Abstract
Objectives
The generation of systemic lupus erythematosus (SLE)-related autoantibodies have been shown to be T cell dependent and antigen driven with HLA-DR restriction. In this study, the initiating antigen(s) and the mechanism of autoantibody diversification were investigated.
Methods
T cell epitopes (T-epitopes) of SmD1 (SmD) were mapped by T-T hybridomas generated from DR3+AE0 mice immunised with SmD and with SmD overlapping peptides. TCRs from the reactive hybridomas were sequenced. The core epitopes were determined. Bacterial mimics were identified by bioinformatics. Sera from DR3+AE0 mice immunised with SmD peptides and their mimics were analysed for their reactivity by ELISA and immunohistochemistry. Samples of blood donors were analysed for HLA-DR and autoantibody specificities.
Results
Multiple HLA-DR3 restricted T-epitopes within SmD were identified. Many T-T hybridomas reacted with more than one epitope. Some of them were crossreactive with other snRNP peptides and with proteins in the Ro60/La/Ro52 complex. The reactive hybridomas used unique TCRs. Multiple T-epitope mimics were identified in commensal and environmental bacteria. Certain bacterial mimics shared both T and B cell epitopes with the related SmD peptide. Bacterial mimics induced autoantibodies to lupus-related antigens and to different tissues. HLA-DR3+ blood donors made significantly more SLE-related autoantibodies.
Conclusions
The unique antigenic structures of the lupus-related autoantigens provide the basis for being targeted and for T and B cell epitope spreading and autoantibody diversification with unique patterns. SLE-related autoantibodies are likely generated from responses to commensal and/or environmental microbes due to incomplete negative selection for autoreactive T cells. The production of SLE-related antibodies is inevitable in normal individuals. The findings in this investigation have significant implications in autoimmunity in general.
INTRODUCTION
Systemic lupus erythematosus (SLE) is an auto-immune disorder affecting multiple organs with complex autoantibodies (auto-Abs).1 The most common auto-Abs are those against ribonucleoproteins such as Sm and RNP within the snRNA particles and Ro/La complexes. There are considerable lag periods up to >9 years between the appearance of these auto-Abs and the diagnosis of SLE.2 Over these lag periods, the auto-Abs specificities become diverse with specific diversification patterns with auto-Ab to specific linked sets of lupus-related autoantigens (auto-Ags).2–4 It is established that the generation of these auto-Abs is antigen (Ag)-driven and T cell dependent. In addition, the generation of these auto-Abs is linked to HLA-DR2 (DR2) and HLA-DR3 (DR3).5–7 Cognizant of these characteristics of lupus-related auto-Abs, we have used mice with the DR3 transgene to interrogate the role of HLA-DR and T cells in the generation of anti-Sm Abs. DR3 transgenic mice have been shown to respond best to immunisation with recombinant SmD1 (SmD) and Ro60.8,9 Immunisation with SmD in DR3 transgenic mice induces a response with auto-Ab diversification to other peptides within the Sm core complex (D1/2/3, B/B’, E, F, and G) as well as to dsDNA.8 In addition, DR3 supports the development of anti-SmD Abs and lupus nephritis with early mortality.10 These results support the important role of HLA-DR3 in the induction and diversification of lupus-related auto-Abs. In order to better understand the origin of auto-Abs to lupus-related auto-Ags, the HLA-DR3 restricted T-epitopes in SmD were mapped. The results provide novel insights into the origin of lupus related auto-Abs, providing an explanation why these auto-Ags are being targeted and the mechanism by which these auto-Abs are diversified. In addition, the novel findings have implications in autoimmunity in general.
METHODS
Peptides and recombinant proteins
15mers spanning the entire sequence of SmD (aal-119), of core SmD epitope peptides with alanine substitutions, and bacterial mimic peptides (HPLC purified with >95% purity) were obtained from ChinaPeptides (Shanghai, China). SmD, SmB, A-RNP Ro60, R052 and La were prepared as previously described.11
Mouse strain and immunisations
Mouse experiments were approved by the Animal Care and Use Committees at the University of Virginia. The HLA-DRB1*0301, HLA-DRA1*0101 (DR3.A0/0E0/0) transgenic (tg) mice were previously described.12 For T-epitope mapping, mice were immunised in the left footpad and base of tail with 100 μg of SmD in IFA. For Ab generation, mice were immunised in the left footpad and base of tail with 100 μg of peptide in CFA. On days 14 and 28 post immunisation, mice received injections of 50 μg of peptide in IFA intraperitoneally. Sera were collected monthly and stored at –20°C.
Generation of smD T-T hybridomas
T-T hybridomas were generated by fusing lymph node cells from DR3+ A0/0E0/0 mice immunised with SmD in IFA with BW5147TCR−/− as described by Kruisbeek.13 For each fusion, cells were suspended in 10 96-well plates. Hybrid cells surviving HAT selection were expanded in 24-well plates. 105 T-T hybridoma cells were incubated with 2.5×105 syngeneic splenic cells in the presence of SmD for 14 hours. Interleukin (IL)-2 in culture supernatants was estimated by ELISA (BD Pharmingen) following manufacturer’s instructions. Reactive hybridomas were cloned by limiting dilution.
Analysis of TCR usage
TCR Vα and Vβ usage by T-T hybridomas was determined by sequencing the TCRs as described Chen et al.14 The consensus sequences of three clones of TCRα and TCRβ were analysed by the IMGT TCR database (http://www.imgt.org/IMGT_vquest/vquest ).
ELIsA, indirect immunofluorescence and immunohistochemistry
These methods are detailed in the online supplemental method section.
