Abstract
Childhood abuse is a potent risk factor for psychopathology, including posttraumatic stress disorder (PTSD). Research has shown high resting vagal tone, a measure of parasympathetic nervous system function, protects abused youth from developing internalizing psychopathology, but potential mechanisms explaining this effect are unknown. We explored fear extinction learning as a possible mechanism underlying the protective effect of vagal tone on PTSD symptoms among abused youth. We measured resting respiratory sinus arrhythmia (RSA) and skin conductance responses (SCR) during a fear conditioning and extinction task in youth with variability in abuse exposure (N= 94; aged 6–18 years). High RSA predicted lower PTSD symptoms and enhanced extinction learning among abused youths. In a moderated-mediation model, extinction learning mediated the association of abuse with PTSD symptoms only among youth with high RSA. These findings highlight extinction learning as a possible mechanism linking high vagal tone to decreased risk for PTSD symptoms among abused youth.
Keywords: Respiratory sinus arrhythmia, resting vagal tone, posttraumatic stress disorder, fear extinction, child abuse
Introduction
Exposure to childhood abuse, such as physical, sexual, and emotional abuse, is a potent risk factor for child psychopathology (McLaughlin et al., 2012), including posttraumatic stress disorder (PTSD) (McLaughlin et al., 2013). Although abuse reflects a severe traumatic stressor, epidemiological and longitudinal studies show that only 15–30% of youth develop PTSD following abuse exposure (McLaughlin et al., 2013). This variability in response to abuse suggests the presence of individual differences that may buffer or magnify risk for PTSD. Identifying protective factors and associated underlying mechanisms is essential for developing novel prevention and intervention approaches. High vagal tone, an index of parasympathetic nervous system function, has consistently been shown to buffer youth from developing psychopathology following exposure to stress and adversity (El-Sheikh & Whitson, 2006), including abuse (McLaughlin, Alves, & Sheridan, 2014; McLaughlin, Rith-Najarian, Dirks, & Sheridan, 2015; Porges, 2007); yet the mechanism of this protective factor is unknown. Given animal work demonstrating that stimulation of the vagal nerve facilitates extinction learning (i.e., attenuation of freezing responses during repeated presentation of the conditioned stimulus in the absence of the unconditioned stimulus; Childs, DeLeon, Nickel, & Kroener, 2017; Peña et al., 2014), the present study investigated whether the protective effect of vagal tone on PTSD symptoms following abuse is mediated by enhanced fear extinction learning.
Autonomic nervous system (ANS) functioning has been shown to moderate the association of adversity and abuse with youth psychopathology in numerous studies (El-Sheikh, Harger, & Whitson, 2001; El-Sheikh & Whitson, 2006; McLaughlin, Alves, et al., 2014). The ANS activates in response to environmental changes and challenges that require adaptation by the organism (Lucini, Di Fede, Parati, & Pagani, 2005; Porges, 1995, 2007). The ANS is a dynamic system that plays an integral role in coordinating the function of multiple organs, including the heart, lungs, kidneys, salivary glands, and facial muscles. Two branches of the ANS include the sympathetic nervous system (SNS), responsible for mobilizing physiological resources in response to environmental challenge, and the parasympathetic nervous system (PNS), which promotes growth and restoration during times of rest and facilitates a return to baseline following stress. Vagal tone is an aspect of the ANS that represents tonic PNS control over heart rate (Berntson et al., 1997; Porges, 2007). Vagal tone reflects the actions of the vagus nerve, which originates in the brain stem and terminates at the sino-atrial node of the heart, and has tonic inhibitory influences that produce a heart rate that is lower than the basal firing rate of the sino-atrial node (Allen, Chambers, & Towers, 2007; Berntson, et al., 1997; Porges, 2007). The polyvagal theory posits the mammalian “vagal brake,” or myelinated portion of the vagus nerve, is responsible for regulating the dual demands of mobilizing resources during threat by decreasing inhibitory control of the heart and facilitating social communication and behaviors by increasing this control during rest (Porges, 1995, 2007). As vagal tone cannot be measured directly, respiratory sinus arrhythmia (RSA) is a noninvasive measure used to estimate vagal tone that reflects the normal variation in heart rate occurring during a respiration cycle (i.e., inhalation compared to exhalation) (Allen, Chambers, & Towers, 2007; Berntson, Cacioppo, & Quigley, 1993). High resting RSA has been conceptualized as a marker for flexible and contextually appropriate modulation of ANS activity and has been associated with a wide range of psychosocial processes (Beauchaine, 2001; Porges, 2007; Thayer, Ahs, Fredrikson, Sollers, & Wager, 2012).
Indeed, high RSA has been linked to a variety of adaptive emotional, behavioral, and social outcomes across development including better social and emotional regulation skills (Calkins & Keane, 2004; Eisenberg et al., 1995; Fabes, Eisenberg, & Eisenbud, 1993), low negative emotionality (Calkins & Keane, 2004), enhanced executive functioning and attention regulation (Mezzacappa, Kindlon, Saul, & Earls, 1998), and lower levels of internalizing and externalizing problems (Calkins, Graziano, & Keane, 2007; Pine et al., 1998; Shannon, Beauchaine, Brenner, Neuhaus, & Gatzke-Kopp, 2007; however see review by Raine, 1996 showing evidence for the association bewten high vagal tone and behavioral disinhibition as an exception). Given the association between vagal tone and improved functioning across multiple domains, it has also been investigated as a protective factor for psychopathology following exposure to adversity (Katz & Gottman, 1995; McLaughlin, Alves, et al., 2014; McLaughlin et al., 2015). Specifically, studies have shown that high RSA buffers youth exposed to parental conflict from exhibiting externalizing (El-Sheikh et al., 2001; Katz & Gottman, 1995) and internalizing (El-Sheikh et al., 2001; El-Sheikh & Whitson, 2006) psychopathology. Similarly, McLaughlin and colleagues showed high resting vagal tone protected adolescents from experiencing internalizing symptoms following numerous forms of childhood adversity, including abuse, community violence, poverty, and peer victimization (McLaughlin, Alves, et al., 2014; McLaughlin et al., 2015). While vagal tone appears to protect abused youth from experiencing internalizing symptoms broadly, we are unaware of prior research examining this buffering effect in relation to PTSD, a common mental health consequence of abuse (McLaughlin et al., 2013). Additionally, the underlying mechanism explaining the protective effect of high resting vagal tone remains unclear.
