Abstract
The schizophrenia and bipolar twin study in Sweden (STAR) is a large nation-wide cohort of monozygotic (MZ) and dizygotic (DZ) same-sex twins with schizophrenia or bipolar disorder and healthy control pairs, extensively characterized with brain imaging, neuropsychological tests, biomarkers, genetic testing, psychiatric symptoms and personality traits. The purpose is to investigate genetic and environmental mechanisms that give rise to schizophrenia and bipolar disorder as well as the intermediate phenotypes. This article describes the design, recruitment, data collection, measures, collected twins’ characteristics, diagnostic procedures as well as ongoing and planned analyses. Identification of biomarkers, genetic and epigenetic variation and the development of specific and common endophenotypes for schizophrenia and bipolar disorder are potential gains from this cohort.
Keywords: psychiatry, etiology, gene-environment interaction, endophenotype, biomarker, neuroimaging
Introduction
Schizophrenia and bipolar disorder are among the most chronic and debilitating of psychiatric syndromes and, with a lifetime prevalence of about 1% each, represent major public health concerns (McGrath et al., 2008; Merikangas et al., 2007). Both are genetic disorders with heritability estimated to approximately 80% in schizophrenia (Cannon et al., 1998; Cardno et al., 1999; Chou et al., 2016; Sullivan et al., 2003) and slightly lower in bipolar disorder (Edvardsen et al., 2008; Kieseppa et al., 2004; Song et al., 2015). Recent work has revealed substantial epidemiological overlap between schizophrenia and bipolar disorder (Lichtenstein et al., 2009), as well as shared associations within genomic regions (Smoller JW, 2013; Sullivan et al., 2012).
Anatomically, patients with schizophrenia show reductions in prefrontal, medial temporal, and superior temporal gray matter volumes and enlarged ventricles (Pantelis et al., 2005; Shenton et al., 2001). Decreased cortical thickness in prefrontal regions has been found to vary with genetic loading for schizophrenia among the non-ill co-twins of patients, while probands have further reductions in gray matter in dorsolateral prefrontal, superior temporal, and parietal regions compared with their non-ill co-twins (Cannon et al., 2002). Decreased cortical thickness in prefrontal regions have also been found in bipolar disorder and unaffected first-degree relatives (Hibar et al., 2018). Neurocognitively, patients with schizophrenia show impairments across all major domains of functioning, including attention, verbal abilities, spatial abilities, learning and memory, and processing speed (Cannon et al., 2000). Across all of these domains, individuals at genetic risk for schizophrenia have been found to perform at a level intermediate between that of probands with schizophrenia and the general population (Cannon et al., 2000). Cognitive impairments are also present in bipolar disorder across several domains although not as pronounced as in schizophrenia (Raucher-Chene et al., 2017).
Elucidating the specific genetic and neural mechanisms influencing susceptibility to and expression of schizophrenia and bipolar disorder, and explaining the nature of the overlap between them, is critical to understand the necessary and sufficient conditions for overt psychosis and to the development of more effective treatment and prevention strategies. To gain traction on these issues, we have established a twin cohort to investigate the inheritance of neural dysfunctions in schizophrenia and bipolar disorder in samples of monozygotic (MZ) and dizygotic (DZ) twin pairs discordant for these two conditions along with healthy control pairs. We use this information to determine which brain abnormalities vary in dose dependent fashion with increasing genetic loading for each of these disorders, to examine DNA variations that may contribute to these abnormalities, to isolate non-genetic influences associated with each illness, and to clarify the extent of overlap between the two syndromes. The goal of the present report is to provide an overview of the sampling approach of the study, assessment methods, and findings to date, along with a brief description of work in progress.
Methods
Study design and population
The schizophrenia and bipolar twin study in Sweden (STAR) was initiated in 2006 as a collaboration project between investigators at the University of California Los Angeles (UCLA) and Department of Medical Epidemiology and Biostatistics (MEB) at Karolinska Institutet. In the recruitment procedure same-sex monozygotic (MZ) or dizygotic (DZ) twins born from 1940 to 1986 were identified through the Swedish Twin Register (Magnusson et al., 2013). The preliminary diagnostic status was ascertained through the Swedish National Patient Register (NPR, National Board of Health and Welfare, Ludvigsson et al., 2011) if at least one twin in the pair had a registered treatment episode of schizophrenia or bipolar disorder according to the International Statistical Classification of Diseases (ICD-8: 295 or 296, ICD-9: 295 or 296 and ICD-10: F20, F30 or F31). The complete pairs had to be alive and residing in Sweden at the time of recruitment. In addition, we invited five control twin pairs for each affected twin pair. Those twin pairs were matched by sex and birth year for each affected pair and were otherwise randomly selected from the Swedish Twin Register. Exclusion criteria included presence of severe somatic disorder (epilepsy or severe neurological symptoms) and ongoing acute psychosis. Parts of the methodology has been described in previous works using data from this twin cohort (Allswede et al., 2018; Fortgang et al., 2016a; Fortgang et al., 2016b; Higier et al., 2014; Johansson et al., 2017; Johansson et al., 2012; Kegel et al., 2017; Mobarrez et al., 2013; Zheutlin et al., 2016).
Information about the study was sent to the psychiatric organization in each Swedish county. The twins were then invited to the research site at the Department of MEB at the Karolinska Institutet and the Karolinska MR-center at the Karolinska Hospital, both sites located in Stockholm, Sweden. If the twin agreed to participate, self-report questionnaires were sent home by mail and they were later on contacted by phone to arrange for the one-day clinical examination procedures that included psychiatric interviews, neuropsychological and neuroimaging assessments and blood- (or saliva) sampling. In 2005, a pilot study was carried out where six MZ twin pairs went through the examination procedure to refine the protocol procedure as well as the clinical examination. The remaining twins in the study were enrolled between the years 2006 and 2012.
Self-report questionnaires
Before coming to the test center, the twins had received questionnaires by mail covering the following main areas: demographics, physical health, circadian rhythms, smoking habits, personality traits (impulsivity, sensation seeking, schizotypy, cyclothymia), stressful life-events, coping and social support. The participants also completed the screening questionnaires of the Structured Clinical Interview for DSM-IV (SCID I, Spitzer et al., 1992) and the Structured clinical interview for DSM-IV axis II personality disorders (SCID II, First et al., 1997). In the demographic survey, information was collected regarding place of birth, origin of biological parents and grandparents, level of education of the twin and parents, employment and marital status. Questions were asked regarding twin resemblance and years living together with the twin partner. In the physical health survey, information on height, weight, exercise habits, physical health and current medication was collected. The following self-report scales were administered regarding personality traits: Morningness Composite Scale (Smith et al., 1989), Fagerstrom Test (Heatherton et al., 1991), Barratt Impulsivness Scale (Patton et al., 1995), Zuckerman Sensation Seeking Scale (Zuckerman and Link, 1968), Schizotypal Personality Questionnare (Raine, 1991) and TEMPS~A (Akiskal et al., 2005). Finally the participants completed the questionnaires Abbreviated Holmes and Rahe Scale (Holmes and Rahe, 1967), Brief COPE Scale (Holmes and Rahe, 1967), Kessler Social Support scale (Schuster et al., 1990).
Clinical examination
All participants were clinically stable at the time of evaluation. A psychiatrist or a physician under psychiatric training, blinded to the diagnosis of the co-twin, administered the interviews of SCID-I and SCDI-II. Any present psychiatric symptoms of psychosis, depression, or expanded mood was rated using the following structured rating scales: Scale for Assessment of Negative Symptoms (SANS, Andreasen, 1983), the Scale for Assessment of Positive Symptoms (SAPS, Andreasen, 1984), the Hamilton Depression Rating Scale (HAM-D, Williams, 1988), and the Young Mania Rating Scale (YMRS, Young et al., 1978). The Global Assessment Function scale (GAF, 1994) was used to determine level of global functioning. Finally the participant was rated according to the NAPLS Outcome scale developed to measure social functioning (Cornblatt et al., 2007).
After the diagnostic interview, the final diagnosis was determined on by an evaluation team, which had access to the files from the clinical interview, register data from previous hospitalizations and hospital records. The final diagnosis was categorized as 1) schizophrenia (ICD-10: F20), 2) schizophrenia with affective features (ICD-10: F25), 3) bipolar disorder or bipolar disorder with psychotic features (ICD-10: F31), 4) major depression or major depression with psychotic features (ICD-10 F32-F33) or 5) not affected by any of the previous diagnoses.
