Abstract
Although prior research has examined how early adversity and chronic stress exposure relate to hypothalamic-pituitary-adrenal (HPA) axis responses to acute stress, to date, no studies have examined how stressors occurring over the entire lifespan predict such responses. To address this issue, we recruited 61 healthy young adults and measured their exposure to 55 different types of acute life events and chronic difficulty occurring over the lifespan. In addition, we characterized differences in participants’ HPA axis responses to acute stress by measuring their salivary cortisol and DHEA responses to the Trier Social Stress Test for Groups. Greater cumulative stress exposure was associated with a blunted cortisol response, but a heightened DHEA response, to the acute stressor. Moreover, it was participants’ exposure to these stressors (i.e., lifetime count), not their perceived severity, which predicted their cortisol and DHEA responses to acute stress. Furthermore, differential effects were observed by stress exposure domain. Notably, only adulthood and marital/partner stressors significantly predicted cortisol responses to acute stress, whereas stress was more uniformly associated with DHEA responses to the acute stressor. These results thus reveal how cumulative stress exposure is associated with HPA axis responsivity to acute stress, while highlighting the fact that different stressors may have substantially different associations with these biological outcomes.
Keywords: cumulative life stress, cortisol, DHEA, allostatic load, health, disease
Introduction
Numerous theories have proposed that acute life events and chronic difficulties occurring across the lifespan can exert a cumulative effect on health (e.g., Boyce & Ellis, 2005; Lupien, McEwen, Gunnar, & Heim, 2009). Cfigonsistent with these theories, a few studies have shown strong associations between cumulative stress exposure and various health-related outcomes, including mental and physical health problems, sleep difficulties, and executive function (Seo et al., 2014; Slavich & Shields, 2018). Cumulative stress exposure shares some features with other indices of stress—such as early adversity and chronic stress—but a critical difference is that cumulative stress exposure includes all of the stressors that a person has experienced throughout his or her entire lifespan. Consequently, cumulative stress exposure tends to be a better predictor of poor health than either early adversity or chronic stress measured alone (Slavich & Shields, 2018). Given the paucity of studies that have actually assessed cumulative stress exposure, though, the biological mechanisms through which cumulative stress exposure affects health remain unclear. To address this issue, we examined whether cumulative life stress exposure is associated with differences in hypothalamic-pituitary-adrenal (HPA) axis responses to acute stress, which has been proposed as a possible mechanism linking stress and health (Boyce & Ellis, 2005).
Cumulative Stress Exposure: Key Conceptual and Measurement Issues
Major life stressors come in many different forms and often have a significant impact on human health (Epel et al., 2018; Shields & Slavich, 2017). These stressors are relatively uncommon, objectively occurring life events and difficulties that cause substantial cognitive upheaval (Slavich, 2016), and they thus differ from daily hassles or perceived stress, which refer to stress related to common day-to-day occurrences or subjective perceptions of stress, respectively. Some of the classifications for major life stress are time dependent (i.e., all stressors occurring over a particular timeframe) without reference to the types of stressors experienced. Common time-dependent stress classifications include recent life stress (i.e., stressors experienced over a recent time period, such as the past two weeks, month, or year) and early adversity (i.e., stressors experienced before age 18)—although in many studies, early adversity refers to childhood-specific stressors, such as abuse by caregivers.
Other classifications for major life stressors are type dependent (i.e., stressors of a particular type, regardless of when they occurred). Common type-dependent stress classifications include acute life events (i.e., time-limited stressors with a clearly defined beginning and endpoint, such as being robbed) and chronic difficulties (i.e., stressors that persist over time without a clearly defined endpoint, such as caring for a spouse with dementia; often referred to as chronic stress). As might already be clear, it is possible for a particular set of stressors studied to contain both type- and time-dependent elements, such as currently ongoing chronic stressors.
Substantial research on stress has been devoted to understanding the health-related effects of these various types of life stress exposure, and this research has been extremely fruitful. For example, a number of studies have found that the more acute life events one experiences, the more likely one is to develop stress-related health problems (e.g., Turner & Lloyd, 1995). In addition, particular life events may be more important for the onset of depression than others (Slavich, Thornton, Torres, Monroe, & Gotlib, 2009). Similarly, greater recent life stress has been associated with worse cognitive function (Shields et al., 2017), and chronic stress predicts accelerated biological aging (Epel et al., 2004).
Another classification for major life stress that has existed in theory for some time (e.g., McEwen, 1998), but which has received very little empirical attention (e.g., Shields & Slavich, 2017), is cumulative stress exposure, defined as the total sum of all acute life events and chronic difficulties that a person has experienced over his or her entire lifespan. This concept draws from acute life events research, which has long found that a greater number of stressful life events experienced over the entire lifespan predicts negative health outcomes (e.g., Turner & Lloyd, 1995). However, this research is often restricted in terms of the number of acute life events assessed, and it ignores chronic difficulties. As a result, although the concept of cumulative stress exposure plays a central role in many theories of stress and health, such as allostatic load (McEwen, 1998; McEwen & Gianaros, 2011), as discussed elsewhere (Slavich & Shields, 2018), very few studies have actually assessed cumulative life stress exposure, due largely to the fact that no instrument has existed for systematically assessing all of the acute life events and chronic difficulties that a person has experienced over his or her life.
The recent development of a validated and reliable measure of cumulative life stress exposure, called the Stress and Adversity Inventory for Adults (i.e., Adult STRAIN; Slavich & Shields, 2018), has addressed this issue by providing researchers with an efficient instrument for assessing all of the acute life events and chronic difficulties that a person has experienced over the lifespan. The STRAIN is a strong predictor of numerous stress-related health outcomes, including poor mental and physical health, cancer-related fatigue, doctor-diagnosed stress-related health problems (e.g., hypertension) and autoimmune disorders, and worse memory and executive function (Bower, Crosswell, & Slavich, 2014; Goldfarb, Shields, Daw, Slavich, & Phelps, 2017; Shields, Moons, & Slavich, 2017; Slavich & Shields, 2018). To date, however, only one study has examined associations between the STRAIN and any biological endpoint (e.g., diurnal cortisol levels; Cuneo et al., 2017), and no studies have examined the important question of how the STRAIN relates to biological responses to stress, which is thought to shape human health.
Stress and the HPA Axis Response
Several systems, including the sympathetic-adrenal-medullary axis and immune system, play important roles in the human stress response, but none have been as extensively studied as the HPA axis. Moreover, the HPA axis’s strong contribution to stress-related health problems makes it an important system to understand (Silverman & Sternberg, 2012). When stressed, the body initiates a cascade of neuroendocrine events that ultimately results in the adrenal glands upregulating the synthesis and release of the catabolic hormone, cortisol, and its indirectly anabolic counterpart, dehydroepiandrosterone (DHEA). These hormones work together to regulate processes such as glucose metabolism and innate immune system activity to prepare the body to handle the energetic and injury-related demands of stressors (Buford & Willoughby, 2008; Kalimi et al., 1994; Silverman & Sternberg, 2012).
