Abstract
BACKGROUND AND AIMS:
Autoimmune hepatitis (AIH) is an important cause of severe liver disease and is associated with both quantitative and qualitative regulatory T cell (Treg) impairments. We have shown that Tregs express CD39, an ectonucleotidase responsible for extracellular nucleotide hydrolysis, culminating in the production of immunosuppressive adenosine. In this study, we describe multiple CD39pos Treg defects that potentially contribute to the impaired immuno regulation that is characteristic of AIH.
METHODS:
We have examined the frequency and phenotype of CD39pos Tregs by flow cytometry and measured their ectonucleotidase activity. The capacity of CD4posCD25high, CD4posCD25highCD39pos and CD4posCD25highCD39neg subsets to suppress both proliferation of effector T cells and IL17 production was evaluated.
RESULTS:
In AIH, CD39pos Tregs are decreased in frequency, exhibit limited adenosine triphosphate (ATP)/adenosine diphosphate (ADP) hydrolysis activity and fail to suppress IL17 production by effector CD4 T cells. Moreover, these CD39pos Tregs display a more pro inflammatory profile in AIH, which is characterised by elevated CD127 positivity, and a greater propensity to produce IFNγ or IL17 upon challenge with pro inflammatory stimuli.
CONCLUSIONS:
In AIH CD39pos Tregs are decreased in number, fail to adequately hydrolyse pro-inflammatory nucleotides and do not suppress efficiently IL17 production by effector CD4 T cells. CD39pos Tregs show plasticity and are unstable upon pro-inflammatory challenge, suggesting that defective immuno-regulation in AIH might result not only from reduced Treg number and function but also from increased conversion of Tregs into effector cells.
Keywords: adenosine, ATP, liver inflammation, Treg
Introduction
Autoimmune hepatitis (AIH) is an inflammatory disease of the liver, characterised by female preponderance, interface hepatitis on histology, hypergammaglobulinaemia and serum autoantibody positivity(1, 2). Several lines of evidence indicate that in AIH numerical and functional regulatory T cell (Treg) defects are likely to play a permissive pathogenic role, allowing effector CD4 and CD8 T lymphocytes to initiate and perpetuate liver damage(3–5).
The reasons for the Treg functional impairment in AIH are unclear. Previous studies in both mice and humans have highlighted a number of mechanisms used by Tregs to mediate suppression, including the release of anti-inflammatory cytokines and the modulation of antigen presenting cell function.
More recently, metabolic disruption of effector cell function by Tregs has also been explored(6). Central to this mode of suppression is the expression by Tregs of the ectoenzyme CD39, which catalyses the degradation of adenosine triphosphate (ATP) and adenosine diphosphate (ADP) into adenosine monophosphate (AMP). AMP is subsequently converted to the immunomodulatory nucleoside adenosine by CD73, an ectoenzyme that works in tandem with CD39(7–9).
At variance with the murine setting, CD39 in humans is expressed not only by conventional Tregs but also by cytokine - i.e. IL4, IL5, IL17 and IFNγ - producing effector memory cells(8, 10). Again unlike mice, CD73 is poorly expressed by human Tregs, suggesting that in humans AMP conversion to adenosine is mediated by paracrine mechanisms or by the presence of CD73 on target or neighbouring cells(10).
Compared to their CD39neg counterpart, CD39pos Tregs have been shown to be phenotypically stable upon pro-inflammatory challenge(10) and to display preferential suppression over Th17 immunity(11).
Defective numbers of CD39pos Tregs have been reported in patients with multiple sclerosis(8, 11), where these cells are also impaired in their ability to suppress IL17 production(11). Defective CD39pos Treg function has been also described in systemic lupus erythematosus(12). Additionally, the presence of a CD39 single nucleotide polymorphism has been reported in Crohn’s disease and found to be associated with low CD39 mRNA expression levels and disease susceptibility(13).
Given the key role of CD39 in governing Treg suppressive function, we aimed to explore whether the impairment of Tregs, previously observed in AIH, resided in alterations of CD39 expression. To this end, we investigated the frequency, phenotypic and functional signature of CD39pos Tregs in AIH as well as their stability upon pro-inflammatory challenge, a feature particularly relevant to the development of immunotherapeutic strategies aimed at reconstituting immuno tolerance through Treg adoptive transfer.
Patients and methods
Patients and controls
Forty-one patients with anti-nuclear and/or anti-smooth muscle antibody positive AIH (25 female) were studied. At the time of, or close to diagnosis a liver biopsy showed interface hepatitis in all. Patients with bile duct changes characteristic of sclerosing cholangitis on retrograde cholangiography were excluded from analysis. The median age of patients included in the study was 14 years (range 6–27 years). Of fourteen patients with active disease, defined by the presence of abnormal aspartate aminotransferase (AST) levels, three were studied before immunosuppressive treatment was started. Twenty-seven patients were studied during drug-induced remission (defined by normalisation of AST levels). Treatment consisted of prednisolone (5–15mg/day) with or without azathioprine (25–150mg/day) or mycophenolate mofetil (MMF; 500–2000mg/day, n=10). Demographic and biochemical data are shown in Table 1. Eight subjects with liver disorders of non-autoimmune and non-viral aetiology served as disease control patients (DC) (7 females, median age 15 years [range 6–25 years]). Of this group, two patients had non-alcoholic fatty liver disease (NAFLD), one α−1 antitrypsin deficiency, one Gilbert syndrome, one Wilson disease, one congenital portosystemic shunt, one Alagille syndrome and one hepatic adenoma. Twenty-five healthy subjects (HS) served as normal controls (15 females, median age 35 years [range 22–50 years]). The age difference between AIH/DC patients and HS derived from ethical constraints in obtaining blood from healthy children. The study was approved by the ethical committee of King’s College Hospital, London and written consent was obtained from each AIH patient and HS enrolled in the study.
Table 1.