One potential mechanism through which vagal tone could exert a protective effect on psychopathology following stress and adversity is by influencing associative learning processes involved in the extinction of conditioned fear. The acquisition of conditioned fear involves the pairing of a neutral stimulus such as a shape (the conditioned stimulus, or CS) with an aversive stimulus such as a shock or loud noise (the unconditioned stimulus, or US). With repeated pairings, the CS begins to elicits a fear response through associative learning mechanisms (LeDoux, 2014). After fear conditioning, fear extinction occurs when the CS is presented repeatedly in the absence of the US. Fear extinction reflects new learning of a CS-no US association that competes with the original fear memory (Milad & Quirk, 2012). In rodents, vagus nerve stimulation when delivered with the CS+ during fear extinction learning promotes more rapid extinction to threat stimuli following fear conditioning (i.e., reduced freezing behaviors) (Peña, Engineer, & McIntyre, 2013). Furthermore, this enhanced extinction response is mediated by plasticity within the infralimbic cortex-amygdala pathway (Peña et al., 2014). The circuitry underlying fear conditioning and extinction is highly conserved across species; the infralimbic cortex in rodents corresponds to the ventromedial prefrontal cortex (PFC) in humans and inhibits the amygdala during extinction learning and retrieval (Milad & Quirk, 2012). Enhanced fear extinction following a conditioned fear response is associated with decreased risk for PTSD (Milad & Quirk, 2012; Vouimba & Maroun, 2011). Together, this evidence suggests that high resting vagal tone may be associated with enhanced extinction learning and, in turn, lower risk for PTSD. This is consistent with a recent conceptualization of vagal tone as reflecting flexible modulation of the amygdala by the medial PFC (Thayer et al., 2012). To date, no studies have investigated these associations in humans or the potential for high resting vagal tone to protect youth with a history of abuse from PTSD symptoms via enhanced fear extinction.
The present study served as a preliminary investigation of several research questions in a sample of youth with and without abuse exposure. First, we were interested in whether resting vagal tone, as measured by RSA, moderates the association between abuse exposure and PTSD symptoms in youth. We aimed to extend prior work demonstrating that high RSA buffers abused youth from internalizing symptoms (McLaughlin et al., 2014), to determine whether this protective effect is also present for PTSD symptoms. Next, we conducted analyses to determine whether high RSA might buffer youth with a history of abuse as compared to non-abused youth from poor fear extinction learning (i.e., enhanced extinction learning will be observed among abused youth with high versus low RSA). In addition, we examined whether enhanced fear extinction was associated with lower PTSD symptoms. Finally, we conducted formal moderated mediation analyses (Hayes, 2013) to explore whether RSA moderated the indirect effect of abuse exposure on PTSD symptoms via fear extinction learning. We predicted that high RSA would be associated with lower PTSD symptoms via fear extinction learning in youth with a history of abuse. Given evidence showing unique patterns of risk across PTSD symptom clusters (Asmundson & Stapleton, 2008; Glover et al., 2011; Stewart, Conrod, Pihl, & Dongier, 1999), we examined associations with total PTSD severity and each symptom cluster (i.e., avoidance, re-experiencing, and arousal) throughout all analyses.
Method
Participants
Participants were 94 youths aged 6 to 18 years old. Data collection occurred between February 2014 and February 2015. As the purpose of the study was to examine emotional learning as a function of child abuse, recruitment efforts were aimed at enrolling youth with and without abuse exposure. As such, we recruited from schools, after-school programs, medical clinics, and the general community as well as neighborhoods with high levels of violent crime, clinics that serve predominantly low-SES clients, and agencies that work with families exposed to violence (e.g., domestic violence shelters, programs for parents mandated to receive intervention from Child Protective Services) in [BLINDED FOR REVIEW].
Procedures
Cardiac data and questionnaires about abuse exposure and psychopathology were collected from all participants prior to completion of the fear conditioning task. Heart period data were acquired during a 10-minute period in which participants were asked to sit quietly without moving.
Participants completed a fear conditioning task validated for children (see Supplemental Figure 1; Shechner et al., 2015). In this task, blue and yellow bell images were used as CS+ and CS− and were counterbalanced across participants. The US was an aversive 96 dB alarm noise. The task included three phases: preconditioning, conditioning, and extinction. During preconditioning phase, participants viewed the CS+ and CS− without the US (four trials each). During the conditioning phase, the CS+ and CS− were presented for 10 trials each, and the CS+ co-terminated with the US in 80% of trials. The extinction phase involved the CS+ and CS− presented without the US (eight trials each). The inter-trial interval ranged from 8 to 12 seconds (mean = 10 seconds). Equipment malfunctions resulted in loss of physiological data from two participants. One participant declined the task, and one participant discontinued during the conditioning phase. The final analytical sample included 90 participants. Differences between youth with and without abuse exposure during the conditioning and extinction phases of the task have been previously published (BLINDED FOR REVIEW, 2016).
Due our specific interest in the role of fear extinction learning, we focused specifically on responses to the CS+ (i.e., the threat cue) during the early fear extinction phase of the task, as fear responses returned by baseline by the later phase of fear extinction (McLaughlin et al., 2016). Moreover, early extinction learning captures the phase of the task where participants exhibit the most variability in the magnitude of fear responses, and is typically the focus in studies of extinction learning (Milad et al., 2009; Milad & Quirk, 2002).
Youth completed questionnaire measures to assess for PTSD symptomatology. Informed consent was obtained from the parent or guardian who attended the session with the participant, and assent was provided by all youth participants. Participants were paid for participation. All procedures were approved by the Institutional Review Board at the University of Washington and performed in accordance with the ethical standards as outlined in the 1964 Declaration of Helsinki.
Measures
Child abuse.
We focused on experiences of child abuse that qualify as a Criterion A traumatic stressor in DSM-5, including physical abuse, sexual abuse, and witnessing domestic violence. Child abuse was assessed using the Childhood Experiences of Care and Abuse interview (CECA; Bifulco, Brown, & Harris, 1994) and the self-report Childhood Trauma Questionnaire (CTQ: Bernstein, Ahluvalia, Pogge, & Handelsman, 1997). The CECA is an interview that assesses caregiving experiences, including physical and sexual abuse. We modified the interview to additionally assess for experiences of witnessing domestic violence (i.e., directly observing violence directed towards caregiver). Inter-rater reliability for reports of abuse is excellent, and there is high agreement between siblings on experiences of abuse (Bifulco et al., 1994). The CTQ is composed of 28 items assessing frequency of abuse during childhood, including physical and sexual abuse. The CTQ exhibits strong convergent and discriminant validity (Bernstein et al., 1997), and demonstrated good internal consistency within the present study (α = 0.79).
A composite (yes/no) indicator of abuse was created from the CECA and the CTQ. Participants were considered abused if they reported (a) physical abuse, sexual abuse, or witnessing more than 2 incidents of domestic violence during the CECA interview or (b) scores on the CTQ physical and sexual abuse subscales that exceed a validated threshold (Walker et al., 1999). Child reported abuse experiences were verified by parent report. A total of 38 (40.4%) participants were classified as abused. Control group participants had no abuse exposure. They were not excluded for exposure to other potential traumas, such as accidents, injuries, and witnessing community violence.
Vagal tone.
