Abstract
Chronic inflammatory diseases are often progressive, resulting not only in physical damage to patients but also social and economic burdens, making early diagnosis of them very critical. Nuclear medicine techniques can enhance the detection of inflammation by providing functional as well as anatomical information when combined with other modalities such as magnetic resonance imaging, computed tomography or ultrasonography. While small molecules and peptides were mainly used for the treatment and imaging of chronic inflammatory diseases in the past, antibodies and their fragments have also been emerging for chronic inflammatory diseases since they show high specificity to their targets and can have various biological half-lives depending on how they are engineered. In addition, imaging using antibodies or their fragments can visualize the in vivo biodistribution of the probes or help monitor therapeutic responses, providing physicians a greater understanding of drug behavior in vivo and another means of monitoring their patients. In this review, we introduce various targets and radiolabeled antibody-based probes for molecular imaging of chronic inflammatory diseases in preclinical and clinical studies. Targets can be classified into three different categories: 1) cell adhesion molecules, 2) surface markers on immune cells, and 3) cytokines or enzymes, and limitations and future directions of using radiolabeled antibodies for imaging inflammatory diseases are also discussed.
Keywords: Antibodies, autoimmune diseases, inflammation, positron emission tomography (PET), single-photon emission computed tomography (SPECT)
Graphical Abstract

Table of Contents:
Chronic inflammatory diseases are often debilitating to their patients, and sometimes difficult to diagnose and monitor. To this end, molecular imaging techniques employing antibodies targeted to various inflammation-specific markers can aid in patient diagnosis and monitoring, as presented in this review.
1. Introduction
Chronic inflammatory diseases can progressively debilitate organs and body systems, and are often accompanied by comorbidities, such as cardiovascular problems, infectious diseases, or cancer.[1] Many chronic inflammatory diseases occur based on autoimmunity. Autoimmune diseases are characterized by the breach of immunological tolerance, which can be triggered by genetic predisposition and environmental factors.[2] In addition, both innate and adaptive immunity may also be involved in the pathophysiology of autoimmune diseases by producing autoantigens and inducing molecular mimicry, ultimately resulting in organ-specific or systemic tissue damages.[2] Rheumatoid arthritis (RA), inflammatory bowel disease (IBD), multiple sclerosis (MS), systemic lupus erythematosus (SLE), autoimmune thyroid disease (AITD) and autoimmune hepatitis are all chronic autoimmune diseases. On the other hand, chronic inflammatory diseases such as atherosclerosis or Alzheimer’s disease are not derived from autoimmunity and are more likely related to aging and degenerative mechanisms.[3] Since diseases in both categories are mostly progressive and destructive, early and accurate detection of the diseases and stratification of patients by level of risk are essential to prevent complications and improve the patients’ prognosis.
Typical diagnostic strategies for inflammatory diseases include physical examination, laboratory analyses, and endoscopy.[2] Furthermore, non-invasive imaging techniques including x-ray, ultrasonography, magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT), and positron emission tomography (PET) are also widely used for the evaluation of disease activity and monitoring treatment response in a clinical setting.[4] In particular, radionuclide-based SPECT and PET imaging have several advantages over other modalities in that they can provide functional molecular information with high contrast and sensitivity in the nano- or pico-molar range.[5] Currently, several radiopharmaceuticals have been developed and used for the diagnosis of chronic inflammatory diseases in clinic. For example, radiolabeled white blood cells such as 99mTc-hexamethylpropyleneamine oxime (HMPAO)-leukocytes have been commonly used for monitoring the migration of immune cells into inflamed sites of IBD or RA.[6] Although 99mTc-HMPAO-leukocytes can detect the extent of disease with high sensitivity, this strategy has disadvantages since the ex vivo labeling techniques are cumbersome and it is hard to achieve long-term observation of cell viability with this method.[7]
18F-fluorodeoxyglucose (FDG) is another radiopharmaceutical extensively used for various inflammatory diseases as well as malignancies by taking advantage of increased glucose metabolism in target cells. However, the usefulness of 18F-FDG may be limited by its non-specificity and vulnerability to physiologic conditions such as glucose level or kidney functions.[8] In this sense, radiolabeled antibody-based molecular imaging has been emerging as a promising strategy not only for noninvasive detection of chronic inflammation but also selection of patients for antibody-based therapy. As a general method for synthesizing antibody-based radiotracers, antibodies are conjugated with chelators using the reactive amine or sulfhydryl groups on the antibodies, and then incubated with radiometal solutions. Detailed methods can be found in several other papers.[9] Since monoclonal antibodies (mAbs) are characterized by high specificity for target molecules and long in vivo circulation half-lives, mAbs labeled with radiometals may detect inflammation effectively and quantitatively in patients with chronic inflammatory diseases.[10] In addition, intact mAb molecules can be engineered into other formats such as antibody fragments (e.g. F(ab’)2, Fab, nanobodies [Nb], and single chain variable fragments [scFv]) with lower molecular weights to achieve faster plasma clearance and potentially enhanced biodistribution.[11] In this review, we will discuss SPECT and PET imaging of chronic inflammatory diseases focusing on radiolabeled antibody-based probes and their targets in three different categories (adhesion molecules, surface markers of immune cells, and cytokines or enzymes) (Figure 1).
Figure 1.

Targets for antibody-based tracers in chronic inflammatory diseases.
2. Targeting cell adhesion molecules
Interaction between cell adhesion molecules with their ligands regulates adherence of leukocytes to other cells or the extracellular matrix (ECM), and mediates migration of immune cells to sites of inflammation.[12] Since the activation of immune cells and subsequent cytokine production (e.g. Tumor necrosis factor-alpha (TNF-α), Interleukin-1, and Interferon-gamma) can increase the expression of adhesion molecules on lymphocytes and endothelial cells during inflammation, adhesion molecules have been promising targets for molecular imaging of autoimmune and inflammatory diseases.[12] Herein, we introduce molecular imaging of adhesion molecules based on radiolabeled antibodies in three different categories: the integrin family, immunoglobulin superfamily, and selectins.
2.1. Integrin family
Integrins are transmembrane molecules found on many cell types that mediate cell adhesion by coupling the ECM to intracellular cytoskeletons and influence cell signaling by activating various pathways including that of the mitogen-activated protein kinase.[12–13] Integrin is a non-covalently bound heterodimer consisting of α and β subunits. Among them, integrin β7 is selectively expressed on lymphocytes in the Peyer’s patches and mesenteric lymph nodes in inflamed guts and can pair with α4 or αE to form a heterodimer.[14] While integrin α4β7 plays a role in the recruitment of lymphocytes by targeting mucosal addressin cell-adhesion molecule-1 present on endothelial cells of the intestine, the αEβ7 integrin is more likely to contribute to retention of lymphocytes by interacting with E-cadherin molecules.[14] In 2014, the US Food and Drug Administration (FDA) therefore approved vedolizumab, an anti-α4β7 antibody, for the treatment of both moderate-to-severe ulcerative colitis and Crohn’s disease.[15]
Dearling et al. employed 64Cu-labeled mAbs (FIB504.64) against the β7 molecule to evaluate the feasibility of immunodetection of lymphocytes in a mouse model of IBD.[14] The accumulation of 64Cu-labeled FIB504.64 (average ± standard deviation, in percent of the injected dose per gram of tissue [%ID/g]) was higher in the gut of mice with colitis (6.49 ± 2.25) than in the mice without colitis (3.64 ± 1.12) or the 64Cu-labeled non-specific antibody group (3.97 ± 0.48, p < 0.05, n = 5–6), indicating β7 as a potential target for the diagnosis and therapy of colitis. Dearling and colleagues also extended their study to clarify target cell populations and to compare the pharmacokinetic properties of three different probes: the F(ab’)2 and Fab fragments of the previous FIB504.64 (anti-β7 antibody), and DATK32 antibodies (anti-α4β7 integrin) in mouse models of colitis.[16] While the uptake of the DATK32 antibodies in the gut of colitis mice slightly increased at the last time point, 48 h post-injection (p.i.), the accumulation of FIB504.64-Fab fragments in the intestine was highest at 4 h p.i. and the uptake of FIB505.64-F(ab’)2 was obvious as early as 1 h p.i. Of note, the ratio of uptake between colitis and normal mice in the large intestine was lower for 64Cu-labeled DATK32 (1.38) than for intact FIB504.64, F(ab’)2, or Fab fragments of FIB504.64 (1.78, 3.15, and 1.84, respectively). These results were also further confirmed by immunohistochemistry results showing a relatively smaller population of α4β7 cells, implicating that targeting the entire population of cells expressing the β7 molecule may be a better option for developing imaging tracers for colitis. Furthermore, the highest intestinal uptake ratio of the F(ab’)2 group suggests its potential as a promising tracer for immunodetection in colitis.
In addition to α4β7, other integrin molecules such as α4β1 (very late antigen-4 [VLA-4]), and αLβ2 (lymphocyte function-associated antigen-1 [LFA-1]) have also been related to autoimmunity.[12] VLA-4 is known to be associated with blood-brain barrier penetration of T cells in MS or experimental autoimmune encephalitis (EAE), an animal model of MS.[17] Natalizumab, a mAb against VLA-4, was accordingly approved in 2004 as an effective therapy against MS.[18] Similarly, the expression of LFA and its ligand ICAM-1 can promote infiltration of autoreactive lymphocytes into synovial tissues in RA or penetration of leukocytes into brain microvascular endothelial cells in MS.[19] Despite the involvement of VLA-4 and LFA-1 in autoimmunity, molecular imaging of autoimmune diseases with radiotracers targeting these integrin molecules has been limited to malignant and non-autoimmune inflammatory diseases. For example, VLA-4 mostly has been targeted for the molecular imaging of malignant diseases such as melanoma[20] or multiple myeloma.[21] Recently, 111In-DOTA-butylamino-NorBIRT (111In-DANBIRT) has been used for targeting LFA-1 on inflammatory cells in atherosclerosis and showed local uptake in atherosclerotic plaque lesions.[22] These integrin molecules certainly merit further investigation as promising targets for radionuclide-based imaging in autoimmune and inflammatory diseases.
2.2. Immunoglobulin superfamily adhesion molecules
Immunoglobulin superfamily adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) were named after their structure containing immunoglobulin-like domains, and play a role in both the migration and adhesion of different types of immune cells at sites of inflammation by interacting with ligands including integrins (e.g. VLA-4).[12] Two peptide-based radiopharmaceuticals targeting VCAM-1 (99mTc-B2702-p,[23] and 18F-4V[24]) were initially developed and the accumulation of the tracers in atherosclerotic plaques was observed in preclinical animal models. Even though they showed high affinity for VCAM-1 and good correlation with the expression levels of inflammatory genes such as CD68, a biomarker of macrophages in inflammatory atherosclerosis, clinical use of these tracers is still limited.[6]
Broisat et al. developed a radiolabeled Nb tracer, 99mTc-labeled cAbVCAM1–5, targeting VCAM-1 for the imaging of atherosclerotic lesions.[25] The tracer showed mouse and human cross reactivity in detection of VCAM-1 and higher lesion-to-control (VCAM-1-negative aortic walls) ratios (4.95 ± 0.85) compared to control cAbBcII10 antibodies (1.66 ± 0.28, p < 0.05, n = 4–6) in ApoE-deficient (ApoE−/−) mice, an animal model of atherosclerosis. The same group also evaluated a potential use of this Nb tracer for monitoring response to statin therapy in mouse models of atherosclerosis.[26] In vivo SPECT/CT imaging showed uptake of 99mTc-cAbVCAM1–5 in the aorta, lymph nodes, and thymus, which correlated well with the expected distribution of VCAM-1 expression in mice with western food intake promoting atherosclerosis. In addition, a significant decrease in the uptake of 99mTc-cAbVCAM1–5 in aortic lesions was observed in 35-wk-old atorvastatin-treated mice (0.87 ± 0.06 vs. 1.11 ± 0.09 in the control group, in the injected dose per volume of tissues [%ID/cm3]; p = 0.035, n = 9), suggesting this radiotracer as a promising agent for sensitive detection of atherosclerotic plaques and monitoring the effects of statin therapy in atherosclerosis.[26]
The same Nb has also been labeled with 18F and used for PET/CT imaging of inflamed atherosclerotic lesions in ApoE−/− mice (n = 3).[27] The accumulation of the 18F-labeled tracer in atherosclerotic plaques was significantly higher than that in the control group and showed good correlation with the extent of atherosclerosis as indicated by lesion-extension scores (Score 0 to 3, 3 is the most serious). Notably, while Nbs radiolabeled with 99mTc typically show high kidney retention, the uptake of 18F-FB-cAbVCAM1–5 in the kidneys was much lower than that of 99mTc-cAbVCAM 1– 5,[26] implicating renal metabolization and excretion of this 18F-labeled Nb.