Neuropsychological assessment
Cognitive testing was performed by a trained neuropsychologist, who administered the following neuropsychological tests: Wechsler Adult Intelligence Scale revised (WAIS-R), subtests Vocabulary design and Block design (verbal and spatial ability, Wechsler, 1981), Wechsler Memory Scale revised (WMS-R), subtests Digit Span, Spatial Span and Visual Reproduction (auditory and spatial attention, working memory and visuospatial memory, Russell, 1975), California Verbal Learning Test (CVLT, verbal memory, Delis et al., 1987), FAS, categories test (verbal fluency, Benton 1976), Trail Making Test (TMT, processing speed) A and B (Reitan, 1985), Dual Task performance (divided attention, Vilkki et al., 1996), and Purdue Pegboard (motor speed, Tiffin and Asher, 1948). Also, a test for the lateral dominance of handedness was performed (Dodrill and Thoreson, 1993) as well as the computerized Stop-Signal task (SST), measuring inhibition (Aron and Poldrack, 2006),
Neuroimaging
Examination with structural magnetic resonance imaging (sMRI) and functional MRI (fMRI) was performed at the Karolinska Institutet MR-center. The MR-session included a structural T1-weighted scan followed by an fMRI-session. MRIs were acquired on a 1.5-T scanner (GE Healthcare, Little Chalfont, Buckinghamshire, UK) using a 3D T1-weighted IRSPGR sequence acquired sagitally (TE=6 msec, flip angle=35°, and no interslice gap). The matrix size was 256×256×256 pixels, which corresponded to a resolution of 1mm3. Scans sensitive to changes in the BOLD signal were acquired while subjects performed spatial working memory and affect labeling tasks (Cannon et al., 2005). Exclusion criteria for the MRI-examination part of the study were any implants of metal in the body, previous severe head injury, previous cerebral hemorrhage, epilepsy or stroke.
In a subsample of six twin pairs discordant for bipolar disorder, positron emission tomography (PET) was performed with the radioligands [11C]raclopride binding dopamine D2 receptor and [11C]AZ10419369 binding to serotonin 5-HT1B receptor. The probands were medication free since more than three months, and the unaffected co-twin had no psychiatric diagnosis.
Biosamples
At the examination site a 10 ml EDTA blood sample for DNA extraction, and 2.5 ml blood for RNA extraction with a Paxgene tube, was taken from each participant and sent to Karolinska Institutet (KI) Biobank (http://ki.se/forskning/ki-biobank) for processing and storage. DNA was extracted from EDTA blood based on a salting out method from Puregene extraction kit using a Gentra robot. The Paxgene tube was handled according to manufacturer’s recommendation and stored at − 80 °C at KI Biobank, until sending the samples to University of California Los Angeles for RNA extraction and analysis.
Saliva was retrieved from a subsample of individuals (n=41) that did not want to participate at the examination site, but were willing to leave a sample of saliva for genetic analyses.
Skin biopsies were taken from six MZ twin pairs discordant for schizophrenia. We have previously collected over 200 skin biopsies in singleton cases with schizophrenia and healthy controls.
Cerebrospinal fluid (CSF) was collected from 25 twin pairs for biomarker analysis and microscopic examination, in an extended assessment procedure, see references for details (Johansson et al., 2017; Johansson et al., 2012; Kegel et al., 2017; Mobarrez et al., 2013). Approximately 0.6 mL was collected in two fractions, for examination with scanning electron microscopy, and 12 mL was stored at − 80 °C in 1.0–1.6 mL aliquots.
Zygosity determination
Zygosity was determined by DNA-analysis using a validated method by Hannelius and coworkers that consists of a highly multiplexed panel of 47 single nucleotide polymorphisms including one sex-specific marker (Hannelius et al., 2007). In single cases, zygosity was determined through survey questions based on experienced twin resemblance.
Birth data
The participants had given informed consent to obtain obstetric records. Information was recorded on birth weight and length, head circumference, gestational age, maternal age, parity, placenta weight, obstetric diagnosis in mother and child, and information on instrumental delivery.
Medical history
Medical history was collected through the NPR, which contains 75-80% of all outpatient diagnoses since 2001, and all inpatient diagnoses since 1987. Both psychiatric and somatic inpatient treatments were recorded. The treating psychiatric hospital was also contacted for those with a diagnosis of schizophrenia or bipolar disorder to get copies of their medical journal with full information on psychiatric care, in accordance with the informed consent. This was especially important for the final diagnostic assessments.
Statistical Analysis
The data have mainly been analyzed using statistical twin design models such as structural equation models (SEM) and co-twin control models. Descriptive statistics for this article was obtained using Statistical Analysis System (SAS 9.4, SAS Institute, Cary, NC).
Ethical considerations
Written informed consent was obtained from all participants in this study. Essential for twin design is participation of both twins in a pair, and recruitment in pairs is desirable. However, in this study, the co-twins were recruited independently and participations were not conditioned on the other twin’s participation. The ethical issues involved getting in contact with the not affected twin sibling, who might not be aware of the affected twin sibling’s diagnosis. We have shown earlier that important themes in sibling relationships, in which one of the siblings suffers from schizophrenia, are closeness and caring, but also distancing, and fear of heredity (Stalberg et al., 2004). In the current study, we have experienced a strong motivation to participate, an altruistic attitude and curiosity from the non-affected twin, but we have also noted a fear of disclosure of similar traits and distancing.
RESULTS
Demographic information
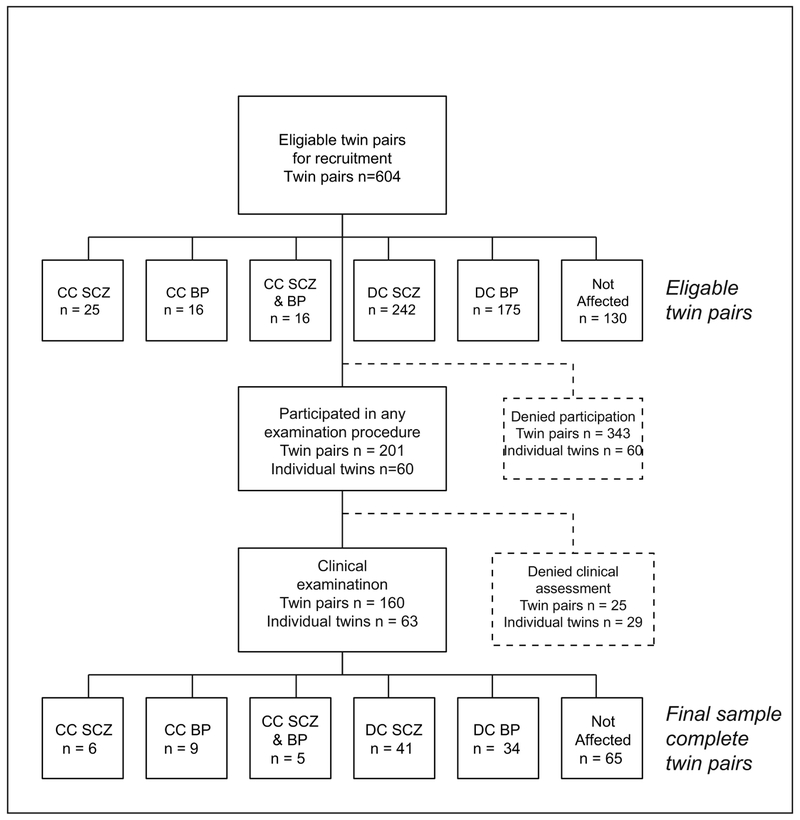
In total, 1208 twins, from 604 same sex twin pairs were initially invited to participate (Figure 1), and from those 462 twins participated in any of the examination procedures, which corresponds to a 38.2% participation rate. For the individual diagnoses, the participation rate was lower in those with a presumptive status of schizophrenia (20.6%) or bipolar disorder (21.2%) compared with not affected twins (58.2%). The proportion of same-sex DZ twins (58.5%) was higher in relation to MZ twins (41.5%). The distribution of males and females was fairly even. The age distribution tended towards higher ages, for the reason that a minimum participation age was set to 24 years at recruitment, as lower ages would increase the risk for disease discordant pairs to transform into concordant pairs, after the clinical examination procedure.
Figure 1.
Flowchart of the recruitment procedure in The Schizophrenia and Bipolar Twin Study in Sweden (STAR).
