, 285–289. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF, 2008. Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci 9, 58–65. [DOI] [PubMed] [Google Scholar]
- Hineno T, Mizobuchi M, Nishimatsu O, Horiguchi J, Kakimoto Y, 1994. Day-night variation of urine volume in Parkinson’s disease. Jpn J. Psychiatry Neurol 48, 583–587. [DOI] [PubMed] [Google Scholar]
- Hughes AT, Piggins HD 2012. Feedback actions of locomotor activity to the circadian clock. Prog. Brain Res 199, 305–336. [DOI] [PubMed] [Google Scholar]
- Itzhacki J, Clesse D, Goumon Y, Van Someren EJ, Mendoza J, 2018. Light rescues circadian behavior and brain dopamine abnormalities in diurnal rodents exposed to a winter-like photoperiod. Brain Struct. Funct 223, 2641–2652. [DOI] [PubMed] [Google Scholar]
- Johnstone DM, Moro C, Stone J, Benabid AL, Mitrofanis J, 2016. Turning On Lights to Stop Neurodegeneration: The Potential of Near Infrared Light Therapy in Alzheimer’s and Parkinson’s Disease. Front. Neurosci 11, 9:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce DS, Feigl B, Kerr G, Roeder L, Zele AJ, 2018. Melanopsin-mediated pupil function is impaired in Parkinson’s disease. Sci. Rep 17, 8(1):7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerman EB, Rimmer DW, Dijk DJ, Kronauer RE, Rizzo JF 3rd, Czeisler CA, Nonphotic entrainment of the human circadian pacemaker. Am. J. Physiol 274, R991–R996. [DOI] [PubMed] [Google Scholar]
- Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P,2003. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 349, 1925–1934. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Rector DM, Roy S, Van Dongen HP, Belenky G, Panksepp J, 2008. Sleep as a fundamental property of neuronal assemblies. Nat. Rev. Neurosci 9, 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Morgia C, Ross-Cisneros FN, Sadun A, Carelli V, 2017. Retinal Ganglion Cells and Circadian Rhythms in Alzheimer’s Disease, Parkinson’s Disease, and Beyond. Front Neurol 8, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SW, Lin CH, Liao KF, Su LT, Sung F Lin C, 2012Association between polypharmacy and dementia in older people: a population-based case-control study in Taiwan. Geriatr. Gerontol. Int 12, 491–498. [DOI] [PubMed] [Google Scholar]
- Landolt HP 2008. Sleep homeostasis: a role for adenosine in humans? Biochem. Pharmacol 75, 2070–2079. [DOI] [PubMed] [Google Scholar]
- LeGates TA, Fernandez DC, Hattar S, 2014. Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci 15, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Emens J, Jackman A, Yuhas K, 2006. Circadian uses of melatonin in humans. Chronobiol. Int 23, 403–412. [DOI] [PubMed] [Google Scholar]
- Li S, Wang Y, Wang F, Hu LF, Liu CF, 2017. A New Perspective for Parkinson’s Disease: Circadian Rhythm. Neurosci. Bull 33, 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AS, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA, 2013. Modification of the relationship of the apolipoprotein E _4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol 70, 1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS, 2004. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu. Rev. Genomics Hum. Genet 5, 407–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen EA, oomans CP, van Putten M, de Kreij SR, van Genugten JH, Sutorius RP, de Rooij KE, van der Velde M, Verhoeve SL, Smit JW, Löwik CW, Smits HH, Guigas B, Aartsma-Rus AM, Meijer JH 2016. Environmental 24-hr Cycles Are Essential for Health. Curr. Biol 26, 1843–1853. [DOI] [PubMed] [Google Scholar]
- Madrid-Navarro J, Escamilla-Sevilla F, Mínguez-Castellanos A, Campos M, Ruiz-Abellán F, Madrid JA, Rol MA, 2018. Multidimensional Circadian Monitoring by Wearable Biosensors in Parkinson’s Disease. Front. Neurol 26, 9:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maetzler W, Liepelt I, Berg D, 2009. Progression of Parkinson’s disease in the clinical phase: potential markers. Lancet Neurol 8, 1158–1171. [DOI] [PubMed] [Google Scholar]
- Martino JK, Freelance CB, Willis GL, 2018. The effect of light exposure on insomnia and nocturnal movement in Parkinson’s disease: an open label, retrospective, longitudinal study. Sleep Med 44, 24–31. [DOI] [PubMed] [Google Scholar]
