Abstract
Background
Many premature infants with respiratory failure are deficient in surfactant, but the relationship to occurrence of bronchopulmonary dysplasia (BPD) is uncertain.
Methods
Tracheal aspirates were collected from 209 treated and control infants enrolled at 7–14 days in the Trial of Late Surfactant. The content of phospholipid, surfactant protein-B, and total protein were determined in large aggregate (active) surfactant.
Results.
At 24 h, surfactant treatment transiently increased surfactant protein-B content (70%, p<0.01) but did not affect recovered airway surfactant or total protein/phospholipid. The level of recovered surfactant during dosing was directly associated with content of surfactant protein-B (r=0.50, p<0.00001) and inversely related to total protein (r=0.39, p<0.0001). For all infants, occurrence of BPD was associated with lower levels of recovered large aggregate surfactant, higher protein content and lower SP-B levels. Tracheal aspirates with lower amounts of recovered surfactant had an increased proportion of small vesicle (inactive) surfactant.
Conclusions.
We conclude that many intubated premature infants are deficient in active surfactant, in part due to increased intra-alveolar metabolism, low SP-B content and protein inhibition, and that the severity of this deficit is predictive of BPD.Late surfactant treatment at the frequency used did not provide a sustained increase in airway surfactant.
Introduction
Bronchopulmonary dysplasia (BPD) of premature infants is currently defined as a continuing requirement for supplemental oxygen and/or respiratory support at 36 weeks post-menstrual age (PMA). BPD is a common form of chronic lung disease in infants born prematurely and is associated with death, childhood respiratory morbidity and neurodevelopmental abnormalities. The pathogenesis of BPD includes lung immaturity with low antioxidant and immune defenses plus exposure to insults of hyperoxia, barotrauma from ventilator support, and infections that damage lung epithelium and elicit inflammation. Sequelae of this injury are arrested lung development, fibrosis, altered airway reactivity and long-term deficits in pulmonary function (1–5).
Therapeutic options for the prevention and treatment of BPD are limited and have not substantially affected the incidence of disease. Vitamin A treatment is associated with a modest reduction of BPD but is not in general use. Caffeine was associated with reduced oxygen use and is routinely used for prevention of apnea. Postnatal dexamethasone therapy improves respiratory status acutely and decreases the incidence of BPD, however, longer courses of this therapy are associated with neurodevelopmental abnormalities (reviewed in (6)). Inhaled nitric oxide (iNO) has demonstrated benefit in some but not all trials (7), and recent evidence by metaanalysis indicates that efficacy of the drug may be limited to non-caucasian infants (8).
Previous reports found that 75% of intubated infants have episodes of dysfunctional surfactant that is associated with lower levels of surfactant proteins, in particular SP-B (decreased 80%) (9–11). In contrast, content of total protein in surfactant was higher in samples with abnormal surface tension properties. These findings are consistent with a critical facilitating role for SP-B (and SP-C), and an inhibitory effect of extraneous proteins, on surfactant function in vivo, and they provided rationale for clinical studies of late surfactant treatment to improve respiratory status of intubated infants.
In two pilot trials of late surfactant treatment, calfactant exposure transiently Increased TA surfactant content of SP-B, improved surfactant function and decreased the respiratory severity score (RSS), as also noted by others (12–14). Limitations of these studies included insufficient information on the amount, as distinguished from function and composition, of surfactant recovered from TA and insufficient statistical power for comparison of surfactant status and a diagnosis of BPD.
In the current study, we hypothesized that deficiency and dysfunction of surfactant early in the postnatal course is associated with increased occurrence of BPD.We determined surfactant content and composition for a subpopulation of control and treated infants from the Trial of Late Surfactant (TOLSURF) study. Our findings indicate associations between surfactant content and composition at <28 days with occurrence of BPD at 36 weeks PMA, implicate increased turnover of surfactant as a major contributor to lower airway content, and suggest racial differences in surfactant status.
Patients and methods
Study population.
The Trial of Late Surfactant (TOLSURF, ClinicalTrials.gov identifier: NCT01022580) was a blinded, randomized, sham-controlled trial performed at 25 US centers and designed to assess effects of late surfactant treatments on respiratory outcome. Infants were enrolled if they were intubated and required ventilatory support between 7 and 14 days of age, representing a population at high risk for BPD. Written informed consent was obtained prior to study enrollment from parents/legal guardians and the study was approved by Institutional Review Boards at all participating centers. Race/ethnicity was ascertained by maternal self-identification. 252 treated infants received up to 5 instilled doses per the manufacturer’s instructions of Infasurf® exogenous surfactant (Calfactant, Forest Pharmaceuticals, St. Louis MO) and 259 controls received a sham instillation procedure. All infants received inhaled nitric oxide (iNO} as described for the NO CLD trial (15). Clinical respiratory parameters were collected and respiratory severity score (RSS, FiO2 x mean airway pressure) was calculated. Respiratory outcomes at near term by physiologic challenge and at 1-year corrected age have been reported (16, 17). Some infants were co-enrolled in the multicenter, observational Prematurity and Respiratory Outcome Program (PROP) (18), and outcomes near term have been described (19).
Tracheal aspirate samples.