CC=Concordant, DC=Discordant, SCZ = Schizophrenia, BP=Bipolar disorder
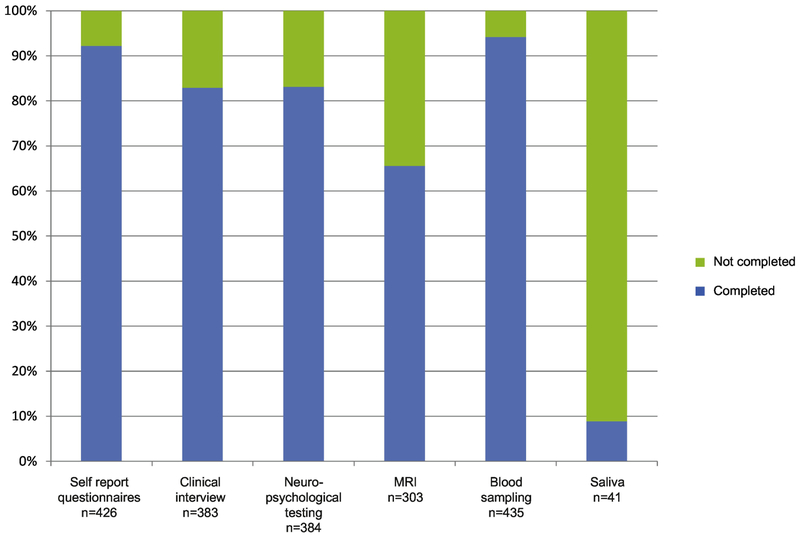
In total 383 (83% of the 462 twins that participated) twins completed the diagnostic interviews, resulting in a final diagnosis (schizophrenia, schizophrenia with affective features, bipolar disorder, major depression) or the status “not affected”. Demographic information on those twins is presented in Table 1, and an overview of the proportion of participants that completed the clinical assessment procedures is presented in Figure 2. The remaining 79 twins (17%) participated by responding to self-assessment questionnaires, and/or leaving a biological sample (blood, saliva or both), for demographic information on those participants, see Supplement table 1.
Table 1.
Characteristics of the twins who participated in the STAR procedures. MZ=Monozygotic, DZ=Dizygotic, CC=Concordant pairs, DC=Discordant pairs, NA= Not affected pairs.
| Complete MZ pairs (n=144) | Complete DZ pairs (n=176) | Non Comple te MZ pairs |
Non comple te DZ pairs |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | CC | DC | NA | CC | DC | NA | |||
| n=383 | n=32 | n=54 | n=58 | n=8 | n=96 | n=72 | n=12 | n=51 | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Sex | |||||||||
| Male | 186 (48.6) |
10 (31.3) |
24 (44.4) |
26 (44.8) |
4 (50.0) |
50 (52.1) |
39 (54.2) |
6 (50.0) |
27 (52.9) |
| Female | 197 (51.4) |
22 (68.8) |
30 (55.6) |
32 (55.2) |
4 (50.0) |
46 (47.9) |
33 (45.8) |
6 (50.0) |
24 (47.1) |
|
Year of birth, categories |
|||||||||
| 1942-51 | 98 (25.6) |
2 (6.3) |
16 (29.6) |
14 (24.1) |
2 (25.0) |
26 (27.1) |
14 (19.4) |
5 (41.7) |
19 (37.3) |
| 1952-61 | 122 (31.9) |
8 (25.0) |
8 (14.8) |
12 (20.7) |
6 (75.0) |
38 (39.6) |
30 (41.7) |
1 (8.3) |
19 (37.3) |
| 1962-71 | 100 (26.1) |
14 (43.8) |
10 (18.5) |
20 (34.5) |
0 (0) |
26 (27.1) |
22 (30.6) |
2 (16.7) |
6 (11.8) |
| 1972-81 | 47 (12.3) |
4 (12.5) |
14 (25.9) |
10 (17.2) |
0 (0) |
4 (4.2) |
6 (8.3) |
3 (25.0) |
6 (11.8) |
| 1982-86 | 16 (4.2) |
4 (12.5) |
6 (11.1) |
2 (3.5) |
0 (0) |
2 (2.1) |
0 (0) |
1 (8.3) |
1 (2.0) |
| Education | |||||||||
| Elementary school or below |
75 (19.6) |
4 (12.5) |
7 (13.0) |
3 (5.2) |
2 (25.0) |
25 (26.0) |
14 (19.4) |
6 (50.0) |
14 (27.5) |
| High school | 165 (43.1) |
23 (71.9) |
19 (35.2) |
32 (55.2) |
3 (37.5) |
33 (34.4) |
31 (43.1) |
2 (16.7) |
22 (43.1) |
| University | 131 (34.2) |
4 (12.5) |
25 (46.3) |
21 (36.2) |
3 (37.5) |
35 (36.5) |
26 (36.1) |
4 (33.3) |
13 (25.5) |
| Unknown | 12 (3.1) |
1 (3.1) |
3 (5.6) |
2 (3.5) |
0 (0) |
3 (3.1) |
1 (1.4) |
0 (0) |
2 (3.9) |
|
Smoking status |
|||||||||
| Smoker | 60 (15.7) |
5 (15.6) |
7 (13.0) |
10 (17.2) |
2 (25.0) |
19 (19.8) |
6 (8.3) |
1 (8.3) |
10 (19.6) |
|
Substance abuse/ dependence |
|||||||||
| Alcohol abuse/ dependence |
57 (14.9) |
6 (18.8) |
13 (24.1) |
4 (6.9) |
1 (12.5) |
18 (18.8) |
8 (11.11) |
2 (16.7) |
5 (9.8) |
| Drug abuse /dependence |
26 (6.8) |
4 (12.5) |
6 (11.1) |
3 (5.2) |
1 (12.5) |
8 (8.3) |
4 (5.6) |
0 (0) |
0 (0) |
|
Diagnosis according to DSM-IV interview |
|||||||||
| Schizophrenia | 38 (9.9) |
6 (18.8) |
5 (9.3) |
0 (0) |
4 (50.0) |
20 (20.8) |
0 (0) |
0 (0) |
3 (5.9) |
| Schizophrenia with affective features |
25 (6.5) |
6 (18.8) |
7 (13.0) |
0 (0) |
1 (12.5) |
9 (9.4) |
0 (0) |
0 (0) |
2 (3.9) |
| Bipolar disorder |
69 (18.0) |
20 (62.5) |
15 (27.8) |
0 (0) |
3 (37.5) |
19 (19.8) |
0 (0) |
2 (16.7) |
10 (19.6) |
| Depression, life-time |
76 (19.8) |
0 (0) |
9 (16.7) |
17 (29.3) |
0 (0) |
13 (13.5) |
24 (33.3) |
3 (25.0) |
10 (19.6) |
| Not affected | 175 (45.7) |
0 (0) |
18 (33.3) |
41 (70.7) |
0 (0) |
35 (36.5) |
48 (66.7) |
7 (58.3) |
26 (51.0) |
|
Age at disease onset, mean (min- max) |
28 (10-60) |
24.5 (15-40) |
25 (12-50) |
- | 29 (16-52) |
29 (16-60) |
- | 41 (15-45) |
28 (14-60) |
|
Age at sampling, mean (min- max) |
50 (24-67) |
46.5 (24-65) |
45 (25-65) |
45 (26-66) |
58.5 (55-66) |
54 (29-65) |
50 (29-67) |
57 (28-65) |
54 (28-66) |
|
Zygosity information |
|||||||||
| DNA | 312 (81.5) |
28 (87.5) |
54 (100) |
54 (93.1) |
8 (100) |
88 (91.7) |
72 (100) |
1 (8.3) |
7 (13.7) |
| Questionnaire | 71 (18.5) |
4 (12.5) |
0 (0) |
4 (6.9) |
0 (0) |
8 (8.3) |
0 (0) |
11 (91.7) |
44 (86.3) |
|
Type of DNA sample |
|||||||||
| Blood | 381 (99.5) |
32 (100) |
54 (100) |
57 (98.28) |
8 (100) |
95 (98.96) |
72 (100) |
12 (100) |
51 (100) |
| Saliva | 9 (2.3) |
4 (12.5) |
2 (3.7) |
0 (0) |
0 (0) |
2 (2.1) |
0 (0) |
0 (0) |
1 (1.96) |
| None | 1 (0) |
0 (0) |
0 (0) |
1 (1.7) |
0 (0) |
1 (1.0) |
0 (0) |
0 (0) |
0 (0) |
|
Years living with twin partner, median (min- max) |
18 (1-38) |
18 (9-34) |
19 (11-24) |
18 (15-25) |
17 (14-21) |
18 (12-32) |
18 (15-25) |
19.5 (1-38) |
18 (9-25) |
|
Cohabitation status with twin partner |
|||||||||
| Still living together |
6 (1.6) |
2 (6.3) |
2 (3.7) |
55 (94.8) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
1 (2.0) |
| Not living together |
366 (95.6) |
29 (90.6) |
49 (90.74) |
1 (1.7) |
8 (100) |
93 (96.9) |
72 (100) |
1 (8.3) |
1 (2.0) |
| Unknown | 11 (2.9) |
1 (3.1) |
3 (5.6) |
2 (3.5) |
0 (0) |
3 (3.1) |
0 (0) |
11 (91.7) |
49 (96.1) |
|
In contact with twin partner |
|||||||||
| Daily | 125 (32.6) |
21 (65.6) |
20 (37.0) |
28 (48.3) |
4 (50.0) |
24 (25.0) |
12 (16.7) |
6 (50.0) |
10 (19.6) |
| Once a week | 126 (32.9) |
6 (18.8) |
17 (31.5) |
23 (39.7) |
0 (0) |
29 (30.2) |
34 (47.2) |
4 (33.3) |
13 (25.5) |
| Once a month |
73 (19.1) |
2 (6.3) |
9 (16.7) |
6 (10.3) |
2 (25.0) |
24 (25.0) |
17 (23.6) |
1 (8.3) |
12 (23.5) |
| Once every 6 months |
22 (5.7) |
0 (0) |
2 (3.7) |
1 (1.7) |
1 (12.5) |
9 (9.4) |
5 (6.9) |
0 (0) |
4 (7.8) |
| Less than every six months |
13 (3.4) |
2 (6.3) |
0 (0) |
0 (0) |
1 (12.5) |
5 (5.2) |
0 (0) |
0 (0) |
5 (9.8) |
| Never | 15 (3.9) |
1 (3.1) |
0 (0) |
0 (0) |
0 (0) |
5 (5.2) |
3 (4.2) |
0 (0) |
6 (11.8) |
| Unknown | 9 (2.3) |
0 (0) |
6 (11.1) |
0 (0) |
0 (0) |
0 (0) |
1 (1.4) |
1 (8.3) |
1 (2.0) |
|
MRI- examination |
303 (79.1) |
26 (81.3) |
47 (87.0) |
54 (93.1) |
7 (87.5) |
74 (77.1) |
54 (75.0) |
8 (66.7) |
33 (64.7) |
| Handedness | |||||||||
| Right | 337 (88.0) |
31 (96.9) |
44 (81.5) |
53 (91.4) |
6 (75.0) |
90 (93.8) |
60 (83.3) |
11 (91.7) |
42 (82.4) |
| Left | 35 (9.1) |
1 (3.1) |
6 (11.1) |
4 (6.9) |
2 (25.0) |
4 (4.2) |
9 (12.5) |
1 (8.3) |
8 (15.7) |
| Ambidextrant | 7 (1.8) |
0 (0) |
