Abstract
This study examined the feasibility of using auditory event-related potentials to evaluate spoken word processing during passive listening in girls with Rett syndrome (n=11) and typical peers (n=33), age 4–12 years. The typical group demonstrated the expected pattern of more negative amplitudes within 200–500ms in response to words than nonwords at left temporal sites. In participants with Rett syndrome, word-nonword differentiation was observed at the right temporal sites. More negative left hemisphere amplitudes in response to words were associated (at trend level) with better receptive language skills and more adaptive behavior. The results indicate that girls with Rett syndrome differentiate known words from novel nonwords, but may do so using potentially atypical neural processes. Brain-behavior correlations support validity of the proposed neural markers of word processing, making passive listening paradigms a promising approach for assessing speech and language processing in participants with limited spoken language skills.
Keywords: auditory, ERP, Rett syndrome, speech, word
1. Introduction
Rett syndrome (RTT) is a neurodevelopmental disorder caused by mutations in the methyl CpG-binding protein 2 gene (MeCP2) occurring in 1 of 10,000 girls (Neul et al., 2010). The MeCP2 protein is highly expressed in the brain, and even slight over- or underexpression results in alterations of brain development and functioning (Chao & Zoghbi, 2012; Collins et al., 2004; Ramocki & Zoghbi, 2008). The phenotype of RTT, which is associated with underexpression of MeCP2 protein, is characterized by regression, loss of purposeful hand skills and replacement with stereotyped movements, abnormal muscle tone, dyspraxia, and limited speech (Hagberg et al., 1983; Neul et al., 2010). Although it is one of the main causes of intellectual disability in girls (Naidu, 1997), the cognitive phenotype in RTT is not well characterized (Berger-Sweeney, 2011; Demeter, 2000), and knowledge about the speech and language processing abilities, particularly post-regression, is limited. This is attributable, in part, to the difficulties of administering conventional standardized psychological assessments, which typically require hand use and/or spoken responses, and therefore are not sufficiently sensitive to capture the full range of functioning (Percy et al., 2010).
Cognitive neuroscience methods using event-related-potentials (ERP), a portion of electroencephalogram time-locked to a stimulus event, have been successfully implemented in studies of individuals with no speech to document language skills (Mills et al., 2004; Yoder et al., 2006), predict developmental outcomes (Kuhl et al., 2013; Molfese, 2000), and provide evidence of treatment effects without relying on overt behaviors by the participant (Yoder et al., 2013). Electrophysiological studies of auditory processing in RTT are limited. Examination of sensory processes relied primarily on detection of pure tone pitch changes and reported delays in auditory responsiveness (Foxe et al., 2016; Stauder et al., 2006). Conversely, studies using more complex, word-level stimuli to investigate higher-order speech and language processing in RTT reported discrimination of familiar voices from novel distractors (Peters et al., 2015) but no evidence of recognizing own name, suggesting general attention to auditory inputs but potentially reduced speech content processing (Peters et al., 2017).
Direct evaluation of auditory comprehension in individuals with limited speech and motor difficulties is challenging. Prior studies in typical populations reported that ERPs elicited during passive exposure to spoken stimuli are sensitive to differences between known words and various contrast stimuli (e.g., nonwords, unknown or backward words; Molfese, 1990; Mills et al., 1993, 1997). Known words elicit more negative amplitudes within 200–500 ms after stimulus onset, and this response can be detected from 13 months of age (Mills et al., 1997; Mills et al., 2004) across multiple bilateral anterior and posterior scalp regions. By 20 months, the topographic distribution becomes more localized to left temporal and parietal electrodes, reflecting increased proficiency with language processing (Mills et al., 1993, 1997; Mills et al., 2004; Mills et al., 2005). The temporal and spatial characteristics of these ERP responses correlated with behavioral measures of language comprehension and production (Mills et al., 1993, 1997) and could be modified through directed training aimed at increasing experience with the stimulus words and their meaning (Mills et al., 2005).