A radiolabeled antibody against VCAM-1 has also been used with other imaging modalities. Liu et al. labeled a scFv targeting VCAM-1 with 99mTc (99mTc-scFv-VCAM1) and Cy5 (Cy5-scFv-VCAM1) for SPECT and optical imaging of atherosclerotic plaques in rabbit and mouse models of atherosclerosis.[28] In SPECT/CT imaging, uptake of the tracer in the aortic arch lesions of experimental rabbits was observed (Figure 2A) and the presence of atherosclerotic plaques was further confirmed by H&E staining. In fluorescence imaging, target-to-background ratios were significantly higher in atherosclerotic mice than control mice at 3 h (1.63 ± 0.09 vs 1.18 ± 0.08, p = 0.003, n = 5) and 6 h (1.39 ± 0.15 vs 1.06 ± 0.05, p = 0.02) while the accumulation was similar at 12 h and 24 h. Compared to a Nb tracer, the optimal in vivo imaging time required for this scFv probe may therefore be elongated since the size of a scFv is larger than that of a Nb (28 kDa vs 15 kDa) and the serum half-life is accordingly longer (194 ± 21 min vs 4.9 min).[28]
Figure 2.
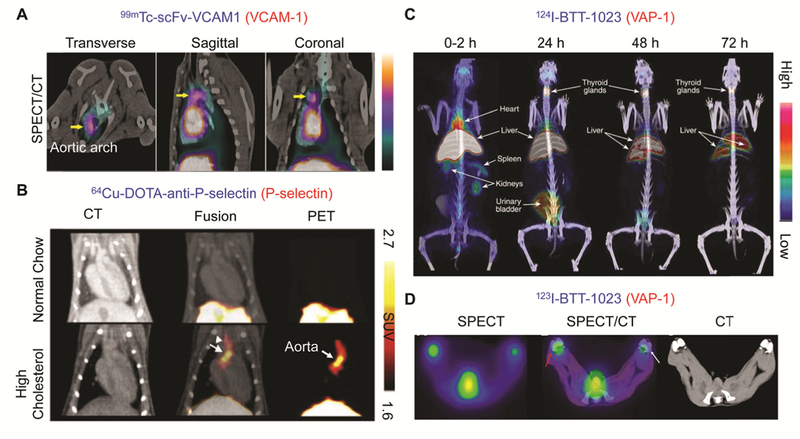
(A) SPECT/CT images of 99mTc-scFv-VCAM1 in a rabbit model of atherosclerosis. The uptake of radiotracer in the aortic arch (yellow arrow) was observed.[28] (B) PET/CT images of 64Cu-DOTA-anti -P-selectin mAbs in control mice (normal chow) and in atherosclerotic mice (high cholesterol).[42b] (C) Whole-body PET/CT images of 124I-BTT-1023 in a healthy rabbit. The accumulation of radiotracer in the liver and thyroid glands was observed. (D) Transaxial SPECT/CT images of 123I-BTT-1023 in rabbit knees at 2 h p.i. Red arrow and white arrow indicate inflamed and control joint, respectively.[48]
In addition to atherosclerosis, high VCAM and ICAM expression has also been found in many autoimmune diseases such as SLE,[29] RA,[30] and AITD.[31] In addition, Sans et al. performed a scintigraphy study with an 123I-labeled anti-VCAM-1 mAb in animal models of colitis.[32] The radiotracer effectively visualized colonic inflammatory lesions and the extent of accumulation correlated well with histological damage, suggesting its potential use as an imaging probe for human IBD. Therefore, even though there have been few studies using VCAM-1 or ICAM-1 as molecular targets for imaging autoimmunity, immunoglobulin superfamily adhesion molecules can be promising markers for molecular imaging of autoimmune diseases.
2.3. Selectins
Selectins are single-chain transmembrane glycoproteins and can be classified into three subtypes (L-, E-, and P-selectins) depending on the cell types on which they are originate.[12] While L-selectin is expressed fundamentally on all leukocytes, E-and P-selectins are highly present on the endothelium and platelets at sites of inflammation induced by immunologic responses such as cytokines (e.g. Interleukin-1, TNF-α).[33] Similar to other adhesion molecules, selectins can mediate migration, localization, and activation of leukocytes at sites of inflammation after binding with glycosylated or sialylated ligands.[12] Therefore, the expression of E-selectin has been demonstrated in several inflammatory diseases[12] and has been targeted for many different imaging modalities including SPECT,[34] MRI,[35] and ultrasound.[36] 111In-labeled anti-E-selectin F(ab’)2 fragments successfully visualized inflamed sites of patients with IBD, suggesting its potential use for evaluating and monitoring patients with IBD [37]. Studies performed by Jamar et al.[34] and Keelan et al.[38] also showed that 99mTc and 111In-labeled mAbs against E-selectin (1.2B6) and its fragment (Fab or F(ab’)2) are promising molecular probes for imaging inflamed synovitis in preclinical RA models and human RA patients. Detailed information about E-selectin imaging with radiolabeled antibodies in RA has been covered in another review.[39]
P-selectin is located in the α-granules of platelets and Weibel-palade bodies of endothelial cells, and is also expressed by inflamed endothelium.[33] P-selectin can be a potential target for imaging atherosclerotic plaques and thrombi since it is involved in the recruitment of monocytes and lymphocytes and increases integrin expression on the arterial wall during inflammation.[40] One of the radiopharmaceuticals for imaging P-selectin is Fucoidan, a polysaccharide which has been utilized for SPECT[41] and PET[42] imaging of thrombi and vulnerable atherosclerotic plaques in preclinical models. Nakamura et al. utilized a 64Cu-DOTA-anti-P-selectin mAb for imaging atherosclerotic plaques in low density lipoprotein receptor-deficient (LDLr−/−) mice.[42b] PET/CT imaging showed selective accumulation of the radiotracer in the aorta of mice fed with a high cholesterol diet (Figure 2B). The results were further confirmed by autoradiography and ex vivo Oil Red O staining of plaques to visualize lipids, indicating that this 64Cu-labeled probe can be useful in detecting atherosclerotic plaques. Furthermore, the presence of P-selectin in platelets allows it to be used as a potential thrombi diagnosis target. Xu et al. expressed the light chain of antibodies against P-selectin (SZ-51) in P.pastoris to generate radiolabeled recombinant proteins (SZ-LC) for imaging thrombi.[43] 99mTc-SZ-LC showed specific binding to activated human platelets and accumulation in the thrombus region induced in dog veins, indicating the feasibility of using this tracer for thrombi diagnosis. The selectin family is consequently a promising imaging target for autoimmune and chronic inflammatory diseases.
2.4. Vascular adhesion protein-1
Vascular adhesion protein-1 (VAP-1) is another glycoprotein on endothelial cells involved in inflammation responses by interacting with other adhesion molecules and producing inflammatory mediators.[44] Even though VAP-1 is one of the highly promising molecules targeted for both imaging and treatment of inflammatory diseases, most of the reported PET imaging studies of this target have been performed using radiolabeled peptides (e.g. 68Ga-DOTAVAP-P1,[45] 68Ga-DOTAVAP-PEG-P1 or 2,[46] and 68Ga-DOTA-Siglec-9[47]). Details of imaging with these probes have been covered by another review.[44] BTT-1023 is a human mAb against VAP-1 and has been used for the treatment of inflammatory diseases.[48] Synovitis was induced chemically in rabbits, and both PET/CT (124I-BTT-1023) and SPECT/CT (123I-BTT-1023) scans were performed.[48] Radiolabeled BTT-1023 was found to have high clearance and distributed to the liver and thyroid, which may be attributed to high VAP-1 expression on hepatic sinusoidal endothelia and the uptake of free iodine in thyroid (Figure 2C). Accumulation of the tracer was detected in inflamed lesions of rabbits with synovitis (Figure 2D), demonstrating the feasibility of using radiolabeled BTT-1023 for imaging of immune responses in inflammatory diseases.
3. Targeting surface markers on immune cells
3.1. Macrophages and monocytes
The activity of macrophages and monocytes is closely associated with the degree of inflammation and the severity of symptoms of autoimmune and inflammatory diseases. For example, in RA, infiltration of activated inflammatory macrophages into synovial tissues may cause matrix degradation, angiogenesis, and fibroblast proliferation.[49] In addition, infiltration of macrophages may result in cartilage and bone destruction, since they are the precursor cells of the osteoclasts responsible for bone resorption.[50] In MS, it is known that macrophages and microglia can induce neuroinflammation and neurodegeneration in the central nervous system (CNS) by secreting pro-inflammatory cytokines, causing oxidative stress, and changing the permeability of the BBB.[51] Currently, SPECT and PET imaging of macrophages and monocytes in inflammatory diseases are mostly based on radiolabeled cells (e.g. 99mTc-HMPAO-labeled monocytes[52]) or small molecules targeting markers of those immune cells (e.g. 11C-PK11195 targeting translocator protein[53] and 99mTc-EC20 targeting the folate receptor[54]). More detailed information about these types of probes can be found in another review.[55] Here, antibody-based molecular imaging of macrophages and monocytes for autoimmune and inflammatory diseases will be described and radiolabeled antibodies for three different targets, including the macrophage mannose receptor, complement receptor of the Ig superfamily, and CD163, will be introduced.
Macrophage mannose receptor
Macrophage mannose receptor (MMR; CD206) is 175 kDa C-type lectin receptor mostly expressed on macrophages, dendritic cells, and selected endothelial cells.[56] Since MMR is involved in phagocytosis, processing, and presentation of self-derived peptides or proteins as well as unopsonized microorganisms or glycoproteins to T cells, expression of this receptor on macrophages may play a role in the pathogenesis of autoimmune diseases.[57] In addition, MMR is associated with the formation of osteoclasts by interacting with mannose-type oligosaccharides present on osteoclast precursor cells, which might affect bone resorption and erosion in RA.[58] Movahedi et al. developed a 99mTc-labeled Nb targeting MMR[59] and Put et al. utilized the probe for SPECT imaging to examine the expression level of MMR in macrophages and osteoclasts in collagen-induced arthritis (CIA), an animal model for RA.[60] The results of quantitative polymerase chain reaction and flow cytometry revealed that expression of MMR was found to be high in macrophages, and relatively low in osteoclasts.[60] The specificity of 99mTc-labeled Nb targeting MMR was confirmed by SPECT/CT imaging of both symptomatic and naïve mice using a control Nb (99mTc-labeled BCII10). In addition, the accumulation of the radiolabeled tracer was significantly higher in arthritic paws than normal paws of mice (Figure 3A). Interestingly, the signal from the radiolabeled Nb was also detected in non-arthritic paws of mice whose other paws had arthritic symptoms, suggesting the potential utility of this radiotracer for early detection of RA before presentation of macroscopic clinical symptoms. Since there are also studies showing that MMR is involved in the pathogenesis of autoimmune thyroid diseases[61] and the expression of MMR is elevated in impaired mucosa of UC patients,[62] MMR also can be a potential imaging target for other autoimmune diseases.