1 (1.9) |
1 (1.7) |
0 (0) |
2 (2.1) |
2 (2.8) |
0 (0) |
1 (2.0) |
| Unknown | 4 (1.0) |
0 (0) |
3 (5.6) |
0 (0) |
0 (0) |
0 (0) |
1 (1.4) |
0 (0) |
0 (0) |
Figure 2:
Proportion of participants in The Schizophrenia and Bipolar Twin Study in Sweden (STAR) who completed the different testing procedures.
After the clinical assessment, 16.4% were diagnosed as schizophrenia with or without affective features, 18.0% were diagnoses as bipolar disorder, 19.8% as major depression, and 45.7% as not affected. The self-reported median age at onset was 27 years for schizophrenia, as well as for bipolar disorder. For depression, the median age at onset was 34 years. Of the twins who completed the diagnostic assessment, the proportion of smokers was relatively high, and 15.7% reported that they smoked one or more cigarettes daily, Table 1. Of the twins with schizophrenia 25.4% reported that they smoked daily, while the corresponding figure for the bipolar twins was 20.3%, and 19.7% for those with a previous depression, while the proportion of smokers were 8.4% in the not affected twins. The proportion of participants that had an ongoing or previous alcohol abuse or dependence was 14.9%, and the corresponding figure for illicit drugs was 6.8%. The majority of the twins (95.6%) were not living with their twin partner as adults, and the majority (65.5%) kept in touch with their twin partner once a week or more often. The median time for living together was 18 years (range 1 to 38 years).
Change of diagnostic status after the clinical assessment
The presumptive status for each twin was based on a register diagnosis from the Swedish National Patient Register that was previously validated (Ludvigsson et al., 2011). The register contains information on hospitalizations for psychiatric diagnoses from 1973, while diagnostic information from outpatient care was collected from 2001. Thus, we were able to compare the lifetime register diagnosis based on the treating physician’s hospital assessments, with the diagnostic outcome from the present clinical assessment based on the SCID-I interview. In 83.0% of the twins, there was no change in diagnostic status after the clinical interview, Table 2. In this sample, the register diagnosis of bipolar disorder was most likely to be changed to not affected after the clinical interview, which occurred in 24 cases (6.3%), Table 2. In individuals who were classified as schizophrenia in the register, ten individuals (2.6%) changed to not affected after the diagnostic assessment. In total 18 individuals (4.7%) switched from schizophrenia to bipolar disorder or the other way around.
Table 2.
Change in diagnostic status for schizophrenia and bipolar disorder based on the register diagnoses and the final diagnostic status after the clinical assessment (n=383).
| Presumptive diagnostic status | Final diagnostic status | Change of diagnoses |
|---|---|---|
| N (%) | ||
| No change of diagnostic status | 318 (83.0) | |
| Bipolar disorder | Not affected | 24 (6.3) |
| Schizophrenia* | Bipolar disorder | 10 (2.6) |
| Schizophrenia* | Not affected | 10 (2.6) |
| Not affected | Bipolar disorder | 9 (2.4) |
| Bipolar disorder | Schizophrenia* | 8 (2.1) |
| Not affected | Schizophrenia* | 4 (1.0) |
| Total number of changes: | 65 (17.0) |
Schizophrenia and schizophrenia with affective features are here included in the category of schizophrenia.
Reasons for not participating in STAR
All twins who decided not to participate in the study were asked for their reason. The most common reason was that either one or both twins in a pair decided not to participate, Table 3. We were not able to get in touch with 17.0% of the presumptive participants for various reasons, such as no available telephone number, the lack of response on our contact attempts, or that the participant had moved to an unknown address, Table 3. It was more common not to have a registered phone number in those with a presumptive status of schizophrenia (10.8%) compared with those who were presumed to be not affected (7.1%).
Table 3.
Reported reasons for not participating in the STAR study
| Presumptive status | ||||
|---|---|---|---|---|
| Reasons for non-participation | Total N=746 |
Schizophren iaa N=213 |
Bipolar disorder N=125 |
Not- Affectedb N=408 |
| Personal | ||||
| One twin in a pair did not want to participate | 267 (35.8) | 87 (40.8) | 54 (43.2) | 126 (30.9) |
| Both twins did not want to participate | 230 (30.8) | 49 (23.0) | 38 (30.4) | 143 (35.0) |
| One twin in a pair was deceased | 18 (2.4) | 10 (4.7) | 1 (1.0) | 7(1.7) |
| Health related | ||||
| One/both twins did not want to participate due to psychiatric symptoms |
25 (3.4) | 13 (6.1) | 4 (3.2) | 8 (2.0) |
| One/both twins did not want to participate due to somatic problems |
73 (9.8) | 11(5.2) | 8 (6.4) | 54(13.2) |
| Twin not possible to localize | ||||
| Did not have a telephone number | 62 (8.3) | 23 (10.8) | 10 (8.0) | 29 (7.1) |
| Was not possible to reach – other reason | 64 (8.6) | 19 (8.9) | 8 (6.4) | 37 (9.1) |
| Unknown address | 1 (0) | 0 (0) | 0 (0) | 1 (0) |
| Unknown | 6 (0) | 1 (0.5) | 2 (1.6) | 3 (1.0) |
Schizophrenia and schizophrenia with affective features are here included in the category of schizophrenia.
Not-affected category includes those with a diagnosis of depression.
Completed projects with STAR data
Until now, there are eleven completed studies based on data from the STAR twin cohort (see Table 4 for overview). Examples of novel findings from those studies are that personality traits related to sociability and verbal function was associated with liability for bipolar disorder but not for schizophrenia (Higier et al., 2014). Personality traits related to impulsivity was found to be a shared endophenotype for schizophrenia, bipolar disorder and depression (Fortgang et al., 2016b). Genes related to memory contributed to schizophrenia risk (Zheutlin et al., 2016). Expression of mRNA related to the complement system was associated with cortical thickness in schizophrenia (Allswede et al., 2018). Structures observed with scanning electron microscopy in CSF was associated with schizophrenia and bipolar disorder and with liability for those disorders (Johansson et al., 2012). Markers related to microglia in CSF was associated with psychotic symptoms in schizophrenia and bipolar disorder (Johansson et al., 2017).