Autoimmune hepatitis patient demographics and laboratory data
| # | % female | Age (years) | AST (nv: <50 IU/L) | Bilirubin (nv: <20pmol/L) | IgG (nv: 6.5–17g/L) | % ANA positive (median) | % SMA positive (median) | |
|---|---|---|---|---|---|---|---|---|
| Active disease | 14 | 71 | 12 (9–18) | 112 (54–1074)** | 25 (8–82)** | 15.6 (2.4–25.9)* | 44 (1/40) | 33 (1/640) |
| • Near presentation† | 9 | 78 | 11 (9–15) | 117 (61–1074) | 39 (8–82) | 19.0 (2.4–25.9) | 60 (1/40) | 40 (1/400) |
| • Relapse | 5 | 60 | 12 (9–18) | 87 (54–224) | 14 (10–24) | 14.0 (10.0–19.5) | 0 | 25 (1/640) |
| Remission | 27 | 55 | 15 (6–27) | 27 (16–46) | 9 (4–42) | 10.7 (0.9–19.0) | 9 (1/60) | 9 (1/120) |
Data are presented as median (range) unless otherwise stated. Abbreviations: #, number; AST, aspartate aminotransferase; nv, normal value; ANA, anti-nuclear antibody; SMA, smooth muscle antibody
4 patients were studied before starting treatment and 5 were studied after treatment initiation but before the normalisation of AST levels
P<0.005
P<0.05 comparing AST, bilirubin and IgG levels in patients with active disease and those at remission
Cell separation
Peripheral blood mononuclear cells (PBMCs) were isolated as described previously (3). Viability of mononuclear cells, determined by trypan blue exclusion, exceeded 98%.
Flow cytometry
PBMCs were stained with allophycocyanin (APC) cychrome-7 (Cy7)-conjugated anti-CD4, APC-conjugated anti-CD73, phycoerythrin (PE)-Cy7-conjugated anti-CD39 (all eBioscience, Hatfield, UK), PE-conjugated anti-CD25, fluorescein isothyocyanate (FITC)-conjugated anti-CD127, PE-conjugated anti-CD45RO and FITC-conjugated anti-CD62L (all BD Biosciences, Discovery Labware, Oxford, UK) monoclonal antibodies. Cells were incubated at 4°C in the dark for 30 minutes and washed with phosphate buffered saline (PBS) supplemented with 1% foetal calf serum (FCS) before analysis by flow cytometry on a Becton Dickinson fluorescence activated cell sorter (FACS-Canto™ II, Beckton Dickinson Immunocytochemistry Systems, San Jose, CA). FACSDiva software was used for analysis. The percentage of cells positive for FOXP3 or intracellular CD152 was determined after fixation and permeabilisation with Cytofix/Cytoperm™ (BD Biosciences) and the addition of APC-conjugated anti-FOXP3 (eBioscience) or APC-conjugated anti-CD152 (BD Biosciences) monoclonal antibodies.
The percentage of IFNγ, IL17, IL10 and transforming growth factor β (TGFβ)-positive cells was determined after exposure to phorbol 12-mystrate 13-acetate (10ng/mL) and ionomycin (500ng/mL; both Sigma Aldrich, Gillingham, UK) and following addition of brefeldin-A (10μg/mL; Sigma Aldrich) for 5 hours. Cells were then stained using PE-conjugated anti-IFNγ, PE-conjugated anti-IL10 (both BD Biosciences), FITC-conjugated anti-IL17 (eBioscience) or Peridinin chlorophyll protein complex (PerCP)-conjugated anti-TGFβ (R&D Systems, Abingdon, UK).
Cell stimulation
PBMCs were seeded at 1×106 cells/ml in 96-well round-bottom plates in RPMI-1640 pre-supplemented with 2mM L-glutamine and 1% Antibiotic-Antimycotic solution (both from Gibco, Invitrogen, Paisley, UK) and 10% FCS. Cells were exposed to anti-CD3/anti-CD28 T cell expander (ratio bead/cell: 1/2; Dynal Invitrogen, Oslo, Norway) and recombinant human IL-2 (30U/mL; EuroCetus; Amterdam, Netherlands), a protocol chosen on the basis of previous experiments(14). To test whether the phenotype of CD39pos Tregs remained stable upon pro-inflammatory challenge, cells were treated with recombinant human IL6 (0.04μg/mL) and IL1β (0.01μg/mL; both R&D Systems) and cultured at 37°C and 5% CO2 for 5 days. Cells were washed in PBS/1% FCS and flow cytometry was performed as above.
Cell purification
For co-culture assays, CD4pos cells were isolated from the total PBMC population using immunomagnetic beads (Dynal Invitrogen) as described(3, 14). CD4pos T cells were then stained with FITC- or APC-Cy7-conjugated anti-CD4 (eBioscience), APC- or PE-conjugated anti-CD25 (BD Bioscences) and PE- or PE-Cy7-conjugated CD39 (eBioscience). The CD4pos cells were then sorted into CD25high, CD25highCD39pos (CD39pos Tregs), CD25highCD39neg (CD39neg Tregs) and CD25neg subsets by fluorescence activated cell sorting (FACS) using a Becton Dickinson cell sorter (FACSAria™ II; Beckton Dickinson Immunocytochemistry Systems). The purity of the CD25high, CD25highCD39pos and CD25highCD39neg populations exceeded 95% and the purity of the CD25neg cells exceeded 98%.
For experiments assessing CD39 enzymatic activity, CD4posCD25pos and CD4posCD25neg populations were isolated immunomagnetically as described previously(3, 14). The purity of immunomagnetically isolated populations exceeded 85%.
Measurement of enzymatic activity
The enzymatic activity of immunomagnetically isolated CD4posCD25neg and CD4posCD25pos cells was measured indirectly by quantifying the concentration of free phosphate using the colorimetric Sensolyte® malachite green phosphate assay kit (AnaSpec, Seraing, Belgium). Populations were washed in saline solution containing 0.9% w/v NaCl – to remove residual phosphate-containing media – and plated at 2×105 cells/ml, before exposure to 10μM ATP (Sigma Aldrich, Gillingham, UK) for 15 minutes. Phosphate concentration was quantified at 600nm using an absorbance plate reader after comparison with a standard curve.
