Abstract
Injury-induced stenosis is a serious vascular complication. We previously reported that p38α (MAPK14), a redox-regulated p38MAPK family member was a negative regulator of the VSMC contractile phenotype in vitro. Here we evaluated the function of VSMC-MAPK14 in vivo in injury-induced neointima hyperplasia and the underlying mechanism using an inducible SMC-MAPK14 knockout mouse line (iSMC-MAPK14-/-). We show that MAPK14 expression and activity were induced in VSMCs after carotid artery ligation injury in mice and ex vivo cultured human saphenous veins. While the vasculature from iSMC-MAPK14-/- mice was indistinguishable from wildtype littermate controls at baseline, these mice exhibited reduced neointima formation following carotid artery ligation injury. Concomitantly, there was an increased VSMC contractile protein expression in the injured vessels and a decrease in proliferating cells. Blockade of MAPK14 through a selective inhibitor suppressed, while activation of MAPK14 by forced expression of an upstream MAPK14 kinase promoted VSMC proliferation in cultured VSMCs. Genome wide RNA array combined with VSMC lineage tracing studies uncovered that vascular injury evoked robust inflammatory responses including the activation of proinflammatory gene expression and accumulation of CD45 positive inflammatory cells, which were attenuated in iSMC-MAPK14-/- mice. Using multiple pharmacological and molecular approaches to manipulate MAPK14 pathway, we further confirmed the critical role of MAPK14 in activating proinflammatory gene expression in cultured VSMCs, which occurs in a p65/NFkB-dependent pathway. Finally, we found that NOX4 contributes to MAPK14 suppression of the VSMC contractile phenotype. Our results revealed that VSMC-MAPK14 is required for injury-induced neointima formation, likely through suppressing VSMC differentiation and promoting VSMC proliferation and inflammation. Our study will provide mechanistic insights into therapeutic strategies for mitigation of vascular stenosis.
Abbreviations: VSMCs, vascular smooth muscle cell(s); MYOCD, myocardin; SRF, serum response factor SRF; HSV, human saphenous vein; HCASMCs, human coronary artery smooth muscle cell(s); HASMCs, human aortic smooth muscle cell(s); RT-PCR, reverse transcription polymerase chain reaction; TUNEL, Terminal deoxynucleotidyl transferase dUTP nick end labeling
Keywords: Oxidative stress, MAPK14, Smooth muscle cell, Neointima formation
Graphical abstract

Highlights
-
•
MAPK14 expression and activity are induced in VSMCs in injured mouse arteries.
-
•
Depletion of VSMC-MAPK14 reduces neointima formation triggered by ligation injury.
-
•
Depletion of VSMC-MAPK14 increases VSMC contractile protein expression and decreases VSMC proliferation and inflammatory response evoked by ligation injury.
-
•
MAPK14 activates proinflammatory gene program via p65/NF-kB-dependent pathway.
-
•
MAPK14 inhibits VSMC contractile gene program via NOX4-dependent pathway.
1. Introduction
Intimal hyperplasia and vascular occlusion contribute to many vascular disorders including atherosclerosis, in-stent restenosis, vein graft failure, and transplant arteriopathy [1], [2], [3]. In response to vascular injury, growth factors, and inflammatory stimuli, vascular smooth muscle cells (VSMCs) switch from a quiescent contractile phenotype to an active synthetic mode. Synthetic VSMCs migrate and proliferate, and their accumulation directly contributes to neointima formation [4], [5], [6]. Activated VSMCs also synthesize and secrete growth and proinflammatory factors and matrix proteins, leading to an extracellular milieu that triggers the infiltration and activation of surrounding proinflammatory and progenitor cells, which also contributes to intima hyperplasia and the subsequent vascular complications [7]. Considerable progress has been made in our understanding of the molecular mechanisms that establish and maintain the VSMC contractile phenotype with the discovery of the Myocardin (MYOCD)/serum response factor (SRF) master transcription switch, epigenetic regulation by TET2, and the TGFβ/SMAD signaling cascade. However, the phenotypic switch of quiescent VSMCs to the synthetic phenotype cannot be explained exclusively through downregulation of these pathways. Significantly, the molecular underpinnings of the activation of the synthetic VSMC phenotype are still poorly understood [8], [9], [10].
Redox signaling contributes to the maintenance of the smooth muscle contractile phenotype and hyperplastic response associated with vascular injury [11], [12], [13], [14]. Stress activated kinases including p38 mitogen activated protein kinase (p38MAPK) are downstream targets of reactive oxygen species producing enzymes such as NADPH oxidases (NOX). For example, NOX4 regulates p38MAPK activity in response to TGFβ to regulate smooth muscle alpha actin expression [15]. Downregulation of SMC-NOX4 inhibits neointima formation [14]. However, the significance of such observations is limited in part due to the lack of a detailed understanding of the role of p38MAPK in vivo in the setting of vascular injury. The p38MAPK family consists of α (MAPK14), β (MAPK11), γ (MAPK12), and δ (MAPK13) isoforms encoded by distinct genes with all isoforms sharing high amino acid homology [16]. MAPK14 is probably the best characterized isoform with a wide spectrum of gene expression profile, high sensitivity to selective inhibitors such as the SB compounds, and functional importance documented by embryonic lethality in the global MAPK14 knockout mice [17], [18], [19]. Classically, p38MAPK is activated by various signals derived from oxidative, metabolic, and inflammatory stresses via upstream MAPK kinases, mainly MKK3 and MKK6 [20]. Outcomes of MAPK14 signal cascade activation are pleiotropic and influence diverse biological processes, including cell proliferation, differentiation, apoptosis, senescence, and inflammation [16]. Functional roles of MAPK14 in vascular pathologies are complex depending on cell context and disease models. For example, mice with MAPK14 deficiency in macrophages were protected from LPS-induced sepsis due to the impairment in the expression of certain proinflammatory cytokines. However, these mice displayed enhanced macrophage apoptosis and progression of advanced atherosclerosis in the ApoE-/- experimental atherosclerosis model [21], [22]. MAPK14 has been frequently implicated as a critical regulator of VSMC inflammation, proliferation, migration, as well as redox-mediated cell death and apoptosis. We previously found that VSMC-MAPK14 is a negative regulator of the VSMC contractile phenotype through modulation of MKL1 nuclear translocation in cultured VSMCs [23]. Most of these results were exclusively derived from in vitro approaches using p38MAPK inhibitors which could also activate AKT pathway, and constitutively active upstream MAPK kinases (MKK3 or MMK6). Direct in vivo analysis of the role of VSMC-MAPK14 is still lacking due to the absence of VSMC-specific genetic knockout mouse model. Because of pleiotropic actions of MAPK14, the function of VSMC-MAPK14 on vascular homeostasis and pathology and the extent of its redox regulation remain an enigma.
In the present study, we generated inducible VSMC-specific MAPK14 knockout mice. Although the vasculature of these knockouts was phenotypically indistinguishable from WT littermates, these mice developed substantially less neointima after ligation injury. We show that MAPK14 deficiency in VSMCs inhibits VSMC de-differentiation and decreases VSMC proliferation and the inflammatory response in the injured vessels. This was confirmed in cultured VSMCs using several molecular and pharmacological approaches to manipulate the activity of MAPK14 pathway. Together with our previous findings, these results demonstrate a key pathological role of VSMC-MAPK14 in injury-induced neointima formation through p65/NFkB and NOX4 regulated signals. Our study may provide important mechanistic insight into the development of therapeutic strategies for limiting injury-induced stenosis through blocking MAPK14 pathway in VSMCs.
2. Materials and methods
2.1. Experimental animals
Mouse breeding has been approved by the Albany Medical College (AMC) Institutional Animal Care and Use Committee (IACUC). Mice carrying Myh11-CreERT2 were bred with ROSA mT/mG mice (Jackson Lab) or MAPK14f/f to generate Myh11-CreERT2-mTmG reporter mice for lineage tracing mature VSMCs, or Myh11-CreERT2-MAPK14f/f for specific deletion of MAPK14 in mature VSMCs, respectively [24], [25]. These mice were subjected to Tamoxifen (TMX) induction at a dose of 33 μg/g body weight in 100 μl sunflower oil intraperitoneally for 5 consecutive days to specifically activate Cre recombinase in mature VSMCs. All mice were viable and born at the expected mendelian ratio. Mice at 10–12 weeks of age were used for ligation surgery after 3 weeks of TMX wash-out time. All mice were with C57BL/6 background.
2.2. Human saphenous veins (HSV) samples collection and ex vivo culture of HSV
The HSV study was conducted in accordance to the protocols approved by AMC Institutional Review Board (IRB). HSV samples were de-identified discarded segments from patients undergoing surgical coronary artery bypass grafting (CABG) at AMC. HSV ex vivo culture was conducted as described [26]. Briefly, HSV samples were cut into 0.5-cm segment rings and cultured in RPMI 1640 supplemented with 30% FBS and 1% Penicillin /Streptomycin Solution for 2 weeks prior to total RNA extraction or tissue processing for immunohistochemistry staining.
2.3. Carotid artery complete ligation injury and tissue isolation
Complete carotid ligations were performed in accordance to the protocol approved by AMC's IACUC. Briefly, Myh11-CreERT2+/--mTmG or Myh11-CreERT2+/--MAPK14f/f male mice at age 10–12 weeks were anesthetized by 1–4% isoflurane inhalation. The left carotid artery was ligated completely immediately proximal to the carotid bifurcation after a midline incision of the neck. The left injured and right uninjured carotid arteries were harvested at 2–3 weeks after surgery for protein/RNA isolation or histopathological assessment. The isolated vessels were fixed in 4% paraformaldehyde PBS solution overnight at 4 °C followed by embedding in either optimal cutting temperature compound (OCT Tissue-Tek, No. 62550) or paraffin.
2.4. Morphometric analysis of carotid arteries
Carotid arteries were isolated at 2–3 weeks after ligation surgery, fixed with 4% paraformaldehyde (PFA) PBS solution overnight at 4 °C, and embedded in paraffin. The paraffin embedded blocks were trimmed till a complete cross section of the vessels was visible. Total of 800 µm immediately below the bifurcation was sectioned and included for measurement. 5 µm-thick sections were prepared. The intimal and medial areas were analyzed by Image J software. Intimal area was calculated as the internal elastic lamina area minus luminal area, the medial area was the external elastic lamina area minus the internal elastic lamina area.