- Mattis J, Sehgal A, 2016. Circadian Rhythms, Sleep, and Disorders of Aging. Trends Endocrinol. Metab 27, 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros CA, Carvalhedo de Bruin PF, Lopes LA, Magalhães MC, de Lourdes Seabra M, de Bruin VM, 2007. Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson’s disease: A randomized, double blind, placebo-controlled study. J. Neurol 254, 459–464. [DOI] [PubMed] [Google Scholar]
- Meissner WG, Frasier M, Gasser T, Goetz G, Lozano A, Piccini P, Obeso JA, Rascol O, Schapira A, Voon V, Weiner DM, Tison F, Bezard E, 2011. Priorities in Parkinson’s disease research. Nat. Rev. Drug Discov 10, 377–393. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE 2005. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res. Brain Res. Rev 49, 429–454. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Salmon PA, 1987. A behavioural method for accelerating re-entrainment of rhythms to new light-dark cycles. Nature 330, 372–373. [DOI] [PubMed] [Google Scholar]
- Musiek ES, Holtzman DM, 2016. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 354, 1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazario LR, da Silva RS, Bonan CD, 2017. Targeting Adenosine Signaling in Parkinson’s Disease: From Pharmacological to Non-pharmacological Approaches. Front. Neurosci 23, 11:658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieoullon A, Cheramy A, Glowinski J, 1977. Nigral and striatal dopamine release under sensory stimuli. Nature 269, 340–342. [DOI] [PubMed] [Google Scholar]
- Niwa F, Kuriyama N, Nakagawa M, Imanishi J, 2011. Circadian rhythm of rest activity and autonomic nervous system activity at different stages in Parkinson’s disease. Auton. Neurosci 165, 195–200. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Goetz CG, Marin C, Kordower JH, Rodriguez M, Hirsch EC, Farrer M, Schapira AH, Halliday G, 2010. Missing pieces in the Parkinson’s disease puzzle. Nat. Med 16, 653–661. [DOI] [PubMed] [Google Scholar]
- Ortuño-Lizarán I, Beach G, Serrano GE, Walker DG, Adler CH, Cuenca N, 2018a. Phosphorylated α-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Mov. Disord 33, 1315–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortuño-Lizarán I, squiva G, Beach TG, Serrano GE, Adler CH, Lax P, Cuenca N, 2018b. Degeneration of human photosensitive retinal ganglion cells may explain sleep and circadian rhythms disorders in Parkinson’s disease. Acta. Neuropathol. Commun 10, 6(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouk K, Aungier J, Morton AJ. (2017) Prolonged day length exposure improves circadian deficits and survival in a transgenic mouse model of Huntington’s disease. Neurobiol Sleep Circadian Rhythms, 2, 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallier PN, Maywood ES, Zheng Z, Chesham JE, Inyushkin AN, Dyball R, Hastings MH, Morton AJ, 2007. Pharmacological imposition of sleep slows cognitive decline and reverses dysregulation of circadian gene expression in a transgenic mouse model of Huntington’s disease. J. Neurosci 27, 7869–7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallier PN, Morton AJ, 2009. Management of sleep/wake cycles improves cognitive function in a transgenic mouse model of Huntington’s disease. Brain Res 1279, 90–98. [DOI] [PubMed] [Google Scholar]
- Pandi-Perumal SR, BaHammam AS, Brown GM, Spence DW, Bharti VK, Kaur C, Hardeland R, Cardinali DP, 2013. Melatonin antioxidative defense: therapeutical implications for aging and neurodegenerative processes. Neurotox. Res 23, 267–300. [DOI] [PubMed] [Google Scholar]
- Paus S, Schmitz-Hübsch T, Wüllner U, Vogel A, Klockgether T, Abele M, 2007. Bright light therapy in PD: a pilot study. Mov. Disord 22, 1495–1498. [DOI] [PubMed] [Google Scholar]
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE, 2017. Parkinson disease. Nat. Rev. Dis. Primers 23, 3:17013. [DOI] [PubMed] [Google Scholar]
- Rahman SA, St Hilaire MA, Lockley SW, 2017. The effects of spectral tuning of evening ambient light on melatonin suppression, alertness and sleep. Physiol. Behav 177, 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid KJ, Baron KG, Lu B, Naylor E, Wolfe L, Zee PC, 2010. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med 11, 934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ, 2008. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA 299, 2642–2655. [DOI] [PubMed] [Google Scholar]