Thin layer chromatography (TLC) was performed as described previously(7), to visualise the hydrolysis of radiolabeled ADP to AMP and its subsequent conversion to adenosine. 2.5×105 immunomagnetically isolated CD4posCD25pos or CD4posCD25neg cells were exposed to 2mCi/ml [C14] ADP (Perkin Elmer, Cambridge, UK) in the presence of 10mM Ca2+ and 5mM Mg2+. Aliquots were collected at reaction-times of 5, 10, 20, 40 and 60 minutes before analysis of [C14]ADP hydrolysis products by TLC. Samples were loaded onto silica gel matrix plates (Sigma Aldrich, Gillingham, UK) and [C14]ADP derivatives were separated using an appropriate solvent mixture(15).
Co-culture assays
Once purified, the CD25neg responder cell populations were seeded overnight in 96-well round-bottom plates in the presence of anti-CD3/anti-CD28 T cell expander (ratio bead/cell: 1/2 Dynal Invitrogen) and recombinant human IL2 (30 U/mL; EuroCetus). CD25high, CD39pos or CD39neg Tregs were then added to autologous CD25neg responder cells at a ratio of 1/8(14). Parallel cultures of CD25neg responder cells in the absence of Tregs were performed. To analyse the proliferation of effector cells, for the final 18 hours of culture, cells were pulsed with 0.25μCi/well 3H-thymidine (Perkin Elmer, Cambridge, UK) and harvested using a multichannel harvester. The amount of incorporated 3H-thymidine was measured using a β-counter. In preliminary experiments, in which cells from 4 AIH patients and 4 HS were tested, proliferation was also analysed using the CellTrace™ carboxy fluorescein succinimidyl ester (CFSE) cell proliferation kit (Molecular Probes, Paisley, UK). For analysis of cytokine production, cells were stained with FITC- or APC-Cy7-conjugated anti-CD4, FITC- or PE- conjugated anti-IL17 (all eBioscience) and APC- or PE- conjugated anti IFNγ (IQ Products, Netherlands, and BD Biosciences) and analysed as described above.
Statistical analysis
The normality of variable distribution was assessed by the Kolmogorov Smirnov goodness of fit test; once the hypothesis of normality was accepted (P<0.05), comparisons were performed using paired or unpaired Student’s t tests for linked or unlinked data respectively. A one-way ANOVA, followed by Tukey’s multiple comparisons test, was used to compare the means of multiple samples. Results are expressed as mean±SEM unless otherwise stated and P values <0.05 were considered significant. Data were analysed using GraphPad Prism® 5 software (GraphPad; San Diego, CA) and SPSS software (IBM; Hampshire, UK).
Results
Enumeration and characterisation of CD4posCD25highCD39pos regulatory T cells
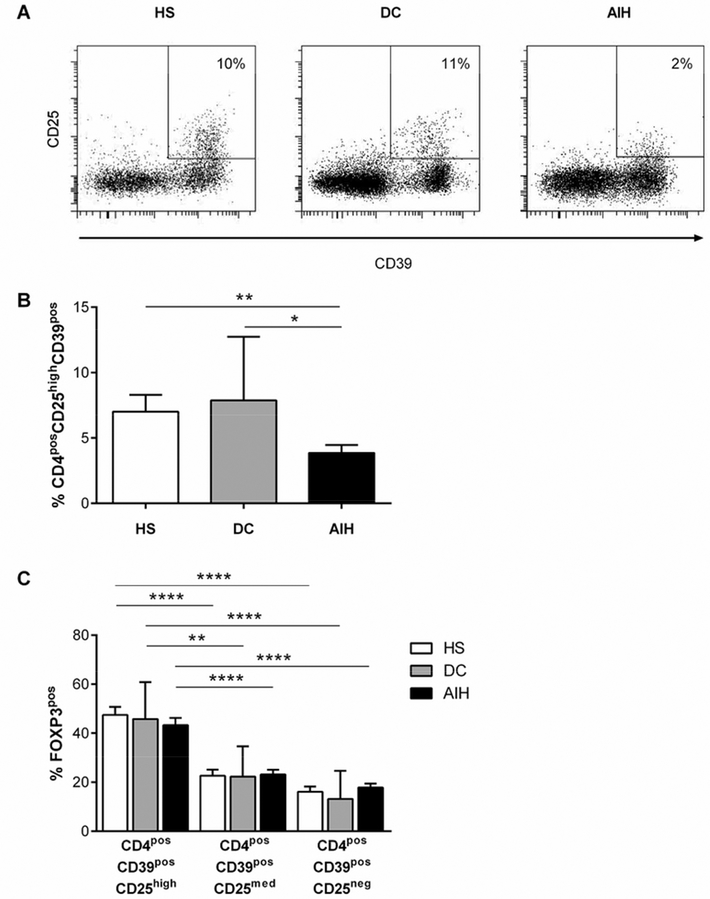
The frequency of circulating CD4posCD39pos cells was similar in AIH patients, DC patients and HS (9.7±2.7, 10.8±2.4 and 11.1±2.0 respectively, P=NS). However, the frequency of CD4posCD39posCD25high (hereafter denoted CD39pos Tregs) was markedly reduced in AIH patients compared to DC patients or HS (Figure 1A,B). The frequency of CD39pos Tregs was similar in AIH patients with active and inactive disease (4.2±1.3 vs 3.5±0.7, P=NS). Male AIH patients had fewer CD39pos Tregs compared to their female counterparts (2.4±0.6 vs 5.1±0.9, P=0.03). The difference between males and females was not observed in the HS population (9.4±2.8 vs 6.8±1.8, P=NS). To test the influence of age on the frequency of CD39pos Tregs, AIH patients were subdivided into those <13 or ≥13 years of age. The frequency of CD39pos Tregs did not differ when this comparison was made (4.7±1.4 vs 3.3±0.3, P=NS). CD25high cells contained higher proportions of cells positive for CD39 compared to the CD25medium(med) and CD25neg populations in HS [CD25high: 43.0±4.8 vs CD25med: 26.0±5.3 (P<0.05) and vs CD25neg: 14.6±3.2 (P<0.001)], DC patients [CD25high: 46.7±8.4 vs CD25med: 23.4±3.6 (P<0.05) and vs CD25neg: 15.2±3.5 (P<0.01)] and AIH patients [CD25high: 40.7±4.6 vs CD25med: 22.8±3.8 (P<0.05) and vs CD25neg: 12.6±2.6 (P<0.001)]. In AIH patients, DC patients and HS, the frequency of CD4posCD39pos cells positive for FOXP3 was greater in the CD25high subset compared to the CD25med or CD25neg populations (Figure 1C).