Table 4. Overview of the hitherto published studies on the STAR twins.
Monozygotic (MZ), Dizygotic (DZ), Concordant (CC), Discordant (DC), Schizophrenia with or without affective features (SCZ), Bipolar disorder (BPD), Depression (DEP), Control-twin (CC). Not applicable (NA). Cerebrospinal fluid (CSF)
| TYPE OF STUDIES | Twins | Diagn oses studie d |
Zyg osity |
Externa l samples |
Summary of study results |
|---|---|---|---|---|---|
|
PSYCHIATRIC SYMPTOMS AND PERSONALITY TRAITS |
- | ||||
| Higier et al. 2014 1 | N=258 | SCZ + BPD |
MZ + DZ |
- | Temperament and cognitive performance were studied in discordant twin pairs and not affected pairs. BPD co- twins scored higher on positive temperaments and had a superior performance on cognitive measures (verbal fluency). Enhanced sociability and verbal fluency were associated with liability for BPD but not SCZ. |
|
Fortgang et al. 2016 2 |
N=420 | SCZ + BPD |
MZ + DZ |
- | Coping with stress (Brief cope questionnaire) was studied in relation to psychopathology. Coping styles showed more phenotypic than genetic overlap across SCZ, BPD, and depression. |
|
Fortgang et al. 2016 3 |
N=420 | SCZ + BPD |
MZ + DZ |
- | Self-reported impulsivity and sensation seeking behavior, and inhibition measured through the computerized Stop Signal Task (SST) was studied in SCZ, BPD, depression, their co-twins, and not affected controls. Impulsivity and inhibition was associated with SCZ and BP but not their co-twins. Sensation seeking was lower in SCZ. Self- reported impulsivity emerged as a shared endophenotype for SCZ, BPD and depression. |
|
GENETIC RISK IN RELATION TO COGNITION AND BRAIN ANATOMY |
- | ||||
| Zheutlin et al. 2016 4 | N=190 | SCZ + BPD |
MZ + DZ |
Indepen dent SCZ twin sample, N=73 |
Gene expression in mononuclear cells from blood was analyzed in relation to cognitive performance; California Verbal Learning Task (CVLT). CVLT performance was related to expression levels of 76 genes. 43 of these genes were differentially expressed in SCZ, indicating that genes influencing memory might contribute to SCZ risk. |
| Allswede et al. 2017 5 | N=129 | SCZ | - | Brain imaging data and mRNA expression in blood was analyzed in SCZ, their co-twins and controls. The mRNA expression levels in genes related to the complement system (C5, SERPING1) were associated with cortical thickness in frontal cortex. The study provides support for involvement of the complement system in the pathogenesis of SCZ. |
|
|
GENETIC STUDIES |
- | ||||
| Bloom et al. 2013 6 | N=10 | SCZ + BPD |
MZ | - | A comparative genomic hybridization array analysis in blood with a genome-wide detection of copy number variants was performed in discordant pairs for SCZ or BPD. No differences identified of the copy number variants within the twin pairs. |
| Hannon et al. 2016 7 | N=36 | SCZ | MZ | Non- twin sample with SCZ (n=1714 ) |
Genome-wide patterns of DNA methylation was analyzed in SCZ, in three independent cohorts. Methylated positions and regions associated with SCZ was independent from smoking. Epigenetic variation contributed to the polygenic nature of SCZ. |
|
MARKERS IN CEREBRO-SPINAL FLUID (CSF) |
|||||
| Johansson et al. 2012 8 | N=37 | SCZ + BPD |
MZ + DZ |
Non- twin controls, n=65 |
Microscopic structures were analyzed in CSF with scanning electron microscopy. Structures were more frequent in twins with SCZ or BPD the co-twins, indicating a partial genetic influence on the occurrence of CSF-structures. |
|
Mobarrez et al. 2013 9 |
N=2 | SCZ | MZ | Non- twin controls, n=4 |
Flow cytometry analysis was used to detect microparticles in CSF originating from lactadherine, platelets, endothelium or leukocytes. The increased number of microparticles suggested increased activity of the mechanisms for membrane shedding in SCZ. |
| Johansson et al. 2017 10 | N=35 | SCZ + BPD |
MZ + DZ |
- | Microglia and neurodegenerative markers were analyzed in CSF in SCZ, BPD, not affected co-twins and healthy controls. Higher CSF sCD14 levels were associated with SCZ, BPD and psychotic symptoms, supporting a role for microglia activation in psychosis. |
| Kegel et al. 2017 11 | N=23 | SCZ + BPD |
MZ + DZ |
- | Kynurenine metabolites and cytokines were analyzed in CSF in SCZ, BPD, not affected co-twins and healthy controls. Kynurenic acid in CSF was associated psychotic symptoms and personality traits. |
Higier RG, Jimenez AM, Hultman CM, et al. Enhanced neurocognitive functioning and positive temperament in twins discordant for bipolar disorder. The American journal of psychiatry. 2014;171(11): 1191-1198.
Fortgang RG, Hultman CM, Cannon TD. Coping Styles in Twins Discordant for Schizophrenia, Bipolar Disorder, and Depression. Clinical psychological science : a journal of the Association for Psychological Science. 2016;4(2):216-228.
Fortgang RG, Hultman CM, van Erp TG, Cannon TD. Multidimensional assessment of impulsivity in schizophrenia, bipolar disorder, and major depressive disorder: testing for shared endophenotypes. Psychol Med. 2016;46(7):1497-1507.
Zheutlin AB, Viehman RW, Fortgang R, et al. Cognitive endophenotypes inform genome-wide expression profiling in schizophrenia. Neuropsychology. 2016;30(1):40-52.
Allswede DM, Zheutlin AB, Chung Y, et al. Complement Gene Expression Correlates with Superior Frontal Cortical Thickness in Humans. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2018;43(3):525-533.
Bloom RJ, Kahler AK, Collins AL, et al. Comprehensive analysis of copy number variation in monozygotic twins discordant for bipolar disorder or schizophrenia. Schizophrenia research. 2013;146(1-3):289-290.
Hannon E, Dempster E, Viana J, et al. An integrated genetic-epigenetic analysis of schizophrenia: evidence for co-localization of genetic associations and differential DNA methylation. Genome biology. 2016;17(1):176.
Johansson V, Nybom R, Wetterberg L, et al. Microscopic particles in two fractions of fresh cerebrospinal fluid in twins with schizophrenia or bipolar disorder and in healthy controls. PloS one. 2012;7(9):e45994.
Mobarrez F, Nybom R, Johansson V, et al. Microparticles and microscopic structures in three fractions of fresh cerebrospinal fluid in schizophrenia: case report of twins. Schizophrenia research. 2013;143(1):192-197.
Johansson V, Jakobsson J, Fortgang RG, et al. Cerebrospinal fluid microglia and neurodegenerative markers in twins concordant and discordant for psychotic disorders. European archives of psychiatry and clinical neuroscience. 2017;267(5):391-402.
Kegel ME, Johansson V, Wetterberg L, et al. Kynurenic acid and psychotic symptoms and personality traits in twins with psychiatric morbidity. Psychiatry research. 2017;247:105-112.
Ongoing projects with STAR data
Currently, we have several ongoing projects based on data from the STAR study, which include collaborations with research groups worldwide. For example, we are studying the hereditary and environmental mechanisms of inflammation in schizophrenia and bipolar disorder, by analyzing inflammatory markers and the microbiome in blood. fMRI data are being analyzed to evaluate functional connectomic differences between probands and co-twins and between co-twins and controls. In a genetic project, we perform whole genome sequencing (WGS) and genome-wide association analyses (GWAS), to study the risk for schizophrenia or bipolar disorder in relation to common and rare somatic mutations. The availability of D2 and 5-HT1B receptors in twins with bipolar disorder and their not affected co-twins are studied with PET scanning technique. Skin biopsies from MZ twins with schizophrenia are under analyses, with the purpose to use patient derived fibroblasts from twins bearing certain genetic alterations of interest, to develop specialized neural cells for experimental studies. Finally, we are updating register data to be able to follow-up on the participants’ psychiatric and somatic disorders longitudinally.