Figure 3.
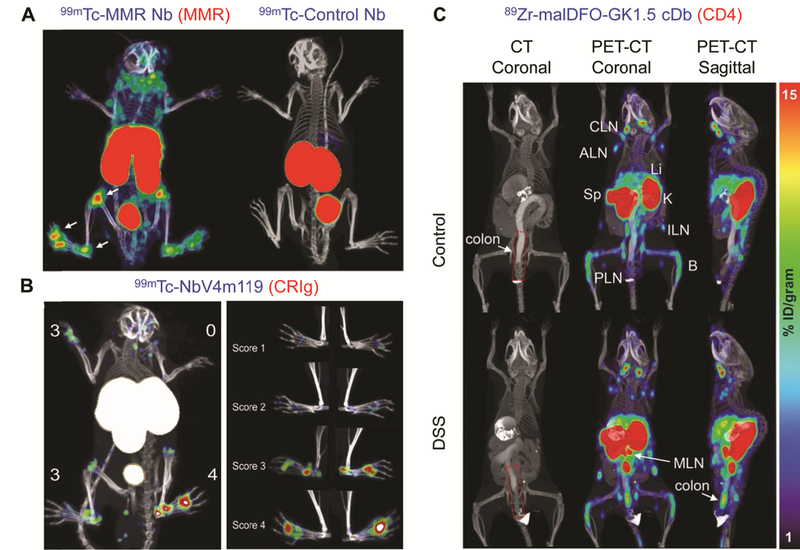
(A) SPECT/CT images of symptomatic paws at 3 h after injection of 99mTc-labeled MMR Nb and control Nb in arthritic mice.[60] The white arrows indicate MMR-positive lesions. (B) SPECT/CT images of arthritic joints and individual limbs correlating with clinical scores at 3 h after injection of 99mTc-labeled NbV4m119.[68] (C) CT (coronal) and PET-CT (coronal and sagittal) images in DSS and control mice 20 h p.i. of 89Zr-malDFO-GK1.5 cDb.[95] The red dotted lines indicate the colon ROI. Abbreviations: cervical lymph nodes (CLN), axillary lymph nodes (ALN), spleen (Sp), liver (Li), kidney (K), inguinal lymph nodes (ILN), popliteal lymph nodes (PLN), bone (B), mesenteric lymph nodes (MLN).
Aside from autoimmune diseases, MMR has been investigated as a target for atherosclerosis since it is known that macrophages also play an important role in the formation of vulnerable plaques and foam cells in atherosclerosis.[63] For example, M1 macrophages (pro-inflammatory macrophages) are involved in lipid accumulation and inflammatory responses while M2 macrophages (alternatively activated macrophages) are associated with plaque stabilization and inflammation resolution.[63] Varasteh et al. successfully observed the expression of MMR on macrophages in atherosclerotic plaques in Apo E-knock out mice with 111In-tilmanocept, a molecule targeting MMR.[64] However, another study performed by Bala et al. demonstrated that the uptake of 99mTc-labeled anti-MMR Nb in aortic lesions showed no significant difference between ApoE−/− mice and control C57BL/6 mice, requiring more consideration when using MMR for the detection of atherosclerosis.[65]
Complement receptor of the Ig superfamily
Complement receptor of the Ig superfamily (CRIg; also called CSIG4 or Z39Ig) is uniquely expressed on specific types of resident tissue macrophage subsets and plays a pivotal role in complement-mediated phagocytosis of C3-opsonized particles.[66] Tanaka et al. revealed that synovial tissues in RA patients include more cells expressing CRIg than normal synovium or tissues from other arthritic diseases such as osteoarthritis or psoriatic arthritis,[67] making CRIg a potential marker for imaging synovial tissues in RA. A CRIg-specific radiolabeled Nb (99mTc-NbV4m119) was developed by Zheng et al. and evaluated for in vivo biodistribution and detection of arthritic lesions in a mouse model of RA.[68] 99mTc-V4m119 was found to accumulate in CRIg-positive macrophages in the liver and inflamed joint lesions with good correlation with the severity of the symptoms (Figure 3B). Of note, 99mTc-NbV4m119 was detected in the knees of mice with arthritis even before the inflammatory symptoms appeared, indicating this tracer has potential for early detection in incipient lesions. The same tracer was also used for monitoring the efficacy of treatment with dexamethasone in arthritic mice.[69] The accumulation of 99mTc-NbV4m119 was observed in inflamed joints of mice with arthritis, and reduced after dexamethasone treatment in most joints, showing the potential of the tracer for monitoring therapeutic responses in autoimmune diseases.
CD163
CD163 is a 130-kDa macrophage-scavenger receptor that can eliminate plasma hemoglobin-haptoglobin complexes and is uniquely expressed on alternatively activated macrophages and a subset of monocyte lineage cells at sites of inflammation.[70] In addition, the soluble form of CD163, originating from the membrane bound receptor, is one of the promising biomarkers for diagnosis of many inflammatory diseases including type 2 diabetes, liver diseases, and sepsis.[71] Eichendorff et al. developed a radiolabeled anti-CD163 antibody, 68Ga-ED2, and investigated the systemic biodistribution of the tracer and expression of CD163 homologues in rats with CIA through PET imaging.[72] They found high uptake of the tracer in the liver and spleen, which corroborated the findings that the expression level of CD163 is high in Kupffer cells, splenic red pulp macrophages, and bone marrow macrophages.[70] The authors found significantly higher uptake in the inflamed rear paw of rats with arthritis than that of normal rats (0.148 ± 0.023 vs 0.081 ± 0.050 %ID/g; p = 0.021, n = 3–6), suggesting increased macrophage infiltration and blood flow into the inflamed area. However, the accumulation of 68Ga-ED2 in paws of rats was still low in both arthritic and normal rats in comparison to the liver or spleen, indicating the need for improvement of both metabolism and tissue penetration of the tracer.
3.2. T lymphocytes
T lymphocytes are highly involved in the initiation and development of autoimmune and inflammatory diseases. For instance, autoreactive T cells recognizing self-antigens and impaired regulatory T cells cause breakdown of tolerance and induce pro-inflammatory responses, resulting in the development of autoimmune diseases such as RA, MS, and type 1 diabetes.[73] Imaging of T lymphocytes with radiolabeled probes has been performed using various methods. For example, direct in vivo labeling of T lymphocytes or cytokines (e.g. 99mTc-HMPAO[74] or 111In-oxine labeled T-lymphocytes[75], 99mTc labeled IL-2[76]), employing nucleoside metabolisms (e.g. 18F-AraG[77], 18F-FAC[78]) or reporter genes (e.g. 18F-FSPG[79]), and targeting surface molecules on the immune cells can be effective strategies for molecular imaging of T lymphocytes. Detailed information about each of these approaches can be found in other reviews.[80] Here, molecular imaging of autoimmune and inflammatory diseases with radiolabeled antibodies targeting CD3 and CD4 will be introduced.
CD3
CD3 is a part of the T cell receptor complex and plays a key role in antigen recognition and T cell signal transduction.[81] CD3-specific monoclonal antibodies have been used for the treatment of various types of inflammatory diseases (e.g. RA, IBD, type 1 diabetes) since they can induce antigenic modulation and apoptosis of autoreactive T cells.[82] In addition, anti-CD3 mAbs can elevate the number and enhance the function of Treg cells and anti-inflammatory cytokines such as TGF-β and IL-10.[83] OKT3 (Muromonab), developed by Kung et al.,[84] was the first monoclonal murine antibody recognizing CD3 molecules expressed on human T lymphocytes. However, due to its murine origin, OKT3 can elicit a high level of immunogenicity and various side effects in humans, requiring the development of more humanized genetically-engineered anti-CD3 mAbs such as visilizumab and teplizumab.[83] In terms of molecular imaging in autoimmune diseases, radiolabeled anti-CD3 mAbs targeting CD3 or T-cell receptor-CD3 complexes can be useful to monitor inflammation activity and therapeutic response, both of which can be associated with the presence of active T lymphocytes.[85] So far, imaging of radiolabeled anti-CD3 antibodies in inflammatory diseases has been mostly limited to SPECT. Several studies using 99mTc labeled anti-CD3 antibodies in RA patients were reviewed by another article.[80a] On the other hand, PET imaging with 89Zr-labeled anti-CD3 mAbs in tumor models has been performed in several studies and successfully detected tumor infiltrating T cells.[86] Therefore, PET imaging with radiolabeled CD3 antibodies in inflammation models merits further investigation.
CD4
CD4+ T lymphocytes can interact with the major histocompatibility complex (MHC) II molecules on antigen-presenting cells, regulate both T and B cell function via helper T cells, and participate in intracellular signal transduction.[80a] It is known that CD4+ T lymphocytes have at least four different subsets, TH1, TH2, TH17 and Treg.[87] Among them, TH1 and TH17 cells play a major role in autoimmunity by producing pro-inflammatory cytokines responsible for inflammation and tissue damage, while Treg cells mainly suppress the immune system and play a role in tissue repair.[88] Since the CD4 molecule is expressed on the surface of helper T cells and macrophages in the synovial tissues of RA patients, there have been many trials using anti-CD4 antibodies for imaging RA.[80a] In 1990, Berker et al. published one of the first scintigraphy studies with 99mTc-labeled anti-CD4 antibodies (MAX.16H5) in RA patients.[89] Furthermore, Kinne et al. confirmed the specificity of a 99mTc-labeled anti-CD4 mAb against CD4+ cells in arthritic lesions of RA patients[90] and also compared the ability to detect infiltrating CD4+ T cells with anti-carcinoembryonic antigen mAbs using SPECT in another study.[91] More detailed information about these studies can be found in another review.[80a] On the other hand, the first clinical study using 99mTc-anti-CD4-Fab (99mTc-EP1645) was performed by Steinhoff et al. in 2014.[92] It was found that 99mTc-EP1645 showed good tolerability and could detect inflamed joints in arthritis patients with a 68% detection rate. As 8% of the positive scintigraphy scans were found in clinically negative joints, this tracer may have potential for early diagnosis of RA before clinical presentation. However, further investigation regarding correlation between clinical presentation and the expression of CD4 in synovial tissues still needs to be conducted.[92]
Radiolabeled anti-CD4 antibodies were also used for imaging CD4+ T cells in inflamed mucosa in preclinical models. Kanwar et al. used 111In-labeled anti-CD4+ antibodies to visualize CD4+ T cells in mice with dextran sulfate sodium (DSS)-induced colitis in SPECT/CT imaging.[93] The accumulation of the tracers in inflamed colon was significantly higher in the group of mice treated with 5% DSS compared to that in the healthy group (p < 0.01). In addition, the mean colon-to-muscle activity ratio showed good correlation (p < 0.01) with the histopathologic score, and with the number of total lymphocytes and CD4+ T cells.[93] Freise et al.[94] developed an anti-CD4 Cys-diabody (GK1.5cDb) probe for immunoPET imaging of CD4+ T cells, and employed it for the detection of DSS-induced colitis in mice.[95] 89Zr-malDFO-GK1.5cDb was used to visualize and quantify CD4+ T cells in the colons and mesenteric lymph nodes of colitis mice with immunoPET. The accumulation of the radiolabeled tracer in the colon of DSS-treated mice was significantly increased and confirmed by ex vivo results (DSS-treated group: 1.8 ± 0.4 vs control group: 0.45 ± 0.12 %ID/organ, p < 0.005, n = 8) (Figure 3C). Interestingly, %ID/g in the colon, cecum, and mesenteric lymph nodes did not show any significant difference between two groups, implicating the impact of the increased weight of the colons in colitis mice. In addition, even though the tracer enabled detection of CD4+ T cells, it is still hard to distinguish each subset of CD4+ T cells (e.g. TH1, TH17, etc), which may need further investigation.[95]
3.3. B lymphocytes
B lymphocytes as well as T lymphocytes are critical in the development of autoimmunity. Since B lymphocytes are involved in the production of autoantibodies, presentation of autoantigens, and activation of the complement cascade, depleting them with antibodies against surface markers on B lymphocytes has been one of the important approaches for the treatment of autoimmune diseases.[73b, 96] In a similar context, current molecular imaging of B lymphocytes in autoimmune and inflammatory diseases is mostly focused on targeting CD20 molecules on the surface of immune cells with anti-CD20 antibodies.[97] Here, we introduce the current status of antibody-based SPECT and PET imaging of B lymphocytes in autoimmunity and relevant diseases, mostly involving imaging of CD20 expression.