Specimens of TA were collected from infants at enrollment before treatment (d 0) and just before receiving each dose of surfactant/sham.The TA sampling procedure involved 2 instillations of 0.5 ml of saline into the trachea, brief ventilation, and suction at the end of the endotracheal tube to recover saline containing lung epithelial lining fluid. Samples were centrifuged at 500×g for 5 min to remove cells and stored at −70 C in the presence of protease inhibitors.
Surfactant isolation and characterization.
TA supernatants were subsequently centrifuged (27,000×g for 60 min) to isolate the large aggregate surfactant (pellet) and a supernatant fraction; surfactant recovery was defined as the total amount of large aggregate PL. Total protein was measured by the Bradford assay (BioRad Laboratories, Hercules CA) and total phospholipids (PL) were assessed by phosphorous assay of extracted PL (20, 21). SP-B was measured in large aggregate surfactant by immunodot assay as previously described (9, 22) and results are expressed as % of PL or total protein by weight.
Statistics.
Data are expressed as mean±sd or median and interquartile range (IQR). Demographic data were evaluated by Student’s t test and Fisher’s Exact Test, and analysis of median data used the Mann-Whitney test. Analysis of quartile data used both chi-squared test and linear regression of mean values for each quartile. Odds ratios (ORs), corresponding to increment change in surfactant parameters, were obtained using logistic regression via function glm in R (23) from a model that additionally adjusted for birth weight, which was associated with BPD (OR 0.85, CI 0.731.01, p=0.07) but not for other clinical variables (gestational age, product of a multiple gestation, gender, and maternal race) that were not significantly associated with 36 weeks outcome in this population. Confidence intervals for the ORs were obtained via profile likelihood methods using the confint function from the R package MASS (24). The statistical software package SAS v9.13 was used for analyses with 0.05 as the significance level in all tests. Reasons for unavailable or censored data included inadequate volume of TA sample or content of PL or total protein.
Results
The study population of 209 infants represents 41% of the infants enrolled in TOLSURF and was chosen based on consecutive enrollment and receipt of TA samples. As shown in Table 1, the control group and surfactant-treated infants were well matched for demographics and severity of early lung disease (RSS at entry) and are representative of the entire population as previously reported (16). All infants were intubated at enrollment and thus were at high risk for BPD or death as reflected in the incidence of 66–68% by physiologic testing at 36 weeks PMA. At 40 weeks, there was a nonsignificant (p=0.20, Table 1) trend toward improved outcome for treated infants, similar to results for the entire cohort (16).
Table 1.
Demographics and outcomes of study infants.
|
Control (n = 102) |
Treated (n = 107) |
|
|---|---|---|
| Gestational Age (wk) | 25.3±1.2 | 25.2±1.2 |
| Birth Weight (g) | 721±172 | 726±180 |
| Age at Randomization (d) | 9.3±2.2 | 9.6±2.3 |
| Male/Female | 54/48 | 61/46 |
| Maternal Race (W/B/H/O)*-% | 51/34/14/3 | 52/39/11/5 |
| RSS at entry | 3.8±2.1 | 3.7±2.1 |
| Death or BPD 36 wk | 67 (65.7%) | 73 (68.2%) |
| Death or BPD 40 wk | 47 (46.1%) | 40 (37.4%) |
| Died | 6 (5.9%) | 5 (4.7%) |
Mean±SD; all NS control vs surfactant-treated.
W, white; B, black; H, Hispanic; O, Native American, Asian/Pacific Islander RSS, respiratory severity score
Surfactant content and composition.
We determined three surfactant parameters for TA samples, which were collected at enrollment (baseline) and just before up to 4 subsequent sham procedures/doses at intervals of 18–96 h (mean/sd 52±12 h). There were no significant differences between groups at study entry for total PL in large aggregate (surfactant recovery), total protein in surfactant as % of PL, and content of SP-B in surfactant expressed as both % PL and % of total protein (Table 2). Moreover, with the TA collection schedule used, there were no differences comparing the baseline levels and mean levels during dosing (Table 2); these results are consistent with relatively short half-lives for both PL and SP-B of exogenous surfactant as previously described (11, 25) Notably, the SP-B content at study entry is comparable to the values previously reported for premature infant surfactants with abnormal minimum surface tension (>5 mN/m) in vitro, indicating that most TOLSURF infants had low SP-B and dysfunctional surfactant (9).
Table 2.
Surfactant parameters at baseline (study entry) and during dosing.
| Baseline | During Dosing | |||
|---|---|---|---|---|
| Control (n=102) |
Treated (n=107) |
Control (n=102) |
Treated (n=107) |
|
| Recovery (μg PL) | 212 (78–436) | 214 (81–405) | 264 (136–434) | 293 (157–448) |
| Total Protein (% PL) | 17.8 (13.0–27.0) | 19.1 (13.9–35.2) | 20.6 (15.2–29.7) | 21.6 (15.6–35.2) |
| SP-B (% PL) | 0.21 (0.12–0.31) | 0.18 (0.10–0.35) | 0.24 (0.16–0.32) | 0.24 (0.17–0.42) |
| SP-B (% protein) | 1.01 (0.58–1.82) | 0.99 (0.52–1.71) | 1.25 (0.80–1.83) | 1.25 (0.69–2.23) |
Median (IQR) values are shown for baseline TA samples (prior to dosing) and for 2–5 (mean 4.2) samples collected 18–72 (mean 52) h after dosing (treated) or sham procedure (control). There are no significant differences between groups or between baseline and the median value during dosing.