Studies of word vs. nonword differentiation in populations with language and communication difficulties reported that two-year-olds with autism spectrum disorder (ASD) who exhibited more adaptive social functioning generated left-lateralized responses differentiating between known and unknown words similar to typical peers, while ERP responses of children with less adaptive functioning were right-lateralized (Kuhl et al., 2013). Furthermore, more negative left parietal amplitudes in response to word stimuli were predictive of better receptive language performance two and four years later. More recently, Sandbank, Yoder, and Key (2017) demonstrated that in preschoolers with ASD, the strength of concurrent associations between performance on a behavioral measure of receptive language and the left temporal ERP responses to the word-nonword contrast was influenced by the number of stimulus words understood by the child. Together, these findings suggest that neural markers of word processing could be a useful index of language skills and a predictor of developmental outcomes in populations with typical or atypical development.
The goal of the current study was to examine the extent of spoken content processing in children with Rett syndrome using ERP markers of word-nonword differentiation during passive listening. Based on findings from prior studies (Mills et al., 1997; Mills et al., 2005; Kuhl et al., 2013), we hypothesized that effective word processing would be reflected in more negative left temporal or parietal auditory responses to words than nonwords within 200–500 ms after stimulus onset. To demonstrate clinical utility of these ERP measures, we evaluated whether they are associated with concurrent behavioral measures of receptive communication skills and adaptive functioning. Greater negative amplitudes to words, or greater amplitude differences between words and nonwords at left temporal or parietal regions were expected to be positively associated with better behavioral performance.
2. Method
2.1. Participants
Eleven girls with RTT, all post-regression, age 4 to 11 years (M age = 5.13 years, SD = 6.50), participated in the study. All participants had confirmed MECP2 mutations. Thirty-three typically developing children, age 4–12 years (M age = 7.75 years, SD = 2.44) served as the comparison group. Two additional participants with RTT and one typical child were excluded due to low number of artifact-free ERP trials.
Ethical approval for this study was obtained from the Institutional Review Board of Vanderbilt University Medical Center. Parents or legal guardians of the participants provided written informed consent, and evidence of assent was documented for all participants. For children with RTT, their parents/caregivers remained present in the testing room for the duration of the study to help identify any dissenting behaviors.
2.2. Procedures
2.2.1. Behavioral assessments
Peabody Picture Vocabulary Test-IV (PPVT, Dunn & Dunn, 2007).
The PPVT–4 is a standardized, individually administered measure of receptive vocabulary. Each form contains training and test items, with four full-color pictures as response options on a page. For each item, the examiner says a word, and the examinee responds by selecting the picture that best illustrates that word’s meaning. A licensed psychologist with extensive experience working with RTT administered the PPVT within a single session, in a private testing room, with the parent/caregiver present.
Aberrant Behavior Checklist (ABC; Aman & Singh, 1986).
The ABC is a questionnaire that evaluates the presence of specific maladaptive behaviors in five categories (irritability, lethargy/withdrawal, inappropriate speech, hyperactivity, and stereotypic behavior). The ABC has been previously used to characterize individuals with a variety of genetic syndromes. The informants were the participants’ primary caregivers who have known the child since birth.
2.2.2. ERP Word-Nonword paradigm
Stimuli.
A set of 10 English words that are typically among the first learned by young children and 10 pronounceable nonwords (see Table 1) matched to words on duration (words: M=424.3 ms, SD=68.18; nonwords: M=472.3 ms, SD=105.01; p=.241) and number of syllables were used in the study. These stimuli were identical to the ones used by Sandbank et al. (2017) and based on the set originally described in Mills et al. (2004). All stimuli were recorded by a young, female, native English speaker and delivered with a neutral intonation.
Table 1.
Stimuli used in the study
| Words | Nonwords |
|---|---|
| Ball | Fipe |
| Book | Jud |
| Bottle | Kobe |
| Car | Lif |
| Cup | Mon |
| Dog | Neem |
| Drink | Neps |
| Milk | Ris |
| Nose | Towd |
| Shoe | Zav |
ERP acquisition.