Human sera and HLA-DR typing
DNA was extracted from the clots and the sera were collected from samples of healthy anonymous blood donors provided by Virginia Blood Services (Richmond, Virginia, USA) without identification. HLA-DR typing was done by the PCR method description by Bunce.15 HLA-DR homozygous cell lines, MGA for DRB1*1501(DR2), QBL for DRB1*0301(DR3) and BSM for DRB1*0410(DR4) were used as positive controls.16
Bioinformatics analysis
The bacterial sequences for analysis were downloaded from NCBI protein database (Taxonomy ID: 2). A software designed by Zhao and Dai (available to academic investigators on request directed to Zhao) divided all the bacterial protein sequences into 15mers with 14 overlapping aa as the bacterial 15mer library. The SmD 15mer peptides with T-epitopes, in which core aa with conservative substitutions are depicted in figure 3A, were stored as the SmD 15mer epitope library. These two libraries were compared. The bacterial 15mers sharing four aa residues identical to the core amino acids in the SmD 15mer library were collected as candidate bacterial mimics. The candidate bacterial mimics were screened by the IEDB tool (http://tools.immuneep-itope.org/mhcii/download/) to identify those bacterial 15mers with binding affinity to HLA-DR3 <5000 μM. The resulting peptides were considered potential bacterial mimics with DR3−restricted SmD T cell mimotopes.
Figure 3.

Identification of multiple potential bacterial mimics of HLA-DR3 restricted 7 SmD core T-epitopes by bioinformatics analysis. (A) Two peptide libraries were constructed. One was a Bacterial Peptides Library, which is generated first by dividing all the bacterial protein sequences from NCBI protein database (Taxonomy ID: 2) into 15mers overlapping with 14 aa. This 15mer library is very large and is reduced by a custom-designed software. In examining the seven 15mer core epitopes (figure 2B), the core epitopes within each 15mers have 11mers with at least four sequential aa that are critical for the reactive hybridoma to bind either DR3 or the reactive TCR. Thus the software were designed to identify in the bacterial library with four aa identical to the seven core T-epitopes. The bacterial 15mer library with the desired aa sequences were then collected and designated as our bacterial peptides library (right panel). The second library is constructed as the collection of the 15mer SmD T-epitopes with conservative substitutions at certain positions and free substitutions at the other positions (left panel). Our software then compared the two libraries to identify peptides with identical sequences. These are the candidate 15mers that contain the bacterial mimics of SmD T-epitopes. All candidate peptides were then analysed for their binding affinity to HLA-DR3 by the IEDB database. Those binding affinities <5000 μM were considered as good mimics for SmD T-epitopes. (B) Summary of numbers of potential bacterial mimics of SmD core T-epitopes and their binding affinities to HLA-DR3 are listed.
RESULTS
Multiple cross-reactive intramolecular T-epitopes in smD
In total, 5000 T-T hybridomas were generated from four fusion experiments with lymph node cells from eight DR3 transgenic mice immunised with SmD. T-T hybridomas reactive with SmD were screened with SmD 20mers with 15 overlapping aa. The hybridomas then were screened with a panel of SmD 15mers with 12 aa overlapping. 135 T-T hybridomas were reactive with diverse SmD T-epitopes (online supplementary material table S1). Two unstable hybridomas were reactive with SmD1–15. Thus, the core T-epitope within SmD1–15 was not determined.
With our protocol, ~90% of the generated hybridomas are monoclonal with a single TCRαβ. In the initial screening, a significant number of the hybridomas were reactive with two or more regions of SmD. As shown in figure 1A, hybridoma D1438–7-21 cloned twice with a single TCRαβ reacted with peptides in SmD1–25 and SmD51–75. The reactive T-epitopes were localised in SmD6–20 and SmD 57–71. Figure 1B summarises hybridomas reactive with two or three SmD T-epitopes. Thus multiple intramolecular HLA-DR3 restricted cross-reactive T epitopes are present within SmD. The presence of multiple intramolecular HLA-DR3 restricted cross-reactive T-epitopes has also been shown in Ro60 online supplementary material table S2.
Figure 1.

T-T hybridomas recognising multiple cross-reactive T-epitopes with SmD and cross-reactive T-epitopes shared by SmD, SmB, A-RNP and Ro60. (A) Hybridoma D1438–7-21 was cloned twice and recognises two different regions within SmD. Further mapping with SmD 15mer peptides overlapping by 12 aa showed H1438–7-21 reacts with SmD6–20 and SmD57–71. These two SmD peptides share cross-reactive T-epitopes although they do not have significant sequence homology. (B) Summary of hybridomas reactive with two or more SmD T epitopes shows that SmD have multiple intramolecular cross-reactive T-epitopes. T-cell epitope in bold means they are the dominant epitope within the cross-reactive epitopes with other epitope(s) inducing <70% of interleukin (IL)-2. (C) Hybridoma F140–9 reacts with SmD66–80. It reacts to SmD, SmB and A-RNP in a dose-dependent manner (left panel). Their responses are DR3 restricted. It reacts much better to SmB. It reacts to Ro60132–277 and Ro6 0 263–406 in a HLA-DR3 restricted manner as shown in the right panel. Thus F140–9 reacts with a T-epitope shared among SmD, SmB, A-RNP and Ro60. (D) Summary of T-T hybridomas reactive with SmD and SmB (four clones), SmD, SmB and A-RNP (three clones) and SmD, SmB, A-RNP and Ro60 (one clone).
Cross-reactive intermolecular T and B cell epitopes in lupus- related auto-Ags
Auto-Abs to multiple proteins within snRNP and to Ro60/La are often detected in individual patients with SLE. The hypothesis that the presence of multiple HLA-D-restricted intermolecular cross-reactive T-epitopes may be responsible for the observed auto-Ab diversification in patients with SLE17 was tested. As shown in figure 1C, the cloned T-T hybridoma F140–9 that expressed a single TCRαβ reacted with SmD66–80, SmD, SmB and A-RNP in a dose-dependent fashion. In fact, this hybridoma reacted better with SmB. In addition, this hybridoma also responded to Ro60 as shown by its response to two recombinant Ro fragments (rRo60132–277 and rRo6 0263–406). Figure 1D summarises our T-T hybridomas reactive with two or more of the lupus related auto-Ags, SmD, SmB, A-RNP and Ro60. Thus there are multiple intermolecular DR3 restricted shared T-epi-topes among the proteins within the snRNP particles and among the snRNP particle and the Ro60 protein. Similarly significant numbers of anti-Ro60 T-T hybridomas also react with T-epitopes on La (online supplementary material table S2). Together with our previous demonstration that the lupus-related Ags have multiple shared B cell epitopes,18 the structure features of lupus-related Ags make them logical targets for intermolecular epitope spreading often observed in SLE.