Continuous cardiac measures were recorded noninvasively according to accepted guidelines (Sherwood et al., 1990). Electrocardiogram (ECG) recordings were obtained with a Biopac ECG amplifier (Goleta, CA) using a modified Lead II configuration (right clavicle, left lower torso, and right leg ground). Biopac MP150 hardware and Acknowledge software were used to acquire the ECG data (sampled at 1.0 kHz). ECG data were scored by trained professionals blind to group status. Signals were visually inspected and scored using Mindware Heart Rate Variability (HRV) Software (Mindware Technologies, Gahannah, OH).
RSA was calculated from interbeat interval time series using spectral analysis conducted in Mindware HRV Software. RSA was calculated for the frequency band 0.12–0.40Hz.
Electrodermal Activity.
Electrodermal activity (EDA) was continuously acquired throughout the fear conditioning task and served as the primary measure of fear extinction learning. EDA was obtained with a Biopac galvanic skin response module (Goleta, CA). Two Ag-AgCl electrodes filled with sodium chloride gel were attached to the distal phalanges of the index and middle finger of the non-dominant hand after the phalanges were cleaned with rubbing alcohol and abraded. The sampling rate was 250 Hz. EDA was analyzed using AcqKnowledge 4.0 software (Biopac Systems, Goleta, CA). Skin conductance responses (SCR) were calculated following standard procedures (Cacioppo, Tassinary, & Berntson, 2007; Dawson, Schell, & Filion, 2007) as the difference from a 1-s pre-CS baseline to peak response to the CS+ in the 1–4 s following stimulus onset, with a minimum response of 0.02 microsiemens (µs) averaged across trials. The latency window of l-4s to identify the SCR peak response following stimulus onset is standard practice in order to obtain the peak SCR response to the CS without inadvertently capturing a non-specific or spontaneous SCR that is not related to the presented stimulus (Cacioppo et al., 2007; Dawson et al., 2007). This latency window also ended before presentation of the US, ensuring that SCR changes were related to the CS and not the US. Seven participants were non-responders during the conditioning phase of the task based on this threshold and were evenly distributed across the abused (n=4) and control groups (n=3). Non-responders were removed from all analyses as extinction learning cannot occur if fear conditioning has not first been established. The pattern of results was unchanged when non-responders were included. Raw SCR data for the pre-conditioning, conditioning, and extinction phases of the task for each group are reported in previously published work on this dataset (see Supplemental Table 2; BLINDED FOR REVIEW, 2016).
PTSD Symptoms.
Child-report version of the UCLA PTSD Reaction Index (PTSD-RI) (Steinberg, Brymer, Decker, & Pynoos, 2004) were used to calculate PTSD symptoms. The PTSD-RI is made up of a 13-item trauma screen that was used to create a composite index of non-abuse trauma exposure and assess the following PTSD symptoms specifically related to the child abuse: re-experiencing, avoidance/numbing, and hyper-arousal. The PTSD-RI produces a PTSD symptom severity score and exhibits good internal consistency and convergent validity (Steinberg et al., 2013). Internal consistency for the overall measure in the present study was excellent (α = 0.95) with good internal consistency observed among the subscales (all a > 0.74).
Data Analysis
To evaluate whether resting RSA moderated the indirect association between abuse and PTSD symptoms through fear extinction learning, we first performed linear regression analyses using the SPSS PROCESS macro (Hayes, 2013) to test the following pathways of the full model: 1) Main effects and interaction of abuse and resting RSA predicting PTSD symptoms; 2) Main effects and interaction of abuse and resting RSA predicting SCR during fear extinction learning; 3) Main effect of SCR during fear extinction learning predicting PTSD symptoms. Average SCR was computed during the early extinction phase of the fear conditioning task (trials 1–8). A square-root transformation was performed on SCR prior to analysis. We examined total PTSD symptoms as well as re-experiencing, avoidance/numbing, and hyper-arousal separately.
Significant interactions were explored following Hayes’ (2013) guidelines for testing regions of significance according to the Johnson-Neyman (J-N) technique (Bauer and Curran, 2005). This procedure uses regression parameters to derive values of a continuous moderator (i.e., resting RSA) at which the conditional effect of the focal predictor (i.e., child abuse) on the dependent variable (i.e., PTSD symptoms or SCR during fear extinction learning) transitions from nonsignificant to significant. Applied to the present study, the J-N technique indicated at what degree of resting RSA those with or without abuse exposure differ significantly in their level of PTSD symptoms or SCR during fear extinction. For all linear regression models, we conducted additional moderation analyses to examine whether effects varied by age. No significant moderation occurred across any regression model, so age was not included as a moderator.
For all significant linear regression models, we used PROCESS (Hayes, 2013) to formally test for moderated mediation using a bootstrapping approach with 10,000 bootstrap resamples that provides confidence intervals for the moderated indirect effects (Hayes, 2013). We also conducted additional sensitivity analyses for the final models by adjusting for socio-economic status (i.e., whether the family’s income was below the poverty line) to determine whether any associations were the result of confounding by socio-demographics. Neither age nor sex were associated with our primary variables of interest, so we did not adjust for either in our final models (see Supplemental Table 1).
Results
Descriptive Statistics and Preliminary Analyses
Demographic information and descriptive statistics for all primary variables separated by abuse exposure are presented (Table 1). Abused and non-abused youths did not differ on sex (p= .56), race (p= .30), age (p= .66), or baseline SCR (i.e., pre-conditioning trials), post-conditioning SCR (i.e., final trial during the conditioning phase), and SCR during extinction learning (ps>.47). Abused youths were more likely to be living in poverty (p= .006) and had greater PTSD symptoms (ps<.001). Raw data Zero-order correlations between primary variables of interest and covariates are presented in Supplemental Table 1.
Table 1.
Distribution of Socio-Demographics and Primary Variables of Interest by Child Abuse Exposure
| Abused (n = 38) | Controls (n = 56) | |||||
| % | N | % | N | χ2 | P-value | |
| Sex | .35 | .56 | ||||
| Female | 52.6 | 20 | 46.4 | 26 | ||
| Male | 47.3 | 18 | 53.6 | 30 | ||
| Race | 4.89 | .30 | ||||
| White | 42.1 | 16 | 57.1 | 32 | ||
| Black | 26.3 | 10 | 10.7 | 6 | ||
| Hispanic | 15.8 | 6 | 12.5 | 7 | ||
| Asian | 7.9 | 3 | 12.5 | 7 | ||
| Biracial or other | 7.9 | 3 | 7.1 | 4 | ||
| Poverty | 51.4 | 18 | 19.6 | 11 | 7.59 | .006 |
| M | (SD) | M | (SD) | t-Value | P-value | |
| Age | 13.77 | 3.48 | 13.44 | 18.65 | .45 | .66 |
| PTSD Symptoms | ||||||
| PTSD Severity | 19.63 | 17.21 | 6.07 | 9.92 | 4.84 | <.001 |
| Avoidance | 7.02 | 6.81 | 1.93 | 3.84 | 4.63 | <.001 |
| Re-experiencing | 5.42 | 6.65 | 1.66 | 3.09 | 3.77 | <.001 |
| Hyper-arousal | 7.18 | 5.89 | 2.48 | 3.81 | 4.70 | <.001 |
| Resting RSA | 7.17 | 1.10 | 7.12 | 1.06 | .26 | .80 |
| Early Extinction CS+ | .11 | .16 | .12 | .20 | .37 | .71 |
Note. Abbreviations: RSA= respiratory sinus arrhythmia; CS+= conditioned stimulus paired with aversive noise; PTSD= posttraumatic stress disorder. Poverty was assessed as percent of families living below the poverty line. PTSD symptoms were assessed with the UCLA PTSD Reaction Index (PTSD-RI), child report.