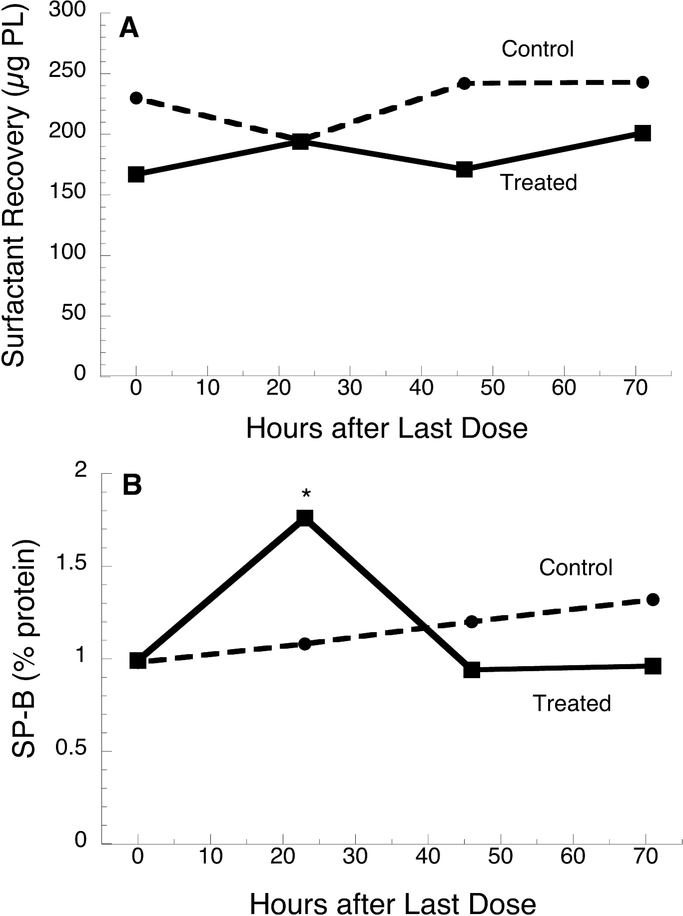
To examine the time course of surfactant levels in more detail, we identified a total of 80 infants (40 treated and 40 control) who had at least one TA collection at a 24 h interval. Surfactant recovery did not differ by interval time (24–72 h) and was similar for control and treated infants (Fig. 1A). The content of SP-B in treated infants was significantly increased by ~70% at 24 h but similar to baseline at 48 and 72 h, consistent with earlier reports (9–11), whereas no increase was observed for control infants at 24 h
Figure 1.
Time course of surfactant recovery (A) and SP-B content (B) for surfactanttreated and control infants. Data are median values for 193 TA samples from 40 treated infants and 186 TA samples from 40 control infants with at least one TA collection at 24 h. *, p<0.01 treated vs control.
(Fig. 1B). There were no differences between control and treated infants in total protein (% of PL) at all time points (data not shown).
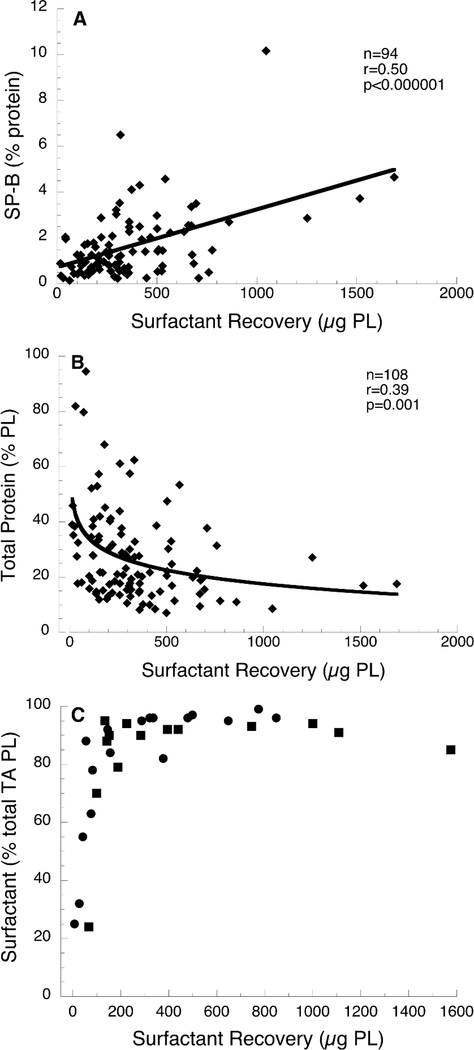
In a separate analysis using data for all TA samples, we examined the relationship for each infant between mean surfactant values during dosing with corresponding baseline values: for surfactant recovery in treated infants, r =0.46 with a regression slope of 0.58 (p=0.001) and in controls r=0.25 and slope 0.17 (p=0.01); for SP-B content in treated infants, r=0.37 with slope of 0.44 (p=<0.0001) and in controls r=0.02 with slope 0.01 (p=NS). The finding of greater slope for treated than control infants indicates that surfactant treatment, as administered in this trial, generally maintains baseline TA surfactant levels and composition during the treatment interval, whereas levels for control infants are more variable with a decrease from baseline for some infants Surfactant recovery, defined as the total PL in isolated large aggregate surfactant, varied by ~100-fold in the TA samples. Increasing surfactant recovery was associated with higher SP-B content (Fig. 2A) and lower level of total protein (Fig. 2B), consistent with improved function with increased surfactant recovery. For all infants, there was a weak inverse correlation between surfactant recovery and mean RSS during dosing (r=0.12, p=0.02, n=209), which reflects the known relationship between surfactant status and lung function.