EEG data were collected using a 128-channel geodesic sensor net (EGI, Inc., Eugene, OR), using 250 Hz sampling rate, 0.1–100 Hz filters, and vertex reference. Impedances were adjusted to <50 KOhm prior to data acquisition. Words and nonwords were presented at 75 dB SPL in random order, with equal probability (6 times each, 120 trials total), with a varied intertrial interval of 1800–2800 ms, which prevented habituation to stimulus onset. The entire session lasted approximately 7 min. No behavioral responses were required. To facilitate cooperation during data acquisition, a developmental age-appropriate video with muted sound was shown to participants. Previous work has shown that muted videos do not interfere with the quality of auditory ERP data in children (Mahajan & McArthur, 2010).
ERP data processing.
During offline processing, data were filtered using a 30 Hz lowpass filter, segmented on stimulus onset to include a 100ms prestimulus baseline and 900ms post-stimulus period. Single trial data were screened for ocular and movement artifacts using an automated algorithm in NetStation followed by a manual review. Data for electrodes with poor signal quality within a trial were reconstructed using spherical spline interpolation procedures (Perrin, Pernier, Bertrand, & Echallier, 1989). A minimum of 10 artifact-free trials per stimulus condition was required for participant inclusion in statistical analyses. The average number of trials retained was comparable (p=.427) between the two stimulus conditions in both groups. However, typical children had significantly more (p<.001) artifact-free trials (words: M=22.64, SD=6.29; nonwords: M=22.03, SD=7.43) than the participants with RTT (words: M=13.36, SD=4.15; nonwords: M=12.55, SD=2.62). The number of trials contributing to the average waveform did not correlate with age in either group.
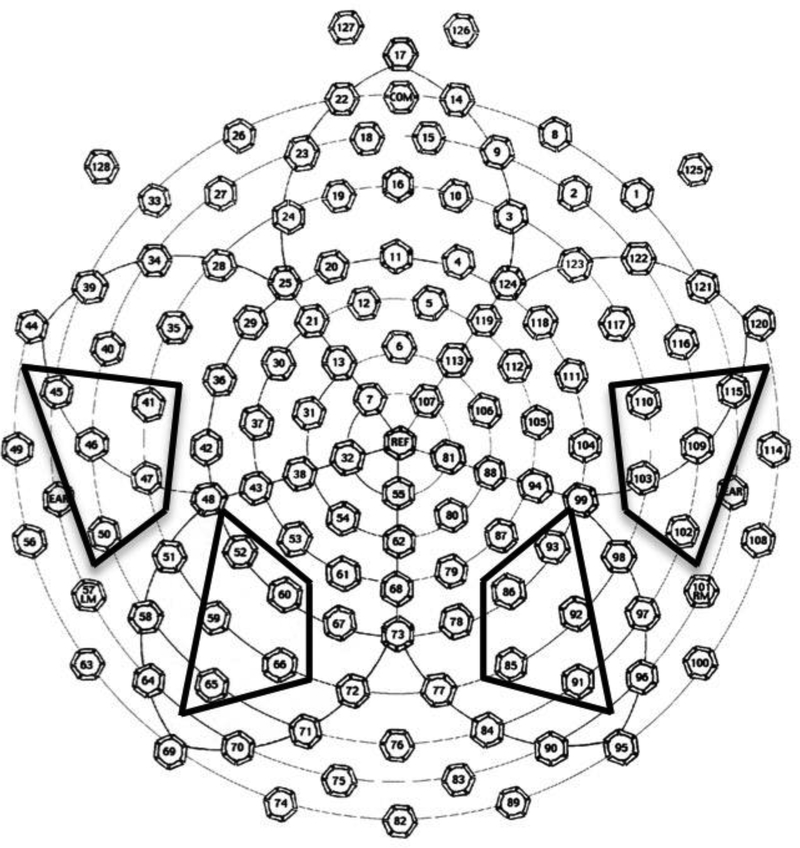
Following artifact screening, individual ERPs for each stimulus category were averaged, re-referenced to an average reference, and baseline-corrected by subtracting the average microvolt value across the 100ms prestimulus interval from the post-stimulus segment. Data from the electrodes corresponding to parietal and temporal regions in the left and right hemisphere were used in the remaining statistical analyses (Figure 1). To capitalize on the rich data set offered by a high-density array, we used a priori determined electrode clusters rather than single electrodes corresponding to the locations of interest because averaging data over several spatially contiguous locations improved signal-to-noise ratio. Word processing was quantified in two ways for each participant within 200–500 ms after stimulus onset: (a) as the mean amplitude for the word and nonword condition and (b) as the within-participant amplitude difference between the nonword and word conditions. These scalp locations and temporal range were selected on the basis of the past research in typically developing children (Mills et al., 1993, 1997; Mills et al., 2004) and children with ASD (Kuhl et al., 2013; Sandbank et al., 2017).
