Abstract
Aim of the study
Rosette-forming glioneuronal tumour (RGNT) of the fourth ventricle is an uncommon tumour. The management is not consensual. Most of the published cases show stable outcome with and without gross total resection and are regarded as having a relatively indolent behaviour.
Material and methods
We present a 32-year-old man with a tumour in the fourth ventricle. He underwent midline suboccipital craniectomy with gross total removal.
Results
The histopathological diagnosis was RGNT grade I. Four years later he presented a radiological progression and received stereotactic radiosurgery. At the last follow-up seven years after surgery, the MRI showed no recurrence.
Conclusions
RGNT should be considered in the differential diagnosis of a posterior fossa tumour and has to be differentiated from other lesions for its indolent course and favourable prognosis. Surgical procedures should be carefully performed to avoid serious surgical morbidities. Stereotactic radiosurgery treatment appears to be a useful treatment in recurrence episodes.
Keywords: rosette-forming glioneuronal tumour, fourth ventricle, posterior fossa, radiosurgery
Introduction
Rosette-forming glioneuronal tumour of the fourth ventricle (RGNT) is an uncommon tumour [1–5]. These tumours comprise both neural and glial components [2]. Initially it was described as dysembryoplastic neuroepithelial tumour of the cerebellum arising in relation to the wall of the fourth ventricle, typically in a midline location [6] with possible extension into surrounding structures. Cases involving other sites of the central nervous system including cerebellum, brainstem, pineal region, optic chiasm, hypothalamus, and cervical spinal cord have been reported [1, 2, 6–9]. In the latest 2016 WHO classification it is recognised as a neural and mixed neuronal glial tumour [10].
Case report
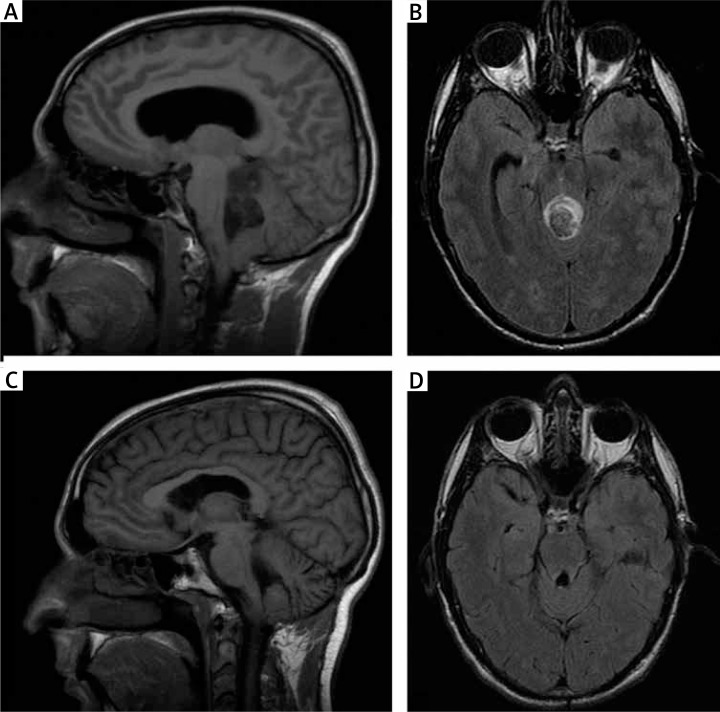
A 32-year-old man was referred because of serious vertigo, dizziness, vomiting, and headache evolving for weeks. On physical examination, left sixth nerve palsy with diplopia, nystagmus, and ataxia was noticed. The initial computerised tomography scan revealed a fourth ventricle mass with intralesional bleeding and calcification areas. The solid component showed patchy enhancement. An obstructive hydrocephalus was also observed. An emergent endoscopic third ventriculostomy and external ventricular drainage insertion were performed to improve the neurological status of the patient. Cranial magnetic resonance imaging (MRI) confirmed a lesion in the fourth ventricle with extent to the supravermian cistern and the posteroinferior third ventricle with haematoma inside and without significant peritumoral oedema. Postcontrast images revealed minimal enhancement (Fig. 1A and 1B).