Figure 2.
Surfactant parameters associated with surfactant recovery. A, SP-B content vs surfactant recovery. Mean values of 2–5 TA collections are shown for 94 treated infants: r=0.50, p=<0.00001. Results were similar for SP-B normalized to PL (r=0.38, p=0.0002). B, Total protein in surfactant vs surfactant recovery. Mean values of 2–5 TA collections are shown for 107 treated infants: r=0.39, p=<0.001. C, Distribution of PL between large aggregate and small aggregate surfactant vs surfactant recovery. Data are for individual TA samples from 14 treated (squares) and 17 control (circles) infants and show that an increasing percentage of total PL is associated with large aggregate surfactant as recovery increases.
Surfactant in TA, as separated by centrifugation, exists as both large aggregate (lamellar bodies, tubular myelin, large vesicles---surface active) and small aggregate (small vesicles---degraded and inactive) forms, with ~95% as large aggregate in normal surfactant (26). We examined the distribution of PL between large and small aggregate forms, as separated by centrifugation, in a subset of TA samples (Fig. 2C). Lower surfactant recovery (<100 μg PL) was associated with a decreased proportion of large aggregate surfactant in an apparent dose-dependent fashion. This finding suggest that in vivo degradation of secreted surfactant contributes to the deficiency of large aggregate surfactant in premature infants.
Surfactant and respiratory outcome.
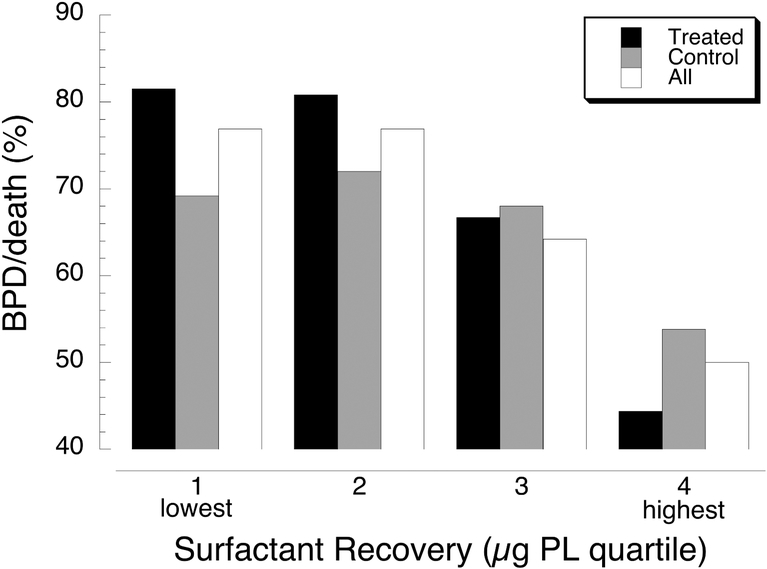
For the TOLSURF study we hypothesized that surfactant deficiency and dysfunction in the first weeks after preterm birth contributed to ongoing lung injury, inflammation, disordered repair and chronic lung disease, and that late surfactant supplementation would reduce injury and improve respiratory outcome. To directly examine the influence of early surfactant status on later outcome, we first determined the incidence of BPD/death by quartiles of the mean amount of recovered surfactant for all available TA samples. For each of the groups, BPD/death was similar for quartiles 1 and 2 and decreased progressively for quartiles 3 and 4 (Fig. 3); this trend was significant (p≤0.02) for all groups. By univariate logistic regression using all infants (treated and control) and adjusting for birth weight, each of the surfactant parameters was associated with BPD at 36 weeks PMA: decreased surfactant recovery (OR 0.85, CI 0.75–0.95, p=0.005), increased total protein (5.63, 1.91–20.3, p=0.004) and lower SP-B (0.85, 0.73–1.01, p=0.13). By multivariate logistic analysis each of these surfactant parameters was independently associated with outcome (data not shown). In TOLSURF, all infants received iNO, and for the whole cohort, infants of self identified black mothers had significantly better outcome than white infants (27). We therefore examined surfactant parameters and respiratory outcome by maternal race (Table 3). In the treated group, infants of black mothers compared to white infants had greater surfactant recovery and increased SP-B during dosing (p=0.06); BPD/death was lower in black infants, as for the whole TOLSURF cohort, but the difference was not significant. By contrast, there were no differences for black versus white infants in the control group (data not shown). These findings support the possibility that iNO preferentially improves surfactant status in infants of black vs white mothers, which may contribute to the racial disparity in BPD outcome observed for infants exposed to iNO (8).
Figure 3.