Figure 1.
Electrode layout of the 128-channel geodesic net (EGI, Inc.) and the left and right temporal and parietal clusters used in data analysis.
2.3. Data Analysis
Differentiation between words and nonwords in children with RTT and typical peers was examined using a repeated measures analysis of variance (ANOVA) with Stimulus (2: word, nonword) x Electrode cluster (2: temporal, parietal) x Hemisphere (2: left, right) within-subject factors and Group (2: RTT, typical) as the between-subject factor. Within-group stimulus-related differences were evaluated using planned comparisons contrasting responses to words and nonwords at each scalp location. Based on the expectation of increased negative amplitudes in response to word compared to nonword stimuli, the significance of the condition differences was tested against zero using one-tailed paired t-tests.
In line with the analytic approaches previously implemented by Kuhl et al. (2013) and Sandbank et al. (2017), brain-behavior associations between mean ERP amplitudes in response to words and age, receptive language skills, and adaptive functioning in RTT group were documented using correlations. Based on the directional prediction of better receptive language and adaptive functioning being associated with more negative ERP amplitudes to words, the significance of these associations was tested using one-tailed tests.
3. Results
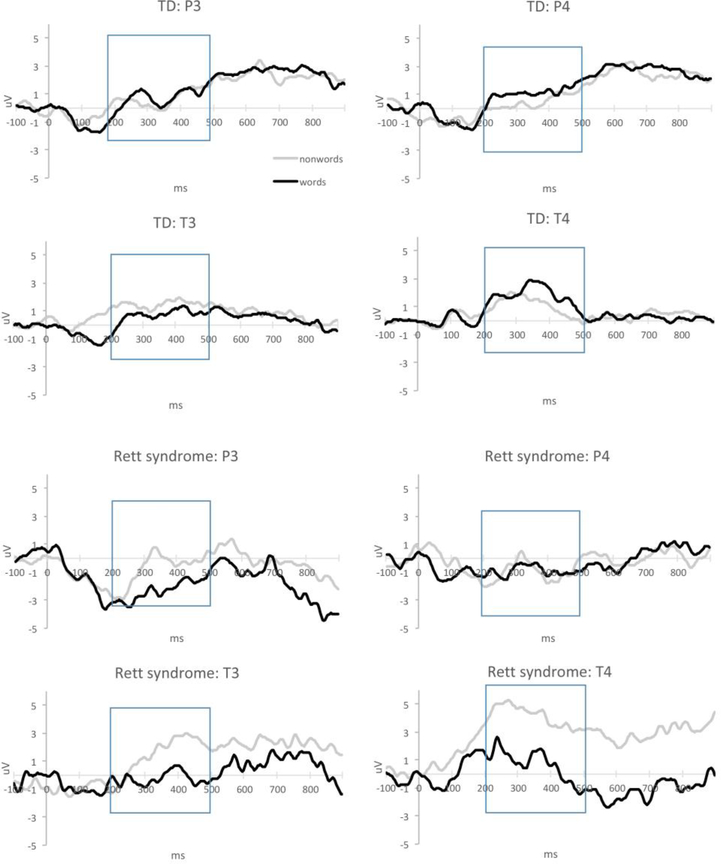
The repeated measures ANOVA identified main effects of Stimulus, F(1,42)=5.806, p=.02, ηp2 =.121, Electrode, F(1,42)=10.573, p=.002, ηp2 =.201, and Group, F(1,42)=4.964, p=.031, ηp2 =.106. These effects were further qualified by interactions of Stimulus x Group, F(1,42)=9.036, p=.004, ηp2 =.177, Electrode x Group, F(1,42)=5.413, p=.025, ηp2 =.114, and Stimulus x Electrode x Hemisphere x Group, F(1,42)=7.623, p=.009, ηp2 =.154. Within-group analyses revealed that typical children elicited more negative ERP amplitudes in response to words than nonwords at left temporal sites, t(32)=2.134, p=.021, d=.37 (Figure 2). In the RTT group, ERP amplitudes to words were significantly more negative than to nonwords at the right temporal locations, t(10)=2.286, p=.023, d=.67. No condition differences reached significance at the other electrode clusters in either group. However, visual inspection did note the typical pattern of more negative amplitudes to words than nonwords at left hemisphere sites in girls with RTT. These differences did not reach significance likely due to high inter-individual variability (see Table 2 for means and standard deviations).