- Rios Romenets S, Creti L, Fichten C, Bailes S, Libman E, Pelletier A, Postuma RB, 2013. Doxepin and cognitive behavioural therapy for insomnia in patients with Parkinson’s disease a randomized study. Parkinsonism Relat. Disord 19, 670–675. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Merrow M, 2016. The Circadian Clock and Human Health. Curr. Biol 26, R432–R443. [DOI] [PubMed] [Google Scholar]
- Rutten S Vriend C, Smit JH, Berendse HW, Hoogendoorn W, van den Heuvel OA, van der Werf YD, 2016. A double-blind randomized controlled trial to assess the effect of bright light therapy on depression in patients with Parkinson’s disease. BMC Psychiatry 16, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhi N, Duffy JF, Horowitz TS, Czeisler CA, 2005. Scheduling of sleep/darkness affects the circadian phase of night shift workers. Neurosci. Lett 384, 316–320. [DOI] [PubMed] [Google Scholar]
- Schapira AHV, Chaudhuri KR, Jenner P, 2017. Non-motor features of Parkinson disease. Nat. Rev. Neurosci 18, 435–450. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Collette F, Leclercq Y, Sterpenich V, Vandewalle G, Berthomier P, Berthomier C, Phillips C, Tinguely G, Darsaud A, Gais S, Schabus M, Desseilles M, Dang-Vu TT, Salmon E, Balteau E, Degueldre C, Luxen A, Maquet P, Cajochen C, Peigneux P, 2009. Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area. Science 324, 516–519. [DOI] [PubMed] [Google Scholar]
- Schroeder AM, Colwell CS 2013. How to fix a broken clock. Trends Pharmacol. Sci 34, 605–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz JM, Redgrave P, Mehring C, Aertsen A, Clements KM, Wickens JR, Reynolds JN, 2009. Short-latency activation of striatal spiny neurons via subcortical visual pathways. J. Neurosci 29, 6336–6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, 2011. Biomarkers for Parkinson’s disease. Sci. Transl. Med 3, 79:79ps14. [DOI] [PubMed] [Google Scholar]
- Speelman AD, van de Warrenburg BP, van Nimwegen M, Petzinger GM, Munneke M, Bloem BR, 2011. How might physical activity benefit patients with Parkinson disease? Nat. Rev. Neurol 7, 528–534. [DOI] [PubMed] [Google Scholar]
- Speelman AD, van Nimwegen M, Bloem BR, Munneke M 2014. Evaluation of implementation of the ParkFit program: A multifaceted intervention aimed to promote physical activity in patients with Parkinson’s disease. Physiotherapy 100, 134–141. [DOI] [PubMed] [Google Scholar]
- Stephenson R, Siderowf A, Stern MB, 2009. Premotor Parkinson’s disease: clinical features and detection strategies. Mov. Disord 24, S665–S670. [DOI] [PubMed] [Google Scholar]
- Struck LK, Rodnitzky RL, Dobson JK, 1990. Circadian fluctuations of contrast sensitivity in Parkinson’s disease. Neurology 40, 467–470. [DOI] [PubMed] [Google Scholar]
- Stuebner E, Vichayanrat E, Low DA, Mathias CJ, Isenmann S, Haensch CA, 2013. Twenty-four hour non-invasive ambulatory blood pressure and heart rate monitoring in Parkinson’s disease. Front. Neurol 49, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulli G, Manoogian ENC, Taub PR, Panda S, 2018Training the Circadian Clock, Clocking the Drugs, and Drugging the Clock to Prevent, Manage, and Treat Chronic Diseases. Trends Pharmacol. Sci 39, 812–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL, 2008. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet 9, 764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL, 2008. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet 9, 764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman JS, Terman M, Lo ES, Cooper B, 2001. Circadian time of morning light administration and therapeutic response in winter depression. Arch. Gen. Psychiatry 58, 69–75. [DOI] [PubMed] [Google Scholar]
- Terman M, 2007. Evolving applications of light therapy. Sleep Med. Rev 11, 497–507. [DOI] [PubMed] [Google Scholar]
- Terman M, Terman JS, 2005. Light therapy for seasonal and nonseasonal depression:efficacy, protocol, safety, and side effects. CNS Spectr 10, 647–663. [DOI] [PubMed] [Google Scholar]