Child Abuse and PSTD Symptoms Moderated by RSA
We first examined whether resting RSA moderated the association between abuse and total PTSD symptoms (Table 2A). We found a main effect of abuse, such that youth with greater exposure to abuse were more likely to experience PTSD symptoms (b=46.60, p=.01). RSA moderated the association between abuse and total PTSD symptoms at a trend level (b=−4.60, p=.08). Due to our a priori interest regarding this interaction term, we conducted exploratory post-hoc J-N testing that indicated the association between abuse and PTSD symptoms only emerged (p<.05) at average to low levels of RSA (i.e., < 7.94), but was not significant for youth with high resting RSA (i.e., ≥7.69) (Figure 1A).
Table 2.
RSA by child abuse predicting PTSD symptoms (A-D) and early fear extinction learning (E)
| A. Prediction of PTSD total symptoms by abuse and RSA | |||
| Predictor | b (SE b) | 95% CI | p |
| Intercept | 12.54(12.11) | [−11.53, 36.61] | .30 |
| Abuse | 46.60(18.59) | [9.66, 83.54] | .01 |
| Resting RSA | −.91(1.68) | [−4.25, 2.44] | .59 |
| Abuse × RSA | −4.59(2.57) | [−9.71, 0.51] | .08 |
| B. Prediction of PTSD avoidance symptoms by abuse and RSA | |||
| Predictor | b (SE b) | 95% CI | p |
| Intercept | 1.29(4.54) | [−7.73, 10.32] | .78 |
| Abuse | 26.90(6.97) | [13.05, 40.75] | <001 |
| Resting RSA | .08(.63) | [−1.17, 1.33] | .90 |
| Abuse × RSA | −3.03(.96) | [−4.94, −1.11] | .002 |
| C. Prediction of PTSD re-experiencing symptoms by abuse and RSA | |||
| Predictor | b (SE b) | 95% CI | p |
| Intercept | 4.06(4.39) | [−4.67, 12.79] | .36 |
| Abuse | 12.27(6.74) | [−1.13, 25.67] | .07 |
| Resting RSA | −.33(.61) | [−1.54, .88] | .59 |
| Abuse × RSA | −1.19(.93) | [−3.04, .66] | .21 |
| D. Prediction of PTSD arousal symptoms by abuse and RSA | |||
| Predictor | b (SE b) | 95% CI | p |
| Intercept | 7.19(4.44) | [−1.64, 16.02] | .11 |
| Abuse | 7.44(6.81) | [−6.11, 20.98] | .28 |
| Resting RSA | −.66(.62) | [−1.88, .57] | .29 |
| Abuse × RSA | −.38(.94) | [−2.25, 1.49] | .69 |
| E. Prediction of early fear extinction response by abuse and resting RSA | |||
| Predictor | b (SE b) | 95% CI | p |
| Intercept | .94(.07) | [.80, 1.09] | <.001 |
| Abuse | .26(.12) | [.02, .51] | .03 |
| Resting RSA | .02(.01) | [−.004, .04] | .12 |
| Abuse × RSA | −.04(.02) | [−0.07, −.004] | .03 |
Note. Abbreviations: RSA= respiratory sinus arrhythmia.
Fig. 1.
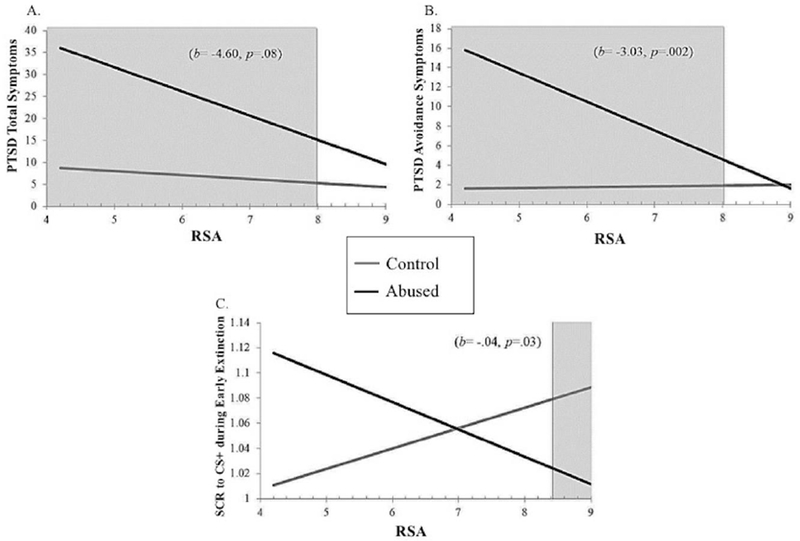
Association between child abuse exposure and child reported (A) PTSD total and (B) avoidance symptoms and (C) skin conductance response (SCR) to the CS+ during early fear extinction learning across observed levels of RSA within the study’s sample. Shaded areas signify Johnson-Neyman regions of significance
We found a similar pattern when examining moderation of the association between abuse and PTSD avoidance symptoms by resting RSA (Table 2B). We found a main effect of abuse such that youth with greater exposure to abuse were more likely to experience PTSD avoidance symptoms (b=26.90, p<.001). RSA significantly moderated the association between abuse and total PTSD avoidance symptoms (b=−3.03, p=.002). Post-hoc J-N testing indicated that the association between abuse and PTSD avoidance symptoms only emerged (p<.05) at average to low levels of resting RSA (i.e., < 8.01), but was not significant for youth with high resting RSA (i.e., ≥8.01) (Figure 1B). There were no interactions between abuse and RSA in predicting re-experiencing (Table 2C) or arousal (Table 2D) symptoms (ps>.21).
In sum, these findings show youth exposed to abuse only experienced greater PTSD symptoms at low levels of RSA and had similar levels of PTSD symptoms compared to control youth at high levels of RSA.