Presence of multiple T cell core epitopes and the polyclonal T cell responses to smD
The core T-epitopes within relevant 15mers of SmD were ascertained by alanine substitutions. For alanine in the peptides, it was substituted by lysine. As shown in figure 2A, hybridoma H425–40 is reactive with SmD31–45. The T-epitope is located within SmD33–42. Within this 9mer, substitutions at SmD36 SmD39 or SmD41 abolished the peptide’s ability to stimulate the hybridoma. Substitutions at SmD33, SmD34 or SmD40 reduced the peptide ability to stimulate the hybridoma by 50%. Figure 2B summarises the results of mapping T-epitopes recognised by the 42 hybridomas reactive with seven 15ers of SmD. Importantly, each hybridoma reacts with a specific epitope. In some T-epitopes, the flanking sequences play major roles in stimulating the T-T hybridomas. The unique nature of each of the T-epitopes suggests that each hybridoma should have a unique TCRαβ. This was confirmed by sequencing the TCRs of 16 hybridomas (figure 2C). Each of the hybridomas has a unique TCRαβ although some TCRα or TCRβ segments are sometimes used by more than one hybridoma. These data indicate that SmD behaves as a conventional Ag with multiple T-epitopes and T cell response to SmD is polyclonal.
Figure 2.
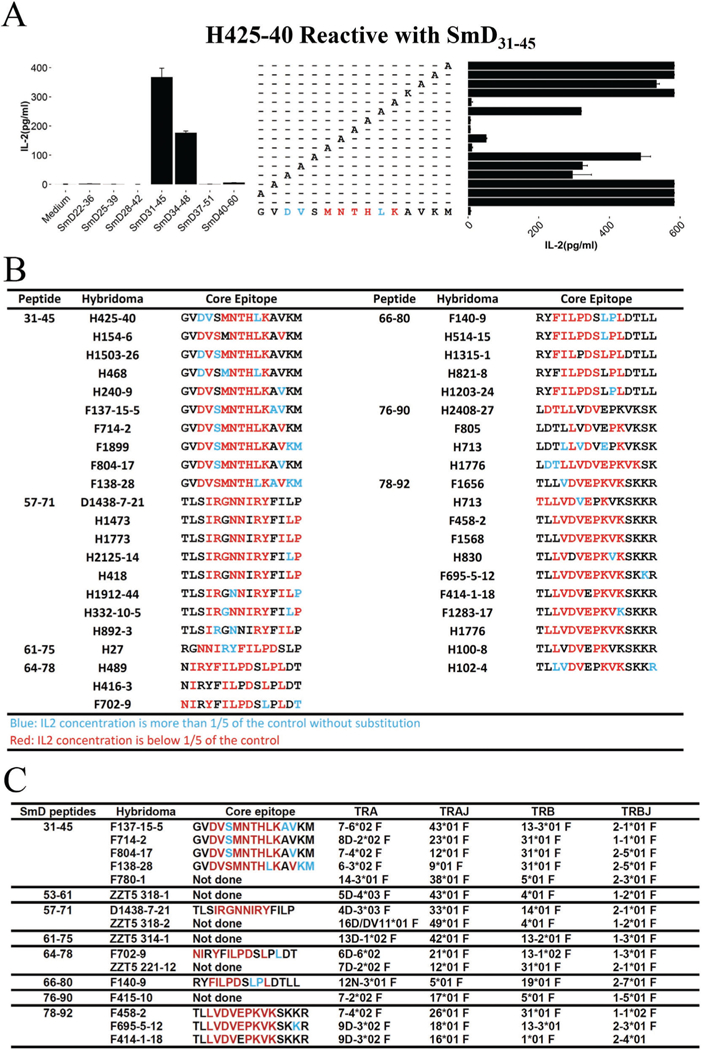
Determination of multiple HLA-DR3 (DR3) restricted cell epitopes on SmD and polyclonal TCR usage for recognising T-cell epitopes. (A) Determination of the core epitope by alanine substitution. Hybridoma H425–40 was incubated with SmD31–45 with alanine-substituted peptides with splenic cells from DR3 transgenic mice as antigen-presenting cells (APC) for 20 hours at 37°C. Supernatants were assayed for interleukin (IL)-2 by ELISA. In the case of alanine, lysine-substituted peptide was used. When the IL-2 responses to the A-substituted peptides were decreased to <20%, the amino acids (aa) were coded by red (M, N, T, H, K at SmD aa36, 37, 38, 39 and 41). When the Il-2 responses were >20%, the aa were coded by blue (SmD aa33, 34 and 40). The core amino acids for SmD31–45 was assigned to be SmD33–41. Hybridoma H425–40 should respond to SmD33–41 with conservative substitutions allowed at aa36, 37, 38, 39 and 40) and with all aa substitutions at other positions. (B) Summary of the core T-epitopes restricted by HLA-DR3 and recognised by T-T hybridomas. Each hydridoma recognises unique T-epitopes. (C) Multiple TCRs are used by T-T hybridomas reactive to HLA-DR3−restricted SmD T epitopes. TCRs are used in responses to the T-epitopes within each SmD 15mer.