BPD or death at 36 weeks PMA by quartile of surfactant recovery. Black bars, 107 treated infants: quartile 1 range 14–152 μg PL, quartile 2 153–287 μg PL, quartile 3 290–442 μg PL, quartile 4 449–1688 μg PL; p for PL vs % BPD/death = 0.002. Gray bars, 102 control infants: quartile 1 range 14–134 μg PL, quartile 2 136–234 μg PL, quartile 3 279–420 μg PL, quartile 4 434–1507 μg PL; p for PL vs % BPD/death = 0.002. White bars, 209 treated plus control infants: quartile 1 14–143 μg PL, quartile 2 144–2 μg PL, quartile 3 287–442 μg PL, quartile 4 445–1688 μg PL; p for PL vs % BPD/death = 0.02.
Table 3.
Surfactant parameters and outcome for treated infants by self-identified maternal race.
| White (n=52) |
Black (n=39) |
P | |
|---|---|---|---|
| Recovery (μg PL) | 246 (124–358) | 313 (222–467) | 0.02 |
| Total Protein (% PL) | 25.9 (15.0–34.8) | 26.9 (16.9–38.4) | 0.60 |
| SP-B (% protein) | 1.23 (0.62–1.96) | 1.40 (0.68–2.48) | 0.35 |
| SP-B (% PL) | 0.22 (0.17–0.35) | 0.37 (0.21–0.45) | 0.06 |
| BPD36/Death | 40/52 (76.9%) | 24/39 (61.5%) | 0.16 |
Median (IQR) values comparing mean infant values during dosing. There were no differences between racial groups for gestational age, birth weight, gender or mean RSS during dosing/sham (data not shown).
Discussion
The critical role of pulmonary surfactant deficiency, and benefit or replacement surfactant, in newborn premature infants related to Respiratory Distress Syndrome is well recognized, however the contribution of surfactant to continuing lung disease in these infants is less well documented. Based on earlier observations of abnormal surfactant function secondary to deficiencies of surfactant proteins in many intubated infants (9–11), we hypothesized that later doses of exogenous surfactant would improve lung function, reduce injury and inflammation, and improve respiratory outcome. The current study in TOLSURF patients describes the transient beneficial impact of treatment, and reports associations between both amount of recovered surfactant and the levels of SP-B and total proteins on occurrence of BPD, which represent new observations in a large cohort of infants with continuing early lung disease. Our findings also implicate increased intra-alveolar degradation of surfactant as contributing to surfactant deficiency in these infants.
Established risk factors for BPD, which were confirmed in TOLSURF infants (27), include the inherent factors of gestational age, birth weight, gender and intrauterine growth restriction; postnatally, both oxygen exposure and mean airway pressure are independent risk factors. In addition, inflammation early in the postnatal course is associated with BPD as indicated by elevated levels of many inflammatory mediators (28). We propose that a deficiency of functional surfactant underlies many BPD risk factors and is a key causal event in lung injury that leads to later BPD by the following scenario. Surfactant production in fetal life and at birth is directly related to gestational age and birth weight and may be influenced by intrauterine conditions, although this has not been investigated. Younger infants are at higher risk of continued surfactant deficiency postnatally, after clearance of exogenous surfactant, which contributes to respiratory failure and need for assisted respiratory support. Treatment with supplemental oxygen and mechanical ventilation further injures the lung epithelium by oxidative stress and barotrauma. This ongoing injury in turn further reduces surfactant production and increases alveolar clearance via degradation and macrophage uptake, setting up a cycle of increasing injury, inflammation and level of respiratory support. The longer-term consequences of the ongoing injury secondary to surfactant deficiency are dysregulated alveogenesis, airway injury with increased reactivity, and vascular dysfunction with development of pulmonary hypertension, conditions that all contribute to the requirement for respiratory support near term (i.e., BPD). This proposed sequence of events for human infants is supported by the observations of reversible surfactant inactivation, decreased lung compliance, plasma protein and inflammatory cell influx, and mortality with conditional knock down of SP-B in mice (29).
Measurement of surfactant levels and composition in intubated infants might provide a useful biomarker for respiratory outcome, but this is not currently a practical approach for routine screening of infants due to the technical complexity of the measurements. Alternatively, a modified approach that involved measurement of PL in both large aggregate surfactant and the TA supernatant, providing data for total recovery of functional surfactant and small:large aggregate distribution (independent of surfactant recovery), is technically more straight forward and worthy of exploring prospectively as a predictor. In addition, it should be noted that surfactant status is likely not related to all occurrences of BPD, which is a disorder that by current definition can include causes such as inadequate chest wall stability, reactive airways, immature respiratory control and pulmonary hypertension in addition to impaired alveolization.
Our study builds on previous findings for surfactant kinetics in infants. Using stable isotope-labeled disaturated phosphatidylcholine (DSPC) in preterm infants, Carnielli et al. (30, 31) found that both pool size and half-life of DSPC in TA were low in infants with RDS and remained lower in infants who required greater ventilator support later. In term infants, both pool size and half-life were reduced by ~50% in infants with pneumonia versus controls, and these parameters were inversely correlated with oxygenation index (32). Bohlin et al (33) used 13C-acetate incorporation and demonstrated lowered surfactant synthesis with respiratory failure in term infants and a correlation between PL amount in TA and disease severity. Most relevant to our study, kinetic measurements in infants at high risk for BPD at 1 month of age indicated higher loss of DSPC from alveolar pools and less recycling of DSPC palmitic acid compared to low-risk controls (34).Collectively, these observations along with our results establish a strong association between alveolar content of functional surfactant and lung disease in infants.