CD20
Rituximab is one of the most widely used drugs for the treatment of autoimmune diseases as well as non-Hodgkin lymphoma by targeting CD20. In 2006, the US FDA approved rituximab for the treatment of RA patients who are resistant to TNF-α therapy.[97] Furthermore, radiolabeled rituximab (e.g. 99mTc-labeled rituximab and 124I-rituximab) has also been used for molecular imaging of autoimmune diseases in several scintigraphy and PET/CT studies, which were included in another review.[97] Recently, radiolabeled rituximab has further been employed for PET imaging of autoimmune diseases in preclinical models and patients. Bruijnen et al. utilized 89Zr-radiolabeled rituximab in patients with RA to evaluate its biodistribution (Figure 4A) and find potential responders to rituximab therapy.[98] The authors found that the accumulation of the radiotracer in hand joints (Figure 4B) had significant correlation with response to rituximab therapy with 90% and 75% positive and negative predictive values, respectively, at target-to-background ratio ≥ 4.0. The probe was also found to accumulate in lymph nodes through PET imaging (Figure 4C) and the specificity of B cell-targeting was further confirmed by quantitative CD22+ (a surrogate B cell marker) cell count in immunohistochemistry analysis of excised lymph node sections.[98] Since B cells are involved in the development of MS in both human and animals, James et al. employed 64Cu-rituximab PET for non-invasive imaging of B cells in humanized mice with EAE.[99] The huCD20tg mouse model whose B cell is humanized was used since targeting human CD20 is more clinically relevant for evaluating rituximab as a molecular probe. The uptake of 64Cu-rituximab was significantly higher in the lumbar spinal cord of huCD20tg mice than in normal groups as early as 1 h p.i. (5.44 ± 0.37 vs 3.33 ± 0.20 %ID/g, p < 0.05, n = 4) (Figure 5A). The results were also corroborated by elevated 64Cu-rituximab accumulation in the spinal cord of huCD20tg EAE mice in ex vivo biodistribution studies and increased number of B220+ (a marker for B cells) cells in immunostaining (Figure 5B, 5C).[99] However, even though the mice used in this study were humanized, the feasibility of using this probe in human MS patients and the range of imaging doses that can be used without inducing B cell depletion still need to be further investigated.
Figure 4.

PET-CT images of 89Zr-rituximab in patients with rheumatoid arthritis (RA). (A) Whole-body (maximum intensity projection) PET image showing increased uptake of the tracer in the liver, spleen, and large joints including shoulders. (B) PET-CT image of an RA patient’s hands/wrists indicating joints with positive uptake of the tracer (red arrow). (C) PET-CT image demonstrating uptake of 89Zr-rituximab in an inguinal lymph node in a patient with RA (red circle).[98]
Figure 5.
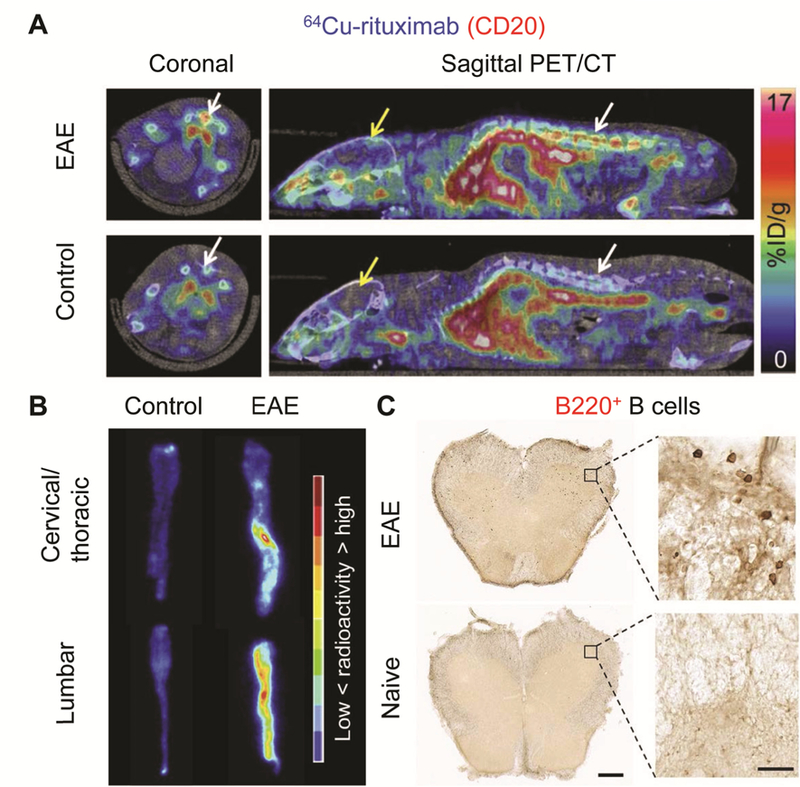
(A) In vivo PET/CT images 1 hour p.i. of 64Cu-rituximab in a mouse model of experimental autoimmune encephalitis (EAE) and control group. White and yellow arrows indicate spinal cord and brain, respectively. (B) Autoradiography images of the spinal cord in control and EAE mice. (C) Detection of B220+ B cells in the spinal cord of mouse with humanized B cells. The scale bar is 25 μm and 500 μm in high and low magnification images, respectively.[99]
Anti-murine or chimeric (tositumumab) or -murine/human (rituximab) CD20 antibodies can cause side effects or problems when re-dosing. In this regard, a new generation of anti-CD20 antibodies such as obinituzumab (humanized) or ofatumumab (fully human) have been developed and recently approved by the FDA for the treatment of B cell lymphoma.[100] The potential of using these new generation drugs for the treatment of autoimmune diseases was described by another review.[101] While there are few studies using these new generation antibodies for imaging autoimmune diseases, there are several studies showing positive results with humanized anti-CD20 antibodies for imaging in lymphoma models. For example, Zettlitz et al. used 89Zr and 124I-labeled engineered antibody fragments (cys-diabody and cys-minibody) of obinutuzumab and performed PET imaging to monitor CD20 expression and internalization after binding with antibodies in transgenic mice and a mouse model of lymphoma expressing human CD20.[102] Also, Yoon et al. utilized 89Zr-labeled obinutuzumab and ofatumumab to observe tumor targeting and biodistribution of these antibodies, and to evaluate their feasibility of being used in anti-CD20 radioimmunotherapy of human lymphoma xenografts in mice.[100] Given these promising results, using these humanized anti-CD20 antibodies for molecular imaging of autoimmune diseases may also be worthwhile.
3.4. Other targets
In addition to macrophages, T and B lymphocytes, other immune cells have also been targeted with radiolabeled mAbs for molecular imaging of chronic inflammatory diseases. BW250/183 is an antibody against granulocytes targeting NCA95 epitopes and immunoscintigraphy with 99mTc-labeled BW250/183 has been performed in IBD patients.[103] However, a study performed by Gyorke et al. suggested that 111In or 99mTc-labeled leukocytes should take priority over radiolabeled anti-granulocyte antibodies for the diagnosis of IBD patients as the method using anti-granulocyte antibodies is less sensitive and can cause patient reactions to the murine antibodies.[103b] Furthermore, class II MHC molecules and CD11b, which are expressed on various inflammatory cells including neutrophils and macrophages, have also been targeted for imaging of chronic inflammatory diseases. Rashidian et al. site-specifically labeled the variable domain of camelid heavy-chain only antibodies targeting class II MHC molecules and CD11b.[104] Both anti-MHC II and CD11b antibody fragments successfully visualized the myeloid cells in paws of mice with induced arthritis. In addition, a 99mTc-labeled anti-CD11b antibody has also been employed for SPECT/CT imaging of atherosclerotic plaques, suggesting the feasibility of this target for imaging of various chronic diseases.[105]
4. Targeting cytokines or enzymes
Targeting cytokines or enzymes unique to inflamed environments is another possible strategy for imaging of inflammatory diseases. There have been several methods for developing radiotracers targeting cytokines or enzymes such as direct labeling of cytokines[10] or employing radioligands targeting cytokine receptors.[106] Even though these peptide and protein-based probes can visualize inflamed lesions effectively, the direct labeling procedure is time-consuming and these probes have limited circulation properties. Therefore, we will focus on radiolabeled antibodies targeting cytokines and enzymes specific for inflammatory conditions, especially tumor necrosis factor-alpha (TNF-α), fibroblast active protein (FAP), and matrix metalloproteinase (MMP).