Multiple bacterial T-epitopes cross-reactive with smD from both commensal and environmental microbiota
It is evident that as shown in figure 2 there are many T-epitopes in SmD. To make our analysis manageable, we chose seven core epitopes in the following peptides: SmD31–45, SmD57–71, SmD61–75, SmD64–78 SmD66–80 SmD76–90 and SmD78–92. UsinS SmD31–45 as an example, the core amino acids is arbitrarily assigned to reside in SmD33–41. The conservative substitutions of aa33, aa34 and aa36 to aa41 are listed in figure 3A. Substitutions with any aa in other positions of this core T-epitope are permitted. A 15mer peptide library that is named library 1 was then constructed to include all 15mers generated from the core SmD T-epitopes. A bacterial 15mer library was constructed from the NCBI bacterial protein database. All known bacterial protein sequences were divided into 15mers with 14 aa overlapping (library 2). These 15mers were screened for the presence of four aa identical to those conserved aa in the seven core T-epitopes. The identified bacterial peptides were then collected as the library of potential bacterial mimic peptides (library 3). The potential mimic peptides were then screened for their binding to HLA-DR3 with the IEDB database. Peptides with binding affinity of IC50 (μM)<5000 were considered candidate peptides with T-epitope mimics. Figure 3B summarises the results of this analysis. It is of note that the parental peptides have middle binding affinity ranks among their mimics. For example, SmD31–45 ranks 259th among the 427 identified molecular mimics. Approximately 10%–15% of the bacterial mimics are from commensal bacteria. Online supplementary table S3 1 provides the identified bacterial mimics for SmD66–80.
Immunogenicity of smd 15mers with core T-epitopes and their bacterial T-epitope mimics
To ascertain the potency of the SmD T cell mimics from bacteria as immunogens, 12 peptides (online supplementary table S4) were randomly chosen from the list of 159 mimics of SmD66–80 (online supplementary table S3). Only one of them activated one of the five hybridomas reactive with SmD66–80 listed in figure 2B. F140–9 could be activated by SmD66–80 M7. However, with the exception of SmD66–80 M11 (M11), four other mimics (SmD66–80 M2, SmD66–80 M5, SmD66–80 M7 and SmD66–80 M9 in figure 4A) induced Abs to lupus-related Ags (figure 4B). These peptides were from commensal or environmental bacteria. It is of note that M9, which is ranked 104th in its affinity among the 159 potential mimics, was a good immunogen. Thus a majority of the mimics are good immunogens, providing evidence that lupus-related auto-Abs can be initiated by many bacterial mimics.
Figure 4.

Immune serum from DR3 mice immunised with selected SmD peptides and SmD66–80 mimics peptides had diverse auto-antibodies (auto-Ab) specificities by ELISA. (A) Five SmD66–80 bacterial mimics were chosen to immunise HLA-DR3 mice. These mimic peptides came from two commensal bacteria (SmD66–80M2, M2 and SmD66–80M5, M5), from two pathogenic bacterial (SmD66–80M7, M7 and SmD66–80M11, M11) and one from environmental bacteria (SmD66–80M9, (M9). They have different binding affinities to HLA-DR3. The red aa are the same ones as the parent SmD peptide, and the blue ones are those with conservative substitutions. Black aa indicates substitutions from other aa. (B) Sera from DR3 transgenic mice immunised with SmD, SmD57–71, SmD66–80, SmD91–119 and M2, M5, M7 and M9 were diluted at 1:100 and used in ELISA for their antibody activities. SmD induced Abs against the six systemic lupus erythematosus (SLE)-related autoantigens (auto-Ags). SmD57–71 induced Ab to SmB, A-RNP and Ro52. SmD66–80 induced Ab to SmD, SmB, A-RNP and Ro52. SmD91–119 induced anti-peptide Ab (data not shown) but did not induce Ab to all six SLE-related Ags. Except for M7, the other three mimics induced Ab to all six Ags. M7 did not induce Ab to Ro60 and La. M11 did not make Ab to any of these auto-Ags (data not shown). (C) Absorption experiments with antisera (1/100 diluted) from mice immunised with M5, M7 and M9 showed shared B cell epitopes among SmD66–80, M3, M5 and M9. All immune sera were obtained 90 days after the initial immunisation.
Five of the seven SmD peptides (SmD31–45, SmD57–71, SmD61–75, SmD66–80 and SmD78–92) and SmD were used to immunise mice. All of them induce auto-Abs to various lupus-related Ags (figure 4B and online supplementary figure S1). Sera from mice immunised with SmD61–75, SmD66–80, or SmD78–92, reacted with SmD and other lupus-related Ags. Sera from mice immunised with SmD57–71 or SmD31–45 were reactive with multiple lupus-related Ags but not with SmD. A similar observation was observed in A/J mice responses to SmD52–66.19 Although SmD91–119 induced anti-peptide antibodies (data not shown), the immune sera did not react with SmD and other lupus-related Ags.
Absorption experiments (figure 4C) with sera showed that M3, M5 and M9 shared a B cell epitope with SmD66–80. These results are remarkable in that some bacterial mimics share both T and B cell epitopes with the relevant SmD auto-peptides, making them potent immunogens. This feature would favour the relevant mimics to initiate lupus-related auto-Abs.
Anti-tissue Ab, anti-nuclear Ab (ANA) and anti-dsdNA Abs in mice immunised with smD peptides and certain mimics.
Sera from DR3 transgenic mice immunised with SmD, or its selected peptides and SmD66–80 bacterial mimics, were interrogated for their reactivity against kidneys, salivary glands and skin. As shown in figure 5A, pre-immune sera and immune sera from mice immunised with SmD91–119 at a dilution of 1:8100 did not stain these tissue samples. Anti-SmD sera stained kidneys and weakly stained skin. Anti-SmD57–71 sera stained kidney tubular cells, salivary gland and skin and this staining was abolished by absorption with the immunogen (data not shown). Anti-SmD66–80 sera stained all three tissues strongly. The staining was partly abolished by absorption with the immunogen. Immune sera from four SmD66–80 mimics stain these tissues with varied patterns. Both M2 and M5 with higher affinities for DR3 than SmD66–80 induced strong Ab staining on all three tissues. Immune sera from mice immunised with SmD66–80 and its mimics contained ANA (figure 5B). Anti-dsDNA Abs were detected in high titres in the immune sera of mice immunised with SmD66–80 M2 (figure 5C). These Abs were not absorbed with the immunogen (data not shown).