Cortisol and DHEA also regulate each other, with DHEA helping to buffer the detrimental effects of cortisol (Kalimi et al., 1994; Maninger, Wolkowitz, Reus, Epel, & Mellon, 2009). Importantly, HPA axis dysregulation in the form of abnormal cortisol and/or DHEA responses to stress has been implicated in numerous physical and mental health problems (Buske-Kirschbaum, Ebrecht, & Hellhammer, 2010; Kamin & Kertes, 2017; Maninger et al., 2009). For example, blunted HPA axis responses to stress may fail to adequately suppress immune system responses to stress, eventually contributing to a sustained state of inflammation that increases a person’s susceptibility to inflammation-related diseases (Silverman & Sternberg, 2012). Consequently, aberrant cortisol and/or DHEA responses to acute stress may represent one key mechanism through which cumulative lifetime stress exposure influences health.
The bulk of prior research examining associations between HPA axis function and health has focused on cortisol (e.g., Silverman & Sternberg, 2012). However, HPA axis functioning is complex, and numerous interactive effects of the HPA axis-governed hormones cortisol and DHEA are relevant for health. For example, although cortisol is primarily anti-inflammatory (Shields & Slavich, 2017), DHEA exerts both anti-glucocorticoid (Kalimi et al., 1994) and anti-inflammatory effects (Maninger et al., 2009), culminating in complex effects on immune system activity (Prall, Larson, & Muehlenbein, 2017). Importantly, relatively lower basal levels of DHEA to cortisol are implicated in some psychiatric disorders (for reviews, see Maninger et al., 2009; Walker et al., 2017). Additionally, DHEA responses to acute stress are associated with better cognitive function following stress (Shields, Lam, Trainor, & Yonelinas, 2016) and are blunted in depressed individuals (Jiang et al., 2017). Because of these and other reasons, DHEA has been proposed as a mechanism underpinning biological resilience to stress (Charney, 2004; Feder, Nestler, & Charney, 2009; Pfau & Russo, 2015; Maninger et al., 2009; Walker et al., 2017). As such, examining associations between life stress exposure and DHEA responses to acute stress may not only help provide a more complete picture of how life stress exposure shapes HPA axis function, it may also help elucidate an additional mechanism through which life stress impacts health (Shields & Slavich, 2017).
Life Stress and Its Effects on the HPA Axis
Given the importance of proper HPA axis function for human health, it might be expected that many studies have examined how life stress exposure is associated with differences in HPA axis responses to an acute stressor. However, although many studies have examined how early adversity (i.e., stressors experienced before age 18) and chronic stress (stressful circumstances that persist over time) are associated with differences in HPA axis responses to acute stress, to our knowledge, no studies have examined associations between cumulative life stress exposure and HPA axis responses to acute stress.
As described above, early adversity differs from cumulative stress exposure in several important ways—for example, early adversity does not include adulthood stressors, and in practice, the assessment of early adversity is often restricted to childhood-specific stressors such as abuse by a caregiver. Nevertheless, prior studies on early adversity and HPA axis responses provide useful background information for the present investigation and, indeed, many studies have been conducted on this topic. As summarized in a recent meta-analysis of 30 studies and more than 4,200 individuals, early adversity is associated with blunted cortisol responses to acute stress (Bunea, Szentágotai-Tătar, & Miu, 2017). We are not aware of any studies that have examined how early adversity relates to DHEA responses to acute stress. Therefore, if cumulative stress exposure has similar effects on the HPA axis as early adversity, we would expect greater cumulative stress exposure to be associated with blunted cortisol responses to acute stress.
Similarly, for reasons outlined in a prior section, the constructs of cumulative stress exposure and chronic stress exposure differ in important ways—for example, chronic stress does not include acute life events, and in practice, the assessment of chronic stress is often restricted to a relatively narrow time period, such as ongoing chronic stressors or those occurring during the past year; cf. Hammen et al., 1987). Nevertheless, prior studies of chronic stress exposure may provide useful information for developing hypotheses regarding the effects of cumulative stress exposure on HPA axis responses. In this context, studies have shown that greater chronic stress is associated with blunted acute stress-induced increases in both cortisol and DHEA-S, which is a metabolite of DHEA (e.g., Lennartsson, Sjörs, & Jonsdottir, 2015; Siegrist, Klein, & Voigt, 1997; Tomiyama, Dallman, & Epel, 2011). To date, no study has examined associations between chronic stress exposure and DHEA (i.e., as opposed to DHEA-S) responses to acute stress. Nonetheless, if cumulative life stress exposure and chronic stress exposure are related to HPA axis responses in a similar fashion, then we would expect greater cumulative life stress exposure to be associated with blunted cortisol and DHEA responses to acute stress.
Present Study
To address the lack of studies examining associations between cumulative lifetime stress exposure and HPA axis responses to acute stress, we recruited healthy young adults and assessed their cumulative exposure to a variety of stressors that they could have experienced over the entire lifespan. These stressors included both acute life events and chronic difficulties known to affect health (Slavich & Shields, 2018). In addition, we characterized each participant’s salivary cortisol and DHEA responses to an acute laboratory stressor. We chose to study DHEA—rather than its sulfated ester, DHEA-S—for two reasons. First, prior work has shown differential effects of stress-related psychiatric disorders on DHEA and cortisol (e.g., Yehuda, Brand, Golier, & Yang, 2006). Second, unlike DHEA-S, DHEA is not flow-rate dependent (Vining & McGinley, 1987), which is advantageous in this context because stress increases salivary flow rate (Rohleder, Wolf, Maldonado, & Kirschbaum, 2006). Examining DHEA rather than DHEA-S thus reduces one possible source of error variance—namely, measurement error due to imprecision in timing of salivary flow rate.
We examined associations between cumulative lifetime stress exposure and both cortisol and DHEA, including whether the associations observed were similar across different types of stress exposure, as would be predicted by classic theories of stress, or whether these associations differed by the specific types of stress experienced. Based on the research summarized above examining the effects of early adversity and chronic stress on cortisol and DHEA-S responses to acute stress, we hypothesized that greater cumulative stress exposure would be associated with blunted cortisol and DHEA responses to the acute laboratory stressor. Moreover, consistent with a stressor characteristics perspective on stress and health, we hypothesized that associations between cumulative stress exposure and these hormones would differ across the different types of lifetime stress exposure that participants experienced.
Method
Participants
Participants were 61 healthy young adults (36 females) with a mean age of 20.62 (range = 18–54, SD = 5.14), who were recruited from a university community. This was a diverse sample, with 50.0% of participants self-identifying as Asian, 20.4% as White, 13.0% as Hispanic/Latino, 9.3% Other/declined to state, 5.6% as African American/Black, and 1.9% as American Indian/Alaska Native. Individuals were excluded if they had any illness or injury over the past week; a history of diabetes, stroke, or any neurological problems; a current or past diagnosis of posttraumatic stress disorder, or were hospitalized for a psychiatric disorder over the past year; had major sleep disturbances over the past six weeks; or consumed more than eight caffeinated beverages a day. Individuals were also excluded if they were pregnant, nursing, or had taken any medications, illegal drugs, or mood-altering medications over the past two months, or if they had taken oral or injected corticosteroids over the past three months. Participants were instructed not to eat, drink anything besides water, use tobacco, brush their teeth or floss, or engage in any exercise for two hours prior to the study. Compliance with these instructions was assessed, and informed consent was obtained prior to participation. All procedures were pre-approved by the Institutional Review Board.