2.5. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
Apoptosis of VSMCs in ligated carotid arteries was detected using a TUNEL Andy Fluor™ 488 Apoptosis Detection Kit (GeneCopoeia A050) according to manufacturer's instructions. Briefly, sections were deparaffinized and rehydrated, permeabilized by Proteinase K solution, incubated with TdT reaction cocktail, and labeled with Andy Fluor™ 488-Streptavidin staining solution. Sections were mounted with histology mounting medium (Sigma) supplemented with 40,6-diamidino-2-phenylin-dole (DAPI; H-1200, VECTASHIELD) for counterstaining DNA. Sections incubated with TdT reaction cocktail without terminal transferase were used as negative controls. Images were taken by a confocal microscope (DMI 4000B; Leica Microsystems, Wet-zlar, German) and quantitated by Image J software as described previously [27].
2.6. siRNA and adenovirus treatment in cultured VSMCs for cell proliferation and RNA/protein extraction
Primary human coronary artery smooth muscle cells (HCASMCs) were purchased from Invitrogen and cultured per the manufacturer's instruction. Human and mouse aortic SMCs (HASMCs and MASMCs) were prepared by the cell culture core at the Department of Molecular and Cellular Physiology, Albany Medical College. MASMCs were maintained in Dulbecco's Modified Eagle's Medium/Nutrient Mixture F-12 Ham (Gibco) supplemented with 10% fetal bovine serum (FBS; Gibco) and HASMCs in Medium 231 (Gibco) supplemented with SMG (Gibco). The source of siRNA to MAPK14 and the negative control siRNA, as well as Adenovirus carrying the constitutively active form of MKK6 (Ad-MKK6) and the negative control empty adenovirus (Ad-empty) were obtained and delivered to cultured VSMCs as described previously [23]. Two different siRNAs to human NOX4 were used (Thermo Fisher Scientific, s224159, s224160). RNA or protein was extracted 48 h or 72 h after the siRNA/virus treatment, respectively. For inhibitor treatment, serum starved VSMCs were pretreated with either SB203580 (Calbiochem, CAS 869185-85-3) or Bay117082 (Selleckchem No. S2913) at a dose of 5 µM for 45 min followed by PDGF (25 ng/ml, R&D, #220-BB-010) induction for 48 h prior to cell counting, or IL1β (4 ng/ml, R&D, #201-LB) for 24 h before RNA isolation. Proliferation assessment was done by cell counting using a hemocytometer as described [28]. HASMCs were pretreated with 5 µM of Bay117082 for 45 min followed by Ad-MKK6 or control virus transduction for 24 h or 72 h before RNA or protein extraction, respectively.
2.7. RNA isolation, qRT-PCR, and RNA-seq analysis
Total RNA from the indicated primary VSMC cultures or homogenized vessels was isolated using a miRNeasy Kit. Complementary DNA (cDNA) was synthesized using an iScript cDNA kit (Bio-Rad). IQ SYBR Green-based qRT-PCR was performed in a MyiQ real-time PCR detection system (Bio-Rad), as described previously [9]. mRNA levels were normalized by 18S ribosomal RNA (as a loading control) and calculated according to ΔΔCT value. Triplicate biological replicates were examined for each group and data were representative of multiple independent experiments (n ≥ 3). Primers for qRT-PCR for the indicated genes were included in supplemental Table 1. RNA-seq analysis and detailed information of library construction was described previously [29]. Briefly, total RNA was isolated from adult mouse aortas and RNA-seq was done at the University of Rochester Medical Center's Genomics Research Center with the polyadenylated RNA fraction at a depth of 20 million reads per replicate using the Illumina HISeq. 2500. Raw sequence reads were pre-processed by using CASAVA 1.8.2 for demultiplexing. Sequence reads were aligned to annotated transcripts on the UCSC Reference Genome (build GRCh37/hg19) via SHRiMP2.2.3. Data was quantitated by utilizing Cufflinks 2.0.2 and Cuffdiff2 (http://cufflinks.cbcb.umd.edu). Finally, the expression value of the all transcripts was presented as FPKM (fragments per kilobase of exon per million fragments mapped).
2.8. Immunohistochemistry and immunofluorescence staining
Freshly harvested tissues were immediately fixed in 4% PFA at 4 °C for 24 h. 10 µm frozen sections or 5 µm paraffin sections were prepared for immunohistochemistry (IHC) and immunofluorescence (IF) staining, respectively. Paraffin embedded sections were deparaffinized and rehydrated. After high pressure-mediated antigen retrieving in an antigen retrieval solution (DAKO, antigen retrieval solution) for 10 min, sections were permeabilized with 0.01% Triton X-100 in PBS, blocked with a blocking solution (Dako, Protein block serum-free, X0909) for 30 min at room temperature, and probed with the indicated primary antibodies for overnight at 4 °C and then horseradish peroxidase (HRP)-conjugated secondary antibody (1:500) or FITC- or TRITC-conjugated secondary antibody (1:500) for 1 h. Slides were finally counterstained with hematoxylin for IHC or DAPI for IF before photographed with a digital microscope or confocal microscope. A concentration and species-matched IgG was served as a negative control. Image J software was used to blindly measure the intensity of IF signal or the percentage of cells positive for the indicated staining. IF for frozen sections was carried out as previously described [30]. Briefly, isolated vessels were embedded in OCT, cryo-sectioned, and fixed in icy cold acetone for 10 min. For carotid artery harvested from mTmG reporter mice, trypsin-mediated antigen retrieval (REF TA-015-TR; Lab Vision Corporation, Fremont, CA) was employed according to manufacturer's instructions to retain the natural mTmG fluorescence followed by immunostaining as described above. Primary antibodies used were as follows: anti-MAPK14 (1:50, p38α/MAPK14, Santa Cruz, sc-535), anti-phospho-MAPK14 (1:50, pp38, Cell Signaling, #9211), anti-MYH11 (1:500, Alfa Aesar, BT-562), anti-CNN1 (1:500, Proteintech, 13938-1-AP), anti-Ki67 (1:500, Abcam, Ab 15580), and anti-CD45 (1:100, BD Pharmigen, 550539).
2.9. Protein extraction and western blot
Freshly harvested tissues were snap frozen in liquid nitrogen, and then homogenized in liquid nitrogen with pellet mixer (VWR, 47747-370). Protein lysates were extracted from homogenized tissue or cultured SMCs by using an ice-cold cell lysis buffer (Cell Signaling, #9803) supplemented with a protease inhibitor cocktail (1%, Sigma, 539131). Protein concentration was measured with the BCA protein assay kit (Bio-Rad, 500). Equal amount of protein was resolved on SDS-PAGE gel for western blotting as described previously [9]. Antibodies used for western blot were as follows: anti-MAPK14 (1:1000, Santa Cruz, sc-535), anti-pMAPK14 (1:1000, Cell Signaling, #9211), anti-MYH11 (1:2000, Alfa Aesar, BT-562), anti-human CNN1 (1:1000, DAKO, M3556), anti-ACTA2 (1:1000, Sigma, A2547), anti-LMOD1 (1:2000, Proteintech, 15117-1-AP), anti-TAGLN (1:1000, Abcam, ab10135), anti-p65 (1:1000, Cell Signaling, #8242), anti-phospho-p65 (1:1000, Cell Signaling, #3033), and anti-TUBA (1:5000, Sigma, T5168).
2.10. Statistical analysis
All experiments were repeated independently at least three times. Data in graphs were presented as mean ± SD. Statistical analysis was determined by an unpaired two-tailed Student's t-test using GraphPad Prism 6 to compare two groups. One-way ANOVA was utilized to compare three groups. P < 0.05 was considered statistically significant. P < 0.05 and P < 0.01 were indicated by * and **, respectively, throughout all the included graphs.
3. Results
3.1. MAPK14 expression and activity are induced in VSMCs under pathological vascular conditions
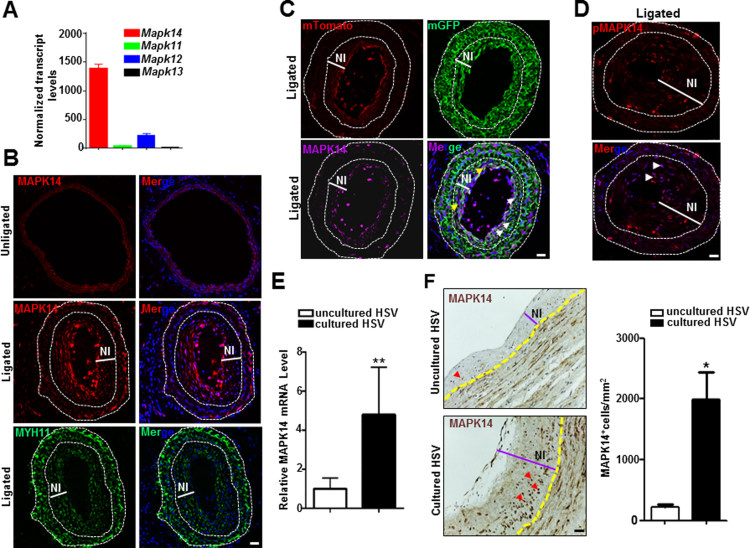
Through a recent RNA-seq study done in adult mouse aortas, we found that MAPK14 was highly expressed in the medial smooth muscle layer compared with other isoforms (Fig. 1A). We next set out to determine which cell types express MAPK14 and possible changes in its expression in a flow induced model of vascular injury through ligation of the left common carotid artery in mice. Three weeks after surgery, tissue sections were prepared from the ligated left and unligated right carotid arteries for immunostaining of MAPK14. MAPK14 protein showed much higher expression in the neointima compared with medial VSMC layers from either injured or uninjured vessels, an expression pattern in contrast to MYH11, the most specific marker of the contractile VSMC phenotype (Fig. 1B). We then sought to determine if neointima cells expressing high levels of MAPK14 protein were derived from a VSMC lineage. To this end, we immunostained for MAPK14 in the ligated carotid arteries from Myh11-CreERT2+/--mTmG reporter mouse, a reporter system that specifically labels mature VSMC with membrane GFP (mGFP) after Tamoxifen (TMX) induction. We found that most of GFP-labeled VSMCs show strong MAPK14 immunoreactivity and that, MAPK14 was also found in GFP negative non-VSMC cells (Fig. 1C). To test whether the highly expressed MAPK14 in the neointima was activated, we stained the vessels for phosphorylated MAPK14 (pMAPK14). There was undetectable pMAPK14 in the unligated vessels (data not shown), consistent with our previous report [23]. In contrast, neointimal cells in the ligated carotid arteries showed evident nuclear-stained pMAPK14, indicating that MAPK14 is activated after vascular injury (Fig. 1D).
Fig. 1.