Child Abuse and Fear Extinction Moderated by RSA
We examined whether resting RSA moderates the association between abuse and SCR during the early extinction phase (Table 2E). Abuse predicted reduced extinction learning (i.e., higher SCR to CS+) (b=0.26, p=.03). We also found a significant interaction between abuse and resting RSA predicting SCR to the CS+ during early extinction (b=−.04,p=03). Post-hoc J-N testing indicated a negative association between abuse and SCR to the CS+ during early extinction at high (i.e., >8.46, p<.05) levels of RSA and positively associated at the trend level for low levels of RSA (i.e., ≤ 5.20, p<.08), but was not significant for moderate levels of RSA (i.e., 4.45 – 8.45; Figure 1C). Results suggest youth exposed to abuse experience enhanced extinction learning only those with high levels of RSA. In contrast, control youth may experience enhanced extinction learning only at low levels of RSA.
Fear Extinction and PTSD Symptoms
We investigated whether SCR during the early fear extinction phase was directly associated with PTSD symptoms. There were no significant associations between either SCR to CS+ or CS− and PTSD total, avoidance, arousal, or re-experiencing symptoms (ps>.15).
Moderated Mediation Models
As a final test of our hypothesis that associations among abuse, fear extinction learning, and PTSD symptoms were moderated by resting RSA, we conducted formal moderated mediation analyses. Because we showed significant associations only when examining total and avoidance PTSD symptoms, we conducted a formal test of moderation of indirect effects by RSA separately for each of these outcomes.
Total PTSD symptoms.
The combined model with abuse, SCR to the CS+ during early fear extinction learning, and resting RSA accounted for 33% of the variance in total PTSD symptoms (R2= .33, F(4, 78)=9.42,p= <.001). There was a marginal effect of RSA moderating the indirect association between abuse and total PTSD symptoms via early fear extinction learning (b= .98, 90% CI: .16, 2.57). Specifically, the indirect association between abuse and total PTSD symptoms through early fear extinction learning was significant only at high (+1 SD) (b= 1.22, 90% CI: .15, 3.09), but not mean (b= .17, 90% CI: −.52, 1.12) or low (−1 SD) (b= −.87, 90% CI: −3.24, .08) levels of RSA. Findings suggest abused youth with high levels of RSA experience enhanced fear extinction learning, which then predicts lower PTSD symptoms.
Sensitivity analyses.
The moderated indirect effect continued to be significant at a trend level once adjusting for poverty status such that the indirect association between abuse and total PTSD symptoms through early fear extinction learning was marginally significant only at high (+1 SD) (b= 1.07, 90% CI: .02, 3.07), but not mean (b= .06, 90% CI: −.68, .86) or low (−1 SD) (b= −.94, 90% CI: −3.55, .08) levels of RSA.
PTSD avoidance symptoms.
The combined model with abuse, SCR during early fear extinction learning to CS+, and resting RSA accounted for 40% of the variance in PTSD avoidance symptoms (R2= .40, F(4, 78)= 12.80, p= <.001). RSA significantly moderated the indirect association between abuse and PTSD avoidance symptoms via early fear extinction learning (b= .51, 95% CI: .05, 1.39). Specifically, the indirect association between abuse and PTSD avoidance symptoms through early fear extinction learning was significant at high (+1 SD) (b= .63, 95% CI: .04, 1.63), but not mean (b= .09, 95% CI: −.46, .53) or low (−1 SD) (b= −.45, 95% CI: −1.72, .18) levels of RSA (Figure 2). This suggests abused youth with high levels of RSA experience enhanced fear extinction learning, and this moderated mediation effect then predicts lower PTSD avoidance symptoms.
Fig. 2.
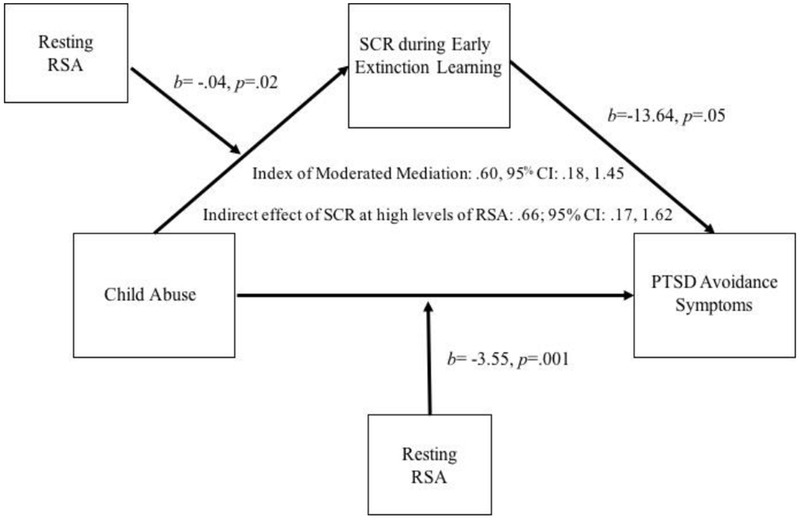
Moderated mediation model showing indirect effect of child abuse on posttraumatic stress disorder (PTSD) avoidance symptoms via skin conductance response (SCR) during early fear extinction learning is moderated by resting respiratory sinus arrhythmia (RSA) adjusting for poverty status
Sensitivity analyses.
The moderated indirect effect predicting PTSD avoidance symptoms remained significant when controlling for poverty status such that the indirect association between abuse and avoidance symptoms through early fear extinction learning was significant at high (+1 SD) (b= .66, 95% CI: .17, 1.62) but not mean (b= .03, 95% CI: −.47, .39) levels of RSA. Additionally, adjusting for poverty resulted in an additional moderated indirect effect at low (−1 SD) (b= −.59, 95% CI: −1.79, −.02) levels of RSA.
Discussion
Childhood abuse is strongly associated with PTSD (McLaughlin et al., 2013). While high vagal tone has been shown to buffer the negative effects of stress and adversity on psychopathology (El-Sheikh et al., 2001; Katz & Gottman, 1995; McLaughlin, Alves, et al., 2014), little research has examined factors that may underlie this association. The present study examined extinction learning as a potential mechanism explaining the protective effects of high vagal tone on PTSD symptoms among children exposed to abuse. We replicated previous literature by demonstrating that high vagal tone was associated with lower PTSD symptoms among abused youths, and we provided novel evidence by showing this association was mediated by enhanced fear extinction learning (i.e., lower SCR during the early phase of extinction learning), particularly for avoidance symptoms. Unexpectedly, we found better extinction learning among control youth with low RSA compared to abused youths. Together, these findings highlight a potential mechanism—enhanced fear extinction learning—underlying the protective effect of high vagal tone on psychopathology following childhood adversity that may inform the development of prevention and intervention approaches.