Figure 2.
Averaged parietal and temporal ERP waveforms for word and nonword conditions in typically developing children (top) and girls with RTT (bottom).
Table 2.
Mean parietal and temporal amplitudes and standard deviation for left and right hemisphere ERP responses across 200–500ms post-stimulus interval.
| Typical development | Rett syndrome | |||||||
|---|---|---|---|---|---|---|---|---|
| Words | Nonwords | Words | Nonwords | |||||
| Scalp Location | M | SD | M | SD | M | SD | M | SD |
| P3 | 0.88 | 2.29 | 0.76 | 2.48 | −2.29 | 4.11 | −0.68 | 3.66 |
| P4 | 1.17 | 2.56 | 0.50 | 2.73 | −0.91 | 3.50 | −0.96 | 3.26 |
| T3 | 0.72 | 2.11 | 1.50 | 1.99 | −0.25 | 3.77 | 1.57 | 2.86 |
| T4 | 1.83 | 2.60 | 1.09 | 2.41 | 0.72 | 4.89 | 4.16 | 5.46 |
Between-group differences were observed in right temporal responses to nonwords, F(1,42)=6.739, p=.013, and left and right parietal responses to words, F(1,42)=10.366, p=.022, and F(1,42)=4.504, p=.040, respectively. Girls with RTT elicited larger (more negative) temporal and smaller parietal amplitudes compared to typical peers. Group differences in the magnitude of nonword-word differentiation (amplitude difference scores) were present only at the right temporal sites, F(1,42)=12.818, p=.001, where girls with RTT exhibited a greater condition difference than typically developing children.
Brain-behavior correlations in RTT group identified no significant associations between ERP amplitudes and age or the number of artifact-free trials. Right temporal amplitudes in response to words did not correlate with behavioral measures of receptive language abilities or adaptive functioning. More negative amplitudes to words at left parietal sites demonstrated a trend-level association with higher PPVT standard scores (r=−.559, p=.059) and reduced lethargy/social withdrawal (r=.427, p=.095), while greater negativity at left temporal locations was related to increased irritability (r=−.434, p=.092). Larger negative amplitudes to words at right parietal sites correlated with reduced hyperactivity (r=.542, p=.043) and irritability (r=.635, p=.018), but lower PPVT standard scores (r=.494, p=.088) and increased lethargy (r=−.442, p=.087) and stereotypy (r=−.412, p=.104).
4. Discussion
The purpose of this investigation was to examine the extent of spoken content processing in girls with RTT compared to typical peers during passive listening to known words and meaningless nonwords. Our results indicated that both participant groups demonstrated the expected more negative ERP amplitudes to words than nonwords, but while in typical children this response was observed at left temporal sites, in girls with RTT consistent evidence of word-nonword discrimination was detected at right temporal sites. Correlational analyses in the RTT group revealed that more typical ERP responses to words (i.e., greater negative amplitudes at left hemisphere sites) were associated, albeit at trend level, with better receptive communication abilities, reduced lethargy/social withdrawal, and increased irritability. These results provide a strong rationale for using ERPs during passive listening to spoken words as a marker of auditory content processing in participants with RTT or other developmental disabilities for whom few optimized standardized behavioral assessments of language exist.