MAPK14 expression and activity were induced under pathological vascular conditions. A) RNA-seq analysis of different p38MAPK family members (n = 5). B) Representative confocal microscopy images of immunofluorescence staining for MAPK14 in uninjured and injured, as well as MYH11 in injured mouse carotid arteries at 3 weeks after ligation injury. Scale bar: 20 µm. C) Representative confocal microscopy image for mGFP and mTomato labeled vessel wall and immunofluorescence staining for MAPK14 isolated from tamoxifen-induced Myh11-CreERT2+/--mTmG reporter mice at 3 weeks after ligation injury. Yellow and white arrowheads indicate non-VSMCs and VSMCs expressing high levels of MAPK14 protein, respectively. Scale bar: 20 µm. D) Representative image of immunofluorescence staining for pMAPK14 in injured mouse carotid artery isolated at 3 weeks after ligation injury. Arrowheads indicate pMAPK14+ cells. Dotted line in above images marks the external or internal elastic lamina. Scale bar: 20 µm. E) qRT-PCR for MAPK14 mRNA in uncultured versus 2 weeks cultured human saphenous vein (HSV) segments (n = 4). **, P < 0.01. F) Representative image of immunohistochemistry staining for MAPK14 in uncultured versus 2 weeks ex vivo cultured HSV segments (Left) and its quantitative analysis (Right, n = 4). Yellow dotted line marks the internal elastic lamina. Red arrows indicate MAPK14+ cells. NI, neointima. Scale bar: 40 µm.
A major late stage complication of HSV bypass graft is neointima hyperplasia which is attributable to extensive VSMC proliferation. We have established an ex vivo HSV culture system to recapitulate this pathological vein remodeling and observed HSV remodeling featuring thickened intimal and medial smooth muscle layers. Interestingly, we found that there was a significant increase in mRNA level of MAPK14 in the ex vivo cultured explants compared with uncultured segments derived from the same donors (Fig. 1E). Consistently, immunostaining showed that MAPK14 protein was markedly increased in the neointima region of the ex vivo cultured explants compared with uncultured controls (Fig. 1F). Taken together, these data demonstrated that both the expression and activity of MAPK14 were induced during pathological vascular remodeling, indicative of the functional importance of MAPK14 in neointima formation.
3.2. Specific deletion of MAPK14 in mature VSMCs inhibits injury-induced neointima formation
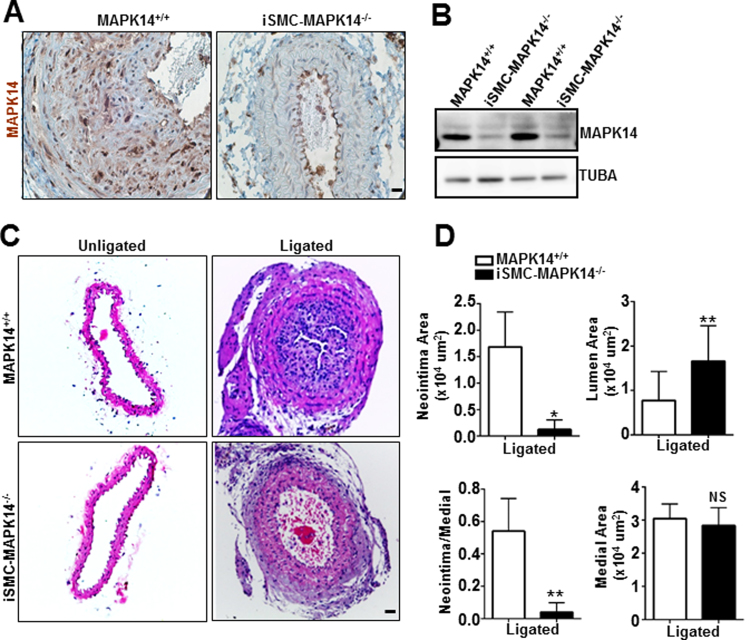
To examine the functional role of VSMC-MAPK14 in injury-induced vascular remodeling, we generated a novel mouse line carrying Myh11-CreERT2 and the floxed MAPK14 allele, Myh11-CreERT2+/--MAPK14f/f. We injected either TMX or oil to these mice to obtain inducible smooth muscle cell-specific MAPK14 knockout (iSMC-MAPK14-/-) or wildtype (WT, MAPK14+/+) control mice, respectively. Immunohistochemistry staining for MAPK14 in carotid arteries demonstrated that the deficiency of MAPK14 specifically occurs in VSMCs but not in adventitial and endothelial cells in the knockout mice (Fig. 2A). Immunostaining also confirmed MAPK14 deletion in SMCs from other organs such as bladder (supplemental Fig. I). Western blot analysis of protein lysates from medial layer SMCs in aortas showed evident reduction of MAPK14 in iSMC-MAPK14-/- compared with WT littermate controls (Fig. 2B). Compared with WT MAPK14+/+ littermate controls, the iSMC-MAPK14-/- mice did not show any visible defect at baseline levels, with comparable body weight, blood pressure, and vessel wall structure (supplemental Fig. II), suggesting that VSMC-MAPK14 is dispensable to adult vessel homeostasis. We next sought to evaluate if mature VSMC-MAPK14 could participate in vascular remodeling in the carotid artery ligation model. 3 weeks after ligation, carotids from iSMC-MAPK14-/- mice showed dramatically reduced neointima formation (Fig. 2C). Quantitative morphometric analysis showed there was decreased neointima area and ratio of neointima to medial layer area but increased lumen area in the injured vessels from knockouts iSMC-MAPK14-/- compared with WT MAPK14+/+ mice (Fig. 2D). The medial VSMC layer areas were not different between the knockouts and WT mice in both injured and uninjured vessels (Fig. 2D and supplemental Fig. IIB). These data demonstrated that mature VSMC-MAPK14 was required for vascular injury-induced neointima formation but dispensable for vessel homeostasis.
Fig. 2.
Specific deletion of MAPK14 in mature VSMCs mitigates injury-induced neointima formation. A) Immunohistochemistry staining of MAPK14 in injured carotid arteries harvested at 3 weeks after ligation injury. Ligation was done in Myh11-CreERT2+/--MAPK14f/f mice at 3 weeks after vehicle or tamoxifen injection for 5 consecutive days. Note: Specific deletion of MAPK14 was only seen in medial layer VSMCs in the vessel wall. Scale bar: 10 µm. B) Western blotting for the indicated proteins in total protein lysates of aortic medial SMC layer isolated from Myh11-CreERT2+/--MAPK14f/f mice 3 weeks after vehicle (for MAPK14+/+) or tamoxifen (for iSMC-MAPK14-/-) injection. C) Representative image of hematoxylin and eosin (HE) staining of sections of carotid arteries isolated from MAPK14+/+ or iSMC-MAPK14-/- adult mice at 3 weeks after ligation injury. Scale bar: 20 µm. D) Quantification morphometric measurements of ligated carotid arteries from MAPK14+/+ (n = 7) and iSMC-MAPK14-/- (n = 8) mice isolated at 3 weeks after ligation injury. Data shown as mean ± SD. *, P < 0.05; **, P < 0.01.
3.3. VSMC-MAPK14 negatively regulates VSMC contractile protein expression
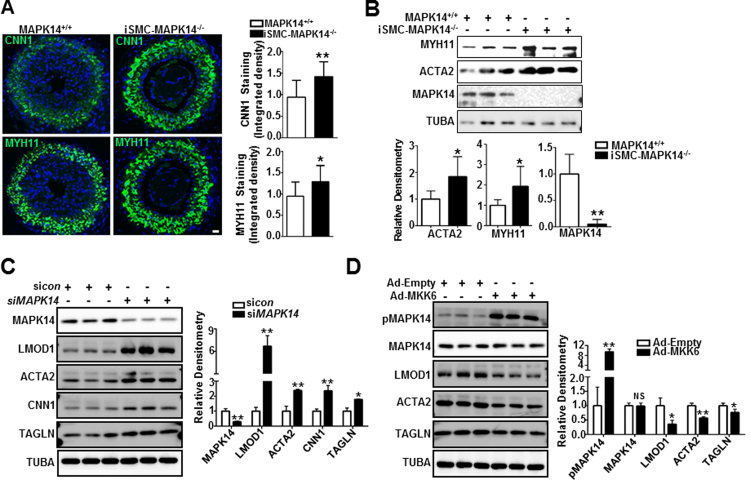
VSMC phenotypically switching from the contractile/differentiated state to a synthetic dedifferentiated state is a prerequisite for neointima formation after vascular injury. We previously reported that MAPK14 negatively regulates VSMC differentiation in cultured VSMCs [23]. Based on these past observations, we wanted to determine whether the loss of MAPK14 in VSMCs could stabilize the contractile phenotype in vivo and therefore inhibit neointima formation following carotid artery ligation. We performed immunofluorescence staining for CNN1 and MYH11, two specific VSMC contractile proteins in the ligated carotid arteries 2 weeks after surgery. Though there is no significant difference in the expression of VSMC contractile proteins in the unligated carotids (supplemental Fig. III), stronger signals of both CNN1 and MYH11 protein were seen in iSMC-MAPK14-/- relative to MAPK14+/+ in the ligated vessels revealed by immunostaining (Fig. 3A, left panel). Quantitative analysis of 12 pairs of injured carotids showed a significant increase in CNN1 and MYH11 protein staining in iSMC-MAPK14-/- compared with WT control mice (Fig. 3A, right panel). Further, western blot of protein lysates of purified medial SMC layer from uninjured aortas showed a significant increase in protein expression of both MYH11 and ACTA2 in iSMC-MAPK14-/- mice (Fig. 3B). In addition, MYH11 mRNA was also significantly elevated in aortas from knockout mice (supplemental Fig. IV). These findings suggest that MAPK14 is a negative regulator of VSMC differentiation in vivo. To extend these findings to human cells, we manipulated MAPK14 pathway in cultured primary human VSMCs. siMAPK14-mediated MAPK14 gene depletion increased whereas activation of MAPK14 pathway via adenovirus transduction of a constitutively active form of MKK6 (Ad-MKK6), an upstream kinase of MAPK14, decreased VSMC contractile protein expression in cultured human VSMCs (Fig. 3C, D). This result is consistent with our previous report [23]. This suggests that MAPK14 is a negative regulator of VSMC differentiation; depletion of MAPK14 in mature VSMCs stabilizes the contractile phenotype following ligation injury which could antagonize injury-induced neointima formation.
Fig. 3.