Figure 1. Characterisation of CD39pos Tregs.
(A) Frequency of CD4posCD25highCD39pos Tregs in one representative autoimmune hepatitis (AIH) patient, one disease control (DC) patient and one healthy subject (HS). Plots show gated CD4pos lymphocyte populations. (B) Frequency of CD4posCD25highCD39pos Tregs in 31 AIH patients, 8 DC patients and 25 HS. (C) Frequency of FOXP3pos cells within CD4posCD39posCD25high, CD4posCD39pos CD25med and CD4posCD39posCD25low populations in 31 AIH patients, 8 DC patients and 25 HS.
AIH patients treated with prednisolone and MMF had a lower frequency of CD39pos Tregs compared to those treated with prednisolone alone (5.6±1.9 vs 1.7±0.5, P=0.01) or with prednisolone and azathioprine (4.7±1.1 vs 1.7±0.5, P=0.03).
Compared to HS and DC patients (Table 2), CD39pos Tregs from AIH patients contained a lower frequency of cells negative for CD127 – the lack of which distinguishes bona fide Tregs from effector T cells(16, 17) – , lower proportions of cells positive for the memory cell marker CD45RO and a similar frequency of cells positive for the Treg function-associated markers FOXP3, CD152 or CD62L. Approximately 10% of CD39pos Tregs expressed CD73 in AIH patients, DC patients and HS.
Table 2.
Phenotypic signature and cytokine profile of CD39posTregs
| HS | DC | AIH | P* | P† | AIH active | AIH remission | P | |
|---|---|---|---|---|---|---|---|---|
| FOXP3pos | 47.4±3.3 | 43.9±7.3 | 43.3±2.9 | NS | NS | 37.7±4.5 | 45.6±3.6 | NS |
| CD127neg | 92.3±1.4 | 95.3±0.9 | 83.0±3.0 | 0.01 | 0.05 | 76.3±4.7 | 85.8±3.6 | NS |
| CD45ROpos | 91.3±2.4 | 93.3±2.0 | 80.8±3.4 | 0.02 | 0.003 | 75.0±8.5 | 83.3±3.3 | NS |
| CD73pos | 13.8±2.9 | 10.3±1.7 | 11.2±2.5 | NS | NS | 9.1±2.5 | 12.0±3.4 | NS |
| CD152pos | 18.3±5.2 | 27.9±5.5 | 20.2±2.3 | NS | NS | 21.4±3.6 | 18.4±2.6 | NS |
| CD62Lpos | 50.0±6.1 | 55.5±7.1 | 49.9±5.0 | NS | NS | 47.2±3.6 | 54.1±12.0 | NS |
| IL17pos | 11.4±2.7 | 12.9±3.3 | 15.7±2.5 | NS | NS | 19.9±4.8 | 14.1±3.0 | NS |
| IFNγpos | 12.3±2.7 | 17.6±2.9 | 9.2±2.0 | NS | NS | 6.1±1.7 | 10.6±2.6 | NS |
| IL10pos | 11.2±2.0 | 21.1±5.0 | 13. 1±2.8 | NS | NS | 11.3±6.1 | 13.5±3.2 | NS |
| TGFβpos | 9.7±2.5 | 11.6±2.8 | 11.2±2.4 | NS | NS | 13.4±2.8 | 10.3±3.1 | NS |
Data are presented as mean±SEM. FOXP3, CD127, CD45RO,CD73, IL17, IFNγ, IL10 and TGFβ data refer to 31 autoimmune hepatitis (AIH) patients (22 inactive, 9 active), 8 disease control (DC) patients and 25 healthy subjects (HS). CD152 and CD62L data refer to 10 AIH patients (4 inactive, 6 active), 8 DC patients and 4 HS. Abbreviations: NS, not significant
P<0.05 comparing HS and AIH patients
P<0.05 comparing DC patients and AIH patients
The frequency of CD39pos Tregs producing the pro inflammatory cytokines IFNγ and IL17 or the anti-inflammatory cytokines TGFβ or IL10 was similar in AIH patients, DC patients and HS (Table 2).
Given that the frequency and the phenotype profile of CD39pos Tregs in HS and DC patients were comparable, only cells from HS were used for the following experimental sections.
Phenotypic stability of CD39pos regulatory T cells
After stimulation of PBMCs with anti-CD3/anti-CD28 T cell expander (Table 3a), the frequency of CD39pos Tregs positive for FOXP3 or CD127 increased to a similar extent in AIH patients and HS. The frequency of CD45ROpos CD39pos Tregs increased in HS, while remaining unchanged upon stimulation in AIH patients. The frequencies of IFNγ and IL17-producing cells within CD39pos Tregs remained stable in HS, while increased in AIH upon stimulation. The increase in the frequency of CD39pos Tregs expressing IL17 or IFNγ was greater in AIH patients compared to HS (Table 3a).
Table 3a.