The mechanism for surfactant deficiency in premature infants with early lung disease could involve a combination of abnormalities. These include: 1) decreased de novo surfactant synthesis by alveolar type 2 cells possibly secondary to epithelial damage, loss of cells, and elevated levels of selected inflammatory mediators (eg., TGF-beta, IL1-beta) that downregulate production, 2) increased degradation of intra-alveolar large aggregate surfactant by elevated levels of phospholipases associated with inflammation, 3) loss of alveolar surfactant secondary to phagocytosis by increased levels of macrophages, and 4) diminished recycling of surfactant PL by injured type 2 cells. In contrast to large aggregate surfactant, the small aggregate form lacks surfactant proteins and is not surface active, and thus is considered to be a nonfunctional metabolic product (26). An increased shift between forms has been observed in animals with acute lung injury and in adults with Acute Respiratory Distress Syndrome; potential mechanisms that have been suggested include 1) increased tidal volume, as would occur with mechanical ventilation, 2) down-regulated production of SP-A, which participates in formation and stability of large aggregate forms, 3) increased intra-alveolar serum proteins, in particular serine proteases, secondary to pulmonary edema, and 4) other secondary effects of lung injury and altered type 2 cell function (35–37).
Late surfactant treatment as administered in TOLSURF did not improve respiratory outcome for infants at 36 weeks PMA despite the observed associations between BPD and levels of recovered surfactant, SP-B and total protein during dosing. We believe that this apparent discrepancy reflects the timing of surfactant doses (mean interval 52 h) and/or the total duration of surfactant treatment (mean ~10 days). These treatment conditions likely did not entirely prevent ongoing injury to the lung epithelium and continuing lung disease at 36 weeks PMA. It should be noted that treated TOLSURF infants appeared to do better at 40 weeks and that there were significantly fewer treated than control infants requiring home respiratory support during the first year (16, 17). Thus, we propose that a treatment approach providing more frequent or nearly continuous surfactant replacement during the vulnerable period would have significant beneficial effects for respiratory outcome. This approach may have to await development of efficient, less invasive approaches for surfactant administration in nonintubated infants.
Based on the positive results of the NO CLD trial (15), all TOLSURF infants received inhaled nitric oxide (iNO) by the NO CLD protocol. In a previous study of NO CLD TA samples, surfactant isolated from treated infants had a lower minimum surface tension than for control infants at higher, but not lower, iNO doses (38). Thus, it is possible that the results from all TOLSURF infants reflects in part surfactant-related effects of iNO; however, we expect that the observed associations between surfactant status and BPD applies to high-risk infants regardless of iNO exposure. By contrast, the apparent racial/ethnic differences observed for surfactant recovery and SP-B in treated infants (Table 3) likely are influenced by iNO exposure. In a recent individual participant metaanalysis of iNO trials, treated infants of black mothers had a significant reduction in BPD/death whereas there was no benefit for treated white infants; of interest, in the placebo group, there was no difference in death or BPD by self-identified maternal race (8). Similarly, in the entire TOLSURF cohort, infants of black mothers had higher rates of survival without BPD compared to infants of white or Hispanic/Latino mothers (37%, 25% and 31%, respectively), a finding that was highly significant in multivariate analysis (27). Thus, benefit from iNO therapy is likely related in part to African genetic ancestry, and possibly race-related socioeconomic factors, and this response may involve better surfactant levels and function.
There are some limitations of the study. Despite a standardized protocol, the collection procedure for TAs likely varied between clinical sites, and the total volume was variable and positively correlated with surfactant recovery. It is possible that some of the variability in surfactant recovery is due to inaccessibility of airways for lavage, possibly due to constriction, secretions and/or epithelial debris, conditions that may promote degradation of airspace surfactant. Nevertheless, low surfactant recovery was strongly associated with low SP-B and increased total protein, consistent with reduced surfactant function. We did not determine levels of SP-A and SP-C in this study, however, based on our previous findings, it is likely that all three surfactant proteins are deficient in infants with low recovery of surfactant (9). TA samples were only collected just prior to each dose/sham procedure, thus our study design did not allow a refined determination of the kinetics of response to late surfactant treatment. Our laboratory studies were limited to a subpopulation (41%) of TOLSURF infants; although these infants were similar to the entire group with regard to demographics and outcomes, they may not necessarily be representative of the entire cohort or all high-risk premature infants. Finally, because TOLSURF only enrolled infants who were <28 weeks and intubated at 7–14 d, it is possible that our results cannot be generalized to include all premature infants; however, this seems unlikely in view of the essential role of surfactant in lung function and disease.