4.1. Tumor necrosis factor-alpha
Tumor necrosis factor-alpha (TNF-α) is one of the major proinflammatory cytokines involved in pathogenesis of many autoimmune diseases including RA,[39] IBD,[107] and AITD.[108] Therefore, anti-TNF-α antibodies binding to membrane-bound or soluble TNF have been used for the treatment of inflammatory diseases by blocking TNF-α action and other inflammatory responses.[39] Furthermore, anti-TNF-α antibodies have also been used in molecular imaging and could potentially help physicians select patient populations for anti-TNF-α therapy and anticipate therapeutic responses to the therapy.[39] For example, both infliximab (chimeric anti-TNF-α antibody)[109] and adalimumab (fully human anti-TNF-α antibody)[110] were radiolabeled with 99mTc and successfully used for scintigraphy studies in RA patients. Detailed information about each study can be found in the review article by Malviya et al.[39] Etanercept is a fully human fusion protein of the TNF receptor and the constant portion of the IgG1 antibody, and particularly binds to soluble TNF-α resulting in its neutralization.[111] Several PET imaging studies have been performed with radiolabeled etanercept.[111–112] Cao et al. used 64Cu-DOTA-etanercept to observe TNF-α levels in a mouse model of 12-O-tetradecanoyl-phorbol-13-acetate-induced acute and chronic inflammation.[111] The radiotracer successfully visualized ear inflammation and changes in TNF-α levels, which were dramatically increased in acute inflammation and lowered in chronic inflammation. Tobinick et al. observed the accumulation of 64Cu-DOTA-etanercept in cerebral ventricles after peripheral perispinal administration of the tracer through microPET imaging in a rat.[112] Since it has been reported that administration of etanercept improved cognitive functions in patients with Alzheimer’s disease,[113] imaging with radiolabeled etanercept may provide useful information about the distribution of the anti-TNF-α antibodies as well as the level of inflammatory cytokines in Alzheimer’s disease.[112]
4.2. Fibroblast activation protein
Fibroblast activation protein (FAP) is a 170 kDa transmembrane glycoprotein that has dipeptidyl peptidase and endopeptidase activity.[114] While FAP expression is low in healthy organs, it shows high upregulation in epithelial carcinoma and sites of tissue remodeling including fibrosis and arthritis.[114] Several radiolabeled antibodies such as 131I-F19 (murine),[115] 131I-sibrotuzumab (humanized F19 mAbs),[116] and 177Lu-labeled ESC11 and ESC14 were used for targeting and imaging malignant tissues.[117] In RA, FAP is mainly expressed on activated fibroblast-like synoviocytes and has been targeted for imaging inflamed tissue and disease activity.[4b] Laverman et al. developed an 111In- and 89Zr-labeled anti-FAP antibody, 28H1, for noninvasive imaging of mice with CIA.[4b] ImmunoSPECT imaging with 111In-28H1 showed excellent targeting of inflamed joints with high resolution, suggesting that FAP is a promising target for the detection of arthritis. Similarly, PET/CT scans with 89Zr-28H1 allowed quantification of the uptake of the tracer and showed good correlation with joint scores compared to 18F-FDG; however, this 89Zr-labeled tracer is less preferred than 111In-28H1 due to increased uptake in the bone.[4b]
Several other studies have been performed with 111In-28H1 to monitor treatment responses in preclinical RA models. For example, Terry et al. conducted a comparison study with different tracers which target fibroblasts (111In-28H1), macrophages (111In-anti-F4/80-A3–1), or integrin αvβ3 (111In-RGD2), to compare their capabilities for monitoring therapeutic responses to etanercept in experimental arthritis.[118] While the uptake of each tracer in inflamed joints before treatment with etanercept was 23 ± 15, 8 ± 4, and 2 ± 1 %ID/g for 111In-28H1, 111In-RGD2, and 111In-anti-F4/80-A3–1, respectively, it significantly decreased to 11 ± 11, 4 ± 4, and 1 ± 0.2 %ID/g after treatment (n = 1–3, 4 joints/mouse, p < 0.001). In addition, arthritis-to-blood ratios of 111In-28H1, 111In-RGD2, and 111In-anti-F4/80-A3–1 antibodies were also significantly higher than that of the control antibody, 111In-DP47GS (p = 0.002), indicating specific uptake of the tracers in inflamed lesions. More recently, van der Geest et al. conjugated 28H1 with 99mTc-S-HYNIC for SPECT/CT imaging of mice with CIA to monitor responses to treatment with liposomes containing prednisolone.[119] 99mTc-S-HYNIC-28H1 was observed in inflamed joints and the uptake showed good correlation with arthritis scores (Figure 6A, 6B). The uptake of the tracer in each paw of liposome-treated mice was significantly lower than arthritic mice with no treatment at 5 and 9 days after treatment (p < 0.02) (Figure 6C). The same group also used the 111In-28H1 antibody for monitoring response to neutralization of IL-22, which is an important cytokine for the development of RA.[120] Anti-IL-22 therapy effectively regulated the progression of arthritis in CIA models when initiated in the early phases of CIA. In SPECT/CT imaging with 111In-28H1, significantly decreased uptake of the tracer was observed in the anti-IL-22 therapy group in a sensitive manner and further confirmed by histological and radiological analysis. Therefore, anti-FAP antibodies have potential to be used not only for imaging inflamed arthritic joints but also detecting changes in disease activity in response to various types of therapies.
Figure 6.

Targeting FAP with 99mTc-S-HYNIC-28H1 in control and liposome-treated (PLP-LCL) mice with CIA. (A) SPECT/CT images of arthritic control and liposome-injected mice at 24 h p.i. of 99mTc-S-HYNIC-28H1. Images were obtained 2, 5, and 9 days after liposomal therapy. (B) Correlation between joint uptake (%ID/g) at 24 h p.i. of 99mTc-S-HYNIC-28H1 and arthritis score of each paw for individual mice. (C) Quantification of joint uptake (%ID/g) based on SPECT analysis 2, 5, and 9 days after PLP-LCL treatment in the control and treatment groups (n = 4 or 5 per group).[119]
4.3. Matrix metalloproteinase
Matrix metalloproteinases (MMPs) are a family of calcium-dependent zinc-containing endopeptidases with subtypes such as the collagenases, the gelatinases, and the membrane-type MMPs.[121] MMPs are involved in the progression of various inflammatory diseases as well as cancer by participating in degradation of the ECM, activation of biological molecules, and regulating the behavior of various cell types.[122] So far, the development of probes for SPECT or PET imaging has been mostly based on small molecules using broad spectrum MMP inhibitors. Gerwien and Hermann et al. demonstrated that MMP-9 derived from immune cells is essential in the development of EAE and is an important marker of leukocyte infiltration of the BBB.[123] PET imaging with 18F-BR-351 allowed successful detection of MMP activity specific to early lesions and leukocyte penetration of the BBB, implicating usefulness of the tracer for monitoring disease activity during initial stages of MS development.[123] In addition, several other radioligands based on MMP inhibitors such as 123I-HO-CGS-27023A[124] and 99mTc-RP805[125] enabled visualization of MMP activity in the arterial wall and MMP expression in atherosclerotic plaques in a mouse model of atherosclerosis.
Recently, 99mTc-labeled MMP-9 antibodies (99mTc-McAbs) were developed by Wang et al. for in vivo SPECT imaging of lesions in a mouse model of atherosclerosis [126]. The accumulation of 99mTc-McAbs in left common carotid lesions was detected 2 h p.i. of the radiotracer, and further confirmed by ex vivo autoradiography and immunostaining studies. While the probes mentioned above mainly target soluble MMPs such as MMP-2 and 9, Kuge et al. developed 99mTc-labeled mAbs targeting membrane-bound type 1 MMP (MT1-MMP), which is responsible for the activation of pro-MMP-2 and pro-MMP-13, and destabilization of atherosclerotic plaques.[127] The uptake of 99mTc-anti-MT1-MMP antibodies in atherosclerotic aorta lesions in rabbit models was 5.4-fold higher than that in the control group and showed positive correlation with MT1-MMP expression. Importantly, the accumulation of 99mTc-anti-MT1-MMP antibodies was highest in the atheromatous lesions with high risk of rupture, indicating that this radiolabeled probe targeting MT1-MMP can be a potential marker for monitoring the risk of destabilization of atherosclerotic plaques.
5. Summary and outlook
Radiolabeled mAbs have high specificity, long circulation, and are easier to prepare than radiolabeled autologous immune cells.[10] By taking advantage of these properties, immunoSPECT and immunoPET can be used in chronic inflammatory diseases not only for evaluation of disease activity but also selection of patient populations for proper treatments or monitoring therapeutic responses.
Nonetheless, radiolabeled antibody-based molecular imaging of chronic inflammatory diseases has also its limitations. First, even though antibodies are molecular probes with high specificity toward their targets, it is difficult to develop tracers targeting molecules specific for chronic inflammatory diseases. Autoimmune diseases, non-autoimmune diseases, acute inflammatory diseases, and infectious diseases share many common mechanisms in their pathophysiology. In addition, general immune responses during inflammation such as increased blood flow also can increase uptake of non-specific IgG antibodies.[10] The combination of all of these factors indicates that finding highly specific biomarkers for autoimmune and inflammatory diseases and efficiently targeting them may be quite difficult. Second, although the accumulation of radiolabeled mAbs in inflamed lesions is significantly higher than that of non-inflamed lesions in many studies, the absolute uptake values of radiotracers when imaging inflammatory diseases are relatively low when compared to accumulations found in other models such as cancer, due to the relatively lower biomarker levels in these diseases. In addition, since antibodies have long circulation in the body, background radioactivity signals from antibodies are normally higher than small molecules, making high-contrast imaging difficult. Lastly, radiolabeled antibodies can face barriers for delivery to sites of inflammation. For example, even though the blood-brain barrier can be damaged during inflammation, delivery of antibodies into the brain is still limited compared to small molecules or peptides. Many studies have been performed to deliver antibodies into the brain via different routes;[128] however, molecular imaging of inflamed lesions in the central nervous system with radiolabeled antibodies still merits further investigation.
Given the studies described above, future exploration of radiolabeled antibodies for imaging chronic inflammatory diseases can take several directions. First, molecular probes targeting FAP at sites of inflammation showed relatively high accumulation compared to targeting other surface markers on immune cells or adhesion molecules, suggesting their high potential for further development and application in various chronic inflammatory diseases.[4b, 118−120]
Second, many antibodies have been modified to overcome their above-mentioned limitations. For example, engineering intact antibodies into fragments (e.g. F(ab’)2, Fab, Nb, scFv) can improve the pharmacokinetic properties of the molecules and expand the application of these probes by decreasing their size and weight. With these engineered agents, it will be important to choose appropriate radionuclides based on the adjusted biological circulation times of the tracers. For example, our group has used 44Sc (t1/2 = 4.0 h) with a relatively shorter physical half-life than 64Cu (t1/2 = 12.7 h) or 89Zr (t1/2 = 78.4 h) for the labeling of cetuximab Fab fragments.[129] In addition, we also employed 61Cu (t1/2 = 3.4 h) to label Fab[130] and F(ab’)2[131] fragments of TRC105, an antibody against CD105, and investigated the potential of the tracers for imaging angiogenesis in murine cancer model. Matching the biological and physical half-lives can not only provide higher-quality imaging, but also limit radiation dose to patients. The physical half-life is not the only property that should be considered when choosing a radionuclide; rather, other characteristics such as decay properties (emission type, energy, etc) need to be taken into account as well. Likewise, the behavior of the free element in vivo should be considered – for example, free zirconium accumulates in bone and may therefore confound imaging results in these tissues. Detailed information about each radiometal for imaging and therapy can be found in another review.[132]
Since murine or chimeric antibodies can induce anti-mouse antibodies in humans, a new generation of humanized or fully human antibodies are being developed.[83] It may also be worthwhile for researchers working in inflammatory diseases to take advantage of imaging tracers being developed for other applications, such as oncology. Indeed, with the rise of immunotherapy treatments for cancer patients, a great number of imaging agents for the immune system are being developed, which can certainly be translated to inflammatory and autoimmune diseases.[80b, 133] For example, our group successfully performed noninvasive PET imaging of lung cancer in mice by targeting CD30, which is expressed on several types of cancer cells as well as immune cells.[134] Since CD30 and its ligand play an important role in the differentiation of TH1 and TH17 cells and have been reported to be involved in the pathogenesis of chronic inflammatory diseases such as RA[135] or CNS autoimmunity,[136] they can be considered as targets for imaging chronic inflammatory diseases.