Figure 5.
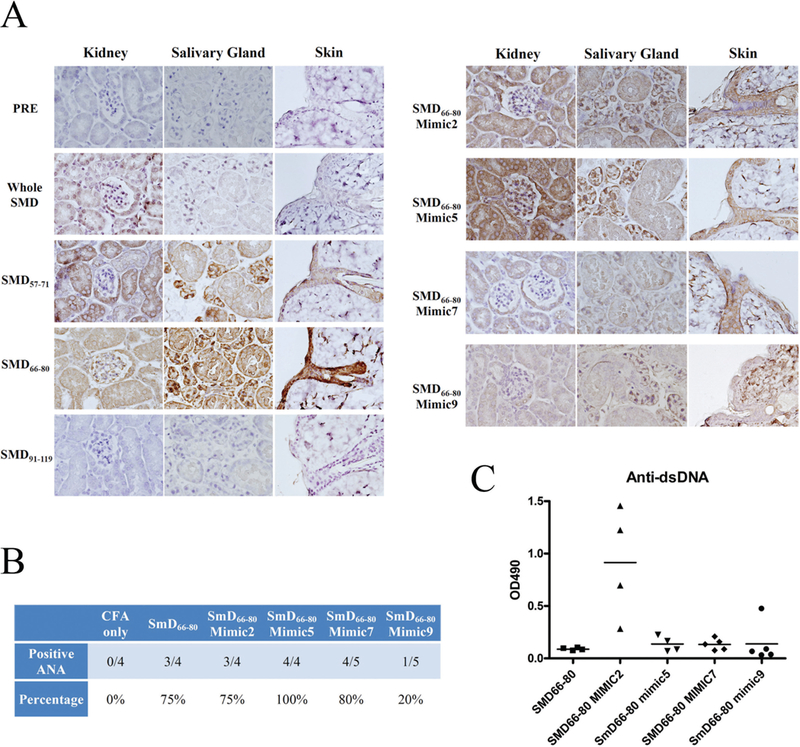
Immune sera from DR3 mice immunised with SmD peptides and SmD66–80 T-epitope mimic peptides had anti-tissue antibodies (Abs), anti-nuclear antibodies (ANA) and anti-dsDNA Abs. (A) Diverse staining patterns on kidney, salivary gland and skin from B6.Rag1−/−mice at 1/8100 dilutions are seen. (B) They have variable ANA positivity against mouse NIH-3T3 cells at 1/30 SLE.2 Overdilutions. (C) Three of four sera from M2 immunised mice had anti-dsDNA Ab. One of the four immune sera from M9 immunised mice had anti-dsDNA Ab. All immune sera tested were obtained 90 days after the initial immunisation.
More auto-Abs to lupus related-Ags in HLA-DR3+ blood donors
DNA extracted from the clot of 169 outdated blood samples from healthy blood donors was used for typing for DR2, DR3 and DR4 by PCR. The sera were used for ELISA assays on various lupus-related Ags and for ANA determination by indirect immunofluorescence (online supplementary table S5). Sera from DR3+ individuals at 1:100 dilutions had higher anti-SmD titres compared with those from individuals who were neither DR3+ nor DR2+ (non-DR3/DR2) with p = 0.036 (figure 6Aa). DR3+ individuals with high titres against SmD had higher Ab titres against SmB, A-RNP and dsDNA (figure 6Ab, Ac). When DR3+ individuals with low titres against SmD were compared with non-DR3/DR2 individuals, DR3+ individuals made more anti-A-RNP Abs (figure 6Ac, Ad). The DR3+ individuals had higher titres of anti-Ro60 Abs (figure 6Ae) and anti-Ro52 Abs (figure 6Af) with p = 0.0007 and 0.012, respectively. DR3+ individuals made significantly more anti-dsDNA Abs compared with individuals negative for DR2, DR3 and DR4 (figure 6B). DR3+ individuals had higher frequencies for positive ANA (figure 6C). The staining patterns vary individually, suggesting that the Abs target different nuclear Ags (figure 6C).
Figure 6.

Normal DR3+ blood donors have more anti-SmD, anti-SmB, anti-A-RNP, anti-Ro60 and anti-Ro52 antibodies (Ab), and anti-dsDNA Ab, and are 15% positive for antinuclear antibodies (ANA) staining. (A) HLA-DR3+donors had higher Ab titres against lupus-related Abs. (a) DR3+ donors have higher anti-SmD Ab than those without DR3 and DR2 (DR3−/DR2−). (b) DR3+ donors with high anti-SmD Ab had significantly higher anti-SmB, anti-A-RNP and anti-dsDNA Ab compared with those with little anti-SmD Ab as shown in c. (c) DR3+ donors with little anti-SmD Ab titres had higher Abs to A-RNP when they were compared with DR2−/DR3− donors as shown in d. (d) DR2−/DR3− donors had little Abs to SmD, SmB, A-RNP and dsDNA. (e) DR3+ donors had more anti-Ro60 Ab compared with DR2−/DR3− donors. (f) DR3+ donors had more anti-Ro52 Ab compared with DR2−/DR3−donors. (g) There was no difference in anti-La Ab in DR3+ donors and DR2−/DR3−donors. (B) DR3+, DR2+ and DR4+donors made anti-dsDNA Ab. There were no significant differences among these six groups. However, DR3+ donors made more anti-dsDNA Ab compared with DR2−/DR3−/DR4− (denoted as non) donors. (C) DR3+ individuals have a higher frequency of ANA. The red colour shows staining on Hela cells by anti-IgG Ab. The blue staining is due to nuclear staining by DAPI. Sera were diluted at 1/100 for ELISA assays and 1/40 for ANA. The two-tailed p value was calculated by unpaired t-test with Welch’s correction.