VSMC-MAPK14 negatively regulates the expression of VSMC contractile proteins. A) Representative image of immunofluorescence staining for CNN1 and MYH11 in the injured carotid arteries from MAPK14+/+ versus iSMC-MAPK14-/- mice isolated at 2 weeks after ligation injury. Quantification of the integrated fluorescent signal of the CNN1 and MYH11 staining in injured carotid arteries from MAPK14+/+ and iSMC-MAPK14-/- mice was analyzed blindly by image J software. Data shown are mean ± SD (n = 12 mice/group). *, P < 0.05, **, P < 0.01. Scale bar: 20 µm. B) Western blotting for the indicated proteins in total protein lysates of aortas isolated from MAPK14+/+ and iSMC-MAPK14-/- mice (Upper) and the densitometric analysis by Image J for the indicated protein normalized to loading control TUBA (Bottom). Data shown are mean ± SD (n = 6). *, P < 0.05, **, P < 0.01. C) Growing human coronary artery smooth muscle cells (HCASMCs) were transfected with siRNA to MAPK14 (siMAPK14) or a scramble control siRNA (sicon) for 72 h followed by western blotting for the indicated proteins (Left) and densitometric analysis for the indicated proteins as descried above (Right). D) Growing human aortic smooth muscle cells (HASMCs) were transduced with adenovirus carrying a constitutively active form of MKK6 (Ad-MKK6) or equal amount of control adenovirus (Ad-Empty, MOI=50) for 72 h followed by western blotting for the indicated proteins (Left) and its quantitative analysis as described above (Right). Results are representative of at least 3 separate experiments and each with 3 biological replicates. Densitometric analysis of the indicated proteins normalized to loading control TUBA is shown. *, P < 0.05, **, P < 0.01.
3.4. Deficiency of MAPK14 in VSMCs suppresses VSMC proliferation
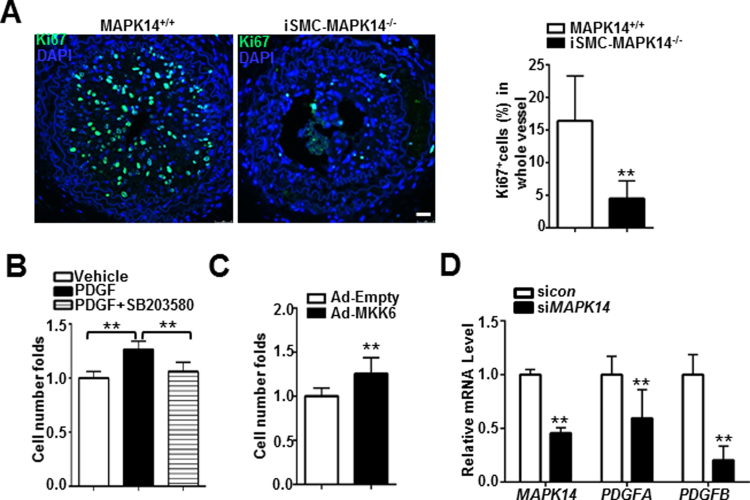
Extensive proliferation of VSMC has been demonstrated as a major contributor to neointima formation in various vascular pathologies [31], [32]. To determine if loss of MAPK14 could influence VSMC proliferation after ligation injury, we performed Ki67 staining in the tissue sections prepared from the ligated carotid arteries isolated at 2 weeks after injury. We found that there was substantially less Ki67 positive cells in the neointima region of the vessels from iSMC-MAPK14-/-mice relative to MAPK14+/+ WT mice (Fig. 4A), suggesting that loss of MAPK14 in VSMCs decreased VSMC proliferation triggered by ligation injury. In a different set of experiments, acute inhibition of MAPK14 using a selective inhibitor of MAPK14, SB203580 was sufficient to suppress PDGF-induced cell proliferation in cultured HCASMCs (Fig. 4B). Conversely, forced expression of constitutively active MMK6 through adenovirus transduction significantly increased human aortic VSMCs (HASMCs) proliferation (Fig. 4C). These in vivo and in vitro data support a positive role of MAPK14 in regulating VSMC proliferation. To begin to elucidate the mechanism responsible for the reduced VSMC proliferation achieved by VSMC deficiency of MAPK14, we sought to examine the expression of genes with recognized roles in cell proliferation. Consistently, we found that depletion of endogenous of MAPK14 in cultured HCASMCs significantly attenuated the mRNA levels of two important growth factors, PDGFA and PDGFB (Fig. 4D). MAPK14 has been reported as a critical regulator of apoptosis [22], [25] and decreased VSMC apoptosis has been shown to contribute directly to neointima formation in multiple knockout mouse models [33], [34]. To determine if loss of MAPK14 could promote VSMC apoptosis and therefore inhibit neointima formation, we performed TUNEL staining in the ligated vessels isolated at different stages after ligation surgery. There was no significant difference in the percentage of apoptotic cells to total cells within the vessel wall of injured carotids isolated at either 2 days (data not shown) or 2 weeks (supplemental Fig. V) after ligation surgery between the knockouts and WT control mice, indicating that MAPK14 has no influence on apoptosis of vascular cells in ligation model. Taken together, these data demonstrated that the deficiency of VSMC-MAPK14 decreases VSMC proliferation as well as gene expression of two important growth factors, PDGFA and PDGFB, which might contribute to the reduced neointima formation in iSMC-MAPK14-/- mice induced by ligation injury.
Fig. 4.
Deficiency of MAPK14 in VSMCs suppresses VSMC proliferation. A) Representative photomicrographs of immunofluorescence staining for a proliferation marker, Ki67 in carotid arteries isolated at 2 weeks after ligation from MAPK14+/+ and iSMC-MAPK14-/- mice (Left). The percentage of Ki67+ cells in injured carotid arteries was shown (right). Data shown are mean ± SD, n = 5. Scale bar: 20 µm. B) HASMCs were starved overnight followed by treatment with 5 μM SB203580 or vehicle control for 30 min prior to stimulation with 50 ng/ml PDGF. Cells were counted by hemocytometer at 24 h after PDGF stimulation. C) Growing HASMCs were transduced with adenovirus carrying a constitutively active form of MKK6 (Ad-MKK6) or equal amount of control adenovirus (Ad-Empty) for 72 h before cell counting as above. Cell proliferation was defined as fold change compared to vehicle control (set to 1). D) HCASMs were transfected with siMAPK14 or siRNA control (sicon) for 72 h before RNA isolation for qRT-PCR of the indicated genes. Data shown in B–D are mean ± SD and are representative of 3 separate experiments. *P < 0.05, *P < 0.01.
3.5. Loss of MAPK14 in VSMCs blunts vascular injury induced-inflammatory response
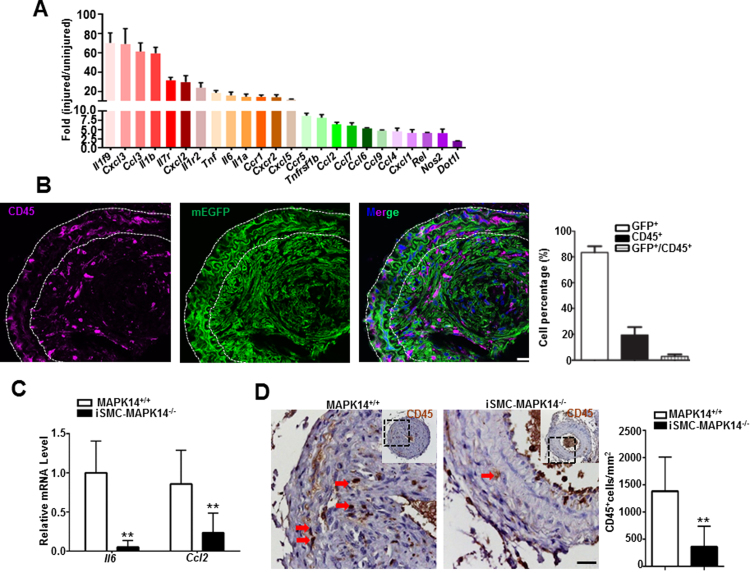
It has been reported that vascular injury triggers an acute inflammatory response, including elevated expression of proinflammatory cytokines and chemokines in the vessel wall, and inflammatory cell infiltration and accumulation [35], [36]. Consistent with this notion, quantitative analysis of the data from a public RNA array study done with the ligation injured carotid arteries [37] revealed a robust induction of a large number of proinflammatory genes, including cytokines, chemokines, and activators of inflammation (Fig. 5A). However, the precise contribution of individual cell lineages to the induction of proinflammatory genes expression is unclear. To more precisely track VSMCs and inflammatory cells in injury-induced vascular remodeling, we isolated injured carotid arteries at 3 weeks after carotid ligation from Myh11Cre-ERT2-mTmG reporter mice induced by TMX and performed immunofluorescence staining for CD45, a leukocyte cell membrane marker. Though the majority of neointima cells were derived from VSMCs which were positive for GFP, there were CD45 positive but GFP negative non-SMC inflammatory cells in both neointima and medial layer regions (Fig. 5B). Such inflammatory cell accumulation was not seen in the unligated carotid arteries (supplemental Fig. VI). Among all these CD45 positive cells in the ligation-injured vessels, very few of them were derived from VSMCs positive for GFP, indicating that VSMC transition to inflammatory cell lineages is not prevalent in this context. Given the central role of MAPK14 in inflammation activation, we hypothesize that deficiency of MAPK14 dampens the above inflammatory response. To test this, we isolated injured vessels at 2 weeks after ligation injury from MAPK14+/+ and iSMC-MAPK14-/- mice for inflammatory response measurements. qRT-PCR showed that mRNA levels of some proinflammatory genes such as Il6 and Ccl2 were significantly decreased in ligated vessels from iSMC-MAPK14-/- mice (Fig. 5C). Consistently, there was a significant reduction of CD45 positive cells in injured vessels from iSMC-MAPK14-/- relative to MAPK14+/+ mice (Fig. 5D). These results demonstrated that VSMC-MAPK14 is critical for ligation-injury triggered inflammation response including proinflammatory gene expression and inflammatory cells infiltration and accumulation.
Fig. 5.
VSMC-MAPK14 knockout mice display impaired inflammatory response upon ligation injury. A) Inflammation-associated genes were determined by RNA microarray in carotid arteries at 5 days after complete ligation injury. Gene expression fold-changes are compared to uninjured carotid arteries and plotted as mean ± SD (n = 4). B) Representative image of immunofluorescence staining for CD45 protein in the injured carotid arteries isolated at 3 weeks after ligation injury from a tamoxifen-induced Myh11-CreERT2+/--mTmG reporter mouse and the quantitation of the indicated cell categories (n = 3). Note: There were considerable numbers of CD45+GFP- cells in the injured vessels at this time point. Scale bar: 20 µm. C) qRT-PCR of the indicated genes in injured carotid arteries from MAPK14+/+ versus iSMC-MAPK14-/- mice at 2 weeks after ligation injury (n = 4). D) Representative image of immunostaining for CD45 protein in injured vessels from MAPK14+/+ and iSMC-MAPK14-/- mice at 3 weeks after ligation injury (Left); quantitation of CD45+ cells in injured vessels (n = 5) (Right). Arrows indicate CD45+ cells. Data shown are mean ± SD. **P < 0.01, *P < 0.05. Scale bar: 20 µm.