Cumulative Life Stress Assessment
We assessed participants’ cumulative life stress exposure using the Stress and Adversity Inventory for Adults (Adult STRAIN v1.6; Slavich & Shields, 2018). The STRAIN is an online stress assessment system that measures individuals’ cumulative exposure to 55 different acute and chronic stressors that are known to affect health (see http://www.strainsetup.com). The STRAIN employs a sophisticated interviewing methodology that includes extensive branching logic. If a person indicates that he or she has experienced a particular stressor, the branching logic then provides numerous follow-up questions to assess the frequency, timing, duration, and perceived severity of the reported stressor. As such, the STRAIN provides important information on stressor exposure, the stressfulness of each exposure, and when each exposure occurred. The two main stress variables used in analyses were participants’ cumulative life stressor count, calculated as the sum of the stressor frequencies, and cumulative life stressor severity, calculated as the sum of the perceived severities of all reported stressors. Stressor count, which we often refer to as stress exposure within the manuscript, can range from 0 to 159, and stressor severity can range from 0 to 275. In addition to producing indices of overall lifetime stress exposure, the Adult STRAIN can calculate twenty subscale scores that index a person’s exposure to stressors occurring across two time periods, two stressor types, eleven life domains, and possessing five social-psychological characteristics, as well as twenty subscale scores that index a person’s stressor severity in each of those same categories. The validity of this question set has been previously demonstrated in the context of predicting many different health outcomes, including mental and physical health complaints, sleep difficulties, cognitive impairment, and doctor-diagnosed general health problems and autoimmune disorders (Slavich & Shields, 2018).
Laboratory-based Stress Manipulation
An experience of acute stress was induced with a standard, commonly used laboratory-based stress task, called the Trier Social Stress Test for Groups (TSST-G), which is an extensively validated acute stress induction (von Dawans, Kirschbaum, & Heinrichs, 2011). Instructions appeared on a computer screen informing each participant that the next task would involve giving a three-minute speech describing why the participant was the ideal candidate for a job of that person’s choice. Participants were also told that the speech would take place in front of a panel of evaluators trained in the evaluation of nonverbal behavior. To make the task more self-relevant (von Dawans et al., 2011), the instructions also told each participant to use his or her actual qualifications in the speech, and that he or she must speak for the full three minutes. The instructions further said that a camera would record the speech, and that a panel of three professors from the psychology, sociology, and communication departments would subsequently conduct a video analysis of their performance to identify nonverbal behaviors that distinguish qualified job applicant from unqualified ones. The instructions then told each participant to use a piece of scratch paper to prepare his or her speech for the next ten minutes. The last sentence of the instructions said that there would be “another task” following the speech. These instructions remained on the screen during the rest of this stressor anticipation phase.
After ten minutes had elapsed, the experimenters opened each participant’s cubicle door, removed each participant’s chair and scratch paper from his or her cubicle, and instructed each participant to stand at the door of his or her cubicle. The experimenters then sat out of the participants’ view. The spatial layout of the testing environment ensured that participants could not see each other. Two evaluators then came into the testing room with a self-standing digital video camera and went to each participant’s cubicle one-by-one in an apparently random fashion, though the order was kept consistent across sessions. When the evaluators came to a participant’s cubicle, the evaluators informed that person to begin his or her speech. If a participant stopped speaking once before the full three minutes allotted to his or her speech had elapsed, the evaluators prompted the participant to continue, stating, “You still have some time left. Please continue!” If a participant stopped a second time before the three minutes had elapsed, the evaluators stared in silence at the participant for twenty seconds or until the participant began talking again; if the full twenty seconds of silence elapsed, the evaluators then asked participants scripted stressful questions, such as, “Why can’t you continue talking?” Immediately after each participant finished the speech task, the evaluators went to another participant’s cubicle and instructed that person to begin in the same fashion.
After all of the participants finished their speeches, the evaluators again went to each of the cubicles one-by-one, in an apparently random fashion. Once at a participant’s cubicle, they instructed each participant to count aloud backwards, from a four-digit number by 16s, as quickly and accurately as possible for 120 seconds. Each participant in a given experimental session was given a different four-digit number, although the different four-digit numbers were kept constant across all study sessions. Thirty seconds and seventy seconds after each participant began counting, one of the evaluators instructed him or her to count faster using scripted statements. If the participant verbally paused, counted too slowly, or made an error, the evaluator instructed the participant to restart. After all participants finished the math task, the evaluators left the room, the experimenters returned the participants’ chairs, the participants returned to their computers, and the experimenters closed the participants’ cubicle doors.
Negative Affect
Prior to learning of the laboratory stressor and immediately following the stressor, participants used an unmarked scale, ranging from 1 (Not at All) to 7 (Very Much), to indicate how much they currently felt numerous negative affective states (i.e., afraid, scared, nervous, negative, distressed, angry, ashamed, disinterested, frustrated, sad, and down). The scores were then averaged to create a negative affect composite. Negative affect was assessed at baseline (α = .87) and immediately after the stress manipulation (α = .93).
Cortisol and DHEA
Participants provided saliva samples using a passive drool method according to the sampling schedule described below. The saliva vials were immediately placed in a freezer kept at −20ºC until they were assayed in duplicate for cortisol and DHEA using high-sensitivity cortisol and DHEA ELISA kits (Salimetrics LLC, State College, PA), according to the manufacturer instructions. For cortisol, the inter-assay CV was 7.45%, the average intra-assay CV was 2.68%, and sensitivity was 0.007 μg/dL; for DHEA, the inter-assay CV was 2.67%, the average intra-assay CV was 2.59%, and sensitivity was 5 pg/mL. Cortisol values are in the units of μg/dL, and DHEA values are in the units of pg/mL. Although DHEA-S is flow-rate dependent, neither cortisol or DHEA are flow-rate dependent, so we did not adjust for flow rate.
Measures for Sensitivity Analyses
Depressive symptoms.
Participants self-reported depressive symptoms using the Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961). The BDI instructs participants to choose one of four statements that best describes the way they have been feeling during the past week, including the current day, for each of 21 items.
Socioeconomic status.
Participants reported the highest education level their father achieved and the highest education level their mother achieved using the following scale: (1) elementary or junior high school, (2) some high school, (3) graduated high school, (4) some college, (5) graduated college, (6) post-graduate or professional degree, or other/decline to state. Other/decline to state options were discarded, and the numeric values assigned to the scales for father’s and mother’s highest education level were averaged to create an index of family socioeconomic status.
Procedure
Participants came to the laboratory at either 12pm or 3pm for 3-hour sessions with 3–4 participants per group.1 Upon arrival, participants were separated into cubicles, provided informed consent, and completed baseline questionnaires. The first (baseline) saliva sample was taken after all of the participants completed the baseline measures. The stressor lasted 20min (not including the 10min anticipation phase). Participants provided the second saliva sample 10min post-stressor offset (30min after stressor onset). Although most studies of stress responses include more than one post-stressor saliva sample, the peak cortisol response to the TSST-G occurs 10min post-stressor offset (von Dawans et al., 2011). As such, we chose this saliva sample collection time in order to capture the peak cortisol response. Participants then completed filler measures for 90min before finally completing the life stress interview (STRAIN). This delay ensured that the acute stressor did not influence the life stress interview.
To verify the delay worked as intended, a separate sample of 30 participants were run through this paradigm in the TSST-G control condition (von Dawans et al., 2011). These control participants did not differ from the acute stress participants in either cumulative stressor count, p = .675, or cumulative stressor severity, p = .819, indicating that the acute stress manipulation did not influence responses to the life stress interview. We intentionally conducted the life stress interview after the acute stress test to prevent the recall of stressful events and difficulties during the interview from influencing participants’ hormone levels. After the life stress interview, participants were asked if they knew the experimenter, any of the evaluators, or any of the other participants, and if they were familiar with any of the tasks/measures in the study. No participant reported knowing any of the other individuals in the study or familiarity with any of the tasks/measures. Participants were then debriefed, thanked, and dismissed.