The findings in the typically developing group replicated prior reports of more negative mean amplitudes to words than nonword stimuli at left temporal sites (Mills et al., 1997; Mills et al., 2005; Kuhl et al., 2013). The same pattern of differences was qualitatively present in girls with RTT syndrome but did not reach significance, while statistically significant condition differences in the same direction were observed at the right temporal scalp locations. These results suggest more diffuse auditory processing of speech and have been previously reported earlier in development of typical infants (Mills et al., 1993, 1997), as well as in toddlers with ASD with lower adaptive functioning (Kuhl et al., 2013).
Increased lateralization of ERP responses indexing word-nonword discrimination has been associated with the size of receptive vocabulary as well as the amount of experience with the word stimuli (Mills et al., 2005; Sandbank et al., 2017). In our participants with RTT syndrome, more typical left hemisphere responses to words (i.e., increased negative amplitudes) correlated (at trend level) with higher PPVT scores, reduced lethargy/social withdrawal, and increased irritability. Conversely, larger right-hemisphere responses to words were related to lower PPVT scores and less adaptive behavior (higher ABC scores), consistent with the idea that right-lateralized or bilateral responses to known vs. unknown words reflect developmentally less mature stage (Mills et al., 1997). Previously, we reported a similar association between increased irritability and more typical brain responses to own name (Peters et al., 2017), which was interpreted to suggest that individuals with RTT who are neurologically less impaired are more likely to exhibit behavioral difficulties such as anxiety (Barnes et al., 2015) or potentially find their communication difficulties more frustrating. Our findings of the cortical responses to spoken words in girls with RTT resembling a developmentally younger brain state are also consistent with the idea that MeCP2 underexpression affects neuronal maturation and experience-dependent remodeling (see Gonzales & LaSalle, 2010 for a review).
The strengths of the current study arise from the application of an established auditory word-nonword paradigm and a priori selection of specific ERP metrics (amplitude at left temporal and parietal sites within 200–500ms) that allowed to formulate falsifiable directional hypotheses (more negative amplitudes to words than nonwords) to test the utility of ERPs as a marker of auditory content processing in RTT. Furthermore, the use of the auditory modality reduced attentional demands (i.e., a participant having to attend to a screen), making the assessment suitable for individuals across ages and functional levels. In our sample, 85% (11/13) of participants with RTT provided usable ERP data. The equiprobable design, where word and nonword stimuli were presented equal number of times, removed the potential confounds of within-session learning making known word comprehension the most likely explanation for the observed condition differences. Finally, our data demonstrated that individual differences in neural responses to words were associated with behavioral characteristics relevant to communicative and adaptive behavior, providing additional support for the validity of ERP responses as an objective measure of speech and language processing in individuals with significant developmental disabilities. The neural evidence of word-nonword differentiation could be used to confirm and extend informant reports of receptive language abilities. The relatively low cost and increasing accessibility of EEG/ERP equipment in a clinical setting may also support the development and use of individualized stimulus sets (e.g., specific words expected to be known or unknown by a participant) to assess vocabulary knowledge as well as to track changes due to maturation or intervention.
Our study is the first to examine spoken word comprehension in RTT, and while yielding novel findings, it also presents several limitations. First, while 11 of 13 participants with RTT provided at least 10 artifact-free trials per condition, their overall data quality was lower than in the typical comparison group. However, we observed no correlations between the number of retained trials and the amplitude of the ERP responses used in the analyses. It is also possible that our artifact removal procedures combining automated and manual review were more conservative than those of previous investigations. Second, two of 11 participants with RTT did not have PPVT data due to test fatigue and lack of attention to visual stimuli, thus reducing the sample size and associated statistical power to detect significant brain-behavior correlations. Nevertheless, the observed trend-level findings were in the predicted direction. Third, while the stimulus words were selected from prior studies and expected to be acquired early in development (<16 months), we did not confirm that individual participants knew each item. In light of the findings by Sandbank et al. (2017), verifying word knowledge prior to collecting ERP data could increase the likelihood of detecting significant and meaningful individual differences. In our study, parents or caregivers of participants with RTT knew the purpose of the task, were present during the ERP procedure (i.e., heard all of the stimuli), and did not identify any of the words as potentially unknown by their child. Finally, the present study was designed to test the feasibility of auditory ERPs as a means to assess spoken content processing (i.e., word-nonword differentiation) in girls with RTT, and thus had limited power to investigate the contributions of additional variables that could account for variability in neural responses (e.g., the specific mutation subtype, presence of seizures, clinical severity, etc.). Future studies with larger samples will need to replicate and extend our results to address these questions.