DISCUSSION
The STAR study, which is one of the largest studies of its kind on MZ and DZ twins with schizophrenia or bipolar disorder, has given us the opportunity to study disease mechanisms in relation to genetic and epigenetic variation, biomarkers, psychiatric symptoms, cognitive ability, and brain function. The classical twin design has enabled us to separate relative contributions from genetic and/or environmental influences on certain traits. This article describes the study design, the recruitment, and examination procedures, and the characteristics of the study participants. It also describes completed and ongoing projects of the STAR study.
The uniqueness of this cohort is the population-based national recruitment of twins. Clinical psychiatrists have used well-validated instruments for the diagnostic assessments. We have assembled a rich collection of information using questionnaire as well as rater-assessed data on psychiatric symptoms and personality traits. The assessment of symptoms allows for studies based on symptom dimensions as well as based on the dichotomous diagnoses. The participants have gone through neuropsychological assessments, neuroimaging examinations, and biological samples have been obtained. We have also access to the medical history for all participants through register linkages, and information on birth data through obstetric records. Each participant has been asked for permission to contact their children, for follow-up purposes in future projects.
Similar twin studies within the field include the Maudsley twin cohort in England following up on twin pairs with a history of psychotic symptoms (Cardno et al., 1999; Cardno et al., 2012). Twin cohorts including twins with schizophrenia or bipolar disorders separately have been established in Finland (Cannon et al., 2000; Kieseppa et al., 2000; Pirkola et al., 2005), and the Netherlands (Baare et al., 2001; Hulshoff Pol et al., 2012), and a Danish twin study has included twins with psychotic spectrum disorders (Hilker, 2015). Similar to STAR those cohorts consists of approximately 300 to 400 twins each and assemble information on neurocognitive measures, brain imaging and genetic data. As schizophrenia and bipolar disorder are rare disorders future projects would benefit from transnational collaboration projects combining data from different cohorts. Challenges include the difficulties in synchronizing data from different cohorts and coordination of the collaboration.
Our twin cohort shows that it has been beneficial to include both schizophrenia and bipolar disorder, but to differentiate between the two disorders may be a challenge. We found that 17% of the participants changed in diagnostic status, when we compared the register based diagnoses with the result from the clinical interview combined with a review of the available clinical information. Around 5% of the participants changed diagnostic status from bipolar disorder to schizophrenia or schizoaffective disorder or conversely. The validation of the diagnoses highlights the difficulty to decide on a definite categorical diagnosis. To include both diagnoses thus enlightens the common features and lifetime changes between schizophrenia and bipolar disorder. Our comprehensive symptom scales enable us to choose a dimensional view on psychotic symptoms for enrichment of the power in future analyses.
At this stage, it is difficult to state which findings from our cohort that are the most innovative. The CSF studies are unique and the future will tell if specific or common biomarkers could be identified. With the expansive development in genetic research in mind, the molecular genetic data from STAR are likely to be more valuable than we could imagine when we started the study over ten years ago. In addition, the skin biopsies might give important contribution to future stem cell studies.
One limitation of this study is the relatively low initial response rate of 38.2%, and the response rate was even lower for the twins with a presumptive status of schizophrenia and bipolar disorder. To motivate participants with severe chronical diseases to participate in extensive examination procedures is difficult. However, as the recruitment was performed nation-wide the population is as representative as possible.
Conclusion
The STAR study in Sweden is a unique cohort of MZ and DZ twins with schizophrenia or bipolar disorder. Here we describe the design, data collection, recruitment, measures as well as past, ongoing and planned analyses in STAR. Until now, this twin cohort has given us the opportunity to address a broad spectrum of research questions by studying the effect of genetic and environmental factors in relation to outcomes for discordant and concordant twin pairs. In addition, we have initiated novel analyses such as WGS among others. The STAR cohort has a great potential for future projects and collaborations opening up for new hypotheses within the field and the possibility to share data between other similar twin cohorts. Potential gains of the study include identification of biomarkers, new leads for prevention of schizophrenia and bipolar disorder, translational modeling and the emergence for drug development.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the study participants, research nurse Karin Dellenwall for blood sampling, Professor Martin Ingvar and Professor Henrik Larsson for imaging expertise and the clinicians who performed the psychiatric assessments.
FUNDINGS
This work was supported by the National Institute of Health (grant number R01 MH052857) to Dr. Cannon, and by ALF, which is a regional agreement on medical training and clinical research between Stockholm County Council and the Karolinska Institutet (grant numbers 20100305, and 20090183) to Dr Hultman. Dr. Cannon is a consultant to Boehringer Ingelheim Pharmaceuticals and Lundbeck A/S. The content of this paper is unrelated to these consulting activities. All other authors have declared that there are no conflicts of interest in relation to the subject of this study.
THE ROLE OF THE FUNDING SOURCES
This work was supported by the National Institute of Health (grant number R01 MH052857) to Dr. Cannon, and by ALF, which is a regional agreement on medical training and clinical research between Stockholm County Council and the Karolinska Institutet (grant numbers 20100305, and 20090183) to Dr Hultman. There is no involvement of the sponsors neither in study design, analysis, and interpretation of the collected data nor in the reporting writing and in the choice of the Journal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTERESTS
Dr. Cannon is a consultant to Boehringer Ingelheim Pharmaceuticals and Lundbeck A/S. The content of this paper is unrelated to these consulting activities. All other authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- Akiskal HS, Mendlowicz MV, Jean-Louis G, Rapaport MH, Kelsoe JR, Gillin JC, Smith TL, 2005. TEMPS-A: validation of a short version of a self-rated instrument designed to measure variations in temperament. J Affect Disord 85(1-2), 45–52. [DOI] [PubMed] [Google Scholar]
- Allswede DM, Zheutlin AB, Chung Y, Anderson K, Hultman CM, Ingvar M, Cannon TD 2018. Complement Gene Expression Correlates with Superior Frontal Cortical Thickness in Humans. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 43(3), 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, 1983. Scale for the Assessment of Negative Symptoms (SANS). University of Iowa, Iowa City. [Google Scholar]
- Andreasen NC, 1984. The scale for the Assessment of Positive Symptoms (SAPS). University of Iowa, Iowa City. [Google Scholar]
- Aron AR, Poldrack RA, 2006. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience 26(9), 2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association AP, 1994. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. American Psychiatric Association, Washington, DC. [Google Scholar]
- Baare WF, van Oel CJ, Hulshoff Pol HE, Schnack HG, Durston S, Sitskoorn MM, Kahn RS, 2001. Volumes of brain structures in twins discordant for schizophrenia. Archives of general psychiatry 58(1), 33–40. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K, 1976. Multilingual Aphasia Examination manual, Iowa City, IA: : University of Iowa. [Google Scholar]