In summary, we estimate that during the first postnatal month at least 50% of the infants in our TOLSURF cohort were deficient in large aggregate surfactant, which was associated with low SP-B and elevated total protein content, and we found that each of these surfactant parameters was associated with adverse respiratory outcome near term. These findings, along with the biological plausibility for surfactant deficiency/dysfunction as a key causal event in the pathogenesis of BPD, suggest that research efforts toward maintaining an effective level of alveolar surfactant are warranted. Late replacement treatment can be considered in candidate infants, particularly if surfactant is administered on a frequent dosing protocol, as there were no serious adverse events associated with surfactant instillation in TOLSURF (16), and treated infants appeared to do better at 40 weeks PMA and at 1 year (17). Surfactant should also be considered as a delivery vehicle for lipophilic lung-targeted drugs in these infants due to its ability to spread rapidly within lung airspaces and reduce systemic exposure. A notable example is the administration of the corticosteroid budesonide in surfactant for prevention of BPD as reported by Yeh et al. (39). In this case, delivery of corticosteroid in surfactant directly to the infant lung suppressed TA cytokines and decreased BPD, and in animals it resulted in efficient intra-pulmonary distribution and undetectable levels of budesonide in brain tissue (39, 40). Further studies of budesonide/surfactant in infants at risk for BPD are planned.
Acknowledgements.
We thank the TOLSURF and PROP Investigators, study coordinators, physicians, nurses, respiratory therapists and the families who participated in the studies.
Sources of Support: This study was supported by grants from the National Health, Lung, and Blood Institute (NHLBI, U01 HL094338, U01 HL094355, U01 HL101798). Ikaria Inc.and ONY Inc. provided drug for the conduct of the parent TOLSURF trial, but neither company had input into study design, data collection, analysis and interpretation or manuscript preparation.
Footnotes
None of the authors states any conflict of interest or financial ties to sponsoring companies.
REFERENCES
- 1.Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005;116:1353–60. [DOI] [PubMed] [Google Scholar]
- 2.Natarajan G, Pappas A, Shankaran S, et al. Outcomes of extremely low birth weight infants with bronchopulmonary dysplasia: impact of the physiologic definition. Early Hum Dev 2012;88:509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–9. [DOI] [PubMed] [Google Scholar]
- 4.Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol 2003;8:73–81. [DOI] [PubMed] [Google Scholar]
- 5.Gibson AM, Reddington C, McBride L, Callanan C, Robertson C, Doyle LW. Lung function in adult survivors of very low birth weight, with and without bronchopulmonary dysplasia. Pediatr Pulmonol 2015;50:987–94. [DOI] [PubMed] [Google Scholar]
- 6.Greenough A, Ahmed N. Perinatal prevention of bronchopulmonary dysplasia. Journal of Perinatal Medicine 2013;41:119–26. [DOI] [PubMed] [Google Scholar]
- 7.Cole FS, Alleyne C, Barks JD, et al. NIH Consensus Development Conference statement: inhaled nitric-oxide therapy for premature infants. Pediatrics 2011;127:363–9. [DOI] [PubMed] [Google Scholar]
- 8.Askie LM, Davies LC, Schreiber MD, Hibbs SM, Ballard PL, Ballard RA. Race Effects of Inhaled Nitric Oxide in Preterm Infants: An Individual Participant Data Meta-Analysis. J Pediatr 2018;193:34–9. [DOI] [PubMed] [Google Scholar]
- 9.Merrill JD, Ballard RA, Cnaan A, et al. Dysfunction of pulmonary surfactant in chronically ventilated premature infants. Pediatr Res 2004;56:1–9. [DOI] [PubMed] [Google Scholar]
- 10.Merrill JD, Ballard PL, Courtney SE, et al. Pilot trial of late booster doses of surfactant for ventilated premature infants. J Perinatol 2011;31:599–606. [DOI] [PubMed] [Google Scholar]
- 11.Keller RL, Merrill JD, Black DM, et al. Late administration of surfactant replacement therapy increases surfactant protein-B content: a randomized pilot study.Pediatr Res 2012;72:613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz LA, Klein JM. Repeat surfactant therapy for postsurfactant slump. J Perinatol 2006;26:414–22. [DOI] [PubMed] [Google Scholar]
- 13.Bissinger R, Carlson C, Hulsey T, Eicher D. Secondary surfactant deficiency in neonates. J Perinatol 2004;24:663–6. [DOI] [PubMed] [Google Scholar]
- 14.Laughon M, Bose C, Moya F, et al. A pilot randomized, controlled trial of later treatment with a peptide-containing, synthetic surfactant for the prevention of bronchopulmonary dysplasia. Pediatrics 2009;123:89–96. [DOI] [PubMed] [Google Scholar]
- 15.Ballard RA, Truog WE, Cnaan A, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med 2006;355:343–53. [DOI] [PubMed] [Google Scholar]