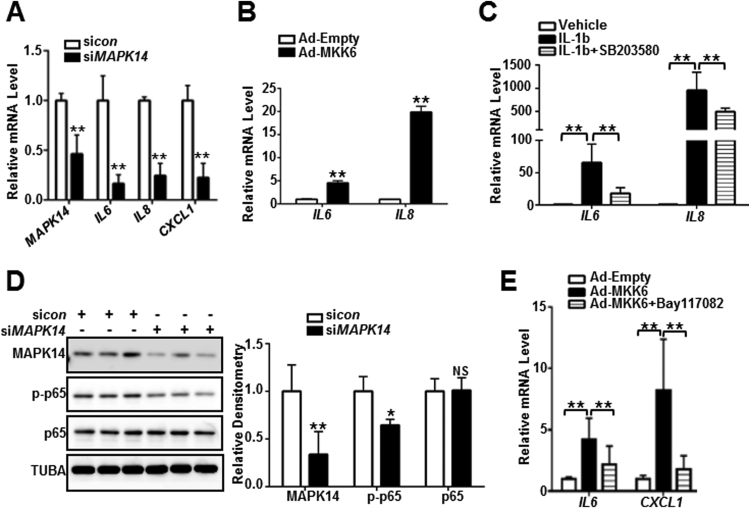
3.6. MAPK14 activated proinflammatory gene expression is p65 /NF-κB-dependent
To delineate the role of MAPK14 in regulating the expression of proinflammatory genes in VSMCs, we performed loss and gain of MAPK14 function studies in cultured primary human VSMCs using multiple approaches. First, we performed siRNA-mediated MAPK14 knockdown experiments. Depletion of MAPK14 in HCASMCs downregulated gene expression of a battery of proinflammatory genes including proinflammatory cytokine and chemokines IL6, IL8, CXCL1 (Fig. 6A). Conversely, activation of MAPK14 pathway by Ad-MKK6 transduction resulted in a dramatic increase in gene expression of IL6 and IL8 (Fig. 6B). Further, blockade of the MAPK14 signal pathway by SB203580 treatment significantly decreased IL1β-induced IL6 and IL8 mRNA expression (Fig. 6C). Similar results were found in mouse aortic VSMCs (MASMCs) (supplemental Fig. VII). These data demonstrated a critical role of the MAPK14 pathway in activating a proinflammatory gene program in cultured VSMCs. Previous studies in other contexts have reported that MAPK14 was able to increase NF-κB activity and activate proinflammatory gene expression [38]. Consistent with this notion, knockdown of MAPK14 through siMAPK14 transfection in HCASMCs resulted in a significant reduction of phosphorylated p65 (pp65) while levels of total p65 protein was not altered, indicating that MAPK14 can activate p65 (Fig. 6D). Further, a selective inhibitor of p65/NF-κB pathway, Bay117082 significantly decreased MKK6-induced proinflammatory genes such as IL6 and CXCL1 (Fig. 6E). Interestingly, though a consistent downregulation of VSMC contractile proteins were observed in Ad-MKK6-transduced human VSMCs, pretreatment of Bay117082 failed to rescue either the suppression of VSMC contractile proteins such as LMOD1 and ACTA2 or the upregulation of PDGFRB induced by MKK6 overexpression (supplemental Fig. VIII). Taken together, these results indicate that MAPK14 activates the proinflammatory gene program through p65/NF-kB-dependent pathway.
Fig. 6.
MAPK14-activated proinflammatory gene program is p65/NF-κB-dependent.
A) HCASMCs were transfected with siMAPK14 or siRNA control (sicon) for 72 h before RNA isolation for qRT-PCR of the indicated genes. B) HASMCs were transduced with Ad-MKK6 or equal amount of control virus (Ad-Empty) for 72 h followed by RNA isolation for qRT-PCR assessment of the indicated genes. C) HASMCs were pretreated with 10 μM of SB203580 for 30 min prior to 4 ng/ml IL-1b stimulation for 24 h. RNA was isolated for the assessment of the indicated genes by qRT-PCR. D) HCASMCs were transfected with siMAPK14 or sicon for 72 h followed by western blotting for the indicated proteins (Left) and its densitometric analysis of protein levels normalized to loading control TUBA (right). E) HASMCs were pretreated with 5 μM of Bay117082 for 45 min prior to Ad-MKK6 or control virus (Ad-empty) transduction for 48 h. RNA was isolated for qRT-PCR assessment of the indicated genes. Results were representative of at least 3 separate experiments and 3 biological replicates were included for each experiment. Data shown are mean ± SD. *P < 0.05, **P < 0.01.
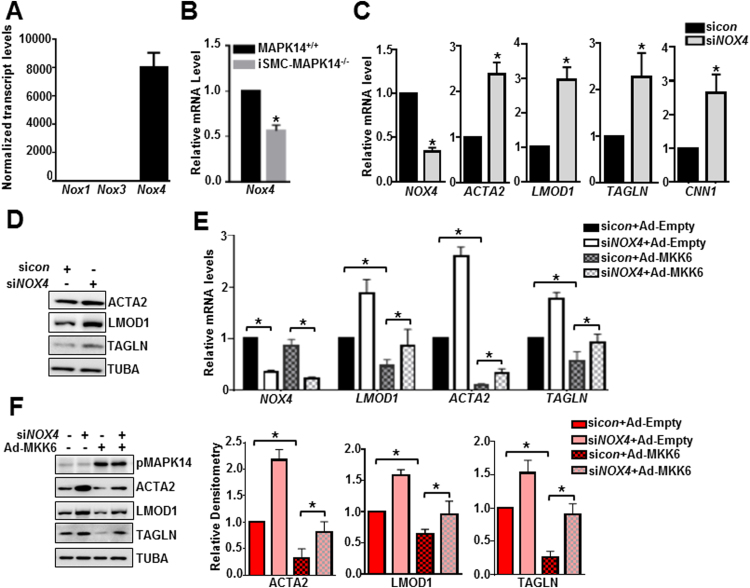
3.7. NOX4 contributes to MAPK14 suppressed VSMC contractile gene program
Increase in redox signaling after vascular injury is as an important contributor to neointima formation [39], [40]. NADPH oxidase (NOX) family members are critical regulators of these redox signaling pathways and contribute to neointima formation [39]. To determine if MAPK14 regulation of VSMC phenotypic modulation requires a redox signal, we first examined the gene expression profile of NOX family members through the aforementioned RNA-seq in mouse aortas. While Nox1 and Nox3 are undetectable in VSMCs, Nox4 was abundantly expressed in these cells (Fig. 7A). Similarly, a separate RNA-seq study (accession number GSE85910) revealed that NOX4 was the only NOX family member expressed in HCASMCs (data not shown). These results suggest that NOX4 might be the key NOX family member in VSMCs. Nox4 mRNA levels were significantly decreased in injured carotid arteries from iSMC-MAPK14-/- mice compared with MAPK14+/+ WT mice at 2 weeks after ligation injury, indicating a positive regulation of MAPK14 on NOX4 expression in vivo (Fig. 7B). To establish NOX4 function in VSMC phenotypes, we performed siRNA study in HCASMCs. siRNA-mediated NOX4 knockdown resulted in an upregulation of VSMC contractile genes at both mRNA and protein levels (Fig. 7C, D), suggesting that NOX4 is a negative regulator of VSMC contractile phenotype. Similar results were obtained with a separate siRNA to NOX4 (data not shown). To determine if NOX4 is a critical mediator for MAPK14 to suppress the VSMC contractile phenotype, we performed a rescue experiment. We depleted NOX4 via siRNA and then transduced Ad-MKK6 to activate MAPK14 pathway in HCASMCs. We found that loss of NOX4 partially rescued MKK6 suppression of VSMC contractile genes at both mRNA and protein levels (Fig. 7E, F). In contrast, depletion of NOX4 via siRNA failed to influence proinflammatory gene program in HCASMCs (supplemental Fig. VIIII). These data indicate that NOX4 contributes to MAPK14 suppression of VSMC contractile but not activation of proinflammatory gene program.
Fig. 7.
NOX4 contributes to MAPK14 suppressed VSMC contractile gene program. A) RNA-seq analysis of NADPH oxidase (NOX) family members (n = 5). B) qRT-PCR analysis of Nox4 in injured carotid arteries at 2 weeks after ligation injury from MAPK14+/+ versus iSMC-MAPK14-/- mice. n = 6. C) HCASMCs were transfected with siNOX4 or siRNA control (sicon) for 72 h before RNA isolation for qRT-PCR of the indicated genes. D) Western blotting for the indicated proteins in HCASMCs transfected with siNOX4 or siRNA control (sicon) for 72 h. E) HCASMCs were treated with siNOX4 for 24 h followed by Ad-MKK6 transduction for 48 h before RNA isolation for qRT-PCR analysis of the indicated genes. F) HCAMSCs treated as (E) followed by western blotting for the indicted proteins (Left) and its densitometric analysis of protein levels normalized to loading control TUBA (Right). Results were representative of at least 3 separate experiments and 3 biological replicates were included for each experiment. Data shown are mean ± SD. *P < 0.05.
4. Discussion
MAPK14 is a stress-induced and pleiotropic pathway. Carefully elucidating its regulation and functionality in a given cell type under a specific pathological condition is essential to manipulate this pathway for translational application. Although many studies have documented that MAPK14 is activated under various pathologies, the real action of VSMC MAPK14 in disease settings in vivo has yet to be definitively characterized using genetic knockout mouse model. In this study, we first showed that both MAPK14 expression and activity were induced in VSMCs in injured mouse arteries and ex vivo cultured human saphenous veins. We then demonstrated that specific deletion of MAPK14 in mature VSMCs reduced neointima formation in a carotid artery ligation injury model. Using combined genetic and pharmacological in vitro and in vivo approaches, we provided the following mechanistic evidence supporting the pathological role of VSMC-MAPK14 in injury-triggered neointima formation: 1) depletion of VSMC-MAPK14 increased VSMC contractile proteins expression but decreased VSMC proliferation; 2) Using unbiased RNA array gene analysis combined with VSMC-specific lineage tracing reporter system, we showed that vascular injury evoked a robust inflammatory response, including proinflammatory gene expression and inflammatory cells accumulation in neointima region; and deficiency of MAPK14 in VSMCs dampens such inflammatory responses; 3) we delineated a MAPK14/p65-dependent pathway mediating proinflammatory gene expression that was not involved in MAPK14 regulated expression of VSMC contractile proteins; 4) we showed that NOX4 is a critical mediator for MAPK14 in suppressing the VSMC contractile gene program with no evident effect on the VSMC inflammatory gene program. Therefore, though MAPK14 has been documented as a double-edged signal transducer under various contexts, our current study clearly demonstrated a pathological role of VSMC-MAPK14 in injury-induced neointima hyperplasia through its diverse detrimental functions on VSMC phenotype. This indicates that specific blockade of VSMC-MAPK14 pathway might represent an effective therapy to mitigate injury-induced stenosis in clinic, and pharmacological therapies targeting NOX4 inhibition should take into consideration of MAPK14 signaling.