- Thenganatt MA, Jankovic J, 2014. Parkinson disease subtypes. JAMA Neurol 71, 499–504. [DOI] [PubMed] [Google Scholar]
- Tsai HY, Chen KC, Yang YK, Chen PS, Yeh TL, Chiu NT, Lee IH, 2011. Sunshine-exposure variation of human striatal dopamine D(2)/D(3) receptor availability in healthy volunteers. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 107–110. [DOI] [PubMed] [Google Scholar]
- Van Maanen A, Meijer AM, van der Heijden KB, Oort FJ 2016. The effects of light therapy on sleep problems: A systematic review and meta-analysis. Sleep Med. Rev 29, 52–62. [DOI] [PubMed] [Google Scholar]
- Van Reeth O, Turek FW, 1989. Stimulated activity mediates phase shifts in the hamster circadian clock induced by dark pulses or benzodiazepines. Nature 339, 49–51. [DOI] [PubMed] [Google Scholar]
- Van Someren EJ, Lijzenga C, Mirmiran M, Swaab DF, 1997. Long-term fitness training improves the circadian rest-activity rhythm in healthy elderly males. J. Biol. Rhythms 12, 146–156. [DOI] [PubMed] [Google Scholar]
- Vetrano DL, Pisciotta MS, Lo Monaco MR, Onder G, Laudisio A, Brandi V, La Carpia D, Guglielmo M, Nacchia A, Fusco D, Ricciardi D, Bentivoglio AR, Bernabei R, Zuccalà G 2015. Association of depressive symptoms with circadian blood pressure alterations in Parkinson’s disease. J. Neurol 262, 2564–2571. [DOI] [PubMed] [Google Scholar]
- Videnovic A, Golombek D 2017. Circadian dysregulation in Parkinson’s disease. Neurobiol. Sleep Circadian Rhythms 2, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnovic A, Golombek D, 2013. Circadian and sleep disorders in Parkinson’s disease. Exp. Neurol 243, 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnovic A, Klerman EB, Wang W, Marconi A, Kuhta T, Zee PC 2017. Timed Light Therapy for Sleep and Daytime Sleepiness Associated With Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol 74, 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnovic A, Lazar AS, Barker RA, Overeem S, 2014. ‘The clocks that time us’--circadian rhythms in neurodegenerative disorders. Nat. Rev. Neurol 10, 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnovic A, Noble C, Reid KJ, Peng J, Turek FW, Marconi A, Rademaker AW, Simuni T, Zadikoff C, Zee PC, 2014. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol 71, 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnovic A, Willis GL, 2016. Circadian system A novel diagnostic and therapeutic target in Parkinson’s disease? Mov. Disord 31, 260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Krack P, Lang AE, Lozano AM, Dujardin K, Schüpbach M, D’Ambrosia J, Thobois S, Tamma F, Herzog J, Speelman JD, Samanta J, Kubu C, Rossignol H, Poon YY, Saint-Cyr JA, Ardouin C, Moro E, 2008A multicentre study on suicide outcomes following subthalamic stimulation for Parkinson’s disease. Brain 131, 2720–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Whittaker DS, Truong D, Mulji AK, Ghiani CA, Loh DH, Colwell CS, 2017. Blue light therapy improves circadian dysfunction as well as motor symptoms in two mouse models of Huntington’s disease. Neurobiol. Sleep Circadian Rhythms 2, 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigren HK, Schepens M, Matto V, Stenberg D, Porkka-Heiskanen T 2007. Glutamatergic stimulation of the basal forebrain elevates extracellular adenosine and increases the subsequent sleep. Neuroscience 147, 811–823. [DOI] [PubMed] [Google Scholar]
- Willis GL, Moore C, Armstrong SM 2012. historical justification for and retrospective analysis of the systematic application of light therapy in Parkinson’s disease. Rev. Neurosci 23, 199–226. [DOI] [PubMed] [Google Scholar]
- Willis GL, Turner EJ, 2007. Primary and secondary features of Parkinson’s disease improve with strategic exposure to bright light: a case series study. Chronobiol. Int 24, 521–537. [DOI] [PubMed] [Google Scholar]
- Yasenkov R, Deboer T, 2012. Circadian modulation of sleep in rodents. Prog. Brain Res 199, 203–218. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Morales-Villagran A, Maidment NT, Behnke EJ, Ackerson LC, Lopez-Rodriguez F, Fried I, Engel J Jr., Wilson CL, 2006. Extracellular adenosine in the human brain during sleep and sleep deprivation: an in vivo microdialysis study. Sleep 29, 455–461. [DOI] [PubMed] [Google Scholar]