- Cannon TD, Glahn DC, Kim J, Van Erp TG, Karlsgodt K, Cohen MS, Nuechterlein KH, Bava S, Shirinyan D, 2005. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Archives of general psychiatry 62(10), 1071–1080. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Huttunen MO, Lonnqvist J, Tuulio-Henriksson A, Pirkola T, Glahn D, Finkelstein J, Hietanen M, Kaprio J, Koskenvuo M, 2000. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. American journal of human genetics 67(2), 369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Kaprio J, Lonnqvist J, Huttunen M, Koskenvuo M, 1998. The genetic epidemiology of schizophrenia in a Finnish twin cohort. A population-based modeling study. Archives of general psychiatry 55(1), 67–74. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Thompson PM, van Erp TG, Toga AW, Poutanen VP, Huttunen M, Lonnqvist J, Standerskjold-Nordenstam CG, Narr KL, Khaledy M, Zoumalan CI, Dail R, Kaprio J, 2001. Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America 99(5), 3228–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardno AG, Marshall EJ, Coid B, Macdonald AM, Ribchester TR, Davies NJ, Venturi P, Jones LA, Lewis SW, Sham PC, Gottesman II, Farmer AE, McGuffin P, Reveley AM, Murray RM, 1999. Heritability estimates for psychotic disorders: the Maudsley twin psychosis series. Archives of general psychiatry 56(2), 162–168. [DOI] [PubMed] [Google Scholar]
- Cardno AG, Rijsdijk FV, West RM, Gottesman II, Craddock N, Murray RM, McGuffin P, 2012. A twin study of schizoaffective-mania, schizoaffective-depression, and other psychotic syndromes. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics 159b(2), 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou IJ, Kuo CF, Huang YS, Grainge MJ, Valdes AM, See LC, Yu KH, Luo SF, Huang LS, Tseng WY, Zhang W, Doherty M, 2016. Familial Aggregation and Heritability of Schizophrenia and Co-aggregation of Psychiatric Illnesses in Affected Families. Schizophrenia bulletin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Auther AM, Niendam T, Smith CW, Zinberg J, Bearden CE, Cannon TD, 2007. Preliminary Findings for Two New Measures of Social and Role Functioning in the Prodromal Phase of Schizophrenia. Schizophrenia bulletin 33(3), 688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Thompkins BAO, Corporation P, Harcourt Brace Jovanovich I, 1987. CVLT, California Verbal Learning Test: Adult Version : Manual. Psychological Corporation. [Google Scholar]
- Dodrill CB, Thoreson NS, 1993. Reliability of the Lateral Dominance Examination. Journal of clinical and experimental neuropsychology 15(2), 183–190. [DOI] [PubMed] [Google Scholar]
- Edvardsen J, Torgersen S, Roysamb E, Lygren S, Skre I, Onstad S, Oien PA, 2008. Heritability of bipolar spectrum disorders. Unity or heterogeneity? J Affect Disord 106(3), 229–240. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS, 1997. Structured Clinical Interview for DSM-IV Axis II Personality Disorders, (SCID-II). American Psychiatric Press, Inc., Washington, D.C. [Google Scholar]
- Fortgang RG, Hultman CM, Cannon TD, 2016a. Coping Styles in Twins Discordant for Schizophrenia, Bipolar Disorder, and Depression. Clinical psychological science : a journal of the Association for Psychological Science 4(2), 216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortgang RG, Hultman CM, van Erp TG, Cannon TD, 2016b. Multidimensional assessment of impulsivity in schizophrenia, bipolar disorder, and major depressive disorder: testing for shared endophenotypes. Psychological medicine 46(7), 1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannelius U, Gherman L, Makela VV, Lindstedt A, Zucchelli M, Lagerberg C, Tybring G, Kere J, Lindgren CM, 2007. Large-scale zygosity testing using single nucleotide polymorphisms. Twin Res Hum Genet 10(4), 604–625. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO, 1991. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hibar DP, Westlye LT, Doan NT, Jahanshad N, Cheung JW, Ching CRK, Versace A, Bilderbeck AC, Uhlmann A, Mwangi B, Kramer B, Overs B, Hartberg CB, Abe C, Dima D, Grotegerd D, Sprooten E, Boen E, Jimenez E, Howells FM, Delvecchio G, Temmingh H, Starke J, Almeida JRC, Goikolea JM, Houenou J, Beard LM, Rauer L, Abramovic L, Bonnin M, Ponteduro MF, Keil M, Rive MM, Yao N, Yalin N, Najt P, Rosa PG, Redlich R, Trost S, Hagenaars S, Fears SC, Alonso-Lana S, van Erp TGM, Nickson T, Chaim-Avancini TM, Meier TB, Elvsashagen T, Haukvik UK, Lee WH, Schene AH, Lloyd AJ, Young AH, Nugent A, Dale AM, Pfennig A, McIntosh AM, Lafer B, Baune BT, Ekman CJ, Zarate CA, Bearden CE, Henry C, Simhandl C, McDonald C, Bourne C, Stein DJ, Wolf DH, Cannon DM, Glahn DC, Veltman DJ, Pomarol-Clotet E, Vieta E, Canales-Rodriguez EJ, Nery FG, Duran FLS, Busatto GF, Roberts G, Pearlson GD, Goodwin GM, Kugel H, Whalley HC, Ruhe HG, Soares JC, Fullerton JM, Rybakowski JK, Savitz J, Chaim KT, Fatjo-Vilas M, Soeiro-de-Souza MG, Boks MP, Zanetti MV, Otaduy MCG, Schaufelberger MS, Alda M, Ingvar M, Phillips ML, Kempton MJ, Bauer M, Landen M, Lawrence NS, van Haren NEM, Horn NR, Freimer NB, Gruber O, Schofield PR, Mitchell PB, Kahn RS, Lenroot R, Machado-Vieira R, Ophoff RA, Sarro S, Frangou S, Satterthwaite TD, Hajek T, Dannlowski U, Malt UF, Arolt V, Gattaz WF, Drevets WC, Caseras X, Agartz I, Thompson PM, Andreassen OA, 2018. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Molecular psychiatry 23(4), 932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higier RG, Jimenez AM, Hultman CM, Borg J, Roman C, Kizling I, Larsson H, Cannon TD, 2014. Enhanced neurocognitive functioning and positive temperament in twins discordant for bipolar disorder. The American journal of psychiatry 171(11), 1191–1198. [DOI] [PubMed] [Google Scholar]
- Hilker R, 2015. The heritability of schizophrenia spectrum disorders - a Danish twin register study, Center for Neuropsychiatric Schizophrenia Research (CNSR) and Center for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS) Psychiatric Center Glostrup, Denmark: Univeristy of Copenhagen, Denmark. [Google Scholar]
- Holmes TH, Rahe RH, 1967. The Social Readjustment Rating Scale. Journal of psychosomatic research 11(2), 213–218. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, van Baal GC, Schnack HG, Brans RG, van der Schot AC, Brouwer RM, van Haren NE, Lepage C, Collins DL, Evans AC, Boomsma DI, Nolen W, Kahn RS, 2012. Overlapping and segregating structural brain abnormalities in twins with schizophrenia or bipolar disorder. Archives of general psychiatry 69(4), 349–359. [DOI] [PubMed] [Google Scholar]
- Johansson V, Jakobsson J, Fortgang RG, Zetterberg H, Blennow K, Cannon TD, Hultman CM, Wetterberg L, Landen M, 2017. Cerebrospinal fluid microglia and neurodegenerative markers in twins concordant and discordant for psychotic disorders. European archives of psychiatry and clinical neuroscience 267(5), 391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson V, Nybom R, Wetterberg L, Hultman CM, Cannon TD, Johansson AG, Ekman CJ, Landen M, 2012. Microscopic particles in two fractions of fresh cerebrospinal fluid in twins with schizophrenia or bipolar disorder and in healthy controls. PloS one 7(9), e45994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegel ME, Johansson V, Wetterberg L, Bhat M, Schwieler L, Cannon TD, Schuppe-Koistinen I, Engberg G, Landen M, Hultman CM, Erhardt S, 2017. Kynurenic acid and psychotic symptoms and personality traits in twins with psychiatric morbidity. Psychiatry research 247, 105–112. [DOI] [PubMed] [Google Scholar]
- Kieseppa T, Partonen T, Haukka J, Kaprio J, Lonnqvist J, 2004. High concordance of bipolar I disorder in a nationwide sample of twins. The American journal of psychiatry 161(10), 1814–1821. [DOI] [PubMed] [Google Scholar]
- Kieseppa T, Partonen T, Kaprio J, Lonnqvist J, 2000. Accuracy of register- and record-based bipolar I disorder diagnoses in Finland; a study of twins. Acta neuropsychiatrica 12(3), 106–109. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM, 2009. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet 373(9659), 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO, 2011. External review and validation of the Swedish national inpatient register. BMC public health 11, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson PK, Almqvist C, Rahman I, Ganna A, Viktorin A, Walum H, Halldner L, Lundstrom S, Ullen F, Langstrom N, Larsson H, Nyman A, Gumpert CH, Rastam M, Anckarsater H, Cnattingius S, Johannesson M, Ingelsson E, Klareskog L, de Faire U, Pedersen NL, Lichtenstein P, 2013. The Swedish Twin Registry: establishment of a biobank and other recent developments. Twin Res Hum Genet 16(1), 317–329. [DOI] [PubMed] [Google Scholar]