Past studies overwhelmingly support the idea that differentiated contractile VSMCs phenotypically switch to a de-differentiated synthetic phenotype and this is a key step in the formation of a neointima, a hallmark of several prominent vascular diseases [4], [5], [6]. This concept has been reinforced recently by authentic lineage tracing studies to fate map formerly differentiated VSMCs in different vascular disease models [31], [32]. Consistent with this notion, we also found that the majority of neointima cells that are derived from mature VSMCs display much decreased VSMC contractile proteins compared with medial layer contractile VSMCs. Of interest, we found that these phenotype-switched neointimal VSMCs exhibited higher expression level and activity of MAPK14 protein. This result together with similar findings ex vivo in cultured HSV explants suggest that activation of a MAPK14 pathway is positively associated with the synthetic VSMC phenotype, which may predispose the vessels to pathological remodeling such as neointima formation. MAPK14 is a stress sensitive pathway subjected to various stimuli for activation. Mechanical injury as well as injury-induced growth factors and inflammatory factors have been reported as local stimuli to activate this pathway [41], [42]. Therefore, the induced MAPK14 activity we observed was expected. However, the induction of MAPK14 expression during VSMC phenotypic modulation is somewhat intriguing. Since a specific kinase of MAPK14, MKK6 has little effect on total MAPK14 protein expression (Fig. 3D), it is unlikely that the induction of MAPK14 during the VSMC phenotypic modulation is attributed to the feedback loop action of the MAPK14 pathway. This was further supported by our recent RNA-seq study wherein the abundance of MAPK14 transcripts was not changed after IL1β stimulation though the activity of MAPK14 was rapidly induced by IL1β in primary cultured human VSMCs (data not shown). The mechanism responsible for the injury-induced MAPK14 expression in VSMC is not clear in our current study. Recent studies have documented that MAPK14 is a direct target of several critical miRs, such as miR17/106, miR125, miR124, and miR128 in cancer cells and neurons [43], [44], [45], [46]. It will be interesting to examine if any of these miRs could be repressed under pathological vascular conditions, which in turn de-represses MAPK14 mRNA expression. On the other hand, emerging evidence demonstrated that some pivotal signal modulators, such as Fbxw7 and β-catenin are regulated by long noncoding RNAs (lncRNAs), a pervasive noncoding RNA class that act via diverse mechanisms, such as competing endogenous RNA or microRNA sponging pathways [47], [48]. Whether the induction of MAPK14 expression during VSMC phenotypic modulation involves lncRNA-mediated events deserves future investigation.
An interesting finding in this study, beyond the populated synthetic/de-differentiated VSMCs, is high expression of MAPK14 also observed in non-VSMC lineage(s) within the neointima region. Though the precise lineage of these non-SMCs is unclear, one would speculate these could be infiltrated inflammatory immune cells, local or bone marrow-derived progenitor cells, or other transdifferentiated cells such as macrophages and mesenchymal stem cells [49], [50]. In line with this notion, lineage tracing showed that a significant fraction of the CD45 positive cells were of non-SMC origins. Synthetic VSMCs per se are proinflammatory in nature and this is further supported by the association of CD45 with a small but significant fraction of cells derived from a SMC lineage [51], [52]. The infiltrated proinflammatory non-VSMC cell types in conjunction with the synthetic and inflammatory VSMCs therefore confer the vasculature a “highly inflamed” status which is geared for the activation of proinflammatory gene expression. Indeed, RNA-array of injured vessels at 5 days revealed a massive induction of an array of inflammatory genes. Some inflammatory cytokines and chemokines, such as CCL2 and TNFa, not only act as chemoattractant to trigger cell infiltration and migration, but also induce VSMC phenotype transition through both autocrine and paracrine fashions and therefore potentiate neointima formation [53], [54]. As such, inhibition of VSMC inflammation might be an important step to limit vascular injury-triggered stenosis clinically.
We found there was increased VSMC contractile protein expression in the injured carotids and medial smooth muscle layer from uninjured aortas in the knockouts. This in vivo data is consistent with our published in vitro findings which we reproduced in our current study [23], demonstrating that MAPK14 is a negative regulator of VSMC contractile phenotype in vitro and in vivo. Previously, we demonstrated a Rho-MRTFA regulatory axis in modulating VSMC contractile gene expression in vitro [23]. Whether this pathway functions similarly in vivo warrants further investigation through the generation of MRTFA-/-/VSMC-MAPK14-/- double knockout mouse. Oxidative stress has been established as critical contributor to various vascular pathologies including neointima formation through various signal cascades. These redox pathways may be derived from multiple sources of reactive oxygen species but NADPH oxidase (NOX) family members have been identified as critical sources. NOXs have been reported to be upregulated in injury-induced intima, and to contribute to enhanced neointima formation, for example in α7nAChR-KO mice [39]. This indicates a pathological role of NOX family in neointima formation. Consistent with this notion, we showed that NOX4 was downregulated in injured vessels of VSMC-MAPK14-/- knockouts, and loss of NOX4 in cultured VSMCs partially rescued MAPK14 suppression of the VSMC contractile gene program but failed to influence its activation of VSMC inflammation. This result is inconsistent with a previous report wherein Nox4 was reported as a critical regulator for the maintenance of the differentiated vascular smooth muscle cell phenotype during in vitro VSMC passage [55]. The discrepancy of NOX4 function on VSMC differentiation is very likely attributed to different contexts. Nevertheless, our current study not only reinforced the contributory role of NOX4 to neointima formation, but also provided a novel mechanism for MAPK14 in suppressing the VSMC contractile phenotype.
It is possible that the induction of VSMC differentiation by MAPK14 deficiency could be an indirect effect from its function on VSMC proliferation. Indeed, we found that depletion of MAPK14 inhibited VSMC proliferation which was accompanied with reduced gene expression of PDGFA and PDGFB, two potent growth factors with recognized role in downregulating VSMC contractile gene expression. In our current study, we provided clear evidence supporting that MAPK14 is critical for VSMC proliferation. However, the underlying molecular mechanism awaits further exploration. MAPK14 has recently been reported as a vital modulator to build a favorable microenvironment to promote lung tumorigenesis through a hyaluronan-dependent pathway [56]. In the vascular system, hyaluronan/CD44 is an important pathway that promotes vascular pathologies such as neointima formation [37], [57]. It is possible that p38MAPK-hyaluronan-dependent pathway might also underlie MAPK14-enhanced VSMC proliferation and thus neointima formation. Finally, a recent study elegantly reported a positive role of MAPK14 in Wnt3a induced beta-catenin nuclear retention through phosphorylation of MEF2, which contributes to increased cell proliferation in vitro [58]. Given that MEF2 is an established substrate of MAPK14, it would be of interest to determine if MAPK14/MEF2/beta-catenin regulatory axis could act similarly in our current context.
MAPK14 is a pivotal activator of the proinflammatory gene program through either the direct activation of key transcription factors such as ATF-2 and C-EBPB, or indirect regulation of the downstream kinases MK2 and ASK1 [59]. Whether these downstream targets of MAPK14 are responsive for MAPK14 regulation on VSMC inflammation, remains to be explored in the future. The central proinflammatory transcription factor, p65, is not a direct substrate of MAPK14. However, activated p38MAPK has been reported to promote NF-κB-dependent proinflammatory gene expression, leading to a senescence-associated secretory phenotype or Bacteroides fragilis enterotoxin-induced mucosal inflammation [38], [60]. Similarly, we found that a selective inhibitor of p65, Bay117082 significantly suppressed MKK6-induced proinflammatory genes and decreased levels of phosphorylated p65 (pp65) while it failed to effect VSMC contractile protein expression and PDGFR. This suggests that MAPK14 might utilize distinct downstream pathways to regulate the proinflammatory, proliferative, and contractile gene programs in VSMCs, which together contribute to the enhanced neointima formation induced by vascular injury.
Germ-line deletion of MAPK14 results in embryonic lethality at midgestation due to a severely defective placenta. Though there was a striking malformation of blood vessels in these knockouts, this defect was found to be secondary to the highly disrupted placental organogenesis [19]. This suggests that MAPK14 might be dispensable for vascular development. In agreement with this notion, although there has been an overwhelming amount of evidence supporting critical roles of MAPK14 in EC pathologies [61], [62], depleting MAPK14 in endothelial cells using Tie2-Cre driver does not lead to any defect in vasculature in mice at baseline [16]. Our current study showed that induced loss of MAPK14 in mature VSMCs failed to impact adult vasculature homeostasis but exhibited strong protection from injury-induced neointima formation. These results support the possibility that therapeutics specifically targeting VSMC-MAPK14 inhibition could be developed to limit injury-induced vascular stenosis without causing any adverse effect on the vasculature. Given that several specific inhibitors of p38MAPK pathway are currently under different phases of clinical trial, utilization of these inhibitors for limiting vascular neointima hyperplasia might be a reasonable strategy [17], [63]. In addition, our current finding was based on injury-induced vascular remodeling, it is conceivable that VSMC-MAPK14 might also contribute to the pathogenesis of other vascular disorders such as atherosclerosis and aneurysm wherein VSMC phenotypic modulation also plays critical roles.
In conclusion, we demonstrated that pathological conditions, such as vascular injury induce MAPK14 expression and activation; loss of VSMC-MAPK14 protects against injury-induced neointima formation, which may be attributed to the increased VSMC contractile protein expression and reduced VSMC proliferation and inflammatory response evoked by the loss of MAPK14 in VSMCs. We also delineated a NF-kB-dependent signaling pathway in mediating the positive regulation of MAPK14 on proinflammatory gene program but not VSMC differentiation. In contrast, MAPK14-mediated regulation of VSMC differentiation required integration with a redox signal relying on NOX4 expression. Given that loss of MAPK14 in ECs or VSMCs is dispensable to homeostasis of adult vessels, our studies thus laid down an important mechanistic foundation for therapeutically targeting injury-induced vascular stenosis through local delivery of MAPK14 inhibitors.