5. Conclusions
The study examined spoken word processing in girls with RTT using auditory ERPs acquired during passive listening. The results indicate that like typically developing children, girls with RTT differentiated known words from novel nonwords, but did so using potentially atypical neural processes as reflected by greater right-hemisphere responses. More typical neural responses over the left hemisphere were associated with higher scores on behavioral measures of receptive communication skills. These results highlight the feasibility of using auditory ERPs to directly evaluate speech and language processes in RTT and other developmental disorders with limited speech. Brain-behavior correlations supported validity of the proposed ERP markers of word processing, making neural measures of functioning a promising approach to documenting outcomes of clinical trials, and for tracking progress related to language and behavioral interventions over time.
Highlights.
Spoken word-nonword discrimination examined during passive listening.
Auditory ERPs recorded in girls with Rett syndrome and typical peers age 4–12 years.
Typical group showed the expected left hemisphere word-nonword response.
The Rett group showed a less mature right hemisphere word-nonword response.
Left hemisphere response correlated with better language skills in Rett syndrome.
Acknowledgements
We would like to thank the participants and their families for their support of the study. This research was supported in part by the International Rett Syndrome Foundation (Grant #3106), as well as by the EKS NICHD (U54HD083211), and NCATS/NIH (UL1TR000445). The content is solely the responsibility of the authors and does not necessarily represent the official views of funding agencies.
Footnotes
Declaration of Interest
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aman MG, Singh NN. Aberrant Behavior Checklist. East Aurora, NY: Slosson; 1986. [Google Scholar]
- Barnes KV, Coughlin FR, O’Leary HM, Bruck N, Bazin GA, Beinecke EB, et al. , 2015. Anxiety-like behavior in Rett syndrome: characteristics and assessment by anxiety scales. Journal of Neurodevelopmental Disorders, 7 (1), 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger-Sweeney J Cognitive deficits in Rett syndrome: what we know and what we need to know to treat them. Neurobiology of Learning and Memory, 2011;96(4), 637–646. [DOI] [PubMed] [Google Scholar]
- Chao HT, Zoghbi HY MeCP2: only 100% will do. Nat. Neurosci 2012;15(2), 176–177. [DOI] [PubMed] [Google Scholar]
- Collins AL, Levenson JM, Vilaythong AP, Richman R, Armstrong DL, Noebels JL, et al. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum. Mol. Genet 2004;13(21), 2679–2689. [DOI] [PubMed] [Google Scholar]
- Demeter K Assessing the developmental level in Rett syndrome: An alternative approach? European Child and Adolescent Psychiatry 2000; 9, 227–233. [DOI] [PubMed] [Google Scholar]
- Dunn DM, Dunn LM (2007). Peabody Picture Vocabulary Test (4th ed.). San Antonio, TX: Pearson. [Google Scholar]
- Foxe JJ, Burke KM, Andrade GN, Djukic A, Frey HP, Molholm S Automatic cortical representation of auditory pitch changes in Rett syndrome. J. Neurodev. Disord 2016;8(1), 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin ES Brodkin JA Blendy SJ Siegel Z Zhou Cellular origins of auditory event-related potential deficits in Rett syndrome. Nat. Neurosci 2014;17(6):804–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales ML, LaSalle JM The role of MeCP2 in brain development and neurodevelopmental disorders. Current Psychiatry Reports 2010; 12(2), 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg B, Aicardi J, Dias K, & Ramos O A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: Report of 35 cases. Annals of Neurology, 1983;14, 471–479. [DOI] [PubMed] [Google Scholar]