- McGrath J, Saha S, Chant D, Welham J, 2008. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiologic reviews 30, 67–76. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC, 2007. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Archives of general psychiatry 64(5), 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobarrez F, Nybom R, Johansson V, Hultman CM, Wallen H, Landen M, Wetterberg L, 2013. Microparticles and microscopic structures in three fractions of fresh cerebrospinal fluid in schizophrenia: case report of twins. Schizophrenia research 143(1), 192–197. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Yucel M, Wood SJ, Velakoulis D, Sun D, Berger G, Stuart GW, Yung A, Phillips L, McGorry PD, 2005. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophrenia bulletin 31(3), 672–696. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES, 1995. Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51(6), 768–774. [DOI] [PubMed] [Google Scholar]
- Pirkola T, Tuulio-Henriksson A, Glahn D, Kieseppa T, Haukka J, Kaprio J, Lonnqvist J, Cannon TD, 2005. Spatial working memory function in twins with schizophrenia and bipolar disorder. Biological psychiatry 58(12), 930–936. [DOI] [PubMed] [Google Scholar]
- Raine A, 1991. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophrenia bulletin 17(4), 555–564. [DOI] [PubMed] [Google Scholar]
- Raucher-Chene D, Achim AM, Kaladjian A, Besche-Richard C, 2017. Verbal fluency in bipolar disorders: A systematic review and meta-analysis. J Affect Disord 207, 359–366. [DOI] [PubMed] [Google Scholar]
- Reitan RM, & Wolfson D, 1985. The Halstead–Reitan Neuropsycholgical Test Battery: Therapy and clinical interpretation. Tucson, AZ: Neuropsychological Press. [Google Scholar]
- Russell EW, 1975. A multiple scoring method for the assessment of complex memory functions. Journal of consulting and clinical psychology 43(6), 800. [Google Scholar]
- Schuster TL, Kessler RC, Aseltine RH Jr., 1990. Supportive interactions, negative interactions, and depressed mood. American journal of community psychology 18(3), 423–438. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW, 2001. A review of MRI findings in schizophrenia. Schizophrenia research 49(1-2), 1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CS, Reilly C, Midkiff K, 1989. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. The Journal of applied psychology 74(5), 728–738. [DOI] [PubMed] [Google Scholar]
- Smoller JW, R. S, Lee PH, Neale B, Nurnberger JI, Santangelo S, Sullivan PF, Perils RH, Purcell SM, Fanous A, Neale MC, Rietschel M, Schulze TG, Thapar A, Anney R, Buitelaar JK, Farone SV, Hoogendijk WJ, Levinson DF, Lesch KP, Riley B, Schachar R, Sonuga-Barke E, Absher D, Agartz I, Akil H, Amin F, Andreassen OA, Anjorin A, Arking D, Asherson P, Azevedo MH, Backlund L, Badner JA, Banaschewski T, Barchas JD, Barnes MR, Bass N, Bauer M, Bellivier F, Bergen SE, Berrettini W, Bettecken T, Biederman J, Binder EB, Black DW, Blackwood DH, Bloss CS, Boehnke M, Boomsma Dl, Breen G, Breuer R, Buccola NG, Bunner WE, Burmeister M, Buxbaum JD, Byerley WF, Sian C, Cantor RM, Chakravarti A, Chambert K, Chicon S, Cloniger CR, Collier DA, Cook E, Coon H, Corvin A, Coryell WH, Craig DW, Craig IW, Curtis D, Czamara D, Daly M, Datta S, Day R, De Geus EJ, Degenhardt F, Devlin B, Srdjan D, Doyle AE, Duan J, Dudbridge F, Edenberg HJ, Elkin A, Etain B, Farmer AE, Ferreira MA, Ferrier IN, Flickinger M, Foroud T, Frank J, Franke B, Fraser C, Freedman R, Freimer NB, Friedl M, Frisén L, Gejman PV, Georgieva L, Gershon ES, Giegling I, Gill M, Gordon SD, Gordon-Smith K, Green EK, Greenwood TA, Gross M, Grozeva D, Guan W, Gurling H, Gustafsson Ó, Hakonarson H, Hamilton SP, Hamshere ML, Hansen TF, Hartmann AM, Hautzinger M, Heath AC, Henders AK, Herms S, Hickie IB, Hipolito M, Hoefels S, Holmans PA, Holsboer F, Hottenga JJ, Hultman CM, Ingason A, Ising M, Jamain S, Jones EG, Jones L, Jones I, Jung-Ying T, Kahler A, Kandaswamy R, Keller MC, Kelsoe JR, Kennedy JL, Kenny E, Kim Y, Kirov GK, Knowles JA, Kohli MA, Koller DL, Konte B, Korszun A, Krasucki R, Kuntsi J, Phoenix K, Landen M, Langstrom N, Lathrop M, Lawrence J, Lawson WB, Leboyer M, Lencz T, Lesch KP, Lewis CM, Li J, Lichtenstein P, Lieberman JA, Lin D, Liu C, Lohoff FW, Loo SK, Lucae S, MacIntyre D, Madden PA, Magnusson P, Mahon PB, Maier W, Malhotra AK, Mattheisen M, Matthews K, Mattingsdal M, McCarroll S, McGhee KA, McGough JJ, McGrath PJ, McGuffin P, McInnis MG, McIntosh A, McKinney R, McClean AW, McMahon FJ, McQuillin A, Medeiros H, Medland SE, Meier S, Melle I, Meng F, Middeldorp CM, Middleton L, Vihra M, Mitchell PB, Montgomery GW, Moran J, Morken G, Morris DW, Moskvina V, Mowry BJ, Muglia P, Mühleisen TW, Muir WJ, Müller-Myhsok B, Myers RM, Nelson SF, Nievergelt CM, Nikolovq I, Nimgaonkar V, Nolen WA, Nöthen MM, Nwulia EA, Nyholt DR, O'Donovan MC, O'Dushlaine C, Oades RD, Olincy A, Olsen L, Ophoff RA, Osby U, Óskarsson H, Owen MJ, Palotie A, Pato MT, Pato CN, Penninx BP, Pergadia ML, Petursson H, Pickard BS, Pimm J, Piven J, Porgeirsson P, Posthuma D, Potash JB, Propping J, Puri V, Quested D, Quinn EM, Rasmussen HB, Raychaudhuri S, Rehnström K, Reif A, Rice J, Rossin L, Rothenberger A, Rouleau G, Ruderfer D, Rujescu D, Sanders AR, Schalling M, Schatzberg AF, Schftner WA, Schellenberg G, Schofield PR, Schork NJ, Schumacher J, Schwarz MM, Scolnick E, Scott LJ, Shi J, Shillling PD, Shyn SI, Sigurdsson E, Silverman JM, Sklar P, Slager SL, Smalley SL, Smit JH, Smith EN, Sonuga-Barke E, St Clair D, State M, Stefansson K, Stefansson H, Steffans M, Steinberg S, Steinhausen HC, Strauss J, Strohmaier J, Stroup TS, Sutcliffe J, Szatmari P, Szelinger S, Thirumalai S, Thompson RC, Tozzi F, Treutlein J, Uhr M, van den Oord EJ, Van Grootheest G, Vieland V, Vincent JB, Visscher PM, Watson SJ, Weissman MM, Werge T, Wienker TF, Willemsen G, Williamson R, Witt SH, Wray NR, Wright A, Xu W, Young AH, Zammit S, Zandi PP, Zhang P, Zitman FG, Zöllner S, Craddock N, Kendler K., 2013. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381(9875), 1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Bergen SE, Kuja-Halkola R, Larsson H, Landen M, Lichtenstein P, 2015. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar disorders 17(2), 184–193. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB, 1992. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Archives of general psychiatry 49(8), 624–629. [DOI] [PubMed] [Google Scholar]
- Stalberg G, Ekerwald H, Hultman CM, 2004. At issue: siblings of patients with schizophrenia: sibling bond, coping patterns, and fear of possible schizophrenia heredity. Schizophrenia bulletin 30(2), 445–458. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Daly MJ, O'Donovan M, 2012. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nature reviews. Genetics 13(8), 537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, Neale MC, 2003. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Archives of general psychiatry 60(12), 1187–1192. [DOI] [PubMed] [Google Scholar]
- Tiffin J, Asher EJ, 1948. The Purdue pegboard; norms and studies of reliability and validity. The Journal of applied psychology 32(3), 234–247. [DOI] [PubMed] [Google Scholar]
- Wechsler D, 1981. WAIS-R : Wechsler adult intelligence scale-revised. New York, N.Y. : Psychological Corporation, [1981] ©1981. [Google Scholar]
- Vilkki J, Virtanen S, Surma-Aho O, Servo A, 1996. Dual task performance after focal cerebral lesions and closed head injuries. Neuropsychologia 34(11), 1051–1056. [DOI] [PubMed] [Google Scholar]
- Williams JB, 1988. A structured interview guide for the Hamilton Depression Rating Scale. Archives of general psychiatry 45(8), 742–747. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A rating scale for mania: reliability, validity and sensitivity. The British journal of psychiatry : the journal of mental science 133, 429–435. [DOI] [PubMed] [Google Scholar]
- Zheutlin AB, Viehman RW, Fortgang R, Borg J, Smith DJ, Suvisaari J, Therman S, Hultman CM, Cannon TD, 2016. Cognitive endophenotypes inform genome-wide expression profiling in schizophrenia. Neuropsychology 30(1), 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M, Link K, 1968. Construct validity for the sensation-seeking scale. J Consult Clin Psychol 32(4), 420–426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.