DISCUSSION
In this investigation, HLA-DR3−restricted T-epitope mapping was carried out in SmD, a major lupus-related auto-Ag that has 100% homology between human and mouse proteins. Multiple HLA-DR3−restricted T-epitopes within SmD were identified. Many T-T hybridomas reacted with more than one epitope, indicating the presence of multiple cross-reactive epitopes. Some of these SmD-reactive hybridomas were also reactive with other snRNP peptides and with proteins in the Ro60/La/Ro52 complex, suggesting the presence of multiple cross-reactive T epitopes among lupus auto-Ags. The reactive hybridomas used unique TCRs. Multiple T-epitope mimics were identified in commensal and environmental bacteria. Certain bacterial mimics shared both T and B cell epitopes with the related SmD peptide. Bacterial mimics induced auto-Abs to lupus-related Ags such as ANA, snRNP Ro60/Ro52, La and dsDNA and to different tissues. HLA-DR3+ blood donors made significantly more SLE-related auto-Abs. These data have significant implications on the patho-genesis of SLE. They extended our previous limited studies11,20 and give further support to our hypothesis that the generation of autoreactive Abs and effector T cells in SLE is induced by environmental T-epitope mimics and is HLA-DR restricted. Accumulation of cross-reactive T cells is a consequence of responses to multiple environmental mimics in hosts with susceptibility genes (HLA-DR3 and others) but not in hosts without these genes. The accumulation of diverse auto-Abs and T cells as a response to these mimics results in varied SLE clinical presentations. AfterCoating peptide therapy, the complexity of these auto-Abs and autoreactive T cells is reduced, leading to remission. Over a period of time after discontinuing therapy, the complexity of auto-Abs and effector T cells returns, leading to a protean clinical presentation in relapses. The mimics reside on a diverse array of environmental antigens and the chances for exposure to these mimics are random, providing a scenario in that SLE is not caused by a single pathogen or commensal microbe. This mechanism has the flavour of a stochastic process.17 As a corollary, cytokines and interferons that are generated in innate immunity play an amplification role in the generation of lupus-related auto-Abs.
The presence of auto-Abs and autoreactive T cells in normal individuals provides evidence that autoimmunity need not lead to autoimmune diseases. In this regard, we have provided evidence that autoimmunity and susceptibility to end-organ damage are under distinct genetic control and that targeted end organs by auto-Abs and autoreactive T cells participate actively in the pathogenesis of autoimmune disorders.21–23 The requirement of interaction between auto-Abs and autoreactive T cells with the targeted end organs for the occurrence of autoimmune diseases should preclude the diagnosis of these diseases with the presence of relevant auto-Abs without any clinical symptoms. Thus far more investigations have focused on the immune system without understanding the roles of the targeted end organs.
Our finding of the presence of multiple cross-reactive intra-molecular and inter-molecular T-epitopes within SmD and among multiple lupus-related auto-Ags may have implications in both organ-specific and systemic autoimmunity. The presence of intra-molecular cross-reactive T-epitopes has been described in thyroid peroxidase, a major auto-Ag in Graves’ disease,24 GAD65 in type 1 diabetes mellitus (DM),25,26 myelin basic protein in experimental autoimmune encephalomyelitis27 and melan-A, a melanocyte differentiation and a melanoma associated Ag.28 Thus the presence of multiple intra-molecular cross-reactive T-epitopes may be the common feature of auto-Ags that are targeted in many autoimmune diseases. The presence of multiple shared T-epitopes among lupus-related auto-Ags provides the basis for the diversification of lupus-related Abs to snRNP and the Ro60/La/Ro52 complex and vice versa. Our observation is congruent with the observation that cloned T cells from patients with mixed connective tissue disease recognise T-epitopes present on U1–70kDa and SmB in a HLA-DR4−restricted manner.29 It is likely that the presence of multiple shared T-epitopes represents a general mechanism for targeting multiple auto-Ags in different autoimmune disorders. For example, patients with type 1 DM have auto-Abs to insulin, GAD65 and protein tyrosine phos-phatase.30 It would be of interest to determine whether these three molecules have shared T-epitopes, explaining why they are targeted together.
TCR polyreactivity31 is the basis for detecting cross-reactive T-epitopes and bacterial molecular mimics that are capable of inducing lupus-related auto-Abs. Our observations agree with the recent observation that the mechanism of negative selection to eliminate autoreactive T cells has been shown to be income-plete and the non-deleted autoreactive T cells are thought to be negatively regulated by Ag-specific Treg cells.32,33 The large number of bacterial mimics of SmD T epitopes with some of them sharing B cell epitopes with relevant SmD peptides and their potency to induce lupus-related auto-Abs would provide the basis for detecting lupus-related auto-Abs in healthy individuals. The ever larger numbers of mimics from viral or fungal organisms that have not been interrogated and the unique T-epi-tope structures of lupus-related Ags would suggest that autoreactive T and B cells against lupus-related Ags are inevitably present in normal individuals. In addition, some of these autoreactive immune cells are likely positively selected for host defense. The identification of a significant number of commensal bacteria harbouring T-epitope mimics that may initiate and perpetuate the production of lupus-related auto-Abs provides credence that manipulation of microbiota is an appropriate therapeutic approach in treating SLE and other autoimmune disorders. Some of these commensal bacteria may have significant immu-nomodulating effects.34,35 They may also serve as a source of antigenic stimulation to initiate and to perpetuate the auto-immune response.