Given that chronic inflammation is associated with the development of cancer through mediation of DNA damage and neoplasia,[137] research on imaging agents for the detection of malignancy in the setting of inflammatory diseases may also help individuals with chronic inflammation and at high risk of cancer. The study performed by Turker et al. using 64Cu-DOTA-cetuximab-F(ab’)2 for the detection of colonic tumors in colitis mice is an excellent example of this approach.[138]
Lastly, developing clinically-relevant animal models for chronic inflammatory diseases is also essential. Currently, animal models of autoimmune diseases such as CIA, EAE, and DSS-induced colitis are established mostly based on the symptoms shown in human diseases and therapeutic responses in a clinical setting. Animal models that can more accurately reflect the elaborate pathophysiology of human autoimmunity such as the presence of autoantibodies or autoreactive immune cells will certainly provide greater insight into these diseases. Models that mimic naturally-occurring autoimmune diseases will allow researchers to develop better early detection imaging tools, as this stage of disease is often difficult to mirror in currently-existing animal models.
Overall, radiolabeled antibodies hold great potential for imaging of chronic inflammatory diseases, even though they do have some intrinsic limitations. Future studies aimed at overcoming these barriers will certainly give more power to immunoSPECT and immunoPET in autoimmune and inflammatory diseases, providing researchers with greater mechanistic understandings of these conditions, and physicians with greater power for diagnosing and monitoring their patients.
Acknowledgements
This work was supported, in part, by the University of Wisconsin - Madison and the National Institutes of Health (P30CA014520, T32GM008505, T32CA009206). Some figures were reproduced and modified under the Creative Commons Attribution License 3.0 from Servier Medical Art (https://creativecommons.org/licenses/by/3.0/).
Biographies
Biographical Sketch

Hye Jin Lee received her Pharm.D. degree from the College of Pharmacy at Korea University in 2017. She is currently a PhD student in pharmaceutical sciences at the University of Wisconsin Madison working under the guidance of Dr. Weibo Cai. She has performed research on drug delivery systems employing nanomaterials and biological carriers, and currently is focused on multimodality imaging with antibody-based agents in cancer and inflammatory diseases.

Emily B. Ehlerding is a PhD candid ate in the Medical Physics department at the University of Wisconsin – Madison, under the supervision of Dr. Weibo Cai. She also received her B.S. in chemistry and physics from Manchester University in North Manchester, IN. She has developed imaging agents, mostly based on positron emission tomography, for many immune targets articularly involved in immunotherapy treatments.

Weibo Cai received his Ph.D. degree from he University of California at San Diego in 2004 and is now a Vilas Distinguished Achieve ment Professor at UW – Madison (http://mi.wisc.edu). His research is primarily focused on molecular imaging and nanotechnology, investing the biomedical application of various agents developed in his laboratory for imaging and therapy of various diseases.
References
- [1].El-Gabalawy H, Guenther LC, Bernstein CN, The Journal of rheumatology. Supplement 2010, 85, 2–10. [DOI] [PubMed] [Google Scholar]
- [2].Wang L, Wang FS, Gershwin ME, Journal of internal medicine 2015, 278, 369–395. [DOI] [PubMed] [Google Scholar]
- [3].Tabas I, Glass CK, Science 2013, 339, 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) Catalano OA, Wu V, Mahmood U, Signore A, Vangel M, Soricelli A, Salvatore M, Gervais D, Rosen BR, American journal of nuclear medicine and molecular imaging 2018, 8, 62–69; [PMC free article] [PubMed] [Google Scholar]; b) Laverman P, van der Geest T, Terry SY, Gerrits D, Walgreen B, Helsen MM, Nayak TK, Freimoser-Grundschober A, Waldhauer I, Hosse RJ, Moessner E, Umana P, Klein C, Oyen WJ, Koenders MI, Boerman C, J Nucl Med 2015, 56, 778–783. [DOI] [PubMed] [Google Scholar]
- [5].Gotthardt M, Bleeker-Rovers CP, Boerman OC, Oyen WJ, Journal of nuclear medicine technology 2013, 41, 157–169. [DOI] [PubMed] [Google Scholar]
- [6].Signore A, Anzola KL, Auletta S, Varani M, Petitti A, Pacilio M, Galli F, Lauri C, Current pharmaceutical design 2018, 24, 743–753. [DOI] [PubMed] [Google Scholar]
- [7].Lee HW, Gangadaran P, Kalimuthu S, Ahn BC, BioMed research international 2016, 2016, 1946585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Glaudemans AW, Israel O, Slart RH, Seminars in nuclear medicine 2015, 45, 500–512. [DOI] [PubMed] [Google Scholar]
- [9].a) Zhang Y, Hong H, Cai W, Current radiop[9] harmaceuticals 2011, 4, 131–139; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sun H, England CG, Hernandez R, Graves SA, Majewski RL, Kamkaew A, Jiang D, Barnhart TE, Yang Y, Cai W, European journal of nuclear medicine and molecular imaging 2016, 43, 2169–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chianelli M, Mather SJ, Martin-Comin J, Signore A, Nuclear medicine communications 1997, 18, 437–455. [DOI] [PubMed] [Google Scholar]
- [11].Chakravarty R, Goel S, Cai W, Theranostics 2014, 4, 386–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McMurray RW, Seminars in arthritis and rheumatism 1996, 25, 215–233. [DOI] [PubMed] [Google Scholar]
- [13].Yee KL, Weaver VM, Hammer DA, IET systems biology 2008, 2, 8–15. [DOI] [PubMed] [Google Scholar]
- [14].Dearling JL, Park EJ, Dunning P, Baker A, Fahey F, Treves ST, Soriano SG, Shimaoka M, Packard AB, Peer D, Inflammatory bowel diseases 2010, 16, 1458–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hagan M, Cross RK, Expert opinion on drug safety 2015, 14, 1473–1479. [DOI] [PubMed] [Google Scholar]
- [16].Dearling JL, Daka A, Veiga N, Peer D, Packard AB, Inflammatory bowel diseases 2016, 22, 529–538. [DOI] [PubMed] [Google Scholar]
- [17].Sheremata WA, Minagar A, Alexander JS, Vollmer T, CNS drugs 2005, 19, 909–922. [DOI] [PubMed] [Google Scholar]
- [18].Schwab N, Schneider-Hohendorf T, Wiendl H, International immunology 2015, 27, 47–53. [DOI] [PubMed] [Google Scholar]
- [19].Anderson ME, Siahaan TJ, Peptides 2003, 24, 487–501. [DOI] [PubMed] [Google Scholar]
- [20].Beaino W, Anderson CJ, J Nucl Med 2014, 55, 1856–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Soodgupta D, Hurchla MA, Jiang M, Zheleznyak A, Weilbaecher KN, Anderson CJ, Tomasson MH, Shokeen M, PloS one 2013, 8, e55841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Meester EJ, Krenning BJ, de Blois RH, Norenberg JP, de Jong M, Bernsen MR, Van der Heiden K, Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Broisat A, Riou LM, Ardisson V, Boturyn D, Dumy P, Fagret D, Ghezzi C, European journal of nuclear medicine and molecular imaging 2007, 34, 830–840. [DOI] [PubMed] [Google Scholar]
- [24].Nahrendorf M, Keliher E, Panizzi P, Zhang H, Hembrador S, Figueiredo JL, Aikawa E, Kelly K, Libby P, Weissleder R, JACC. Cardiovascular imaging 2009, 2, 1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Broisat A, Hernot S, Toczek J, De Vos J, Riou LM, Martin S, Ahmadi M, Thielens N, Wernery U, Caveliers V, Muyldermans S, Lahoutte T, Fagret D, Ghezzi C, Devoogdt N, Circulation research 2012, 110, 927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Broisat A, Toczek J, Dumas LS, Ahmadi M, Bacot S, Perret P, Slimani L, Barone-Rochette G, Soubies A, Devoogdt N, Lahoutte T, Fagret D, Riou LM, Ghezzi C, J Nucl Med 2014, 55, 1678–1684. [DOI] [PubMed] [Google Scholar]
- [27].Bala G, Blykers A, Xavier C, Descamps B, Broisat A, Ghezzi C, Fagret D, Van Camp G, Caveliers V, Vanhove C, Lahoutte T, Droogmans S, Cosyns B, Devoogdt N, Hernot S, European heart journal cardiovascular Imaging 2016, 17, 1001–1008. [DOI] [PubMed] [Google Scholar]
- [28].Liu C, Zhang X, Song Y, Wang Y, Zhang F, Zhang Y, Zhang Y, Lan X, Atherosclerosis 2016, 254, 263–270. [DOI] [PubMed] [Google Scholar]
- [29].Belmont HM, Buyon J, Giorno R, Abramson S, Arthritis and rheumatism 1994, 37, 376–383. [DOI] [PubMed] [Google Scholar]
- [30].Ishikawa H, Nishibayashi Y, Kita K, Ohno O, Imura S, Hirata S, Bulletin 1993, 53, 23–28. [PubMed] [Google Scholar]
- [31].Ciampolillo A, Napolitano G, Mirakian R, Miyasaki A, Giorgino R, Bottazzo GF, Clin Exp Immunol 1993, 94, 478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sans M, Fuster D, Vazquez A, Setoain FJ, Piera C, Pique JM, Panes J, European journal of gastroenterology & hepatology 2001, 13, 31–38. [DOI] [PubMed] [Google Scholar]
- [33].Ley K, Trends in molecular medicine 2003, 9, 263–268. [DOI] [PubMed] [Google Scholar]
- [34].Jamar F, Houssiau FA, Devogelaer JP, Chapman PT, Haskard DO, Beaujean V, Beckers C, Manicourt DH, Peters AM, Rheumatology (Oxford) 2002, 41, 53–61. [DOI] [PubMed] [Google Scholar]
- [35].Reynolds PR, Larkman DJ, Haskard DO, Hajnal JV, Kennea NL, George AJ, Edwards AD, Radiology 2006, 241, 469–476. [DOI] [PubMed] [Google Scholar]
- [36].Machtaler S, Knieling F, Luong R, Tian L, Willmann JK, Theranostics 2015, 5, 1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bhatti M, Chapman P, Peters M, Haskard D, Hodgson HJ, Gut 1998, 43, 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Keelan ET, Harrison AA, Chapman PT, Binns RM, Peters AM, Haskard DO, J Nucl Med 1994, 35, 276–281. [PubMed] [Google Scholar]
- [39].Malviya G, Conti F, Chianelli M, Scopinaro F, Dierckx RA, Signore A, European journal of nuclear medicine and molecular imaging 2010, 37, 386–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].a) Bevilacqua MP, Nelson RM, The Journal of clinical investigation 1993, 91, 379–387; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang HB, Wang JT, Zhang L, Geng ZH, Xu WL, Xu T, Huo Y, Zhu X, Plow EF, Chen M, Geng JG, Nature immunology 2007, 8, 882–892. [DOI] [PubMed] [Google Scholar]