Fig. 1.
A–B) Preoperative MRI. T1-weighted sagittal and T2-weighted axial images demonstrating the tumour mass with a cystic component and extension into the floor of the fourth ventricle and to the supravermian cistern. Partial obstruction of the fourth ventricle and secondary obstructive hydrocephalus is also observed. C–D) Two-year postoperative MRI. No apparent residual tumour is shown at T1-weighted sagittal and T2-weighted axial images
The patient underwent midline suboccipital craniectomy with multimodal intraoperative neurophysiological monitoring. The solid part of the tumour was exposed through telovelar split approach. Intraoperative findings were suggestive of an intra-axial solid tumour arising from the rhomboid fossa. It was scarcely vascular, soft, and suckable. Gross total removal of the solid part was performed.
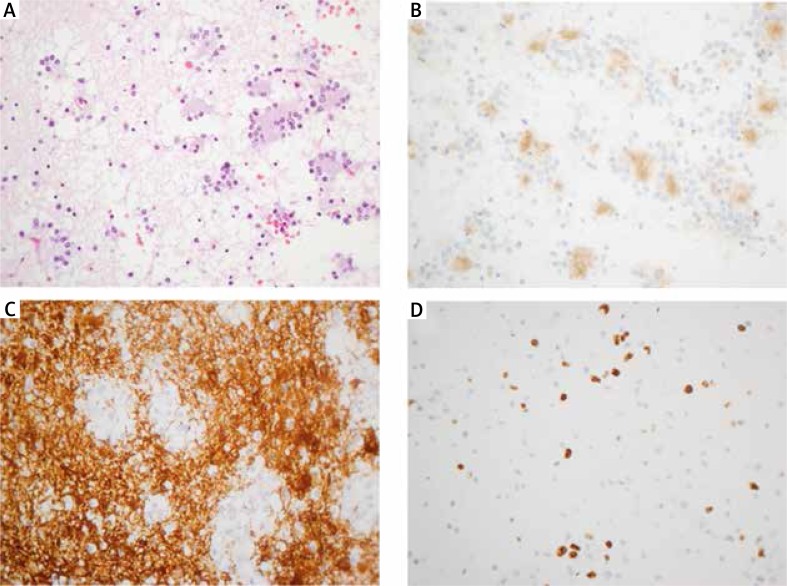
Microscopically the tumour was composed of low-density cellular proliferation with both astrocytic and neurocytic components. The neurocytic cells formed neurocytic rosettes with eosinophilic centres and perivascular pseudorosettes. The cores of these neurocytic rosettes showed positive immunostaining for synaptophysin and negative for epithelial membrane antigen (EMA). The astrocytic component showed glial fibrillary acidic protein (GFAP) positivity. There were no necrosis areas or vascular proliferation. Ki67 proliferative index was very low (less than 1%) in both glial and neuronal components. Based on these features, a diagnosis of RGNT grade I was made (Fig. 2).
Fig. 2.
Microscopic features of the rosette-forming glioneuronal tumour. A) Histological features of the tumour showing both a neurocytic and an astrocytic component. Neurocytic rosettes are formed by the neurocytic components. B) The eosinophilic core at the centre of the neurocytic rosettes displays strong positive staining with synaptophysin. C) The astrocytic components of the tumour showed that the tumour cells had bipolar and spindle processes with positive immunostaining of GFAP. D) The MIB-1 labelling index was about 5–7%. Original magnifications 400×
Postoperatively the patient showed a left facial nerve palsy, nystagmus, and signs of ataxia. Postoperative brain MRI showed postsurgical changes with apparently gross total resection. He required rehabilitation treatment, and he improved subsequently. MRI two years after surgery revealed no residual tumour (Fig. 1C and 1D). T2-weighted images showed gliotic changes in the tectal area and a high-intensity area in both olivary nuclei. This olivary lesion probably represented a hypertrophic olivary degeneration related to a lesion in the Guillain-Mollaret triangle.