Acknowledgements
We thank Mrs. Diane Singer for providing us the primary human and mouse VSMC cultures, and Frances L. Jourd'heuil for AVF sections. We thank the University of Rochester Genomics Research Center for performing the RNA-seq experiments. We thank Dr. Kinya Otsu from Department of Internal Medicine and Therapeutics, Osaka University Graduate School of Medicine in Japan, and Dr. Stefan Offermanns from Institute of Pharmacology, University of Heidelberg in Germany for kindly providing MAPK14f/f and Myh11-CreERT2 mouse lines, respectively. We thank the following surgeons at Albany Medical Center for providing the precious human samples: David Conti, MD, Reynold Lopez-Soler, MD and Ph.D., Nikolaos Chandolias, MD from AMC Surgery Group-Transplantation Department, and Paul Kreienberg, MD from the Vascular Group, PLLC, Albany.
Acknowledgments
Sources of funding
This work is supported by National Institutes of Health R01HL122686, Paul Teschan Research Fund #2017-07, and Albany Medical College faculty startup funding to X.L; R01HL49426 to H.A.S; American Heart Association 16GRNT31280002 to D.J.
Disclosures
None.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2019.101137.
Appendix A. Supplementary material
Supplementary material
Supplementary material
References
- 1.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 2.Kearney M., Pieczek A., Haley L., Losordo D.W., Andres V., Schainfeld R., Rosenfield K., Isner J.M. Histopathology of in-stent restenosis in patients with peripheral artery disease. Circulation. 1997;95:1998–2002. doi: 10.1161/01.cir.95.8.1998. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell R.N., Libby P. Vascular remodeling in transplant vasculopathy. Circ. Res. 2007;100:967–978. doi: 10.1161/01.RES.0000261982.76892.09. [DOI] [PubMed] [Google Scholar]
- 4.Thyberg J. Phenotypic modulation of smooth muscle cells during formation of neointimal thickenings following vascular injury. Histol. Histopathol. 1998;13:871–891. doi: 10.14670/HH-13.871. [DOI] [PubMed] [Google Scholar]
- 5.Owens G.K., Kumar M.S., Wamhoff B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 6.Bennett M.R., Sinha S., Owens G.K. Vascular smooth muscle cells in atherosclerosis. Circ. Res. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geary R.L., Wong J.M., Rossini A., Schwartz S.M., Adams L.D. Expression profiling identifies 147 genes contributing to a unique primate neointimal smooth muscle cell phenotype. Arterioscler. Thromb. Vasc. Biol. 2002;22:2010–2016. doi: 10.1161/01.atv.0000038147.93527.35. [DOI] [PubMed] [Google Scholar]
- 8.Miano J.M. Myocardin in biology and disease. J. Biomed. Res. 2015;29:3–19. doi: 10.7555/JBR.29.20140151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long X., Miano J.M. Transforming growth factor-beta1 (tgf-beta1) utilizes distinct pathways for the transcriptional activation of microrna 143/145 in human coronary artery smooth muscle cells. J. Biol. Chem. 2011;286:30119–30129. doi: 10.1074/jbc.M111.258814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu R., Jin Y., Tang W.H., Qin L., Zhang X., Tellides G., Hwa J., Yu J., Martin K.A. Ten-eleven translocation-2 (tet2) is a master regulator of smooth muscle cell plasticity. Circulation. 2013;128:2047–2057. doi: 10.1161/CIRCULATIONAHA.113.002887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong X., Hou X., Jourd'heuil D., Weisbrod R.M., Cohen R.A. Upregulation of nox4 by tgf{beta}1 oxidizes serca and inhibits no in arterial smooth muscle of the prediabetic zucker rat. Circ. Res. 2010;107:975–983. doi: 10.1161/CIRCRESAHA.110.221242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Marco E., Gray S.P., Kennedy K., Szyndralewiez C., Lyle A.N., Lassegue B., Griendling K.K., Cooper M.E., Schmidt H., Jandeleit-Dahm K.A.M. Nox4-derived reactive oxygen species limit fibrosis and inhibit proliferation of vascular smooth muscle cells in diabetic atherosclerosis. Free Radic. Biol. Med. 2016;97:556–567. doi: 10.1016/j.freeradbiomed.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginnan R., Jourd'heuil F.L., Guikema B., Simons M., Singer H.A., Jourd'heuil D. Nadph oxidase 4 is required for interleukin-1beta-mediated activation of protein kinase cdelta and downstream activation of c-jun n-terminal kinase signaling in smooth muscle. Free Radic. Biol. Med. 2013;54:125–134. doi: 10.1016/j.freeradbiomed.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong X., Khandelwal A.R., Qin Z., Wu X., Chen L., Ago T., Sadoshima J., Cohen R.A. Role of smooth muscle nox4-based nadph oxidase in neointimal hyperplasia. J. Mol. Cell. Cardiol. 2015;89:185–194. doi: 10.1016/j.yjmcc.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Martin-Garrido A., Brown D.I., Lyle A.N., Dikalova A., Seidel-Rogol B., Lassegue B., San Martin A., Griendling K.K. Nadph oxidase 4 mediates tgf-beta-induced smooth muscle alpha-actin via p38mapk and serum response factor. Free Radic. Biol. Med. 2011;50:354–362. doi: 10.1016/j.freeradbiomed.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta J., Nebreda A.R. Roles of p38alpha mitogen-activated protein kinase in mouse models of inflammatory diseases and cancer. FEBS J. 2015;282:1841–1857. doi: 10.1111/febs.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corre I., Paris F., Huot J. The p38 pathway, a major pleiotropic cascade that transduces stress and metastatic signals in endothelial cells. Oncotarget. 2017;8:55684–55714. doi: 10.18632/oncotarget.18264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuadrado A., Nebreda A.R. Mechanisms and functions of p38 mapk signalling. Biochem. J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 19.Adams R.H., Porras A., Alonso G., Jones M., Vintersten K., Panelli S., Valladares A., Perez L., Klein R., Nebreda A.R. Essential role of p38alpha map kinase in placental but not embryonic cardiovascular development. Mol. Cell. 2000;6:109–116. [PubMed] [Google Scholar]
- 20.Cuenda A., Rousseau S. P38 map-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta. 2007;1773:1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Kang Y.J., Chen J., Otsuka M., Mols J., Ren S., Wang Y., Han J. Macrophage deletion of p38alpha partially impairs lipopolysaccharide-induced cellular activation. J. Immunol. 2008;180:5075–5082. doi: 10.4049/jimmunol.180.7.5075. (Baltim., Md.: 1950) [DOI] [PubMed] [Google Scholar]
- 22.Seimon T.A., Wang Y., Han S., Senokuchi T., Schrijvers D.M., Kuriakose G., Tall A.R., Tabas I.A. Macrophage deficiency of p38alpha mapk promotes apoptosis and plaque necrosis in advanced atherosclerotic lesions in mice. J. Clin. Investig. 2009;119:886–898. doi: 10.1172/JCI37262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long X., Cowan S.L., Miano J.M. Mitogen-activated protein kinase 14 is a novel negative regulatory switch for the vascular smooth muscle cell contractile gene program. Arterioscler. Thromb. Vasc. Biol. 2013;33:378–386. doi: 10.1161/ATVBAHA.112.300645. [DOI] [PubMed] [Google Scholar]
- 24.Wirth A., Benyo Z., Lukasova M., Leutgeb B., Wettschureck N., Gorbey S., Orsy P., Horvath B., Maser-Gluth C., Greiner E., Lemmer B., Schutz G., Gutkind J.S., Offermanns S. G12-g13-larg-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat. Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 25.Nishida K., Yamaguchi O., Hirotani S., Hikoso S., Higuchi Y., Watanabe T., Takeda T., Osuka S., Morita T., Kondoh G., Uno Y., Kashiwase K., Taniike M., Nakai A., Matsumura Y., Miyazaki J., Sudo T., Hongo K., Kusakari Y., Kurihara S., Chien K.R., Takeda J., Hori M., Otsu K. P38alpha mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol. Cell. Biol. 2004;24:10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai Y., Nagel D.J., Zhou Q., Cygnar K.D., Zhao H., Li F., Pi X., Knight P.A., Yan C. Role of camp-phosphodiesterase 1c signaling in regulating growth factor receptor stability, vascular smooth muscle cell growth, migration, and neointimal hyperplasia. Circ. Res. 2015;116:1120–1132. doi: 10.1161/CIRCRESAHA.116.304408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long X., Slivano O.J., Cowan S.L., Georger M.A., Lee T.H., Miano J.M. Smooth muscle calponin: an unconventional carg-dependent gene that antagonizes neointimal formation. Arterioscler. Thromb. Vasc. Biol. 2011;31:2172–2180. doi: 10.1161/ATVBAHA.111.232785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao J., Wu W., Zhang W., Lu Y.W., Tou E., Ye J., Gao P., Jourd'heuil D., Singer H.A., Wu M., Long X. Selective expression of tspan2 in vascular smooth muscle is independently regulated by tgf-beta1/smad and myocardin/serum response factor. FASEB J. 2017 doi: 10.1096/fj.201601021R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell R.D., Long X., Lin M., Bergmann J.H., Nanda V., Cowan S.L., Zhou Q., Han Y., Spector D.L., Zheng D., Miano J.M. Identification and initial functional characterization of a human vascular cell-enriched long noncoding rna. Arterioscler. Thromb. Vasc. Biol. 2014;34:1249–1259. doi: 10.1161/ATVBAHA.114.303240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao J., Jourd'heuil F.L., Xue M., Conti D., Lopez-Soler R.I., Ginnan R., Asif A., Singer H.A., Jourd'heuil D., Long X. Dual function for mature vascular smooth muscle cells during arteriovenous fistula remodeling. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chappell J., Harman J.L., Narasimhan V.M., Yu H., Foote K., Simons B.D., Bennett M.R., Jorgensen H.F. Extensive proliferation of a subset of differentiated, yet plastic, medial vascular smooth muscle cells contributes to neointimal formation in mouse injury and atherosclerosis models. Circ. Res. 2016;119:1313–1323. doi: 10.1161/CIRCRESAHA.116.309799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herring B.P., Hoggatt A.M., Burlak C., Offermanns S. Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury. Vasc. Cell. 2014;6:21. doi: 10.1186/2045-824X-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jourd'heuil F.L., Xu H., Reilly T., McKellar K., El Alaoui C., Steppich J., Liu Y.F., Zhao W., Ginnan R., Conti D., Lopez-Soler R., Asif A., Keller R.K., Schwarz J.J., Thanh Thuy L.T., Kawada N., Long X., Singer H.A., Jourd'heuil D. The hemoglobin homolog cytoglobin in smooth muscle inhibits apoptosis and regulates vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 2017;37:1944–1955. doi: 10.1161/ATVBAHA.117.309410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leeper N.J., Raiesdana A., Kojima Y., Kundu R.K., Cheng H., Maegdefessel L., Toh R., Ahn G.O., Ali Z.A., Anderson D.R., Miller C.L., Roberts S.C., Spin J.M., de Almeida P.E., Wu J.C., Xu B., Cheng K., Quertermous M., Kundu S., Kortekaas K.E., Berzin E., Downing K.P., Dalman R.L., Tsao P.S., Schadt E.E., Owens G.K., Quertermous T. Loss of cdkn2b promotes p53-dependent smooth muscle cell apoptosis and aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 2013;33:e1–e10. doi: 10.1161/ATVBAHA.112.300399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herring B.P., Hoggatt A.M., Griffith S.L., McClintick J.N., Gallagher P.J. Inflammation and vascular smooth muscle cell dedifferentiation following carotid artery ligation. Physiol. Genom. 2017;49:115–126. doi: 10.1152/physiolgenomics.00095.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fedorov A., Kostareva A., Raud J., Roy J., Hedin U., Razuvaev A. Early changes of gene expression profiles in the rat model of arterial injury. J. Vasc. Interv. Radiol.: JVIR. 2014;25:789–796. doi: 10.1016/j.jvir.2013.11.031. (e787) [DOI] [PubMed] [Google Scholar]