- [41].Rouzet F, Bachelet-Violette L, Alsac JM, Suzuki M, Meulemans A, Louedec L, Petiet A, Jandrot-Perrus M, Chaubet F, Michel JB, Le Guludec D, Letourneur D, J Nucl Med 2011, 52, 1433–1440. [DOI] [PubMed] [Google Scholar]
- [42].a) Li X, Bauer W, Israel I, Kreissl MC, Weirather J, Richter D, Bauer E, Herold V, Jakob P, Buck A, Frantz S, Samnick S, Arteriosclerosis, thrombosis, and vascular biology 2014, 34, 1661–1667; [DOI] [PubMed] [Google Scholar]; b) Nakamura I, Hasegawa K, Wada Y, Hirase T, Node K, Watanabe Y, Biochem Biophys Res Commun 2013, 433, 47–51. [DOI] [PubMed] [Google Scholar]
- [43].Xu Y, Li J, Fang W, Yu M, Ru B, Thrombosis research 2008, 123, 306–315. [DOI] [PubMed] [Google Scholar]
- [44].Hong H, Chen F, Zhang Y, Cai W, Adv Drug Deliv Rev 2014, 76, 2–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lankinen P, Makinen TJ, Poyhonen TA, Virsu P, Salomaki S, Hakanen AJ, Jalkanen S, Aro HT, Roivainen A, European journal of nuclear medicine and molecular imaging 2008, 35, 352–364. [DOI] [PubMed] [Google Scholar]
- [46].a) Autio A, Henttinen T, Sipila HJ, Jalkanen S, Roivainen A, EJNMMI research 2011, 1, 10; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Silvola J, Autio A, Luoto P, Jalkanen S, Roivainen A, Clinical physiology and functional imaging 2010, 30, 75–78. [DOI] [PubMed] [Google Scholar]
- [47].a) Aalto K, Autio A, Kiss EA, Elima K Nymalm Y, Veres TZ, Marttila-Ichihara F, Elovaara H, Saanijoki T, Crocker PR, Maksimow M, Bligt E, Salminen TA, Salmi M, Roivainen A, Jalkanen S, Blood 2011, 118, 3725–3733; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Retamal J, Sorensen J, Lubberink M, Suarez-Sipmann F, Borges JB, Feinstein R, Jalkanen S, Antoni G, Hedenstierna G, Roivainen A, Larsson A, Velikyan I, American journal of nuclear medicine and molecular imaging 2016, 6, 18–31. [PMC free article] [PubMed] [Google Scholar]
- [48].Autio A, Vainio PJ, Suilamo S, Mali A, Vainio J, Saanijoki T, Noponen T, Ahtinen H, Luoto P, Teras M, Jalkanen S, Roivainen A, J Nucl Med 2013, 54, 1315–1319. [DOI] [PubMed] [Google Scholar]
- [49].Navegantes KC, de Souza Gomes R, Pereira PAT, Czaikoski PG, Azevedo CHM, Monteiro MC, J Transl Med 2017, 15, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Davignon JL, Hayder M, Baron M, Boyer JF, Constantin A, Apparailly F, Poupot R, Cantagrel A, Rheumatology (Oxford) 2013, 52, 590–598. [DOI] [PubMed] [Google Scholar]
- [51].a) Airas L, Rissanen E, Rinne J, Mult Scler 2017, 23, 496–504; [DOI] [PubMed] [Google Scholar]; b) da Fonseca AC, Matias D, Garcia C, Amaral R, Geraldo LH, Freitas C, Lima FR, Frontiers in cellular neuroscience 2014, 8, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].van Hemert FJ, Thurlings R, Dohmen SE, Voermans C, Tak PP, van Eck-Smit BL, Bennink RJ, Nuclear medicine and biology 2007, 34, 933–938. [DOI] [PubMed] [Google Scholar]
- [53].a) Vowinckel E, Reutens D, Becher B, Verge G, Evans A, Owens T, Antel JP, J Neurosci Res 1997, 50, 345–353; [DOI] [PubMed] [Google Scholar]; b) Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, Price G, Wegner F, Giovannoni G, Miller DH, Perkin GD, Smith T, Hewson AK, Bydder G, Kreutzberg GW, Jones T, Cuzner ML, Myers R, Brain : a journal of neurology 2000, 123 (Pt 11), 2321–2337. [DOI] [PubMed] [Google Scholar]
- [54].Turk MJ, Breur GJ, Widmer WR, Paulos CM, Xu LC, Grote LA, Low PS, Arthritis and rheumatism 2002, 46, 1947–1955. [DOI] [PubMed] [Google Scholar]
- [55].Van De Wiele C, Sathekge M, Maes A, The quarterly journal of nuclear medicine and molecular imaging : official publication of the Italian Association of Nuclear Medicine (AIMN) [and] the International Association of Radiopharmacology (IAR), [and] Section of the So 2014, 58, 269–275. [PubMed] [Google Scholar]
- [56].Taylor PR, Gordon S, Martinez-Pomares L, Trends Immunol 2005, 26, 104–110. [DOI] [PubMed] [Google Scholar]
- [57].a) Gazi U, Martinez-Pomares L, Immunobiology 2009, 214, 554–561; [DOI] [PubMed] [Google Scholar]; b) Fairweather D, Cihakova D, J Autoimmun 2009, 33, 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].a) Morishima S, Morita I, Tokushima T, Kawashima H, Miyasaka M, Omura K, Murota S, J Endocrinol 2003, 176, 285–292; [DOI] [PubMed] [Google Scholar]; b) Schett G, Arthritis Res Ther 2007, 9, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Movahedi K, Schoonooghe S, Laoui D, Houbracken I, Waelput W, Breckpot K, Bouwens L, Lahoutte T, De Baetselier P, Raes G, Devoogdt N, Van Ginderachter JA, Cancer Res 2012, 72, 4165–4177. [DOI] [PubMed] [Google Scholar]
- [60].Put S, Schoonooghe S, Devoogdt N, Schurgers E, Avau A, Mitera T, D’Huyvetter M, De Baetselier P, Raes G, Lahoutte T, Matthys P, J Nucl Med 2013, 54, 807–814. [DOI] [PubMed] [Google Scholar]
- [61].Chazenbalk GD, Pichurin PN, Guo J, Rapoport B, McLachlan SM, Clin Exp Immunol 2005, 139, 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Cosin-Roger J, Ortiz-Masia D, Calatayud S, Hernandez C, Alvarez A, Hinojosa J, Esplugues JV, Barrachina MD, PloS one 2013, 8, e78128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bobryshev YV, Ivanova EA, Chistiakov DA, Nikiforov NG, Orekhov AN, BioMed research international 2016, 2016, 9582430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Varasteh Z, Hyafil F, Anizan N, Diallo D, Aid-Launais R, Mohanta S, Li Y, Braeuer M, Steiger K, Vigne J, Qin Z, Nekolla SG, Fabre JE, Doring Y, Le Guludec D, Habenicht A, Vera DR, Schwaiger M, EJNMMI research 2017, 7, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bala G, Baudhuin H, Remory I, Gillis K, Debie P, Krasniqi A, Lahoutte T, Raes G, Devoogdt N, Cosyns B, Hernot S, Mol Imaging Biol 2018, 20, 260–267. [DOI] [PubMed] [Google Scholar]
- [66].Helmy KY, Katschke KJ Jr., Gorgani NN, Kljavin NM, Elliott JM, Diehl L, Scales SJ, Ghilardi N, van Lookeren Campagne M, Cell 2006, 124, 915–927. [DOI] [PubMed] [Google Scholar]
- [67].Tanaka M, Nagai T, Tsuneyoshi Y, Sunahara N, Matsuda T, Nakamura T, Tsuyama S, Hasui K, FitzGerald O, Matsuyama T, Clin Exp Immunol 2008, 154, 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zheng F, Put S, Bouwens L, Lahoutte T, Matthys P, Muyldermans S, De Baetselier P, Devoogdt N, Raes G, Schoonooghe S, J Nucl Med 2014, 55, 824–829. [DOI] [PubMed] [Google Scholar]
- [69].Zheng F, Perlman H, Matthys P, Wen Y, Lahoutte T, Muyldermans S, Lu S, De Baetselier P, Schoonooghe S, Devoogdt N, Raes G, Scientific reports 2016, 6, 35966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Polfliet MM, Fabriek BO, Daniels WP, Dijkstra CD, van den Berg TK, Immunobiology 2006, 211, 419–425. [DOI] [PubMed] [Google Scholar]
- [71].Moller HJ, Scand J Clin Lab Invest 2012, 72, 1–13. [DOI] [PubMed] [Google Scholar]
- [72].Eichendorff S, Svendsen P, Bender D, Keiding S, Christensen EI, Deleuran B, Moestrup SK, Mol Imaging Biol 2015, 17, 87–93. [DOI] [PubMed] [Google Scholar]
- [73].a) Skapenko A, Leipe J, Lipsky PE, Schulze-Koops H, Arthritis Res Ther 2005, 7 Suppl 2, S4–14; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF, Immunological reviews 2008, 223, 284–299. [DOI] [PubMed] [Google Scholar]
- [74].Fulgenzi A, Casati R, Colombo FR, Gasparini M, Ferrero E, Bondanza A, Gerundini P, Ferrero ME, Nuclear medicine and biology 2004, 31, 631–638. [DOI] [PubMed] [Google Scholar]
- [75].van Montfrans C, Bennink RJ, de Bruin K, de Jonge W, Verberne HJ, Ten Kate FJ, van Deventer SJ, Te Velde AA, J Nucl Med 2004, 45, 1759–1765. [PubMed] [Google Scholar]
- [76].Annovazzi A, Bonanno E, Arca M, D’Alessandria C, Marcoccia A, Spagnoli LG, Violi F, Scopinaro F, De Toma G, Signore A, European journal of nuclear medicine and molecular imaging 2006, 33, 117–126. [DOI] [PubMed] [Google Scholar]
- [77].Namavari M, Chang YF, Kusler B, Yaghoubi S, Mitchell BS, Gambhir SS, Mol Imaging Biol 2011, 13, 812–818. [DOI] [PubMed] [Google Scholar]
- [78].Salas JR, Chen BY, Wong A, Cheng D, Van Arnam JS, Witte ON, Clark PM, J Nucl Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hoehne A, James ML, Alam IS, Ronald JA, Schneider B, D’Souza A, Witney TH, Andrews LE, Cropper HC, Behera D, Gowrishankar G, Ding Z, Wyss-Coray T, Chin FT, Biswal S, Gambhir SS, J Neuroinflammation 2018, 15, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].a) Malviya G, Galli F, Sonni I, Signore A, The quarterly journal of nuclear medicine and molecular imaging : official publication of the Italian Association of Nuclear Medicine (AIMN) [and] the International Association of Radiopharmacology (IAR), [and] Section of the So 2014, 58, 237–257; [PubMed] [Google Scholar]; b) Wei W, Jiang D, Ehlerding EB, Luo Q, Cai W Trends in cancer 2018, 4, 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Malviya G, Galli F, Sonni I, Pacilio M, Signore A, The quarterly journal of nuclear medicine and molecular imaging : official publication of the Italian Association of Nuclear Medicine (AIMN) [and] the International Association of Radiopharmacology (IAR), [and] Section of the So 2010, 54, 654–676. [PubMed] [Google Scholar]
- [82].Chatenoud L, Bluestone JA, Nature reviews. Immunology 2007, 7, 622–632. [DOI] [PubMed] [Google Scholar]
- [83].Kuhn C, Weiner HL, Immunotherapy 2016, 8, 889–906. [DOI] [PubMed] [Google Scholar]
- [84].Kung P, Goldstein G, Reinherz EL, Schlossman SF, Science 1979, 206, 347–349. [PubMed] [Google Scholar]
- [85].a) Marcus C, Thakur ML, Huynh TV, Louie JS, Leibling M, Minami C, Diggles L, Nuclear medicine communications 1994, 15, 824–830; [DOI] [PubMed] [Google Scholar]; b) Lopes FP, de Azevedo MN, Marchiori E, da Fonseca LM, de Souza SA, Gutfilen B, Rheumatology (Oxford) 2010, 49, 933–939. [DOI] [PubMed] [Google Scholar]