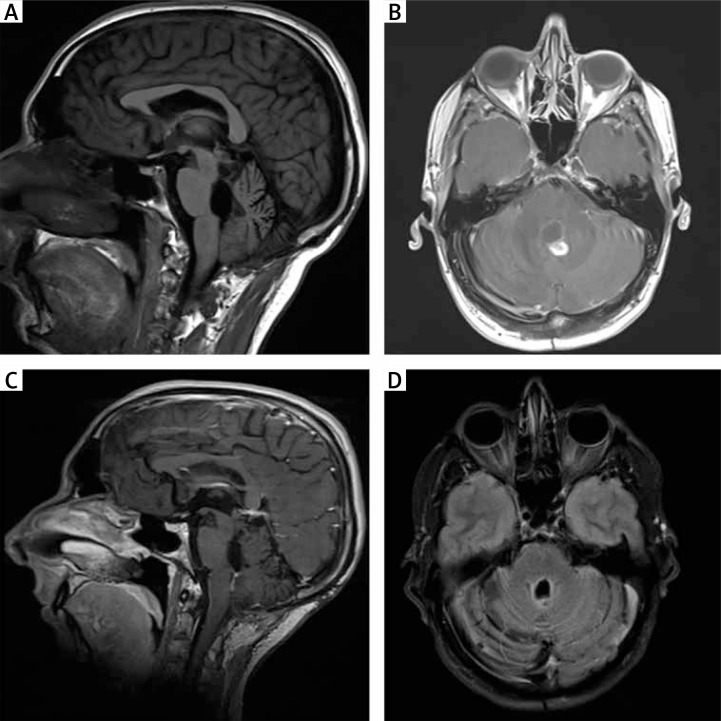
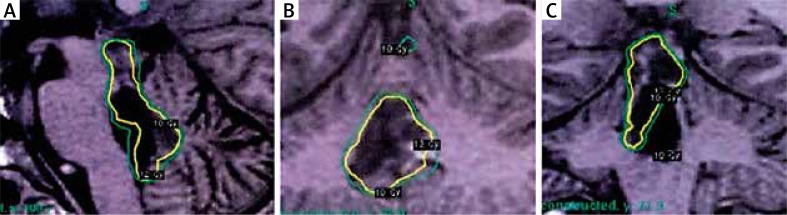
Control MRI after four years revealed a nodular enhancing 10-mm lesion with increased angiogenesis close to the roof of the fourth ventricle, without perilesional oedema, and a second lesion 13 mm in size, close to the posterior edge of the quadrigeminal lamina (Fig. 3A and 3B). The patient evolved without evidence of progressive clinical deterioration. Base on the radiological findings, he received radiosurgery. The treatment dose was 24 Gy, and it was delivered using a gamma knife through 34 shots in one fraction. The tissue volume included in the isodose area was 5.32 cc (Fig. 4). The treatment was well tolerated. A new control MRI scan after radiosurgery showed smaller size of the lesions without enhancement after the administration of contrast.
Fig. 3.
A–B) MRI scans 48 months after the initial procedure. T1-weighted sagittal and T1-weighted axial contrast enhanced images reveal a nodular lesion close to the roof of the fourth ventricle. C–D) T1-weighted sagittal and T2-weighted axial MRI images two years after radiosurgery show stabilisation of the lesions
Fig. 4.
Treatment planning for gamma knife radiosurgery. A) Sagittal view. B) Axial view. C) Coronal view
At the latest follow-up seven years after the initial diagnosis, the patient was neurologically and radiologically stable (Fig. 3C and 3D).
Discussion
The term RGNT was first described by Komori in 2002 [11]. Until recently only about 47 cases were reported in the literature with only short follow-up [2, 7]. A review of the literature published by Hakan [9] in 2016 reported only four cases of recurrence. We observed a recurrence in our patient four years after total resection.