Percentage of FOX3pos, CD127neg, CD45ROpos or CD73pos within CD39pos cells and frequency of IFNγ or IL17 producing CD39pos cells after stimulation of PBMCs with anti-CD3/CD28 T cell expander
| HS | AIH | |||||||
|---|---|---|---|---|---|---|---|---|
| Frequency at BL | Frequency after stimulation | P | Size of change | Frequency at BL | Frequency after stimulation | P | Size of change | |
| FOXP3pos | 33.2±3.8 | 81.3±9.2 | <0.001 | 48.1±9.1 | 35.3±6.9 | 73.4±10.0 | 0.03 | 25.4±10.2 |
| CD127pos | 1.8±0.8 | 5.7±1.4 | 0.01 | 3.3±1.6 | 2.6±1.8 | 11.6±2.9 | 0.01 | 6.0±2.2 |
| CD45ROpos | 87.3±5.1 | 98.7±0.8 | 0.03 | 11. 4±4.4 | 90.5±5.2 | 98.1±1.1 | NS | 5.1±3.6 |
| CD73pos | 24.0±5.3 | 10.4±3.6 | NS | −13.6±6.8 | 17.5±7.7 | 27.2±13.9 | NS | 6.5±12.4 |
| IFNγpos | 7.3±2.6 | 6.6±2.4 | NS | −0.7±2.9 | 8.3±5.3 | 28.3±15.1 | NS | 20.0±11.5* |
| IL17pos | 8.0±1.2 | 8.8±2.0 | NS | 0.9±1.9 | 3.8±1.8 | 12.3±3.7 | 0.03 | 5.7±2.3* |
Data are presented as mean±SEM and refer to 6 autoimmune hepatitis (AIH) patients with inactive disease and 9 healthy subjects (HS).
Abbreviations: BL, baseline; PBMCs, peripheral blood mononuclear cells; NS, not significant
P<0.05 when comparing magnitude of change in frequencies between HS and AIH patient
Interestingly, in AIH the size of the increase in frequency of IFNγpos CD39pos Tregs correlated with serum AST concentration (r2=0.82, P<0.05).
Exposure of PBMCs to IL1β and IL6 (Table 3b) increased the frequency of FOXP3pos CD39pos Tregs in HS but not AIH patients, but had no effect on the frequency of CD39pos cells expressing CD127 or CD45RO. The frequency of CD73pos CD39pos Tregs decreased significantly in HS, while it increased in AIH patients, though not significantly. While the frequency of CD39pos Tregs producing IFNγ increased to a similar extent in both AIH patients and HS, the frequency of those producing IL17 increased in AIH but remained stable in health.
Table 3b.
Percentage of FOX3pos, CD127neg, CD45ROpos or CD73pos within CD39pos cells and frequency of IFNγ or IL17 producing CD39pos cells after exposure of PBMCs to IL1β and IL6
| HS | AIH | |||||||
|---|---|---|---|---|---|---|---|---|
| Frequency at BL | Frequency after stimulation | P | Size of change | Frequency at BL | Frequency after stimulation | P | Size of change | |
| FOXP3pos | 33.2±3.8 | 73.9±5.1 | <0.001 | 40.7±7.7 | 35.3±6.9 | 62.7±13.1 | NS | 27.4±11.1 |
| CD127pos | 1.8±0.8 | 1.3±0.8 | NS | −0.5±1.2 | 2.6±1.8 | 14.3±12.0 | NS | 11.7±10.3 |
| CD45ROpos | 87.3±5.1 | 90.9±2.7 | NS | 3.6±4.7 | 90.5±5.2 | 81.5±6.3 | NS | −9.0±7.2 |
| CD73pos | 24.0±5.3 | 6.4±2.0 | 0.01 | −17.6±6.0 | 17.5±7.7 | 25.9±7.7 | NS | 8.3±11.9* |
| IFNγpos | 7.3±2.6 | 68.9±15.0 | 0.004 | 54.0±13.4 | 8.3±5.3 | 69.0±10.1 | 0.002 | 60.7±10.4 |
| IL17pos | 8.0±1.2 | 14.9±4.4 | NS | 5.3±5.3 | 3.8±1.8 | 17.9±2.7 | 0.003 | 14.1 ±2.6 |
Data are presented as mean±SEM and refer to 6 autoimmune hepatitis (AIH) patients with inactive disease and 9 healthy subjects (HS).
Abbreviations: BL, baseline; PBMCs, peripheral blood mononuclear cells; NS, not significant
P<0.05 when comparing magnitude of change in frequencies between HS and AIH patient
ATP/ADP hydrolysis
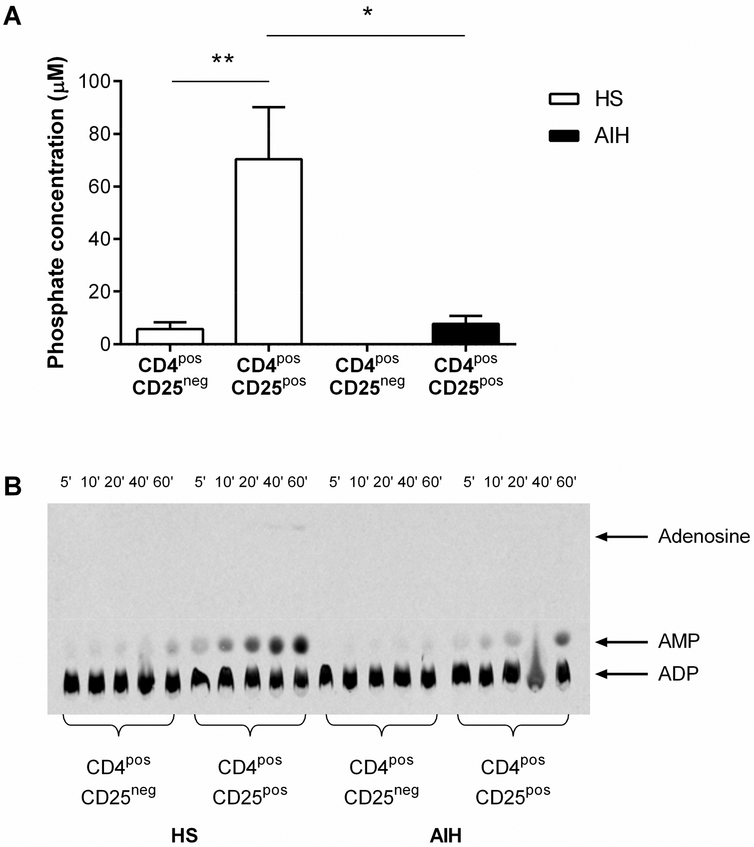
Immunomagnetically isolated CD4posCD25pos Tregs from AIH patients were less able to hydrolyse exogenous ATP compared to HS (Figure 2A). In HS, but not AIH patients, the CD25pos cells generated greater concentrations of phosphate compared to CD25neg cells (Figure 2A), as reflected by higher CD39 expression (3.65±0.40 vs 24.53 ±4.61, P=0.002)
Figure 2. CD39 enzymatic activity of Tregs in AIH and HS.