Data Reduction and Analysis
Preliminary analyses were conducted using paired t tests and primary analyses were conducted using linear mixed models, nesting participants within sessions in order to account for shared variance due to groups—rather than participants—being randomly assigned to experimental conditions. The natural logarithm transformation was applied when variables evidenced significant skew (i.e., baseline and post-stressor cortisol and DHEA). One participant was excluded from the cortisol analyses due to excessively high baseline cortisol (|Value| > 3 SDs × Mean after log transformation). In graphs and analyses, we describe the responses of these hormones, indexed as the mean residuals from regressing post-stressor values on baseline values (i.e., residual changes from pre- to post-stressor). Due to a moderate correlation between the responses of these two hormones (r = .420, p < .001), we controlled for changes in the other hormone in primary and secondary analyses to isolate the specific associations that cumulative stress exposure had with each hormone’s response. This entailed that the scatterplots depicted in the figures are correlations of residuals (i.e., partial correlations) from models nesting participants within sessions and controlling for the response of the other hormone. The values presented in the figures were standardized to better illustrate the magnitude of associations. Age, sex, and body mass index (BMI) were included in analyses including covariates, given that these factors influence stress-related physiology (Kajantie & Phillips, 2006; O’Connor et al., 2009).
Results
The key demographic and clinical characteristics of the sample are presented in Table 1.
Table 1.
Demographic and Clinical Characteristics of the Sample
| Mean | (SD) | N | (%) | |
|---|---|---|---|---|
| Age | 20.62 | (5.14) | ||
| Sex | ||||
| Female | 36 | (59.0) | ||
| Male | 19 | (31.1) | ||
| Other/Decline to state | 6 | (9.8) | ||
| Race/Ethnicity | ||||
| Asian | 30 | (49.2) | ||
| White | 11 | (18.0) | ||
| Hispanic/Latino | 9 | (14.8) | ||
| Other/Decline to state | 6 | (9.8) | ||
| Black or African American | 3 | (4.9) | ||
| American Indian or Alaska Native | 2 | (3.3) | ||
| Native Hawaiian or Pacific Islander | 0 | (0) | ||
| Highest education level a parent achieved | ||||
| Elementary or junior high school | 3 | (4.9) | ||
| Some high school | 2 | (3.3) | ||
| Graduated high school | 3 | (11.5) | ||
| Some college | 6 | (9.8) | ||
| Graduated college | 10 | (16.4) | ||
| Post-graduate or professional degree | 18 | (29.5) | ||
| Other/Decline to state | 15 | (24.6) | ||
| Body Mass Index | 22.31 | (3.48) | ||
| Beck Depression Inventory Score | 5.49 | (4.32) | ||
| Lifetime Stressor Exposure Count | 19.28 | (10.89) | ||
| Lifetime Stressor Exposure Severity | 44.37 | (24.61) |
Note: highest education level a parent achieved represents the highest level achieved by either parent. Analyses including socioeconomic status, however, used an average of the highest level achieved by both parents.
Preliminary Analyses
As a manipulation check, we first examined whether negative affect, cortisol, and DHEA increased from baseline to post-stressor. Confirming the success of the laboratory stressor, and as described in Table 2, participants increased in negative affect from baseline to post-stressor, t(60) = 3.44, p = .001. Similarly, we found that cortisol levels increased from baseline to post-stressor, t(57) = 5.59, p < .001, and that DHEA levels increased from baseline to post-stressor, t(58) = 4.03, p < .001. Thus, the stress manipulation successfully produced an emotional and physiological stress response.
Table 2.
Descriptive Statistics for the Stress-Responsive Variables
| Baseline | Post-Stressor | |||
|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | |
| Negative Affect | 2.23 | (0.87) | 2.72 | (1.33) |
| Cortisol (nmol/L) | 4.99 | (3.21) | 11.77 | (11.04) |
| DHEA (ug/dL) | 276.66 | (316.88) | 371.97 | (352.17) |
Note: Transformed cortisol and DHEA values were used in all analyses.
Primary Analyses
Next, we tested our primary hypotheses concerning associations of cumulative stress exposure with cortisol and DHEA responses to acute stress. The results are presented below; all models are provided in the Supplementary Material.
Cortisol and DHEA responses.
As hypothesized, greater cumulative stress exposure over the life course was related to a blunted cortisol response to the acute laboratory-based stressor, β = −.25, p = .033 (Figure 1a). Surprisingly, though, greater cumulative stress exposure was associated with a heightened DHEA response to the acute laboratory stressor, β = .32, p < .001 (Figure 1b). Controlling for age, sex, and BMI did not alter these results, as greater cumulative stress exposure remained a significant predictor of both blunted cortisol, β = −.31, p = .030, and heightened DHEA responses, β = .41, p = .003, while adjusting for these factors. In sum, greater cumulative stress exposure predicted heightened DHEA—and blunted cortisol—responses to the acute laboratory stressor, and these associations were robust to statistical adjustment for possible confounds.
Figure 1.
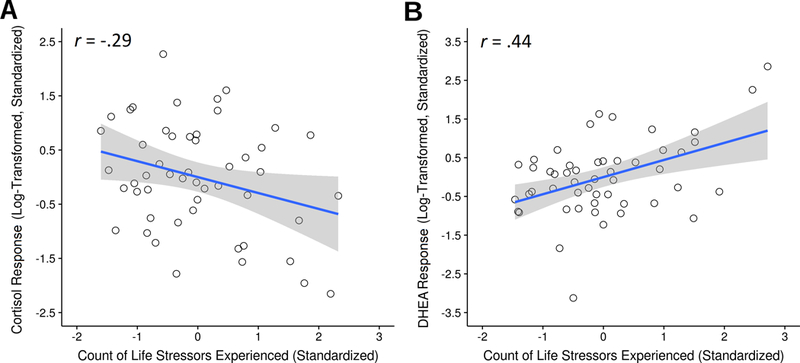
Greater cumulative life exposure was significantly associated with participants’ cortisol and DHEA responses to acute stress. More specifically, greater cumulative stress exposure predicted blunted cortisol but heightened DHEA responses to the acute laboratory-based stress task. Depicted values are standardized to better illustrate the magnitudes of associations.
Secondary Analyses
To further explicate these findings, we conducted three sets of secondary analyses—namely, sensitivity analyses, analyses of lifetime stressor exposure (i.e., count) versus severity, and analyses examining associations with HPA axis responses by stressor type.
Sensitivity analyses.
We examined whether socioeconomic status, race, or depressive symptoms might explain the observed results, given known associations of these variables with life stress exposure. Each model controlled for relevant covariates listed above. In these analyses, socioeconomic status was related to blunted cortisol responses to acute stress, β = −.40, p < .001, and marginally related to heightened DHEA responses to acute stress, β = .18, p = .09, but cumulative lifetime stress exposure remained significantly associated with blunted cortisol responses, β = −.35, p < .001, and heightened DHEA responses, β = .48, p < .001, in these fully adjusted models. Interestingly, race/ethnicity was not related to participants’ cortisol or DHEA responses to acute stress, ps > .165; moreover, in these fully adjusted models, cumulative stress exposure remained a significant predictor of both blunted cortisol, β = −.35, p = .012, and heightened DHEA responses to acute stress, β = .42, p < .001. Finally, depressive symptoms were not significantly related to participants’ cortisol or DHEA responses to acute stress, ps > .069, and in these models, cumulative stress exposure remained a significant predictor of both blunted cortisol, β = −.38, p = .007, and heightened DHEA responses to acute stress, β = .46, p < .001. In sum, socioeconomic status, race/ethnicity, and depressive symptoms did not explain the associations between cumulative life stress exposure and HPA axis responses to acute stress.