- [86].a) Beckford Vera DR, Smith CC, Bixby LM, Glatt DM, Dunn SS, Saito R, Kim WY, Serody JS, Vincent BG, Parrott MC, PloS one 2018, 13, e0193832; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Larimer BM, Wehrenberg-Klee E, Caraballo A, Mahmood U, J Nucl Med 2016, 57, 1607–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Zhu J, Paul WE, Blood 2008, 112, 1557–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Dittel BN, Brain, behavior, and immunity 2008, 22, 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Becker W, Emmrich F, Horneff G, Burmester G, Seiler F, Schwarz A, Kalden J, Wolf F, European journal of nuclear medicine 1990, 17, 156–159. [DOI] [PubMed] [Google Scholar]
- [90].Kinne RW, Becker W, Schwab J, Horneff G, Schwarz A, Kalden JR, Emmrich F, Burmester GR, Wolf F, Nuclear medicine communications 1993, 14, 667–675. [DOI] [PubMed] [Google Scholar]
- [91].Kinne RW, Becker W, Schwab J, Schwarz A, Kalden JR, Emmrich F, Burmester GR, Wolf F, European journal of nuclear medicine 1994, 21, 176–180. [DOI] [PubMed] [Google Scholar]
- [92].Steinhoff K, Pierer M, Siegert J, Pigla U, Laub R, Hesse S, Seidel W, Sorger D, Seese A, Kuenstler JU, Pietzsch HJ, Lincke T, Rullmann M, Emmrich F, Sabri O, Nuclear medicine and biology 2014, 41, 350–354. [DOI] [PubMed] [Google Scholar]
- [93].Kanwar B, Gao DW, Hwang AB, Grenert JP, Williams SP, Franc B, McCune JM, Journal of immunological methods 2008, 329, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Freise AC, Zettlitz KA, Salazar FB, Lu X, Tavare R, Wu AM, Mol Imaging Biol 2017, 19, 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Freise AC, Zettlitz KA, Salazar FB, Tavare R, Tsai WK, Hadjioannou A, Rozengurt N, Braun J, Wu AM, J Nucl Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Hampe CS, Scientifica 2012, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Iodice V, Lagana B, Lauri C, Capriotti G, Germano V, D’Amelio R, Picchianti Diamanti A, The quarterly journal of nuclear medicine and molecular imaging : official publication of the Italian Association of Nuclear Medicine (AIMN) [and] the International Association of Radiopharmacology (IAR), [and] Section of the So 2014, 58, 258–268. [PubMed] [Google Scholar]
- [98].Bruijnen S, Tsang ASM, Raterman H, Ramwadhdoebe T, Vugts D, van Dongen G, Huisman M, Hoekstra O, Tak PP, Voskuyl A, van der Laken C, Arthritis Res Ther 2016, 18, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].James ML, Hoehne A, Mayer AT, Lechtenberg K, Moreno M, Gowrishankar G, Ilovich O, Natarajan A, Johnson EM, Nguyen J, Quach L, Han M, Buckwalter M, Chandra S, Gambhir SS, J Nucl Med 2017, 58, 1845–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Yoon JT, Longtine MS, Marquez-Nostra BV, Wahl RL, J Nucl Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Du FH, Mills EA, Mao-Draayer Y, Auto- immunity highlights 2017, 8, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zettlitz KA, Tavare R, Knowles SM, Steward KK, Timmerman JM, Wu AM, Clinical cancer research : an official journal of the American Association for Cancer Research 2017, 23, 7242–7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].a) Papos M, Nagy F, Narai G, Rajtar M, Szantai G Lang J, Csernay L, Digestive diseases and sciences 1996, 41, 412–420; [DOI] [PubMed] [Google Scholar]; b) Gyorke T, Duffek L, Bartfai K, Mako E, Karlinger K, Mester A, Tarjan Z, European journal of radiology 2000, 35, 183–192. [DOI] [PubMed] [Google Scholar]
- [104].Rashidian M, Keliher EJ, Bilate AM, Duarte JN, Wojtkiewicz GR, Jacobsen JT, Cragnolini J, Swee LK, Victora GD, Weissleder R, Ploegh HL, Proceedings of the National Academy of Sciences of the United States of America 2015, 112, 6146–6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Liu G, Hu Y, Xiao J, Li X, Li Y, Tan H, Zhao Y, Cheng D, Shi H, Scientific reports 2016, 6, 20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Liu Z, Wyffels L, Barber C, Wan L, Xu H, Hui MM, Furenlid LR, Woolfenden JM, Nuclear medicine and biology 2012, 39, 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Levin AD, Wildenberg ME, van den Brink GR, Journal of Crohn’s & colitis 2016, 10, 989–997. [DOI] [PubMed] [Google Scholar]
- [108].Rebelo Pinto Edos S, Lopes FP, de Souza SA, da Fonseca LM, Vaisman M, Gutfilen B, Teixeira Pde F. dos Santos, Hormone and metabolic research = Hormon-und Stoffwechselforschung = Hormones et metabolisme 2013, 45, 765–768. [DOI] [PubMed] [Google Scholar]
- [109].Chianelli M, D’Alessandria C, Conti F, Priori R, Valesini G, Annovazzi A, Signore A, The quarterly journal of nuclear medicine and molecular imaging : official publication of the Italian Association of Nuclear Medicine (AIMN) [and] the International Association of Radiopharmacology (IAR), [and] Section of the So 2006, 50, 217–225. [PubMed] [Google Scholar]
- [110].Barrera P, Oyen WJ, Boerman OC, van Riel PL, Annals of the rheumatic diseases 2003, 62, 825–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Cao Q, Cai W, Li ZB, Chen K, He L, Li HC, Hui M, Chen X, European journal of nuclear medicine and molecular imaging 2007, 34, 1832–1842. [DOI] [PubMed] [Google Scholar]
- [112].Tobinick EL, Chen K, Chen X, BMC research notes 2009, 2, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Tobinick EL, Gross H, J Neuroinflammation 2008, 5, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Hamson EJ, Keane FM, Tholen S, Schilling O, Gorrell MD, Proteomics. Clinical applications 2014, 8, 454–463. [DOI] [PubMed] [Google Scholar]
- [115].Welt S, Divgi CR, Scott AM, Garin-Chesa P, Finn RD, Graham M, Carswell EA, Cohen A, Larson SM, Old LJ, et al. , Journal of clinical oncology : official journal of the American Society of Clinical Oncology 1994, 12, 1193–1203. [DOI] [PubMed] [Google Scholar]
- [116].Scott AM, Wiseman G, Welt S, Adjei A, Lee FT, Hopkins W, Divgi CR, Hanson LH, Mitchell P, Gansen DN, Larson SM, Ingle JN, Hoffman EW, Tanswell P, Ritter G, Cohen LS, Bette P, Arvay L, Amelsberg A, Vlock D, Rettig WJ, Old LJ, Clinical cancer research : an official journal of the American Association for Cancer Research 2003, 9, 1639–1647. [PubMed] [Google Scholar]
- [117].Fischer E, Chaitanya K, Wuest T, Wadle A, Scott AM, van den Broek M, Schibli R, Bauer S, Renner C, Clinical cancer research : an official journal of the American Association for Cancer Research 2012, 18, 6208–6218. [DOI] [PubMed] [Google Scholar]
- [118].Terry SY, Koenders MI, Franssen GM, Nayak TK, Freimoser-Grundschober A, Klein C, Oyen WJ, Boerman OC, Laverman P, J Nucl Med 2016, 57, 467–472. [DOI] [PubMed] [Google Scholar]
- [119].van der Geest T, Laverman P, Gerrits D, Walgreen B, Helsen MM, Klein C, Nayak TK, Storm G, Metselaar JM, Koenders MI, Boerman OC, J Nucl Med 2017, 58, 151–155. [DOI] [PubMed] [Google Scholar]
- [120].van der Geest T, Roeleveld DM, Walgreen B, Helsen MM, Nayak TK, Klein C, Hegen M,Storm G, Metselaar JM, van den Berg WB, van der Kraan PM, Laverman P, Boerman OC, Koenders MI, Rheumatology (Oxford) 2018, 57, 737–747. [DOI] [PubMed] [Google Scholar]
- [121].Verma RP, Hansch C, Bioorganic & medicinal chemistry 2007, 15, 2223–2268. [DOI] [PubMed] [Google Scholar]
- [122].Van Lint P, Libert C, Journal of leukocyte biology 2007, 82, 1375–1381. [DOI] [PubMed] [Google Scholar]
- [123].Gerwien H, Hermann S, Zhang X, Korpos E, Song J, Kopka K, Faust A, Wenning C, Gross CC, Honold L, Melzer N, Opdenakker G, Wiendl H, Schafers M, Sorokin L, Science translational medicine 2016, 8, 364ra152. [DOI] [PubMed] [Google Scholar]
- [124].Schafers M, Riemann B, Kopka K, Breyholz HJ, Wagner S, Schafers KP, Law MP, Schober O, Levkau B, Circulation 2004, 109, 2554–2559. [DOI] [PubMed] [Google Scholar]
- [125].Razavian M, Nie L, Challa A, Zhang J, Golestani R, Jung JJ, Robinson S, Sadeghi MM, Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology 2014, 21, 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wang Z, Deng G, Zhang Z, Huang H, Zhao Y, Nuclear medicine communications 2017, 38, 299–305. [DOI] [PubMed] [Google Scholar]
- [127].Kuge Y, Takai N, Ogawa Y, Temma T, Zhao Y, Nishigori K, Ishino S, Kamihashi J, Kiyono Y, Shiomi M, Saji H, European journal of nuclear medicine and molecular imaging 2010, 37, 2093–2104. [DOI] [PubMed] [Google Scholar]
- [128].Hersh DS, Wadajkar AS, Roberts N, Perez JG, Connolly NP, Frenkel V, Winkles JA, Woodworth GF, Kim AJ, Current pharmaceutical design 2016, 22, 1177–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Chakravarty R, Goel S, Valdovinos HF, Hernandez R, Hong H, Nickles RJ, Cai W, Bioconjug Chem 2014, 25, 2197–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Zhang Y, Hong H, Orbay H, Valdovinos HF, Nayak TR, Theuer CP, Barnhart TE, Cai W, European journal of nuclear medicine and molecular imaging 2013, 40, 759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Hong H, Zhang Y, Orbay H, Valdovinos HF, Nayak TR, Bean J, Theuer CP, Barnhart TE, Cai W, Molecular pharmaceutics 2013, 10, 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Bhattacharyya S, Dixit M, Dalton transactions 2011, 40, 6112–6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Ehlerding EB, England CG, McNeel DG, Cai W, J Nucl Med 2016, 57, 1487–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Kang L, Jiang D, Ehlerding EB, Barnhart TE, Ni D, Engle JW, Wang R, Huang P, Xu X, Cai W, Molecular pharmaceutics 2018, 15, 1627–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Barbieri A, Dolcino M, Tinazzi E, Rigo A, Argentino G, Patuzzo G, Ottria A, Beri R, Puccetti A, Lunardi C, Journal of immunology research 2015, 2015, 729654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Shinoda K, Sun X, Oyamada A, Yamada H, Muta H, Podack ER, Kira J, Yoshikai Y, J Autoimmun 2015, 57, 14–23. [DOI] [PubMed] [Google Scholar]
- [137].Coussens LM, Werb Z, Nature 2002, 420, 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Turker NS, Heidari P, Kucherlapati R, Kucherlapati M, Mahmood U, Theranostics 2014, 4, 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]