High vagal tone has been shown to protect against broadband internalizing and externalizing psychopathology among children exposed to interpersonal stressors including parental marital conflict, abuse, and other forms of victimization (El-Sheikh et al., 2001; El-Sheikh & Whitson, 2006; Katz & Gottman, 1995; McLaughlin, Alves, et al., 2014; McLaughlin et al., 2015). We extended this literature by showing high resting RSA was associated with lower PTSD symptoms among abused youths, a common sequela of abuse exposure (McLaughlin et al., 2012, 2013). Similar to past work, our findings fit within the polyvagal theory that argues flexible and rapid vagal regulation via the vagal brake facilities contextually appropriate behavior in response to environmental challenge that may promote a return to baseline following stress (Porges, 2007). Appropriate engagement of this vagal brake may be a particularly important protective factor for PTSD symptoms given research showing increased susceptibility to environmental stressors experienced by individuals with preexisting ANS dysregulation, such as SNS reactivity (Busso, McLaughlin, & Sheridan, 2014; Friedman, 2007; Guthrie & Bryant, 2005; Pole et al., 2009), and improved psychological outcomes following interventions targeting ANS function among trauma survivors (Descilo et al., 2010).
Animal research has suggested that enhanced vagal function may be related to associative learning processes, including fear extinction (Peña et al., 2014, 2013). Our findings are the first to provide evidence in humans that high vagal tone is associated with enhanced fear extinction learning, but only among youth who have experienced significant trauma. Specifically, we demonstrated that youth with a history of abuse extinguished fear to previously conditioned threat cues more quickly if they had high RSA versus low RSA. These findings are consistent with conceptual models positing that high vagal tone reflects the ability of the medial PFC to flexibly modulate amygdala reactivity (Thayer et al., 2012), a circuit well-documented to underlie fear extinction and other emotion regulation processes (Lane et al., 2009; Milad & Quirk, 2012; Neumann et al., 2006). Specifically, ventromedial PFC recruitment serves to inhibit the amygdala and promote extinction and better retention of associative learning between previously threatening cues and the absence of threat (Milad & Quirk, 2012). Of note, previously published work in this sample demonstrated that youth exposed to abuse demonstrate reduced SCR to the CS+ during conditioning than youth who had not been abused (BLINDED FOR REVIEW, 2016). It is possible that differences in the magnitude of initial conditioning contribute to the patterns observed here. However, because the present study examined vagal tone as a moderator of the link between abuse and extinction learning, abuse-related differences during conditioning are unlikely to have a meaningful impact on the interpretation of the present findings. While our findings support research suggesting close integration between vagal tone and mPFC-amygdala circuitry, future work examining these processes across multiple levels (i.e., neural, ANS, behavioral function) are necessary to determine how robust these associations are and whether the links between vagal tone and extinction learning only emerge in the context of trauma and adversity exposure.
When examining abuse history, fear extinction learning, RSA, and PTSD symptom outcomes within an omnibus moderated mediation model, we found extinction learning mediated the association between abuse and PTSD only among youth with high vagal tone. This finding was robust when adjusting for poverty status and was particularly strong when predicting PTSD avoidance symptoms. Exposure-based therapies, the primary treatment modality for PTSD, rely heavily on extinction based mechanisms, as avoidance of feared stimuli is tied to the inability to extinguish fear due to lack of habituation to the feared stimulus (Dorsey, Briggs, & Woods, 2011). Vagus nerve stimulation during fear extinction has been associated with enhanced extinction learning and retention as well as increased neural plasticity in mPFC-amygdala circuitry in rodents (Peña et al., 2013). In humans, vagus nerve stimulation has been studied as a treatment for depression (Daban, Martinez-Aran, Cruz, & Vieta, 2008) and shown to increase PFC-amygdala connectivity compared to sham treatment among depressed adults (Liu et al., 2016). While results predicting PTSD avoidance symptoms were encouraging, models predicting total PTSD symptoms were significant only at the 90th percentile confidence interval with and without adjusting for poverty. Given the preliminary nature of this research question, relatively small sample size, and number of paths being examined in the moderated mediation models, larger samples may be necessary to observe significant effects. Nevertheless, the present study offers evidence that vagus nerve stimulation, or other methods for increasing vagal tone (e.g., mindfulness training, yoga; Descilo et al., 2010; Ditto, Eclache, & Goldman, 2006), as an adjunct to exposure therapy among individuals with PTSD may be a promising area of future research.
While results were generally consistent with the broader literature, two unexpected findings emerged. First, we observed a cross-over interaction where high RSA not only buffered abused youths against poor fear extinction learning, but actually enhanced extinction learning compared to control youth. Furthermore, we observed a marginal effect for better extinction learning among control youth with low RSA. While caution is warranted in interpreting this pattern until replicated, associations between low RSA and positive psychological or behavioral outcomes may relate to contextual factors pursuant to a differential susceptibility framework (Belsky & Pluess, 2009). The differential susceptibility framework posits that characteristics may function as a protective or risk factor depending on the individual’s context (i.e., abusive versus non-abusive environments). Indeed, one study found better mental health outcomes among children with low vagal tone only in the context of little marital conflict (El-Sheikh et al., 2001). Relatedly, previous studies have typically utilized simple slope methodology in favor of the more sensitive and specific J-N regions of significance testing when probing interactive effects of RSA and environmental stress. It is possible that future work may indeed find differential effects of high/low RSA dependent upon environmental context when utilizing regions of significance probing techniques Second, while we did not find a direct association between fear extinction learning and PTSD symptoms within in our sample, there is a robust literature showing extinction learning recall, (i.e., response to the CS+ on a subsequent day), is disrupted among those with PTSD as opposed to the initial extinction learning itself (Milad & Quirk, 2012). The present study did not include a measure of extinction learning recall on a subsequent day, so we were unable to examine whether extinction recall may also underlie the association between high vagal tone and lower PTSD symptoms among maltreated youth. Future research utilizing both extinction learning and recall phases following fear conditioning along with replication of this effect in larger samples is clearly warranted.
While the present investigation represents a novel contribution to our understanding of the mechanisms underlying the protective effects of vagal tone, our findings should be interpreted in light of several limitations. The primary limitation is our use of a cross-sectional study design that does not allow us to determine the temporal associations among abuse exposure, vagal tone, fear extinction and PTSD symptoms. Replication of these findings in prospective studies is needed. Additionally, our relatively small sample size may have hindered our ability to find significant moderated indirect effects when predicting total PTSD symptoms, particularly after adjustment for covariates. Given the overlap between poverty and trauma exposure, these sensitivity analyses are important to isolate the effects of trauma that are independent of other forms of adversity that are strongly linked to poverty (McLaughlin & Sheridan, 2016; McLaughlin, Sheridan, & Lambert, 2014). This overlap presents challenges in disentangling the unique effects of particular forms of adversity in small samples, however. Our sample was recruited based on exposure to trauma, including abuse and domestic violence. We did not have sufficient variability in other forms of trauma to determine whether the pattern of findings was similar for less severe traumatic events. Determining whether these findings extend to other forms of trauma and adversity is a key question for future research. While we utilized self-report versus verified reports (i.e., police or child protective service reports) of child abuse exposure to determine group classification, relying on documented reports of abuse also carries limitations including substantial underreporting of abuse (Widom, Weiler, & Cottler, 1999). In order to reduce the risk of misclassifying youth, we relied on multiple assessment methods (i.e., validated cut-offs on self-report and interview) as well as verified any child-reported abuse exposure with a caregiver. Finally, we utilized a symptom-based measure of PTSD as opposed to a diagnostic interview. Determining whether our findings are replicated in studies that examine PTSD disorder onset is another important goal for future research.