(A) Ability of immunomagnetically isolated CD4posCD25pos and CD4posCD25neg cells from 3 autoimmune hepatitis (AIH) patients and 4 healthy subjects (HS) to produce free phosphate – the bi-product of ATP hydrolysis – after the addition of exogenous ATP. (B) CD39 ADPase enzymatic activity of immunomagnetically isolated CD4posCD25pos and CD4posCD25neg cells was assessed by thin layer chromatography at 5, 10, 20, 40 and 60 minute time-points following incubation with 14C-radiolabelled ADP substrate. Image representative of 3 independent experiments.
Analysis of [C14]-radiolabelled ADP hydrolysis by TLC (Figure 2B) revealed that CD4posCD25pos cells from HS were able to hydrolyse ADP into AMP and, at the longer reaction time of 60 minutes, these cells could generate extracellular nucleosides.
In contrast, AMP generation was less pronounced in CD4posCD25pos cells from AIH patients. CD4posCD25neg cells in health and AIH degraded ADP less efficiently than the CD4posCD25pos populations, failing to produce extracellular adenosine.
Suppressive ability of CD39pos Tregs
Preliminary experiments, in which both 3H-thymidine and CFSE were used to analyse the suppressive ability of immunomagnetically isolated CD4posCD25pos cells, confirmed reports(3, 4, 14) that Tregs from AIH patients are less able to suppress the proliferation of autologous CD4posCD25neg responder cells compared to HS (Supplementary Figure 1). As CFSE and 3H-thymidine-based assays gave comparable results, given the requirement for fewer cells, 3H-thymidine was used to measure the proliferation of FACS sorted populations, which had comparatively low yield compared to those magnetically isolated.
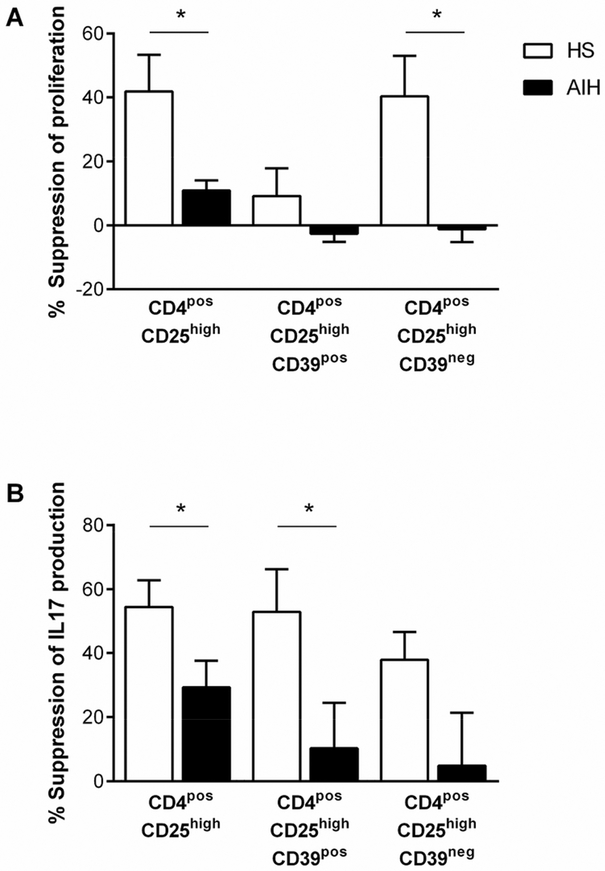
CD4posCD25high Tregs from HS and AIH patients and CD39neg Tregs from HS were able to suppress the proliferation of responder T cells (one-sample t-tests when comparing suppression in the presence and absence of Tregs; P=0.04, P=0.04, P=0.05 respectively. Percent suppression of proliferation by CD25high cells was lower in AIH patients than in HS. In HS, CD39pos Tregs were poor suppressors of proliferation compared to conventional CD4posCD25high Tregs and CD39neg Tregs (Figure 3A).
Figure 3. Suppressive ability of CD39pos Tregs.
The ability of FACS-sorted CD4posCD25high, CD4posCD25highCD39pos and CD4posCD25highCD39neg Treg populations to suppress the proliferation (A) or IL17 production (B) of CD4posCD25neg responder cells. For suppression of proliferation, data refer to 4 healthy subjects (HS) and 4 autoimmune hepatitis (AIH) patients. For suppression of IL17 production, data refer to 6 HS and 10 AIH patients.
In HS, CD4posCD25high, CD39pos and CD39neg Tregs were able to suppress the production of IL17 (one sample t-tests; P=0.001, P=0.01, P=0.007 respectively), while in AIH, only the CD25high population was able to suppress IL17 production (one sample t-test; P=0.006). Both the CD25high and CD39pos Tregs were less able to suppress IL17 production in AIH patients compared to HS (Figure 3B).