Autoreactive T cells and auto-Abs to auto-Ags in other autoimmune diseases may be generated by similar mechanisms as those to lupus-related Ags. Our observations and those by others provide an explanation for the observed side effects of autoimmune disorders in patients treated with checkpoint inhibitors as their anti-cancer therapies.36 In this regard, it is of interest to note that the most common endocrine disease in cancer immunotherapies is thyroid dysfunction.37 It is no coincidence that the most common endocrine disorder of autoimmune origin is autoimmune thyroiditis. These observations suggest that screening patients with a familial history of multiple autoimmune disorders and for the presence of auto-Abs may identify the population at risk. Similarly, our findings have significant implications in post-vaccination autoimmunity. It has been postulated that autoimmune/inflammatory syndrome may be due to the immunogenic components of the vaccines and/or adjuvants that are often present in vaccines to enhance their immunological potency.38,39 The molecular basis for vaccine-induced autoimmunity is likely due to the presence of T-epitope mimics of autoimmune disease-related auto-Ags in addition to the presence of adjuvants that are used to boost the immunoge-nicity of the vaccines. In this regard, Tetanus Toxoid (TT) has been implicated to be temporally associated with the development of SLE.40,41 Recently we have obtained preliminary data showing the cross-reactivity between TT and SmD. Several of the HLA-DR3−restricted TT T-epitopes appear to be capable to induce auto-Ab to SmD and/or Ro60 in mice with the human leukocyte antigen-DR isotype (HLA-DR)3 transgene (data not shown). Further studies are needed to firmly establish the casual effect of TT in the pathogenesis of SLE. However, these preliminary results may provide an explanation for the above-cited clinical observations. A systematic review and meta-analysis reveals that vaccinations with human papillomavirus (HPV), hepatitis B virus (HBV), influenza vaccine and anthrax are also linked to increased risk of SLE and rheumatoid arthritis.3,4 Analyses on the T-epitopes of these vaccine immunogenic components that are restricted by relevant HLA-DR types may provide information regarding the pathogenesis of autoimmune diseases associated with these vaccines. Elimination of these T-epitopes may result in safer vaccines.
Supplementary Material
Key messages.
Lupus-related auto-antibodies (auto-Abs) are T cell driven and HLA-DR restricted but the mechanisms for their initiation and diversification are not known.
T cell responses to SmD, a major lupus-related auto-Ag, utilize multiple TCRs and are HLA-DR restricted.
Multiple intramolecular cross-reactive T cell epitopes and intermolecular cross-reactive T cell epitopes are identified with SmD and among various systemic lupus erythematosus related autoantigens (auto-Ags), providing the molecular basis why these auto-Ags are being targeted and the basis for auto-Ab diversification.
The prevalence of T cell epitope mimics from microbes is consistent with the conclusions that auto-Abs and autoreactive T cells are initiated by multiple environmental Ags and their detection is inevitable in normal individuals.
Autoimmunity should be separated from endorgan damage in lupus and other autoimmune diseases, suggesting that autoimmune diseases should not be diagnosed without related clinical presentations.
Acknowledgements
Some of the data was presented in abstract at the American College of Rheumatology’s annual meeting in 2016. The help in preparation for this manuscript by Lena Garrison is acknowledged.
Funding This research was supported by grants from the National Institutes of Health: specifically, R01 AR047988 from the National Institute of Arthritis and Skin and Musculoskeletal Diseases and from the National Institute of Diabetes and Kidney Diseases R01 DK105833. It was also supported by the Alliance for Lupus Research TIL33261 5 and Lupus Research Alliance DIA481517.
Footnotes
Disclaimer The opinions in this manuscript do not reflect those of the granting agencies.
Competing interests None declared.
Patient consent Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Tsokos GC. Systemic lupus erythematosus. N EnglJ Med 2011;365:21 10–21. [DOI] [PubMed] [Google Scholar]
- 2.Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003;349:1526–33. [DOI] [PubMed] [Google Scholar]
- 3.Craft J Antibodies to snRNPs in systemic lupus erythematosus. Rheum Dis Clin North Am 1992;18:311–35. [PubMed] [Google Scholar]
- 4.Harley JB, Scofield RH, Reichlin M. Anti-Ro in Sjögren’s syndrome and systemic lupus erythematosus. Rheum Dis Clin North Am 1992;18:337–58. [PubMed] [Google Scholar]
- 5.Graham RR, Ortmann W, Rodine P, et al. Specific combinations of HLA-DR2 and DR3 class II haplotypes contribute graded risk for disease susceptibility and autoantibodies in human SLE. Eur J Hum Genet 2007;15:823–30. [DOI] [PubMed] [Google Scholar]
- 6.McHugh NJ, Owen P, Cox B, et al. MHC class II, tumour necrosis factor alpha, and lymphotoxin alpha gene haplotype associations with serological subsets of systemic lupus erythematosus. Ann Rheum Dis 2006;65:488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rood MJ, van Krugten MV, Zanelli E, et al. TNF-308A and HLA-DR3 alleles contribute independently to susceptibility to systemic lupus erythematosus. Arthritis Rheurri 2000;43:129–34. [DOI] [PubMed] [Google Scholar]
- 8.Jiang C, Deshmukh US, Gaskin F, et al. Differential responses to Smith D autoantigen by mice with HLA-DR and HLA-DQ transgenes: dominant responses by HLA-DR3 transgenic mice with diversification of autoantibodies to small nuclear ribonucleoprotein, double-stranded DNA, and nuclear antigens. J Immunol 2010;184:1085–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paisansinsup T, Deshmukh US, Chowdhaiy VR, et al. HLA class II Influences the Immune response and antibody diversification to Ro60/Sjögren’s syndrome-A: heightened antibody responses and epitope spreading in mice expressing HLA-DR molecules. J Immunol 2002;168:5876–84. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhary VR, Dai C, Tilahun AY, et al. A Central Role for HLA-DR3 in Anti-Smith Antibody Responses and Glomerulonephritis in a Transgenic Mouse Model of Spontaneous Lupus. J Immunol 2015;195:4660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deshmukh US, Sim DL, Dai C, et al. HLA-DR3 restricted T cell epitope mimicry in induction of autoimmune response to lupus-associated antigen SmD. J Autoimmun 2011;37:254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajagopalan G, lijima K, Singh M, et al. Intranasal exposure to bacterial superantigens induces airway inflammation in HLA class II transgenic mice. Infect Immun 2006;74:1284–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kruisbeek AM. Production of mouse T cell hybridomas. CurrProtocImmunol 2001;Chapter 3:Unit 3.14. [DOI] [PubMed] [Google Scholar]