Count vs. severity of cumulative stressor exposure.
Next, we examined how cumulative lifetime stressor count versus participants’ experienced severity of those stressors predicted their HPA axis responses to the acute laboratory stressor. We did this by regressing HPA axis responses on both count and severity simultaneously and then comparing the slopes. Cumulative stressor count was a significantly better predictor (β = −.42) of cortisol responses, t(51) = 2.05, p = .045, and a marginally better predictor (β = .36) of DHEA responses, t(51) = 1.86, p = .069, than the perceived severity of those stressors (β = .19 and β = −.05, respectively). Therefore, greater lifetime stress exposure itself, rather than the perceived severity of those exposures, was more predictive of participants’ biological responses to the acute laboratory-based social stressor.
Cumulative stress exposure by stressor type.
Finally, given that the Adult STRAIN provides several ways to decompose cumulative life stress exposure, we next examined associations between several different stressor types and social-psychological characteristics. Although we expected differences across different types of stress, we did not develop a priori hypotheses regarding these differences given the distinct lack of existing data on this topic. As expected, though, associations between cumulative stress exposure and participants’ cortisol responses to the laboratory-based social stressor were not uniform across the different stressor types. Associations between cumulative stress exposure and cortisol responses to the acute stressor were significant only for adulthood stress exposure (β = −.25, p = .029) and marital/partner stress exposure (β = −.27, p = .022), though all types of stress tended to show similar associations (see Figure 2a). In contrast, associations between cumulative lifetime stress exposure and participants’ DHEA responses varied more greatly across the different stressor types. Unlike cortisol, however, most of the cumulative stress exposure categories were significantly related to DHEA responses (see Figure 2b).
Figure 2.
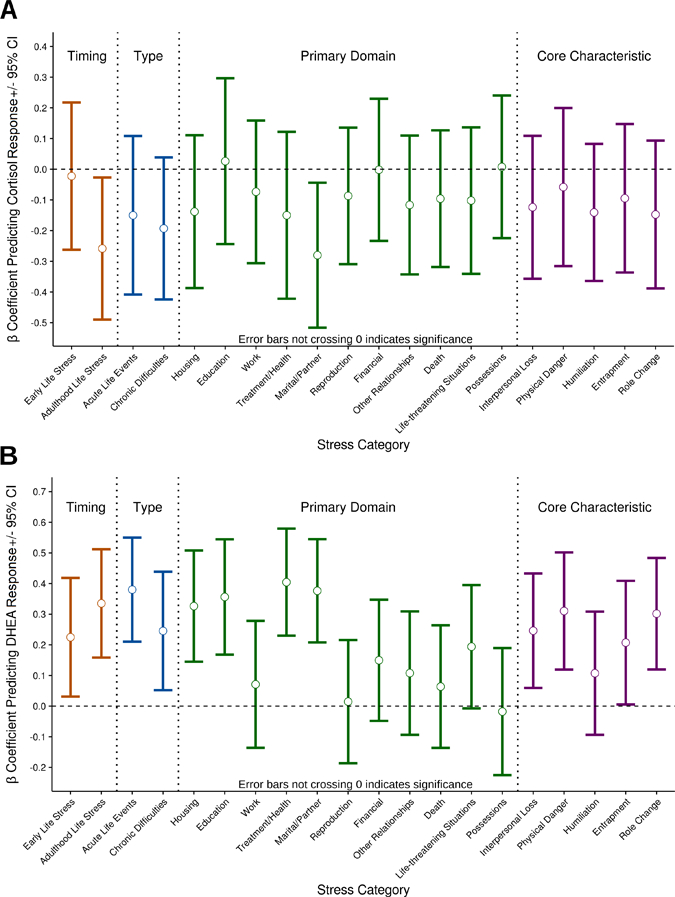
Associations between different types of cumulative lifetime stress exposure and participants’ (A) cortisol responses and (B) DHEA responses to acute stress. Error bars represent 95% confidence intervals; as such, if a bar does not cross zero, it indicates that the coefficient is significant. Associations between cumulative stress exposure and individuals’ cortisol responses were similar across different types of lifetime stress exposure assessed, with the strongest effects being evident for adulthood and marital/partner stressors. In contrast, the effects of cumulative life stress exposure on DHEA responses differed more greatly across the different stressor categories; unlike cortisol, however, most of the stress exposure categories were significantly related to DHEA responses.
Discussion
Despite substantial interest in how life stress affects health, very few studies have assessed lifetime stress exposure. As a result, little is known about how stress exposure occurring over the entire lifespan is associated with cortisol and DHEA responses to acute stress, which may in turn have implications for disease risk and development. We addressed this important issue in the present study by showing that greater cumulative lifetime stress exposure was associated with a blunted cortisol response, but a heightened DHEA response, to an acute laboratory-based social stressor. Moreover, these associations were independent of participants’ socioeconomic status, race/ethnicity, and depressive symptoms. Decomposing these associations revealed that it was participants’ exposure to these stressors, not the perceived severity of such exposures, that was more strongly associated with individuals’ stress-induced cortisol and DHEA responses.
Although prior research has shown that the Adult STRAIN is a strong predictor of numerous health-related outcomes (e.g., Slavich & Shields, 2018), the biological mechanisms underlying these associations have been unclear. The results of the present study begin to address this issue by suggesting that the STRAIN may be associated with these outcomes at least in part through stress-related changes in hormonal activity. Exactly how these stress-related biological effects might lead to poor health remains unknown. Therefore, examining whether alterations in cortisol or DHEA responses to acute stress mediate the effects of lifetime stress exposure on health represents an important avenue for future research.
Our results are consistent with prior work examining associations between chronic stress and cortisol responses, which has found that chronic stress is associated with cortisol responses to acute stress, although the directionality is not always clear (see Miller, Chen, & Zhou, 2007). We found that cumulative stress exposure predicted blunted cortisol responses to acute stress, similar to some findings in the chronic stress literature (e.g., Tomiyama et al., 2011). In contrast, no studies have examined how repeated or chronic stress is associated with DHEA responses to acute stress, though some work has examined effects of repeated or chronic stress on stress-induced DHEA-S responses (e.g., Lennartsson et al., 2015; Maninger, Capitanio, Mason, Ruys, & Mendoza, 2010). Our findings extend this work, showing that greater cumulative stress exposure predicts blunted cortisol but heightened DHEA responses to acute stress.