The present study represents the first investigation of fear extinction learning as a potential mechanism underlying the protective effect of vagal tone on PTSD symptoms among youth exposed to abuse. We replicated and extended prior research by showing that high vagal tone was associated with lower PTSD symptoms, particularly avoidance symptoms, among abused youth. We provided novel evidence that abused children and adolescents with high RSA demonstrate enhanced fear extinction learning. We also found better extinction learning among control youth with low RSA compared to abused youths which may be related to potential differential susceptibility processes. Finally, we showed a significant moderated indirect effect such that more rapid fear extinction learning was associated with lower PTSD symptoms, particularly avoidance symptoms, among youth with both an abuse history and high RSA. Interventions that improve vagal tone, especially when paired with existing evidence-based interventions targeting fear extinction mechanisms (e.g., trauma-focused cognitive behavioral therapy), may bolster the effects of existing approaches for treating and preventing PTSD among abused children.
Supplementary Material
Supplemental Fig. 1 Fear conditioning task
Acknowledgments:
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (T32 HD057822-06: JLJ; F32 HD089514: MLR), the National Institute of Mental Health (R01-MH103291: KAM; F32 MH108238: ABM; K23MH112872-01: JLJ), a Brain and Behavior Research Foundation NARSAD Young Investigator Grant (KAM), and a Jacobs Foundation Early Career Research Fellowship (KAM).
References
- Allen JJ, Chambers AS, & Towers DN (2007). The many metrics of cardiac chronotropy A pragmatic primer and a brief comparison of metrics. Biological Psychology, 74(2), 243–262. [DOI] [PubMed] [Google Scholar]
- Asmundson GJG, & Stapleton JA (2008). Associations Between Dimensions of Anxiety Sensitivity and PTSD Symptom Clusters in Active-Duty Police Officers. Cognitive Behaviour Therapy, 37, 66–75. 10.1080/16506070801969005 [DOI] [PubMed] [Google Scholar]
- Beauchaine T (2001). Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology, 13(02), 183–214. [DOI] [PubMed] [Google Scholar]
- Belsky J, & Pluess M (2009). Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin, 135(6), 885. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, & Handelsman L (1997). Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child & Adolescent Psychiatry, 36(3), 340–348. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, … van der Molen MW (1997). Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology, 34(6), 623–648. [DOI] [PubMed] [Google Scholar]
- Berntson Gary G., Cacioppo JT, & Quigley KS (1993). Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology, 30(2), 183–196. [DOI] [PubMed] [Google Scholar]
- Bifulco A, Brown GW, & Harris TO (1994). Childhood Experience of Care and Abuse (CECA): a retrospective interview measure. Journal of Child Psychology and Psychiatry, 35(8), 1419–1435. [DOI] [PubMed] [Google Scholar]
- Busso DS, McLaughlin KA, & Sheridan MA (2014). MEDIA EXPOSURE AND SYMPATHETIC NERVOUS SYSTEM REACTIVITY PREDICT PTSD SYMPTOMS AFTER THE BOSTON MARATHON BOMBINGS: Research Article: Vulnerability to PTSD Symptoms Following a Terrorist Attack. Depression and Anxiety, 31(7), 551–558. 10.1002/da.22282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Tassinary LG, & Berntson G (2007). Handbook of psychophysiology. Cambridge University Press. [Google Scholar]
- Calkins SD, Graziano PA, & Keane SP (2007). Cardiac vagal regulation differentiates among children at risk for behavior problems. Biological Psychology, 74(2), 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, & Keane SP (2004). Cardiac vagal regulation across the preschool period: Stability, continuity, and implications for childhood adjustment. Developmental Psychobiology, 45(3), 101–112. [DOI] [PubMed] [Google Scholar]
- Childs JE, DeLeon J, Nickel E, & Kroener S (2017). Vagus nerve stimulation reduces cocaine seeking and alters plasticity in the extinction network. Learning & Memory, 24(1), 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daban C, Martinez-Aran A, Cruz N, & Vieta E (2008). Safety and efficacy of Vagus Nerve Stimulation in treatment-resistant depression. A systematic review. Journal of Affective Disorders, 110(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, & Filion DL (2007). The electrodermal system. Handbook of Psychophysiology, 2, 200–223. [Google Scholar]
- Descilo T, Vedamurtachar A, Gerbarg PL, Nagaraja D, Gangadhar BN, Damodaran B, … Brown RP (2010). Effects of a yoga breath intervention alone and in combination with an exposure therapy for post-traumatic stress disorder and depression in survivors of the 2004 South-East Asia tsunami. Acta Psychiatrica Scandinavica, 121(4), 289–300. [DOI] [PubMed] [Google Scholar]
- Ditto B, Eclache M, & Goldman N (2006). Short-term autonomic and cardiovascular effects of mindfulness body scan meditation. Annals of Behavioral Medicine, 32(3), 227–234. 10.1207/sl5324796abm3203_9 [DOI] [PubMed] [Google Scholar]
- Dorsey S, Briggs EC, & Woods BA (2011). Cognitive-behavioral treatment for posttraumatic stress disorder in children and adolescents. Child and Adolescent Psychiatric Clinics of North America, 20(2), 255–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Murphy B, Maszk P, Smith M, & Karbon M (1995). The Role of Emotionality and Regulation in Children’s Social Functioning: A Longitudinal Study. Child Development, 66(5), 1360–1384. 10.1111/j.1467-8624.1995.tb00940.x [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Harger J, & Whitson SM (2001). Exposure to interparental conflict and children’s adjustment and physical health: The moderating role of vagal tone. Child Development, 72(6), 1617–1636. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, & Whitson SA (2006). Longitudinal relations between marital conflict and child adjustment: Vagal regulation as a protective factor. Journal of Family Psychology, 20(1), 30. [DOI] [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N, & Eisenbud L (1993). Behavioral and physiological correlates of children’s reactions to others in distress. Developmental Psychology, 29(4), 655–663. https://doi.org/10.1037/0012-1649.29.4.655 [Google Scholar]
- Friedman BH (2007). An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology, 74(2), 185–199. [DOI] [PubMed] [Google Scholar]
- Glover EM, Phifer JE, Crain DF, Norrholm SD, Davis M, Bradley B, … Jovanovic T (2011). Tools for translational neuroscience: PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depression and Anxiety, 28(12), 1058–1066. 10.1002/da.20880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie RM, & Bryant RA (2005). Auditory startle response in firefighters before and after trauma exposure. American Journal of Psychiatry, 162(2), 283–290. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY, US: Guilford Press. [Google Scholar]
- Katz LF, & Gottman JM (1995). Vagal tone protects children from marital conflict. Development and Psychopathology, 7(1), 83–92. [Google Scholar]