This tumour appears to have a high propensity for significant adherence to the ventricular wall and infiltration into surrounding structures [12]. RGNT has a slight female predominance, and most patients are in their third decade of life [1, 6, 9].
Headache and cerebellar signs such as ataxia and nystagmus are the most common initial clinical symptoms. Diplopia, dizziness, vomiting, and sings and symptoms due to increased intracranial pressure may also be seen. A three ventricular hydrocephalus is frequent, and it may require emergent insertion of an external ventricular drain. Fortunately, most of the tumours are slow growing lesions and the development of ventriculomegaly may be clinically compensated [9].
Radiologically, RGNT presents as iso- to hypo-intense on T1-weighted and hyperintense on T2-weighted MRI images. The tumour is well circumscribed, with solid and cystic components. Variable degrees of contrast enhancement and calcification may also be seen [1, 13]. Secondary hydrocephalus can be found. Satellite lesions have been detected.
The management is not consensual because the incidence of these tumours is very low (Table 1). However, surgical removal aiming at gross total removal seems preferable [8, 9]. An aggressive approach can increase surgical morbidity, so subtotal removal may be chosen. Postoperative neurological deficits are noted in almost 50% of cases: the most common postoperative deficits are ataxia, cranial nerve palsy, and diplopia [9].
Table 1.
Summary of publications
| Author | Type | Treatment | Follow-up |
|---|---|---|---|
| Zanabria et al. 2013 | Case report | Surgery | |
| Zhang et al. 2013 | Case base update, 41 cases | Surgery or biopsy Two cases: radiotherapy | 20 months |
| Schalamann et al. 2014 | Meta-analysis, 85 cases | Surgery Radiotherapy in 3 patients | 1, 2 years |
| Garcia Cabeza et al. 2015 | Case report | Surgery/Temozolomide/Radiotherapy | 2 years |
| Allison et al. 2015 | Case report | Surgery/Radiotherapy | |
| Beuriat el al. 2016 | Case base update, 32 cases | Surgery Two cases: biopsy, chemotherapy and radiotherapy | 20 months |
| Hakan et al. 2016 | Case report | Surgery | 32 months |
The histopathological features are mixed glial and neuronal elements [1]. The neurocytic components are composed of neurocytic and perivascular rosettes dispersed in a microcystic myxoid matrix admixed with pilocytic astrocytes [3]. The presence of Rosenthal fibres and eosinophilic granular bodies in the glial component should orient the diagnosis to a RGNT [8].
Hypertrophic olivary degeneration was evident in our patient two years after the surgery. We have found another case reported in the literature with these radiological changes [12]. The Guillain-Mollaret triangle is a triangular circuit connecting the dentate nucleus of the cerebellum on one side with the red nucleus and inferior olive contralaterally. Hypertrophic olivary degeneration is trans-synaptic degeneration and it occurs because of interruption of the pathways in the circuit of the Guillain-Mollaret triangle.
Most of the published cases show stable outcome with and without gross total resection. RGNT are regarded as having relatively indolent behaviour [1, 4, 5] with no evidence of progression at an average of two years of follow-up [14]. No adjuvant therapy seems to be needed even in cases of subtotal resection [11]. However, there are few cases in the literature that present progression [4, 5, 14] after receiving focal radiotherapy.
We present an eight-years follow-up case with total surgical resection, recurrence, and good response after stereotactic radiosurgery. Long-term follow-up studies are required in order to better determine the prognosis and the best therapeutic strategies in these patients.
Conclusions
Despite its rarity, RGNT should always be considered in the differential diagnosis of tumours occurring in the fourth ventricle in a young adult. It has an indolent course and favourable prognosis. A suboccipital craniotomy is the treatment of choice for symptomatic tumours. Surgical procedures should be carefully performed to avoid serious surgical morbidities associated with the lesion invading vital structures. The telovelar approach may minimise the risk of complications. Radiotherapy may be considered for patients with tumour recurrence. A long follow-up is needed because recurrence or metastatic progression exists especially in subtotal resection cases.