Discussion
In the current study we show that Tregs expressing the ectonucleotidase CD39 are present at low levels and are also dysfunctional in AIH.
Phenotypic analysis has indicated that the expression of CD39 is associated with classical Treg features, i.e. high CD25 and FOXP3 and low CD127 expression. CD39pos Tregs effectively suppress CD4 T cell IL17 production while exerting poor control over target cell proliferation, suggesting that this Treg subgroup may have a specific role in dampening Th17 immunity. Low frequencies of CD39pos Tregs and inability to control adequately IL17 mediated immuno reactivity have been described also in patients with MS (8, 11). Moreover, low CD39 expression has been reported in IBD, where it is associated to a CD39 polymorphism (13), suggesting a genetically encoded defect of immune regulation in this condition. Future studies should explore whether CD39 polymorphisms account for the observed Treg/effector cell imbalance in AIH, and therefore contribute to disease initiation and/or perpetuation.
A comparison between health and disease has revealed that CD39pos Tregs from AIH patients are impaired in number, in their ability to hydrolyse ATP and ADP and in their suppressive function, indicating that in AIH CD39pos Treg impairment occurs at multiple levels.
A potential limitation of this study is the use of a heterogenous AIH population, including patients under different treatment regimens. This has been overcome to some extent by the size of the patient group which has enabled us to observe interesting and novel associations. We have, for example, noted that the frequency of CD39pos Tregs was markedly decreased in AIH patients receiving prednisolone and MMF compared to those treated with prednisolone alone or in combination with azathioprine, raising the possibility that these treatment regimens differentially impact the frequency of this regulatory T cell subset. Alternatively, the lower CD39pos Treg frequencies observed in the MMF treated group may reflect the fact that these patients have a particularly severe form of disease characterised by a more marked impairment in immune regulation and the mechanisms governing it. Also of note, CD39pos Treg defects were particularly pronounced in male AIH patients. Since this gender difference was not observed in healthy subjects, it is possible that hormonal differences, particularly the presence of oestrogen, can partially overcome the CD39pos Treg defect in AIH.
In AIH patients, particularly in those with active disease, CD39pos Tregs contain low proportions of CD127neg lymphocytes, suggesting that CD39pos Tregs from AIH patients, in addition to being numerically defective, are also skewed towards a pro-inflammatory profile. Lower CD127neg cell frequencies within CD39pos Tregs are paralleled by lower proportions of CD45ROpos cells. Though the reasons for the CD45RO decrease are unclear, it should be recalled that in humans CD39 is mainly expressed on memory cells(10); therefore low frequencies of CD45ROpos lymphocytes may reflect the numerical CD39pos Treg impairment.
In AIH, CD39pos Tregs are also less able to hydrolyse ATP and ADP, this defect ultimately resulting in reduced production of AMP and immunosuppressive adenosine. Persistently high levels of pro-inflammatory ATP and ADP may contribute to the perpetuation of inflammation. Inefficient CD39 hydrolytic activity is likely to account for the decreased ability of Tregs to control CD4 effector cell function, in particular the production of IL17, which is involved in AIH liver damage(18, 19).
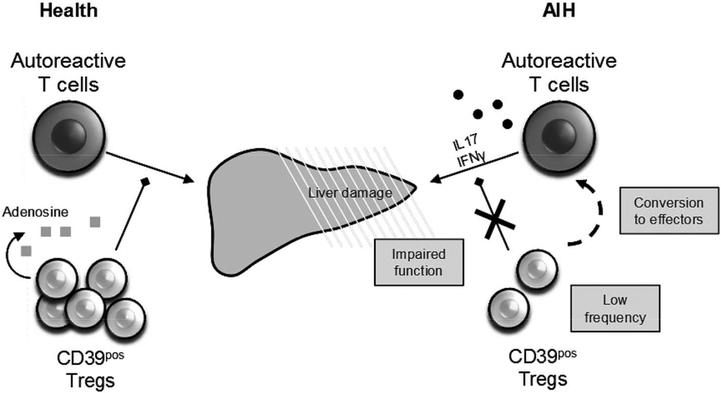
Expression of CD39 has previously been linked to Treg lineage stability(10, 11). Characterisation of CD39pos Treg phenotype before and after stimulation with anti-CD3/anti-CD28, a classical T cell stimulus, and with IL6 and IL1β, cytokines mimicking the pro inflammatory environment in AIH, has shown that CD39pos Tregs from AIH patients are less stable than in health, as they undergo a marked increase in the production of IL17 and IFNγ. These data suggest that Treg impairment in AIH might derive from an increased rate of Treg conversion into effector cells (Figure 4).
Figure 4. Impairment of CD39pos Tregs in AIH.
In health, CD39pos Tregs produce adenosine and adequately control autoreactive T cell effector functions. In AIH, multiple CD39pos Treg defects – a reduction in frequency, an inability to suppress IL17 production by effector cells, and a propensity to convert to IFNγ or IL17 producing effectors – contribute to impaired immunosuppression and autoimmune liver damage.
Of note, in contrast to HS, CD73 expression by CD39pos Tregs in AIH patients remained elevated after pro-inflammatory challenge. That CD73 is strictly linked to activation was previously shown in a study by Doherty et al, who reported high CD73 expression levels on CD4 T cells from Crohn’s patients with more active disease(20).
The findings of a reduced stability of CD39pos Tregs in AIH patients should be taken into consideration when developing immunotherapeutic strategies aimed at re-establishing immune-homeostasis through adoptive Treg transfer. Thus, protocols for Treg expansion should include treatment with agents/molecules (e.g. retinoic acid and/or rapamycin) aimed at boosting Treg properties while inhibiting their conversion to pathogenic Th1 and Th17 effector cells. Since CD39pos Tregs exhibit potent IL17-suppressive properties in health, possible mechanisms for boosting CD39 expression in AIH should be explored. A potential candidate is retinoic acid, which is able to boost CD39 expression by naïve T cells (Robson, unpublished observation).