The finding that cumulative stress exposure was associated with decreased cortisol but increased DHEA responses to stress could be seen as peculiar, given that both hormones are secreted as products or outputs of the HPA axis. However, it is important to note that these hormones are heavily involved in the regulation of each other (Kalimi et al., 1994). Importantly, glucocorticoid administration suppresses DHEA (Kalimi et al., 1994), whereas DHEA administration suppresses cortisol (Wolf et al., 1997). As such, although both DHEA and cortisol production increase in response to adrenocorticotropic hormone (ACTH), to the extent that DHEA increases in response to stress, cortisol is likely to show a relatively lesser increase over time than it would in response to the same initial ACTH stimulation if the DHEA response was lower. This fine-tuned interplay of DHEA and cortisol may be important for understanding disorders with HPA axis abnormalities, as persons with depression, schizophrenia, and post-traumatic stress disorder all show abnormalities in the ratio of cortisol to DHEA (Ritsner et al., 2004; Yehuda et al., 2006; Young, Gallagher, & Porter, 2002). Our results show that cumulative stress exposure may be one factor potentially contributing to alterations in this ratio, though future research is needed to evaluate the relevance of these associations for health.
Consistent with a stressor characteristics perspective (e.g., Slavich, O’Donovan, Epel, & Kemeny, 2010; Slavich et al., 2009), we found that the effects of cumulative stress exposure were not uniform across different stressor types; instead, stressors were differentially associated with participants’ HPA axis responses. Lifetime stress exposure and cortisol associations were relatively consistent and strongest for stressors occurring in adulthood and involving romantic relationships, which is in accord with prior work suggesting that recent social stressors (e.g., rejection by a romantic partner) are particularly strongly related to health outcomes, such as the development of depression (Slavich & Irwin, 2014; Slavich et al., 2009). It is possible, therefore, that stress-related blunting of cortisol responsivity is one mechanisms underlying these effects (see Carroll, Ginty, Whittaker, Lovallo, & de Rooij, 2017). An interesting lack of association, however, was observed between early adversity—which a recent meta-analysis showed is, on average, associated with moderately blunted cortisol reactivity (Bunea et al., 2017)—and cortisol responses to acute stress. One reason for this difference may be that early adversity is often conceptualized as childhood-specific stressors, such as childhood maltreatment (e.g., Carpenter et al., 2007), or all stressors occurring prior to age 13 or 15, whereas the STRAIN considers early adversity as all stressors experienced prior to 18 years of age. Alternatively, it is possible that early adversity may interact with recent stressors to modulate HPA axis function (e.g., Starr et al., 2017).
Associations between the different stressor types and participants’ DHEA responses were more variable than cortisol (see Figure 2), although when compared directly, more of the stress-DHEA associations were statistically significant. Notably, one of the few stressor types not associated with DHEA responses was work-related stress, whereas prior research has found that perceived stress at work (Lennartsson, Theorell, Kushnir, Bergquist, & Jonsdottir, 2013) and occupational burnout (Lennartsson et al., 2015) are associated with blunted DHEA-S responses to acute stress. These differences may be explained by differences between stress type (i.e., the STRAIN assesses objective exposure, whereas perceived stress and burnout are subjective), analyte (DHEA vs. DHEA-S), or sample differences (e.g., young adults who may have lacked much work experience). Nevertheless, the reasons behind these variable associations with DHEA across stressor types are unknown; these data suggest that taking a more fine-grained approach to life stress assessment may be warranted when examining the effects of stress on DHEA.
At the same time, it should be noted that the effects of lifetime stress exposure domains and characteristics are complex and could potentially be moderated by several factors, including timing of stress exposure, total number of stressors experienced, and the temporal ordering of stressors experienced. Therefore, we do not view these stressor characteristics analyses as definitive. Rather, we suggest that these results provide rationale to study different stressor domains and characteristics on a more fine-grained level.
More broadly, these findings provide new information relating to “allostatic load” models of stress and health (e.g., McEwen, 1998), in which cumulative lifetime stress exposure is thought to alter the regulatory dynamics of biological systems that affect health. For example, McEwen (1998) proposed that one form of allostatic load involves an inadequate stress response. Consistent with the idea from allostatic load that the body achieves stability through change, and consistent with the co-regulatory roles of DHEA and cortisol, we found that greater cumulative stress exposure predicted blunted cortisol, but heightened DHEA responses to acute stress.
Although the present data do not address the role this biological response pattern may play in human health, prior research indicates that this pattern may be important for disease risk. In particular, the response pattern we observed (i.e., higher DHEA and lower cortisol) is consistent with baseline differences in the cortisol-to-DHEA ratio between individuals with posttraumatic stress disorder (PTSD) and healthy controls, as individuals with PTSD show heightened DHEA and lowered cortisol levels (Yehuda et al., 2006). However, there may be important health differences between biological responses to stress and basal levels (Tomiyama et al., 2012). Although the effects of cortisol responses to acute stress on health are becoming clearer (e.g., Aguilera, 2011; Tomiyama et al., 2012), to date no study has examined the role that DHEA responses to acute stress play in general mental or physical health. Nonetheless, some studies have found that DHEA administration protects against detrimental health effects of stress exposure (e.g., Hu, Cardounel, Gursoy, Anderson, & Kalimi, 2000). However, it is difficult to integrate the seemingly protective role of DHEA with the finding that DHEA is increased both in general and relative to cortisol in PTSD and depression (Gill, Vythilingam, & Page, 2008; Yehuda et al., 2006); therefore, the health relevance of a stronger DHEA but weaker cortisol response to acute stress is unknown. In sum, future research is needed to examine the role these dynamics play in shaping health.
Several limitations of this study should be noted. First, although we controlled for several person factors and potential health-related confounds, the sample consisted of relatively young, healthy adults, thus limiting the generalizability of these results to other populations. Second, we employed a well validated system for assessing lifetime stress exposure, but future studies could use stress assessment methods that yield independent judgments of stressor count and severity in order to limit the possible influence of reporting biases on the observed associations. Third, our data suggest that different stressors are differentially associated with individual’s biological responses to acute stress, but additional research is needed to replicate these effects and to explain why some stressors are associated with stronger HPA-axis responses than others. Fourth, although we adjusted for a group stress manipulation using linear mixed models that nested participants within sessions, we did not match each group on gender, and it is possible that there may be some interactive effects between the sex composition of the groups and stress that were unaccounted for by our models. At the same time, it should be noted that the gender composition of the groups did not systematically covary with cumulative stress exposure, r = .07, and controlling for the gender composition of groups did not affect our results (data not shown); therefore, this limitation is not responsible for our most important findings. Fifth, we measured cortisol and DHEA at only two timepoints, which differs from much prior work and precluded us from examining stress reactivity in its entirety. This lack of complete information on total HPA axis output in response to the stressor may in fact be responsible for some of the anomalous findings observed, such as the absence of an association with early adversity. Sixth, we did not assess cortisol recovery, which has been associated with chronic stress in prior research (e.g., Matthews, Gump, & Owens, 2001) and may thus show different associations with cumulative stress exposure than what we observed. Finally, although the life stress interview used in this study assesses past stressor exposure, this study was cross-sectional; consequently, it was not possible to ensure that the stressors experienced preceded the alterations in cortisol and DHEA that were observed. Future research could address this limitation by assessing cumulative stress exposure and HPA axis responses to acute stress longitudinally.
Notwithstanding these limitations, this study is one of the first to assess individuals’ cumulative life stress exposure, and it is the first to examine associations between these stress exposure profiles and individuals’ cortisol and DHEA responses to an acute laboratory stressor. We found that greater cumulative stress exposure was associated with blunted cortisol—but heightened DHEA—responses to acute stress, and that these associations were robust to statistical adjustment for possible confounds. These associations were not due to factors such as socioeconomic status or depression. Moreover, these associations were primarily driven by the stressor exposure itself and not by the perceived severity of those exposures. Finally, the present data show that these associations differ by stressor type and timing of exposure. Future research should examine possible mechanisms underlying these associations, replicate these associations in other populations, and examine the relevance of these associations for human health and disease.