- 14.Chen F, Rowen L, Hood L, et al. Differential transcriptional regulation of individual TCR V beta segments before gene rearrangement. J Immunol 2001;166:1771–80. [DOI] [PubMed] [Google Scholar]
- 15.Bunce M PCR-sequence-specific primer typing of HLA class I and class II alleles. Methods Mol Biol 2003;210:143–71. [DOI] [PubMed] [Google Scholar]
- 16.Hansen JA, Fu SM, Antonelli P, et al. B-Lymphoid cell lines derived fromHLA-D homozygous donors. Immunogenetics 1979;8:51–64. [Google Scholar]
- 17.Fu SM, Deshmukh US, Gaskin F. Pathogenesis of systemic lupus erythematosus revisited 2011: end organ resistance to damage, autoantibody initiation and diversification, and HLA-DR. J Autoimmun 2011;37:104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pal R, Deshmukh US, Ohyama Y, et al. Evidence for multiple shared antigenic determinants within Ro60 and other lupus-related ribonucleoprotein autoantigens in human autoimmune responses. J Immunol 2005;175:7669–77. [DOI] [PubMed] [Google Scholar]
- 19.Deshmukh US, Bagavant H, Sim D, et al. A SmD peptide induces better antibody responses to other proteins within the small nuclear ribonucleoprotein complex than to SmD protein via intermolecular epitope spreading. J Immunol 2007;178:2565–71. [DOI] [PubMed] [Google Scholar]
- 20.Szymula A, Rosenthal J, Szczerba BM, et al. T cell epitope mimicry between Sjögren’s syndrome Antigen A (SSA)/Ro60 and oral, gut, skin and vaginal bacteria. Clin Immunol 2014;152(1–2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waters ST, McDuffie M, Bagavant H, et al. Breaking tolerance to double stranded DNA, nucleosome, and other nuclear antigens is not required for the pathogenesis of lupus glomerulonephritis. J Exp Med 2004;199:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ge Y, Jiang C, Sung SS, et al. Cgnz1 allele confers kidney resistance to damage preventing progression of immune complex-mediated acute lupus glomerulonephritis. J Exp Med 2013;210:2387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai C, Deng Y, Quinlan A, et al. Genetics of systemic lupus erythematosus: immune responses and end organ resistance to damage. Curr Opin Immunol 2014;31:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quaratino S, Thorpe CJ, Travers PJ, et al. Similar antigenic surfaces, rather than sequence homology, dictate T-cell epitope molecular mimicry. Proc Natl Acad Sci U S A 1995;92:10398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao CC, Sytwu HK, Chen EL, et al. The role of MHC class II molecules In susceptibility to type I diabetes: Identification of peptide epitopes and characterization of the T cell repertoire. Proc Natl Acad Sci U S A 1999;96:9299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Wang B, Frelinger JA, et al. T-cell promiscuity in autoimmune diabetes. Diabetes 2008;57:2099–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kono DH, Urban JL, Horvath SJ, et al. Two minor determinants of myelin basic protein induce experimental allergic encephalomyelitis in SJL/J mice. J Exp Med 1988;168:213–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dutoit V, Rubio-Godoy V, Pittet MJ, et al. Degeneracy of antigen recognition as the molecular basis for the high frequency of naive A2/Melan-a peptide multimer(+) CD8(+) T cells in humans. J Exp Med 2002;196:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Silva-Udawatta M, Kumar SR, Greidinger EL, et al. Cloned human TCR from patients with autoimmune disease can respond to two structurally distinct autoantigens. J Immunol 2004;172:3940–7. [DOI] [PubMed] [Google Scholar]
- 30.Taplin CE, Barker JM. Autoantibodies in type 1 diabetes. Autoimmunity 2008;41:11–18. [DOI] [PubMed] [Google Scholar]
- 31.Wucherpfennig KW, Allen PM, Celada F, et al. Polyspecificity of T cell and B cell receptor recognition. Semin Immunol 2007;19:216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legoux FP, Lim JB, Cauley AW, et al. CD4+ T cell tolerance to tissue-restricted self antigens is mediated by antigen-specific regulatory T cells rather than deletion. Immunity 2015;43:896–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Legoux FP, Lim JB, Cauley AW, et al. CD4+ T cell tolerance to tissue-restricted self antigens is mediated by antigen-specific regulatory T cells Rather than deletion. Immunity 2015;43:896–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geva-Zatorsky N, Sefik E, Kua L, et al. Mining the human gut microbiota for immunomodulatory organisms. Cell 2017;168:928–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schirmer M, Smeekens SP, Vlamakis H, et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 2016;167:1 125–36. [DOI] [PubMed] [Google Scholar]
- 36.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68. [DOI] [PubMed] [Google Scholar]
- 37.Villa NM, Farahmand A, Du L, et al. Endocrinopathies with use of cancer immunotherapies. Clin Endocrinol 2018;88:327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang B, Shao X, Wang D, et al. Vaccinations and risk of systemic lupus erythematosus and rheumatoid arthritis: A systematic review and meta-analysis. Autoimmun Rev 2017;16:756–65. [DOI] [PubMed] [Google Scholar]
- 39.Soriano A, Nesher G, Shoenfeld Y. Predicting post-vaccination autoimmunity: who might be at risk? Pharmacol Res 2015;92:18–22. [DOI] [PubMed] [Google Scholar]
- 40.Fox RA. Disseminated lupus erythematosus: an allergic disease? Arch Path 1943;36:311–5. [Google Scholar]
- 41.Older SA, Battafarano DF, Enzenauer RJ, et al. Can immunization precipitate connective tissue disease? Report of five cases of systemic lupus erythematosus and review of the literature. Semin Arthritis Rheum 1999;29:131–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