Previous investigations have shown accumulation of CD39pos Tregs in the liver of patients with chronic hepatitis B infection (21). In the experimental cancer setting, hepatic growth of melanoma metastatases is inhibited in CD39null mice, while CD39pos T-regs inhibit anti-tumour immunity (22). Future studies should examine the frequency and the tissue localisation of liver infiltrating CD39pos Tregs in AIH and explore whether defective CD39 expression by circulating Tregs is also reflected in the inflamed liver. These findings would have important implications for the development of adoptive Treg therapy for AIH.
In conclusion, this study has shown that in AIH there is a numerical decrease in CD39pos Tregs. These CD39pos Tregs are impaired in their enzymatic and suppressive abilities and, upon pro-inflammatory challenge, are less stable than in health. Defective immune-regulation in AIH may derive not only from impaired Treg number and function but also from an increased rate of Treg conversion into effector cells.
Supplementary Material
Acknowledgements
The authors acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
Grant Support: CR Grant is supported by an Alex P Mowat PhD Studentship, King’s College Hospital Charity, UK. R Liberal is supported by a Doctoral Grant from the Science and Technology Foundation, Science and Higher Education Ministry, Portugal. MS Longhi was supported by the Roger Dobson Fund, King’s College Hospital Charity, UK when this project was started and she is currently supported by a Clinician Scientist Fellowship from the Medical Research Council, UK.
Abbreviations:
- ADP
adenosine diphosphate
- AIH
autoimmune hepatitis
- AMP
adenosine monophosphate
- ANA
anti-nuclear antibody
- AST
aspartate aminotransferase
- ATP
adenosine triphosphate
- SMA
smooth muscle antibody
- Treg
regulatory T cell
Footnotes
Disclosures: the authors have nothing to disclose
References
- 1.Longhi MS, Ma Y, Mieli-Vergani G, Vergani D. Aetiopathogenesis of autoimmune hepatitis. J Autoimmun 2010;34:7–14. [DOI] [PubMed] [Google Scholar]
- 2.Gregorio GV, Portmann B, Reid F, Donaldson PT, Doherty DG, McCartney M, Mowat AP, et al. Autoimmune hepatitis in childhood: a 20-year experience. Hepatology 1997;25:541–547. [DOI] [PubMed] [Google Scholar]
- 3.Longhi MS, Ma Y, Bogdanos DP, Cheeseman P, Mieli-Vergani G, Vergani D. Impairment of CD4(+)CD25(+) regulatory T-cells in autoimmune liver disease. J Hepatol 2004;41:31–37. [DOI] [PubMed] [Google Scholar]
- 4.Ferri S, Longhi MS, De Molo C, Lalanne C, Muratori P, Granito A, Hussain MJ, et al. A multifaceted imbalance of T cells with regulatory function characterizes type 1 autoimmune hepatitis. Hepatology 2010;52:999–1007. [DOI] [PubMed] [Google Scholar]
- 5.Longhi MS, Hussain MJ, Kwok WW, Mieli-Vergani G, Ma Y, Vergani D. Autoantigen-specific regulatory T cells, a potential tool for immune-tolerance reconstitution in type-2 autoimmune hepatitis. Hepatology 2011;53:536–547. [DOI] [PubMed] [Google Scholar]
- 6.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008;8:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007;204:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 2007;110:1225–1232. [DOI] [PubMed] [Google Scholar]
- 9.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5’-adenosine monophosphate to adenosine. J Immunol 2006;177:6780–6786. [DOI] [PubMed] [Google Scholar]
- 10.Dwyer KM, Hanidziar D, Putheti P, Hill PA, Pommey S, McRae JL, Winterhalter A, et al. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant 2010;10:2410–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farrelly C, Tubridy N, et al. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol 2009;183:7602–7610. [DOI] [PubMed] [Google Scholar]
- 12.Loza MJ, Anderson AS, O’Rourke KS, Wood J, Khan IU. T-cell specific defect in expression of the NTPDase CD39 as a biomarker for lupus. Cell Immunol 2011;271:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman DJ, Kunzli BM, YI AR, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, et al. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A 2009;106:16788–16793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longhi MS, Hussain MJ, Mitry RR, Arora SK, Mieli-Vergani G, Vergani D, Ma Y. Functional study of CD4+CD25+ regulatory T cells in health and autoimmune hepatitis. J Immunol 2006;176:4484–4491. [DOI] [PubMed] [Google Scholar]
- 15.Beldi G, Wu Y, Banz Y, Nowak M, Miller L, Enjyoji K, Haschemi A, et al. Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. Hepatology 2008;48:841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 2006;203:1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 2006;203:1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao L, Tang Y, You Z, Wang Q, Liang S, Han X, Qiu D, et al. Interleukin-17 contributes to the pathogenesis of autoimmune hepatitis through inducing hepatic interleukin-6 expression. PLoS One 2011;6:e18909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H, Huang J, Liu Y, Ai G, Yan W, Wang X, Ning Q. IL-17 contributes to autoimmune hepatitis. J Huazhong Univ Sci Technolog Med Sci 2010;30:443–446. [DOI] [PubMed] [Google Scholar]
- 20.Doherty GA, Bai A, Hanidziar D, Longhi MS, Lawlor GO, Putheti P, Csizmadia E, et al. CD73 is a phenotypic marker of effector memory Th17 cells in inflammatory bowel disease. Eur J Immunol 2012;42:3062–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Y, Jiang L, Zheng Y, Ni B, Wu Y. Expression of CD39 on FoxP3+ T regulatory cells correlates with progression of HBV infection. BMC Immunol 2012;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun X, Wu Y, Gao W, Enjyoji K, Csizmadia E, Muller CE, Murakami T, et al. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology 2010;139:1030–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.