Supplementary Material
Acknowledgments
We would like to thank the numerous research assistants who helped collect these data.
Funding
This study was supported by a University of California, Davis Provost’s Undergraduate Fellowship to Jovian C. W. Lam; a University of California, Davis Department of Psychology Summer Grant in Aid of Research, and a University of California, Davis Provost’s Dissertation Year Fellowship to Grant S. Shields; NIH grant R01 MH103322 to Brian C. Trainor; a Society in Science—Branco Weiss Fellowship, NARSAD Young Investigator Grant #23958 from the Brain & Behavior Research Foundation, and NIH grant K08 MH103443 to George M. Slavich; and NIH grant R01 MH059352 to Andrew P. Yonelinas.
Footnotes
Conflict of Interest
The authors have declared that they have no conflicts of interest.
Time of day the study began was unrelated to cortisol or DHEA responses to acute stress (ps > .448), and controlling for it did not affect the results. Similarly, hours since waking was unrelated to cortisol or DHEA responses to acute stress (ps > .466), and controlling for it did not affect the results.
References
- Aguilera G (2011). HPA axis responsiveness to stress: Implications for healthy aging. Experimental Gerontology, 46, 90–95. 10.1016/j.exger.2010.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, & Erbaugh J (1961). An inventory for measuring depression. Archives of General Psychiatry, 4, 561–571. 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- Bower JE, Crosswell AD, & Slavich GM (2014). Childhood adversity and cumulative life stress: Risk factors for cancer-related fatigue. Clinical Psychological Science, 2, 108–115. 10.1177/2167702613496243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, & Ellis BJ (2005). Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology, 17, 271–301. 10.1017/S0954579405050145 [DOI] [PubMed] [Google Scholar]
- Buford TW, & Willoughby DS (2008). Impact of DHEA (S) and cortisol on immune function in aging: A brief review. Applied Physiology, Nutrition, and Metabolism, 33, 429–433. 10.1139/H08-013 [DOI] [PubMed] [Google Scholar]
- Bunea IM, Szentágotai-Tătar A, & Miu AC (2017). Early-life adversity and cortisol response to social stress: A meta-analysis. Translational Psychiatry, 7:1274 10.1038/s41398-017-0032-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Ebrecht M, & Hellhammer DH (2010). Blunted HPA axis responsiveness to stress in atopic patients is associated with the acuity and severeness of allergic inflammation. Brain, Behavior, & Immunity, 24, 1347–1353. 10.1016/j.bbi.2010.06.013 [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Price LH (2007). Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry, 62, 1080–1087. 10.1016/j.biopsych.2007.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D, Ginty AT, Whittaker AC, Lovallo WR, & de Rooij SR (2017). The behavioural, cognitive, and neural corollaries of blunted cardiovascular and cortisol reactions to acute psychological stress. Neuroscience & Biobehavioral Reviews, 77, 74–86. 10.1016/j.neubiorev.2017.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS (2004). Psychobiological mechanisms of resilience and vulnerability. American Journal of Psychiatry, 161, 195–216. 10.1176/foc.2.3.368 [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, Kaplan Z (2006). Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biological Psychiatry, 59, 1208–1218. 10.1016/j.biopsych.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Cuneo MG, Schrepf A, Slavich GM, Thaker PH, Goodheart M, Bender D, Cole SW, Sood AK, & Lutgendorf SK (2017). Diurnal cortisol rhythms, fatigue and psychosocial factors in five-year survivors of ovarian cancer. Psychoneuroendocrinology, 84, 139–142. 10.1016/j.psyneuen.2017.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, & Cawthon RM (2004). Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences of the United States of America, 101, 17312–17315. 10.1073/pnas.0407162101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Crosswell AD, Mayer SE, Prather AA, Slavich GM, Puterman E, & Mendes WB (2018). More than a feeling: A unified view of stress measurement for population science. Frontiers in Neuroendocrinology, 49, 146–169. 10.1016/j.yfrne.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, & Charney DS (2009). Psychobiology and molecular genetics of resilience. Nature Reviews Neuroscience, 10, 446–457. 10.1038/nrn2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J, Vythilingam M, & Page GG (2008). Low cortisol, high DHEA, and high levels of stimulated TNF‐α, and IL‐6 in women with PTSD. Journal of Traumatic Stress, 21, 530–539. 10.1002/jts.20372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb EV, Shields GS, Daw ND, Slavich GM, & Phelps EA (2017). Low lifetime stress exposure is associated with reduced stimulus–response memory. Learning & Memory, 24, 162–168. 10.1101/lm.045179.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, Adrian C, Gordon D, Burge D, Jaenicke C & Hiroto D (1987). Children of depressed mothers: Maternal strain and symptom predictors of dysfunction. Journal of Abnormal Psychology, 96, 190–198. 10.1037/0021-843X.96.3.190 [DOI] [PubMed] [Google Scholar]
- Hu Y, Cardounel A, Gursoy E, Anderson P, & Kalimi M (2000). Anti-stress effects of dehydroepiandrosterone: Protection of rats against repeated immobilization stress-induced weight loss, glucocorticoid receptor production, and lipid peroxidation. Biochemical Pharmacology, 59, 753–762. 10.1016/S0006-2952(99)00385-8 [DOI] [PubMed] [Google Scholar]
- Jiang X, Zhong W, An H, Fu M, Chen Y, Zhang Z, & Xiao Z (2017). Attenuated DHEA and DHEA-S response to acute psychosocial stress in individuals with depressive disorders. Journal of Affective Disorders, 215, 118–124. 10.1016/j.jad.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Kajantie E, & Phillips DI (2006). The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology, 31, 151–178. 10.1016/j.psyneuen.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Kalimi M, Shafagoj Y, Loria R, Padgett D, & Regelson W (1994). Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA). Molecular and Cellular Biochemistry, 131, 99–104. 10.1007/BF00925945 [DOI] [PubMed] [Google Scholar]
- Kamin HS, & Kertes DA (2017). Cortisol and DHEA in development and psychopathology. Hormones and Behavior, 89, 69–85. 10.1016/j.yhbeh.2016.11.018. [DOI] [PubMed] [Google Scholar]
- Lennartsson AK, Sjörs A, & Jonsdottir IH (2015). Indication of attenuated DHEA-S response during acute psychosocial stress in patients with clinical burnout. Journal of Psychosomatic Research, 79, 107–111. 10.1016/j.jpsychores.2015.05.011 [DOI] [PubMed] [Google Scholar]