References
- 1.Allison KSJ, O´Donovan DG, Jena R, Coss JJ, Santarius TS. Rosette-forming glioneuronal tumor with dissemination throughout the ventricular system: a case report. Clin Neuropathol. 2015;34:64–69. doi: 10.5414/NP300682. [DOI] [PubMed] [Google Scholar]
- 2.García Cabezas S, Serrano Blanch R, Sanchez-Sanchez R, Palacios Eito A. Rosette-forming glioneuronal tumor (RGNT) of the fourth ventricle: a highly aggressive case. Brain Tumor Pathol. 2015;32:124–130. doi: 10.1007/s10014-014-0195-z. [DOI] [PubMed] [Google Scholar]
- 3.Nai AR, Gopalakrishnan CV, Kapilamoorthy TR, Radhakrishnan N. Rosette forming glioneuronal tumor of the fourth ventricle in squash cytology smear. J Cytol. 2015;31:215–257. doi: 10.4103/0970-9371.151138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlamann A, von Bueren A, Hagel C, Zwiener I, Seidel C, Kortmann RD, Müller K. An individual patient data meta-analysis on characteristics and outcome of patients with papillary glioneuronal tumor, rosette glioneuronal tumor with neuropil-like island and rosette forming glioneuronal tumor of the fourth ventricle. PLoS ONE. 2014;9:e101211. doi: 10.1371/journal.pone.0101211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zanabria Ortiz R, Domínguez Báez JJ, Lazo Fernández E, Sánchez Medina Y, Gómez Perals LF, Pérez del Rosario P. Rosette-forming glioneuronal tumor of the fourth ventricle. Case report and literature review. Neurocirugia (Astur) 2013;24:172–177. doi: 10.1016/j.neucir.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Yin B, Lui L, Chen XR, LI K, Geng DY. Rosette-forming glioneuronal tumor of the fourth ventricle. J Neuroradiol. 2012;39:129–130. doi: 10.1016/j.neurad.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Bera G, Das A, Chatterjee S, Chatterjee U. Rossete-forming Glioneuronal Tumor: A rare posterior fossa tumor in an adolescent. J Pediatric Neurosci. 2007;12:168–191. doi: 10.4103/jpn.JPN_12_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beuriat PA, Tauziede-Espariat A, Pages M, Varlet P, Di Rocco F. Rosette-forming glioneuronal tumor outside the fourth ventricle: a case-based update. Childs Nerv Syst. 2016;32:65–68. doi: 10.1007/s00381-015-2922-0. [DOI] [PubMed] [Google Scholar]
- 9.Hakan T, Aker FV. Rosette-forming glioneuronal tumour of the fourth ventricle: case report and review of the literature. Folia Neuropathol. 2016;54:80–87. doi: 10.5114/fn.2016.58919. [DOI] [PubMed] [Google Scholar]
- 10.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 11.Komori T, Scheithauer BW, Hirosi T. A rosette-forming glioneuronal tumor of the fourth ventricle. Infratentorial form of dysembrioplastic neuroepithelial tumor? Am J Surg Pathol. 2002;26:582–591. doi: 10.1097/00000478-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Fushimi Y, Miyasaki A, Taki H, Aoyama K, Hirato J, Kanagaki M, Togashi K. Rosette-forming glioneuronal tumor of the fourth ventricle with bilateral olivary degeneration. Jpn J Radiol. 2011;29:445. doi: 10.1007/s11604-011-0566-x. [DOI] [PubMed] [Google Scholar]
- 13.Hainfellner JA. Rosette forming glioneuronal tumour of the fourth ventricle. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. 4th ed. Lyon: International Agency for Research on Cancer; 2007. pp. 113–114. [Google Scholar]
- 14.Zhang J, Babu R, McLendon RE, Friedman AH, Adamson C. A comprehensive analysis of 41 patients with rosette-forming glioneuronal tumors of the fourth ventricle. J Clin Neurosci. 2013;20:335–341. doi: 10.1016/j.jocn.2012.09.003. [DOI] [PubMed] [Google Scholar]