- Lane RD, McRae K, Reiman EM, Chen K, Ahem GL, & Thayer JF (2009). Neural correlates of heart rate variability during emotion. Neuroimage, 44(1), 213–222. [DOI] [PubMed] [Google Scholar]
- LeDoux JE (2014). Coming to terms with fear. Proceedings of the National Academy of Sciences, 111(8), 2871–2878. 10.1073/pnas.1400335111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Fang J, Wang Z, Rong P, Hong Y, Fan Y, … others (2016). Transcutaneous vagus nerve stimulation modulates amygdala functional connectivity in patients with depression. Journal of Affective Disorders, 205, 319–326. [DOI] [PubMed] [Google Scholar]
- Lucini D, Di Fede G, Parati G, & Pagani M (2005). Impact of chronic psychosocial stress on autonomic cardiovascular regulation in otherwise healthy subjects. Hypertension, 46(5), 1201–1206. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Alves S, & Sheridan MA (2014). Vagal regulation and internalizing psychopathology among adolescents exposed to childhood adversity. Developmental Psychobiology, 56(5), 1036–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2012). Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Archives of General Psychiatry, 69(11), 1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Koenen KC, Hill ED, Petukhova M, Sampson NA, Zaslavsky AM, & Kessler RC (2013). Trauma exposure and posttraumatic stress disorder in a national sample of adolescents. Journal of the American Academy of Child & Adolescent Psychiatry, 52(8), 815–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Rith-Najarian L, Dirks MA, & Sheridan MA (2015). Low vagal tone magnifies the association between psychosocial stress exposure and internalizing psychopathology in adolescents. Journal of Clinical Child & Adolescent Psychology, 44(2), 314–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, & Sheridan MA (2016). Beyond cumulative risk: a dimensional approach to childhood adversity. Current Directions in Psychological Science, 25(4), 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Gold AL, Duys A, Lambert HK, Peverill M, … Pine DS (2016). Maltreatment exposure, brain structure, and fear conditioning in children and adolescents. Neuropsychopharmacology. Retrieved from http://www.nature.com/npp/journal/vaop/ncurrent/full/npp2015365a.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Lambert HK (2014). Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neuroscience & Biobehavioral Reviews, 47, 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzacappa E, Kindlon D, Saul JP, & Earls F (1998). Executive and Motivational Control of Performance Task Behavior, and Autonomic Heart-rate Regulation in Children: Physiologic Validation of Two-factor Solution Inhibitory Control. Journal of Child Psychology and Psychiatry, 39(4), 525–531. [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, … Rauch SL (2009). Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biological Psychiatry, 66(12), 1075–1082. https://doi.org/10.1016/j.biopsych.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, & Quirk GJ (2002). Neurons in medial prefrontal cortex signal memory for fear extinction. Nature, 420(6911), 70. [DOI] [PubMed] [Google Scholar]
- Milad MR, & Quirk GJ (2012). Fear extinction as a model for translational neuroscience: ten years of progress. Annual Review of Psychology, 63, 129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann SA, Brown SM, Ferrell RE, Flory JD, Manuck SB, & Hariri AR (2006). Human choline transporter gene variation is associated with corticolimbic reactivity and autonomic-cholinergic function. Biological Psychiatry, 60(10), 1155–1162. [DOI] [PubMed] [Google Scholar]
- Peña DF, Childs JE, Willett S, Vital A, McIntyre CK, & Kroener S (2014). Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Frontiers in Behavioral Neuroscience, 8 Retrieved from https://books.google.com/books?hl=en&lr=&id=-wXmCgAAQBAJ&oi=fnd&pg=PA60&dq=pena+vagal+tone+rats&ots=ZDA8DNWquX&sig=GhM0KKKQmGQf9BX4G9Q6_zsVGH0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña DF, Engineer ND, & McIntyre CK (2013). Rapid remission of conditioned fear expression with extinction training paired with vagus nerve stimulation. Biological Psychiatry, 73(11), 1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Wasserman GA, Miller L, Coplan JD, Bagiella E, Kovelenku P, … Sloan RP (1998). Heart period variability and psychopathology in urban boys at risk for delinquency. Psychophysiology, 35(5), 521–529. [DOI] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Otte C, Henn-Hasse C, Metzler TJ, & Marmar CR (2009). Prospective prediction of posttraumatic stress disorder symptoms using fear potentiated auditory startle responses. Biological Psychiatry, 65(3), 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW (1995). Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology, 32(4), 301–318. [DOI] [PubMed] [Google Scholar]
- Porges SW (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A (1996). Autonomic nervous system factors underlying disinhibited, antisocial, and violent behavior biosocial perspectives and treatment implications. Annals of the New York Academy of Sciences, 794(1), 46–59. [DOI] [PubMed] [Google Scholar]
- Shannon KE, Beauchaine TP, Brenner SL, Neuhaus E, & Gatzke-Kopp L (2007). Familial and temperamental predictors of resilience in children at risk for conduct disorder and depression. Development and Psychopathology, 19(03), 701–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, Britton JC, Ronkin EG, Jarcho JM, Mash JA, Michalska KJ, … Pine DS (2015). Fear conditioning and extinction in anxious and nonanxious youth and adults: examining a novel developmentally appropriate fear-conditioning task. Depression and Anxiety, 32(4), 277–288. 10.1002/da.22318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, & van Doornen LJP (1990). Methodological Guidelines for Impedance Cardiography. Psychophysiology, 27(1), 1–23. https://doi.org/10.1111/j.1469-8986.1990.tb02171.x [DOI] [PubMed] [Google Scholar]
- Steinberg AM, Brymer MJ, Decker KB, & Pynoos RS (2004). The University of California at Los Angeles Post-traumatic Stress Disorder Reaction Index. Current Psychiatry Reports, 6(2), 96–100. [DOI] [PubMed] [Google Scholar]
- Steinberg AM, Brymer MJ, Kim S, Briggs EC, Ippen CG, Ostrowski SA, … Pynoos RS (2013). Psychometric Properties of the UCLA PTSD Reaction Index: Part I. Journal of Traumatic Stress, 26(1), 1–9. 10.1002/jts.21780 [DOI] [PubMed] [Google Scholar]
- Stewart SH, Conrod PJ, Pihl RO, & Dongier M (1999). Relations between posttraumatic stress symptom dimensions and substance dependence in a community-recruited sample of substance-abusing women. Psychology of Addictive Behaviors, 13(2), 78–88. https://doi.org/10.1037/0893-164X.13.2.78 [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Söbers JJ, & Wager TD (2012). A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews, 36(2), 747–756. [DOI] [PubMed] [Google Scholar]
- Vouimba R-M, & Maroun M (2011). Learning-induced changes in mPFC-BLA connections after fear conditioning, extinction, and reinstatement of fear. Neuropsychopharmacology, 36(11), 2276–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1 Fear conditioning task