- Lennartsson AK, Theorell T, Kushnir MM, Bergquist J, & Jonsdottir IH (2013). Perceived stress at work is associated with attenuated DHEA-S response during acute psychosocial stress. Psychoneuroendocrinology, 38, 1650–1657. 10.1016/j.psyneuen.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10, 434–445. 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Maninger N, Capitanio JP, Mason WA, Ruys JD, & Mendoza SP (2010). Acute and chronic stress increase DHEAS concentrations in rhesus monkeys. Psychoneuroendocrinology, 35, 1055–1062. 10.1016/j.psyneuen.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, & Mellon SH (2009). Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Frontiers in Neuroendocrinology, 30, 65–91. 10.1016/j.yfrne.2008.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Gump BB, & Owens JF (2001). Chronic stress influences cardiovascular and neuroendocrine responses during acute stress and recovery, especially in men. Health Psychology, 20, 403–410. 10.1037/0278-6133.20.6.403 [DOI] [PubMed] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ (2011). Stress- and allostasis-induced brain plasticity. Annual Review of Medicine, 62, 431–445. 10.1146/annurev-med-052209-100430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Zhou ES (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133, 25–45. 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Monroe SM, Slavich GM, Torres LD, & Gotlib IH (2007). Major life events and major chronic difficulties are differentially associated with history of major depressive episodes. Journal of Abnormal Psychology, 116, 116–124. 10.1037/0021-843X.116.1.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME,. Thomas KS (2009). To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain, Behavior, and Immunity, 23, 887–897. 10.1016/j.bbi.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau ML, & Russo SJ (2015). Peripheral and central mechanisms of stress resilience. Neurobiology of Stress, 1, 66–79. 10.1016/j.ynstr.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prall SP, Larson EE, & Muehlenbein MP (2017). The role of dehydroepiandrosterone on functional innate immune responses to acute stress. Stress and Health, 33, 656–664. 10.1002/smi.2752 [DOI] [PubMed] [Google Scholar]
- Ritsner M, Maayan R, Gibel A, Strous RD, Modai I, & Weizman A (2004). Elevation of the cortisol/dehydroepiandrosterone ratio in schizophrenia patients. European Neuropsychopharmacology, 14, 267–273. 10.1016/j.euroneuro.2003.09.003 [DOI] [PubMed] [Google Scholar]
- Rohleder N, Wolf JM, Maldonado EF, & Kirschbaum C (2006). The psychosocial stress‐induced increase in salivary alpha‐amylase is independent of saliva flow rate. Psychophysiology, 43, 645–652. 10.1111/j.1469-8986.2006.00457.x [DOI] [PubMed] [Google Scholar]
- Seo D, Tsou KA, Ansell EB, Potenza MN, & Sinha R (2014). Cumulative adversity sensitizes neural response to acute stress: Association with health symptoms. Neuropsychopharmacology, 39, 670–680. 10.1038/npp.2013.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist J, Klein D, & Voigt KH (1997). Linking sociological with physiological data: The model of effort-reward imbalance at work. Acta Physiologica Scandinavica Supplementum, 640, 112–116. [PubMed] [Google Scholar]
- Silverman MN, & Sternberg EM (2012). Glucocorticoid regulation of inflammation and its functional correlates: From HPA axis to glucocorticoid receptor dysfunction. Annals of New York Academy of Sciences, 1261, 55–63. 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Doty D, Shields RH, Gower G, Slavich GM, & Yonelinas AP (2017). Recent life stress exposure is associated with poorer long-term memory, working memory, and self-reported memory. Stress, 20, 598–607. 10.1080/10253890.2017.1380620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Lam JCW, Trainor BC, & Yonelinas AP (2016). Exposure to acute stress enhances decision-making competence: Evidence for the role of DHEA. Psychoneuroendocrinology, 67, 51–60. 10.1016/j.psyneuen.2016.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Moons WG, & Slavich GM (2017). Better executive function under stress mitigates the effects of recent life stress exposure on health in young adults. Stress, 20, 92–102. 10.1080/10253890.2017.1286322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, & Slavich GM (2017). Lifetime stress exposure and health: A review of contemporary assessment methods and biological mechanisms. Social and Personality Psychology Compass, 11:8 10.1111/spc3.12335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM (2016). Life stress and health: A review of conceptual issues and recent findings. Teaching of Psychology, 43, 346–355. 10.1177/0098628316662768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, & Irwin MR (2014). From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin, 140, 774–815. 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, O’Donovan A, Epel ES, & Kemeny ME (2010). Black sheep get the blues: A psychobiological model of social rejection and depression. Neuroscience and Biobehavioral Reviews, 35, 39–45. 10.1016/j.neubiorev.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, & Shields GS (2018). Assessing lifetime stress exposure using the Stress and Adversity Inventory for Adults (Adult STRAIN): An overview and initial validation. Psychosomatic Medicine, 80, 17–27. 10.1097/PSY.0000000000000534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Thornton T, Torres LD, Monroe SM, & Gotlib IH (2009). Targeted rejection predicts hastened onset of major depression. Journal of Social and Clinical Psychology, 28, 223–243. 10.1521/jscp.2009.28.2.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr LR, Dienes K, Stroud CB, Shaw ZA, Li YI, Mlawer F, & Huang M (2017). Childhood adversity moderates the influence of proximal episodic stress on the cortisol awakening response and depressive symptoms in adolescents. Development and Psychopathology, 29, 1877–1893. 10.1017/S0954579417001468 [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, Dallman MF, & Epel ES (2011). Comfort food is comforting to those most stressed: Evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology, 36, 1513–1519. 10.1016/j.psyneuen.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama AJ, O’Donovan A, Lin J, Puterman E, Lazaro A, Chan J, Epel E (2012). Does cellular aging relate to patterns of allostasis? An examination of basal and stress reactive HPA axis activity and telomere length. Physiology & Behavior, 106, 40–45. 10.1016/j.physbeh.2011.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RJ, & Lloyd DA (1995). Lifetime traumas and mental health: The significance of cumulative adversity. Journal of Health and Social Behavior, 360–376. 10.2307/2137325 [DOI] [PubMed] [Google Scholar]
- Vining RF, & McGinley RA (1987). The measurement of hormones in saliva: Possibilities and pitfalls. Journal of Steroid Biochemistry, 27, 81–94. 10.1016/0022-4731(87)90297-4 [DOI] [PubMed] [Google Scholar]
- von Dawans B, Kirschbaum C, & Heinrichs M (2011). The Trier Social Stress Test for Groups (TSST-G): A new research tool for controlled simultaneous social stress exposure in a group format. Psychoneuroendocrinology, 36, 514–522. 10.1016/j.psyneuen.2010.08.004 [DOI] [PubMed] [Google Scholar]
- Walker FR, Pfingst K, Carnevali L, Sgoifo A, & Nalivaiko E (2017). In the search for integrative biomarker of resilience to psychological stress. Neuroscience & Biobehavioral Reviews, 74, 310–320. 10.1016/j.neubiorev.2016.05.003 [DOI] [PubMed] [Google Scholar]
- Wolf OT, Köster B, Kirschbaum C, Pietrowsky R, Kern W, Hellhammer DH, Fehm HL (1997). A single administration of dehydroepiandrosterone does not enhance memory performance in young healthy adults, but immediately reduces cortisol levels. Biological Psychiatry, 42, 845–848. 10.1016/S0006-3223(97)00323-5 [DOI] [PubMed] [Google Scholar]
- Yehuda R, Brand SR, Golier JA, & Yang RK (2006). Clinical correlates of DHEA associated with post‐traumatic stress disorder. Acta Psychiatrica Scandinavica, 114, 187–193. 10.1111/j.1600-0447.2006.00801.x [DOI] [PubMed] [Google Scholar]
- Young AH, Gallagher P, & Porter RJ (2002). Elevation of the cortisol-dehydroepiandrosterone ratio in drug-free depressed patients. American Journal of Psychiatry, 159, 1237–1239. 10.1176/appi.ajp.159.7.1237 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