- Hagberg B, & Witt-Engerstrom I Rett syndrome: A suggested staging system for describing impairment profile with increasing age towards adolescence. American Journal of Medical Genetics. Supplement, 1986;1, 47–59. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Coffey-Corina S, Padden D, Munson J, Estes A, Dawson G Brain responses to words in 2-year-olds with autism predict developmental outcomes at age 6. PLoS One 2013;8(5), e64967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan Y, McArthur G The effect of a movie soundtrack on auditory event-related potentials in children, adolescents, and adults. Clin Neurophys, 2011;122(5), 934–941. [DOI] [PubMed] [Google Scholar]
- Mills DL, Prat C, Zangl R, Stager CL, Neville HJ, Werker JF Language experience and the organization of brain activity to phonetically similar words: ERP evidence from 14- and 20-month-olds. J. Cogn. Neurosci 2004;16 (8), 1452–1464. [DOI] [PubMed] [Google Scholar]
- Mills DL, Coffey-Corina SA, Neville HJ Language acquisition and cerebral specialization in 20-month-old infants. Journal of Cognitive Neuroscience, 1993;5(3), 317–334. [DOI] [PubMed] [Google Scholar]
- Mills DL, Coffey-Corina S, Neville HJ Language comprehension and cerebral specialization from 13 to 20 months. Developmental Neuropsychology, 1997;13(3), 397–445. [Google Scholar]
- Mills D, Plunkett K, Prat C, Schafer G Watching the infant brain learn words: effects of vocabulary size and experience. Cognitive Development, 2005;20(1), 19–31. [Google Scholar]
- Molfese DL Auditory evoked responses recorded from 16-month-old human infants to words they did and did not know. Brain Lang, 1990;38(3), 345–363. [DOI] [PubMed] [Google Scholar]
- Molfese DL Predicting dyslexia at 8 years of age using neonatal brain responses. Brain Lang. 2000;72 (3), 238–245. [DOI] [PubMed] [Google Scholar]
- Naidu S Rett syndrome: Natural history and underlying disease mechanisms. European Child and Adolescent Psychiatry, 6(Suppl. 1), 1997;14–17. [PubMed] [Google Scholar]
- Neul JL, Kaufmann WE, Glaze DG, Christodoulou J, Clarke AJ, Bahi-Buisson N, et al. Rett syndrome: revised diagnostic criteria and nomenclature. Ann. Neurol 2010;68 (6), 944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy AK, Neul JL, Glaze DG, Motil KJ, Skinner SA, Khwaja O, et al. Rett syndrome diagnostic criteria: lessons from the natural history study. Ann. Neurol 2010; 68(6):951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology, 1989; 72(2), 184–187. [DOI] [PubMed] [Google Scholar]
- Peters SU, Gordon RL, Key AP Induced gamma oscillations differentiate familiar and novel voices in children with MECP2 duplication and Rett syndromes. J. Child Neurol 2015;30 (2), 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SU, Katzenstein A, Jones D, Key A Distinguishing response to names in Rett and MECP2 Duplication syndrome: An ERP study of auditory social information processing. Brain Research, 2017;1675, 71–77. [DOI] [PubMed] [Google Scholar]
- Ramocki MB, Zoghbi HY Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature 2008;455 (7215), 912–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbank M, Yoder P, Key A Word processing in children with autism spectrum disorders: evidence from event-related potentials. Journal of Speech, Language, and Hearing Research, 2017;60(12), 3441–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauder JE, Smeets EE, van Mil SG, Curfs LG The development of visual- and auditory processing in Rett syndrome: an ERP study. Brain Dev. 2006;28 (8), 487–494. [DOI] [PubMed] [Google Scholar]
- Yoder PJ, Camarata S, Camarata M, Williams SM Association between differentiated processing of syllables and comprehension of grammatical morphology in children with Down syndrome. American Journal of Mental Retardation, 2006;111(2), 138–152. [DOI] [PubMed] [Google Scholar]
- Yoder PJ, Molfese D, Murray MM, Key A Normative topographic ERP analyses of speed of speech processing and grammar before and after grammatical treatment. Developmental Neuropsychology, 2013;38(8), 514–533. [DOI] [PMC free article] [PubMed] [Google Scholar]