- 16.Ballard RA, Keller RL, Black DM, et al. Randomized Trial of Late Surfactant Treatment in Ventilated Preterm Infants Receiving Inhaled Nitric Oxide. J Pediatr 2016;168:23–9 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller RL, Eichenwald EC, Hibbs AM, et al. The Randomized, Controlled Trial of Late Surfactant: Effects on Respiratory Outcomes at 1-Year Corrected Age. J Pediatr 2017;183:19–25 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pryhuber GS, Maitre NL, Ballard RA, et al. Prematurity and respiratory outcomes program (PROP): study protocol of a prospective multicenter study of respiratory outcomes of preterm infants in the United States. BMC pediatrics 2015;15:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poindexter BB, Feng R, Schmidt B, et al. Comparisons and Limitations of Current Definitions of Bronchopulmonary Dysplasia for the Prematurity and Respiratory Outcomes Program. Annals of the American Thoracic Society 2015;12:1822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Med Sci 1959;37:911–7. [DOI] [PubMed] [Google Scholar]
- 21.Dittmer JC, Wells MA. Quantitative and qualitative analysis of lipids and lipid components In: Methods in Enzymology Lowenstein JM (Ed) Academic Press, Inc, New York: 1969:482–7. [Google Scholar]
- 22.Ballard PL, Merrill JD, Godinez RI, Godinez MH, Truog WE, Ballard RA. Surfactant protein profile of pulmonary surfactant in premature infants. Am J Respir Crit Care Med 2003;168:1123–8. [DOI] [PubMed] [Google Scholar]
- 23.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: ISBN 3–90005107-0, URL http://www.r-project.org/; 2008. [Google Scholar]
- 24.Venables WN, Ripley BD. Modern Applied Statistics. Fourth Edition ed. New York: Springer; 2002. [Google Scholar]
- 25.Torresin M, Zimmermann LJ, Cogo PE, et al. Exogenous surfactant kinetics in infant respiratory distress syndrome: A novel method with stable isotopes. Am J Respir Crit Care Med 2000;161:1584–9. [DOI] [PubMed] [Google Scholar]
- 26.Yamada T, Ikegami M, Jobe AH. Effects of surfactant subfractions on preterm rabbit lung function. Pediatr Res 1990;27:592–8. [DOI] [PubMed] [Google Scholar]
- 27.Wai KC, Kohn MA, Ballard RA, et al. Early Cumulative Supplemental Oxygen Predicts Bronchopulmonary Dysplasia in High Risk Extremely Low Gestational Age Newborns. J Pediatr 2016;177:97–102 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhandari A, Bhandari V. Biomarkers in bronchopulmonary dysplasia. Paediatric Respiratory Reviews 2013;14:173–9. [DOI] [PubMed] [Google Scholar]
- 29.Ikegami M, Whitsett JA, Martis PC, Weaver TE. Reversibility of lung inflammation caused by SP-B deficiency. Am J Physiol Lung Cell Mol Physiol 2005;289:L962–70. [DOI] [PubMed] [Google Scholar]
- 30.Verlato G, Cogo PE, Balzani M, et al. Surfactant status in preterm neonates recovering from respiratory distress syndrome. Pediatrics 2008;122:102–8. [DOI] [PubMed] [Google Scholar]
- 31.Carnielli VP, Zimmermann LJ, Hamvas A, Cogo PE. Pulmonary surfactant kinetics of the newborn infant: novel insights from studies with stable isotopes. J Perinatol 2009;29 Suppl 2:S29–37. [DOI] [PubMed] [Google Scholar]
- 32.Facco M, Nespeca M, Simonato M, et al. In vivo effect of pneumonia on surfactant disaturated-phosphatidylcholine kinetics in newborn infants. PLoS One 2014;9:e93612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bohlin K, Merchak A, Spence K, Patterson BW, Hamvas A. Endogenous surfactant metabolism in newborn infants with and without respiratory failure. Pediatr Res 2003;54:185–91. [DOI] [PubMed] [Google Scholar]
- 34.Cogo PE, Toffolo GM, Gucciardi A, Benetazzo A, Cobelli C, Carnielli VP. Surfactant disaturated phosphatidylcholine kinetics in infants with bronchopulmonary dysplasia measured with stable isotopes and a two-compartment model. J Appl Physiol (1985) 2005;99:323–9. [DOI] [PubMed] [Google Scholar]
- 35.Ueda T, Ikegami M, Jobe A. Surfactant subtypes. In vitro conversion, in vivo function, and effects of serum proteins. Am J Respir Crit Care Med 1994;149:1254–9. [DOI] [PubMed] [Google Scholar]
- 36.Veldhuizen RA, Marcou J, Yao LJ, McCaig L, Ito Y, Lewis JF. Alveolar surfactant aggregate conversion in ventilated normal and injured rabbits. Am J Physiol 1996;270:L152–8. [DOI] [PubMed] [Google Scholar]
- 37.Veldhuizen RA, McCaig LA, Akino T, Lewis JF. Pulmonary surfactant subfractions in patients with the acute respiratory distress syndrome. Am J Respir Crit Care Med 1995;152:1867–71. [DOI] [PubMed] [Google Scholar]
- 38.Ballard PL, Merrill JD, Truog WE, et al. Surfactant function and composition in premature infants treated with inhaled nitric oxide. Pediatrics 2007;120:346–53. [DOI] [PubMed] [Google Scholar]
- 39.Yeh TF, Chen CM, Wu SY, et al. Intratracheal Administration of Budesonide/Surfactant to Prevent Bronchopulmonary Dysplasia. Am J Respir Crit Care Med 2016;193:86–95. [DOI] [PubMed] [Google Scholar]
- 40.Roberts JK, Stockmann C, Dahl MJ, et al. Pharmacokinetics of Budesonide Administered with Surfactant in Premature Lambs: Implications for Neonatal Clinical Trials. Current Clinical Pharmacology 2016;11:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]