- 37.Kiene L.S., Homann S., Suvorava T., Rabausch B., Muller J., Kojda G., Kretschmer I., Twarock S., Dai G., Deenen R., Hartwig S., Lehr S., Kohrer K., Savani R.C., Grandoch M., Fischer J.W. Deletion of hyaluronan synthase 3 inhibits neointimal hyperplasia in mice. Arterioscler. Thromb. Vasc. Biol. 2016;36:e9–e16. doi: 10.1161/ATVBAHA.115.306607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freund A., Patil C.K., Campisi J. P38mapk is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li D.J., Fu H., Tong J., Li Y.H., Qu L.F., Wang P., Shen F.M. Cholinergic anti-inflammatory pathway inhibits neointimal hyperplasia by suppressing inflammation and oxidative stress. Redox Biol. 2018;15:22–33. doi: 10.1016/j.redox.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bahnson E.S., Koo N., Cantu-Medellin N., Tsui A.Y., Havelka G.E., Vercammen J.M., Jiang Q., Kelley E.E., Kibbe M.R. Nitric oxide inhibits neointimal hyperplasia following vascular injury via differential, cell-specific modulation of sod-1 in the arterial wall. Nitric oxide: Biol. Chem. 2015;44:8–17. doi: 10.1016/j.niox.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacob T., Ascher E., Alapat D., Olevskaia Y., Hingorani A. Activation of p38mapk signaling cascade in a vsmc injury model: role of p38mapk inhibitors in limiting vsmc proliferation. Eur. J. Vasc. Endovasc. Surg.: Off. J. Eur. Soc. Vasc. Surg. 2005;29:470–478. doi: 10.1016/j.ejvs.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 42.Ohashi N., Matsumori A., Furukawa Y., Ono K., Okada M., Iwasaki A., Miyamoto T., Nakano A., Sasayama S. Role of p38 mitogen-activated protein kinase in neointimal hyperplasia after vascular injury. Arterioscler. Thromb. Vasc. Biol. 2000;20:2521–2526. doi: 10.1161/01.atv.20.12.2521. [DOI] [PubMed] [Google Scholar]
- 43.Lawson S.K., Dobrikova E.Y., Shveygert M., Gromeier M. P38alpha mitogen-activated protein kinase depletion and repression of signal transduction to translation machinery by mir-124 and -128 in neurons. Mol. Cell. Biol. 2013;33:127–135. doi: 10.1128/MCB.00695-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naka-Kaneda H., Nakamura S., Igarashi M., Aoi H., Kanki H., Tsuyama J., Tsutsumi S., Aburatani H., Shimazaki T., Okano H. The mir-17/106-p38 axis is a key regulator of the neurogenic-to-gliogenic transition in developing neural stem/progenitor cells. Proc. Natl. Acad. Sci. USA. 2014;111:1604–1609. doi: 10.1073/pnas.1315567111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan G., Niu J., Shi Y., Ouyang H., Wu Z.H. Nf-kappab-dependent microrna-125b up-regulation promotes cell survival by targeting p38alpha upon ultraviolet radiation. J. Biol. Chem. 2012;287:33036–33047. doi: 10.1074/jbc.M112.383273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mateescu B., Batista L., Cardon M., Gruosso T., de Feraudy Y., Mariani O., Nicolas A., Meyniel J.P., Cottu P., Sastre-Garau X., Mechta-Grigoriou F. Mir-141 and mir-200a act on ovarian tumorigenesis by controlling oxidative stress response. Nat. Med. 2011;17:1627–1635. doi: 10.1038/nm.2512. [DOI] [PubMed] [Google Scholar]
- 47.Zhang P., Cao L., Fan P., Mei Y., Wu M. LncRNA-MIF, a c-myc-activated long non-coding rna, suppresses glycolysis by promoting fbxw7-mediated c-myc degradation. EMBO Rep. 2016;17:1204–1220. doi: 10.15252/embr.201642067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huan J., Xing L., Lin Q., Xui H., Qin X. Long noncoding rna crnde activates wnt/beta-catenin signaling pathway through acting as a molecular sponge of microrna-136 in human breast cancer. Am. J. Transl. Res. 2017;9:1977–1989. [PMC free article] [PubMed] [Google Scholar]
- 49.Daniel J.M., Bielenberg W., Stieger P., Weinert S., Tillmanns H., Sedding D.G. Time-course analysis on the differentiation of bone marrow-derived progenitor cells into smooth muscle cells during neointima formation. Arterioscler. Thromb. Vasc. Biol. 2010;30:1890–1896. doi: 10.1161/ATVBAHA.110.209692. [DOI] [PubMed] [Google Scholar]
- 50.Shankman L.S., Gomez D., Cherepanova O.A., Salmon M., Alencar G.F., Haskins R.M., Swiatlowska P., Newman A.A., Greene E.S., Straub A.C., Isakson B., Randolph G.J., Owens G.K. Klf4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat. Med. 2015;21:628–637. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang X., Coriolan D., Murthy V., Schultz K., Golenbock D.T., Beasley D. Proinflammatory phenotype of vascular smooth muscle cells: role of efficient toll-like receptor 4 signaling. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H1069–H1076. doi: 10.1152/ajpheart.00143.2005. [DOI] [PubMed] [Google Scholar]
- 52.Orr A.W., Hastings N.E., Blackman B.R., Wamhoff B.R. Complex regulation and function of the inflammatory smooth muscle cell phenotype in atherosclerosis. J. Vasc. Res. 2010;47:168–180. doi: 10.1159/000250095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moehle C.W., Bhamidipati C.M., Alexander M.R., Mehta G.S., Irvine J.N., Salmon M., Upchurch G.R., Jr., Kron I.L., Owens G.K., Ailawadi G. Bone marrow-derived mcp1 required for experimental aortic aneurysm formation and smooth muscle phenotypic modulation. J. Thorac. Cardiovasc. Surg. 2011;142:1567–1574. doi: 10.1016/j.jtcvs.2011.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ali M.S., Starke R.M., Jabbour P.M., Tjoumakaris S.I., Gonzalez L.F., Rosenwasser R.H., Owens G.K., Koch W.J., Greig N.H., Dumont A.S. Tnf-alpha induces phenotypic modulation in cerebral vascular smooth muscle cells: implications for cerebral aneurysm pathology. J. Cereb. Blood Flow. Metab.: Off. J. Int. Soc. Cereb. Blood Flow. Metab. 2013;33:1564–1573. doi: 10.1038/jcbfm.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clempus R.E., Sorescu D., Dikalova A.E., Pounkova L., Jo P., Sorescu G.P., Schmidt H.H., Lassegue B., Griendling K.K. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler. Thromb. Vasc. Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brichkina A., Bertero T., Loh H.M., Nguyen N.T., Emelyanov A., Rigade S., Ilie M., Hofman P., Gaggioli C., Bulavin D.V. P38mapk builds a hyaluronan cancer niche to drive lung tumorigenesis. Genes Dev. 2016;30:2623–2636. doi: 10.1101/gad.290346.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kashima Y., Takahashi M., Shiba Y., Itano N., Izawa A., Koyama J., Nakayama J., Taniguchi S., Kimata K., Ikeda U. Crucial role of hyaluronan in neointimal formation after vascular injury. PloS One. 2013;8:e58760. doi: 10.1371/journal.pone.0058760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ehyai S., Dionyssiou M.G., Gordon J.W., Williams D., Siu K.W., McDermott J.C. A p38 mitogen-activated protein kinase-regulated myocyte enhancer factor 2-beta-catenin interaction enhances canonical wnt signaling. Mol. Cell. Biol. 2015;36:330–346. doi: 10.1128/MCB.00832-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bachstetter A.D., Van Eldik L.J. The p38 map kinase family as regulators of proinflammatory cytokine production in degenerative diseases of the CNS. Aging Dis. 2010;1:199–211. [PMC free article] [PubMed] [Google Scholar]
- 60.Yoon Y.M., Lee J.Y., Yoo D., Sim Y.S., Kim Y.J., Oh Y.K., Kang J.S., Kim S., Kim J.S., Kim J.M. Bacteroides fragilis enterotoxin induces human beta-defensin-2 expression in intestinal epithelial cells via a mitogen-activated protein kinase/i kappab kinase/nf-kappab-dependent pathway. Infect. Immun. 2010;78:2024–2033. doi: 10.1128/IAI.00118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Z., Lv Y., Zhang Y., Liu F., Zhu L., Pan S., Qiu C., Guo Y., Yang T., Wang J. Matrine-type alkaloids inhibit advanced glycation end products induced reactive oxygen species-mediated apoptosis of aortic endothelial cells in vivo and in vitro by targeting mkk3 and p38mapk signaling. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.007441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coleman P.R., Chang G., Hutas G., Grimshaw M., Vadas M.A., Gamble J.R. Age-associated stresses induce an anti-inflammatory senescent phenotype in endothelial cells. Aging. 2013;5:913–924. doi: 10.18632/aging.100622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patnaik A., Haluska P., Tolcher A.W., Erlichman C., Papadopoulos K.P., Lensing J.L., Beeram M., Molina J.R., Rasco D.W., Arcos R.R., Kelly C.S., Wijayawardana S.R., Zhang X., Stancato L.F., Bell R., Shi P., Kulanthaivel P., Pitou C., Mulle L.B., Farrington D.L., Chan E.M., Goetz M.P. A first-in-human phase i study of the oral p38 mapk inhibitor, ralimetinib (ly2228820 dimesylate), in patients with advanced cancer. Clin. Cancer Res.: Off. J. Am. Assoc. Cancer Res. 2016;22:1095–1102. doi: 10.1158/1078-0432.CCR-15-1718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material